Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Almost any inflammatory dermatological condition occurring on nongenital skin can afflict the vulva. Histopathologic diagnosis in the vulva, however, is often complicated by its unique anatomy (where there is increased occlusion, friction, and moisture, causing a variety of secondary changes), delayed seeking of medical attention due to patient embarrassment, and the use of self-treatment regimens which may not be readily divulged to the treating clinician. Moreover, vulvar dermatoses exhibit varying histologic features depending on the course of the disease and when the biopsy was taken. A patient may have endured multiple trials of recalcitrant treatments before a biopsy is eventually obtained. In the setting of vulvar inflammatory dermatoses, it must be emphasized that clinical information is of utmost importance to arrive at an accurate diagnosis.
The initial histologic approach to any inflammatory disorder of the skin or mucosa requires knowledge of the precise anatomic location and an appreciation of the alterations in the regional mucocutaneous anatomy. The mons pubis and labia majora closely resemble skin from other anatomic regions of the body and are composed of a slightly rugose, keratinizing, stratified epithelium containing all of the cutaneous adnexal structures and a richly vascular dermis. Areas of compact stratum corneum, in addition to the conventional basket-weave stratum corneum, are a normal finding in the mons pubis/labia majora. The labia minora, in contrast, have a stratified, glycogen-rich squamous epithelium. Adnexal structures are absent. On each side, the mucocutaneous junction between the labium majus and labium minus (also known as Hart’s line) may exhibit a focal zone of parakeratosis and should not be mistaken for a pathologic abnormality. The subjacent dermis is highly vascular and contains erectile tissue.
The next step in evaluating an inflammatory process is the identification at low power of the major tissue reaction pattern , followed by the pattern of inflammation . Detailed descriptions of this approach, championed initially in the teachings of Wallace Clark, have been further popularized and refined by Ackerman, Weedon, and LeBoit. The tissue reaction pattern is a distinctive group of morphologic findings which allows the observer to place a biopsy within a specific group of cutaneous diseases. This rational framework was also adopted by the 2006 International Society for the Study of Vulvovaginal Disease (ISSVD) classification of vulvar dermatoses ( Table 1.1 ). The pattern of inflammation refers to the distribution of the inflammatory infiltrate within the dermis and subcutis ( Box 1.1 ). Once the major tissue reaction pattern and pattern of inflammation are identified, the pathologist can generate a working differential diagnosis ( Table 1.1 ). In 2011, the ISSVD published a separate clinically oriented framework to classify vulvar dermatoses, allowing the clinician to generate a differential diagnosis based on macroscopic features ( Table 1.2 ). This 2011 ISSVD framework serves as an adjunct tool to aid in diagnosis and was meant to supplement, not supplant, the 2006 classification scheme.
| Pattern | Characteristic Morphologic Feature | Clinical Correlation |
|---|---|---|
| Spongiotic (Eczematous) Pattern | Epidermal edema | Atopic dermatitis Allergic dermatitis Irritant contact dermatitis |
| Acanthotic Pattern | Epidermal hyperplasia | Psoriasis Lichen simplex chronicus
|
| Lichenoid Pattern | Band-like lymphocytic infiltrate at dermal–epidermal junction with basal keratinocyte damage | Lichen sclerosus Lichen planus Erythema multiforme |
| Dermal Homogenization/ Sclerosis Pattern | Dermal sclerosis | Lichen sclerosus |
| Vesiculobullous Pattern | Subepidermal or intraepidermal blister formation | Pemphigoid, cicatricial type Linear IgA disease |
| Acantholytic Pattern | Clefting of epithelial cells due to breakage of intercellular junctions | Hailey-Hailey disease Darier disease Papular genitocrural acantholysis |
| Granulomatous Pattern | Granulomas | Crohn disease Melkersson-Rosenthal syndrome Tuberculosis |
| Vasculopathic Pattern | Vascular injury | Aphthous ulcers (Non-sexually related acute genital ulcers, aka Lipschutz ulcer) Behcet disease Plasma cell vulvitis |
Superficial perivascular inflammation
Superficial and deep perivascular inflammation
Folliculitis and perifolliculitis
Panniculitis
|
|
|
|
|
|
|
|
The above approach can be applied to the most common inflammatory disorders of the vulva, which should allow the pathologist to categorize lesions and generate a rational differential diagnosis. This, in turn, will allow the clinician to develop a meaningful treatment plan.
The terms “eczema” and “eczematous dermatidities” are clinical terms used to describe a variety of lesions that share similar clinical and histologic features (spongiotic dermatitis), but often are etiologically unrelated. Eczematous dermatitis can be due to intrinsic factors (atopic dermatitis and seborrheic dermatitis) or be triggered by extrinsic agents (irritant contact dermatitis [ICD]).
All eczematous dermatidities demonstrate epidermal spongiosis during their evolution, clinically seen as crusted patches and plaques, papules and vesicles, and, at the far end of the spectrum, frank bullous lesions and, rarely, ulceration. The histologic changes of spongiotic dermatitis typically affect the epidermis and sometimes upper dermis, while the lower dermis, follicular infundibula, and acrosyringia are spared. The cardinal histologic feature of the spongiotic (eczematous) epidermal reaction pattern is spongiosis, seen histologically as an increase in the intercellular space between keratinocytes, due to the accumulation of edema fluid within the epidermis. As the vulvar skin is often confined by clothing, secondary changes such as crusting are also frequently found. If the severity of the response increases, there is widening of the intercellular space, eventuating in desmosomal rupture, followed by formation of vesicles within the epidermis. These vesicles may contain fluid, lymphocytes, Langerhans cells, and acantholytic or ruptured keratinocytes. Due to the laxity of the vulvar skin, vesicle formation is generally less common than in other parts of the body. In very severe cases, erosions and ulceration may occur. Chronic lesions can become progressively lichenified and evolve into lesions that resemble lichen simplex chronicus (LSC). At this stage, changes related to the inciting event will likely have dissipated.
Although absolute histologic distinction between the various categories of spongiotic dermatitis is not possible, there are some features that may be utilized to favor a more specific clinical-pathologic correlate. In many cases, arrival at the correct diagnosis is contingent on the clinical history. The most important differential considerations in genital skin will be discussed.
