Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
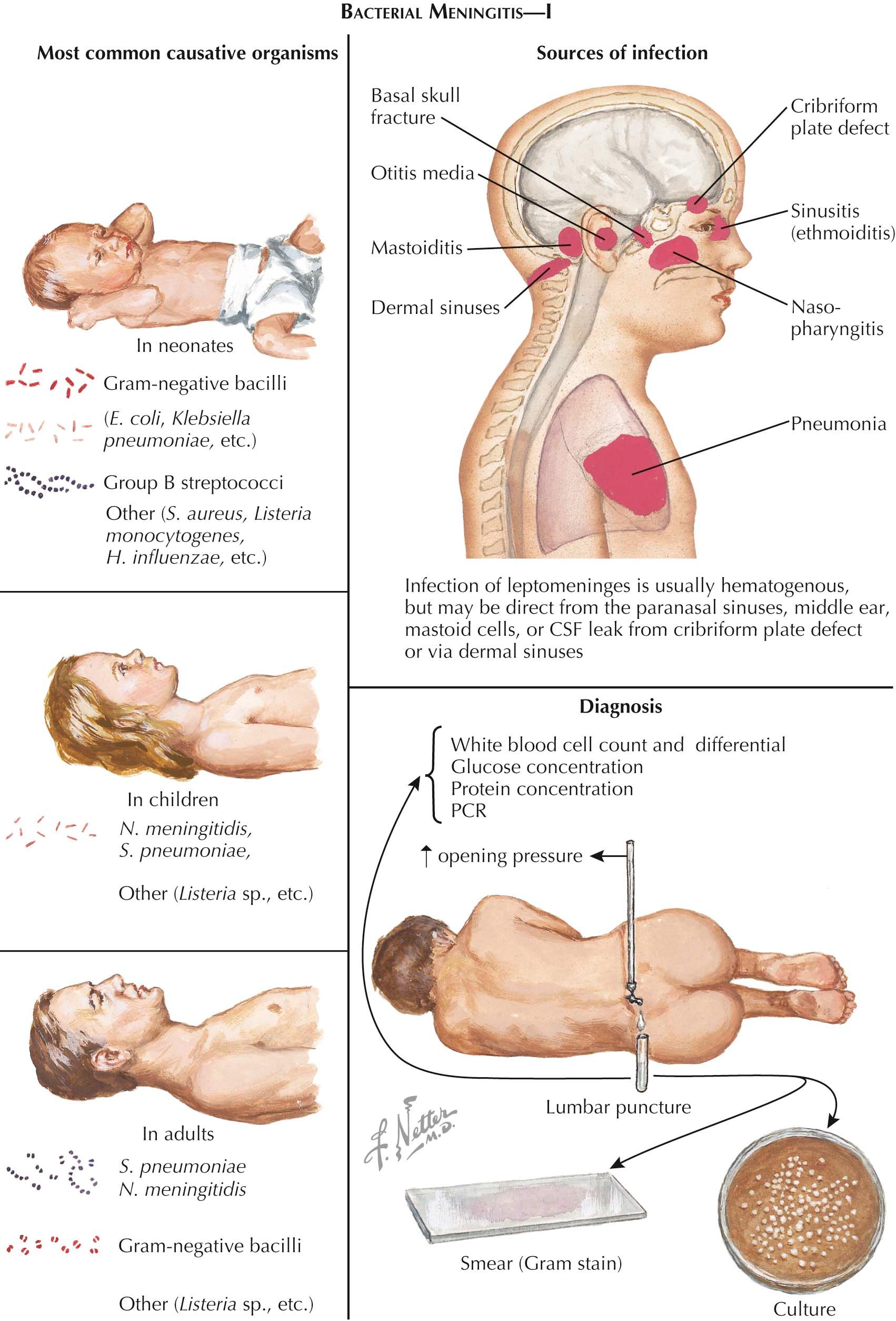
Pathophysiology. Bacterial meningitis is initially an acute purulent infection of the meninges and subarachnoid space that is followed by an inflammatory reaction in the subarachnoid space, the brain parenchyma, and the cerebral arteries (arteritis) and veins (dural sinus thrombosis and thrombophlebitis). Meningitis is most often the result of bacterial invasion of the subarachnoid space from hematogenous dissemination. Bacterial meningitis may be preceded by colonization of the nasopharynx by the organism or develop as a complication of pneumonia, acute otitis media, acute sinusitis, endocarditis, skull fracture, a neurosurgical procedure, or the use of a catheter to decrease intracranial pressure or administer chemotherapeutic or antimicrobial agents.
The meningeal pathogen can be predicted by the patient's age. In neonates, the most common pathogens are group B streptococci ( Streptococcus agalactiae ), gram-negative bacilli, and Listeria monocytogenes . In children, adolescents, and adults, the most common causative organisms of community-acquired bacterial meningitis are Neisseria meningitidis and Streptococcus pneumoniae . Listeria monocytogenes is a causative organism of meningitis in individuals with impaired cell-mediated immunity due to organ transplantation, chronic illness, pregnancy, acquired immunodeficiency syndrome, malignancy, immunosuppressive therapy, or age. When meningitis complicates acute otitis media, mastoiditis, or sinusitis, the causative organisms are Streptococci spp., gram-negative anaerobes, S. aureus , Haemophilus sp., or Enterobacteriaceae. Meningitis in the postneurosurgical patient and the patient with a ventriculostomy or other indwelling catheter may be due to staphylococci, gram-negative bacilli, or anaerobes.
