Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The inferior vena cava (IVC) and its tributaries are frequent sites of vascular pathology. Diseases of organs that drain into the IVC may first become clinically apparent when the cava becomes involved. IVC imaging and intervention are prominent components of current interventional radiology practice.
The IVC is formed by the confluence of the common iliac veins at the level of the L5 vertebral body ( Fig. 13-1 ). In the abdomen, the IVC is usually located to the right of the midline and the aorta, anterior to the lumbar and lower thoracic spine. The IVC is a posterior structure for much of its course. The retrohepatic IVC resides in a groove or tunnel in the bare area of the liver encompassed posteriorly by suspensory ligaments of the liver and the diaphragm. The IVC exits the abdomen through a diaphragmatic hiatus, with a slight anterior course before draining through the inferoposterior wall of the right atrium. Frequently there is a membranous lip at the junction of the IVC with the right atrium, termed the eustachian valve ( Fig. 13-2 ). The supradiaphragmatic portion of the IVC is frequently intrapericardial.
The IVC typically has an oval shape in cross-section, but is easily deformed by adjacent abdominal or retroperitoneal masses. The average diameter of the infrarenal IVC is approximately 23 mm, although the intrarenal segment is usually slightly larger. The IVC is a valveless elastic structure that responds to increased venous volume or pressure by dilatation, and responds to decreased volume or increased intraabdominal pressure by collapsing. The dynamic nature of the IVC should be considered when interpreting imaging studies or contemplating interventions.
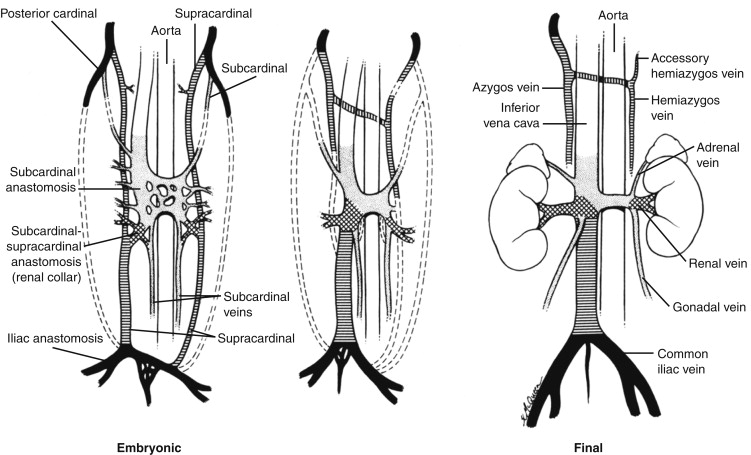
The IVC is a single, right-sided structure in approximately 97% of individuals ( Table 13-1 ). The embryology of the IVC is complex in that the antecedent structures are paired and segmented. Anomalies of the IVC can be explained by aberrations of regression of these segments. The three pairs of fetal veins that become the IVC are the posterior cardinal, the subcardinal, and the supracardinal (see Fig. 13-1 ). The posterior cardinal veins normally involute completely, although persistence on the right results in a retrocaval right ureter. The subcardinal veins form the intrahepatic IVC, and contribute to the renal veins and suprarenal segment of the IVC. Regression of the right subcardinal vein results in azygos or hemiazygos continuation of the IVC ( Fig. 13-3 ). The infrarenal IVC and the azygos veins are derived from the supracardinal veins. Duplication of the infrarenal IVC results from failure of regression of the left supracardinal vein, while a left-sided IVC results from regression of the right supracardinal vein ( Fig. 13-4 ). When there is caval duplication, each iliac vein is usually isolated and drains through its own IVC, although communication at the normal level of the iliac vein confluence may also occur. The left side of a duplicated IVC drains into the left renal vein, which then usually crosses the aorta in the normal location to join the right IVC, forming a normal single suprarenal IVC. When there is only a single left-sided IVC, both iliac veins drain into the IVC, which usually crosses the aorta at the level of the left renal vein to form a normally located suprarenal IVC (see Fig. 13-4 ). Thus, unless there is an associated anomaly of the subcardinal veins, duplicated or left-sided IVCs usually revert to a normal location above the level of the renal veins.
| Variant | Incidence (%) |
|---|---|
| Duplicated IVC | 1.0 |
| Left-sided IVC | 0.5 |
| Absent IVC | 0.1 |
| Azygos/hemiazygos continuation of the IVC | 0.5 |
| Circumaortic left renal vein | 7 |
| Retroaortic left renal vein | 3 |
| Multiple right renal veins | 28 |
The major tributaries of the IVC are the hepatic, renal, gonadal, and common iliac veins (see Fig. 13-1 ). Smaller tributaries include the lumbar, right adrenal, and phrenic veins. The common and external iliac veins are discussed in Chapter 16 , and the hepatic veins in Chapter 14 .
