Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The common wart (verruca vulgaris) is caused by infection with human papillomavirus (HPV) ( Fig. 18.1 ). HPV is a DNA virus of the papovavirus family. The number of known HPV genotypes currently stands at more than 200, classified according to the extent of their DNA homology (DNA hybridization) ( Table 18.1 ). In order for an HPV type to be regarded as ‘new’, sequences in selected genomic regions must exhibit more than 10% divergence compared to any of the known HPV types. Monoclonal antibodies to intact viruses have been produced and can demonstrate individual types of HPV; antibodies to viral components are only group specific ( Fig. 18.2 ). Advances in molecular pathology have resulted in improved and more specific methods of HPV detection and classification, including in situ polymerase chain reaction (PCR), nonisotopic in situ hybridization (NISH), rolling circle amplification and next-generation sequencing. Five genera exist, namely, α, β, γ, µ, and ν. The α HPV types are predominantly mucosotropic, and are often classified as low-risk (e.g., HPV6, HPV11) or high-risk (e.g., HPV16, HPV18) based on their association with the development of cancer, including cervical and anogenital neoplasms and some carcinomas of the oral cavity. Although the other four genera are associated with cutaneous infection, it is the β HPV types that are of particular relevance in the etiopathogenesis of nonmelanoma skin cancer in the setting of epidermodysplasia verruciformis (EV).
| HPV type | Associated clinical lesions |
|---|---|
| 1 | Deep plantar warts, common warts |
| 2 | Common warts, flat warts |
| 3 | Flat warts |
| 4 | Common warts, plantar warts |
| 5 | Epidermodysplasia verruciformis (EV) |
| 6 | Genital warts, laryngeal papilloma |
| 7 | Butcher warts |
| 8 | EV |
| 9 | EV, keratoacanthoma |
| 10 | Flat warts |
| 11 | Laryngeal papillomas, genital warts |
| 12 | EV |
| 13 | Focal epithelial hyperplasia |
| 14, 15 | EV |
| 16 | Genital warts, bowenoid papulosis, cervical dysplasia, cervical carcinoma, digital, squamous cell carcinoma, nongenital Bowen disease |
| 17 | EV |
| 18 | Genital warts, bowenoid papulosis, cervical dysplasia, cervical carcinoma |
| 19–25 | EV, keratoacanthoma |
| 26–29 | Common warts, flat warts |
| 30 | Laryngeal carcinoma, genital warts |
| 31–32 | Genital warts, bowenoid papulosis, cervical dysplasia, cervical carcinoma |
| 33 | Cervical carcinoma |
| 34 | Bowenoid papulosis, Bowen disease |
| 35 | Cervical dysplasia, cervical carcinoma |
| 36 | EV |
| 37 | EV, keratoacanthoma |
| 38 | EV |
| 39 | Bowenoid papulosis, cervical carcinoma |
| 41 | Flat warts |
| 42 | Genital warts, bowenoid papulosis, cervical dysplasia, cervical carcinoma |
| 43, 44 | Genital warts, laryngeal papillomas |
| 46, 47 | EV |
| 48 | Bowenoid papulosis, Bowen disease |
| 49, 50 | EV |
| 51–54 | Genital warts, bowenoid papulosis, cervical dysplasia, cervical carcinoma |
| 55 | Genital warts, laryngeal papillomas |
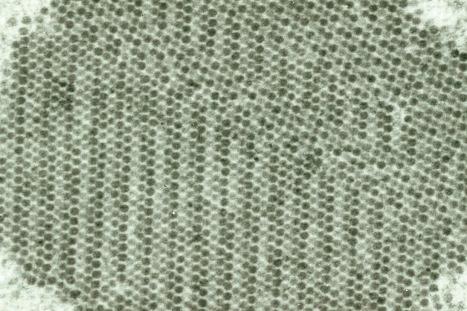
Papillomaviruses, which are small and nonenveloped and show icosahedral symmetry, contain circular double-stranded DNA composed of approximately 8000 base pairs. The viral particle, which has a diameter of approximately 55 nm, contains 72 capsomeres. The HPV genome is divided into three functional regions: a late region, an early region, and a noncoding 1000 base pair upstream regulatory region (URR). The URR is located immediately upstream of the E6 open reading frame (ORF) and contains sequences regulating expression of all ORFs, including promoter elements and transcriptional enhancer sequences. In excess of 20 messenger RNAs are expressed, usually in a differentiation-specific and cell-specific manner. Genes in the early region (E1, E2, E4, E5, E6, E7) are responsible for transcription, replication, and cellular transformation. The E4 ORF is highly expressed in differentiated HPV-infected epithelial cells. Some forms of E4 encode a protein capable of disrupting the cytokeratin network, resulting in the phenomenon of koilocytosis. The E4 ORF represents a region of maximal divergence between different HPV types. Each viral genotype is most often detected in lesions at specific anatomical sites or shows distinct histologic characteristics.
HPV infection in man results in a variety of cutaneous lesions including verruca vulgaris, filiform warts, verruca plana, plantar warts, anogenital warts, and bowenoid papulosis. Mucosal lesions include oral warts and condylomata, focal epithelial hyperplasia or Heck disease, nasal and conjunctival papillomas, laryngeal papillomatosis, and cervical lesions. HPV infection may be asymptomatic or result in a carrier status. One study showed that cutaneous HPV infections commonly persist on healthy skin over several years, and that persistence does not appear to be associated with age, sex, a history of warts, immunosuppressive therapy, or HPV type.
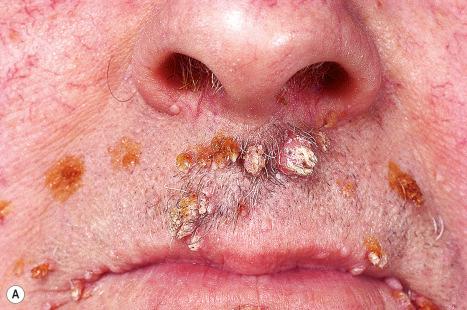
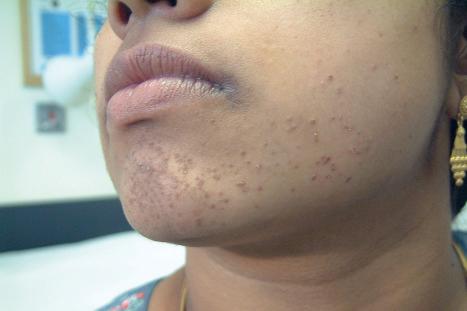
Common warts are caused by HPV types 1, 2, 4, 7, and 26–29. In immunosuppressed patients, HPV subtypes 75, 76, and 77 may be pathogenetic. A case with extensive, recalcitrant verrucae linked to infection with HPV type 57 has been reported. Rarely, HPV subtypes associated with genital warts such as 6 and 11 have been found in common warts in children. HPV16, a common genital HPV type with oncogenic potential, was detected in 6.6% of lesions in a series of 45 immunocompetent patients with nongenital cutaneous warts. Conversely, verrucae vulgaris may sometimes occur on the vulva. HPV type 2 has been detected in such cases. It is important to note that these ‘nonvenereal’ genital lesions may occur in girls less than 5 years of age, as an erroneous clinical or histologic diagnosis of condylomata acuminata could lead to allegations of sexual abuse.
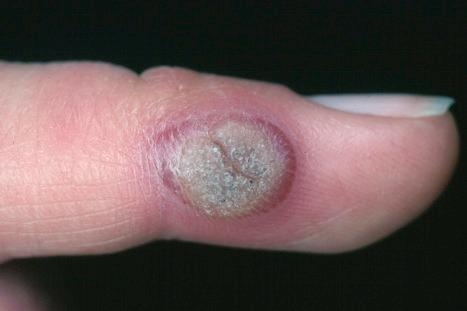
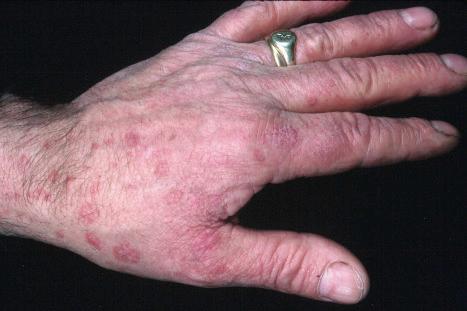
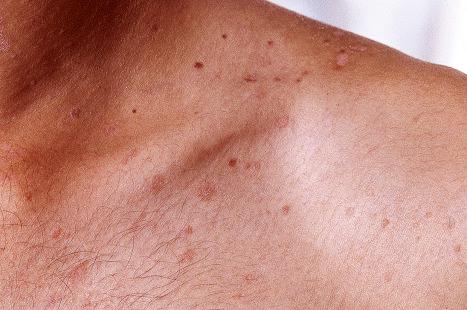
Warts are very common lesions, particularly in children. Adults are also frequently affected. In a survey of 2180 adults, 3.5% had warts. Butchers and slaughterhouse workers have an increased risk. Common warts may occur anywhere on the skin and in people of any age, but are most common on the backs of the hands and the fingers and on the knees of young children, where they appear as firm keratotic papules 1–10 mm across ( Fig. 18.3 ). Koebnerization is common (hence, kissing lesions on fingers). In other sites they may appear more filiform and less firm ( Fig. 18.4 ). The latter are particularly seen on the lips, nostrils, and eyelids. Giant periungual lesions have been described. Warts may also present as a cutaneous horn. They persist for a few months up to several years and often regress spontaneously, particularly in children.
Chronically immunosuppressed patients (e.g., following renal transplantation) often have a large number of warts ( Fig. 18.5 ). Chronicity is associated with increasing numbers of lesions. EV-like lesions due to HPV5 have also been described in human immunodeficiency virus (HIV)-positive patients and following renal transplantation. Numerous warts may be seen in other immunosuppressed patients (e.g., with non-Hodgkin lymphoma, leukemia, Hodgkin lymphoma, and HIV infection). In patients with acquired immunodeficiency syndrome (AIDS), warts may regress following antiretroviral therapy (ART).
In the skin, it is the inter-appendageal epidermis and the stem cells of the bulge region of the hair follicle that are the apparent targets of the virions. In situ hybridization studies of HPV lesions have shown that viral DNA synthesis in the epidermis occurs in the superficial prickle cell layer, and full virus assembly with capsid production occurs in the granular cell layer. HPV DNA has been demonstrated in apparently normal skin up to 15 mm from a virus-associated lesion. The requirement for growth in very well-differentiated epithelia may explain the difficulty of culturing HPV and why host destruction of the lesions may be protracted. Immune mechanisms are presumed to be less effective against organisms or altered cells that are situated superficially with no direct blood supply.
Regression of HPV lesions is usually spontaneous but may not occur for several years. Cell-mediated immunity seems to be important in effecting the regression since lymphocytes are seen infiltrating the wart epithelium at this stage. Other features of regression include liquefactive basal cell degeneration, epidermal degeneration, and vascular thrombosis. Toll-like receptors (TLRs) have been identified as important role players in viral recognition and the initiation of an antiviral host immune response. TLR3, TLR9, interferon-beta (IFN-β), and tumor necrosis factor-alpha (TNF-α) appear to play an important role in the skin's innate immune response to HPV infection. Langerhans cells and Langerhans-like dendritic cells may exert direct antiviral activity. This is facilitated via the expression of TLRs such as TLR3, which may in turn trigger the release of IFN-inducible chemokines, including CXCL9, a monokine induced by IFN-γ.
Following regression of the wart(s), an individual is usually immune to further HPV infection. Patients with a deficiency in cell-mediated immunity – whether primary or acquired, iatrogenic or virally induced (HIV/AIDS) – are particularly susceptible to the development of warts, which tend not to involute spontaneously and can be a particularly refractory therapeutic problem.
Transmission of HPV is by inoculation of infected desquamated cells through close contact at points of minor trauma; hence, common warts are seen most often on the hands. Periungual warts are particularly associated with nail biting and plantar warts are especially related to prolonged immersion in water.
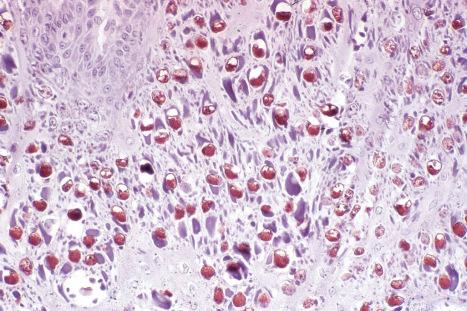
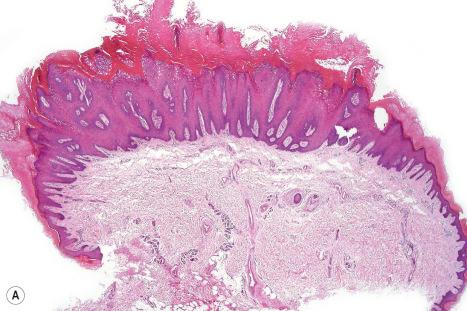
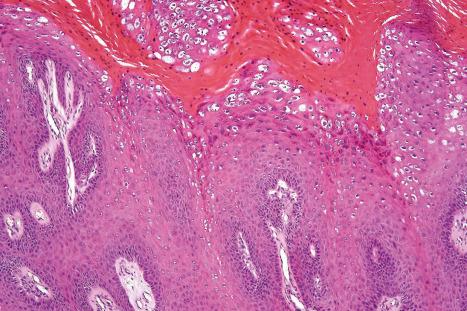
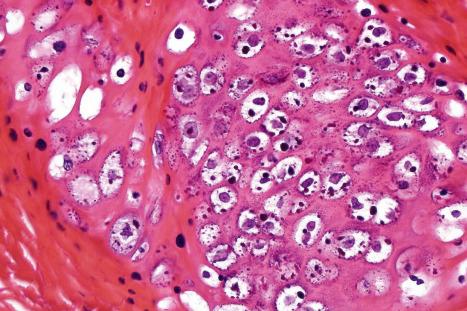
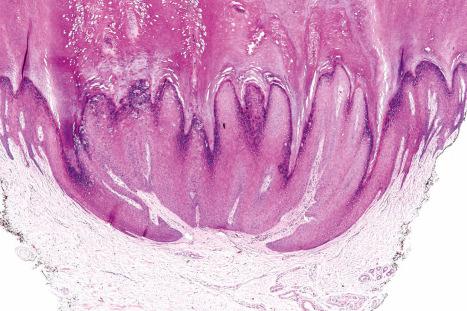
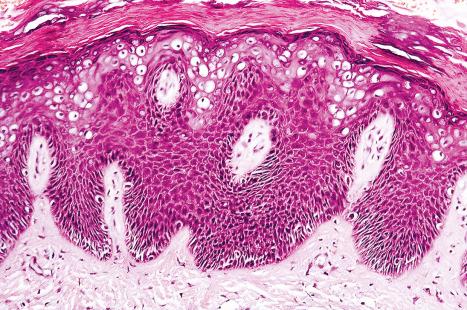
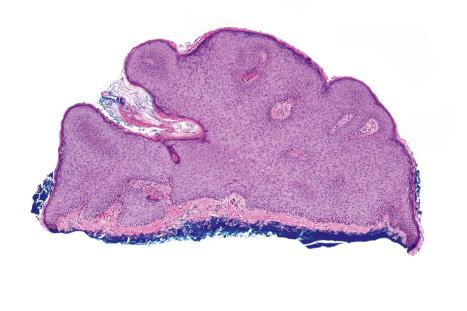
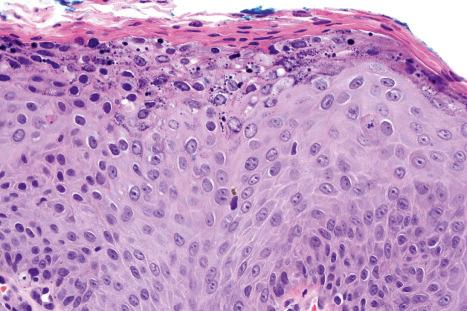
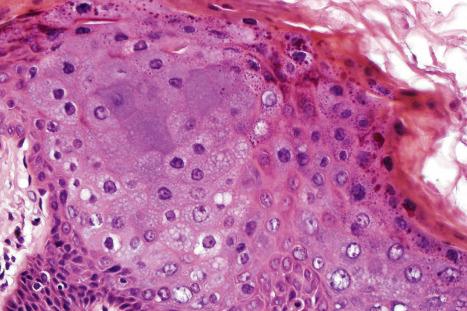
Common warts show filiform acanthosis with vertical tiers of parakeratosis over the tips of the exophytic component ( Fig. 18.6 ). There is also marked orthokeratosis. A downward extension of the acanthosis produces a curvilinear deep margin and curved distortion of the adjacent rete ridges in the uninvolved epidermis. There is a prominent granular cell layer within which are enlarged clumps of irregular basophilic keratohyalin ( Fig. 18.7 ). These are seen best in the concavities between the papillomatotic epithelial papillae. Large cells with prominent vacuolated cytoplasm and a small pyknotic nucleus are seen in the upper layers of the epidermis (koilocytes) ( Fig. 18.8 ). Koilocytes are, however, more frequently observed in genital warts (see below). Connective tissue and tortuous small blood vessels may invade the filiform projections ( Fig. 18.9 ). In some cases, involvement of the superficial portion of the hair follicles by HPV results in focal changes identical to a trichilemmoma or an inverted follicular keratosis. However, not all of these lesions are induced by HPV as has been suggested.
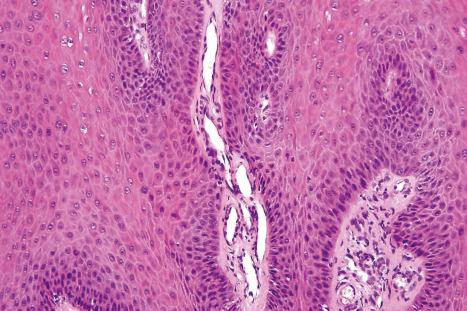
Ordinary common warts are only exceptionally associated with in situ or invasive squamous cell carcinoma. HPV16 has been associated with periungual Bowen disease and squamous carcinoma. The role of HPV in cutaneous neoplasia is discussed further in Chapter 22 . Molecular studies have implicated cutaneous HPV infection as a carcinogenic cofactor in association with solar ultraviolet radiation in the evolution of nonmelanoma skin cancer.
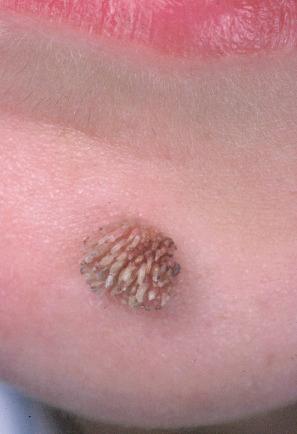
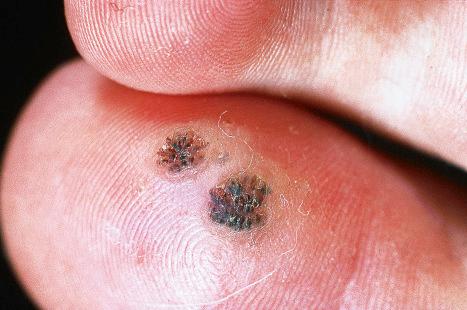
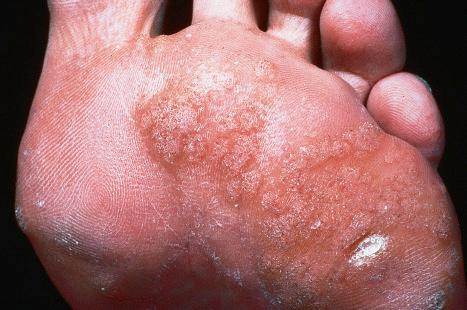
Plantar warts occur on the sole of the foot; they are only slightly elevated and appear as a horny plug surrounded by a ring of hyperkeratotic skin ( Fig. 18.10 ). Often, they are covered with black dots representing thrombosed capillaries ( Fig. 18.11 ). They are most common in children and are frequently seen over pressure points. Most plantar warts are caused by HPV1 and are painful; however, HPV4 may produce a confluent or mosaic pattern of similar small warts (‘mosaic plantar warts’) and these are painless ( Fig. 18.12 ). They may also be seen on the palms and in the periungual region. There have been reports from Japan of unusual plantar warts produced by HPV60.
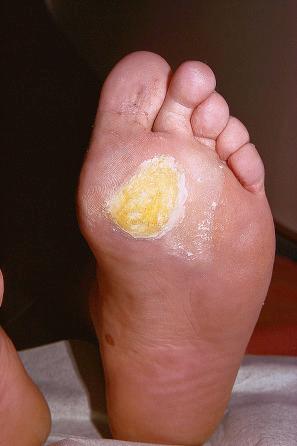
The lesions may be nodular, ridged, or pigmented. A cystic variant has also been described. The cystic variant has the features of an epidermoid cyst and may rarely be multiple. Most are associated with HPV60, but an association with HPV57 has also been reported. Epidermoid cysts induced by HPV may also be seen outside acral locations. Pigmented warts are caused by HPV4, 60, or 65 and may contain fibrillar intracytoplasmic inclusion bodies. A case of a large plantar wart caused by HPV66 has been documented. A further subtype of HPV associated with palmoplantar warts is HPV63.
Plantar warts usually regress within a few months in children, but may persist longer in adults. Rarely, chronic plantar warts may be associated with the development of verrucous carcinoma (carcinoma cuniculatum) (see Chapter 22 ).
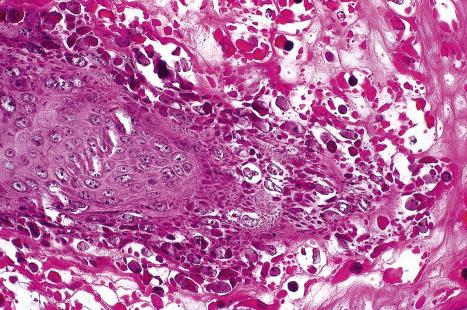
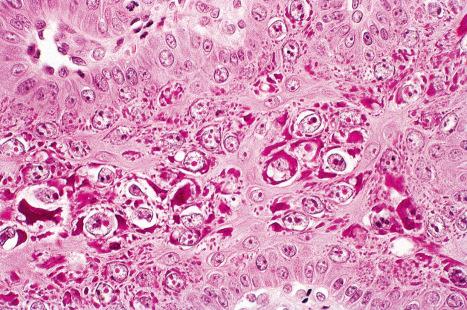
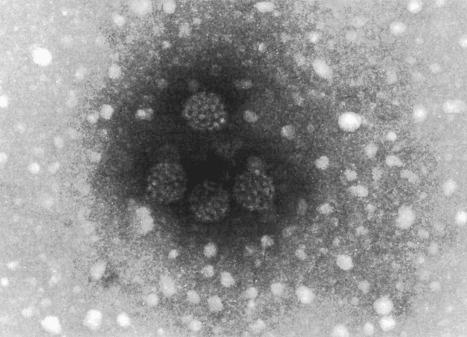
Plantar warts are almost entirely endophytic, with a central parakeratotic plug surrounded by multiple deep extensions of acanthotic epidermis ( Fig. 18.13 ). The depth and complexity of these downgrowths have been likened to an anthill, giving rise to the term ‘myrmecia’. Vacuolation is more prominent in the plantar wart and, in the active growing phase, large eosinophilic (and to a lesser extent, basophilic) cytoplasmic inclusions are present, which represent disordered growth of giant keratohyalin granules ( Fig. 18.14 ). The large eosinophilic cytoplasmic inclusions are usually seen in infections caused by HPV1 and to a lesser extent in those caused by HPV60 and HPV65. In warts induced by HPV4, the infected keratinocytes show prominent cytoplasmic vacuolar change with almost no keratohyalin granules. Intranuclear inclusions may also be evident ( Fig. 18.15 ). HPV can be demonstrated in the nuclei of these cells via electron microscopy ( Fig. 18.16 ). Melanin granules are discernible within the cytoplasm of HPV60-induced pigmented plantar warts.
Regressive changes are the same as those described in common warts and consist of thrombosis of superficial blood vessels, necrosis, and a mixed inflammatory cell infiltrate. A recently described multiplexed PCR-based assay may have merit in both HPV genotyping and in monitoring treatment efficacy.
Plane warts (verrucae plana), usually caused by HPV2, 3, or 10, are flat, smooth, and a few millimeters in diameter with typically little change in color from the adjacent skin, although they may appear gray-yellow or pale brown ( Fig. 18.17 ). HPV5 is rarely implicated in HIV-infected patients. Plane warts may also result from HPV types 26–29 and 41 infection. They affect the face, backs of the hands, and the shins. There may be only a few present, but occasionally they are very numerous and become confluent in areas of scratching (koebnerization). Plane warts are common in children and may be seen in women, but are not usually found in males after puberty except in association with HIV infection. They may regress spontaneously after a few weeks or months, or may persist for years. Signs of regression include pruritus, an erythematous, edematous appearance, depigmented haloes, and an eruption of multiple tiny plane warts. Cell-mediated immunity plays a key role in the spontaneous regression of plane warts in immunocompetent individuals. Multiple plane warts may evolve as a cutaneous manifestation of immune reconstitution inflammatory syndrome (IRIS) in HIV-infected patients receiving highly active ART. Exacerbation of lesions has been reported following facial laser resurfacing.
Plane warts are acanthotic and show orthokeratosis with an open pattern reminiscent of ‘chicken wire’ (‘basket weave’ hyperkeratosis). Parakeratosis is not a feature and there is little papillary configuration to the acanthosis ( Fig. 18.18 ). Keratinocytes of the upper part of the stratum spinosum show striking cytoplasmic vacuolation with margination of the keratohyalin granules and tonofilaments. Regression is characterized by keratinocyte necrosis (apoptosis), individual cell keratinization, parakeratosis, lymphocytic exocytosis with spongiosis, and a superficial perivascular chronic inflammatory cell infiltrate. The lymphocytes encountered in regressing lesions have been found to express the cytotoxic granule granzyme-B. Extravasation of erythrocytes may be a feature and edema of the papillary dermis is frequently present.
A majority of sexually active individuals will have detectable HPV infection at least once during their lifetime. An estimated 14 million people are infected annually with genital HPVs. A recent systematic review of the literature concerning anogenital warts revealed a median incidence of 137 and 120.5 per 100 000 among males and females, respectively.
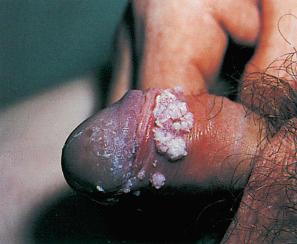
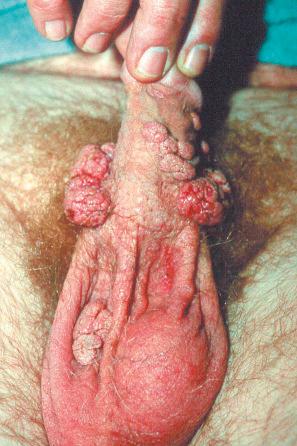
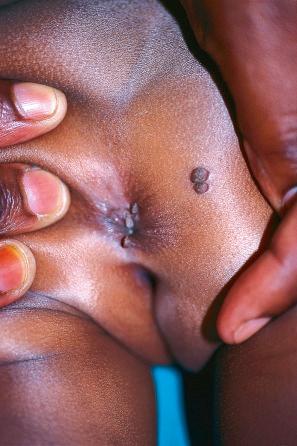
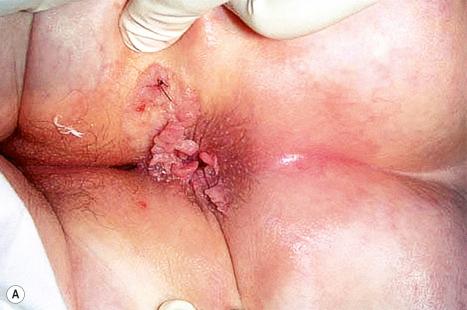
Condylomata acuminata are particularly caused by HPV types 2, 6, 11, 16, 18, 30–33, 35, 39, 41–45, 51–56, and 59 and develop as a consequence of the trauma accompanying sexual intercourse. More frequent transmission has been reported from females to males than from males to females. HPV6 and 11 alone account for more than 90% of these lesions, with HPV6 present in about two-thirds of cases and the remaining one-third caused by HPV11. The incubation period is variable (usually between 2 and 3 months). Condylomata acuminata occur on the glans penis and prepuce or shaft as soft, fleshy, sometimes filiform plaques and may extend into the meatus ( Figs 18.19 and 18.20 ). On the shaft, they are less exophytic. Vulval lesions may be bulky and macerated, and may extend into the introitus ( Fig. 18.21 ). Similar fleshy and filiform soft masses occur perianally, more often in males ( Fig. 18.22 ). Anal squamous carcinoma has also been shown to contain HPV6, 16, and 18 in a significant proportion of cases ( Fig. 18.23 ). The rate of local recurrence is about 30%. The lesions are uncommon in children (where they may be a sign of sexual abuse) and are seen most often in young adults (second and third decades), frequently in association with other genital infections. Childhood condylomata regress spontaneously in more than 50% of cases. Genital warts are common among HIV-infected individuals.