Clinical Manifestations. The signs and symptoms of meningitis in the neonate include irritability, lethargy, poor feeding, vomiting, diarrhea, temperature instability, respiratory distress, apnea, seizures, and a bulging fontanel. The signs and symptoms of bacterial meningitis in children, adolescents, and adults include fever, vomiting, photophobia, headache, nuchal rigidity (meningismus), and a decreased level of consciousness ranging from lethargy to stupor, obtundation, or coma.
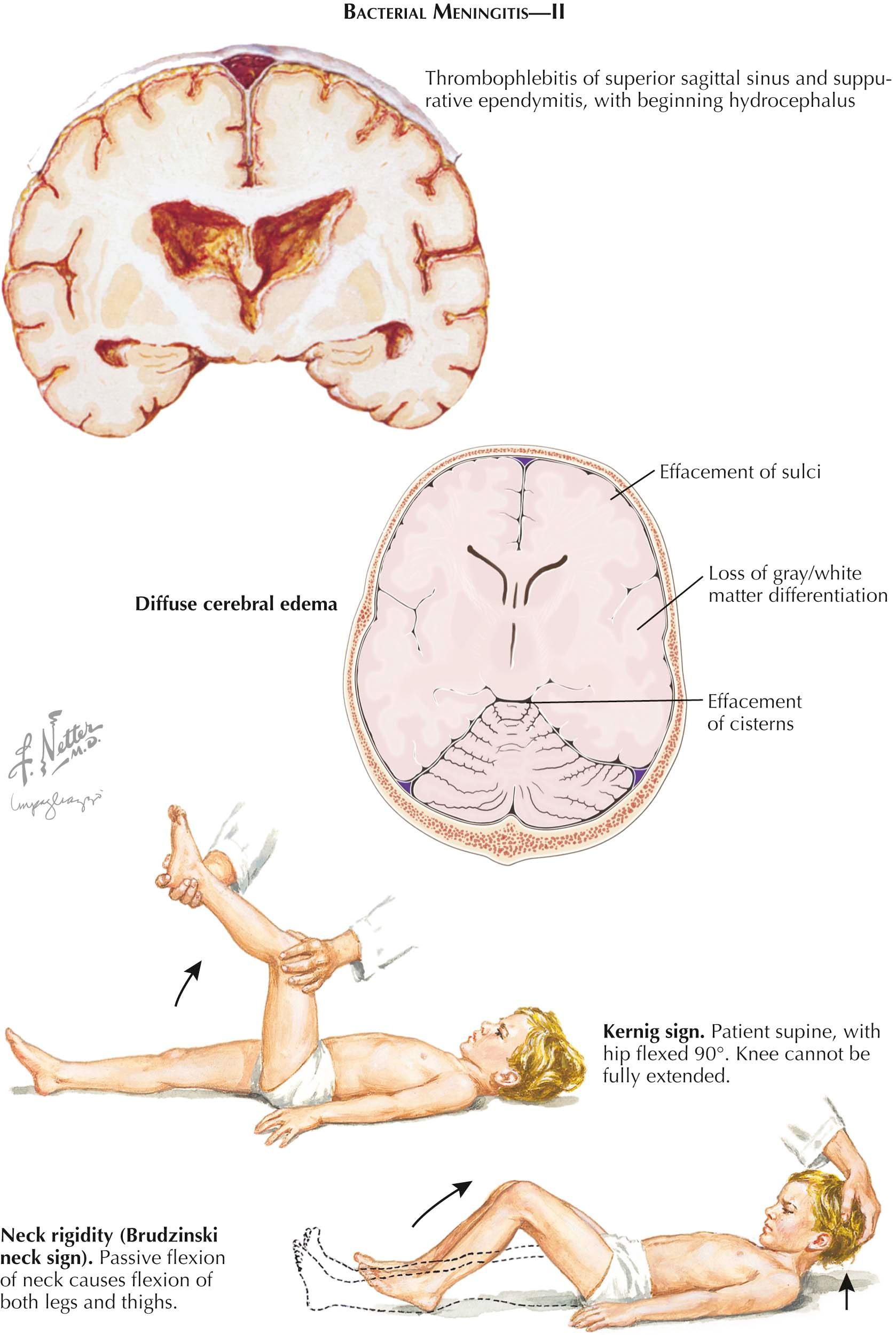
On physical examination, the classic sign of bacterial meningitis is meningismus, but this sign is not invariably present. The neck resists passive flexion. Kernig sign and Brudzinski sign are also signs of meningeal irritation (see Plate 11-2 ). Both signs are elicited with the patient in the supine position. To elicit Kernig sign, the thigh is flexed on the abdomen with the knee flexed. Attempts to passively extend the leg elicit pain and are met with resistance when meningeal irritation is present. Brudzinski sign is positive when passive flexion of the neck results in spontaneous flexion of the hips and knees. The presence of a petechial rash on the trunk and lower extremities, in the mucous membranes and conjunctiva, and occasionally on the palms and soles is typical of the rash of meningococcemia. A petechial rash is not seen in all cases of meningococcal meningitis, and a petechial rash is rarely seen in H. influenzae , pneumococcal, and staphylococcal meningitis. Patients with enteroviral meningitis may also have a rash, but this is an erythematous maculopapular rash that involves the face and neck early in infection.