The most common pattern of renal vein anatomy is a single vein from each kidney, with the left renal vein passing anteriorly between the aorta and the superior mesenteric artery (SMA) to join the IVC opposite the right renal vein at the level of the L2 vertebral body. The orifice of the normal left renal vein is anterior, while that of the right is posterior. The right renal vein is shorter than the left, with average lengths of 3 cm and 7 cm, respectively ( Fig. 13-5 ). Renal veins rarely have valves, but commonly connect to other retroperitoneal veins such as the lumbar, azygos, and gonadal veins. In patients with portal hypertension these connections may enlarge to allow drainage of portal blood from the splenic and short gastric veins into the left renal vein.
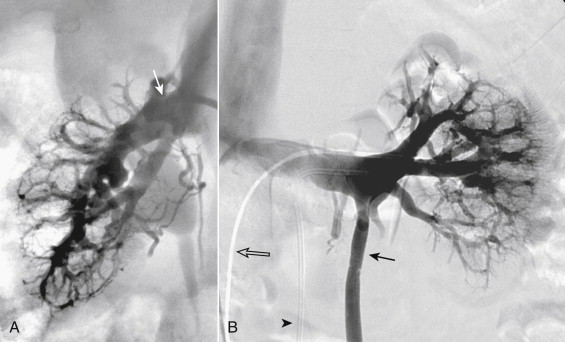
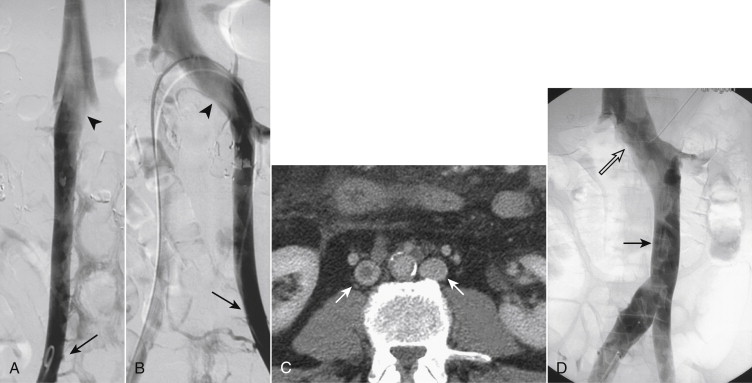
Variations in renal vein anatomy are present in almost 40% of individuals (see Table 13-1 ) due to the complex embryologic relationships of the kidneys and the veins. In the fetus, the subcardinal and supracardinal veins form a web of veins that surround the aorta. As the kidneys rise out of the pelvis between the sixth and ninth weeks of gestation, their blood supply is constantly changing. Persistence of any of the venous elements may result in an anomaly, of which multiple right renal veins are the most common (28%) ( Fig. 13-6 ). The next most common anomaly is a circumaortic left renal vein (5%-7%), in which the left renal vein has both a preaortic and a retroaortic component. The latter may enter the IVC close to the level of the normal preaortic vein, or as low as the confluence of the iliac veins. In 3% of individuals a single left renal vein passes behind (retroaortic) the aorta to reach the IVC.
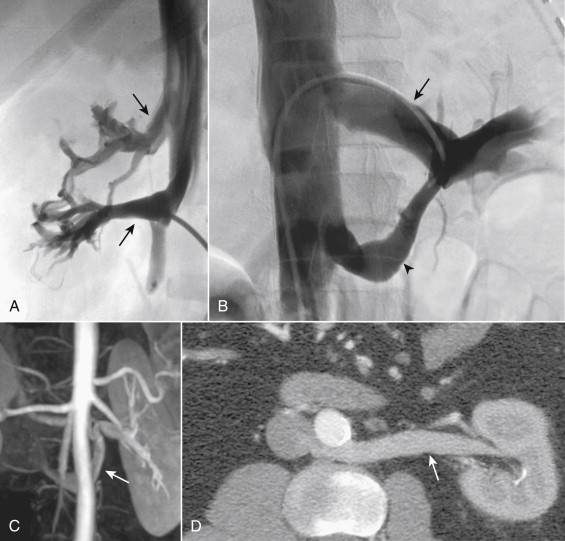
The gonadal veins ascend from the pelvis anterior to the psoas muscle as companions of the gonadal arteries and the ureters. There are multiple small anastomoses between the gonadal veins and other retroperitoneal veins along the entire length of the vessels. This fact is crucial when considering gonadal vein interventions. The right gonadal vein drains into the anterior surface of the IVC just below, or at the level of, the right renal vein in most individuals (see Fig. 13-1 ). In fewer than 10% the right gonadal vein empties directly into the right renal vein. In the majority (>99%) of individuals the left gonadal vein empties into the left renal vein just before the renal vein crosses the aorta. Rarely, the left gonadal vein empties directly into the IVC. Usually, there is a valve present just at or below the orifice of the gonadal veins.