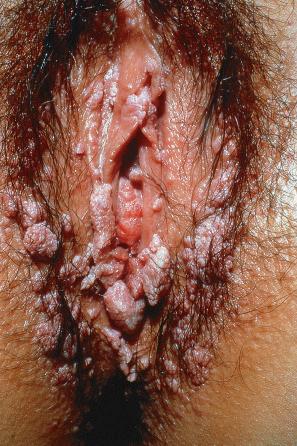
It is important to note that a significant proportion of genital HPV infections are asymptomatic. The female partners of male patients with condyloma acuminata have been shown to have an increased risk of cervical HPV infection and intraepithelial neoplasia (squamous intraepithelial lesion/cervical intraepithelial neoplasia [SIL/CIN]). Cervical neoplasia associated with pre-existent condylomata acuminata has also been related to a background of immunosuppression, at least in some patients. The worldwide HPV prevalence in cervical carcinomas is reported to be 99.7%. HPV16, 18, 31–33, 35, 39, 42, and 51–54 are most commonly associated with cancers of the cervix, vulva, and penis. Patients with condylomata acuminata are at increased risk for developing not only carcinomas of the vulva, vagina, penis, and anus, but also certain nonanogenital squamous cell carcinomas. Routine vaccination with a quadrivalent vaccine against HPV types 6, 11, 16, and 18 has led to a significant reduction in the burden of vulval and cervical carcinomas, genital warts, and anogenital intraepithelial neoplasia.
A large, exuberant, and locally destructive variant of condyloma (Buschke-Löwenstein tumor) may rarely be encountered ( Fig. 18.24 ). This is associated with HPV types 6, 11, or 16. It is likely that this giant variant represents a variant of verrucous carcinoma but the issue has been controversial (see Chapter 22 ). Juvenile laryngeal papillomas containing HPV6 and 11 can be seen in children born to mothers with condylomata acuminata. They may show malignant progression if irradiated.
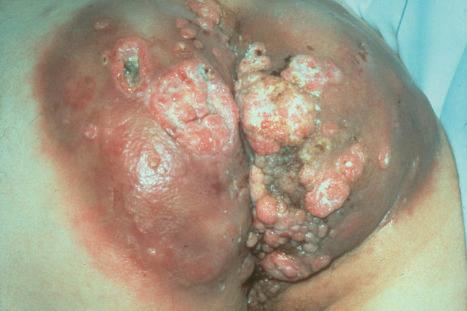
Malignant transformation of condyloma acuminatum is uncommon, but it is seen more often than in other lesions associated with HPV except for EV.
Condylomata acuminata are characterized by marked acanthosis with a solid or trabecular pattern and a broad rounded exophytic growth ( Fig. 18.25 ). There is a sharp, fairly regular, deep margin. The surface of the lesion is hyperkeratotic and parakeratotic. Superficial vacuolated keratinocytes (koilocytes) are characteristic ( Fig. 18.26 ) and coarse keratohyaline granules may be present. The vacuolated epithelium is often most marked in the declivities. Condylomata that are treated with podophyllin prior to removal demonstrate marked epidermal pallor and increased mitoses and necrotic keratinocytes in the lower half of the epidermis. These changes may lead to a misdiagnosis of malignancy. Giant condyloma acuminatum (anogenital verrucous carcinoma, Buschke-Löwenstein tumor) occurs most frequently on the genitalia, and is larger and more cauliflower-like. It shows some tendency to endophytic growth, but without any suggestion of frank infiltration. It can recur locally, but metastasizes very rarely. Most experts regard this lesion as a variant of verrucous carcinoma. Anal condylomata may develop bowenoid features, and occasionally invasive tumor supervenes.
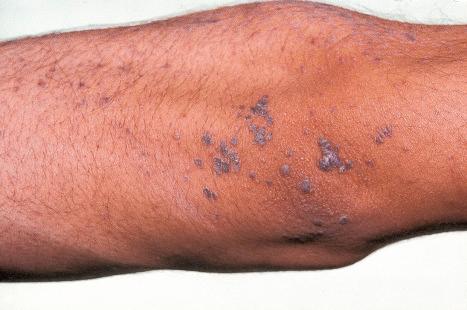
Bowenoid papulosis (koilocytosis with intraepithelial neoplasia) is a clinicopathological entity that bears marked histologic similarity to koilocytosis, SIL/CIN, and Bowen disease. Although the term is no longer used by the International Society for Study of Vulval Disease (ISSVD) and some have questioned the validity thereof, many clinicians believe that it represents a distinctive clinicopathological entity, and we have decided to describe it in this chapter. Clinically, it is quite different from genital Bowen disease in that multiple small papules develop over a short time scale in young people. Prognosis is uncertain; many patients do not show evidence of progression, but a small proportion may develop invasive tumor and, on occasion, this may have metastatic potential. It is usually associated with HPV16 or 18, but occasionally HPV types 31–35, 39, 42, 49, and 51–54 are detected. Although uncommon, some cases may be associated with mixed infection by different HPV types. A unique HIV-associated case with genital and extragenital (lip) lesions caused by two separate HPV types (HPV16 and HPV32, respectively) has been described. E6 and E7 viral oncoproteins of high-risk HPV types induce overexpression of p16 and human telomerase reverse transcriptase.
Bowenoid papulosis most often presents as multiple reddish-brown, sometimes lichenoid, discrete papules, but occasionally these become a confluent plaque. Papules, on average 4 mm in diameter, are found on the penis, vulva, perianal region, and perineum. Extragenital sites of occurrence include the face, neck, and fingers. The lesions are sometimes pigmented. A case of oral bowenoid papulosis in an HIV-infected male has been reported. Bowenoid papulosis manifests in young, sexually active adults in contrast to true Bowen disease, which occurs in an older age group. Genital Bowen disease is, however, also often associated with HPV16. The occurrence in childhood should raise suspicion of sexual abuse. Genital bowenoid papulosis has been associated with periungual bowenoid dysplasia. Bowenoid papulosis with concurrent Bowen disease has been reported in a patient with systemic lupus erythematosus (SLE).
Spontaneous regression is uncommon. As progression to frank invasive carcinoma in bowenoid papulosis is rare, these lesions are best managed conservatively. However, bowenoid papulosis may be resistant to treatment and may be characterized by a prolonged course in immunosuppressed patients. Bowenoid papulosis has also been associated with oral warts and lingual carcinoma. Patients with bowenoid papulosis and HPV infection may be primarily immunosuppressed due to diminished T-helper (Th) cell levels (non-HIV-associated). The condition may also occur in organ transplant recipients. Penile bowenoid papulosis is associated with a high risk of the consort developing cervical dysplasia. Consequently, female patients and consorts should regularly have cervical smears.
A bowenoid papulosis lesion consists of a well-circumscribed area of acanthosis producing a raised plaque or dome, which is hyperkeratotic and sometimes shows superficial epithelial vacuolation. The keratinocytes may show nuclear hyperchromatism and pleomorphism. There is variable dyskeratosis.
These histologic features of atypia, associated with numerous mitoses, including atypical forms, are similar to those of true Bowen disease. The distinction rests in the circumscribed elevated plaquelike pattern, the age of the patient, and the size and multiplicity of lesions. Immunohistochemistry for p16 reveals strong, diffuse staining of the full thickness of the lesional epidermis.
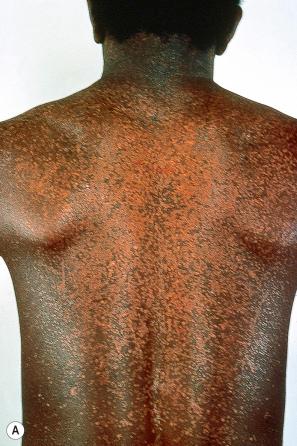
EV is a rare inherited condition characterized by selective susceptibility to skin infection with certain HPV types, defects in cell-mediated immunity, and an increased risk for the development of cutaneous malignancies, especially squamous cell carcinomas. The lesions of affected individuals are the result of infection with a wide range of HPV subtypes including 3, 5, 8–10, 12, 14, 15, 17, 19–25, 28, 29, 36–38, 46, 47, 49, 50, 51, and 59. The vast majority of these are β-HPV genotypes. The more common flat warts, caused by HPV3 and HPV10, may also occur in these patients but have an extensive distribution pattern; they may form plaques and can be persistent. These are seen most often on the arms, legs, face, and the dorsum of the hands ( Figs 18.27–18.29 ). The specific EV subtypes of HPV cause reddish, or pigmented or depigmented, scaly flat macular plane warts, mainly on the trunk, but also on the face, neck, and arms. Clinically, they resemble pityriasis versicolor ( Fig. 18.30 ). Some patients, especially those who are dark-skinned, may present with seborrheic keratosis-like changes. Spiny hyperkeratosis of the fingers is a rare manifestation. The occurrence of palmar pits is another rarely reported finding. Involvement of mucosal epithelium is not a feature of EV.
Susceptibility to EV is usually inherited in an autosomal recessive manner although X-linked recessive inheritance has been reported in one family. The lesions persist throughout life, and after some years (usually more than 20) they may show nuclear atypia resembling Bowen disease, and frank carcinoma sometimes develops. Basal cell carcinoma can also occur. The tumors develop particularly on sun-exposed skin and are most often associated with HPV5 or 8. Patients who develop invasive squamous carcinoma in association with EV do so at a younger age than those who develop this tumor not in association with EV (27 years compared with 67 years in one study). Such tumors, which are often multiple, are usually associated with a good prognosis unless they are treated with radiotherapy when they may be associated with metastatic disease, which has a high mortality. A large, locally aggressive squamous cell carcinoma of the nose related to HPV22b infection has been recorded, with detection of the virus both within the EV lesions and the malignant neoplasm.
EV-like disease, often referred to as acquired EV, has been reported in patients with a background of immunosuppression in such conditions as SLE, Hodgkin lymphoma, and HIV infection, and rarely in patients following renal transplantation, small bowel transplantation, peripheral blood stem cell transplantation, and bendamustine chemotherapy. An EV-like eruption has been documented in association with idiopathic CD4 lymphopenia and even with CD8 lymphopenia. Remission of lesions has been recorded in an HIV-positive patient following immune restoration due to ART.
EV therefore represents an unusual condition in which HPV infection, inherited predisposition (possibly a defect in cell-mediated immunity), and exposure to the sun all play a role (co-carcinogens).
The pathogenesis of EV is not yet fully understood. The EV-related HPV may be present in the general population, but the characteristic lesions only occur in predisposed individuals ( Fig. 18.31 ). It has been established that invalidating mutations in either of two adjacent novel transmembrane channel (TMC) genes termed EVER1 (or TMC6 ) and EVER2 (or TMC8 ) are responsible for most cases of EV. This susceptibility locus for EV has been mapped to the long arm of chromosome 17 (17q25.3). The EVER1 and EVER2 transmembrane proteins encoded by these genes are located in the endoplasmic reticulum. The proteins form a complex that interacts with zinc transporter 1 (ZnT-1), resulting in altered intracellular zinc distribution in keratinocytes. It has been proposed that EVER proteins in keratinocytes may serve as restriction factors for EV-specific HPV types. There have, however, been reports of cases lacking EVER1 and EVER2 mutations.
There appears to be a specific abnormal T-cell response to HPV-infected keratinocytes. The immune defect most often associated is a reduction in the number and function of Th cells, but patients with EV do not show general immunodeficiency or susceptibility to other infections. There have nevertheless been rare reports of EV in association with common variable immunodeficiency syndrome. It has also recently been described in two siblings with an autosomal recessive form of severe combined immunodeficiency linked to a mutation in CORO1A . A similar case of EV in association with severe immunodeficiency, lymphoma, and disseminated molluscum contagiosum (MC) infection appears to have been reported previously. EV has also been documented in a patient with a malignant thymoma. Although EV is not usually seen in patients with iatrogenic immunosuppression, some cases have been recorded. Humoral immunity is characteristically normal, although a case with isolated IgM deficiency has been documented. EV-associated HPVs have been detected in the amniotic fluid, placenta, and cervical scrapes of a pregnant patient with EV, thereby suggesting that vertical transmission of EV HPVs may play a role.
Histologically, EV is characterized by hyperkeratosis, hypergranulosis, and acanthosis ( Figs 18.32 and 18.33 ). The keratinocytes are vacuolated and show a striking blue-gray pallor on staining with hematoxylin and eosin (H&E). They are arranged in clusters or columns, the pallor being most conspicuous in the superficial granular cell layer. Identical focal changes may be occasionally seen in samples removed for other reasons in patients without the disease. The latter are usually but not exclusively observed, in sun-damaged skin of elderly patients. A recently reported case of HIV-associated acquired EV showed unique cornoid lamella-like structures. As the lesions progress to atypicality, the nuclei of the keratinocytes become larger and hyperchromatic, and cellular maturation is more disorderly. The dysplastic changes may also affect appendageal epithelium, particularly that of sweat ducts. The atypia eventually amounts to carcinoma in situ, and in 30–50% of patients the lesions progress to invasive carcinoma. Cutaneous neoplasia in EV is largely associated with HPV5 and 8. The E6 oncoprotein of HPV5 inhibits transactivation of SMAD3, which is an important component of the transforming growth factor (TGF)-β1 signaling pathway. Most carcinomas are squamous in type, but some show features reminiscent of sweat gland differentiation. The sweat ducts may show markedly disordered growth and atypia. These stages in the progression to frank carcinoma only occur on sun-exposed skin. In contrast to cervical cancer where the viral genome is integrated into the host DNA, in EV it remains extrachromosomal (episomal). Ultraviolet B radiation has been shown to modulate the noncoding region promotor activity of HPV5 and 8 in infected keratinocytes. Merkel cell carcinoma has rarely been documented in association with EV. A unique HIV-associated case developed multiple low-grade cutaneous sarcomas with a fibroblastic phenotype.
Swollen keratinocytes, as described above as a diagnostic feature of EV, have been recorded as a manifestation of immunosuppression, particularly HIV infection. Focal histologic features of EV have rarely been documented as an incidental finding in a variety of benign skin lesions in the absence of clinical evidence of underlying EV, including an intradermal nevus, a pigmented seborrheic keratosis, and an acantholytic acanthoma. The term ‘EV acanthoma’ has been proposed for these isolated, incidental cutaneous lesions.
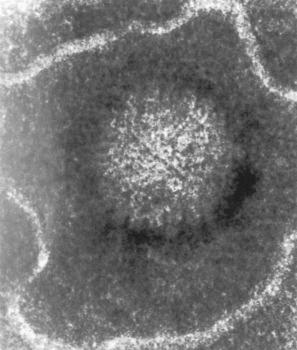
Herpes simplex virus (HSV) has two subtypes: HSV-1 and HSV-2. There is considerable homology between the two genomes, about 50% of sequences being highly conserved. Humans are the natural hosts for HSV-1 and HSV-2 and therefore also represent the viral reservoir. Herpes viruses are double-stranded DNA viruses with a complex capsid and glycoprotein envelope ( Fig. 18.34 ).
HSV-1 usually causes herpes labialis (90%), whereas HSV-2 most often causes herpes genitalis. Although HSV-2 previously accounted for approximately 90% of herpes genitalis cases, more recent epidemiological evidence reflects an increase in the proportion of cases attributable to HSV-1 (22–29%) and a diminished number of HSV-2 positive cases (68–71%). In some European cohort studies, HSV-1 infection has been a more common cause of genital herpes than HSV-2 infection. This trend may be attributable to the practice of oral sex. Both HSV-1 and HSV-2 are transmitted through mucosal surfaces or traumatized skin by exposure to contaminated secretions.
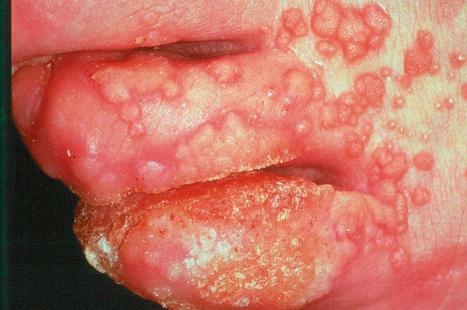
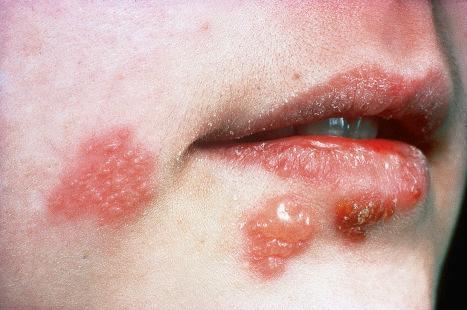
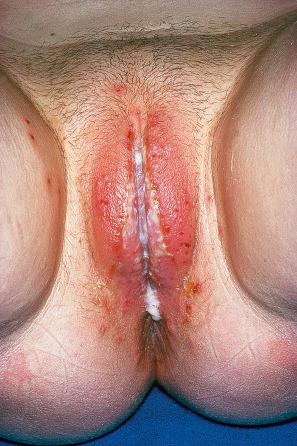
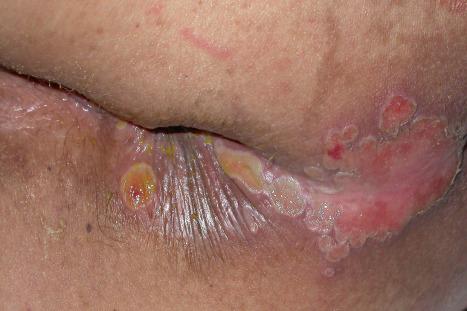
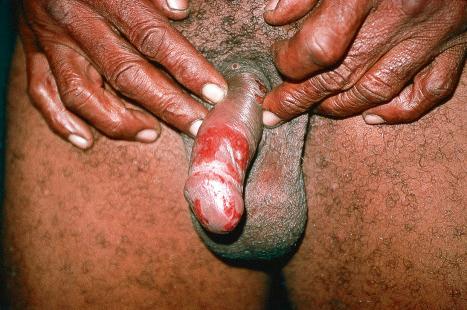
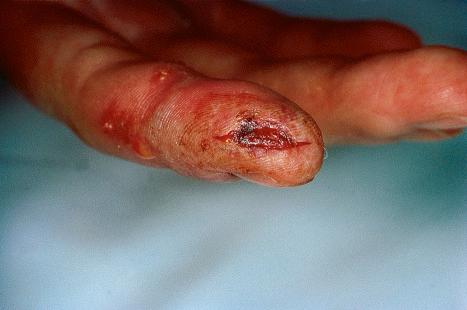
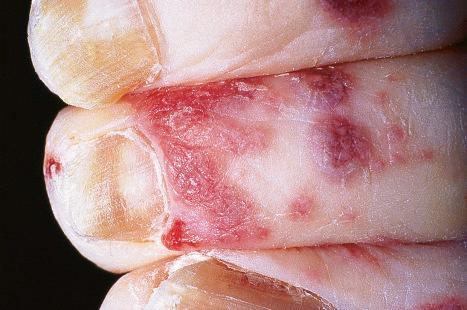
A first-episode infection – i.e., in someone who is seronegative (first-episode primary infection) or who has serum antibodies to the heterologous HSV type (first-episode, nonprimary infection) – may be associated with constitutional symptoms of fever and malaise. These symptoms are often worse in women with genital herpes, perhaps because of the wider area of epithelium involved and the greater viral load. The lesions may be found in the mouth, pharynx, lips, penis, vulva, vagina, or cervix ( Figs 18.35–18.39 ). HSV infections are also being seen more frequently in perianal and anorectal sites. Involvement of a finger in the form of a herpetic whitlow is most often seen in healthcare workers, especially dental practitioners ( Fig. 18.40 ). Primary HSV-1 infection, however, is asymptomatic in about 90% of patients, and primary HSV-2 in about 75%. It is important to remember that infection is for life. Herpes compunctorum is a rare form of HSV infection acquired as a result of tattooing. HSV may also be transmitted during close contact sports such as wrestling and rugby (herpes gladiatorum and herpes rugbiorum, respectively).
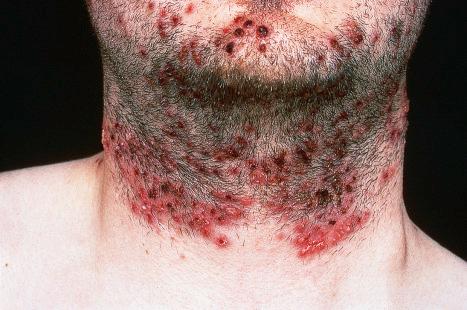
At the original inoculation site there is no detectable change for 3–5 days. The lesions that develop vary with site, but all are associated with the development of small grouped vesicles, often on an erythematous base. On mucosal surfaces, the vesicles rupture early and are superseded by grayish-yellow plaques or ulcers. In skin, grouped vesicles are seen on an erythematous base and then evolve into grouped pustules, which rupture and result in a crusted ulcer. The lesions are typically painful and sting or itch. The distribution of the lesions is characteristically wider than the initial site of inoculation, involving the area of innervation by the sensory nerve to that site. Occasionally, a separate area of lesions may develop away from the initial inoculation site, after transmission along a different branch of the same nerve. These initial cutaneous lesions only develop after involvement of the nerve and the ganglion and subsequent return of the virus to the epithelium. The lesions may then extend peripherally to involve adjacent skin or mucosa. This first episode of infection lasts for around 15 days. The epithelial lesions then resolve completely, but the virus persists, becoming latent within the ganglia of the corresponding sensory nerve. HSV is the most frequent infective cause of erythema multiforme.
Recurrent HSV lesions are usually less florid than the first infection and are not usually associated with general symptoms. They may be precipitated by sunlight, fever, menstruation, pregnancy, HIV infection, emotional stress, or local trauma. The incidence of recurrent orofacial herpes varies from 16% to 45%, while that of recurrent genital herpetic infection varies from about 50% to 65% of patients. Repeated recurrence is usual with genital HSV-2 and common with orofacial HSV-1, but with gradually decreasing frequency. ‘Reinfection’ with the heterologous type resembles a less severe first-episode primary infection.
Antibodies to HSV-1 are found in about 70% to 90% of adults, suggesting very wide contact with the virus, with a subclinical infection or an unrecognized oropharyngitis in childhood. Worldwide, an estimated 16% of people aged 15–49 years are infected with HSV-2. The seroprevalence of HSV-2 infection varies geographically. The seroprevalence in Asian countries ranges from 10% to 30%, whereas more than 80% of female commercial sex workers in parts of sub-Saharan Africa are infected. The United States saw a 30% rise in the prevalence of HSV-2 infection between the late 1970s and the early 1990s. Although HSV-1 occurs most commonly above the waist and HSV-2 below, these are preferential rather than obligatory sites. It has been noted that 10% to 15% of first-episode genital herpes is associated with pharyngeal lesions, emphasizing not only the frequency of orogenital contact, but that both viruses can affect either orofacial or genital epithelia.
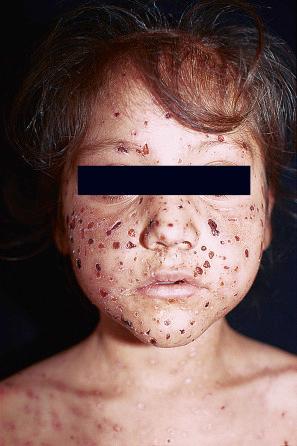
Occasionally, a first-episode primary infection in an atopic patient may result in extensive vesicular crops, so-called Kaposi varicelliform eruption (eczema herpeticum) ( Fig. 18.41 ). These lesions pustulate, ulcerate, and crust, as in the usual herpetic infection, but involve more or less the whole skin surface. Systemic symptoms may be severe, with fever and dehydration. The condition may occasionally prove fatal. Recurrent attacks may occur, but they are usually short and less severe. This manifestation of herpetic infection may also complicate Darier disease, Hailey-Hailey disease, Grover disease, pemphigus foliaceus, and other bullous dermatoses. Historically, a similar clinical picture was occasionally caused by vaccinia virus (eczema vaccinatum) as a complication of vaccination against smallpox. Histologically, the lesions of Kaposi varicelliform eruption are the same as those of the more localized form of herpetic infection. Disseminated disease may be seen in the immunosuppressed ( Fig. 18.42 ).
HSV infection in pregnancy may be associated with both severe maternal illness and fetal involvement via transplacental spread. Congenital infection is rare, but neonatal herpes simplex infection is seen in 10% of babies born to women with an active herpetic lesion after the 32nd week of pregnancy. Fatal disseminated infection with HSV-1 has been reported in preterm twins born to a mother with active herpetic gingivostomatitis during pregnancy. Congenital HSV infection forms part of the TORCH (toxoplasmosis, other infections, rubella, cytomegalovirus [CMV] and herpes simplex) complex. Lesions in congenital herpes infection can be extensively bullous, with severe erythroderma and loss of body fluid through exudation. The reported mortality rate in infants with disseminated infection is approximately 57%. Neonatal infection usually presents as a relatively mild oropharyngitis, and many are probably undiagnosed.
Immunosuppressed patients with underlying HIV/AIDS may present with extensive genital and perineal involvement. Not uncommonly, this is accompanied by concomitant CMV infection, and on rare occasions dual HSV-2 and CMV infection may even give rise to fungating anogenital lesions. Herpes simplex is also a potential cutaneous manifestation of IRIS among individuals receiving ART.
The double-stranded DNA of herpes virus is enclosed in an icosahedral protein shell (capsid), which in turn is invested by a complex envelope of lipid and glycoproteins. The latter are important in the attachment and penetration of cells. The complete virus measures about 150–1200 nm in diameter. Viral replication occurs within the nucleus where a basophilic Feulgen-positive inclusion body, including viral DNA, may be found as well as an eosinophilic inclusion body, which represents a focal deficiency of viral DNA, a so-called ‘scar’ of viral infection. Heparan sulfate moieties act as receptors to which HSV-1 and HSV-2 bind. HSV-1 encodes a complement-interacting glycoprotein (gC) and an IgG Fc binding glycoprotein (gE). These glycoproteins mediate immune evasion. Glycoproteins C and D (gC and gD) play an important role in the attachment of HSV-1 to host cell surface heparan sulfate receptors, whereas glycoprotein B (gB) is responsible for HSV-2 cellular attachment, entry, and cell-to-cell spread.
The most characteristic feature in the pathogenesis of herpetic infections is the early involvement of sensory nerves within which the virus, without its lipid/glycoprotein envelope, is transported to the ganglia. Further replication (associated with cell lysis) occurs within the ganglia, and the complete virus then migrates to the skin around the site of inoculation via the peripheral sensory nerves. This process of viral migration also occurs at times of recurrence. The state of the virus during the latent periods, which may last for many months or years, is not clear. It may continue as a more or less intact virus, but in virtually suspended animation without cell death, or it may persist as episomes or be incorporated into the cell genome. Immune mechanisms are responsible for both latency and reactivation of the virus.
Although immune defects (particularly of cell-mediated immunity and including HIV infection) are associated with a high incidence of severe and extensive herpetic infections, a state of raised immunity (either humoral or cellular) does not preclude recurrent lesions; indeed, recurrent lesions are usually associated with very high titers of IgG antibody. It is thought that humoral immunity may impede neuronal extension and reduce the likelihood of encephalitis, but cell-mediated immunity is effective in limiting the local cutaneous extension of the lesions and accelerates healing. Low levels of IFN-γ may contribute to reactivation of infections. Genital ulceration due to HSV-2 increases the risk of acquiring HIV infection; conversely, clinical trials have shown that HIV-1 viral load is reduced following suppression of HSV-2 infection.