Diagnosis. The gold standard for the diagnosis of bacterial meningitis is analysis of the cerebrospinal fluid. A computed tomography (CT) scan should be obtained in the patient with any of the following: an altered level of consciousness, papilledema, a focal neurologic deficit, new-onset seizure activity, immunocompromised state, a dilated or poorly reactive pupil, signs of a posterior fossa mass lesion (cranial nerve abnormalities, cerebellar deficit, and a wide-based ataxic gait), or a risk for neurocysticercosis. The classic abnormalities in bacterial meningitis on examination of the cerebrospinal fluid are the following: (1) an opening pressure greater than 180 mm H 2 O, (2) an increased white blood cell count with a predominance of polymorphonuclear leukocytes, (3) a decreased glucose concentration (less than 40 mg/dL), (4) an increased protein concentration, and (5) a positive Gram stain and bacterial culture. Gram stain is positive in identifying the organism in 60% to 90% of cases of bacterial meningitis. The probability of detecting bacteria on a Gram-stained specimen depends on the number of organisms present. The cerebrospinal fluid (CSF) 16S ribosomal ribonucleic acid (rRNA) conserved-sequence broad-based bacterial polymerase chain reaction (PCR) detects bacterial nucleic acid in CSF. There are also meningeal pathogen–specific PCRs available to identify the nucleic acid of a specific meningeal pathogen. The PCR is most useful in rapidly distinguishing between bacterial and viral meningitis. The PCR will not replace bacterial culture because culture is essential for antimicrobial sensitivity testing.
The classic cerebrospinal fluid abnormalities in viral meningitis are (1) a normal opening pressure, (2) a lymphocytic pleocytosis, (3) a normal glucose concentration, and (4) a normal or slightly elevated protein concentration. Enteroviruses can either be isolated in CSF culture or detected in CSF by the reverse-transcriptase polymerase chain reaction (RT-PCR). Herpes simplex virus type-2 deoxyribonucleic acid (DNA) can be detected in CSF by PCR. Human immunodeficiency virus-1 (HIV-1) RNA can be detected and measured in CSF, and the virus can be cultured from CSF. Viral immunoglobulin M (IgM) antibodies can be detected in CSF.