Atopic dermatitis is a chronic, pruritic process which occurs in individuals (more often men) with a personal or family history of atopic diathesis (including asthma, allergic rhinitis, allergic conjunctivitis). The disease usually first manifests during childhood as an erythematous papulovesicular rash involving the flexural and extensor surfaces of the extremities. The lesions evolve towards scaly lichenified patches and plaques over time. The vulva is infrequently involved.
Atopic dermatitis features the classic histologic changes of spongiotic dermatoses. In acute lesions, there is intercellular, and to some extent, intracellular edema of the lower epidermis with epidermal and perivascular infiltration by lymphocytes. Perivascular eosinophils are rare. Subacute lesions exhibit increasing psoriasiform epidermal hyperplasia with parakeratosis ( Fig. 1.1 ), and a decrease in the extent of spongiosis. Chronic lesions display more marked psoriasiform epidermal hyperplasia and lichenification with very mild to absent spongiosis. Further lichenification produces prominent hyperkeratosis with “vertical streaking” of collagen in the papillary dermis, leading ultimately to LSC.
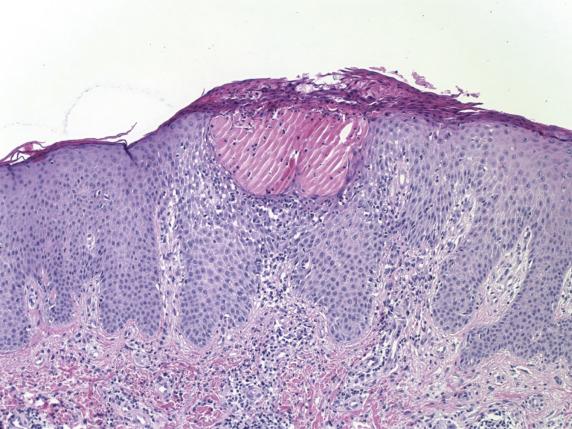
The distinction between atopic dermatitis, allergic contact dermatitis (ACD), and ICD (drug, ingestant, scabetic infestation, others) is difficult without clinicopathologic correlation. Direct immunofluorescence can be performed if there is a vesiculobullous lesion associated with eosinophils, to rule out pemphigus vegetans or bullous pemphigoid . Chronic lesions can resemble psoriasis histologically. Unlike the regular pattern of epidermal hyperplasia found in psoriasis, the rete ridges in advanced stages of a chronic eczematous dermatitis are generally irregular in both length and width.
Most cases of atopic dermatitis resolve spontaneously by the age of 30. Treatment is aimed towards hydration of the skin, minimizing triggering factors and symptoms, and decreasing inflammation. The latter is usually achieved with topical steroids. Nonsteroidal immunosuppressive agents such as tacrolimus are also considered as they have fewer disadvantages compared with chronic steroid use.
Chronic pruritic dermatitis in individuals with personal or family history of atopic diathesis (atopic dermatitis, asthma, allergic rhinitis, allergic conjunctivitis)
Common
∼20% of infants and young children symptomatic, 90% before age 5
Predisposition to infection due to compromised cutaneous barrier
Increased risk in males
Increased risk in people of African or Asian descent
Erythematous papulovesicular rash in flexural and extensor surfaces of arms and legs
Sometimes vulvar involvement, particularly in children
Scaly lichenified patches and plaques
Secondary infection may occur
Generally spontaneous remission throughout childhood
Few cases persist over age 30
Treatment includes hydration of skin, minimizing trigger factors, relief of pruritus, and decreasing inflammation
Topical steroids and nonsteroidal immune modulators
Intercellular edema of lower epidermis
Exocytosis of lymphocytes
Perivascular lymphohistiocytic infiltrate with occasional eosinophils and rarely neutrophils
Epidermis of normal thickness with normal basket-weave stratum corneum
Can have edema of papillary dermis
Psoriasiform epidermal hyperplasia with parakeratosis
Serum crust can form, made of proteinaceous debris, neutrophils, and parakeratotic cells
Variable lymphocyte exocytosis
Marked psoriasiform epidermal hyperplasia
Compact hyperkeratosis, hypergranulosis, and less parakeratosis
Mild to absent spongiosis and lymphocyte exocytosis
Can resemble lichen simplex chronicus
Contact dermatitis
Spongiotic hypersensitivity reaction
Pemphigus vegetans or bullous pemphigoid
ACD is a type IV hypersensitivity reaction initiated by contact with an allergen to which an individual has been previously exposed and sensitized. Lesions typically develop within 12–48 hours following exposure to the allergen. Numerous chemical, biological, and physical agents can elicit this reaction pattern ( Table 1.3 ). Exposure to the offending agent may be related to the patient’s occupation or daily hygiene routines. Topical medications, fragrances, and preservatives are the most common causes of ACD. Patch testing with an allergist can be helpful to identify the offending agent.
| Endogenous | Urine, feces, sperm |
|---|---|
| Exogenous | Detergents, soaps/shampoos, perfume/deodorants, fabrics/wools, textile dyes, sanitary napkins, antiseptics/disinfectants, spermicides, latex, nickel, cosmetic fillers (silicone, hyaluronic acid) |
| Drugs | Antibiotics/Neosporin, corticosteroids, anesthetics (benzocaine in Vagisil), imiquimod, podophyllin |
ACD is more frequent in females and in white persons. Common findings include a pruritic papulovesicular eruption, discretely patterned crusted plaques, or, occasionally, bizarre-appearing patterns, depending on the extent of contact with the agent.
In acute episodes, the spongiosis is limited to the lower epithelium, and the stratum corneum remains uninvolved. With persistent allergen exposure, there is formation of coalescing vesicles at various levels of the epidermis, imparting a “Swiss-cheese” appearance ( Fig. 1.2 ). Intraepithelial inflammatory cells are usually lymphocytes (rarely eosinophils). Chronic forms feature progression from a spongiotic to a lichenoid pattern like in atopic dermatitis.