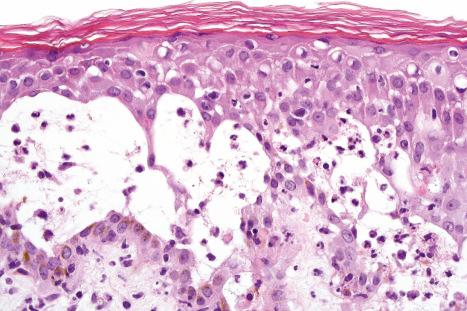
Histologically, the early change of HSV infection is increasing edema of the keratinocytes, which progresses to so-called ballooning degeneration. Some adjacent keratinocytes fuse so that they appear large and multinucleate. A number of cells show acantholysis, while others rupture as a result of extreme balloon degeneration (reticular degeneration). The result of acantholysis and balloon degeneration is an irregular intraepidermal vesicle containing groups of keratinocytes, many of which may be multinucleate ( Figs 18.43–18.47 ). The nuclei of keratinocytes may contain basophilic and/or pale ground-glass inclusions. As a vesicle expands, it involves the full thickness of the epidermis and may not be so clearly intraepidermal. HSV infection of the hair follicle epithelium can result in herpes folliculitis. Massive necrosis of the epidermis and superficial part of the hair follicle including sebaceous glands eventually develops. Involvement of hair follicles is, in fact, very common in most infections. In cases with very prominent superficial secondary changes, the distinctive findings of the infection are sometimes only evident in the infundibular portion of the hair follicle.
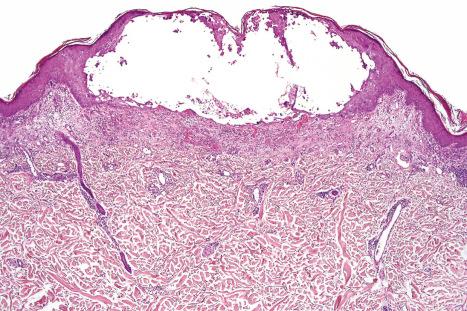
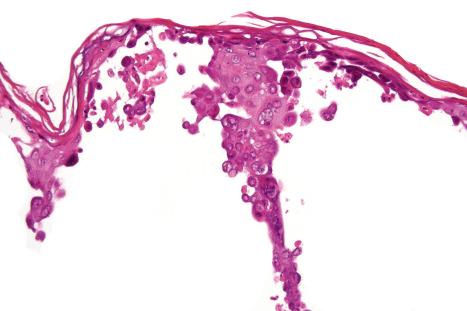
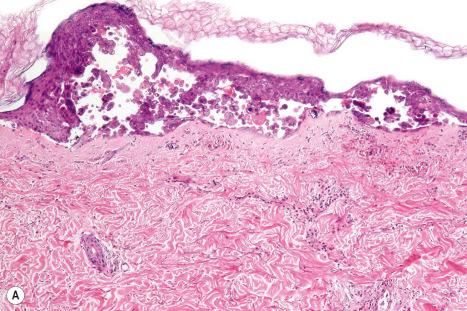
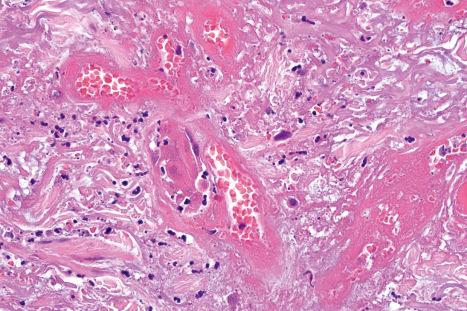
The underlying dermis is usually intensely infiltrated by mixed inflammatory cells. The infiltrate shows perineural accentuation, and occasionally a superficial leukocytoclastic vasculitis is present ( Fig. 18.48 ). Dermal nerve twigs may exhibit a perineural inflammatory infiltrate composed of lymphocytes and neutrophils, sometimes associated with intraneural involvement. Schwann cell hypertrophy and frank neuronal necrosis are occasionally encountered. Infection is sometimes associated with a prominent lymphoid infiltrate, resulting in a pseudolymphoma. Such cases may be associated with frequent CD30-positive cells, potentially mimicking a CD30-positive cutaneous lymphoproliferative disorder. HSV infection is also capable of inciting an inflammatory infiltrate rich in CD56-positive cells, thereby mimicking a NK-/T-cell lymphoma. An HIV/AIDS-associated case of scrotal HSV-2 infection with an atypical plasma cell infiltrate mimicking a plasmacytoma also has been reported.
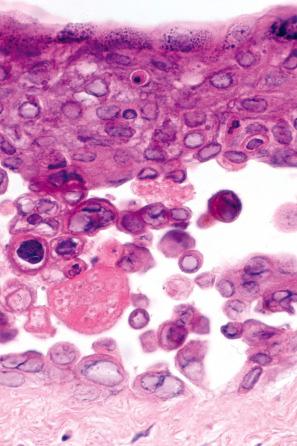
The features of an ulcerated herpetic lesion are not diagnostic unless the epithelial margins retain the characteristic features of intracellular edema, multinucleate epithelial cells, and inclusion bodies. Careful scrutiny of the surface exudate may nevertheless reveal isolated degenerate epithelial cells whose nuclei contain ghost outlines of herpetic viral inclusions. This should prompt examination of the adjacent intact epidermis for more characteristic features of HSV infection. The viable multinucleate cells are the diagnostic feature of the Tzanck test, a Giemsa-stained smear of vesicle contents. Biopsies of anogenital HSV-related ulcers in HIV-infected patients may show evidence of concomitant CMV infection. In the past, laboratory diagnosis of herpes infection was confirmed by growth in tissue culture, electron microscopy, immunofluorescent demonstration of viral-specific protein, or viral DNA hybridization. Nowadays, the diagnosis of HSV-1 or HSV-2 infection is confirmed by PCR or immunohistochemistry ( Fig. 18.49 ).
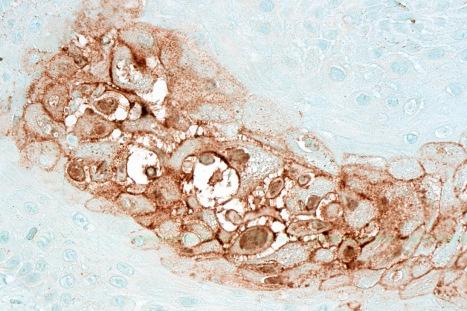
Varicella-zoster virus (VZV), also referred to as herpes varicella virus, is similar morphologically to HSV, and is the causative agent of varicella and zoster.
Varicella, or chickenpox, which is highly contagious, is most often an infection of children and is characterized by a disseminated vesicular eruption in crops. The major route of dissemination is by airborne droplets from the respiratory tract. In the immunocompetent, spread via the cutaneous lesions seems to be of little importance. Varicella is endemic in the temperate climates and manifests predominantly in winter and spring.
The incubation period is around 2 weeks and is followed by a rash, which is most pronounced on the trunk. The rash starts as red macules 2–4 mm across, which progress rapidly to fragile vesicles said to resemble ‘dew drops on rose petals’; these become pustular and rapidly show crusting ( Fig. 18.50 ). Lesions in varying stages are present at any one time. There is often considerable pruritus, and the associated scratching may result in secondary infection with Staphylococcus aureus or Streptococcus pyogenes . Mucosal lesions are frequently also present. Systemic effects in children are mild whereas they are almost invariably severe in adults, neonates, and immunocompromised patients. Recurrent varicella is rare.
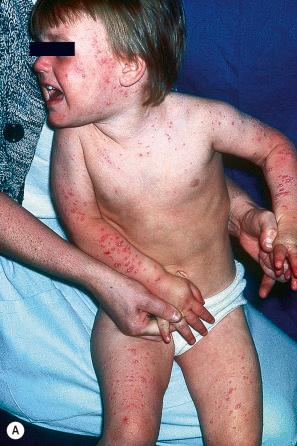
Complications include pneumonitis, meningitis, encephalitis, myelitis, and purpura fulminans. In the last, symmetrical hemorrhagic and necrotic lesions are seen on the legs following typical chickenpox, and the condition is associated with disseminated intravascular coagulation (DIC). Acquired deficiencies in protein S or protein C have been implicated in the pathogenesis of varicella-associated purpura fulminans. Less common complications include orchitis, hepatitis, glomerulonephritis, arthritis, myocarditis, and rhabdomyolysis. Necrotizing fasciitis (NF) is a potentially life-threatening complication of childhood varicella.
Varicella occurring in pregnancy may have serious consequences for both mother and fetus. Varicella pneumonia may lead to maternal death. Congenital varicella syndrome occurs in approximately 2% of neonates born to mothers who acquire chickenpox during the first two trimesters. Manifestations of this syndrome include dermatomal cutaneous lesions, ocular involvement, neurological complications, and skeletal abnormalities. Generalized neonatal varicella, which carries a mortality of around 20% if untreated, is the result of a maternal varicella rash appearing in a mother without antibodies between the last 4 or 5 days of pregnancy and the first 2 days following delivery of the baby.
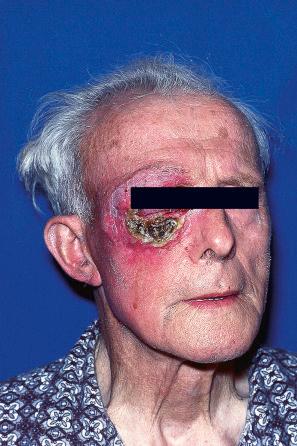
Herpes zoster, or shingles, occurs particularly in adults, usually the elderly, and most often presents as a girdle-like vesicular eruption in the thoracic or lumbar region, or with facial lesions as a result of trigeminal nerve involvement ( Figs 18.51 and 18.52 ). It is analogous to a recurrent episode of herpes simplex where the virus remains latent in the ganglia of sensory nerves. It is, however, worth noting that although rare, co-infection with VZV and HSV has been reported. Herpes zoster is thought to develop as a consequence of partial immunity. The eruption is preceded by paresthesia or pain in the dermatome supplied by a sensory nerve. This is followed, usually after 2–4 days, by the development of an edematous erythematous plaque on which groups of vesicles arise. As in chickenpox, these rapidly become pustules, which may coalesce to form bullae, occasionally hemorrhagic ( Fig. 18.53 ). The areas become crusted and this may very occasionally be followed by scarring and keloid formation. The lesions are usually painful, and this may persist for months or years as postherpetic neuralgia. The development of a vaccine for the prevention of herpes zoster in older adults holds promise. Involvement of the ophthalmic division of the trigeminal nerve (herpes zoster ophthalmicus) is an important manifestation in the elderly, which can have serious complications ( Fig. 18.54 ). Involvement of the mucocutaneous division of the seventh cranial nerve or the eighth cranial nerve leads to Ramsay Hunt syndrome, which is characterized by lesions in the auricular canal, facial paralysis, and auditory and vestibular symptoms. Rarely, noncontiguous multidermatomal cutaneous involvement (zoster multiplex) may occur. Although uncommon, herpes zoster may occur in children who have received varicella vaccine, a live attenuated virus. Cervical and sacral dermatomes are most commonly affected in these children, and potential complications include secondary bacterial infection, scarring, and depigmentation.
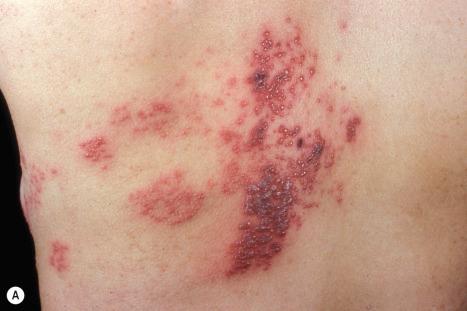
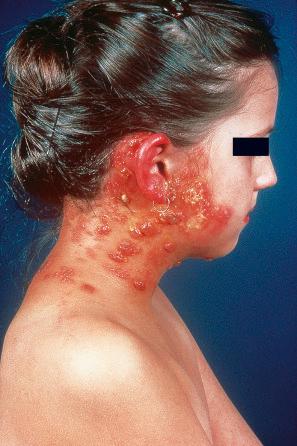
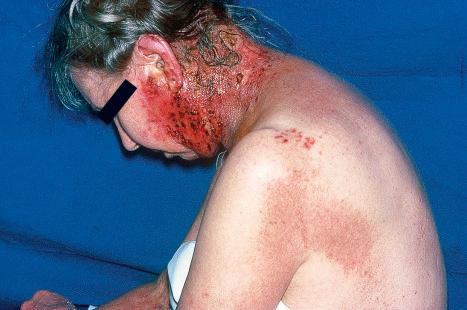
Reactivation of latent VZV may be associated with a deficiency in cell-mediated immunity, as immunity to chickenpox per se is normally lifelong. Patients with Hodgkin lymphoma, non-Hodgkin lymphoma, or SLE, and those treated with irradiation or chemotherapy, are particularly at risk and often develop a more serious illness. In more severe cell-mediated immunodeficiency, the zoster may become widely disseminated and sometimes proves fatal. Disseminated cutaneous lesions most often present as vesicles, pustules, hemorrhagic bullae, ulcers, and black eschars, although occasionally patients may develop verrucous, hyperkeratotic lesions ( Fig. 18.55 ). Visceral involvement is most often seen in the lung, liver, and brain. Cerebral disease most often presents as progressive leukoencephalitis. Cerebral vasculitis may occur as a result of direct invasion of cerebral arteries by VZV, leading to hemiparesis or hemiplegia. A nonimmune individual may contract chickenpox from a person with herpes zoster. Herpes zoster occurs in as many as 40% to 50% of patients in the first year following bone marrow transplantation, but the lesions increasingly resemble varicella as the time after transplantation increases. This suggests that T-cells specific for VZV are less well represented as time goes by.
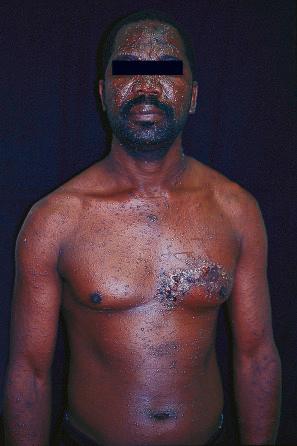
VZV infection in patients with AIDS may present with unusual manifestations, including verrucous skin lesions resembling viral warts and disseminated varicella in the absence of skin lesions. Verrucous VZV infection, however, has also been reported in a renal transplant recipient. Herpes zoster is a well-recognized cutaneous manifestation of IRIS occurring in HIV-infected patients (including children) receiving highly active ART.
Although inhalation of viral particles is the usual route of infection, direct cutaneous inoculation may occur. Initial contact is followed by viremia before the cutaneous lesions develop. During the viremic stage, VZV is transported to the skin by T-cells. Cell-free viral replication takes place in the skin and facilitates person-to-person spread. Even when the resolution of the lesions is clinically complete, the virus may remain latent in the ganglia of sensory nerves. IgG, IgM, and IgA antibodies develop soon after the vesicles; some IgG antibody is detectable thereafter throughout life, but the other antibodies disappear. It has been noted that cell-mediated immunity is depressed during an episode of chickenpox and for at least the first few days of zoster.
VZV has the ability to interfere with the expression of major histocompatibility complex (MHC) class I and class II proteins required for CD4 and CD8 T-cell recognition, leading to delayed clearance of virus-infected cells. VZV glycoprotein E (gE) is essential for viral replication and also plays a role in spread of the virus from cell to cell, secondary envelopment, and viral entry. ORF 66 (ORF66) of VZV encodes a protein kinase which modulates apoptosis and interferon pathways, down-regulates MHC class I protein expression on cell surfaces, and facilitates tropism of VZV for T-cells. VZV has evolved a diverse array of immunomodulatory mechanisms, including the ability to transiently evade immune recognition. The virus also has an effect on cell cycle regulatory pathways. These are just some of many aspects of the complex pathogenesis and immunobiology of VZV infection. A more detailed account is beyond the remit of this text, and the reader is thus referred to reference numbers 3, 32, 39, 37, and 40.
Histologically, the cutaneous lesions of VZV, whether in varicella or zoster form, are generally indistinguishable from those of herpes simplex, although it has been suggested that inflammation is more profound in the latter. Follicular epithelial involvement may also serve as a further point of distinction (see below). The intraepidermal blisters associated with intracellular edema and multinucleate epithelial cells with inclusion bodies are characteristic. The dermal infiltrate and fibrinopurulent exudate are seen regularly, but are not diagnostic. There is often more intraepidermal and dermal hemorrhage than in herpes simplex infection.
The wartlike cutaneous lesions encountered in patients with AIDS show hyperkeratosis, verruciform acanthosis, and virally induced cytopathic alterations, often with minimal or absent cytolysis of the infected epidermal keratinocytes, and little by way of a dermal inflammatory infiltrate. Cytopathic changes may be very minimal, and a high index of suspicion and the use of multiple sections with or without ancillary tests such as PCR are often necessary to establish a diagnosis. Immunosuppressed individuals with VZV infection may have a protracted clinical course during which biopsies reveal a lichenoid inflammatory reaction pattern rather than cytolysis of keratinocytes.
Biopsies from early herpes zoster lesions may exhibit VZV-infected cells in the hair follicles, suggesting that VZV spreads from dorsal root ganglia or trigeminal ganglia to an area of skin innervated by myelinated nerves, the latter terminating at the level of the follicular isthmus. Exclusive involvement of folliculosebaceous units in this manner may predate the evolution of more characteristic vesicular lesions, and serves as a point of distinction from recurrent herpes simplex. In the latter, there is axonal transport of the virus from sensory ganglia to the skin via terminal nonmyelinated nerve twigs. Very rarely, VZV infection may manifest with dermal vasculitis in the absence of associated epidermal involvement. Another rare occurrence is exclusive involvement of epithelium of the eccrine apparatus (herpetic syringitis), manifesting with isolated nodular skin lesions in the absence of epidermal viropathic changes. A number of cutaneous reactions have been described at the sites of healed herpes zoster scars, a phenomenon referred to as Wolf isotopic response. These include pseudolymphomatous cutaneous lymphoid hyperplasia, granulomatous vasculitis, granulomatous folliculitis, granuloma annulare, lichen planus, reactive perforating collagenosis, lichen sclerosus, and cutaneous Rosai-Dorfman disease. Active lesions may also harbor a pseudolymphomatous dermal lymphoid infiltrate.
As with HSV, the presence of multinucleate cells is valuable in the Tzanck test. In shingles, the spinal ganglia may show necrosis with inflammation, and intranuclear inclusions are sometimes evident. Otherwise, diagnosis is usually based on clinical criteria, but can be confirmed by electron microscopy, immunofluorescence of blister contents, growth in tissue culture, PCR, in situ hybridization, or immunohistochemistry ( Fig. 18.56 ). Serological tests are less useful than rapid antigen-detection methods and are only of value later, when a rising titer can be demonstrated.
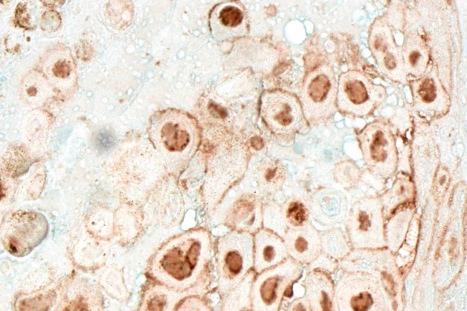
Antibody studies suggest that most people have been exposed to CMV. Generally, an asymptomatic infection ensues. CMV infection, however, may result in clinical features under a variety of circumstances. These include neonatal lesions, an infectious mononucleosis-like disease in adults, or a manifestation of disseminated disease in immunocompromised patients. An in-depth review of the literature revealed that severe visceral CMV infection in apparently immunocompetent individuals might not be as uncommon as previously considered. Clinical lesions in the skin, however, are distinctly uncommon.
CMV is the most frequently transmitted viral infection in utero, and is the most common cause of congenital infection worldwide. The incidence of reported infection ranges from 0.2% to 2.2% of live births. Less than 10% of affected infants and neonates will actually develop clinical lesions. Clinical manifestations have been grouped with other neonatal infections under the rubric ‘TORCH syndrome’, which includes toxoplasmosis, other infections (e.g., syphilis), rubella, CMV, and herpes simplex.
Affected newborn babies and neonates may develop a wide range of lesions including jaundice, hepatosplenomegaly, microcephaly, sensorineural deafness, chorioretinitis, pneumonia, direct hyperbilirubinemia, thrombocytopenia with petechiae, purpura, and ‘blueberry muffin’ lesions. The last consist of blue-red or violaceous papules and nodules and represent foci of dermal erythropoiesis. The mortality in this syndrome is of the order of 20% to 30%. Other reported pediatric manifestations of CMV infections include scleredema, the Gianotti-Crosti syndrome, perineal ulceration, the juvenile variant of papular-purpuric gloves and socks syndrome, acute hemorrhagic edema of infancy, and the fetal inflammatory response syndrome (FIRS).
Adults, particularly females, most often in the third decade, may develop a heterophil agglutinin-negative infectious mononucleosis-like syndrome in which a short-lived rubelliform eruption has been described. Patients are also at risk of developing an ampicillin-related allergic dermatosis (cf., infectious mononucleosis).
CMV infection may also be a feature of immunosuppression. Generalized CMV infection is not an uncommon finding at autopsy in AIDS patients. CMV is frequently detected in association with toxoplasmosis and Pneumocystis jiroveci infection, and has also been described in patients with concomitant cutaneous herpes simplex, bacillary angiomatosis (BA), Mycobacterium avium complex, mucormycosis, Kaposi sarcoma, and even acanthamebiasis. CMV infection is a potential manifestation of IRIS in HIV-infected patients receiving ART. CMV infection is said to occur in 20% to 60% of organ transplant recipients, with cutaneous manifestations occurring in 10% to 20% of patients with systemic infection. Chronic cutaneous CMV infection has been described in a patient with severe combined immunodeficiency syndrome. Skin lesions in AIDS- and non-AIDS-associated immunocompromised patients do not appear to differ clinically or histologically.
Reported cutaneous manifestations include ulcers on the genitalia, anus, perineum, buttocks, and thighs (sometimes in association with herpes simplex), purpuric eruptions, petechiae, erythema nodosum, cutaneous vasculitis, hyperpigmented nodules and plaques, lesions resembling prurigo nodularis, erythema multiforme (including the persistent form of the latter), nodular auricular lesions, epidermolysis, urticaria, pustules, vesiculobullous lesions, and a generalized, pruritic erythematous maculopapular eruption. Cutaneous CMV infection has also been detected in a patient with febrile ulceronecrotic Mucha-Habermann disease. There are isolated reports of CMV infection in association with eruptive pseudoangiomatosis (EPA) and reactive perforating collagenosis. A possible link to scleroderma has also been suggested. In one study, graft-versus-host disease (GVHD)-like histologic changes were observed in biopsies of clinically normal skin from allogeneic bone marrow transplant recipients who had peaks of CMV antigen in the blood within 100 days of transplantation. There are rare reports of cutaneous CMV infection complicating pre-existing dermatoses, including herpes zoster scars, pustular psoriasis, and pemphigus.
In addition to maternally derived infections, there is also some evidence to suggest a venereal mode of spread. In the immunocompromised patient, CMV infections represent an acquired phenomenon or reactivation of a latent focus. In immunosuppressed transplant recipients, infection occurs predominantly after the first month post-transplantation and is the result of either primary infection, reinfection, or reactivation of latent disease. A high antigen-specific T-cell response to CMV is responsible for chemokine-mediated endothelial cell damage.
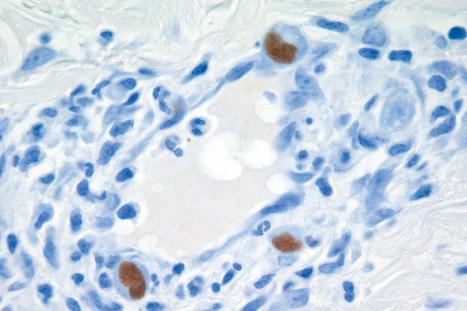
The histologic hallmark is the presence of large, often purple-staining, intranuclear inclusions surrounded by a clear halo ( Fig. 18.57 ). Smaller basophilic, periodic acid-Schiff (PAS)-positive intracytoplasmic inclusions may also be evident. These have been described within enlarged endothelial cells of dermal blood vessels, sometimes accompanied by the features of leukocytoclastic vasculitis. Inclusions may sometimes be identified in dermal fibrocytes, macrophages, and eccrine ductal epithelial cells, the last rarely associated with syringosquamous metaplasia. They have also been identified within the endothelial cells of blood vessels and histiocytes in the inflammatory bed deep to cutaneous ulcers ( Fig. 18.57 ). Cutaneous nerve involvement (CMV neuritis) has been reported in perineal ulcers. In histologic specimens from immunocompromised hosts, care should be taken to rule out CMV infection in association with other infective disorders, such as HSV infection or BA.
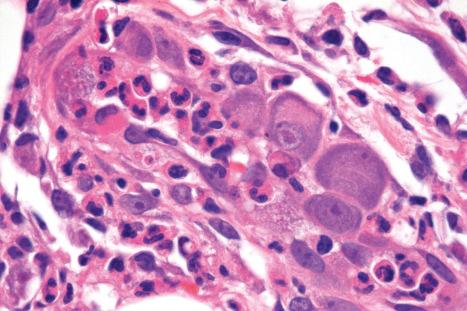
Vesiculobullous lesions are characterized by spongiosis and reticular degeneration, accompanied by epidermal multinucleate giant cells, which may contain viral inclusion bodies. The diagnosis of CMV infection can be confirmed by immunohistochemistry, in situ hybridization, or PCR ( Fig. 18.58 ).
Exanthem subitum (also known as roseola infantum or sixth disease) is generally a benign febrile disease of infancy and early childhood usually caused by infection with human herpesvirus 6 (HHV-6), and HHV-6B in particular. Infection with HHV-7, a closely related β-herpesvirus, however, may also manifest as exanthem subitum. Usual clinical features include high fever and a cutaneous eruption that resembles rubella or measles. Encephalitis and febrile seizures are potential complications. A case with vesicular lesions has been reported. Erythema and crusting at a bacille Calmette-Guérin (BCG) inoculation site has been reported in a patient with exanthem subitum. The diagnosis is confirmed by serology or the detection of the causative virus in body fluid or tissue samples, usually by real-time PCR.
A similar exanthematous rash caused by HHV-6 infection has been reported in leukemic patients and hematopoietic stem cell transplant recipients; a possible link to GVHD has also been suggested. HHV-7 is also recognized as an important pathogen in transplant recipients. Other reported clinical associations and cutaneous manifestations of HHV-6 infection include papular-purpuric ‘gloves and socks’ syndrome, erythema elevatum diutinum, an infectious mononucleosis-like syndrome, Gianotti-Crosti syndrome, drug hypersensitivity syndrome, and more recently, pityriasis rosea. HHV-6 DNA has also been detected in lesions of Langerhans cell histiocytosis. HHV-7 has been implicated in pityriasis rosea and drug-induced hypersensitivity syndrome.
The histopathological findings in exanthem subitum are rather non-specific and include papillary dermal edema and a superficial perivascular mononuclear inflammatory cell infiltrate. Rare cases with a vesicular presentation may show mononuclear inflammatory cell exocytosis into the epidermis, with microscopic intraepidermal spongiotic vesiculation. Intranuclear inclusions (as seen in HSV infection or VZV infection) are absent. The diagnosis can be confirmed by immunofluorescence microscopy, using an antibody to HHV-6.
Measles is a highly contagious, predominantly pediatric viral infection caused by measles virus. The latter is an enveloped, negative-sense, single-stranded virus belonging to the Morbillivirus genus in the Paramyxoviridae family. It is estimated that between 7 and 8 million people died annually from measles in the pre-vaccine era. The widespread use of vaccines against measles for more than five decades, however, led to a marked reduction in the disease. Recent years have nevertheless seen a resurgence of the infection, with a number of outbreaks recorded mainly in the Northern Hemisphere. Currently, an estimated 120 000 to 134 000 deaths are thought to occur annually, with some 400 measles-associated deaths worldwide per day. The United Kingdom saw some 477 cases of measles in the first 9 months of 2016, with 65% of the patients aged 15 years or older. Factors contributing to the reemergence of the disease include parental vaccine hesitancy, international travel to areas where measles is endemic, an influx of unvaccinated refugees to certain European countries, and poor vaccine coverage, with transmission between unvaccinated or incompletely immunized individuals. One of the main causes of parental vaccine hesitancy has been the unfounded suggestion of a link between autism and the administration of the MMR (measles, mumps, rubella) vaccine. Additional risk factors include immunosuppression (due to underlying HIV/AIDS, leukemia or malnutrition), and a loss of passive immunization in infants prior to an age for routine antimeasles vaccination.