In patients with a clinical presentation of meningitis and a CSF lymphocytic pleocytosis with a decreased glucose concentration, fungal infections, Mycobacterium tuberculosis , sarcoidosis, and lymphoma/leptomeningeal metastases are in the differential diagnosis. A subarachnoid hemorrhage manifests with headache and a sudden transient loss of consciousness. Examination of the spinal fluid will reveal red blood cells and xanthochromia, although it may take several hours for xanthochromia to appear.
Treatment. When bacterial meningitis is suspected, dexamethasone and empiric antimicrobial therapy is begun immediately. The choice of empiric antimicrobial therapy depends on the suspected meningeal pathogen, which is determined by the age of the patient and predisposing or associated conditions. Once the organism is identified and the results of antimicrobial sensitivity testing are known, antimicrobial therapy is modified accordingly.
Complications. The major complications of bacterial meningitis are focal and diffuse brain edema, hydrocephalus, arterial cerebrovascular complications (ischemic and/or hemorrhagic stroke), septic sinus thrombosis with thrombophlebitis, hearing loss and vestibulopathy, and seizures. It is the complications of bacterial meningitis that cause the acute and chronic neurologic deficits.
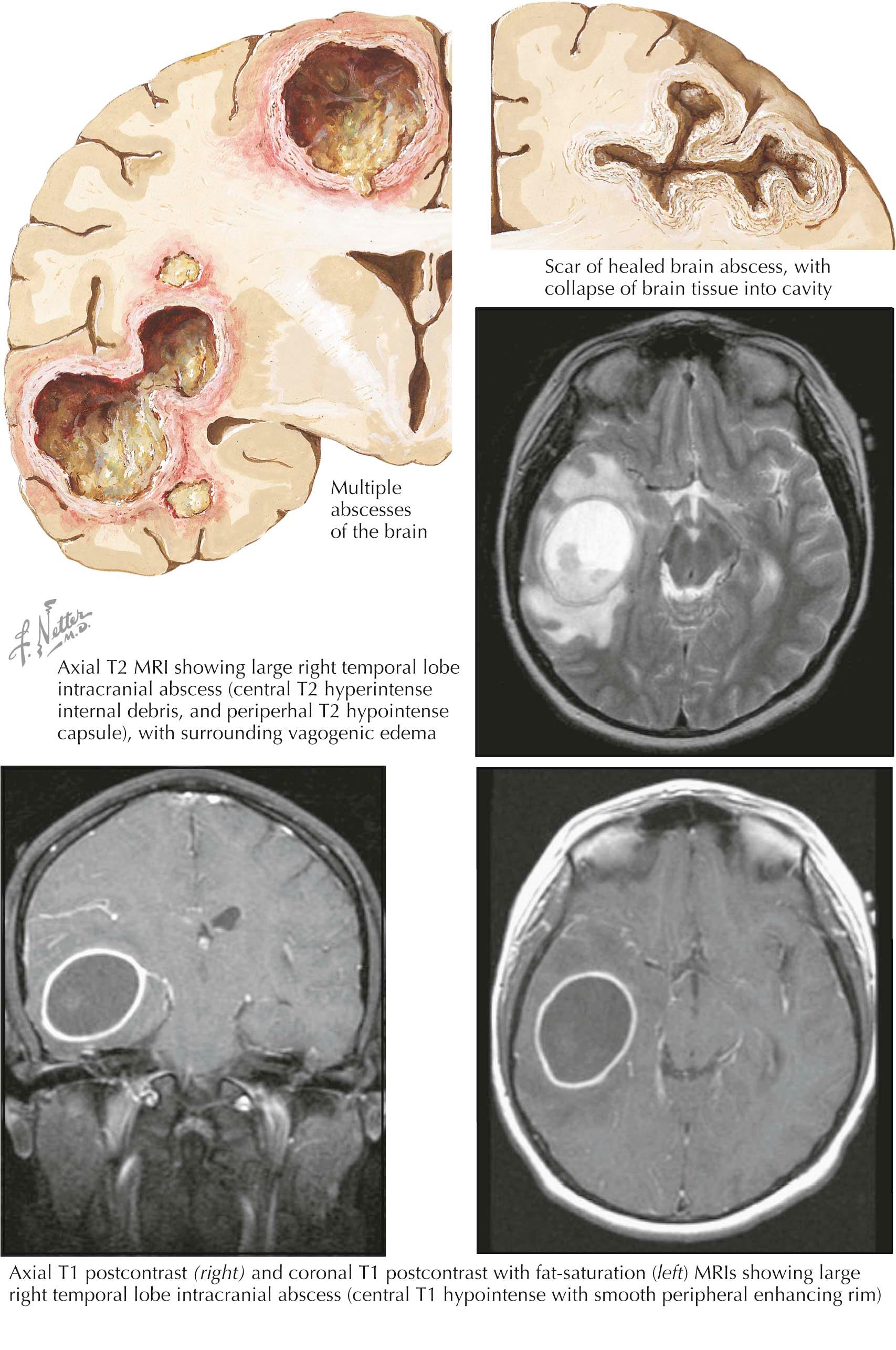
A brain abscess is a focal suppurative process that develops in the brain parenchyma in one of the following ways: (1) by direct spread from a contiguous cranial site of infection (paranasal sinusitis, mastoiditis, otitis media, or dental infections), (2) after cranial trauma, or (3) as a result of hematogenous spread from a remote site of infection (cyanotic congenital heart disease, endocarditis, lung abscess, intra-abdominal infection). The most common etiologic organisms of a brain abscess are streptococci, Bacteroides species, staphylococci (after trauma or craniotomy), Fusobacterium species, Haemophilus species, Enterobacteriaceae, and Pseudomonas aeruginosa . A brain abscess manifests with fever, headache, and a focal neurologic deficit. Headache is the most common symptom, but fever is not invariably present. As the area of cerebral edema surrounding the brain abscess increases, signs of increased intracranial pressure develop.