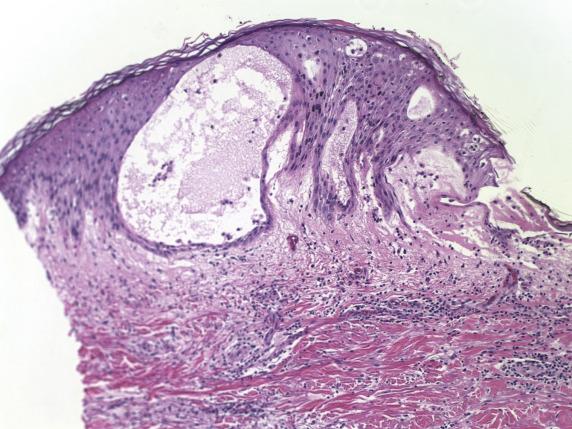
See atopic dermatitis section.
Removal of, or protection from the offending agent is paramount. Without it, the disease will persist, leading to longer and more severe episodes, and to complications such as infection. In addition, treatment includes short-term topical corticosteroid therapy to reduce inflammation, and emollients to allow the reestablishment of the barrier function of the skin.
Type IV hypersensitivity reaction initiated by contact with allergen to which an individual has been previously sensitized
Common (4%–7% of all dermatologic consultations)
Relapse and chronicity from reexposure to offending antigen, particularly occupational exposure
May impact individual occupational choices
Approximately twice as common in females as in males
More frequent in whites than in other racial groups
Pruritic papulovesicular eruption
Circumscribed crusted plaques
Occasionally bizarre-appearing patterns
Excellent prognosis if removal or protection from offending agent
Emollients and short-term topical steroid therapy
Spongiotic microvesicles within various levels of epidermis (“Swiss-cheese” appearance)
Exocytosis of lymphocytes and eosinophils (eosinophilic spongiosis)
Unchanged stratum corneum in acute reaction
Epidermal hyperplasia and diminution of spongiosis if persistent allergen
Atopic dermatitis
Eczematous hypersensitivity reaction
Pemphigus vegetans or bullous pemphigoid
In contrast to ACD, ICD is caused by the direct effect of an irritant compound on an epithelial surface, and therefore occurs rapidly (minutes to hours) after exposure to the agent, not requiring prior sensitization. Offending agents can be similar to that seen with ACD ( Table 1.3 ).
Females are more frequently affected than males, with no particular genetic predisposition. A large range of clinical appearances may be seen, including erythema, eczematous changes, vesiculobullous lesions, and epidermal necrosis. Lesions tend to be confined to the areas of contact with the offending agent, thus often sparing the genitocrural folds.
In contrast to ACD, the agent responsible for ICD produces direct injury to the epidermis. The degree and severity of the epidermal injury varies based on the nature, potency, and concentration of the irritant. Histologically, common changes include ballooning degeneration of the surface keratinocytes with subsequent necrosis and surrounding epidermal spongiosis. Neutrophil infiltration is seen in areas of epidermal necrosis ( Fig. 1.3 ). The underlying superficial dermis contains perivascular inflammation composed of lymphocytes, macrophages and, frequently, neutrophils. Interstitial edema and vasodilatation are also present ( Fig. 1.4 ).
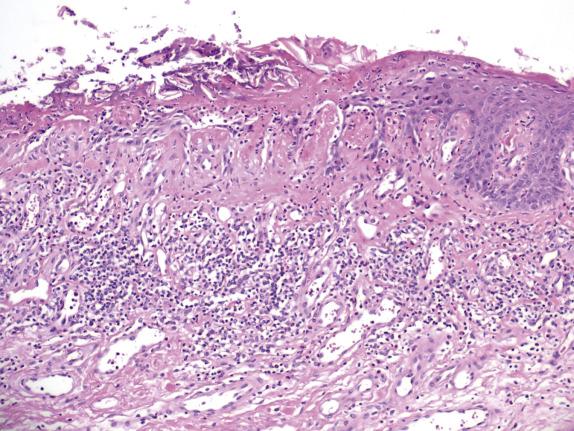
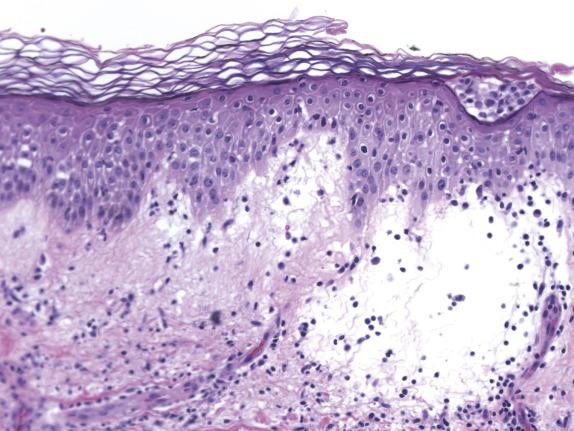
See atopic dermatitis section.
Removal of, or protection from, the offending agent is paramount to prevent recurrences. Management aims to restore the skin’s barrier function. Topical applications containing ceramides may be particularly helpful. Corticosteroids and immune modulators are in general not recommended.
Dermatitis initiated by direct effect of an irritant compound on epithelial surface
Common
Significant long-term sequelae possible if chronic exposure
More common in women than men, secondary to environmental rather than genetic factors
No age or racial predilection
Wide range of clinical appearances, including erythema, eczematous changes, vesiculobullous lesions, and epidermal necrosis
Lesions often well-demarcated (confined to areas of contact with offending agent and sparing genitocrural folds)
Excellent prognosis if removal of, or protection from, offending agent
Emollients reestablish cutaneous barrier function
Topical steroids and immune modulators not helpful
Ballooning degeneration and necrosis of surface keratinocytes
Neutrophil exocytosis in areas of epidermal necrosis
Interstitial edema and vasodilatation
Chronic cases can form pseudoverrucous papules, nodules, and dermal fibrosis
Allergic contact dermatitis (ACD)
Eczematous hypersensitivity reaction
Pemphigus vegetans or bullous pemphigoid
Seborrheic dermatitis is a common eczematous dermatitis occurring in areas of the skin that have the greatest number of sebaceous glands (scalp, face, chest, upper back, axillae, and anogenital skin). Not surprisingly, men are more commonly affected than women. Most patients present during the fourth to fifth decade; however, an infantile form (in babies during first three months) has been described. The affected vulvar skin, more commonly the genitocrural folds, is erythematous with scaling “bran-like” papules and plaques with a “greasy yellow” appearance. Immunosuppressed patients, especially those with human immunodeficiency virus (HIV) infection, have a higher prevalence of this disease. The pathogenesis is unknown; however, Malassezia species (Pityrosporum) has been implicated as an etiologic factor; in many patients, flares of the disease correlate with proliferation of Malassezia spp. and respond to antifungal agents. It is thought that the yeast triggers an immunologic response affecting the hair-bearing epidermis.