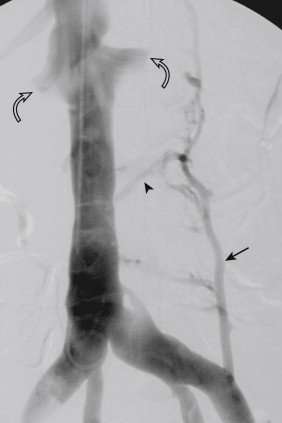
Four to five pairs of lumbar veins drain the vertebral column and the surrounding musculature. These veins empty into the posterolateral aspect of the IVC at the levels of the L4-L1 vertebral bodies. The lumbar veins anastomose with the ascending lumbar veins, paired structures that lie deep to the psoas muscles, parallel to the IVC ( Fig. 13-7 ). The ascending lumbar veins originate from the superior aspect of the common iliac veins. In the thorax, the ascending lumbar veins become the azygos vein on the right and the hemiazygos vein on the left. The ascending lumbar veins interconnect with other retroperitoneal veins, such as the intercostal and renal veins.
The pelvic structures drain through veins named in a manner analogous to the arterial supply. The superior gluteal, inferior gluteal, and obturator veins coalesce into the internal iliac veins, which drain into the common iliac vein. The visceral structures of the pelvis drain by the middle and inferior rectal (also known as hemorrhoidal), vesical, uterine, vaginal, and prostatic veins. These veins are all interconnected with each other, so that precise labeling of structures is not always possible. In addition, the middle hemorrhoidal vein anastomoses with the portal venous system through the superior hemorrhoidal vein.
The testicle drains initially into the pampiniform plexus, a complex of venous sinuses contained in the scrotum. The anterior pampiniform plexus drains into the internal spermatic vein, the middle plexus drains around the ductus deferens, and the posterior plexus drains along the posterior edge of the spermatic cord into branches of the internal pudendal veins. The three components of the pampiniform plexus anastomose and allow collateral drainage.
The internal spermatic (gonadal) vein traverses the retroperitoneum as described earlier and enters the renal vein on the left and the IVC on the right. The internal spermatic vein is a single vessel in only about 50% of individuals. Valves are usually present in these veins (see Fig. 13-1 ). Rarely, the internal spermatic vein may communicate with the portal veins.
The uterus has a prominent venous plexus that drains through the broad ligaments to the uterine veins. The uterine veins primarily drain into the internal iliac veins, but also through the gonadal veins and through the perineum along the vagina to the labial veins. In pregnancy, the uterine plexus dilates enormously and is often associated with prominent gonadal and labial veins. The ovarian (gonadal) veins drain into the IVC at the level of the renal veins. Valves are present in 85% of left and 95% of right ovarian veins. In a manner analogous to the internal spermatic veins, the ovarian veins may be single or multiple, and have multiple communications with other retroperitoneal veins ( Fig. 13-8 ).
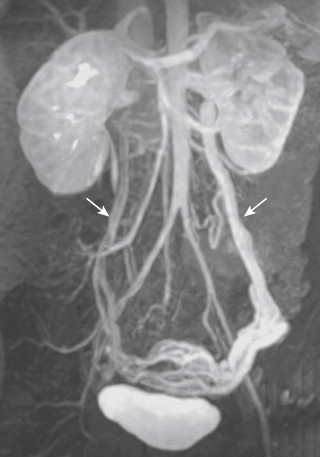
In most individuals, the adrenal glands are each drained by a single vein. Both adrenal veins communicate with renal capsular and retroperitoneal veins. Multiple adrenal veins are the exception, but do occur. The right adrenal vein empties directly into the IVC 2-5 cm above the right renal vein, usually between T11 and L1 (see Fig. 13-1 ). The orifice of the vein is located on the right posterolateral wall of the IVC in three fourths of patients and on the left in about one fourth. The vein usually courses inferiorly toward the gland but may run superiorly in about 15% of patients. Rarely (approximately 8%) a small accessory hepatic vein drains into the right adrenal vein, or vice versa. The left adrenal vein drains into the superior aspect of the left renal vein 3-5 cm from the IVC. The left inferior phrenic vein forms a common trunk with the left adrenal vein before it joins the renal vein. The location of the left adrenal vein is extremely constant, but in unusual cases the vein may drain directly into the IVC. In 2% of patients, two left adrenal veins may be found.
The collateral drainage of the IVC varies with the level of the occlusion. Infrarenal obstruction results in drainage of the lower extremities via ascending lumbar, paraspinal, gonadal, inferior epigastric, and abdominal wall veins ( Fig. 13-9 ). Retrograde or obstructed flow in the internal iliac veins results in drainage through gonadal, ureteric, and the inferior mesenteric veins (the latter through anastomoses between the hemorrhoidal veins). Occlusion of the IVC between the renal and hepatic veins can be drained by all of the collateral routes described for infrarenal obstruction. In addition, the azygos and hemiazygos veins assume an important role, particularly for drainage of the renal veins. Obstruction at the level of the suprahepatic IVC (above the hepatic vein orifices) results in collateral flow through all of the routes described, except the inferior mesenteric vein.