Magnetic resonance imaging (MRI) with contrast administration is the neuroimaging procedure of choice, because MRI is better able to demonstrate an abscess that is in the cerebritis stage than a cranial CT scan. On T1-weighted MRI after the administration of intravenous gadolinium, the abscess appears as a central area of hypointensity with a smooth peripheral enhancing rim. On T2-weighted MRI, the abscess appears as a hyperintense lesion surrounded by a hypointense capsule. A lumbar puncture is contraindicated. Aerobic and anaerobic blood cultures can be obtained, and a careful physical examination may identify the source of infection. Definitive diagnosis is made by CT- or MRI-guided stereotactic aspiration of the abscess for Gram staining and culture. Empiric antimicrobial therapy is typically started before the results of Gram stain and culture are known and is based on the possible causative organism if the source of infection is known. Empiric therapy is modified once the results of Gram stain and bacterial culture and antimicrobial sensitivity testing is known. Corticosteroids are recommended in patients with significant edema but only for a short period of time because they decrease antibiotic penetration into the abscess cavity. Prophylactic antiepileptic medications are recommended because a brain abscess is an epileptogenic focus.
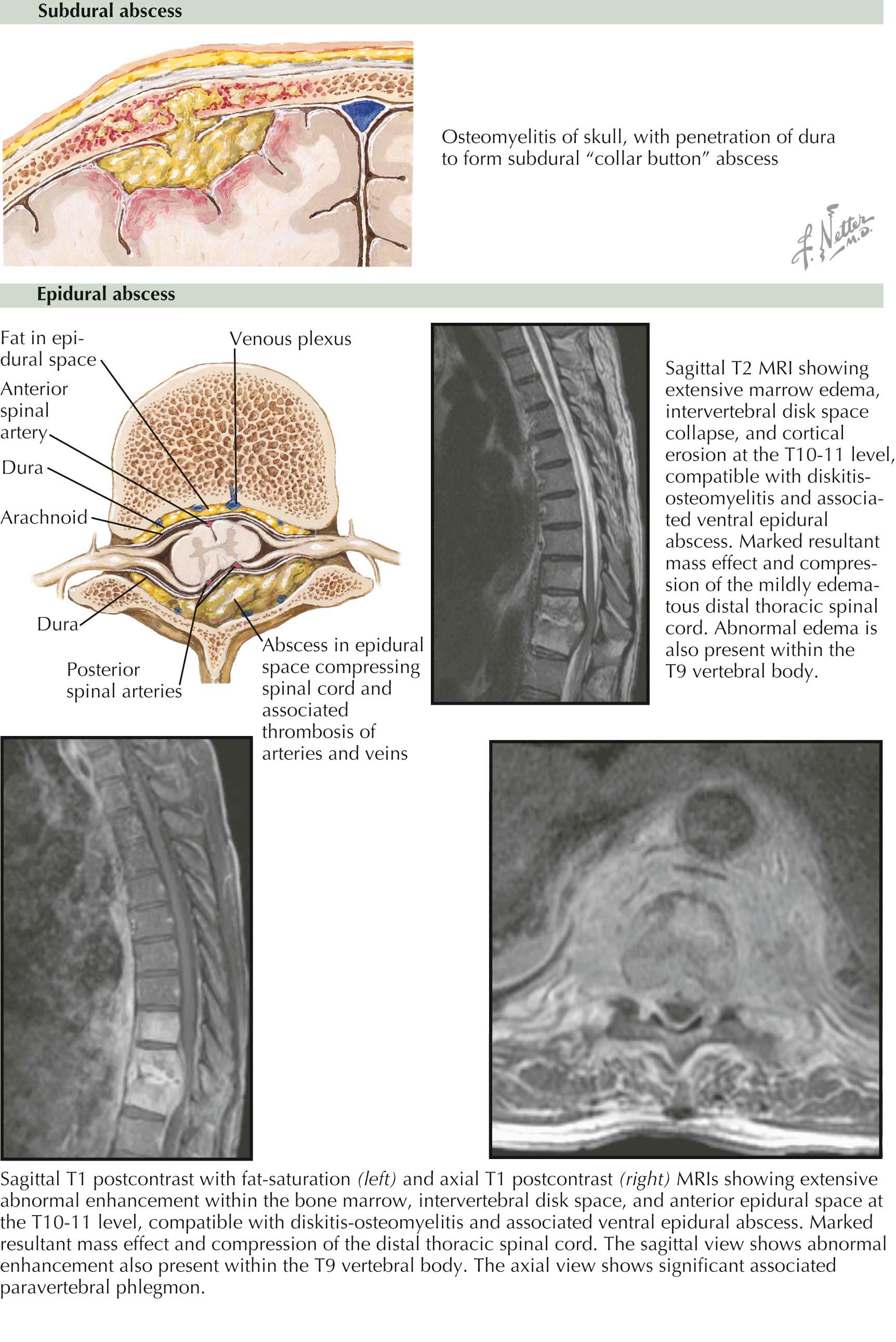
A subdural empyema is a collection of pus in the space between the dura and the arachnoid. Paranasal sinusitis is the most common predisposing condition associated with a subdural empyema, but otitis media, mastoiditis, and a neurosurgical procedure may also be complicated by a subdural empyema. A subdural empyema that is a complication of sinusitis, otitis media, or mastoiditis is usually due to aerobic, microaerophilic, or anaerobic streptococci. Subdural empyemas that are a complication of a neurosurgical procedure are often due to staphylococci. The initial signs and symptoms of a subdural empyema are due to increased intracranial pressure from an expanding infectious mass lesion. Headache and fever are the initial symptoms, followed by focal neurologic deficits, seizures, and a decrease in the level of consciousness. A subdural