In its acute form, seborrheic dermatitis features mild spongiosis with a parakeratotic scale crust containing scattered neutrophils ( Fig. 1.5 ). The papillary dermis is mildly edematous with a mixed perivascular inflammatory infiltrate (composed of lymphocytes, histiocytes and, rarely, neutrophils) involving the superficial plexus. As lesions progress, psoriasiform hyperplasia appears and becomes extensive with parafollicular accentuation of the parakeratotic scale (“shoulder parakeratosis”) ( Fig. 1.6 ). Neutrophils are commonly observed within the scale crust and within spongiotic foci. In chronic lesions, the psoriasiform hyperplasia is prominent and the parakeratotic scale crust may extend into the interfollicular epidermis.
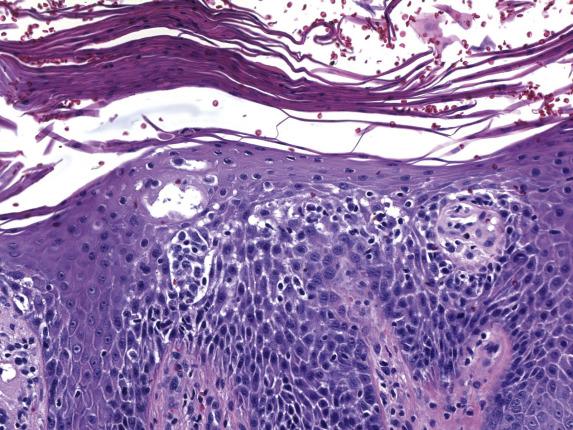
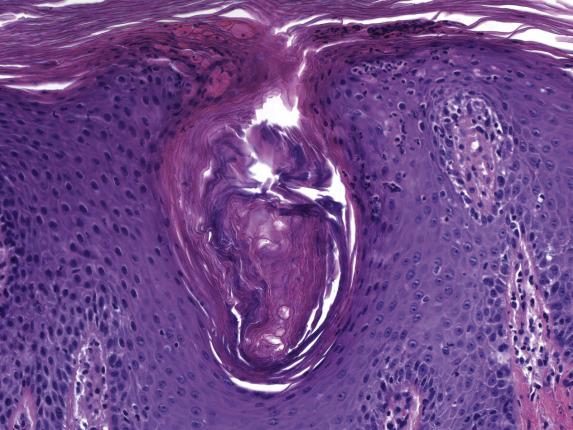
The presence of neutrophilic parakeratosis and psoriasiform epidermal hyperplasia raises consideration of psoriasis . Features in favor of seborrheic dermatitis are irregular elongation of the epidermal rete, accentuation of parakeratosis and scale crusts about the follicular ostia, and predilection of the follicular and epidermal spongiosis toward the upper layers of the epidermis. However, distinction can be difficult. Neutrophil-rich parakeratosis is also a feature observed in many impetiginized eczematous dermatitis . A tissue Gram stain is a helpful adjunct in excluding this possibility.
Seborrheic dermatitis generally exhibits a mild chronic clinical course with little discomfort. Treatment is directed towards removal of the scales with keratolytics, followed by sparing use of topical corticosteroids. Topical antifungal agents (i.e., ketoconazole, ciclopirox) constitute an important adjunct in therapy and tend to reduce or eliminate the need for steroid therapy over time when used consistently. Resistance to therapy may be a sign of immunodeficiency, particularly HIV infection.
Common eczematous dermatitis occurring on skin with greatest number of sebaceous glands
Common; approximately 5% prevalence
Cosmetic
More common in men than women
No racial predisposition
Common in fourth to fifth decade
Self-limited infantile form in first 3 months of life
Erythematous skin, with papules and plaques with fissures causing “bran-like” scale and “greasy yellow” appearance
Most affect the genitocrural folds
Extensive therapy-resistant seborrheic dermatitis may indicate underlying HIV infection
Mild clinical course with little discomfort other than cosmetic concerns
Removal of scale with keratolytics followed by sparing use of corticosteroids and antifungal agents
Mild spongiosis with neutrophilic parakeratosis
Papillary dermal edema and lymphohistiocytic perivascular infiltrate
Substantial psoriasiform hyperplasia and “shoulder parakeratosis” (perifollicular accentuation of parakeratosis)
Psoriasis
Impetiginized eczema
The acanthotic reaction pattern is defined by an increase in the number of keratinocytes in the epidermis, leading to overall thickening of the epidermis. “Psoriasiform hyperplasia” and historically “squamous cell hyperplasia,” are terms that have also been used to describe this reaction pattern.
Psoriasis is the prototype of a group of dermatoses characterized by epidermal hyperplasia.
Psoriasis is a chronic dermatitis characterized by a hyperproliferative epidermis. It is a common disorder, affecting 2%–4% of whites (less frequently other ethnicities). The disease usually manifests during early adulthood. Vulvar involvement can be a manifestation of the classic, generalized form or of the so-called inverse psoriasis which affects intertriginous areas. The most common clinical vulvar presentation is a pruritic, bilaterally symmetrical, erythematous, well-demarcated, nonscaly, macular eruption or plaque, as opposed to patches and plaques with a “silvery” scale typical of other anatomic regions. The anatomic distribution is wide, and most patients that present with a vulvar psoriasiform lesion will have evidence of lesions elsewhere on the body. These lesions tend to concentrate in areas of persistent trauma or friction (elbows, knees, sacrum, scalp, and intertriginous areas). Other triggers include drugs and infections. Superinfection by yeast, fungi, and bacteria is possible.