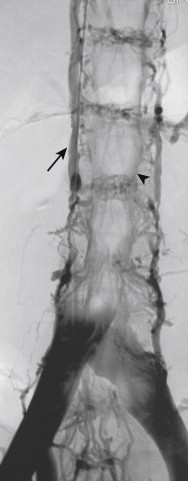
Renal vein obstruction on the right is drained via lumbar veins and the azygos vein. The ureteric vein may also hypertrophy in these patients. Renal vein obstruction on the left is drained by the lumbar veins, hemiazygos vein, and the left gonadal vein.
The rich network of intercommunicating veins in the pelvis allows collateral drainage of occluded gonadal veins through transpelvic, ascending lumbar, and internal iliac veins. Obstruction of the adrenal veins results in drainage through small retroperitoneal collaterals such as renal capsular veins.
Evaluation of the IVC with ultrasound is inexpensive and widely available. The intrahepatic portion of the IVC can be consistently visualized. Duplex ultrasound can provide information about direction of flow. However, the depth of the vessel within the abdomen, bowel gas, and obesity make imaging of the infrarenal IVC with ultrasound more difficult. Renal vein anatomy can be difficult to evaluate for similar reasons.
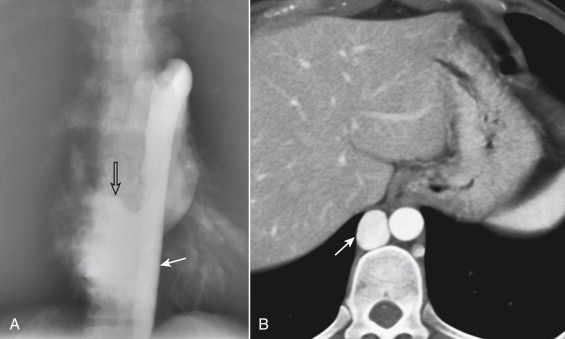
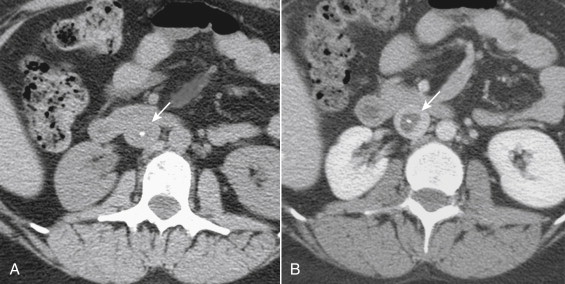
Imaging of the IVC with multidetector computed tomography (CT) is simple and highly accurate for most forms of pathology. For dedicated CT of the IVC, a triple-phase study consisting of a noncontrast scan, an arterial-phase acquisition, and a delayed acquisition (1-2 minutes) during the venous enhancement phase should be used. Contrast can be injected through an upper extremity vein. The collimation can be thicker than that used for arterial studies, because the venous structures are larger in diameter. The noncontrast scan is useful for detection of high-attenuation abnormalities such as high-density acute thrombus or calcified chronic occlusions ( Fig. 13-10 ). The late phase scan is necessary because mixing of opacified blood from the renal veins during the arterial phase can result in pseudo filling defects. Variant IVC and renal vein anatomy is depicted with sensitivity and specificity that exceeds 95%, particularly when studies are viewed on postprocessing workstations that allow reconstruction in multiple planes (see Fig. 13-6 ).
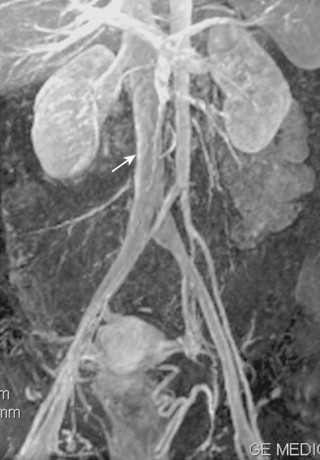
Magnetic resonance imaging (MRI) of the IVC and renal veins with venographic sequences (MRV) has similar accuracy, sensitivity, and specificity as contrast-enhanced CT. Anatomic sequences in at least two planes (axial and coronal) should be obtained, followed by flow sequences. Thick-sliced (5 mm) two-dimensional time-of-flight (2-D TOF) sequences acquired in the axial plane with superior saturation provide excellent images, although slow or retrograde flow in obstructed segments will suffer from signal loss. Gadolinium-enhanced breath-hold 3-D acquisitions of the IVC in the coronal plane are not susceptible to signal loss. These sequences are similar to those used for evaluation of the abdominal aorta. Imaging of the venous system is accomplished by repeating the same sequence after the arterial phase ( Fig. 13-11 ).