empyema is a life-threatening infection because patients may have a rapid progression of neurologic deficits and altered level of consciousness. A subdural empyema is readily imaged by computed tomography (CT) scan or magnetic resonance imaging (MRI) with contrast administration. The definitive step in the management of subdural empyema is surgical drainage and antimicrobial therapy. Empiric therapy with a combination of a third- or fourth-generation cephalosporin plus vancomycin and metronidazole is begun and then modified when the results of Gram's stain and bacterial cultures and sensitivities are known.
A spinal epidural abscess develops in the space outside the dura mater but within the spinal canal as a result of the hematogenous spread of infection from a remote site of infection or by direct extension from a contiguous infection, such as vertebral osteomyelitis, decubitus ulcers, or infected abdominal wounds. Neurologic deficits are the result of direct mechanical compression of the spinal cord and/or inflammatory thrombosis of the intraspinal vessels with subsequent ischemia and infarction. The initial symptom is back pain. Fever may be present. Back pain is followed by radicular pain, then weakness, and then paralysis of appendicular musculature, loss of sensation below the level of the lesion, and loss of bowel and bladder control. MRI is the procedure of choice to demonstrate a spinal epidural abscess and a contiguous area of infection when present. If there is evidence of compression of the spinal cord from the epidural abscess, an emergency decompression with evacuation of pus and granulation tissue is performed. This also allows for identification of the causative organism and guides antimicrobial therapy. Empiric antimicrobial therapy is directed at the most common causative organisms, which are staphylococci ( Staphylococcus aureus and coagulase-negative staphylococci) and gram-negative bacilli.
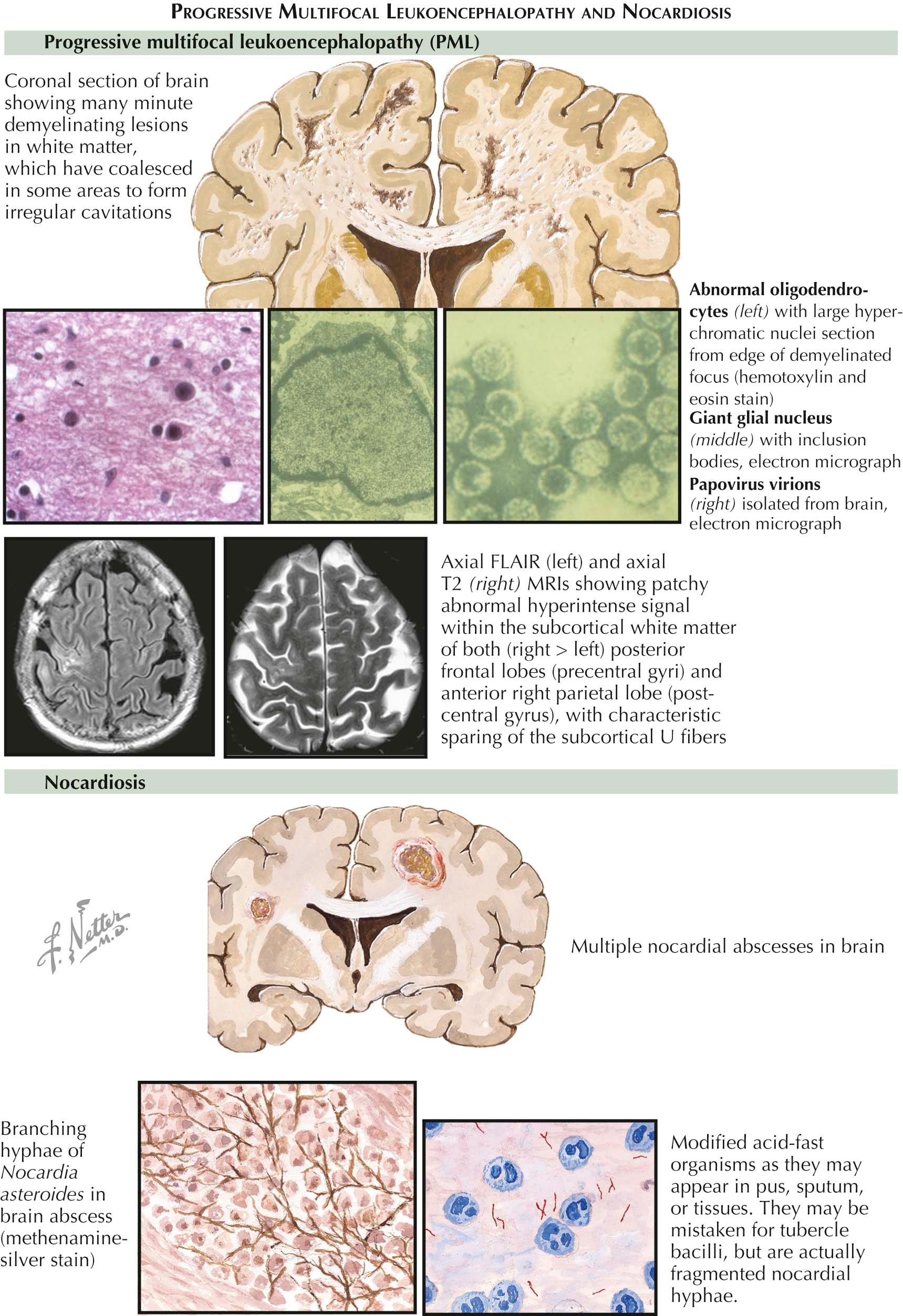
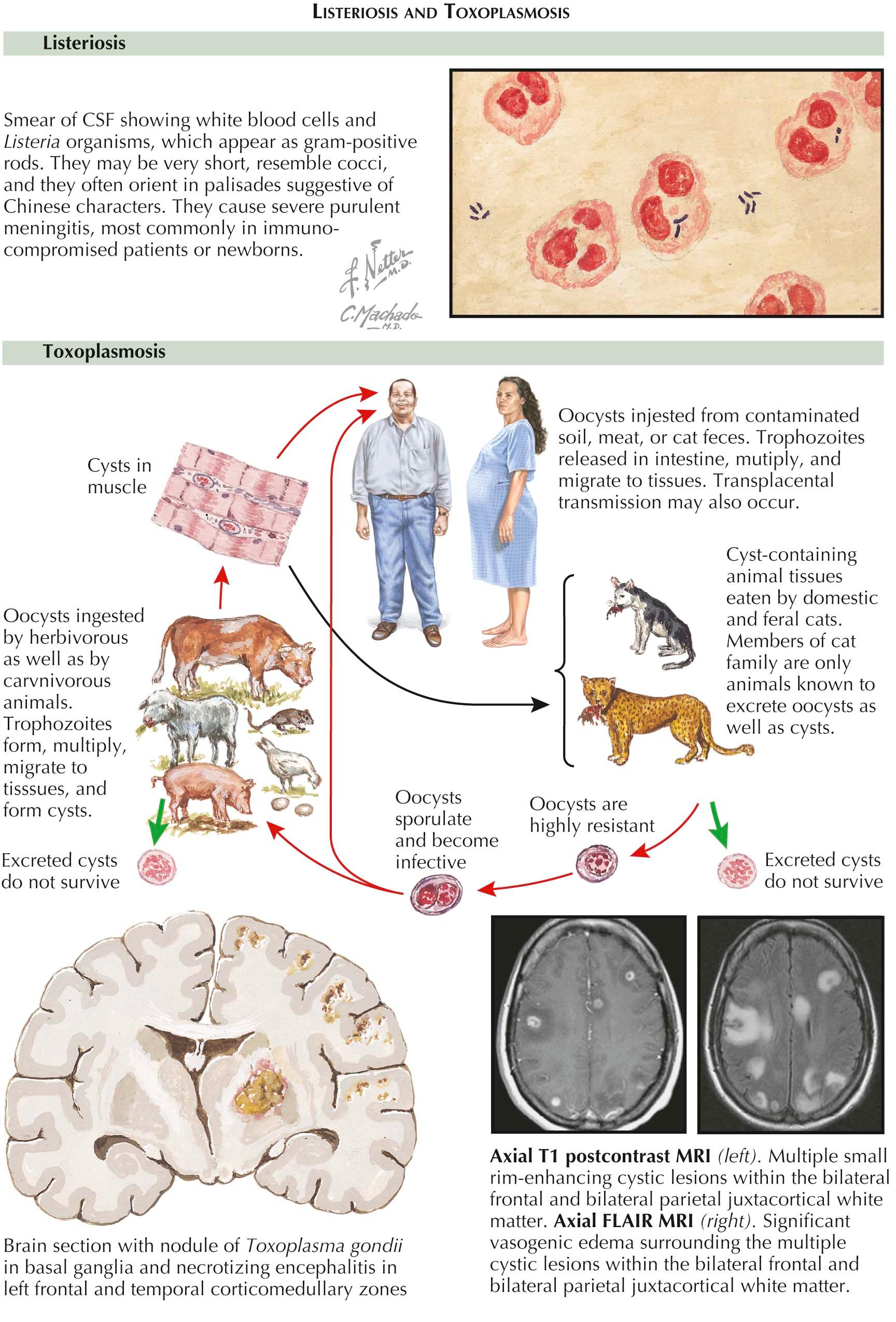
There are four central nervous system (CNS) infections unique to the immunocompromised host. They are progressive multifocal leukoencephalopathy, a brain abscess due to Nocardia asteroides , meningitis due to Listeria monocytogenes, and toxoplasmosis.