Patients usually develop symptoms 7–14 days after exposure to the virus, and present initially with fever, cough, coryza, and conjunctivitis, often accompanied by malaise and a loss of appetite. An erythematous maculopapular skin rash in a characteristic cephalocaudal distribution develops some 4 days later ( Fig. 18.59 ). Patients are infective from 4 days before until 4 days after the onset of the rash. Whitish lesions referred to as Koplik spots appear on the buccal mucosa 2–3 days prior to the rash. The latter generally last for 3–5 days and are said to be pathognomonic of measles. The non-specific nature of the initial symptoms and the reduced incidence of the infection since the introduction of routine immunization may result in the diagnosis being overlooked. Although a majority of patients recover from the infection, a mortality rate of up to 10% has been recorded. Potential complications which may arise in the first 4–6 weeks after acute infection include viral pneumonia, secondary bacterial pneumonia, laryngotracheobronchitis, otitis media, corneal ulceration, stomatitis, and encephalitis. Subacute sclerozing panencephalitis is a much feared delayed complication of measles.
Membrane fusion induced by morbillivirus glycoproteins constitutes a critical step for viral entry and replication in the host, and accounts for the predominantly lymphotropic virus's ability to breach host epithelial barriers. Resultant syncytia formation potentiates further cell-to-cell spread of the virus. The cellular tropism of the measles virus is determined by expression of the cellular receptors CD150 and poliovirus receptor-like 4 (PVRL4) on subsets of activated immune cells (dendritic cells, macrophages, B-cells, T-cells) and on epithelial cells, respectively.
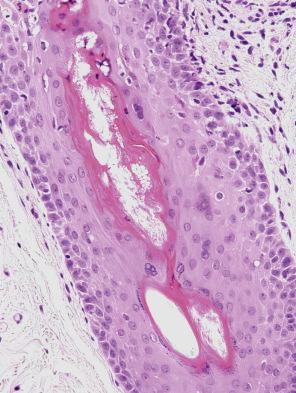
A characteristic finding in skin biopsies is the presence of multinucleate syncytial-type epithelial cells within the epidermis and particularly hair follicles ( Fig. 18.60 ). Apoptotic keratinocytes are not infrequently observed in the epidermis and they are quite prominent in pilosebaceous follicles, the latter being an important clue to the diagnosis ( Fig. 18.61 ). Associated parakeratosis and a mild superficial perivascular dermal mononuclear inflammatory cell infiltrate may also be seen. Additional findings which have been described include intradermal syncytial giant cells, and intravascular fibrin thrombi in the presence of a mixed dermal inflammatory infiltrate harboring numerous eosinophilic leukocytes.
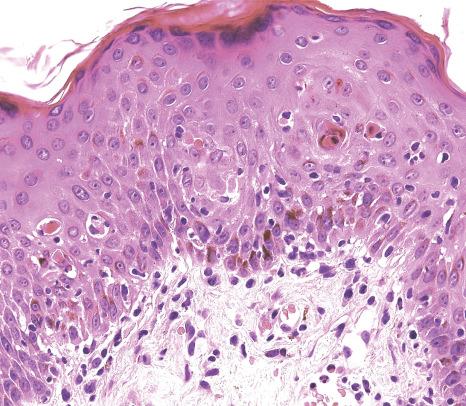
Since keratinocyte apoptosis and a superficial perivascular lymphocytic infiltrate may be observed in viral exanthems other than measles, a heightened index of suspicion is required. Careful scrutiny of additional serial histologic sections, however, should enable identification of the characteristic multinucleate epithelial giant cells encountered in measles. Although infection with HSV or VSV is frequently associated with epidermal and follicular epithelial cell multinucleation, the clinical features (including vesiculation) and presence of Cowdry-type intranuclear viral inclusions in the aforementioned herpes virus infections should readily facilitate their distinction from measles. Uncommonly, multinucleate epidermal keratinocytes may also be encountered in noninfective dermatoses, including lichen simplex chronicus, prurigo nodularis, lichen planus, and dermatitis artefacta; this phenomenon has been ascribed to chronic rubbing.
In 1969, Cherry et al. reported a series of four infants who developed an eruption of small hemangioma-like papules, which blanched on pressure. The lesions had an abrupt onset and apparently evolved in association with an acute echovirus infection, resolving spontaneously within a few days. This uncommon condition was later referred to as EPA and was initially regarded as an exanthem unique to infants and children. The more recent literature, however, reveals that more than half of all recorded cases have occurred in adults, often as small outbreaks, and especially in the Mediterranean region during the summer months. The condition is similar to or synonymous with the entity referred to in Japan as erythema punctatum Higuchi, which has been linked to mosquito bites. In children, the eruption is frequently preceded by an upper respiratory tract infection or, less commonly, gastroenteritis. Prodromal constitutional symptoms such as malaise, fever, headache, vomiting, or diarrhea are encountered more frequently in pediatric patients than in adults. Rare cases have occurred in iatrogenically immunosuppressed individuals. Isolated cases have been linked to the ingestion of a herbal medicine or food allergen.
The acute eruption comprises numerous small, asymptomatic, bright red angiomatoid papules. The individual lesions, which measure between 2 and 5 mm in diameter, are surrounded by a distinctive pale halo and characteristically blanch on pressure. The face, trunk, and limbs are sites of predilection. Spontaneous resolution usually takes place within 3 to 10 days. Relapse, however, has been reported in around 70% of cases in some series. Exceptionally, EPA may persist for months.
Although there is often strong circumstantial evidence to suggest that the eruption is precipitated by a viral infection, further investigation seldom leads to the identification of a specific pathogen. Isolated cases, however, have been linked to CMV infection and infection with Epstein-Barr virus. Several cases have occurred following arthropod bites, especially those of mosquitos. Lesions of EPA have also been induced experimentally by mosquito bites. Although a vector-borne infectious agent seems probable in a subset of patients, the authors of a recent meta-analysis concluded that there was insufficient epidemiological evidence to either substantiate or refute an infectious etiology. Since there is no true vascular proliferation, the authors who initially described the condition proposed that the cutaneous lesions were the result of either a direct viral effect on vascular endothelial cells or binding of antigen-antibody complexes to the endothelium. The latter is unlikely as there is no evidence of vasculitis. The surrounding white ring observed clinically around each lesion has been ascribed to vasoconstriction peripheral to the central zone of vasodilatation. It seems likely that EPA represents an unusual reaction pattern in response to a number of different viruses.
The histopathological picture predominantly shows dilated blood vessels in the papillary dermis and upper reticular dermis. These vessels are lined by plump endothelial cells, which usually assume a hobnail-like appearance ( Fig. 18.62 ). A sparse perivascular lymphoid infiltrate is sometimes evident. Importantly, there is no increase in dermal vascular density. Although some authors have noted the presence of intravascular neutrophils, true vasculitis is conspicuously absent.
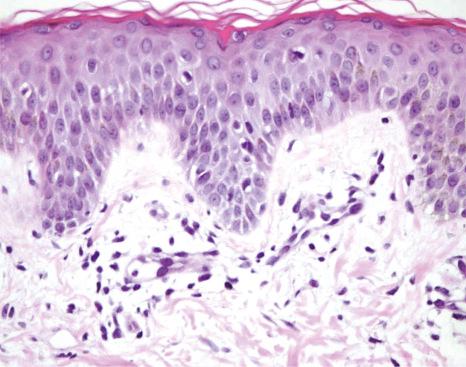
Although eruptive pyogenic granulomas, BA, bartonellosis, and multiple glomeruloid hemangiomas may enter the clinical differential diagnosis, each of the aforementioned conditions has distinctive histology and is associated with a true angiomatous dermal vascular proliferation. Furthermore, the individual lesions are not surrounded by a peripheral white halo.
Hobnail hemangioma (targetoid hemosiderotic hemangioma) is characterized clinically by a perilesional halo and histologically by hobnail-like vascular endothelial cells. Unlike EPA, however, lesions are usually single; the surrounding halo is pigmented rather than pale, and there is a true dermal vascular proliferation. Telangiectases lack protrusion of plump endothelial cells into the dilated vessel lumina. Spider angiomas comprise centrally located dilated dermal arterioles with thin branches; they, too, are devoid of the prominent endothelium observed in EPA.
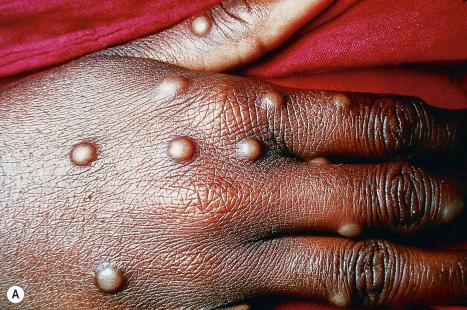
The orthopox viruses are DNA in type and cause variola, vaccinia, cowpox, and monkeypox.
Variola, or smallpox, has not been diagnosed endemically since 1977 and until relatively recently appeared to be of historical interest only. For more than a decade, however, there has been renewed interest in smallpox as a potential agent in bioterrorism. The highly virulent pathogen is human-specific. It was endemic in parts of Africa, South America, and Asia, with only occasional cases seen in Europe and North America. Transmission of the virus, which is capable of retaining viability in dried exudate, is by inhalation. The disease typically has an incubation period of 12 days, followed by a prodromal phase of high fever, headache, and vomiting, and 3–4 days later by a transient erythematous and petechial rash. This is in turn followed by the characteristic eruptive lesions ( Fig. 18.63 ). These lesions are most common on the face and limbs. They begin as papules, which become vesicular and then pustular and crusted. Healing is usually associated with a pitted scar. Mortality varied from 2% to 50%, depending on the severity of the infection. Although death was previously attributed to secondary bacterial sepsis, it has since come to light that it was probably the direct result of the cytopathic effects of the smallpox virus itself. It has been suggested that the scarring observed in survivors of the disease might have been the result of destruction of sebaceous glands, although other mechanisms have also been proposed.
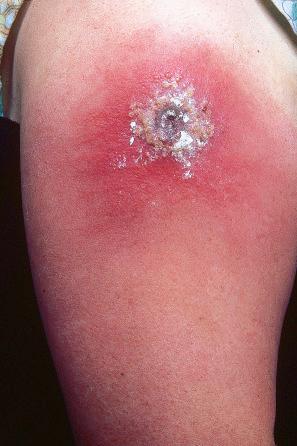
Vaccinia virus is closely related antigenically to variola virus, but is probably derived from cowpox virus. It was used for immunization against variola and no doubt was effective because of its similar antigenicity. This skin inoculation results in a single vesicle, which becomes pustular and crusts, like variola ( Fig. 18.64 ). It also heals similarly, leaving a scar. Since it was accepted that variola had been eradicated in the wild, for many years vaccination was no longer thought necessary except in laboratory workers at special risk. Public fear of smallpox as a potential biological weapon or bioterrorism agent in the United States, however, led to the reintroduction of a smallpox vaccination campaign in that country. The vaccination procedure is not without risk. Generalized vaccinia was occasionally seen and the vaccine was responsible for some cases of Kaposi varicelliform eruption (eczema vaccinatum). Vaccinia necrosum, encephalitis, and myocarditis were also rare complications. Occasional cases of generalized vaccinia following smallpox vaccination have been recorded.
Despite the name, the reservoir for cowpox virus is not cattle, but wild animals such as hedgehogs and badgers. Cattle and man are both infected accidentally, although man may acquire the disease from cows. Cats, and more recently rats, have been identified as additional sources of infection. It is endemic to parts of Eurasia. The occurrence of cowpox among young people in Europe has been attributed to the cessation of smallpox vaccination. Although this rare zoonotic disease generally results in a self-limiting infection, more severe illness may occur in immunocompromised individuals and those with eczema. The incubation period after inoculation is usually 5–7 days; a papule then develops, which rapidly becomes pustular. The pustule is surrounded by a zone of erythema and edema. Eschars or necrotic ulcers may be seen. The lesions are often multiple and can occur on the hands, arms, or face ( Figs 18.65 and 18.66 ). A severe generalized or varicelliform eruption in association with atopy has been reported. Sporotrichoid spread has been documented. Lymphangitis, lymphadenitis, and fever are almost invariably present. Healing and recovery occur in 3–4 weeks. A fatal case of a cowpox-like illness has been recorded. One reported case developed facial cellulitis and necrotizing lymphadenitis with abscess formation after inoculation of the virus in the nasal respiratory epithelium.
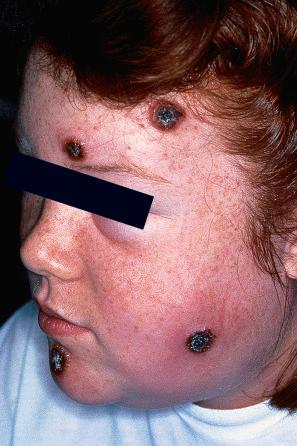
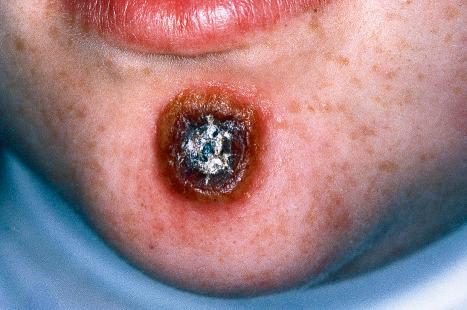
Monkeypox is an uncommon zoonosis attributable to monkeypox virus, a member of the orthopox virus group. The first recorded cases occurred in the Democratic Republic of Congo (DRC; formerly Zaire) in 1970. Although sporadic cases are encountered in remote villages in the tropical rain forests of West and Central Africa, an outbreak of the disease occurred in the state of Wisconsin in the United States in 2003, spreading to neighboring states. This outbreak was traced to a shipment of exotic animals from West Africa. Patients appeared to have come into direct contact with ill prairie dogs that were being kept or sold as pets. It was established that these animals had been exposed to infected rodents imported from Ghana. Person-to-person spread may also occur. Prior smallpox vaccination may confer some protection against monkeypox infection. The rise in the number of cases of human monkeypox in the DRC in recent years may in part be due to the cessation of routine smallpox vaccination. The condition presents with fever, rigors, and a skin rash, sometimes accompanied by lymphadenopathy. The cutaneous lesions progress from papules to vesicopustules to resolving eschars. Encephalitis may also occur, and fatalities have been recorded. Monkeypox may be confused clinically with chickenpox.
Orthopox viruses are large and have a discrete DNA compartment (nucleoid) and a complex capsid and lipoprotein coat containing characteristic tubular structures. They are brick-shaped and their outer tubular structures are irregularly arranged. These viruses are all transmitted by inoculation except variola, which usually gains entry by inhalation. The virus is able to resist dehydration outside the host and therefore inhalation or inoculation of dust or inoculation from shared facilities is quite possible, as well as direct inoculation from an active lesion.
Evidence has shown that these viruses secrete interleukin (IL)-18 binding proteins, resulting in viral dissemination or persistent infection. Variola virus encodes CrmB, a TNF receptor homologue, which serves as a specific binding protein for a number of chemokines that mediate recruitment of inflammatory cells to the skin and mucosae, sites of viral entry, and viral replication. Binding of chemokines is potentiated via the C-terminal domain of CrmB, referred to as smallpox virus-encoded chemokine receptor (SECRET). In primate animal models, multiorgan failure ensues in the presence of a high viral burden in the affected tissues. A hemorrhagic diathesis and depletion of T-cell dependent zones in lymphoid tissues may also be encountered. A cytokine storm arises due to elaboration of cytokines such as IL-6 and IFN-γ. The vaccinia virus is able to evade the host immune response by impairing dendritic cell maturation, with subsequent inhibition of T-cell activation. The large vaccinia viral genome also encodes proteins, which when secreted from the infected host cell, are capable of binding and neutralizing interferons, cytokines, chemokines, and complement. Others function within the host cell, leading to inhibition of cellular signaling pathways and apoptosis. Cowpox virus encodes an array of immunomodulatory proteins capable of modulating the host immune response. Among these are CPXV012 and CPXV203, which have been shown to disrupt antigen presentation. Monkeypox virus infection is also associated with the production of immunomodulatory proteins.
After inoculation, the viruses proliferate within keratinocytes and basal cells. This leads to severe intracellular edema with resultant ballooning degeneration and consequent reticular degeneration due to cell rupture, giving rise to multilocular vesicles and subsequent infiltration by polymorphs. In variola, vaccinia, and monkeypox, there is often extensive epidermal necrosis. Variable degrees of hyperkeratosis and acanthosis are present. Cytoplasmic eosinophilic inclusion bodies may be seen in the keratinocytes of all four diseases. The small inclusions of variola are called Guarnieri bodies. They are surrounded by a clear halo and are located close to the nucleus ( Fig. 18.67 ). Similar bodies are seen in vaccinia and monkeypox. Those of cowpox, however, are slightly larger, but are still predominantly seen in the cytoplasm. A dermal chronic inflammatory cell infiltrate consisting of lymphocytes is usually present. CD30-positive cells may be very prominent, and confusion with a CD30-positive lymphoproliferative disorder is possible if close attention is not paid to the epidermal changes and the presence of inclusions.
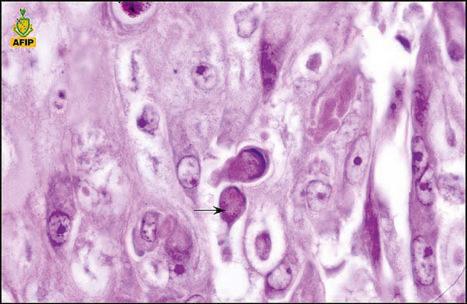
Diagnosis is based upon clinical information, but confirmation can be obtained by electron microscopy, isolation of the viruses in tissue culture, or, more recently, with the aid of PCR or in situ hybridization studies. Variola virus is detectable in formalin-fixed, paraffin-embedded tissue after decades of prolonged archival storage.
Milker nodule (or paravaccinia) is caused by a parapox virus, distinct from that which causes cowpox. It occurs as a localized lesion on the udders of cows and causes little systemic disturbance. It may be recurrent in the same herd. It is usually acquired by man by inoculation, but since the virus is viable in a dried state, indirect fomite infection is possible. Small outbreaks involving several patients have been recorded. The incubation period is around 5 days; some two to five red papules then develop, which gradually become bluish tender nodules. The overlying epidermis is at first tense and shiny, but becomes opaque and gray ( Fig. 18.68 ). The center of the lesion is crusted and slightly depressed. The surrounding skin often shows lymphangitis, but despite this the lesion has the appearance of a tumor and is well circumscribed. There are few systemic symptoms, but there may be an associated short-lived papulovesicular eruption on the upper limbs and occasionally on the legs. The main nodular lesions resolve, without scarring, in 4–6 weeks.
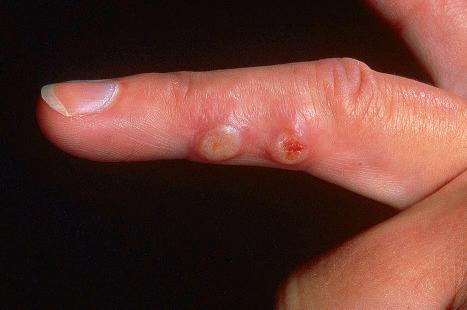
Milker nodule virus measures 160–260 nm and is ellipsoid in shape. It is characterized by spirally arranged tubules. The histologic features are indistinguishable from those seen in orf infection (see below). A CD30-positive atypical dermal lymphoid infiltrate may sometimes be encountered, and confusion with a CD30-positive lymphoproliferative disease is possible. In the latter, the CD30-positive cells are usually in clumps while in Milker nodule the CD30-positive cells are scattered between other inflammatory cells. This is, however, not entirely consistent, and in some cases clusters of CD30-positive cells may be seen.
Ecthyma contagiosum (orf, contagious pustular dermatosis) is caused by an epitheliotropic DNA parapoxvirus morphologically identical to that causing milker nodule. The infection is endemic in sheep in which it causes crusted pustules of the lips and perioral area ( Fig. 18.69 ). This underlies the accurate descriptive term of ‘scabby mouth’ used by Australian farmers. It is transmitted by inoculation from sheep to sheep, although it is said that the virus can persist in pastures. It is particularly likely to arise when the pasture is dry and results in minor abrasions of the labial mucosa of the sheep. Transmission to man, most often males and usually sheep-handlers, is usually by direct inoculation from infected lesions, but it may also result from contact with contaminated objects such as fences and shears. Orf may also be seen in goats. In one English study, 23% of individuals employed or living on a sheep farm reported having had the condition. Nonoccupational acquisition of the infection has been described following a sacrificial feast.
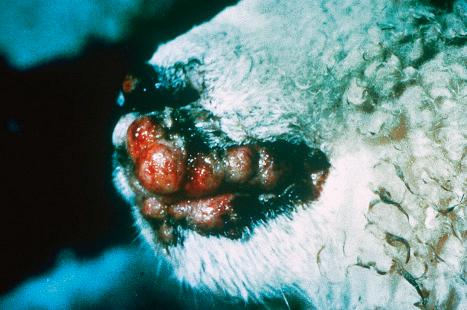
After an incubation period of 5–6 days, the lesion (usually a solitary, small, firm, red-blue papule) develops. It is most common on the hand or forearm ( Figs 18.70 and 18.71 ). Less commonly, lesions may occur on the facial and perianal regions. The papule becomes a flat-topped hemorrhagic blister or pustule, later crusting over its depressed center. By this stage, it may be 2–5 cm in diameter. Clinically, it may be mistaken for a lobular capillary hemangioma (pyogenic granuloma) or keratoacanthoma. The lesions are surrounded by a zone of erythema, which may be associated with itching and tenderness. The main lesion usually resolves without scarring in 3 weeks, although on rare occasions digital deformity may occur. Lymphangitis, lymphadenitis, and mild fever occasionally develop. In addition, some patients develop a transient maculopapular eruption on the trunk or erythema multiforme-like lesions on the limbs. Other potential complications include erysipelas, Pseudomonas aeruginosa infection, a papulovesicular eruption, and a bullous pemphigoid (BP)-like bullous eruption. Ocular involvement may be a rare manifestation. Giant lesions are sometimes encountered. Giant orf lesions were reported in an organ transplant recipient. Lesions occurring in immunosuppressed organ transplant recipients also tend to be more extensive, are less likely to involute spontaneously, and may be resistant to treatment.
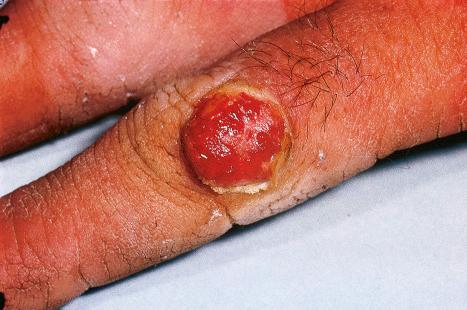
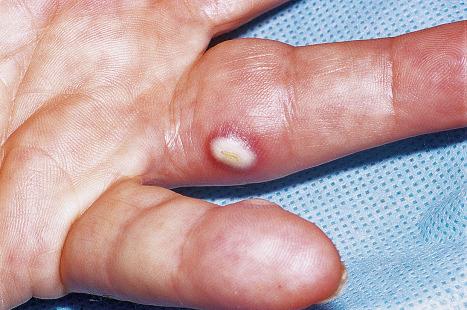
The histologic features are quite characteristic. The overall appearance of the lesion is that of a symmetrical nodule. There is a parakeratotic crust and acanthosis with thin epidermal strands extending quite deeply into the adjacent dermis. Viral cytopathic changes including cytoplasmic and nuclear vacuolation are usually conspicuous. Reticular degeneration with intraepidermal vesiculation is often evident ( Fig. 18.72 ).
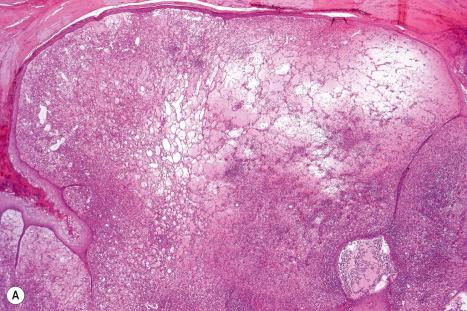
If the biopsy is taken from an early lesion, typical 3–5 µm intracytoplasmic eosinophilic inclusions may be seen ( Fig. 18.73 ). Sometimes intranuclear inclusions may also be evident. They can be rendered more conspicuous with Lendrum phloxine tartazine ( Fig. 18.74 ). Similar inclusions may also be seen in the cytoplasm of the endothelial cells lining blood vessels, among the underlying heavy chronic inflammatory cell infiltrate. The latter comprises lymphocytes and histiocytes although neutrophils and occasional eosinophils may be evident. The dermis is often very edematous, and characteristic of orf (and milker nodule) is the presence of massive capillary proliferation and dilatation. This is attributable to the production of virus-encoded homologues of ovine vascular endothelial growth factor (VEGF). In addition, orf virus encodes a homologue of IL-10, which appears to impair dendritic cell function and monocyte proliferation. A prominent, CD30-positive reactive dermal lymphoid infiltrate may occur.
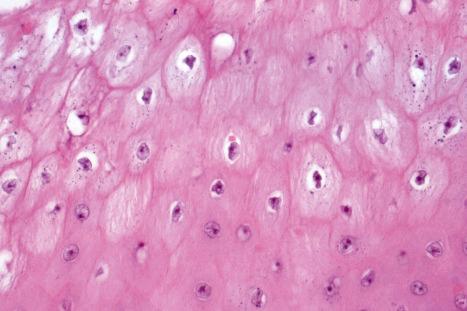
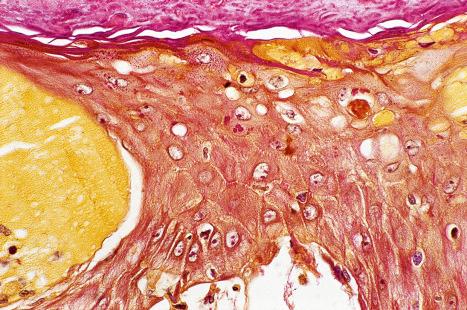
The immunobullous (BP-like) lesions are subepidermal in location and contain a mixed inflammatory cell infiltrate, which includes polymorphonuclear leukocytes and eosinophils. A study of two cases showed that orf virus DNA was absent from the blistering lesions, while direct immunofluorescence microscopy revealed linear deposition of IgG and complement C3 along the dermal–epidermal junction in perilesional skin. Although the precise autoantigen has yet to be identified, the condition appears to be distinct from true BP and other immunobullous conditions such as epidermolysis bullosa acquisita.
The diagnosis of orf may be confirmed immunocytochemically or by PCR, including real-time PCR. The Tzanck test has also been used as a diagnostic aid. Ultrastructural studies reveal that the orf virus is best visualized in negatively stained preparations ( Fig. 18.75 ).
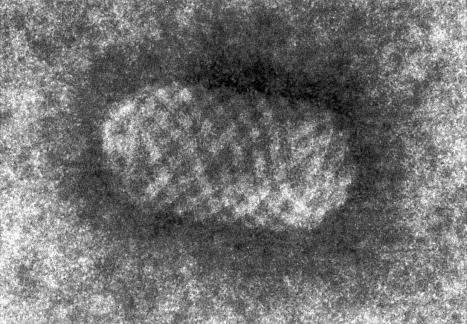
Parapoxvirus infections are known to occur in animal species other than common domestic ruminants, including wild animals such as white-tailed deer, red deer, reindeer, musk ox, cervids, and even seals. There have been sporadic reports of infections among humans following direct or indirect exposure to some of the aforementioned animals, e.g., via an accidental cut to the finger while dressing the carcass of a hunted animal. Usual presentation is with a nonhealing violaceous nodule on the injured digit.