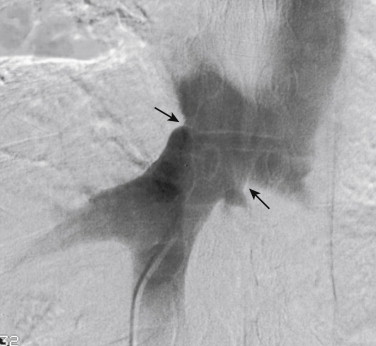
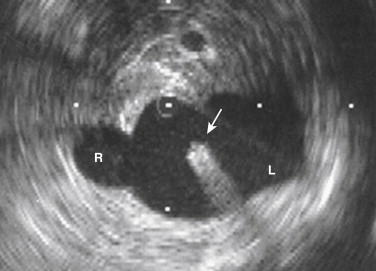
Cavography should be performed with a pigtail catheter positioned just at or slightly below the confluence of the common iliac veins (see Figs. 13-4 and 13-7 ). When an abnormality is suspected higher in the IVC, the catheter can be repositioned at that level after the initial injection. Power injection of contrast (20-25 mL/sec for 2 seconds) and rapid filming (3-4 frames/sec) are necessary to adequately opacify the IVC. When the right femoral approach is used for catheter placement, reflux of contrast into the proximal left common iliac vein is indicative of a conventional single IVC. Injection in the left common iliac vein is advocated by some interventionalists to exclude a duplicated IVC. An unexpectedly small infrarenal IVC at cavography from either side also suggests the presence of a duplicated IVC (see Fig. 13-4 ). Brisk unopacified inflow of renal vein blood produces a changing flame-shaped filling defect in the IVC contrast that points toward the heart. This should not be confused with a renal vein thrombus; repeat injection with the pigtail in the intrarenal IVC will usually resolve this question. In patients with elevated right heart pressures, contrast may reflux into hepatic and renal veins. Whenever the identity or location of a tributary vein is in doubt, selective injection with a visceral catheter should be performed. The projection most commonly used for cavography is anteroposterior, but the lateral view is useful when evaluating for obstruction or intraluminal masses. In patients with renal insufficiency or contrast allergies, either CO 2 or gadolinium can be used as appropriate. Alternatively, intravascular ultrasound (IVUS) with a low-frequency probe can be used to image the IVC and guide interventions ( Fig. 13-12 ). The major imaging IVUS landmarks of the IVC are the confluence of the iliac veins, the renal and hepatic veins, and the cavoatrial junction.
The renal veins have very high flow rates, which makes selective renal venography challenging. When the primary goals are localization of the renal veins or sampling, selective catheter shapes such as Cobra-2 or Simmons (1 for the right renal vein, 2 for the left) can be used. For diagnostic venography, catheters with multiple side holes should be used so that large volumes of contrast can be injected without traumatizing the vein. A multi-side hole straight or pigtail catheter directed into the renal veins with a deflecting guidewire is a useful combination for renal venography. When extensive filling of the intrarenal veins is required, injection of 10 μmg of epinephrine into the renal artery temporarily reduces venous outflow (see Fig. 13-5 ). Typical renal venography injection rates are 10-15 mL/sec for 2 seconds, with rapid filming (3-4 frames/sec).
Selective gonadal venography is simplified by the consistent location of the left gonadal vein orifice in the left renal vein. A left renal venogram often localizes the orifice of the left gonadal vein (see Fig 13-5 B ). Injection during the Valsalva maneuver or tilting the venography table in reverse Trendelenburg may be necessary to unmask reflux into the gonadal vein. A competent valve at the gonadal vein orifice can make selective placement of a catheter difficult. The right gonadal vein orifice is more difficult to localize as it originates from the IVC. When a femoral venous access is used, a Cobra-2 catheter can be used to select the left gonadal vein, and a recurved catheter such as a Simmons-1 can be used for the right ( Fig. 13-13 ). From a jugular approach, a Headhunter-1 or similar angled catheter can be used to select each vein. Many angiographers prefer a jugular venous access for gonadal venography, particularly when an intervention is anticipated. A steerable angled hydrophilic guidewire is helpful to advance the catheter into the gonadal vein. Hand injection of 5-15 mL of contrast is usually sufficient to opacify a normal gonadal vein. Higher volumes may be necessary in dilated veins. Shielding of the gonads is not possible in female patients but should be considered in males.