Progressive Multifocal Leukoencephalopathy. Progressive multifocal leukoencephalopathy (PML) is a disease caused by the JC virus, a polyoma virus that is acquired in childhood, establishes latent infection in the kidneys and lymphoid organs, and reactivates in the setting of cellular immunosuppression. Because PML is a viral infection of oligodendrocytes causing focal areas of demyelination, the clinical presentation is that of focal or multifocal neurologic deficits, including hemianopsia, hemiparesis, or aphasia. On neuroimaging, the lesions are located in the subcortical hemispheric white matter, sparing the U fibers, and are typically not contrast enhancing and not surrounded by edema. The spinal fluid is similarly noninflammatory. There may be a slight increase in the white blood cell count and a mild elevation in the protein concentration. The diagnosis is made by demonstration of JC virus deoxyribonucleic acid (DNA) by polymerase chain reaction (PCR) of cerebrospinal fluid (CSF) or by brain biopsy. There is no specific antiviral therapy, and treatment is directed at reversing the immunosuppression.
Nocardiosis. Nocardia asteroides is a gram-positive bacterium that is found in soil and decaying vegetables. This bacterium is a causative organism of a brain abscess in individuals with impaired cell-mediated immunity. Risk factors include organ transplantation, immunosuppressive therapy, pulmonary alveolar proteinosis, sarcoidosis, and pregnancy. Unlike the primary management of the majority of bacterial brain abscesses by stereotactic aspiration guided by computed tomography (CT) or magnetic resonance imaging (MRI), a brain abscess due to Nocardia asteroides requires surgical excision through a craniotomy. These are thick-walled multiloculated brain abscesses. The infection is treated with trimethoprim-sulfamethoxazole or sulfonamide. Nocardial brain abscesses are relatively rare in human immunodeficiency virus (HIV)-positive individuals, because many HIV-positive individuals take trimethoprim-sulfamethoxazole to prevent Pneumocystis carinii .
Listeriosis. Listeria monocytogenes is a gram-positive bacterium that causes meningitis in immunocompromised individuals from organ transplantation, malignancies, chronic corticosteroid therapy, immunosuppressive therapy, diabetes mellitus, and pregnancy. Increasing age is also a risk factor for Listeria monocytogenes meningitis due to the natural decrease in cell-mediated immunity. Infection is acquired from soft cheeses, unpasteurized milk, hot dogs, deli meats, and cole slaw. In addition to meningitis, Listeria monocytogenes is one of the causative organisms of a brainstem encephalitis (rhomboencephalitis). Patients typically have headache, nausea, vomiting, and fever, followed by brainstem symptoms and signs, the most common of which is a unilateral facial nerve palsy. This is followed by dysarthria, vertigo, dysphagia, and hemiataxia. Spinal fluid analysis demonstrates a CSF pleocytosis with a predominance of neutrophils but also a mixture of lymphocytes and monocytes. The spinal fluid may also show a predominance of lymphocytes or monocytes. The glucose concentration may be decreased or normal. The organism can be grown in culture of CSF. In rhomboencephalitis, a lesion of increased signal intensity on T2-weighted and fluid attenuated inversion recovery (FLAIR) imaging can be seen in the pons and medulla. Therapy of meningitis due to Listeria monocytogenes is with ampicillin. In patients who are obtunded, gentamicin is added. Rhomboencephalitis is treated with a combination of ampicillin and gentamicin.
Toxoplasmosis. Toxoplasma gondii is a parasite that is acquired by ingesting the oocysts from contaminated soil, meat, or cat feces; however, Toxoplasma encephalitis is the result of reactivation of latent infection. HIV-infected individuals and patients receiving immunosuppressive therapy for lymphoproliferative disorders are at greatest risk for this infection. Patients present with headache, fever, an altered level of consciousness, focal neurologic deficits, and/or seizures. Neuroimaging demonstrates one or more focal or multifocal ring-enhancing lesions with edema. Diagnosis begins with serology for anti- Toxoplasma immunoglobulin G (IgG). In an HIV-infected individual with multiple enhancing lesions with edema and a positive anti- Toxoplasma IgG, a treatment trial is often initiated with a combination of pyrimethamine and sulfadiazine. If clinical and radiographic improvement occurs with treatment, a presumptive diagnosis of Toxoplasma encephalitis is made. Clinical improvement is expected in 90% of patients by day 7 of therapy, and if this does not occur, additional diagnostic studies are warranted because primary central nervous system lymphoma and tuberculous abscesses may have a similar clinical and radiographic appearance in HIV-infected individuals. As primary central nervous system lymphoma is the leading disease in the differential diagnosis, cerebrospinal fluid can be sent for the detection of Epstein-Barr virus deoxyribonucleic acid (DNA) by PCR. If spinal fluid analysis is not safe due to the degree of edema, a stereotactic CT-guided brain biopsy is recommended.