There is histopathological overlap with milker nodule and sheep- or goat-derived orf, including the presence of intraepidermal eoninophilic intracytoplasmic inclusions and a prominent superficial dermal vascular proliferation. The ultrastructural characteristics of the viruses are also identical to those responsible for milker nodule and orf acquired from domestic ruminants. The diagnosis can be confirmed by immunohistochemistry or PCR.
MC is a self-limiting epidermal papular condition caused by a poxvirus of the Molluscipox virus genus ( Fig. 18.76 ). The genome encodes 163 proteins of which 103 are closely related to variola virus. Recent studies have shown that MC virus has a number of immune evasion strategies, including inhibition of NF-κB activation and apoptosis.
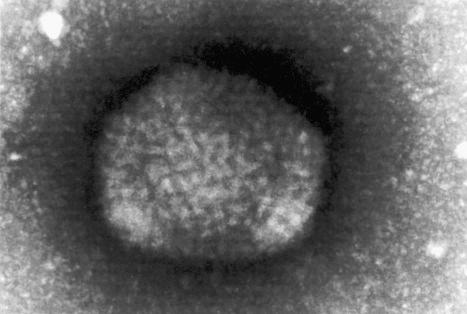
MC occurs on the face, trunk, and limbs of young children, who are infected by direct cutaneous contact or inoculation from fomites, and in young adults on genitalia and surrounding skin after transmission by sexual contact. The palms and soles are usually unaffected, but rare cases have been reported; subungual involvement, however, is exceptional. Although the condition is worldwide, it is more prevalent in tropical regions. The earlier literature indicated that lesions were particularly common in Fiji and Papua New Guinea, where 1 child in 10 had or had had the condition; most infected children were under 5 years of age. Subsequent studies from India and the United States have shown that around 80% of children with MC are younger than 8–10 years of age. There have been rare reports of neonatal or congenital infection.
It has been suggested that MC may be more common in people with atopic dermatitis. In the United States and the United Kingdom, the incidence in adults attending sexually transmitted disease (STD) clinics is in the range of 1 for every 40–60 cases of gonorrhea. Transmission can also occur between wrestlers (molluscum gladiatorum), between doctor and patient, and through joint use of equipment and bathing facilities. The use of public swimming pools appears of particular significance in children. MC may also be acquired through tattooing. The disease has an increased incidence in patients with impaired immunity. In the latter, lesions tend to be more extensive, generalized, and persistent. Although man was initially thought to be the only host to the virus, there have been isolated reports of MC in animals such as certain bird species, the chimpanzee, and possibly the red kangaroo.
The incubation period ranges from 2 to 7 weeks but may be as long as 6 months. Individual lesions are smooth, shiny, pearly, firm, umbilicated papules up to 5 mm across or occasionally larger ( Figs 18.77 and 18.78 ). Mucous membrane involvement is seen occasionally. The lesions are usually quite characteristic in appearance, but may be confused occasionally with a fibrous histiocytoma, intradermal melanocytic nevus, keratoacanthoma, syringoma, basal cell carcinoma, common wart, and even cutaneous cryptococcosis. They are often multiple, especially in patients with immune deficiency. Although uncommon, giant lesions may also occur, especially in immunocompromised patients. HIV-infected individuals can manifest with exclusive involvement of facial and perioral skin, as well as the eyelids. MC may be a cutaneous manifestation of IRIS in HIV-infected patients receiving highly active ART. A number of recent reports have highlighted an increased susceptibility to MC among patients on treatment with immunosuppressive drugs, including methotrexate, ciclosporine, anticancer chemotherapy, and newer therapeutic agents such as kinase inhibitors and TNF-α antagonists.
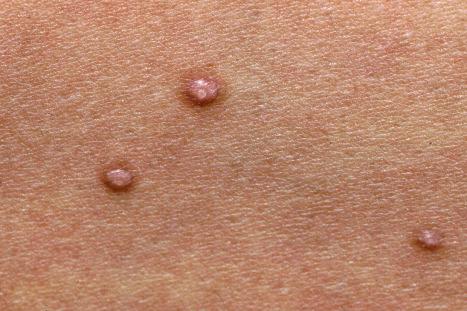
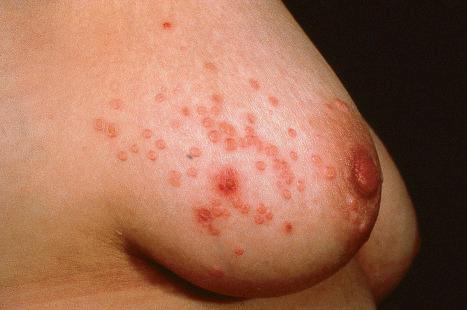
Symptoms of itching, tenderness, and pain are uncommon, but approximately 10% of patients develop an eczematous dermatitis around the molluscum papule. The papule itself may become secondarily infected and then resemble a furuncle. The individual lesion usually persists for 2 months, but sometimes lasts much longer. Since the patient may have numerous lesions at different stages of development, MC can be present for years in some patients. Pitted scarring sometimes occurs in atopic individuals. Systemic lesions have not been described.
In MC, intracellular edema with reticular degeneration and vesiculation are not features, in contrast to those caused by the other pox viruses. The characteristic feature is the presence of lobulated, endophytic hyperplasia, producing a circumscribed, somewhat crateriform intradermal pseudotumor ( Fig. 18.79 ). The keratinocytes contain a very large intracytoplasmic inclusion, which compresses the nucleus against the cell membrane. Although initially eosinophilic in size, the inclusion gradually develops a marked basophilia ( Fig. 18.80 ). The latter can be recognized in a cytological preparation from the surface of the lesion; this may serve as a quick diagnostic test. Their presence may be rendered more conspicuous by the use of Lendrum phloxine tartrazine reaction ( Fig. 18.81 ). Rarely, primitive follicular induction may be encountered in the basal epidermis in the vicinity of MC lesions, and is likely a virally induced reactive phenomenon. In those cases lacking obvious molluscum bodies on initial examination, the presence of an edematous or fibromyxoid stroma, or keratinocyte alterations such as nucleolar prominence, cytoplasmic amphophilia, and clear cytoplasmic vacuolation, should prompt scrutiny of deeper tissue sections.
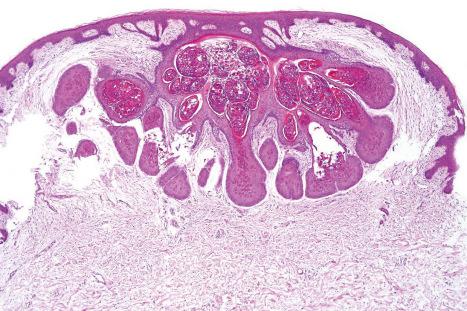
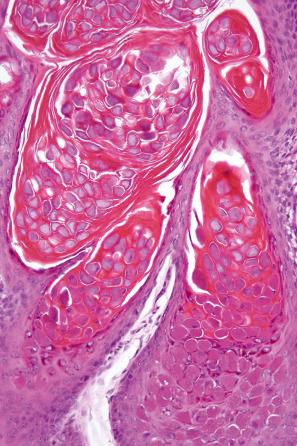
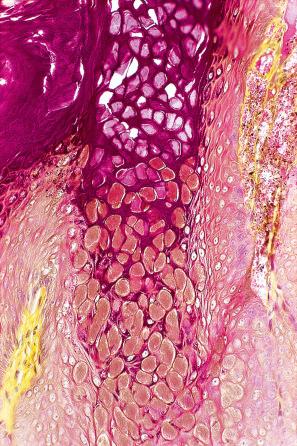
Usually, there is no dermal infiltrate, but when it does occur, allegedly in response to virus or inclusion bodies entering the dermis, the lymphocytic infiltrate is so intense that lymphoma may enter the differential diagnosis if the characteristic inclusion bodies are not obvious ( Figs 18.82 and 18.83 ). This dermal lymphoid infiltrate may harbor large, atypical CD30-positive lymphoid cells, potentially mimicking a CD30-positive cutaneous lymphoproliferative disorder. This is particularly seen in regressing lesions containing few inclusion bodies, and the histologic diagnosis may be very difficult. Langerhans cell hyperplasia has also been reported.
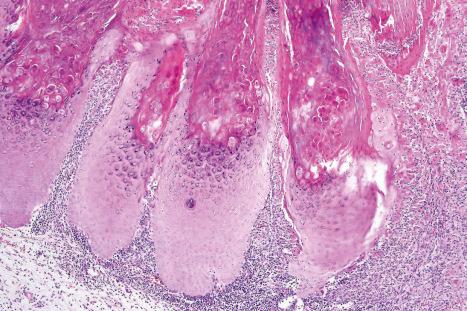
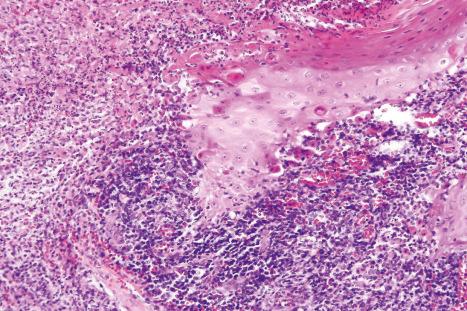
The presence of metaplastic bone formation in otherwise typical lesions of MC has been reported. MC has also been noted in association with epidermal cysts, melanocytic nevi, melanoma, sebaceous hyperplasia, soft fibromas, lupus erythematosus, leukemia cutis, eosinophilic cellulitis, and even Kaposi sarcoma.
This is a viral illness caused most often by coxsackievirus A16 and less often by enterovirus 71. It usually affects young children, shows seasonal variation (being more common in summer and autumn), and presents as small epidemics. A number of outbreaks have been documented in the East, especially Taiwan and Mainland China. Outbreaks have also occurred in Malaysia, Singapore, and India. Children in their first 4 years of life are most susceptible. Other viruses implicated include echovirus 19, and very rarely, echovirus 4. More recently, there have been outbreaks caused by coxsackievirus A6 in Europe, North America, and Asia. Molecular phylogenetic analysis has shown that two distinct genogroups of coxsackievirus A16 exist, namely, A and B. Phylogenetic evidence has also revealed that intertypic recombination between enterovirus 71 and coxsackievirus A16 may play a role in the emergence of subgenotypes of enterovirus 71.
Hand, foot, and mouth disease has an incubation period of 3–7 days. Following a prodrome of headache, fever, malaise, abdominal pain, and sometimes diarrhea, patients develop oral ulcers and blisters, most commonly on the inner cheeks and lips, accompanied by small erythematous papules, which soon evolve into grayish vesicles on the soles, palms, and ventral surfaces and sides of the fingers and toes ( Figs 18.84–18.87 ). Lesions are less commonly found on the perineum, buttocks, trunk, and extremities. The eruption is self-limiting. Atypical manifestations include eczema coxsackium in children with atopic dermatitis, and onychomadesis. Complications only occur in approximately 6% of cases attributable to coxsackievirus A16, notably aseptic meningitis.
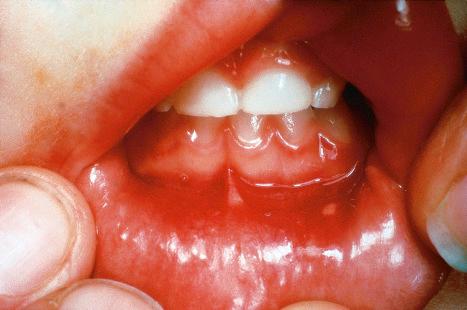
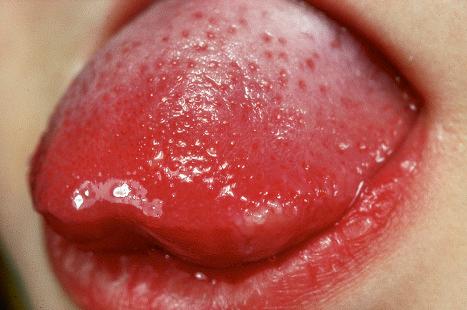
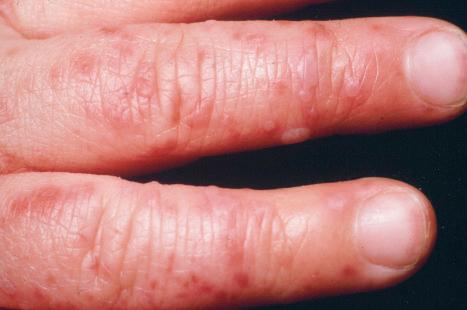
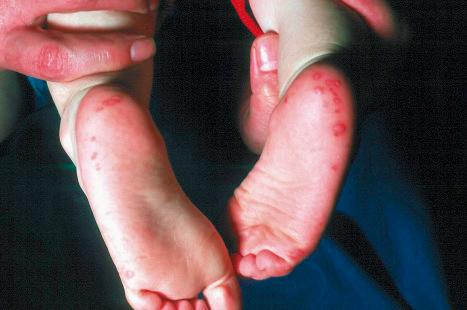
Hand, foot, and mouth disease due to enterovirus 71 is a more serious illness, with complications occurring in approximately one-third of patients. These include central nervous system (CNS) lesions predominantly affecting the cerebellum. Encephalitis, aseptic meningitis, and a poliomyelitis-like condition with acute flaccid paralysis can also occur. Concomitant infections may sometimes be seen. Although a mortality rate of around 8% was recorded in the past, more recent data from China reflect lower fatality rates, ranging from 0.03% to 1.8%. Death is usually attributable to cardiopulmonary decompensation and acute pulmonary edema. The mechanism of pulmonary edema is unclear, since it does not appear to be the direct result of viral myocarditis in most cases; it has been postulated that increased pulmonary vascular permeability secondary to brainstem lesions or a systemic inflammatory response to encephalitis may play a role. In one outbreak in Singapore, however, most fatalities were the result of interstitial pneumonitis, either alone or in association with myocarditis or encephalitis.
The virus is transmitted by direct contact with nasal and pharyngeal secretions, feces, and blood. Histologically, the blister is intraepidermal and develops as a consequence of marked inter- and intracellular edema ( Figs 18.88 and 18.89 ). There may be associated papillary dermal edema. Viral inclusions and giant cells are not a feature. Enterovirus 71 may induce apoptosis of infected host cells via a virus-encoded protein.
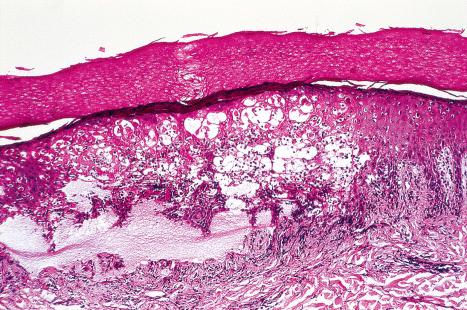
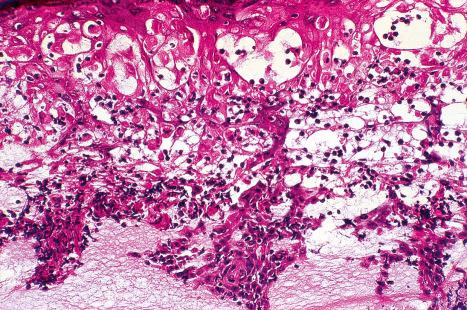
A real-time reverse transcriptase PCR method that allows for the differentiation of enterovirus 71 from coxsackievirus A16 has been described.
The hemorrhagic fever (HF) viruses are a special group of highly infectious RNA viruses transmitted to humans by arthropods (mosquitoes, ticks) or rodents, resulting in systemic illness and a generalized bleeding diathesis. The viral etiological agents may be grouped according to family:
Flaviviridae, which are responsible for infections such as yellow fever, dengue fever, Omsk HF, and Kyasanur Forest disease,
Bunyaviridae, which cause Rift Valley fever, Crimean–Congo HF, and hantavirus infections (e.g., HF with renal syndrome),
Arenaviridae, which are responsible for Lassa fever, Argentine HF, Bolivian HF, Venezuelan HF, Brazilian HF, Lujo virus infection, etcetera,
Filoviridae, which cause Marburg virus disease and Ebola.
There is marked diversity in the severity of illness and the mortality within this group of diseases; dengue fever, for example, it has a mortality of around 5%, whereas the mortality associated with Ebola may be as high as 50% to 90%. Healthcare workers exposed to infected patients are at particular risk of contracting the disease, as seen during the 2008 Lujo virus outbreak in Southern Africa, and the more recent West African Ebola epidemic of 2013–2016. Ebola may also be transmitted by the handling of bushmeat, close contact with infected animals (e.g., chimpanzees, fruit bats), and direct contact with the blood, bodily fluids, or skin of infected patients or their corpses. Increased international travel has resulted in some infections (e.g., dengue) presenting in travelers upon their return to nonendemic countries. Most viral HFs manifest as an acute febrile illness, often with myalgias. Conjunctival injection and periorbital edema may occur. Diagnosis is usually confirmed by virus-specific enzyme-linked immunosorbent assay (ELISA) and PCR methods. Although some earlier classifications of viral HF included chikungunya (caused by chikungunya fever virus, a member of the Togaviridae), hemorrhagic manifestations are relatively rare and the disease has thus been omitted from most contemporary HF classifications.
A detailed discussion of all of the conditions listed above is not possible, and the reader is referred to references for excellent overviews on the subject. The further discussion here will focus on the potential mucocutaneous manifestations of viral HFs in general.
A conspicuous but non-specific, diffuse, nonpruritic maculopapular skin rash is typically encountered in filovirus infections such as Marburg virus disease and Ebola Desquamation may take place during the recovery phase in nonfatal cases. A similar although less prominent eruption may be seen on the trunk and limbs of patients with Rift Valley fever. Dengue HF is frequently associated with a morbilliform rash, often with islands of spared skin. Omsk HF, Kyasanur Forest disease, Argentine HF, and Bolivian HF are associated with a papulovesicular eruption involving the palate. Around 30% of patients with dengue HF have mucosal involvement, most often of the conjunctiva.
Thrombocytopenia is a characteristic sequel in the majority of viral HFs and manifests as petechial hemorrhages on the skin and in relation to the mucous membranes. Ecchymoses may develop in severe infections (e.g., Crimean–Congo HF) and are usually located over pressure points. There may also be bleeding from venipuncture sites. DIC is known to complicate the clinical course of some infections, especially Rift Valley fever. Jaundice is a typical feature of yellow fever but may also be encountered in Rift Valley fever, Crimean–Congo HF, and the filoviral HFs.
Although Sindbis fever is not strictly one of the viral HFs, this relatively mild, self-limiting togavirus infection may rarely be associated with hemorrhagic skin lesions, and has been included here for the sake of completeness. In usual cases of Sindbis fever, the exanthem is papular or vesicular, occurring in crops lasting up to 10 days. Lesions tend to be concentrated over the buttocks, legs, palms, and soles.
Although the precise pathogenesis of all viral HFs has yet to be fully elucidated, the general trend is to target dendritic reticulum cells, monocytes, lymphocytes, hepatocytes, and vascular endothelial cells. The profound and diverse pathophysiological effects are mediated via the release of a host of proinflammatory cytokines from these infected cells, resulting in a cytokine storm, increased vascular permeability, shock, tissue necrosis, hemorrhage, and coagulopathy. Many of these viruses are capable of suppressing innate and adaptive immune responses in the infected host, leading to rapid local and systemic dissemination.
The dermatopathological manifestations of this group of infections are poorly documented, largely because of the infectious and hemorrhagic risks associated with biopsy. In most cases, the findings are relatively non-specific. Consequently, skin biopsy is of questionable diagnostic or prognostic value. The cutaneous histologic features of dengue HF are perhaps the best studied among the HFs. In general, HF cases with a maculopapular eruption show mild lymphocytic vasculitis, with a mild perivascular mononuclear inflammatory cell infiltrate, endothelial swelling, and minor perivascular erythrocytic extravasation ( Fig. 18.90 ). Keratinocyte dyskeratosis may also be seen (personal observation). Viral antigens may be demonstrated by immunohistochemistry if specific antibodies are used. Extensive dermal hemorrhage is seen in ecchymotic lesions. Intravascular fibrin-platelet thrombi characterize cases complicated by DIC.
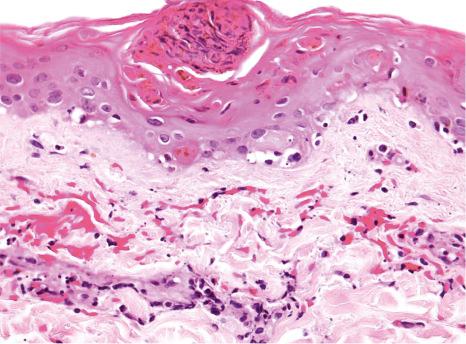
It is important to remember that the HF viruses are not the only infective agents that may be associated with pyrexia and hemorrhage. Other viruses such as smallpox virus and herpes simplex also may produce an HF picture (e.g., hemorrhagic smallpox). Additional infective agents that may cause HF are rickettsiae (e.g., Rickettsia rickettsii ), chlamydiae (e.g., Chlamydia psittaci ), bacteria (e.g., Neisseria meningitidis , Yersinia pestis ), fungi (e.g., Aspergillus ), spirochetes (e.g., Leptospira icterohaemorrhagiae , Borrelia recurrentis ), and protozoa (e.g., Plasmodium falciparum ).
Trichodysplasia spinulosa (TS), also known as virus-associated trichodysplasia spinulosa, is a rare, relatively recently recognized, and novel viral infection of the hair follicle in immunocompromised patients. In 2010 it was linked to a newly discovered human polyomavirus, now termed TS-associated polyomavirus (TSPyV). The condition is mainly seen in solid organ transplant patients, particularly renal transplant, but may also occur in patients with chemotherapy-associated immunosuppression. It is likely that TS is the same entity as pilomatrix dysplasia and ciclosporine-induced folliculodystrophy.
TS is characterized by multiple small, skin-colored to erythematous spiky follicular papules which are asymptomatic or mildly pruritic and have predilection for the face and ears. Lesions can also occur on the trunk and limbs, although they tend to be more sparse in these locations, and may coalesce. Focal alopecia can occur. When the immunosuppression is reduced the condition improves or regresses.
TS is induced by active TSPyV infection of the hair follicle. Electron microscopy studies have demonstrated intranuclear viral particles of variable size, from 30 to 46 nm. There is clustering of infected follicular cells, which express the putative transforming TSPyV early large tumor (LT) antigen. The latter is thought to result in hyperproliferation, pRB phosphorylation, and up-regulation of p16 and p21.
Histologically, anagen hair follicles appear dysmorphic, with distension, dilatation, and hyperkeratosis of the infundibula and abnormal maturation with marked inner root sheath (IRS) differentiation. The follicles are overpopulated by IRS cells, a process has been described ‘as if the hair follicles were entirely devoted to producing inner root sheath’. Prominent cells with eosinophilic cytoplasm and numerous trichohyaline granules are seen ( Fig. 18.91 ). The outer root sheath persists beyond the isthmus and the bulbs are abnormal, lacking fully formed papillae. Often, there are only few matrical cells as they are replaced by large numbers of cells with eosinophilic cytoplasm. There is usually no transition between the IRS and a fully cornified hair shaft. The diagnosis may be confirmed by immunohistochemistry.
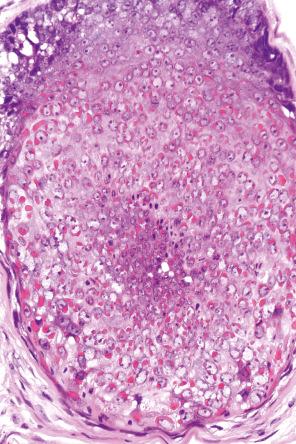
The differential diagnosis includes keratosis pilaris and lichen spinulosus. Keratosis pilaris mainly involves the proximal limbs, rarely involves the face, and it has very subtle microscopic features, mainly hyperkeratosis and plugging of the infundibula. Lichen spinulosus mainly occurs in children, has predilection for the proximal limbs, neck, and buttocks, and it is characterized histologically by hyperkeratosis in the infundibular part of the hair follicles.
The skin has a normal commensal population of bacteria in which Staphylococcus epidermidis predominates. Other resident Gram-positive bacteria include Micrococcus spp. and Corynebacterium spp. The free fatty acids and other lipids derived from the stratum corneum and sebum have an antibacterial role, yet 10% to 20% of the normal population are cutaneous carriers of S. aureus and approximately 10% are pharyngeal carriers of group A β-hemolytic streptococci ( Streptococcus pyogenes ). This ‘carrier’ state may precede infective lesions in the host or may be the origin of infections in others. S. aureus and S. pyogenes are the most common agents in superficial bacterial infections of the skin, but even organisms of low virulence, such as S. epidermidis , can become pathogenic with a sufficiently large inoculum or in an immunocompromised host. S. aureus toxin production is also responsible for bullous impetigo, the staphylococcal scalded skin syndrome (SSSS), and the toxic shock syndrome.
Impetigo is the most superficial pyogenic (pyoderma) bacterial skin infection and is highly infectious. It is exceedingly common and occurs most often in childhood, but may be seen in the elderly and in patients with immunodeficiency states. It is estimated that more than 162 million children worldwide suffer from this condition at any given time. The median childhood prevalence is around 12%. The highest disease burden, however, appears to be among underprivileged children from marginalized communities in high-income countries. Impetigo is typically subdivided into nonbullous (simple) impetigo and bullous impetigo and usually follows the contamination of minor skin abrasions and insect bites by S. aureus or S. pyogenes . Scabies is an important risk factor for impetigo, especially among children.
In simple impetigo, the lesions present as small superficial vesicles, which rapidly burst and are replaced by a characteristic, adherent, thick yellowish dirty crust with a margin of erythema ( Figs 18.92–18.94 ). The mouth, nose, and extremities are particularly affected. Regional lymphadenopathy is sometimes present. Simple impetigo may occur in endemic or epidemic form and often spreads to involve siblings and schoolmates. It is seen more often in warm, humid conditions. Although S. aureus remains the more frequent cause in North America and S. pyogenes was the more important etiological agent of impetigo in developing countries in the past, S. aureus is now the most commonly isolated pathogen in many parts of the world. In some cases, however, the two bacteria appear to coexist. Streptococcal impetigo may occasionally progress to cellulitis or precede acute glomerulonephritis, erythema nodosum, or erythema multiforme.
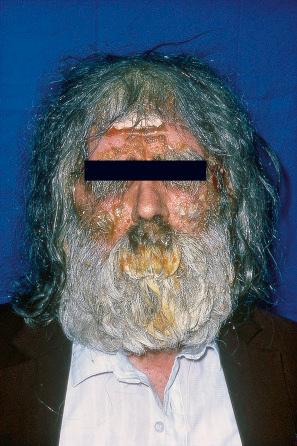
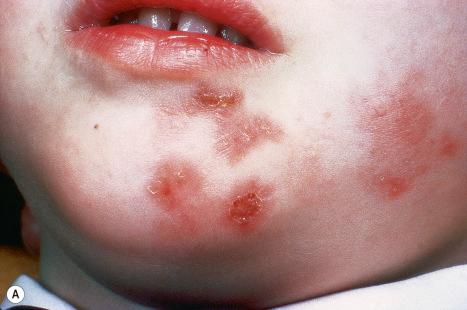
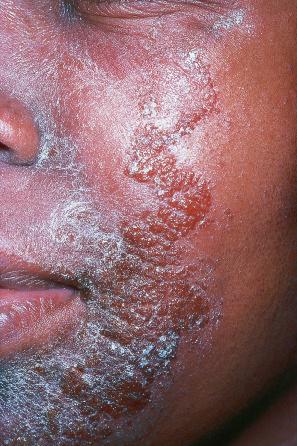
Bullous impetigo is primarily a staphylococcal-mediated disease exclusively due to phage group II S. aureus . Superficial blisters up to 2 cm across are the initial features ( Fig. 18.95 ). The contents are at first clear, but rapidly become cloudy and then develop a thin seropurulent crust; erythema is not marked. The lesions do not usually involve mucosae. There may be mild constitutional symptoms, and the lesions resolve in 2–3 weeks.