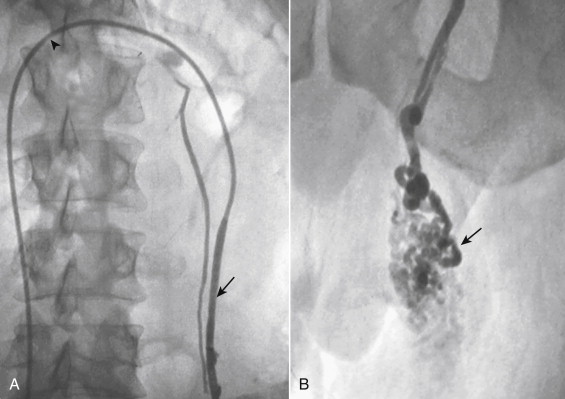
Adrenal venography is usually only required to confirm catheter location during adrenal vein sampling ( Fig. 13-14 ). Review of abdominal CT scans before the procedure can help localize the right renal vein. A single side hole is added near the tip of the catheter to facilitate aspiration of blood. The left adrenal vein is easily catheterized with a 5-French Simmons-2 or similar long recurved catheter. The tip of the catheter is placed in the left renal vein beyond the ostium of the left phrenic vein. The catheter is then slowly retracted so that the tip pushes against the superior wall of the renal vein. As the catheter is withdrawn, the tip will engage the phrenic vein, and allow selection of the adrenal vein. On occasion, a microcatheter is used to subselect the adrenal vein. On the right, a steeply angled 5-French catheter is used to find the right adrenal vein above the right renal vein. Small hepatic branches can be easily confused with the adrenal gland. A few milliliters of contrast should be slowly and gently injected to avoid rupture of the delicate adrenal veins. Patients may report a mild ache or sensation of fullness in the back with adrenal vein injections. This characteristic sensation can be used to help determine when the correct vein has been selected. Adrenal venography has many appearances; a well-defined wedge-shaped structure with arborizing veins, a delta pattern of veins, a triangular pattern of veins, spiculated veins in the shape of the gland, or even a nonspecific-appearing vein in the expected location of the adrenal vein. On the right, collateral drainage into a hepatic vein suggests that the selected vein is an accessory hepatic rather than adrenal vein.
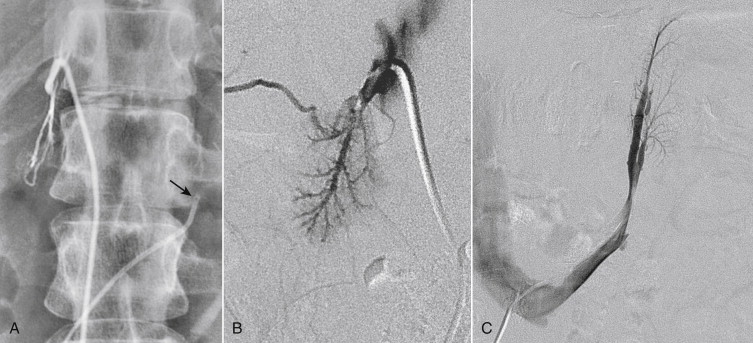
The usual location (>90%) of IVC occlusion is in the infrarenal segment. Obstruction can be caused by intrinsic or extrinsic pathology ( Box 13-1 ). The most common etiology of intrinsic obstruction is thrombosis, typically extension of iliac vein thrombus ( Box 13-2 ). Isolated thrombotic occlusion of the IVC is unusual in the absence of a translumbar IVC line, an IVC filter, trauma, or surgery. Delayed thrombosis can occur following liver transplantation due to stenosis of the caval anastomoses.
Thrombosis
Stenosis
Tumor
Primary
Invasion from adjacent organ/retroperitoneum
Iatrogenic
Enlarged liver
Hypertrophy
Tumor
Regenerating nodules
Compression by retroperitoneal mass
Adenopathy
Tumor
Aortic aneurysm
Pregnant uterus
Retroperitoneal fibrosis
Surgical ligation, clip
Abdominal compartment syndrome
Hypercoagulable state
Instrumentation
Central venous access catheter (transfemoral or direct inferior vena cava [IVC])
Partial IVC interruption (filter or clip)
Surgery
Extrinsic compression
Tumor (primary IVC or invasion)
Chemotherapy
Extrinsic compression of the IVC can be caused by tumor, adenopathy, aortic aneurysm, pericaval fibrosis, surgical ligation, and hepatic enlargement (see Fig. 13-9 ). In pregnant patients, positional obstruction of the IVC can be caused by the enlarged uterus. This is usually relieved when the patient is in the left lateral decubitus position.
The symptoms of IVC obstruction vary with the rapidity of the occlusion, the status of collateral veins, and the flow within the venous system. Gradual occlusion of the IVC may be asymptomatic if the collateral veins are intact and enlarged. Acute occlusion usually results in sudden onset of bilateral lower extremity edema. The swelling subsides, sometimes completely, as collateral veins become recruited and enlarge. In some patients, sudden interruption of venous return from the lower extremities may result in hypotension. This rare complication is most likely to occur in a patient with an IVC filter that becomes acutely occluded by a large embolus. In patients with disrupted or thrombosed collateral veins, even gradual occlusion may cause symptoms. Exercise, or creation of a lower extremity arteriovenous fistula, may unmask a previously asymptomatic obstruction by virtue of the increased venous flow.
On physical examination, the finding of bilateral lower extremity edema and dilated abdominal wall veins should suggest occlusion of the IVC. Patients with longstanding IVC occlusion can develop typical venous stasis changes of the lower extremities, such as brawny discoloration of the skin, woody edema, and ulcers.