Psoriasis, like many other dermatoses, has a range of microscopic changes that evolve over time. Early lesions have a nonspecific spongiotic epidermal reaction pattern that includes superficial dermal vascular congestion, edema, and lymphocyte-rich perivascular inflammation. As lesions progress, a characteristic (“psoriasiform”) pattern of epithelial hyperplasia appears, characterized by regular acanthosis, elongation of the rete ridges, thinning of the suprapapillary plates, and loss of the granular cell layer (hypogranulosis) ( Fig. 1.7 ). Other typical findings include mounds of neutrophil-rich parakeratosis and increased basal mitotic activity. Confluence of neutrophilic aggregates within the parakeratosis is seen in mature lesions. Intraepidermal neutrophil collections located in the stratus corneum are termed “Munroe microabscesses,” whereas those in the spinous layer are called “spongiform pustules of Kogoj” ( Fig. 1.8 ). The accompanying epidermal hyperplasia has a regular appearance, characteristically with intervening thinned suprapapillary plates and subjacent dilated capillaries, frequently in immediate apposition to the overlying epidermis ( Fig. 1.9 ).
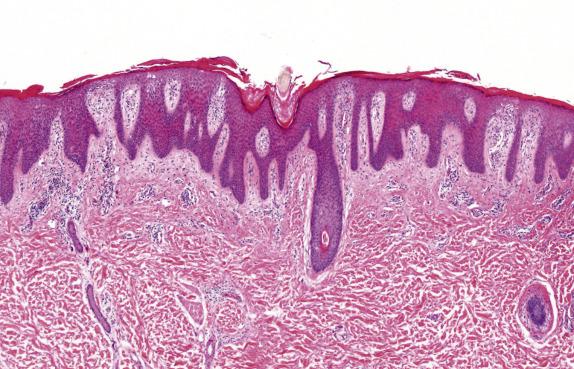
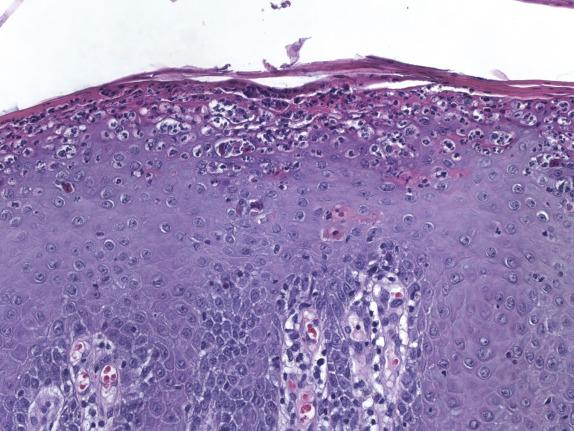
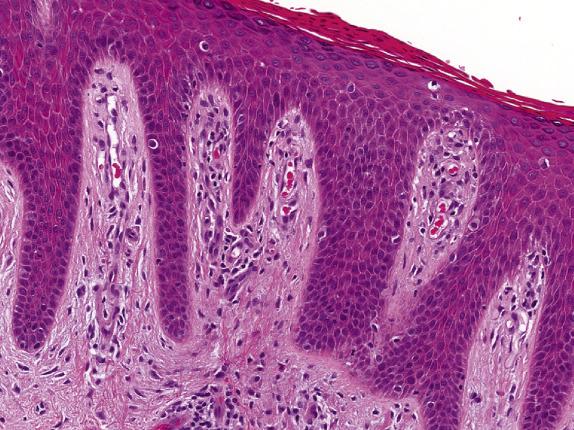
Late-stage lichen simplex chronicus can be distinguished from psoriasis by the presence of thicker suprapapillary plates, hypergranulosis, and thickened vertically oriented collagen bundles within the papillary dermis. Distinction of psoriasis from seborrheic dermatitis can be very difficult, particularly at early stages; the presence of spongiosis involving the rete ridges is not typical of psoriasis, and favors seborrheic dermatitis instead. Chronic candidiasis and dermatophytoses may feature neutrophil exocytosis and psoriasiform epidermal hyperplasia. Therefore, special stains should be routinely considered to exclude the presence of fungal elements.
Psoriasis is a chronic condition characterized by frequent relapses. Complications include psoriatic arthritis (seen in up to 30% of patients) and infections. Thus, long-term management is required, focusing on balancing the extent of disease involvement with the potential side-effects of therapy. Topical treatment, in the form of emollients, corticosteroids, tars, vitamin D analogs, and tacrolimus, is utilized for localized plaques. Systemic therapies for severe disease include methotrexate, retinoids, and cyclosporine. Phototherapy and photochemotherapy (psoralen with ultraviolet A) are also used.
Chronic dermatitis characterized by hyperproliferative epidermis and regular epidermal hyperplasia
2%–4% prevalence rate in whites
Significant cosmetic impact on quality of life
Psoriatic arthritis (5%–30%)
No gender predisposition
Low prevalence in Asians, Native Americans, and Africans
Earlier onset in females than males
Onset before age 40 in ∼75% of patients
Circumscribed, well-demarcated erythematous patches and plaques
Wide anatomic distribution, but mostly in areas of persistent trauma or friction
Long-term management due to chronic course of disease
Topical therapies for localized disease
Systemic therapies for severe disease, including methotrexate, retinoids, cyclosporine, phototherapy, and photochemotherapy (psoralen with ultraviolet A)
Epidermal spongiosis
Papillary dermal vascular congestion and edema
Sparse perivascular dermatitis
Regular acanthosis with increased basal mitotic activity
Mounds of neutrophil-rich parakeratosis
Hypogranulosis
Marked regular psoriasiform epidermal hyperplasia with thinned suprapapillary plates
Confluent neutrophil-rich parakeratosis, “Munroe microabscesses,” and “spongiform pustules of Kogoj”
Papillary dermal vascular ectasia
Lymphocytic-rich perivascular dermatitis
Lichen simplex chronicus
Seborrheic dermatitis
Dermatophytosis and candidiasis
LSC is a condition in which scaly plaques are formed in response to repetitive irritation of affected sites by rubbing or scratching. Women are more commonly affected, usually between the third and fifth decades of life. Anatomic sites of predilection include the perianal and genital regions, and the posterior neck, forearms, and pretibial areas. Within the vulvar region, LSC can present as a primary or secondary condition. Heat, sweat, and friction can be a primary cause of skin irritation that can ignite the itch/scratch cycle leading to the features of LSC ( Fig. 1.10 ). Secondary causes can be numerous and are important for the pathologist to identify as the underlying cause of LSC, especially when premalignant or malignant in nature.