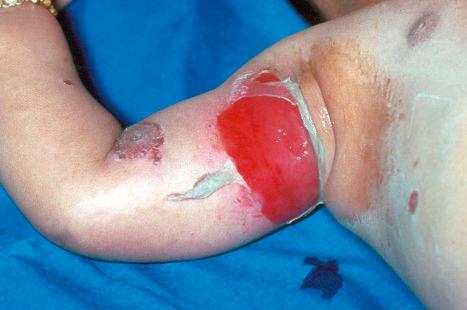
Ecthyma is probably a variant of impetigo and occurs predominantly on the lower limbs of children, but may occur in adults and at other sites ( Figs 18.96 and 18.97 ). It presents with thick crusting, overlying punched-out ulceration, and resultant scarring. S. pyogenes is the usual cause. It is more common in tropical climates, where it occurs in all age groups. Minor trauma or scabies infestation may determine the site of the lesions. It is possible that vasculitis and necrosis induced by bacterial toxins determine the different presentation.
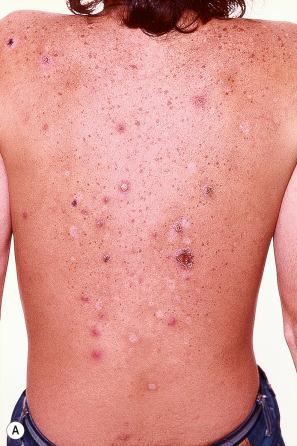
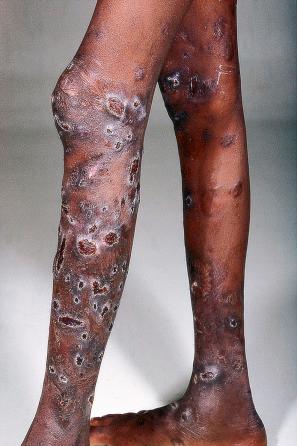
Ecthyma gangrenosum is usually a complication of P. aeruginosa septicemia that occurs in immunodeficient patients, particularly those with a neutropenia. It has also been reported in association with hypogammaglobulinemia. There have been rare reports of ecthyma gangrenosum occurring in the absence of neutropenia or septicemia. The condition has also been reported following toxic epidermal necrolysis (TEN). Lesions, which may be single or multiple, begin as painless erythematous macules that become indurated, bullous, or pustular. Annular lesions have been described. The lesions soon become gangrenous and covered by a characteristic gray-black eschar and erythematous halo. Lesions are especially seen on the gluteal and perineal regions and on the limbs. The mortality is high, particularly in those with multiple lesions. Organisms other than P. aeruginosa have been implicated in the evolution of the disease.
The carrier state or inoculation by a contaminated object is a necessary precondition to superficial infection of the skin. The organisms become attached to the traumatized area, binding strongly to fibronectin and possibly type IV collagen and laminin, which are abundant in the exudate. The innate virulence of the organisms and the host defense capability determine the subsequent progress of the infection, but this is facilitated if the bacteria produce coagulase, hyaluronidase, or lipases. The form of S. aureus responsible for bullous impetigo is of serotype II and mainly of phage type 71 and produces exfoliative toxins A and B; the latter are also involved in the SSSS (see below). During the past decade, community-acquired methicillin-resistant S. aureus (CA-MRSA) emerged as a major cause of impetigo.
An early lesion of impetigo is characterized by a split in the epidermis just beneath the stratum granulosum ( Fig. 18.98 ). The resultant vesicle becomes filled with neutrophils, Gram-positive cocci, and occasional acantholytic cells ( Fig. 18.99 ). The underlying dermis contains a mixed neutrophil and lymphocyte infiltrate. Neutrophils may be seen in the spongiotic stratum spinosum beneath the vesicle in the process of migrating from the dermis as a chemotactic response to the causative bacteria. In conditions of impaired neutrophil function, impetigo may be common and more extensive.
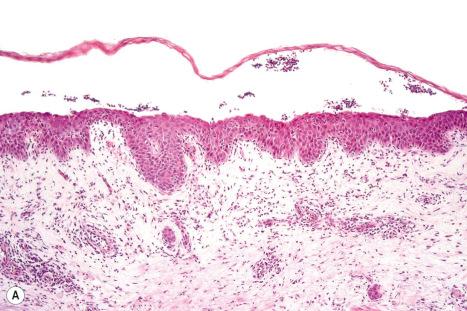
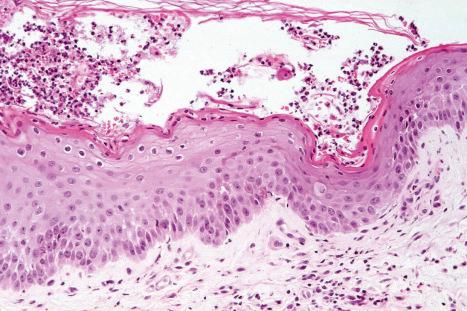
In echthyma, there is a sharply circumscribed area of ulceration with a heavy neutrophil infiltrate and overlying adherent crust ( Fig. 18.100 ).
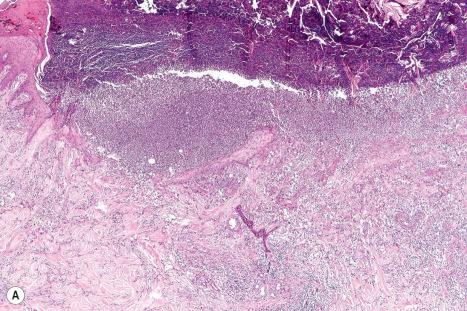
Ecthyma gangrenosum is characterized by epidermal necrosis with hemorrhage and dermal infarction, usually accompanied by a mixed inflammatory cell infiltrate of lymphocytes, histiocytes, and neutrophils. Less commonly, a dearth of inflammatory cells is noted. Gram-negative bacilli may be seen in the dermis and involving the media and adventitia of venules. Vasculitis and thrombosis may be present.
The diagnosis is usually made on clinical grounds and supported by culture of the causative organisms. Rarely, a biopsy is necessary.
The lesion may be confused histologically with a superficial variant of pemphigus, particularly as the latter can become secondarily infected and there may be a few acantholytic cells in impetigo. Antibodies in a pemphigus-like pattern may be demonstrated in bullous impetigo and distinction from pemphigus foliaceus may therefore be a problem. Generally, the presence of numerous neutrophils and the recognition of Gram-positive cocci is sufficiently characteristic to confirm impetigo, as acantholytic cells are very scanty. Distinction from subcorneal pustular dermatosis and pustular psoriasis may be considered histologically, but the lack of acanthosis, although not conclusive, should point toward impetigo.
Staphylococcal scalded skin syndrome (SSSS) is so named because of the presence of staphylococcal toxin and the resemblance of the established lesion to a scald. It may present in epidemic form as well as sporadically. It has a relatively abrupt onset and occurs almost entirely, but not exclusively in neonates and young children, who develop first a macular scarlatiniform eruption in association with a staphylococcal infection. This is often associated with fever, irritability, and skin tenderness. The eruption then spreads from its usual original sites on the face, axillae, and groins to involve large areas of the skin surface. Conjunctivitis is often also present. Mucous membranes, however, are not affected. At the same time, the skin becomes edematous and the surface fragile so that it can be sheared off in thin wrinkled sheets, likened to peeling wet wallpaper, leaving a glistening red surface, and the child becomes sick and feverish ( Fig. 18.101 ). SSSS is therefore associated with a positive Nikolsky sign.
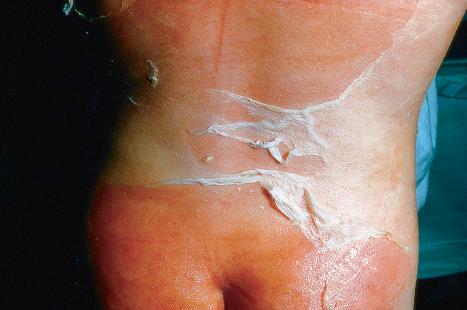
Following blistering, desquamation occurs and the skin often returns to normal by 2–3 weeks. Scarring is not usually a feature. The associated source of infection may be an upper respiratory tract infection, conjunctivitis, or umbilical sepsis, or it may be more occult, such as in the middle ear, in the pharynx, or at the site of minor surgery. The condition is rarely seen at other ages, such as the first day after birth or even at birth, indicating an infection acquired in labor or in utero, and it may be seen in older children. Numerous adult cases have been reported, although many of these patients were either immunocompromised (including HIV infection) or in renal failure with a possible inability to excrete the toxin. The condition does occur rarely in previously healthy adults or those with relatively minor conditions ( Fig. 18.102 ). It is also a potential complication among hematopoietic stem cell recipients.

The disease in neonates (Ritter disease) is usually self-limiting with rapid resolution of the skin blisters and complete recovery. However, there is a mortality of less than 10% (generally 2–4%) due to progression of the staphylococcal infection or the complications of exfoliation. In the adult, this rare condition has shown a mortality rate of between 40% and 63%. Recovery is facilitated by antistaphylococcal antibiotics and attention to fluid balance.
An abortive form, the scarlatiniform variant (staphylococcal scarlet fever), in which the initial erythroderma evolves into the desquamative phase in the absence of blistering, has been described. This may be particularly associated with occult bone and joint infections or contaminated wounds.
In SSSS the organism is of group II and usually phage type 71, but phage types 3A, 3B, 3C, and 55 have also been found. These bacteria all produce an epidermolytic toxin (formerly referred to as exfoliatin), of which there are two antigenic types: exfoliative toxin A (ETA) and exfoliative toxin B (ETB). ETA is associated with a chromosomal gene while ETB is plasmid encoded. These serine proteases have indistinguishable activity and manifest exquisite pathological sensitivity by inducing blistering only at the level of the superficial epidermis. Their high substrate specificity is associated with selective recognition and hydrolysis of desmosomal proteins, notably desmoglein 1. The effect of the toxins is determined by their site of production:
Toxins produced by the appropriate staphylococcal infection of mucosae or surgical wounds enter the circulation (toxemia) and produce a generalized change in the skin, the SSSS. The intensity is determined by the rapidity with which the toxins are metabolized and excreted renally and by the presence of antibodies against the toxins.
If the same staphylococcus infects the skin directly, local release of toxin results in bullous impetigo.
Staphylococcal strains also produce toxic shock syndrome toxin (TSST-1) and a variety of enterotoxins (A–E). These are more frequently associated with staphylococcal scarlet fever and the toxic shock syndrome.
The toxin in both SSSS and bullous impetigo causes a split in the epidermis at the level of the granular layer ( Fig. 18.103 ). Ultrastructurally, the level of separation is in the midgranular layer. It is associated with separation of the cell membranes at the desmosomes, and the interdesmosomal regions appear less dense. The cells on either side of the cleft appear normal. It has been shown that the epidermolytic toxins (ETA and/or ETB) act as serine proteases and bind desmoglein 1, a desmosomal adhesion molecule. The cleavage and subsequent inactivation thereof induces blistering.
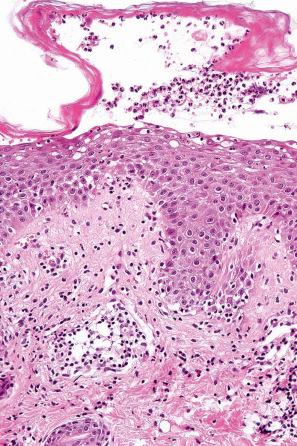
Histologically, the clefts through the granular layer of the epidermis in SSSS are associated with only a scanty inflammatory infiltrate in the epidermis or dermis, and there may be some dermal edema and dilatation of the superficial vascular plexus. The adjacent epidermis does not show necrosis. Acantholysis is variably present ( Fig. 18.104 ).
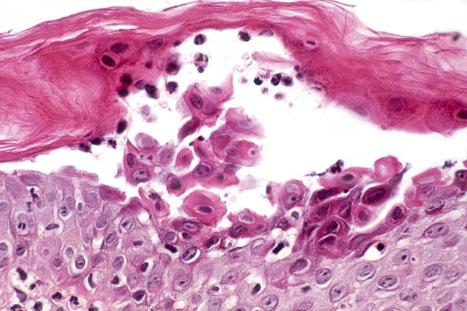
The main clinical differential diagnosis is from TEN, a severe variant of erythema multiforme usually due to a drug reaction. This, however, is uncommon in children. In TEN the skin changes are very widespread and mucosal involvement is common, whereas SSSS extends from the face and flexures and does not involve mucosae. The contrasting histology of the two conditions can be used with a frozen section technique to give a rapid diagnosis :
TEN is characterized by a subepidermal blister with a necrotic epidermal roof and a moderately intense lymphocytic infiltrate in the dermis.
SSSS is characterized by separation through the granular cell layer.
Alternatively, a Tzanck smear can be used, which reveals necrotic keratinocytes with inflammatory cells in TEN and viable acantholytic keratinocytes without inflammatory cells in SSSS.
Erysipelas is classically a S. pyogenes (group A β-hemolytic streptococcal) infection of the skin of the face, characterized by a sharply outlined edematous, erythematous, tender, and painful plaque ( Fig. 18.105 ). The outer margin is elevated and contrasts with the adjacent normal skin. Toward the edge of the lesion there may be vesicles and hemorrhagic bullae. Other sites commonly affected include the feet and hands. Although S. pyogenes is the most common organism, it is not always possible to culture it, and other streptococci (group C or G) or S. aureus may be isolated, in particular, methicillin-resistant S. aureus (MRSA).
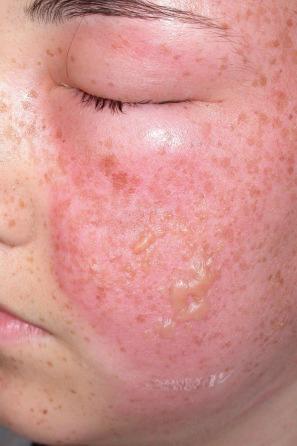
The classical presentation of erysipelas is more often on the face, but it has been suggested that the features of this condition are changing because it is becoming more common and is predominantly affecting the lower limbs. A seasonal variation in incidence is not uniformly found.
The superficial infection of erysipelas is associated with fever and malaise. It is similar in its presentation, etiology, and associated symptoms to cellulitis, a spreading infection affecting deeper tissues. This lesion is again clearly demarcated, hot, red, and painful, but without the elevated margin of erysipelas. An associated lymphangitis and lymphadenitis are common. The lesions may progress to pustulation, ulceration, and necrosis; the last may involve underlying fascia and muscle, resulting in NF. Osteoarticular complications such as bursitis, arthritis, tendinitis, and osteitis have been reported. Positive blood cultures may be found in 4.6% of patients.
Cellulitis is similar to erysipelas, but tends to involve the deeper tissues and is seen most often on the legs, where it often complicates tinea pedis or chronic lymphedema ( Fig. 18.106 ). Other potential risk factors include diabetes mellitus, leukemia, postsaphenous venectomy, and peripheral vascular disease. Patients with dry skin may also be susceptible. Cellulitis is characterized by an expanding area of erythema. Involvement of lymphatics in both erysipelas and cellulitis is characteristic, resulting in the edema which is sometimes associated with vesiculation ( Fig. 18.107 ); infective damage to lymphatics in cellulitis may be the reason this condition can become recurrent. Approximately 25% of patients will experience more than one episode of cellulitis within 3 years. Symptoms and signs of systemic illness may be encountered in up to 40% of cases. In a systematic review of 1578 cases, 7.9% of patients had positive blood cultures.
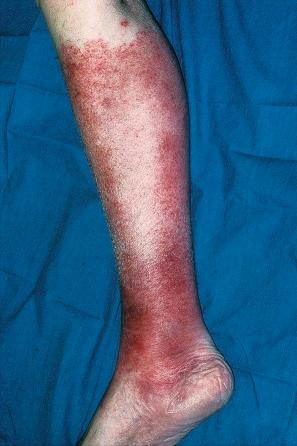
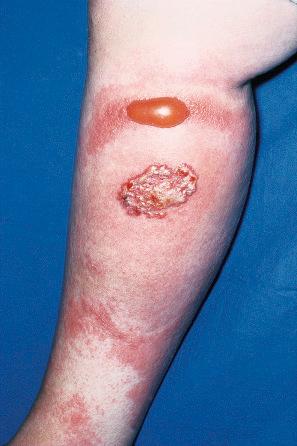
The term hemorrhagic cellulitis has been applied to an uncommon clinical syndrome caused by Gram-positive or Gram-negative organisms of noncutaneous origin. Patients manifest with the abrupt onset of painful erythema on the lower extremities, followed by dermal hemorrhage and sloughing of the overlying epidermis. There is usually a satisfactory response to a combination of antibiotics and corticosteroid therapy. Virulent marine bacteria such as Vibrio vulnificus may result in this form of bullous and hemorrhagic cellulitis; the disease carries an estimated mortality of 15%.
Minor trauma to the skin is important in the development of both erysipelas and cellulitis, but peripheral vascular disease, diabetes, lymphedema, and alcohol abuse are additional predisposing factors.
In both erysipelas and cellulitis, S. pyogenes is the most common organism and the lesions are initiated by inoculation of minor abrasions or splits in the epidermis. An increased proportion of infections due to MRSA, however, has emerged during the past one and a half decades. Proliferation of the organisms is associated with the production of enzymes (streptolysins, deoxyribonuclease B, hyaluronidase) which may be detected in rising titers and may therefore be useful diagnostically. These enzymes are also important in facilitating the extension of the bacteria in the skin. Lymphatic involvement with obstruction is common, resulting in edema, and is associated with lymphangitis and lymphadenitis. In the more aggressive forms of cellulitis, it is likely that other organisms (certainly S. aureus and some anaerobes) may be causative or synergistic. A synergistic role for S. aureus (especially methicillin-resistant strains) and S. pyogenes has been implicated in the development of bullous erysipelas. Group G streptococci predominated over group A β-hemolytic streptococci in a Finish series of 90 patients with acute cellulitis. There have been rare reports of severe cellulitis due to organisms such as Streptococcus pneumoniae , Haemophilus influenzae , and Nocardia otitidiscaviarum . In those forms of cellulitis in which necrosis is a more prominent feature, bacterial toxins are an important mechanism.
Histologically, the conspicuous features of erysipelas and cellulitis are dermal edema and lymphatic dilatation. There is also a diffuse, heavy neutrophil infiltration with a limited localization around blood vessels. Vascular and lymphatic dilatation and red cell extravasation are variable features. In later stages, some lymphocytes and histiocytes are also seen and granulation tissue may be present deep to the zone of subepidermal edema. When clinical vesicles or bullae are noted, there is a corresponding severe papillary edema merging with subepidermal vesiculation.
In hemorrhagic cellulitis, the bacterial lipopolysaccharide-induced or bacterial mitogen-induced release of TNF-α is thought to result in injury to endothelial cells and epidermal keratinocytes. DNA fragmentation and cell lysis may be the consequence of neutrophil degranulation and anti-DNase activation. Digestion of vascular basement membrane as a result of V. vulnificus metalloprotease production leads to the formation of hemorrhagic bullae. Histologically, there is necrosis of epidermal keratinocytes, necrotizing vasculitis affecting dermal blood vessels, and large numbers of bacteria.
It is important to remember that not all cases of cellulitis are bacterial in origin; deep fungal infections such as cryptococcosis and aspergillus may present with cellulitis in immunocompromised hosts. Appropriate histochemical stains and the referral of additional specimens for microbiological examination should facilitate correct identification of the etiological agent.
NF is an uncommon, rapidly progressive, and potentially fatal bacterial infection of the subcutaneous soft tissues. NF may evolve following a surgical procedure (e.g., esthetic liposuction, cesarean section, laparoscopic appendicectomy, excision of a skin lesion or even cardiac catheterization), minor trauma, seemingly insignificant scratches, in the presence of a chronic wound, or even in apparently intact skin. NF occurs predominantly in middle-aged individuals, although the pediatric population may also be affected. Patients with underlying diabetes mellitus, chronic alcoholism, cirrhosis, and iatrogenic immunosuppression are particularly susceptible. NF is a well-recognized complication of childhood varicella. Many reported cases have developed after intramuscular injection of non-steroidal anti-inflammatory drugs (NSAIDs), which may, in turn, mask the symptoms of evolving NF; an association with the intake of oral NSAIDs has also been documented. Rare cases have occurred following the bite of a spider. NF may be a rare complication of fistulating Crohn disease. Reported mortality ranges from 3.4% to 53%. An increased fatality rate may be encountered in the elderly and in those with worsening symptoms and signs within the first 48 hours of hospital admission.
Although group A β-hemolytic streptococci (so-called ‘flesh-eating’ bacteria) were first recognized as a prime etiological agent, a number of other aerobic and even anaerobic pathogens have been implicated. It has become increasingly apparent that NF very often is a polymicrobial condition. In some series, Staphylococcus aureus is the most frequently cultured organism. Less often, other streptococci have been identified; these include group B and group G β-hemolytic streptococci, Streptococcus pneumoniae , anaerobic streptococci ( Peptostreptococcus spp.), and S. dysgalactiae subsp. equisimilis ; the latter is a recently recognized cause of NF and shares approximately 70% of its genome with group A Streptococcus , but lacks some of the virulence factors of the latter organism. Other bacteria implicated include marine organisms ( Vibrio vulnificus , V. parahaemolyticus , Photobacterium damsela ), members of the family Enterobacteriaceae, Serratia marcescens , Pseudomonas spp., Clostridium spp., and Bacteroides spp. There have also been isolated reports of NF due to Haemophilus influenzae serotype f, Myroides odoratus and Aeromonas sorbia . Candida albicans is an exceptionally uncommon cause of NF. Meleney postoperative progressive synergistic gangrene (Meleney gangrene) is synonymous with polymicrobial NF arising as a complication of surgical trauma. The latter condition may be clinically indistinguishable from postsurgical cutaneous amebiasis.
The clinical presentation may be fulminant, acute, or subacute. NF commences as an ill-defined area of erythema, accompanied by tenderness, swelling, and increased temperature. It is, therefore, not uncommon for evolving NF to be mistaken for cellulitis or an insignificant wound infection, especially when the hallmark cutaneous necrosis is not established. This may result in a potentially life-threatening delay in diagnosis and aggressive surgical debridement, and a high index of suspicion, therefore, is required. The clinical features of established NF include severe pain, indurated edema, skin necrosis, cyanosis, bullae (which may be hemorrhagic, especially in cases caused by Vibrio spp.), crepitation, muscle weakness, and malodorous exudates ( Fig. 18.108 ). Anesthesia may be a late sign. Patients often have other systemic manifestations of severe sepsis, including hypotension, tachycardia, tachypnea, oliguria, and mental confusion. In NF caused by streptococcal species, the latter signs are usually attributable to streptococcal toxic shock syndrome. Radiographs may reveal gas in the affected soft tissues, although this is only seen in approximately 25% of cases. NF occurs mainly on the extremities, although almost any site may be affected, including the abdominal wall, chest wall, eyelids and periorbital region, and the head and neck region. Periumbilical NF may occur in newborn infants. The Waterhouse-Friderichsen syndrome is a potential complication of NF.
Fournier gangrene is a clinical variant of NF which involves the penis, scrotum, perineum, and abdominal wall in men and (less often) the vulva in women. An obliterative endarteritic process affecting the small branches of the superficial branch of the internal pudendal artery may play a key pathogenetic role. Because of a response to corticosteroids, Fournier gangrene may be perceived as a localized vasculitis. In addition to the usual risk factors such as diabetes mellitus or immunosuppression, rare associations include vasectomy or unhygienic ritual circumcision. Hypertension, alcoholism, and advanced age are further risk factors. The reported mortality of this polymicrobial synergistic necrotizing infection is in the order of 16–20%, although this ranges from 3% to as high as 80% in various series. Extent of infection is a significant predictor of clinical outcome.
NF due to invasive group A β-hemolytic streptococcal infection is associated predominantly with M types 1 and 3, which produce either pyrogenic exotoxin A or B, or both. Tissue invasion is facilitated by CD44-mediated cell signaling with subsequent manipulation of the host cytoskeleton. Superantigens and Th1 cytokines appear to play a critical role in severe group A invasive streptococcal infections. Streptococcal cysteine protease SpeB inactivates the antimicrobial peptide cathelicidin LL-37 at the bacterial surface. Impaired recruitment of polymorphonuclear leukocytes to the site of the infection has been linked to the streptococcal peptidase ScpC which degrades IL-8. It has been shown that a hyper-virulent phenotype ensues as a result of the organism's destruction of its own covRS two-component system; the latter exercises a negative regulatory effect on numerous virulence factor genes. Protein S deficiency may be responsible for the necrosis. S. aureus may potentiate the β-hemolytic streptococcal infection in NF.
An adequately sized specimen including subcutaneous soft tissue is essential for diagnosis. The histologic appearances are those of a severe necrotizing process with edema, necrosis, and inflammation involving skin and subcutaneous tissue, including fascial planes ( Figs 18.109 and 18.110 ). Deep biopsies or debridement specimens containing underlying skeletal muscle may exhibit concomitant myonecrosis. Vascular thrombosis is encountered at all levels, and secondary vasculitic alterations are not uncommon. Hyaline necrosis of sweat glands has been described. The presence of large numbers of bacteria often results in diffuse basophilia of the tissue on low-power examination. A Gram or Brown-Hopps stain confirms the latter ( Fig. 18.111 ).
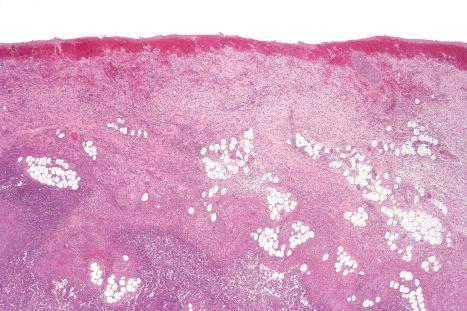
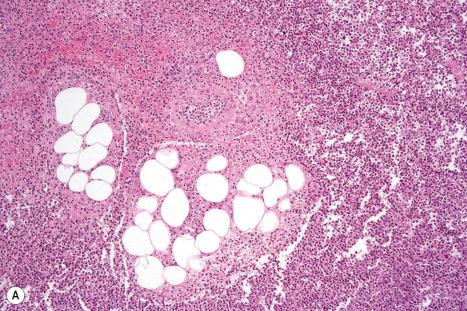
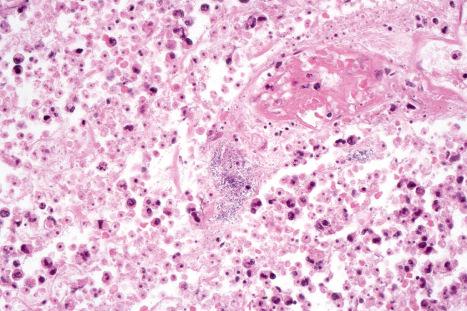
Although the histologic picture is sufficiently distinctive to facilitate a diagnosis of NF, microbiological examination (including aerobic and anaerobic tissue culture) is of paramount importance in the identification of the specific infective etiological agent(s). Intraoperative frozen section has a particularly useful role to play, not only in early diagnosis but also in assessing the viability of surgical margins at the time of debridement. PCR detection of streptococcal pyrogenic exotoxin B may be useful in confirming group A streptococcal infection when cultures are negative or unavailable.
Necrotizing (gangrenous) cellulitis has a similar etiopathogenesis to NF but shows no extension of the necrotizing inflammatory process into subcutaneous tissue planes. This diagnosis should be made with caution and only when there is a complete absence of subcutaneous involvement in a specimen that is of sufficient depth. NF is invariably associated with necrotizing inflammation of the dermis. Furthermore, necrotizing cellulitis may be a harbinger of impending NF.
Angioinvasive deep fungal infections such as aspergillosis, hyalohyphomycosis, and mucormycosis may be associated with extensive cutaneous and subcutaneous tissue necrosis. The causative organisms in such cases, however, are often visible on careful examination of routine H&E sections, and are readily identified with the aid of appropriate histochemical stains.
Tissue autolysis with bacterial overgrowth may closely mimic NF, especially if tissue is obtained from a patient with a relatively minor cutaneous infection and the specimen was not placed in the appropriate formalin fixative prior to submission to the laboratory.