Obstruction of the IVC can be suggested on duplex ultrasound examination when there is stasis of flow, loss of the normal respiratory variation, or thrombosis of the iliofemoral veins bilaterally ( Fig. 13-15 ). A blunted or absent Doppler response to Valsalva is suggestive of central occlusion when the finding is bilateral. Direct interrogation of the IVC may be possible, but it cannot be reliably performed in the infrarenal segment in all patients.
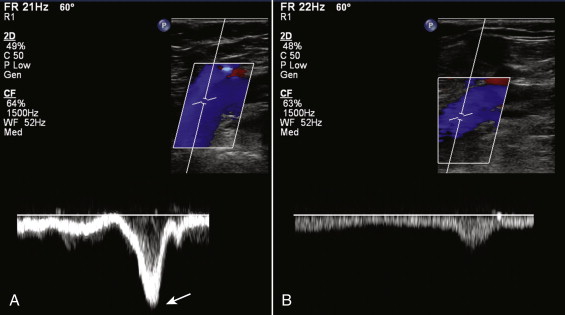
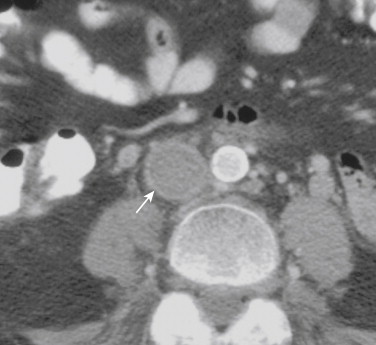
Cross-sectional imaging with CT and MR allows diagnosis of IVC obstruction as well as evaluation of the adjacent soft tissues for possible causes. Expansion of the IVC implies an intrinsic occlusion, whereas compression is almost always associated with an obvious extrinsic mass lesion. High-attenuation material in the IVC on a noncontrast CT can be seen with acute thrombus. Enhancement of the wall of the IVC with a low-attenuation lumen after administration of contrast is diagnostic of an intrinsic occlusion (typically bland thrombus) ( Fig. 13-16 ). Contrast may be visualized around an intraluminal filling defect at the proximal and distal extent of an occlusion, or with incomplete obstruction. Intraluminal tumor may enhance with contrast (see Tumors). The extent of an occlusion may be overestimated if stagnant blood caudad to an obstruction does not enhance with contrast. Delayed scans help avoid this pitfall.
Diagnostic cavography provides less information about the etiology of an occlusion than ultrasound, CT, or MRI, but may be necessary to localize the pathology as intrinsic or extrinsic and determine the extent. Intraluminal occlusions have a characteristic appearance with contrast on both sides of a filling defect ( Fig. 13-17 ). Extrinsic compression causes broadening or effacement of the lumen (see Fig. 13-9 ). Rarely, IVUS is required to make a conclusive diagnosis. Stenosis of the IVC can be evaluated with measurement of a pressure gradient across the lesion. In the supine patient, the normal gradient should be less than 3 mm Hg. When performing cavography in a patient with obstruction, contrast should be injected as close to the lesion as possible to avoid overestimating the extent of occlusion. Contrast injected in a remote location such as a femoral vein preferentially opacifies the collateral veins rather than the obstructed IVC. Patients should not be instructed to perform Valsalva during injection of contrast, because the IVC lumen may be artifactually obliterated by the transient increase in intraabdominal pressure.
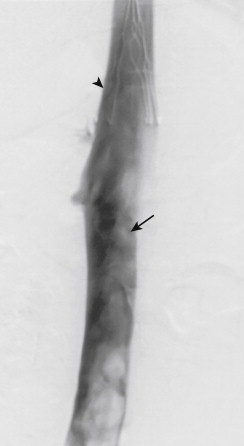
Acute thrombosis of the IVC is often linked to iliofemoral thrombosis, occlusion of an IVC filter, or a hypercoagulable condition. Therapy for IVC thrombosis is usually the same as that for lower extremity deep venous thrombosis. Anticoagulation, elevation of the extremities, and compression stockings are the traditional medical treatments. Surgical thrombectomy is rarely indicated. Catheter-directed thrombolysis or catheter thrombectomy with pharmacomechanical or mechanical techniques can provide rapid relief of symptoms. (This technique is discussed in Chapter 16 .) One of the goals of thrombolysis or thrombectomy is the unmasking of underlying IVC pathology that may have precipitated the thrombosis ( Fig. 13-18 ). There is a small risk (1%) of major pulmonary embolism (PE) during interventions for caval thrombus. Optional or temporary IVC filters may have a role in selected patients as a preventive measure.