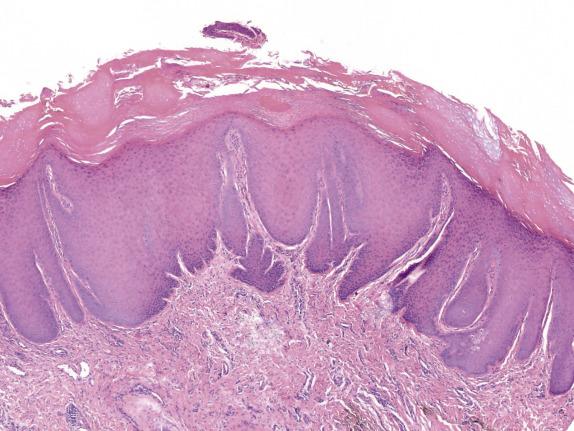
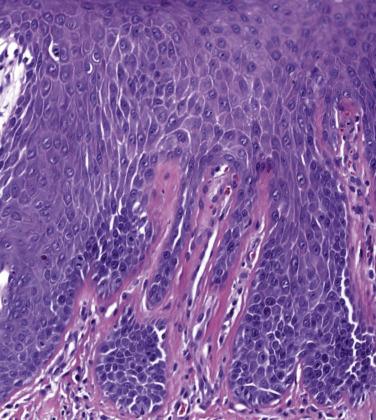
LSC is characterized by epidermal acanthosis (hyperplasia) with psoriasiform appearance. The epithelium shows broad compact orthokeratosis with hypergranulosis; parakeratosis is present, often with a patchy distribution ( Fig. 1.11 ). Focal erosion or ulceration is common. The rete ridges are thick without suprapapillary plate thinning. A characteristic feature is the presence of thickened eosinophilic bundles of collagen arranged in vertical streaks within the papillary dermis ( Fig. 1.12 ), although compared to the skin this feature is less developed in the mucocutaneous surfaces of the vulva and perineum.
Superimposed changes of LSC can be observed in virtually all dermatoses, therefore careful evaluation to exclude a primary dermatosis is essential before rendering a diagnosis of idiopathic LSC; this particularly applies to instances in which primary treatment is unsuccessful. Superimposed LSC on high grade squamous intraepithelial lesion / vulvar intraepithelial neoplasia of usual type (HSIL/u-VIN) , a human papillomavirus (HPV) associated premalignant skin lesion, is an important differential. Close examination of the lower epithelial layers is key: nuclear enlargement, hyperchromasia, loss of polarity, overlapping, and loss of maturation with mitoses above the basal membrane are features indicative of HSIL. Importantly, HSIL with superimposed LSC will show rather abrupt maturation of the dysplastic epithelium, which may lead to not only misinterpretation as idiopathic LSC but also as a low-grade squamous intraepithelial lesion (LSIL/VIN1) or differentiated VIN. A VIN3 lesion with superimposed LSC can also resemble a VIN2 lesion, but this distinction may not carry clinical consequences. In equivocal cases, strong, nuclear, and cytoplasmic block staining of p16 immunohistochemistry will help point to a diagnosis of HSIL. Atypical verruciform lesions of the vulva also present with hyperkeratotic lesions that show irregular epidermal hyperplasia. Verruciform proliferations of the vulva, including the so-called verruciform LSC, are rare and still poorly understood. Importantly, some are regarded as neoplastic in nature. The most important is verrucous carcinoma , which is locally aggressive. Unlike LSC, verrucous carcinoma presents clinically as a large exophytic mass. Histologically, they show expansile invasion into the vulvar stroma in the form of bulbous nests with broad-fronts and smooth edges. While LSC, if tangentially sectioned, may give this appearance, the depth and complexity of the growth of verrucous carcinoma are usually obvious and exceed that of tangential cutting. Other verruciform lesions that deserve distinction from LSC include entities historically known as vulvar acanthosis with altered differentiation (VAAD) and atypical verruciform hyperplasia , recently grouped into the term differentiated vulvar intraepithelial lesion (DEVIL) . DEVIL presents clinically as plaques or areas of exophytic cauliflower-like growth, corresponding histologically to epithelial acanthosis with verruciform growth lacking cytologic atypia (see Chapter 2 ). The finding of frequent PIK3CA mutations and absence of TP53 mutations, added to the observation of DEVIL-type lesions preceding or accompanying verrucous carcinoma, has led to consideration of this constellation of atypical verruciform proliferations as a third category of squamous preinvasive neoplasia. It is important to note that verrucous carcinoma and DEVIL have bland cytomorphology throughout, and their distinction from LSC must be based on the severity and distribution of the epithelial proliferation. The distinction between DEVIL-type lesions and LSC can be quite difficult. In fact, the term “verruciform LSC” has been used to describe LSC lesions with prominent exophytic growth, and these lesions have been found to harbor PIK3CA mutations linking them to DEVIL. From the diagnostic perspective, it is important to suspect DEVIL if the lesion is exophytic and produces verruciform growth, and if it persists despite treatment or recurs over time. Complete excision and close monitoring may be prudent in this scenario.
Identification and treatment of any underlying process, and cessation of the traumatic stimulus, are imperative in the treatment of this condition, something that may be quite difficult in the long-term. Emollients, topical steroids, and simple barrier occlusion may help break the itch–scratch cycle. Behavioral modification and psychological/psychiatric intervention can be beneficial, particularly if no underlying etiologic factor is identified and medical management is not effective.