NF is distinguished from pyoderma gangrenosum and Sweet syndrome by the absence of true tissue necrosis and demonstrable bacterial organisms by culture or appropriate stains in the latter two conditions. Although there is frequent dermal infiltration by polymorphonuclear leukocytes in pyoderma gangrenosum and invariable neutrophilic dermatosis in Sweet syndrome, the acute inflammatory changes in both conditions are generally centered on the dermis rather than the subcutis. Vasculitic alterations may occur in both pyoderma gangrenosum and NF but are usually absent in Sweet syndrome.
Extravasation of anthracycline chemotherapeutic agents such as doxorubicin may be associated with extensive necrosis of skin and subcutaneous tissues, with a resultant histologic picture distinctly reminiscent of NF. The clinical history and negative Gram stain, however, argue against NF in such cases.
Infection of hair follicles is probably the commonest form of skin infection. It is usually due to S. aureus (impetigo of Bockhart) and, although disfiguring, is self-limiting. Pustular folliculitis usually implies infection of the ostium and upper part of the follicle. It presents as numerous small red and tender pustules, which discharge pus and quickly resolve without scarring ( Fig. 18.112 ). Staphylococcal carriers tend to have recurrent infections. The role of community-associated CA-MRSA in cutaneous infections, including folliculitis, has been emphasized in recent years.
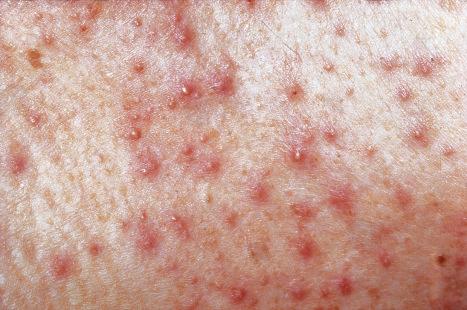
P. aeruginosa is a well-recognized cause of epidemics of folliculitis associated with swimming pools, whirlpools, or spa baths. These shared facilities can be infected by Pseudomonas if they became alkaline and if the chlorine content drops. Nevertheless, moisture and occlusion are necessary to affect normal skin. For this reason, lesions of this type are found only under the area covered by bathing costumes. Other Gram-negative bacteria such as Klebsiella spp., Escherichia coli , Enterobacter spp., and Proteus spp. have been implicated in the pathogenesis of folliculitis in patients receiving long-term antibiotic therapy for treatment of acne or rosacea. Extensive folliculitis may be an early manifestation of HIV infection. Micrococcus spp., which are considered commensal organisms, may be a cause of folliculitis in patients with HIV infection. Folliculitis due to Acinetobacter baumanii has been reported in a patient with AIDS.
A furuncle or boil is a more exuberant form of suppurative folliculitis. It is common in young adults and usually affects the skin of the face, neck, buttocks, and axillae ( Figs 18.113–18.115 ). Lesions can be up to 2 cm across and the inflammation is not confined within the follicle, but is associated with much surrounding erythema and often systemic symptoms. After discharge of the pustular necrotic core, the lesion heals rapidly, but with scarring. A deep folliculitis due to S. aureus may affect the beard area; this form is termed sycosis or folliculitis barbae. CA-MRSA is strongly associated with recurrent furunculosis in the United States. Nasal carriage of S. aureus occurs in 60% of individuals and represents a major risk factor for the development of recurrent furunculosis.
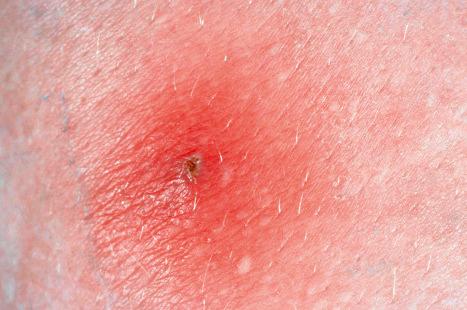
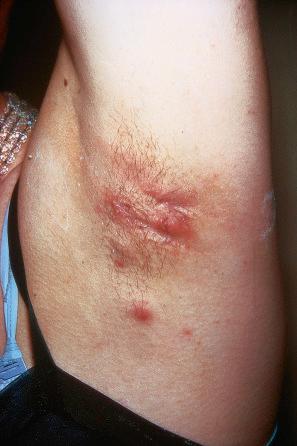
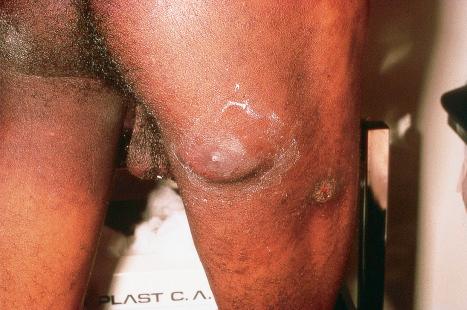
A carbuncle is a variant of a furuncle with multiple tracks and routes of discharge. It is most commonly seen in older men and may be associated with systemic symptoms.
Acute paronychia is comparable to a folliculitis in that it is a painful suppurative infection of the nail fold, most commonly caused by S. aureus ; it heals rapidly on release of the pus ( Fig. 18.116 ). A rare scarring alopecia in which S. aureus has been implicated is termed folliculitis decalvans. However, an etiological link to the organism has not been demonstrated. Although the scalp is predominantly affected, lesions may also be found in the axillae and pubic region ( Fig. 18.117 ). The latter condition is discussed in greater detail in the relevant chapter on diseases of the hair.
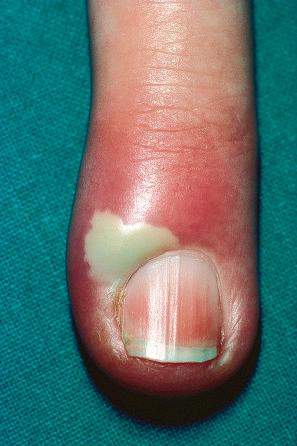
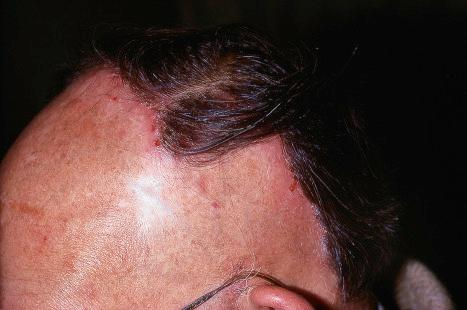
Many cases of superficial suppurative folliculitis are associated with S. aureus . The infection is not due to a break in the epithelium, but growth occurs within the ostium of the follicle and may progress more deeply around the hair shaft. There is an associated accumulation of neutrophils, forming an abscess associated with spongiosis and infiltration of the adjacent follicular epithelium. The superficial suppurative folliculitis may discharge through the ostium and rapidly resolve. Alternatively, it may progress more deeply and rupture through the follicular epithelium; the abscess then extends into perifollicular dermis and surrounds the whole follicle. The follicular epithelium and hair shaft with pus then form the purulent necrotic core of the furuncle or boil. Healing is preceded by a lymphohistiocytic or even granulomatous phase and is followed by scarring and loss of hair in the involved area. A carbuncle is associated with more persistent suppuration, much more fibrosis, and granulation tissue. Panton-Valentine leukocidin-producing strains of S. aureus have been linked to the evolution of deep-seated, often multiple furuncles.
Although most of the suppurative forms of folliculitis are due to S. aureus , other causal conditions include dermatophytosis, herpes simplex, and syphilis; the features of these infections are described under the appropriate headings elsewhere in this chapter.
This deep and scarring folliculitis and perifolliculitis (sometimes known as acne keloidalis or acne keloidalis nuchae) occurs on the back of the neck (lower occipital/nuchal region) of postpubertal males. It occurs more commonly in black than in white men. Although it was initially thought not to develop in females, rare cases have been reported. It presents in the early stages with inflamed papules and pustules, but no consistent organisms are found. Patients may complain of itching, burning, or pain and advanced lesions may be foul smelling. Each lesion is complicated by dense scarring, producing a keloid-like appearance. Scarring alopecia is a complication. The condition has been reported in Caucasian organ transplant recipients on ciclosporine therapy. Isolated cases occurring in association with keratosis follicularis spinulosa decalvans have been documented.
The pathogenesis is unknown. Any bacteria identified probably represent a secondary phenomenon. It is doubtful whether poor hygiene is a factor. There may be an element of pseudofolliculitis (see below) since the condition is common in Afro-Caribbeans and Africans and is worsened by close shaving of the neck. The use of pomades and wearing tight collars is said to exacerbate the condition. The term acne keloidalis is a misnomer, as the disorder has nothing to do with acne vulgaris or its variants and keloid formation is not usually seen.
The follicle, which may contain pus extending through the epithelium, is initially surrounded by a lymphocytic and neutrophil infiltrate; later, there are large numbers of plasma cells. The perifollicular inflammation is maximal at the level of the isthmus and lower infundibulum. The condition is more often seen at a later stage, however, when there is marked fibrosis accompanying free, broken hair shafts, many of which are surrounded by a foreign body giant cell reaction. Hyalinization as seen in a true keloid is only very occasionally a feature. Complete loss of the sebaceous glands is a frequent occurrence.
Pseudofolliculitis (pseudofolliculitis barbae, pseudofolliculitis cutis) presents with an acneiform papular and pustular eruption on the beard area. Comedones are not a feature. It develops as a consequence of the reentry of a terminal hair shaft through the epidermis and occurs most often in males with curly hair, but is also seen in women in the pubic region following cosmetic shaving. Pseudofolliculitis occurs predominantly in patients of African, African-American, and Hispanic origin. The pathogenesis appears to be multifactorial, and seems to relate to the shape of the hair follicle, the hair cuticle, and the direction of hair growth. The presence of curly hair and a single-nucleotide substitution in the gene encoding keratin 75 act in concert to confer an increased risk for the development of the condition. The penetration is facilitated by the sharp ends produced on hairs by shaving and the curliness of the hair bringing the cut end back into contact with the skin surface. Alternatively, the penetration may occur laterally through the superficial part of the follicular infundibulum following partial retraction of the hair after close shaving. A hypertrophic form of the disease has been described in renal transplant recipients.
The process is not a true folliculitis, and is not usually associated with infection. The reentry of the hair shaft provokes a foreign body granulomatous reaction with accompanying fibrosis. The inflammation is predominantly histiocytic with occasional multinucleate giant cells. Secondary infection may result in superimposed suppuration. Resolution occurs rapidly, with slight scarring, once the hair shaft is removed.
Meningococcal septicemia (meningococcemia) is due to the Gram-positive diplococcus Neisseria meningitidis . In its acute form, this is a very serious condition with a high mortality, which affects children in seasonal epidemics. The infection may occasionally be encountered in adults. Occurrence in the neonatal period, however, is rare. The organism is spread via droplet inhalation from upper respiratory tract infections. Meningitis is the most frequently encountered manifestation of meningococcal infection. Children with acute meningococcal septicemia develop widespread purpura that shows a predilection for the trunk and limbs. Ecchymoses may also be a feature. In the more chronic variant (chronic meningococcemia), patients present with vasculitis-like lesions, particularly nodules, and palpable purpura.
The histologic features are essentially those of a leukocytoclastic vasculitis. Superimposed DIC may also be present. The hemorrhagic skin lesions and vascular thromboses are attributable to up-regulation of tissue factor leading to coagulation, and by inhibition of fibrinolysis by plasminogen activator inhibitor. Impairment of the protein C anticoagulation pathway also plays an important role. Experimental evidence has revealed that adhesion of the organism to the dermal microvascular endothelium leads to local vascular damage, thrombosis, and purpura. Capsular polysaccharides and lipooligosaccharides play key roles in the evasion of killing by host complement.
Diplococci may be demonstrable in Gram-stained sections, especially in biopsies obtained from purpuric lesions. Culture or Gram staining of aspirates or biopsies of skin lesions may facilitate early diagnosis. Diagnosis can also be confirmed on skin biopsy with the aid of a PCR-based method.
This common venereal disease is due to infection with the Gram-negative intracellular diplococcus Neisseria gonorrhoeae , which especially affects the mucous membranes. In males, this results particularly in purulent urethritis, although gonococcal proctitis, epididymitis, prostatitis, and oropharyngitis may also be seen. Females most often develop endocervicitis. Urethritis and proctitis can also be features.
Systemic gonococcal infection most commonly affects the skin, but may also result in arthritis and less often endocarditis or meningitis. Patients present with small numbers of erythematous macules that progress to painful papular, petechial, papulovesicular, or pustular lesions that particularly affect the distal limbs ( Fig. 18.118 ). They measure from 1–2 mm up to 2 cm in diameter. Lesions may appear frankly vasculitic. Cutaneous lesions can be a presenting feature of disseminated infection during pregnancy. Rarely, however, patients present with primary cutaneous (genital and extragenital) involvement. Cellulitis, pustules, ulcers, and furuncle-like lesions have been documented. Lobular capillary hemangioma (pyogenic granuloma)-like lesions of the penile shaft have also been described. Primary digital gonorrhea has been recorded.
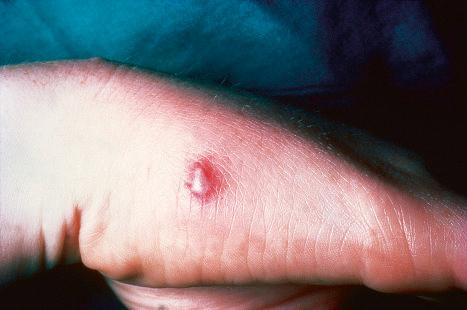
Disseminated lesions show variable epidermal changes ranging from edema accompanied by a neutrophil infiltrate with purpura, to vesiculation, pustulation, and eventually necrosis. In the dermis, the histologic features are essentially those of a neutrophil-mediated acute vasculitis, often accompanied by thrombosis. Very occasionally, Gram-negative diplococci may be identified.
Plague is an acute, febrile infectious disease caused by Yersinia pestis , a nonmotile, bipolar Gram-negative aerobic bacillus and one of the most deadly pathogens known to man. Since the 1990s, the majority of human cases have been reported from Africa. The disease has a high incidence in Uganda, the DRC, South Africa, Madagascar, and parts of India. Foci of plague have also been reported from the United States and in parts of South America. Rodents act as the reservoir, and the infection is usually transmitted to humans via the bite of a flea; aerosol spread may also occur, leading to pneumonic plague. Y. pestis is well recognized as a potential bioweapon and bioterrorism agent. There are three distinct clinicopathological forms of the disease: bubonic plague, primary pneumonic plague, and primary septicemic plague.
Bubonic plague accounts for the vast majority of cases. After a short incubation period of approximately 2–4 days, the disease manifests abruptly with pyrexia, chills, tachycardia, and tachypnea, and the formation of a so-called bubo – a painful, pathologically enlarged unilateral group of infected lymph nodes, usually in the groin or axilla ( Fig. 18.119 ). Cervical and axillary buboes are more common in children than in adults. Septicemia and secondary pneumonic plague may follow in untreated cases. Minor (‘ambulatory’) forms of bubonic plague also exist.
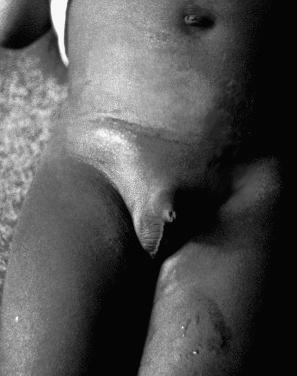
Primary pneumonic plague is acquired by inhalation of the organisms, whereas primary septicemic plague tends to occur after the bite of an infected flea on the head and neck region. Untreated, both of these forms of plague carry a mortality of around 90%.
Cutaneous manifestations of Y. pestis infection are seen predominantly in bubonic plague. A small vesicle, pustule, papule, or necrotic lesion may develop at the site of the flea bite. The skin overlying the bubo is erythematous and edematous and may undergo hemorrhage and necrosis, resulting in the formation of fistulae. Roseolar, scarlatiniform, vesicopustular, and erythema multiforme-like eruptions may occur elsewhere on the body. Petechial hemorrhages and ecchymoses characterize severe cases. Cutaneous ulceration and necrosis may ensue, hence the term ‘black death’.
The lymph nodes comprising the buboes show severe acute hemorrhagic lymphadenitis in the presence of large amphophilic or ‘ground-glass’ aggregates of bacilli. Their characteristic ‘safety-pin’ appearance is discernible on sections stained with the Gramor Giemsa methods. Extranodal extension results in ulceration of the overlying skin. Subsequent septicemic illness may be complicated by DIC. In the skin, the latter manifests with intradermal hemorrhage, thrombotic vascular occlusion, and multiple cutaneous infarcts. The diagnosis may be confirmed by microscopy, culture, immunofluorescence, ELISA, or PCR. An immunohistochemical method for detection of the organism in formalin-fixed, paraffin-embedded tissue has been described.
Anthrax is a zoonotic infection caused by Bacillus anthracis , an encapsulated, spore-forming, Gram-positive bacillus. Although the condition is relatively uncommon in humans, epidemic outbreaks still occur in tropical and subtropical regions of the world (including Africa and South America), southern Europe, Turkey, the Middle East, and India. Anthrax is very rarely seen in developed countries. Cutaneous anthrax accounts for more than 95% of cases; pulmonary and gastrointestinal forms generally account for the remainder, and are associated with a high mortality. Recent years, however, have seen the emergence of a fourth form of the disease – injection (injectional) anthrax. The latter occurs among users of intravenous drugs, notably heroin. All of the cases recorded thus far appear have occurred in Europe, and the infection is attributed to contamination of the heroin.
In its conventional form, the condition is usually acquired after contact with infected animals (herbivores) or contaminated animal products. It has also been described in rural Turkish children who were subjected to the ritual smearing of cow's blood on their foreheads. Under ideal conditions, spores may survive in the soil or in animal products for many years. There is ongoing interest in the organism as an agent of bioterrorism.
Cutaneous anthrax occurs after inoculation of B. anthracis into abraded skin. An erythematous macule or papule develops after an incubation period of 2–3 weeks. This later evolves into a pruritic vesicle. A characteristic black eschar develops after breakdown of the blister ( Figs 18.120 and 18.121 ). There is often pronounced edema and erythema of the surrounding skin, sometimes accompanied by the formation of bullae. The infection may present with periorbital involvement. Lymphadenitis is sometimes seen. Septicemia may arise in untreated cases; this carries a mortality of 10% to 20%. Toxemic shock and renal failure have also been reported. A rare form of the disease termed malignant edema presents with severe, rapidly spreading edema, lymphangitis, lymphadenitis, and systemic symptoms. Hemorrhagic and necrotic vesicles may evolve in these cases. Complications documented among patients with injection anthrax have included mulitorgan failure, compartment syndrome, NF, and lethal cervical cellulitis. Injection anthrax carries a mortality of up to 37%.
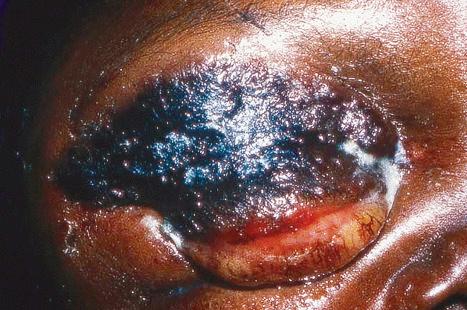
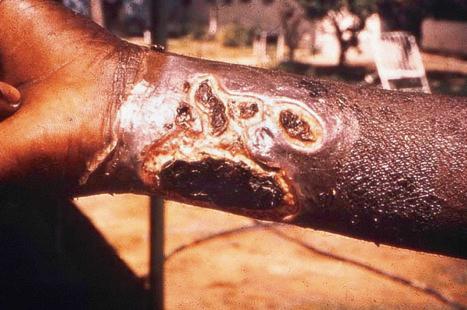
B. anthracis is associated with potent virulence factors, namely, a poly- d -glutamic acid capsule with antiphagocytic properties, and a plasmid-encoded polypeptide protein exotoxin consisting of three components: edema factor (EF), protective antigenic factor (PA), and lethal factor (LF). Anthrax toxin receptor 2 (ANTXR2) and capillary morphogenesis protein 2 (CMG2) are the major receptors for anthrax toxins. Binding of PA to cellular receptors facilitates translocation of EF and LF into host cells, with subsequent alterations in cell signaling pathways. EF leads to increased vascular permeability via the production or release of inflammatory mediators, including neurokinins, prostanoids, and histamine.
The histologic picture is dominated by massive subepidermal edema ( Fig. 18.122 ). Intraepidermal edema results in coalescent intercellular vacuoles. The epidermis is often attenuated. The dermis is expanded by a dense inflammatory infiltrate consisting of large numbers of polymorphonuclear leukocytes, admixed with lymphocytes and histiocytes. The process often extends into subcutaneous fat. Vasodilatation may be prominent. Hemorrhage and fibrin deposition occur in the deep dermis and subcutis ( Fig. 18.123 ). Thrombotic vascular occlusion and fibrinoid necrosis of blood vessel walls may be encountered in the deep dermis and subcutaneous fat ( Fig. 18.124 ). A Gram stain will reveal considerable numbers of large Gram-positive bacilli predominantly in the superficial dermis ( Fig. 18.125 ). The diagnosis is confirmed by culture. Serological investigations are also available. Immunohistochemical methods of detection may be used on tissue specimens.
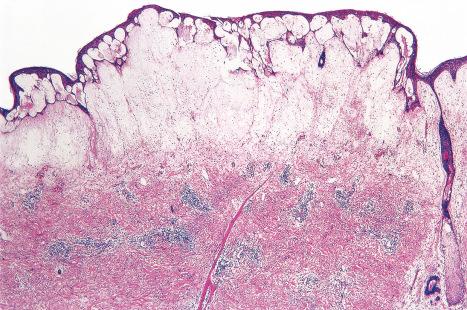
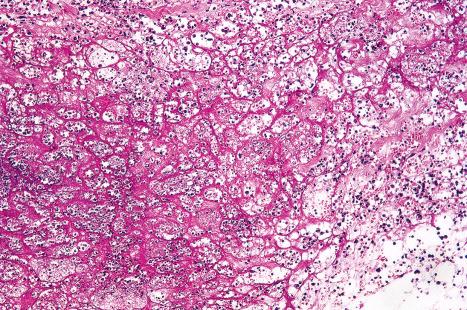
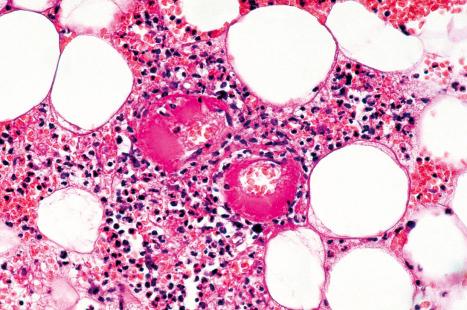
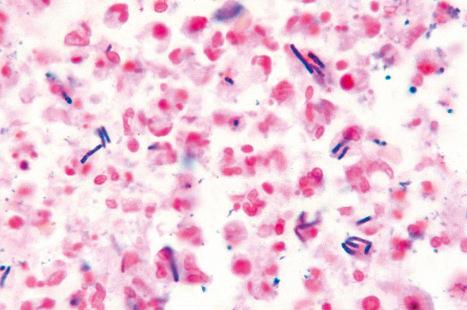
Brucellosis is a zoonotic infection by Brucella spp. such as B. melitensis , B. abortus , B. canis , and B. suis . The organism is a Gram-negative bacillus, and infection is acquired either by ingesting contaminated, unpasteurized milk/milk products, or by handling infected animal products (contact brucellosis). Human-to-human transmission does not occur. Brucellosis results in either an acute febrile illness or a chronic systemic disease characterized by fever, malaise, sweats, arthralgia, myalgia, and/or hepatosplenomegaly. Although the disease is endemic in several countries bordering the Mediterranean Sea, the majority of reports of human infection appear to have come from Turkey and the Middle East. The mortality is low and most patients recover within 3 months.
Cutaneous manifestations occur in approximately 6% or less of cases; however, this figure approached 14% in a series from Turkey. A number of cutaneous manifestations have been recorded, including a disseminated papulonodular eruption, a diffuse maculopapular rash, erythema nodosum-like subcutaneous nodules, purpura, leukocytoclastic vasculitis, erythema multiforme, urticaria-like papules and plaques, multiple abscesses, cutaneous ulcers, ecchymoses and (rarely) livedo reticularis, palmar erythema, or an even large mass mimicking a soft tissue tumor. Vasculitic skin lesions may form part of an infection-induced systemic thrombotic microangiopathy mimicking Henoch-Schönlein purpura. There is a single case report of Brucella -associated Stevens-Johnson syndrome. Contact brucellosis manifests with erythema or pruritus, usually on the forearm or hand. In some cases, this may progress to a follicular, vesicular, or pustular eruption. A rare case of contiguous skin involvement secondary to underlying Brucella chronic osteomyelitis has been recorded. A factitious case of brucellosis caused by autoinoculation has also been reported; the patient developed bacteremia and ulcerating cutaneous abscesses.
The histologic features vary according to the type of cutaneous lesion biopsied. Biopsies obtained from the erythematous papular and maculopapular lesions show a perivascular and periadnexal infiltrate of lymphocytes and histiocytes, sometimes accompanied by epithelioid histiocytes and multinucleated giant cells. Erythrocyte extravasation is sometimes seen, and inflammatory cells may infiltrate the overlying epidermis. Erythema nodosum-like nodules are characterized by a perivascular lymphohistiocytic infiltrate centered on the deep dermis and superficial subcutis. There is associated vascular endothelial swelling and luminal thrombosis, sometimes with foci of necrosis. Accompanying granulomatous inflammation is not uncommon. Erythema multiforme lesions and leukocytoclastic vasculitic lesions exhibit the usual histologic changes associated with these conditions.
Brucella organisms are rarely visualized in histologic material. The diagnosis is therefore confirmed by culture, serology or, PCR.
Cat scratch disease is a not uncommon, usually self-limiting illness which, as its name implies, usually follows (after 3–5 days) a scratch or bite by a cat (usually a kitten). On rare occasions, it has been reported following a similar injury caused by a dog or even by a monkey. It occurs equally in males and females, most often during the first two decades. Cat scratch disease shows seasonal variation, occurring usually in autumn and winter. The causative agent is Bartonella henselae (formerly Rochalimaea henselae ), a weakly Gram-negative bacillus measuring 1–2 µm in length. The condition was first described in 1950, yet the nature of the infective etiological agent remained elusive until relatively recently. In the past, a variety of infectious agents had been suggested as responsible for cat scratch disease, including mycobacteria, Chlamydia , herpes-like viruses, and latterly, Afipia felis . The cat represents the primary reservoir for the bacillus in addition to being its principal vector. Although transmission among cats is via the cat flea, the latter does not appear to play a direct role in transmission to humans.
The primary skin lesion – a macule, papule, vesicle, pustule, or nodule – develops most commonly on the arm or hand, followed by the head, leg, conjunctiva, trunk or neck, in decreasing order of frequency. Sometimes more than one member of a family may be affected. The cutaneous lesions measure 1–5 mm or more in diameter and may sometimes resemble an insect bite.
Other rare cutaneous manifestations include a nonpruritic macular or maculopapular rash, erythema nodosum, urticaria, erythema marginatum, erythema annulare, and thrombocytopenic purpura. A case with cutaneous lesions resembling those of Sweet syndrome has been reported. Fever and malaise are occasional symptoms. Less common additional features include headache, nausea, vomiting, arthralgia, and splenomegaly.
Patients invariably develop lymphadenopathy in the drainage region, usually within 1–3 weeks of the initial lesion. The enlarged nodes are tender and often persistent, with lymphadenopathy lasting up to 2 months or more. Suppuration is not uncommon. Conjunctival or eyelid lesions may be associated with preauricular lymphadenopathy (Parinaud syndrome). The only consistently abnormal laboratory function test is a moderately raised erythrocyte sedimentation rate. Purported cases of disseminated cat scratch disease occurring as a manifestation of AIDS probably represent examples of advanced BA (see below).
Atypical manifestations of cat scratch disease may occur in as many as 25% of cases. These include nonthrombocytopenic purpura and bone, liver, spleen, pulmonary, endocardial, and/or neurological involvement, presumably due to bacteremia. A vasculitic pathogenesis has also been proposed. CNS lesions have significant morbidity and include coma, encephalitis, convulsions, pareses, cerebellar signs, and abnormal tendon jerks. Hepatosplenic infection results in peliosis.