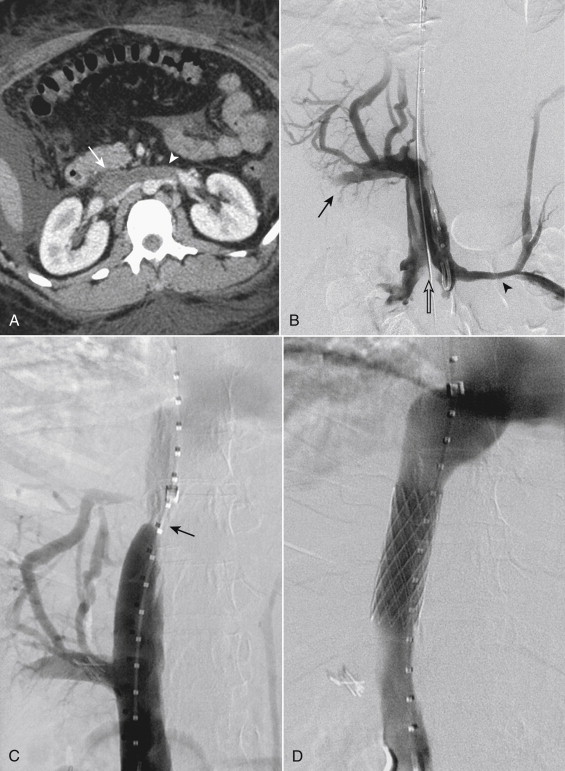
Symptomatic stenoses of the IVC or other occlusive lesion can be stented to prevent recurrent thrombosis. A variety of stents have been used for this purpose, including both balloon-expandable and self-expanding. Stents with diameters in the range of 12-30 mm are required. The long-term results of IVC stents for benign stenoses in the absence of a hypercoagulable state have been excellent when large-diameter stents can be placed. The reported patency is 80% at 5 years.
Chronic occlusions of the IVC can be successfully recanalized using standard techniques. Evaluation with CT before the procedure is helpful to plan the procedure. The longer the occlusion, the more difficult it will be to recanalize successfully. Complete absence of a visible IVC remnant on CT is discouraging, but does not exclude successful recanalization. Simultaneous jugular and femoral vein access is often necessary to provide a visual target during recanalization and assist in delivering devices across the lesion. Stent placement is almost always necessary. Large-diameter self-expanding stents are preferred in this location. Longstanding occlusions may not initially dilate beyond 10 or 12 mm diameter due to atresia of the IVC remnant. These sometimes respond to gentle angioplasty and dilate further at a later date. Stents can be used in the presence of a chronically occluded IVC filter. In this situation, the stents are placed through the filter, pushing the filter elements to the side. Surgical approaches with embolectomy, bypass, and hybrid techniques that include stent placement have a 5-year primary patency of 40%-50%. Large studies with long-term results of endovascular approaches are not available, but should have similar if not slightly better patency.
Treatment of symptomatic extrinsic compression of the IVC (massive lower extremity and scrotal/labial edema, abdominal wall edema, and ascites) should be directed, when possible, to the mass or lesion causing the compression. Resection of tumors, shrinkage of nodal masses with chemotherapy or radiation, or patience (especially in the case of compression of the IVC by a pregnant uterus) may resolve symptoms. Diuretics, correction of hypoalbuminemia, compression stockings, and anticoagulation or antiplatelet therapy should be instituted when possible. When conservative measures fail or are not feasible, stent placement is an excellent option for patients with malignant or other terminal illnesses who seek improvement in the quality of their remaining life ( Fig. 13-19 ). Large self-expanding stents should be used, sized to the normal diameter IVC. The stent should not protrude into the right atrium if possible, because this may induce arrhythmias and the motion may fracture the stents. Approximately two thirds of patients experience significant symptomatic relief. The average life expectancy of these patients when the compression is due to malignancy is well less than 6 months.
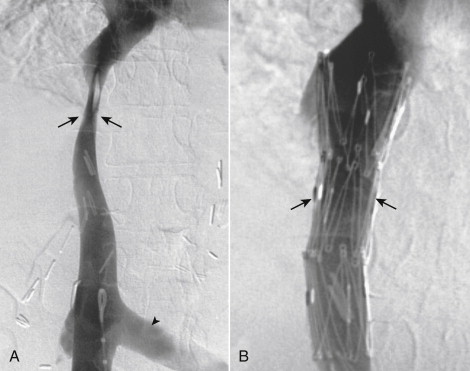
Vena cava filters prevent thrombotic pulmonary emboli (PE) by trapping the thrombus in the vena cava. Filters do not prevent the formation of thrombus, enhance anticoagulation, or treat PE that has already occurred. The conventional treatment for deep venous thrombosis (DVT) and PE is anticoagulation. Filters should be used when patients with venous thromboembolism (VTE) cannot be anticoagulated or when patients at high risk for development of DVT cannot be screened, monitored, or receive prophylaxis. Filters placed in patients who are at risk for but do not yet have VTE are considered prophylactic, a controversial but very common indication. There is no role for filters in stable patients with VTE who can be treated with anticoagulation ( Box 13-3 ). Patients who receive filters should resume conventional treatment appropriate for their degree of VTE as soon as practical.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here