Condition in which skin becomes thickened with scaly plaques in response to repetitive rubbing and/or scratching of affected sites
Can be primary (idiopathic) or secondary to an underlying dermatosis that causes itching
Common
Cosmetic
Superinfection if abraded and ulcerated lesions
More common in women than men
No racial predilection
Peak incidence between 30 and 50 years
Hyperpigmented scaly plaques at sites of repetitive rubbing
Perianal and genital regions, posterior neck, forearms, and pretibial areas most common
Cessation of the itch–scratch cycle
Emollients, topical steroids, and barrier occlusion
Behavior modification and psychopharmacologic agents in select patients
Compact orthokeratosis and focal parakeratosis
Hypergranulosis with subjacent irregular psoriasiform epidermal hyperplasia
Thick eosinophilic bundles of collagen in “vertical streaks” in papillary dermis
Variable perivascular inflammation
Signs of dermatitis, lichen sclerosus, or other dermatoses may be present
High-grade squamous intraepithelial lesion
Verrucous carcinoma
Atypical verruciform lesions (DEVIL)
This tissue reaction pattern is characterized by basal keratinocyte damage via T-cell-mediated cytotoxic damage or induction of apoptosis. By convention, the damaged keratinocytes are termed dyskeratotic cells , “ Civatte bodies” if they are confined to the epidermis, and “ colloid bodies” when they descend into the papillary dermis. In addition to basal keratinocyte damage, vacuolar change may also be present, sometimes more prominent than cell death in certain dermatoses. Vacuolar change consists of intercellular keratinocyte vacuole formation and edema with separation from the basement membrane zone. The distribution of the inflammatory infiltrate is an important characteristic. The infiltrate may appose the basal layer or obscure the dermoepidermal junction (DEJ). The density and composition of the infiltrate also vary according to the specific disorder. The presence and quantity of displacement of melanin into the dermis (“pigment incontinence”) should also be noted. Lastly, the phase of the lichenoid epidermal reaction pattern is important, as it can point towards a specific histopathologic diagnosis. For instance, in acute cytotoxic reactions the epidermis is minimally affected, as in the early lesions of erythema multiforme (EM) (erythema multiforme-like epidermal reaction pattern). In more chronic lesions, such as those found in lichen planus (LP), the epidermis shows signs of reaction such as premature terminal differentiation (lichen planus-like epidermal reaction pattern). Over time, there may be supervening epidermal hyperplasia, which may be regular (psoriasiform epidermal reaction pattern) as in secondary syphilis, or irregular, as observed in discoid lupus erythematosus. The final or end-stage epidermal reaction pattern is found in those dermatoses featuring epidermal atrophy (lichen sclerosus [LS]). Integration of all these findings with the clinical picture will allow the pathologist to make a specific histopathologic diagnosis in most cases.
Lichen planus (LP) is a T-cell mediated inflammatory dermatosis that affects keratinized and nonkeratinized epithelium. While idiopathic in nature, LP is sometimes associated with other disorders such as ulcerative colitis, hepatitis B/C, immunodeficiency, cirrhosis, and peptic ulcer disease. Several clinical variants exist, but there are three most important to the vulva: classic (papulosquamous), hypertrophic, and erosive, each with its own unique clinical and histologic features ( Table 1.4 ). Erosive lesions are a more common presentation at anogenital sites; they appear clinically as ulcerated and friable patches in the mucosa. Other types of LP clinically appear as reticulate lacy lesions. When chronic, erosive lesions may evolve to scarring.
| Pattern | Classic (Papulosquamous) | Hypertrophic | Erosive |
|---|---|---|---|
| Location | Keratinized skin | Keratinized skin | Nonkeratinized skin Can include vaginal involvement |
| Clinical Features | Violaceous-pink flat-topped papule | Raised hyperkeratotic red papules and plaques | Well-demarcated Glazed erythema Hyperkeratotic border Reticulate lacy lesions |
| Histopathology | Lichenoid infiltrate at epidermal–dermal junction “Wedge-shaped” hypergranulosis Dyskeratotic and vacuolar changes at basilar layer |
Lichenoid infiltrate Acanthosis Parakeratosis Hypergranulosis Hyperkeratosis Pseudoepitheliomatous hyperplasia |
Lichenoid infiltrate Superimposed erosive ulcer |
| Differential Diagnosis | Lichenoid drug eruption Early lichen sclerosus Lichen simplex chronicus |
Squamous cell carcinoma | Bullous disorders Differentiated vulvar intraepithelial neoplasia (dVIN) |
| Treatment/Prognosis | Self-limiting | Self-limiting | Ultra-potent steroids Chronic, poor response to therapy |
Evolved lesions of classic (papulosquamous) LP feature a band-like infiltrate composed of lymphocytes with an admixture of macrophages, which adhere to the basal aspect of the epidermis ( Fig. 1.13 ). The associated basal cell damage is seen as abrupt maturation (“squamatization”) of basal keratinocytes, dyskeratotic cells throughout the epidermis (Fig.1.14), and eosinophilic colloid bodies in the papillary dermis. The infiltrate does not obscure the interface and does not extend into the suprabasilar epidermis. Plasma cells may be prominent in mucosal epithelium or at mucocutaneous interfaces. Other hallmarks of LP include irregular acanthosis, hyperkeratosis, “ wedge-shaped ” hypergranulosis (adjacent to acrosyringia or acrotrichia), and clefts which appear at the dermoepidermal junction (“ Max–Joseph” spaces). Importantly, parakeratosis is not a feature of LP and its presence should suggest other disorders. As chronicity ensues, dermal fibrosis becomes apparent and the epidermis becomes progressively flattened and thinned. Hypertrophic LP features the same lichenoid infiltrate with accompanying hypergranulosis and acanthosis. The latter can sometimes be quite exuberant, producing the so-called “pseudoepitheliomatous hyperplasia,” which can be mistaken for a premalignant or malignant process. Erosive LP , as the term implies, exhibits a superficial ulcerative surface with a lichenoid stromal infiltrate. If present, the edge of the lesion will show changes of classic LP ( Fig. 1.15 ).