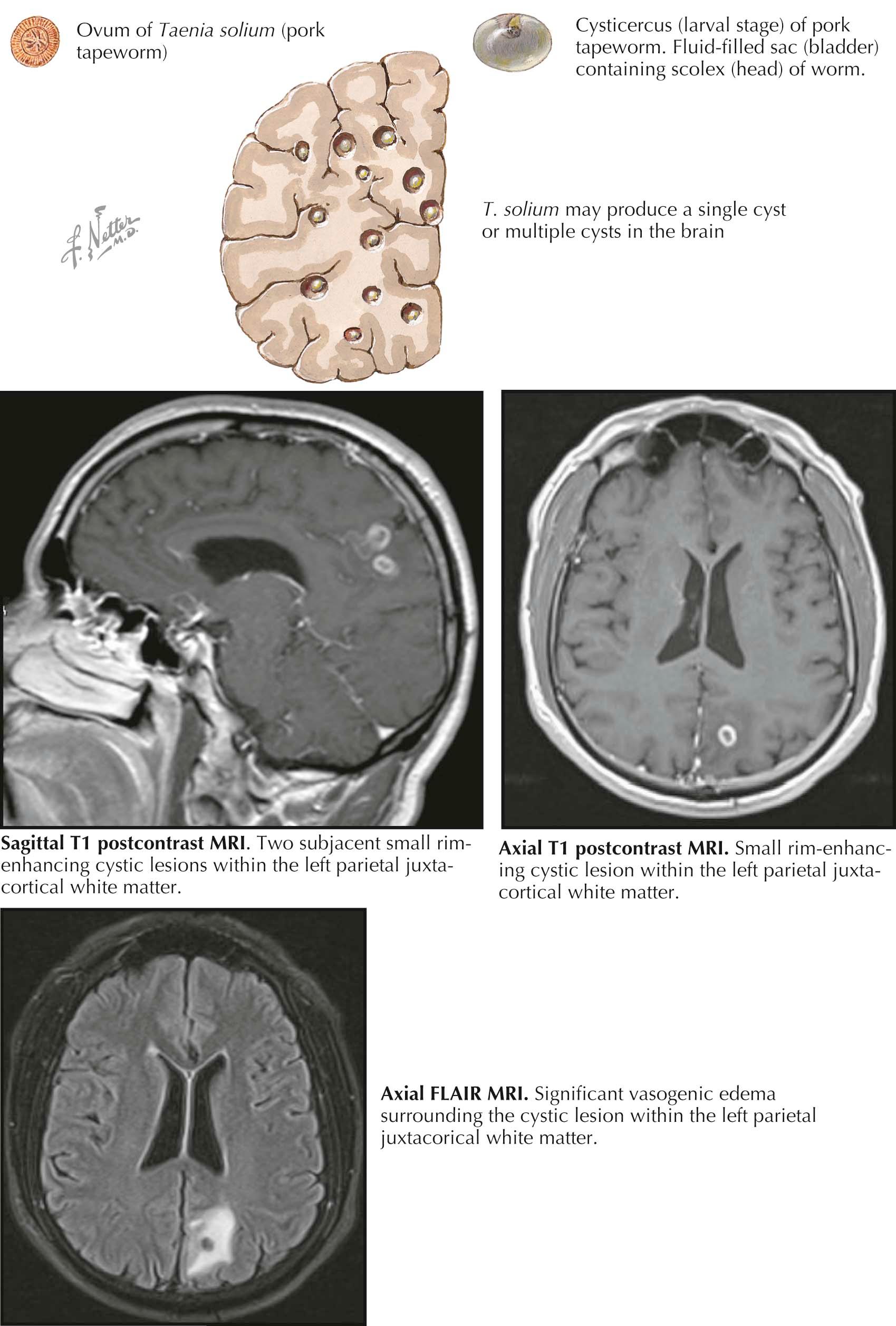
Neurocysticercosis is a parasitic infection of the central nervous system that is acquired by either the ingestion of undercooked pork contaminated by larva of the tapeworm Taenia solium or by fecal-oral transmission of the eggs of the tapeworm as a result of exposure to feces of asymptomatic Taenia carriers. The embryos of the eggs develop and hatch in the intestine and then enter the bloodstream. Larvae migrate to the central nervous system (CNS). The most common clinical manifestation is a seizure, and neurocysticercosis is the most common cause of acquired epilepsy in the developing world. The clinical presentation is affected by the number and location of cysts in the brain parenchyma, the basilar or perimesencephalic cisterns, and the subarachnoid space. Cysts may also be attached to the choroid plexus or the ventricular wall. As such, the presentation may be that of headache, signs of increased intracranial pressure, or focal neurologic deficits. Cysticercal cysts evolve through four stages: the vesicular stage, the colloidal stage, the granular stage, and the stage of calcification. The appearance of the cyst on computed tomography (CT) and magnetic resonance (MR) scan depends on the stage. In the vesicular stage, the cyst contains living larvae and has the appearance of a nonenhancing cystic lesion without edema. In the colloidal stage, the larva is degenerating, and a CT scan demonstrates a ring-enhancing lesion with edema. On CT scan, but better demonstrated on MR scan, cysts in the vesicular stage and those in the colloidal stage contain live active cysts that have the appearance of a nodule, which is the invaginated scolex. In the granular stage, the larva continues to degenerate and the cyst develops a ring enhancement. Finally, a calcified lesion is seen on neuroimaging. The most definitive neuroimaging evidence of neurocysticercosis is a cystic lesion showing the scolex. The diagnosis is supported by a serum immunoblot assay that detects anticysticercal antibodies. In every stage, with the exception of the vesicular stage, the parasite is in the process of dying. Patients most typically become symptomatic with a seizure when the cyst has evolved to a calcified lesion, but this stage does not require anticysticidal therapy. Patients may also become symptomatic in the earlier stages, when the parasite elicits an inflammatory response and the lesion becomes surrounded by edema. The decision to treat cysts in the other stages must take into account that during therapy with anticysticidal agents there is a risk of a strong inflammatory reaction, with an increase in cerebral edema. Prednisone is started either before or with the first dose of anticysticidal therapy and continued throughout the course of treatment. Cysticidal drug therapy appears to be most efficacious in patients with cysticerci in the colloidal and vesicular stages.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here