The histopathological features are those of dermal necrobiosis. The necrobiotic foci are round, triangular, or stellate and are surrounded by a palisade of histiocytes with occasional multinucleate giant cells ( Figs 18.126 and 18.127 ). Around the periphery of the histiocytic zone is a lymphocytic infiltrate in which eosinophils may be conspicuous. Nuclear debris may sometimes be prominent. The bacteria may often be identified using the Warthin-Starry reaction or the Brown-Hopps modified Gram stain within histiocytes or lying free. Bacteria may also be detected immunocytochemically. In the adjacent dermis, the blood vessels are surrounded by an infiltrate consisting of lymphocytes, plasma cells, and histiocytes. A granulomatous reaction has been described, but this is an uncommon phenomenon.
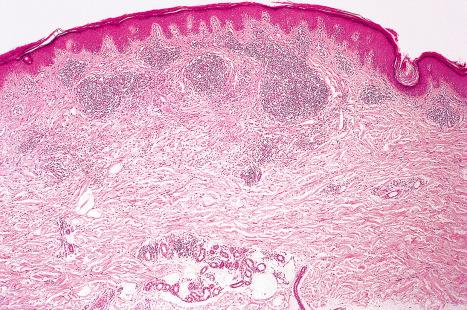
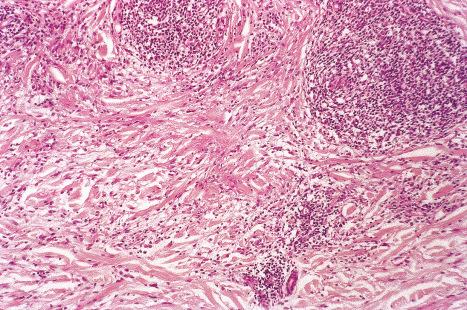
In early lesions, the lymph nodes show subcapsular foci of necrosis associated with a neutrophil polymorph infiltrate. An established infection is characterized by extensive necrotic lesions often involving the follicular germinal centers and associated with karyorrhexis. A peripheral rim of epithelioid cells and occasional giant cells is characteristic. These features are not in themselves diagnostic because they may be seen in a variety of conditions including lymphogranuloma venereum, yersiniosis, and fungal infections.
Previously, the diagnosis of cat scratch disease was confirmed by a positive delayed hypersensitivity reaction to the cat scratch disease antigen. Since B. henselae is difficult to culture, serology, and currently PCR have been advocated as more reliable diagnostic modalities. The organism may be identified in skin swabs obtained from the primary inoculation site.
Although a major epidemic of trench fever was documented during the First World War, more recent outbreaks of this condition have been described among homeless people, in whom there is a high seroprevalence of this bacteremic illness. Outbreaks have also occurred in overpopulated Central African refugee camps. Trench fever is caused by Bartonella quintana ; human body lice ( Pediculus humanus var. corporis ) are the known vectors. Head lice ( P. humanus var. capitis ) might also play a role in the transmission of the disease. B. quintana may also cause chronic bacteremia, endocarditis, and BA (see below).
Patients present with non-specific symptoms and signs including headache, malaise, pyrexia, rigors, tachycardia, myalgia, arthralgia, and injected conjunctivae. An erythematous macular or papular skin rash may occur. The rash is often seen on the trunk and usually lasts no more than a day or two. The disease is rarely fatal, except in some debilitated patients. Relapsing illness sometimes occurs, and the organism may remain latent in the host for a number of years following the acute infection.
The histopathological features in the skin are non-specific. There is a perivascular lymphocytic infiltrate, without evidence of vascular thrombosis. Organisms are not usually demonstrable in routinely stained skin biopsy specimens. Dermal vascular proliferation and neutrophilic infiltration (as seen in BA) are not features of trench fever. The diagnosis is confirmed by serology, culture, or PCR.
Bartonellosis (Carrión disease) is a biphasic disease caused by Bartonella bacilliformis , an organism that is closely related to B. henselae and B. quintana . The initial stage of infection (hematic phase) is referred to as Oroya fever. Patients are acutely ill with pyrexia, rigors, myalgia, and a severe hemolytic anemia. The last is attributable to infection of the circulating erythrocytes and can be confirmed with the aid of a blood smear. The case fatality rate among untreated patients may exceed 80% during acute outbreaks. Later, the disease enters an eruptive phase characterized by the evolution of numerous papular, nodular, or verrucous vascular skin lesions, referred to as verruga peruana (Peruvian wart, cutaneous verrucous disease). These occur predominantly on the face and extremities ( Fig. 18.128 ). Atypical cases may present with verrucous skin lesions as the sole manifestation. Most lesions resolve spontaneously. Genital lesions and nasal mucosal involvement have been recorded.
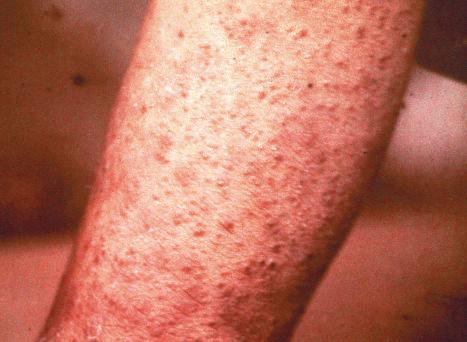
The condition is endemic in the higher altitude regions of Peru, where it was first described in the nineteenth century. Carrión disease also occurs in Ecuador and Colombia. Outbreaks have also been recorded in nonendemic parts of Peru. It has been suggested that the condition may be underreported in some endemic areas because of the existence of mild infection by less virulent strains of B. bacilliformis . The sand fly, Lutzomyia ( Phlebotomus ) verrucarum is the apparent vector.
There is infection of endothelial cells and circulating erythrocytes following introduction of B. bacilliformis via the bite of the vector. In the cutaneous verruga peruana lesions, organisms are detectable in the extracellular spaces, where they induce angiogenesis by producing putative microbial-encoded or microbial-induced angiogenic factors. In vitro studies have shown that B. bacilliformis exercises a mitogenic effect on human vascular endothelial cells. GroEL produced by the organism regulates endothelial cell growth. Others have observed that infection leads to the production of angiopoietin-2 by endothelial cells, and the production of VEGF by epidermal cells. The dermal angiomatous proliferation appears to occur in concert with the reactivation of latent B. bacilliformis organisms.
Histopathological examination of the verrucous lesions reveals an exuberant intradermal capillary proliferation lined by swollen endothelial cells, often accompanied by a neutrophilic infiltrate ( Fig. 18.129 ). Some of the superficial and peripheral vessels may be dilated, whereas deep dermal or subcutaneous nodules tend to have a more compact vascular and endothelial cell proliferation. Occasional cases harbor a cytologically atypical endothelial proliferation, resulting in potential confusion with malignant vascular tumors. There is a background mixed inflammatory cell infiltrate of variable intensity comprising neutrophils, histiocytes, lymphocytes, and plasma cells. Careful examination of the endothelial cells in early lesions may reveal characteristic intracytoplasmic aggregates of B. bacilliformis , referred to as Rocha-Lima inclusions. These may be highlighted with the aid of a Giemsa preparation. Ultrastructurally, the endothelial inclusions represent degraded bacteria and extracellular matrix components contained within cell surface invaginations. Bacteria are conspicuously absent from late lesions.
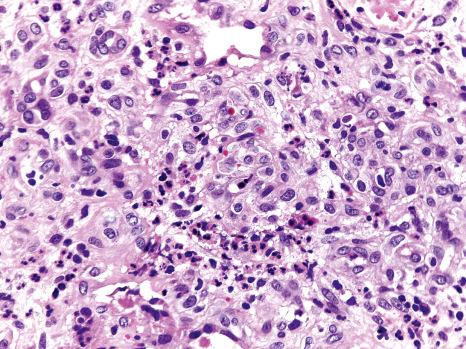
Verruga peruana should be distinguished from Kaposi sarcoma, BA, lobular capillary hemangioma (pyogenic granuloma), and true epithelioid vascular neoplasms (such as epithelioid hemangioma and epithelioid hemangioendothelioma).
Bacillary angiomatosis (BA) is a vasoproliferative lesion that may be readily confused with pyogenic granuloma or Kaposi sarcoma and is seen predominantly (but not exclusively) in the skin. Lesions have also been described in the bones, soft tissues, liver, lymph nodes, and spleen. Patients may have systemic manifestations including fever, malaise, hepatosplenomegaly, and lymphadenopathy. Although it was originally thought to be a disease specific to AIDS, it has also been described in other immunocompromised states (e.g., renal transplant recipients) and even in apparently normal individuals. Patients present with widespread, numerous (sometimes hundreds) of blood-red, smooth-surfaced, superficial papules and skin-colored or dusky subcutaneous nodules ( Figs 18.130 and 18.131 ). The condition may be caused either by Bartonella henselae (the organism responsible for cat scratch disease) or less frequently by B. quintana (the cause of trench fever).
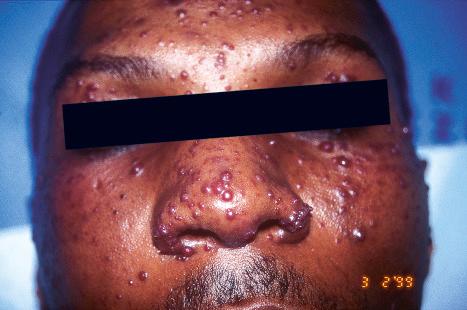
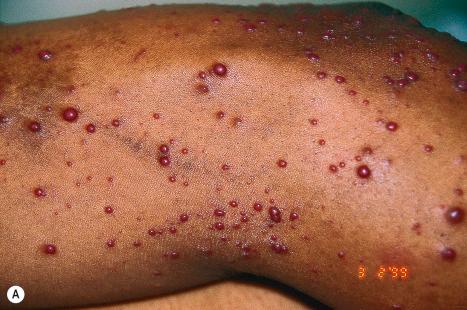
B. henselae infection is acquired via a cat bite or scratch, or cat flea bites, whereas B. quintana infection is transmitted by body lice. Patients with BA, however, seldom seem able to corroborate this history. A PCR-based study from Johannesburg revealed a 10% rate of Bartonella bacteremia among 188 attendees at an HIV clinic, only one of whom exhibited clinical features of BA. Vascular endothelial cells are the prime target of the organisms following initial intracellular colonization of erythrocytes. The VirB type IV secretion system of Bartonella plays a crucial role in not only establishing intraerythrocytic infection but also in mediating the organism's interaction with endothelial cells. Angiogenesis is potentiated by a combination of mechanisms, including inhibition of apoptosis, release of chemokines such as IL-8, and activation of hypoxia-inducible factor-1 (HIF-1).
Histology reveals lobules of capillaries with prominent, often cuboidal vascular endothelial cells, sometimes surrounding ectatic vessels among which are dispersed neutrophil polymorphs showing leukocytoclasis and purplish granules of bacilli, which can be identified best by the Warthin-Starry reaction ( Figs 18.132–18.135 ). Giemsa may also be used to identify the organisms, but since both the former and the latter stains are difficult to interpret and often to perform, a PCR method has been developed (see below). Sometimes solid endothelial cell proliferation is evident. Atypia and mitoses may be present. Superficial lesions have a polypoid configuration, and there may be an associated epidermal collarette reminiscent of a pyogenic granuloma. Ulceration is seen occasionally. Associated pseudoepitheliomatous epidermal hyperplasia has been described.
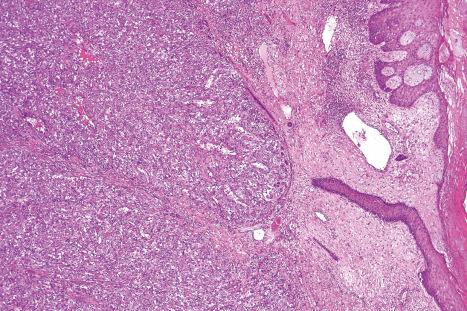
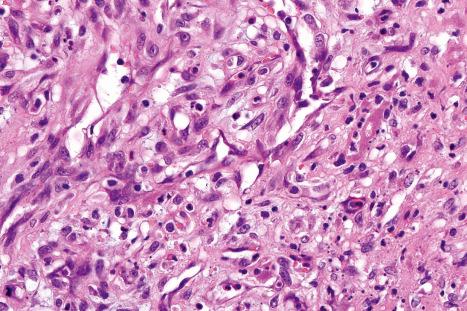
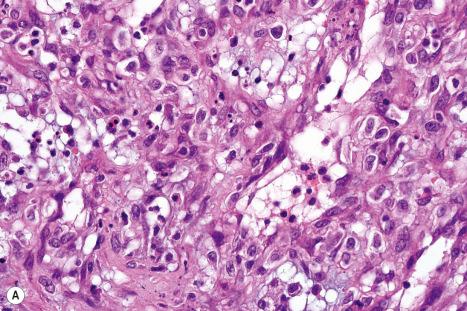
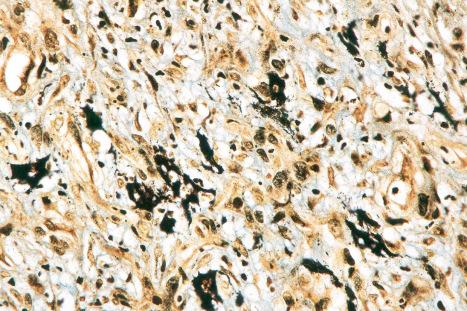
Although collagen dissection by spindled endothelial cells is encountered at the periphery of some lesions, hemosiderin deposition and hyaline globules as seen in Kaposi sarcoma are not evident. Late, involuting lesions show extensive fibrosis of the vascularized dermis, and little by way of a polymorphonuclear leukocytic infiltrate with karyorrhexis. Such cases require a high index of suspicion, as the bacteria may be difficult to demonstrate. Since most patients with this condition are immunocompromised HIV-infected individuals, it is prudent to examine sections carefully for additional opportunistic pathogens, such as CMV or mycobacteria.
The endothelial cells can be labeled with antibodies to factor VIII-related antigen, CD31 and CD34. Histologically, liver and splenic involvement is seen as peliosis. Typical bacteria are, however, also present. The recognition and distinction of this infection from Kaposi sarcoma and other vasoproliferative lesions is of great importance, particularly as it readily responds to antibiotic therapy.
Ultrastructurally, the organisms appear as aggregates of bacilli within the dermis. The bacteria have trilaminar walls.
BA must be distinguished from verruga peruana, pyogenic granuloma, epithelioid hemangioma, and Kaposi sarcoma. Pyogenic granuloma is not associated with Bartonella infection. The lobular capillary hemangioma (pyogenic granuloma)-like variant of Kaposi sarcoma in particular may be confused with BA. Although rare, BA with concurrent Kaposi sarcoma has been described. PCR or immunohistochemistry for detection of HHV-8 is a useful means of differentiating Kaposi sarcoma from BA; the former is invariably associated with HHV-8 whereas the latter has been found to be HHV-8 negative. Furthermore, PCR may be used to confirm the presence of Bartonella spp. in suspected cases of BA since these organisms are difficult to culture.
Lyme disease (Lyme borreliosis) is a generalized infection due to the spirochete Borrelia burgdorferi ( B. burgdorferi sensu lato complex), of which there are three main pathogenic genospecies in humans: B. burgdorferi sensu stricto , B. garinii , and B. afzelii . Two additional species described in Europe are B. valaisiana and B. spielmanii . It is the most frequently diagnosed tick-borne zoonotic illness in North America and Europe. The Centers for Disease Control and Prevention (CDC) reported a 40% increase in the annual incidence of this emerging zoonosis in the United States between 2001 and 2002; more than 40 000 cases were documented during this period. A subsequent CDC survey revealed that more than 64 000 cases were reported in that country during the period 2003 to 2005. This trend has also been observed in parts of the United Kingdom and Europe. Although Lyme disease remains a relatively uncommon condition in the United Kingdom, a 3.6-fold increase in the number of annual cases was nevertheless recorded in that country in 2011 as compared with pre-2004 data.
The disease affects most organ systems of the body. Lyme disease has been divided into three stages, I–III.
The skin lesion of the primary stage (erythema chronicum migrans, erythema migrans) consists initially of a small erythematous papule at the site of an insect bite and expands centrifugally as a flat ring ( Fig. 18.136 ). It is the commonest manifestation of the disease and develops on average 1–3 weeks after the bite. Occasionally, target lesions are described. Necrotic lesions are rare. With extension, the macules may develop a bluish or violet hue. If untreated, the ring may spread to a diameter of 50 cm before clearing. Lesional clearing is associated with a characteristic ‘bull's-eye’ appearance. Although lesions are usually asymptomatic, patients may complain of pruritus, burning, or rarely, pain. The lower extremity and trunk are most often affected. Multiple lesions (usually 2 or 3) may occur. There are rare reports of vesiculobullous forms of erythema migrans.
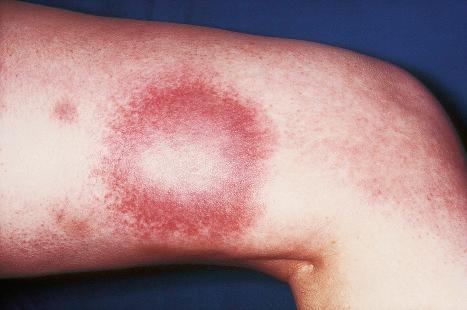
Approximately 50% of patients have secondary lesions, which are smaller. The palms, soles, and mucous membranes are usually unaffected. Erythema chronicum migrans may occasionally recur. Other cutaneous manifestations that have been described in the early stage of Lyme disease include granuloma annulare, papular urticaria, and Henoch-Schönlein purpura. Age at presentation is exceedingly variable, ranging from 15 months to 80 years. Infection rates are, however, highest among children aged 5 to 15 years, and in adults over the age of 50 years. The sex incidence is equal, and lesions present most often from May to September.
Systemic symptoms (due to a spirochetemia) tend to occur early in the disease and include chills, fever, general malaise and lethargy, arthralgia, myalgia, headache, and neck stiffness. Physical examination may reveal lymphadenopathy, splenomegaly, hepatitis, and orchitis. B. garinii and B. afzelii are the pathogens most often implicated in cases of Lyme disease reported from Europe, and some authors have described differences in the clinical presentation of erythema chronicum migrans caused by these two organisms. Erythemas associated with B. garinii tend to evolve more rapidly, are often larger and homogeneous, are more often located on the trunk than the extremities, and are more frequently associated with systemic symptoms when compared with B. afzelii erythemas, which are usually annular. Patients with B. garinii infection also tend to be older, and there is a shorter incubation period.
Lymphocytoma cutis (borrelial lymphocytoma), a B-cell response, may present in the acute stage and most often affects the lower ear lobes and nipples. It is, however, more often a feature of the third stage of the illness.
Stage II disease primarily affects the cardiovascular and nervous system (meningopolyradiculitis; Garin-Bujadoux-Bannwarth syndrome). It may involve both the peripheral and central nervous systems, and tends to present 1–2 months after the primary infection; symptoms include meningism, nerve palsies (especially Bell palsy), and cerebral symptoms, including personality changes, drowsiness, or stupor. Neurological involvement is said to occur in 11% of cases. There have been isolated reports of orbital myositis, neurosensory hearing loss, parkinsonism, and spontaneous brain hemorrhage. Ischemic stroke may occur as a result of cerebral vasculitis. Cardiac involvement is encountered in 4% to 8% of patients, who may present with myocarditis and conduction defects.
Arthritis, which characterizes the third stage, presents as a recurrent, asymmetrical, and oligoarticular process involving the large joints (especially the knee) or as a migratory polyarthritis lasting up to a week in any one particular joint. Arthritis may occur in 45% to 60% of patients. Cutaneous lesions and peripheral nervous system involvement are also frequently encountered in the third stage. The typical skin lesions of late Lyme disease are acrodermatitis chronica atrophicans, which characteristically presents as a red or violet discoloration of swollen peripheral skin, and lymphadenosis benigna cutis (borrelial lymphocytoma). Lesions are often bilateral. Patients may also develop sclerodermatous changes. Lichen sclerosus et atrophicus-like lesions have also been described. In the late atrophic stages of acrodermatitis chronica atrophicans, the skin may resemble crumpled tissue paper. Nodular or bandlike juxta-articular fibrous nodules are not uncommon, and may regress with appropriate antibiotic therapy. Acrodermatitis chronica atrophicans occurs mainly in Europe, where B. afzelii is the overwhelmingly predominant etiological genospecies. B. afzelii is not endemic in North America, possibly accounting for the striking geographic distribution of the condition.
There have been rare reports suggesting a possible association between B. burgdorferi infection and anetoderma. A case with acquired cutis laxa has also been described. The conflicting role of B. burgdorferi in the pathogenesis of morphea and lichen sclerosus is discussed elsewhere.
Although the major clinical features are similar among European and North American cases, certain differences exist. The occurrence of acrodermatitis chronica atrophicans as an apparently European phenomenon has already been alluded to. Borrelial lymphocytoma and meningoradiculoneuritis are also observed more frequently in Europe, while multiple erythema migrans due to hematogenous dissemination in early Lyme disease is said to occur less frequently outside of North America. A European series of 54 cases, however, showed that 46% of patients had multiple erythema migrans lesions.
Reinfection may occur in a small but significant proportion of patients following treatment with antibiotics. This usually manifests as a recurrent episode of erythema migrans at a different cutaneous site from the previous lesion. A Swedish study showed that women were more susceptible to reinfection than men, ascribing this phenomenon to gender differences in immune reactivity, especially in the postmenopausal age group.
Erythema chronicum migrans was first described in association with tick bites; cases were subsequently reported following mosquito bites and thorn pricks, or without preceding trauma. In a proportion of cases, an encephalitis was noted and the disease was termed ‘tick-borne meningopolyneuritis’. In the 1970s, several cases were reported in the United States, and because of a clustering effect near Lyme, Connecticut, the term Lyme disease was coined (these cases had a high proportion of arthritis). The Ixodes tick has been known to be the vector for some time, but the actual etiological agent, a spirochete, was only identified in the 1980s after spirochetes were found in Ixodes dammini ticks in an endemic disease area. In Europe, Ixodes ricinus has been incriminated. Ixodes ticks are found widely in the temperate regions of the Northern hemisphere. The increased frequency of infections during the spring and summer months coincides with the time when the nymph stage of the tick is active.
Patients recovering from the disease have been shown to have antibodies to the spirochetes in their serum. Spirochetes have also been identified from biopsy sites, and cultured from or detected by PCR performed on specimens of blood, cerebrospinal fluid, synovial fluid, and skin. Antibody-based tests for confirmation of the diagnosis are limited by the fact that results are more likely to be negative in the early stages of the infection. Immunosuppression does not appear to alter clinical presentation, treatment response, or anti- B. burgdorferi antibody production.
Borreliae have developed strategies to evade or inactivate host immune defenses via a variety of mechanisms. These include complement regulator-acquiring surface proteins (CRASPs), which confer complement resistance. Borrelial CRASPs are capable of binding FHL-1/reconectin and factor H, which are two major regulators of the alternative complement pathway. The selective up-regulation of host matrix metalloproteinase-9 by B. burgdorferi in skin lesions of erythema chronicum migrans may play a role in the local spread of the organism and its dissemination to other organs. In the early stage of Lyme disease, B. burgdorferi antigens induce a strong host immune response in which the production of cytokines such as IFN-γ, TNF-α, and TGF-β 1 predominates. By contrast, chronic neuroborreliosis is associated with a lack of TNF-α and TGF-β 1 responses. Studies performed on lesional skin have revealed high levels of the T-cell-active chemokines CXCL9 and CXCL10 in erythema chronicum migrans and acrodermatitis chronica atrophicans. Borrelial lymphocytoma, on the other hand, is associated with high levels of CXCL13, a B-cell-active chemokine.
The central component of the initial lesion shows the typical appearance of an insect bite reaction. Histology reveals a polymorphic inflammatory cell infiltrate including neutrophils, eosinophils, histiocytes, lymphocytes, and mast cells. Vascular proliferation and dermal necrosis may additionally be present. A superficial and deep dermal perivascular lymphocytic infiltrate characteristic of an annular erythema then develops. A biopsy from the periphery is non-specific, showing a perivascular and interstitial infiltrate of lymphocytes, mast cells, and plasma cells in both the superficial and deep dermis ( Figs 18.137 and 18.138 ). Unusual histologic features, however, have been reported in some cases and present a potential diagnostic pitfall; these include spongiosis, focal interface dermatitis, an infiltrate confined to the superficial vascular plexus, and an absence of plasma cells.
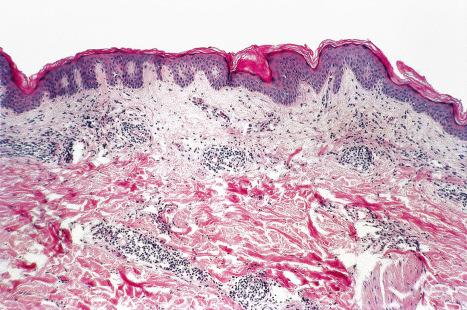
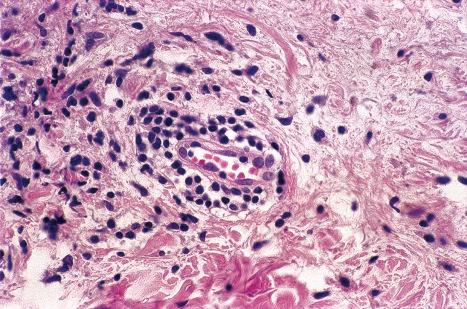
Identification of spirochetes by a silver stain is diagnostic. An immunohistochemical method for demonstrating the etiological agent has been described. PCR may also be used to detect the organisms in formalin-fixed, paraffin-embedded tissue specimens. One study concluded that focus floating microscopy was more sensitive than PCR in detecting Borrelia spirochetes in the lesional tissue of erythema chronicum migrans, borrelial lymphocytomas, and acrodermatitis chronica atrophicans. Clonal or pseudoclonal IgH gene rearrangements have been documented in DNA extracted from erythema migrans cutaneous lesions. Care should therefore be exercised when interpreting PCR results in such cases; some authors have advocated duplicate or triplicate testing.
The borrelial lymphocytoma consists of a dense (polyclonal) dermal infiltrate composed of lymphocytes, plasma cells with macrophages, and scattered eosinophils. Although it has been suggested that germinal center formation, when present, helps to exclude a cutaneous B-cell lymphoma, this is not necessarily true; cutaneous lymphomas of marginal zone type typically display germinal centers. The association between B. burgdorferi infection and cutaneous B-cell lymphoma is discussed elsewhere.
Acrodermatitis chronica atrophicans is characterized by vascular dilatation in the mid and upper dermis accompanied by a dense infiltrate of lymphocytes, plasma cells, macrophages, and mast cells. Scattered groups of ‘vacuoles’ may be seen in the dermis; this phenomenon is thought to be attributable to lymphedema. The epidermis, which is usually hyperkeratotic, may be acanthotic or atrophic with loss of ridge pattern. In some patients, the appearances are reminiscent of lichen sclerosus or eosinophilic fasciitis. Occasionally, the histologic features may overlap with scleromyxedema. The juxta-articular fibrous nodules are characterized by fibrosis of the superficial subcutaneous tissue, with hyaline collagen bundles encircling clusters of adipocytes. There is an accompanying perivascular and interstitial inflammatory infiltrate comprising lymphocytes and plasma cells. Smaller periarticular fibrous nodules on the fingers show disorganized bundles of thickened dermal collagen.
The triad of meningitis, cranial neuropathy, and radiculopathy has been said to be unique for Lyme disease. CNS lesions include cortical, perivascular chronic inflammatory cell infiltrates, mild spongiform changes, and gliosis. Plasma cells, however, are said to be absent. The similarity of the late CNS changes of Lyme disease and meningovascular syphilis has been stressed. Chronic leptomeningeal inflammation may also be evident. Peripheral nervous system lesions are characterized by nerve and ganglion lymphocyte and occasional plasma cell infiltration. Adjacent vessels may show endarteritis obliterans.
Endocardial lesions are characterized by a lymphocytic and plasma cell infiltrate; deep specimens show an interstitial myocarditis. Focal myonecrosis may also be evident.
Histologic examination of the synovium may show periadventitial cell onion-skinning proliferation and chronic inflammation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here