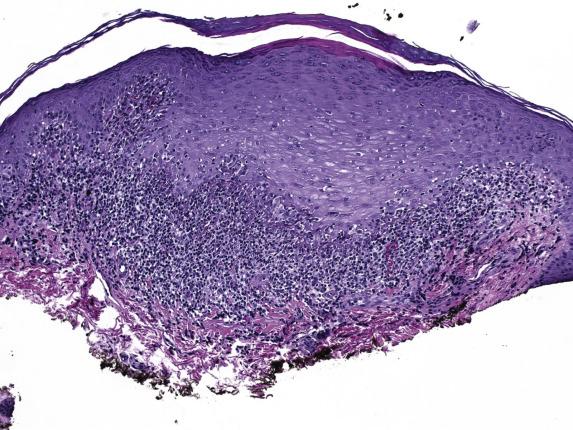
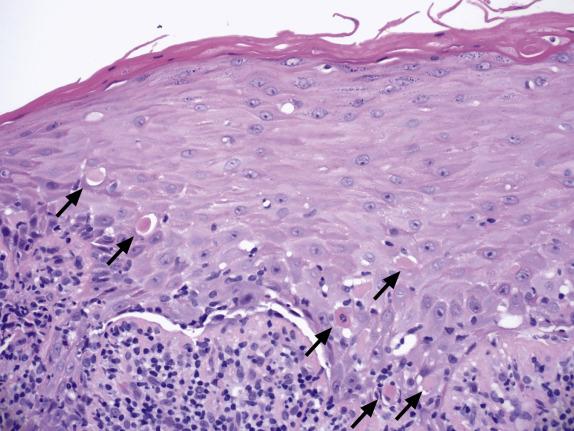
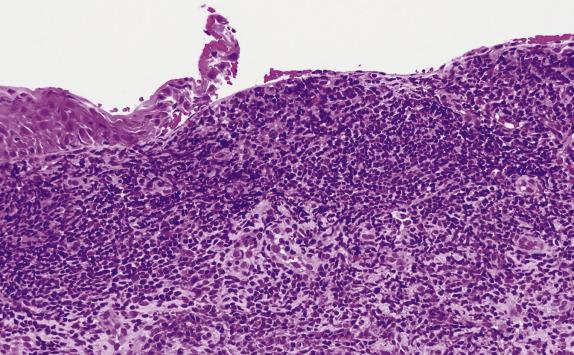
Immunofluorescence shows irregular fibrin deposits in the DEJ. IgM deposits around necrotic keratinocytes are common and, when prominent, are helpful to confirm the diagnosis of LP. IgG, C3, and IgA deposits can also be present.
The differential diagnosis of LP in the vulva depends on the variant of LP . Classic/papulosquamous LP features a band-like infiltrate and attention should be drawn to the epidermal reaction pattern and composition of the infiltrate because, although uncommon in flexural or mucosal sites, lichenoid drug eruptions may be histologically indistinguishable from LP. Helpful clues in favor of lichenoid drug eruption are: presence of eosinophils within the infiltrate, involvement of the deep vascular plexus, and the presence of parakeratosis. Early lesions of LS are virtually indistinguishable from LP, and a descriptive diagnosis of “lichenoid dermatosis” followed by a differential may be prudent. The diagnosis of LS can, nonetheless, be raised if signs of chronicity are identified (effacement and flattening of the rete pegs, homogenization of the papillary dermis). If the infiltrate is rich in plasma cells, plasma cell (zoon) vulvitis may be considered; however, in this disorder the epithelium displays atrophy and hypogranulosis, unlike LP.
Hypertrophic LP may raise the differential diagnosis of a neoplastic proliferation such as a squamous cell carcinoma due to their overlying pseudoepitheliomatous hyperplasia and lichenoid-type host response. The presence of epithelial atypia and irregular inward growth should raise the possibility of malignancy. Long-standing lesions may overlap morphologically with LSC; the presence of basal cell damage, particularly at the base of the rete ridges, is indicative of LP. Lichenoid keratosis or LP -like keratosis is a solitary lesion that is essentially a T-cell-mediated regression of a variety of benign “keratoses” including warts , seborrheic keratosis , and lentigines . These may be present alongside the inflammatory infiltrate and be a helpful histologic clue to the diagnosis. As a pitfall, the inflammation associated with regression of pigmented lesions (including melanoma ) and atypical keratinocytic proliferations (including squamous cell carcinoma in situ and basal cell carcinoma ) may have a striking lichenoid appearance. Any lichenoid infiltrate containing numerous melanophages should be treated with caution, and additional level sections and immunohistochemical stains for melanocytic markers may be useful in evaluating a clinically ambiguous lesion. Likewise, the presence of large epithelioid cells or keratin plugs should prompt consideration for epithelial markers to exclude epithelial neoplasia.
Erosive LP requires distinction from other ulcerative lesions, particularly aphthous ulcers and infections (fungi, syphilis, herpes). In addition to correlating with the clinical history, it is very important to examine the edge of the eroded area to identify classic LP changes, or microorganisms or viral cytopathic changes in the epithelium. A dense plasma cell infiltrate should raise the possibility of syphilis. A neutrophilic perivascular infiltrate is more in keeping with aphthous ulcers.
Most cases of LP have a self-limiting course; indeed, over two-thirds of patients experience spontaneous remission in the 12–18 months following diagnosis. Treatment is largely symptomatic and consists of immunosuppressive agents, either topical or intralesional steroids (for local to regionally confined lesions), or systemic corticosteroids, retinoids, and cyclosporine (for cases with widespread involvement).
Idiopathic T-cell mediated skin disorder
Prototype of the lichenoid-type reaction
1% worldwide
In vulva: erosive subtype>papulosquamous>hypertrophic
Occasional vulvar scarring as consequence of erosive subtype
Female predominance
No racial predisposition
Rare in childhood; typically 30–60-year range
No difference in age distribution between variants
Predilection for wrists, ankles, and genitalia, but may be widespread, including mucous membranes, nails, and hair
Erosive
Well-demarcated glazed erythema
Hyperkeratotic border
Reticular lacy lesions
Hypertrophic:
Raised hyperkeratotic plaque
Classic (papulosquamous):
Violaceous papules
Pruritic or asymptomatic
Associated with ulcerative colitis, hepatitis B/C, immunodeficiency disorders, cirrhosis, and peptic ulcer disease
Generally self-limited disorder (12–18 months)
Topical or intralesional steroids for regionally confined lesions
Systemic steroids, retinoids, or cyclosporine for widespread involvement
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here