Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The diagnosis and management of luminal gastrointestinal (GI) tract infections has been an essential component of the practice of gastroenterology since the birth of the subspecialty. The emergence of endoscopy with mucosal biopsy as a safe and accurate diagnostic tool for patients with suspected infection has elevated the GI endoscopist to a key partner in the management team. The importance of the endoscopist may be best appreciated for patients who develop GI complications related to immune suppression, such as after organ transplantation, during chemotherapy, or with human immunodeficiency virus (HIV) infection. In these settings, infections are frequent, and the differential diagnosis and diagnostic approach to GI symptoms often differ from the normal host. This chapter provides an overview of GI infections from the endoscopist's perspective based on organ systems because the clinical presentation generally points to the site of gut involvement and dictates the diagnostic strategy. Several common themes are applicable to most GI infections:
The clinical presentation is dictated by the infecting pathogen.
The specific immunodeficiency state, its pathophysiology, and severity dictate the spectrum of complicating pathogens.
The severity and chronicity of infection for an immunosuppressed patient are dictated by the cause, duration, and type of immunodeficiency.
The endoscopic features of any GI infection are variable and overlapping, making definitive diagnosis by biopsy essential when other tests such as stool culture are negative. Biopsy of normal mucosa may be necessary in some situations.
The frequency of luminal GI infections, spectrum of pathogens, severity of infection, and organ involvement are dictated by the combination of exposure, predilection (host factors), and organ-specific tropism of the infecting pathogen ( Box 41.1 ). Most infections are transmitted by the fecal-oral route via contaminated food or water and are typically self-limited. With the increase in international travel, geography plays an increasingly important role in the prevalence of some GI infections. Traveler's diarrhea, usually caused by enterotoxigenic Escherichia coli, is characteristically seen with travel to developing countries. These travelers are at increased risk of other causes of diarrhea including invasive bacteria like Campylobacter and parasites like E. histolytica .
Exposures
Immunodeficiency
Transplant and type
AIDS
Cancer
Geographic location
Hospital setting
Medication exposures
Pathogens are endemic in certain portions of the world and in specific regions within countries. For example, in the United States, histoplasmosis is endemic in the Midwest and Mississippi Valley. Mycobacterium tuberculosis (TB) is endemic in third world countries, and involvement of the GI tract is well recognized. Medications can also be an important risk factor. Antibiotics are the major risk factor for C. difficile infection (CDI). There is increasing evidence that acid suppressing medications increase the risk of most enteric infections, perhaps even CDI.
For immunosuppressed patients, the incidence and severity of infection are often linked to the cause and degree of the immunodeficiency state. In addition, they are subject to opportunistic infections, defined as infection by a microorganism that does not typically cause disease. Patients undergoing solid organ transplantation are at a heightened risk of infection early after transplantation because of profound medication-induced immunodeficiency. It is also during this time that latent infections such as cytomegalovirus (CMV) and Strongyloides may become manifest. Over time, however, as drug-induced immunosuppression decreases as drugs are tapered, the incidence of infections decreases. Moreover, infections are more commonly observed after heart transplantation than liver transplantation because more potent and prolonged drug-induced immunosuppression is required to prevent cardiac rejection.
For patients infected with HIV, the incidence of GI infections increases markedly as immune function deteriorates, and the infection risk can be accurately stratified by the absolute CD4 lymphocyte count and HIV-1 RNA levels as higher HIV levels and lower CD4 lymphocyte counts correlate with the risk of opportunistic infections.
Rarely, opportunistic infections have been observed in the apparently normal host, but in contrast to an immunodeficient patient, these infections are typically self-limited. Generally, the more severe the immunodeficiency state required for development of an opportunistic infection, the less likely the pathogen will be observed in the normal host. For example, until the advent of transplantation, CMV was a rare pathogen, and its identification in any patient suggests some type of immune dysfunction. CMV is regarded as one of the most common opportunistic infections owing to the high prevalence of prior exposure to CMV, as reflected by seropositivity rates of more than 90% in developed countries, and to the fact that CMV disease generally occurs from recrudescence of latent infection during periods of profound immunosuppression.
In summary, a careful history regarding potential exposure, cause and stage of immunodeficiency, if present, and specific epidemiologic factors germane to the clinical presentation will often help determine the potential causes of infection.
The pathogenic mechanisms of luminal GI infections include: (1) exposure to a pathogen, (2) reactivation of prior infection (recrudescence), (3) overgrowth of a commensal organism, and/or (4) local spread or dissemination. The specific organ involved with any infectious process, other than local spread, is dictated by the organ-specific tropism of the infecting pathogen. Candida and herpes simplex virus (HSV) almost exclusively infect squamous epithelium, whereas Campylobacter jejuni and Shigella species are colonic pathogens. Inherent to any discussion of pathogenesis is the issue of host-related factors. Numerous nonspecific and immune-based defense mechanisms both prevent and attenuate GI infections. These defenses may be altered by disease, medications, organ dysfunction or organ absence (e.g., splenectomy), or as a part of the aging process. Saliva provides an effective physical barrier because of its physical properties. Moreover, immunoglobulins that are present in saliva and in intestinal secretions provide an important early line of defense. Gastric acid is a barrier to enteropathogens, and hypochlorhydria has been shown to be a risk factor for the development of cholera and other GI infections. GI motility propels ingested pathogens through the gut and prevents stasis, which can lead to bacterial overgrowth. Inherent antibacterial proteins secreted by Paneth cells, termed defensins, seem to play a key role in the host response to bacterial infections of the gut. The normal gut microbiota also plays a vital role in the prevention of infection such as CDI; its role in the prevention of other infections is unknown.
The mucosal immune system is composed of inflammatory cells, most notably T cells. After exposure to a foreign antigen, these cells differentiate into helper or cytotoxic cells depending on whether the cells express the CD4 or CD8 receptor. The release of cytokines by these cells plays a crucial role in limiting infection but can also result in tissue damage. The critical role of the mucosal immune system in preventing and controlling infections is best shown by the array and severity of luminal GI infections that occur in AIDS, in which there is a progressive loss of CD4 lymphocytes from both the systemic circulation and the mucosal-based immune system. Following HIV infection, there is a rapid, substantial and perhaps irreversible loss of mucosa associated lymphoid tissue based CD4 T-cells. Loss of these cells predisposes to small intestinal infections by opportunistic infections such as cryptosporidiosis and microsporidiosis. Likewise, lymphocyte dysfunction, either medication-induced or as part of an immunodeficiency state, predisposes to symptomatic primary infection or recrudescence (e.g., CMV).
In immune compromised patients, most viral GI infections considered here result from recrudescence of infection rather than recent exposure (primary infection). Normally, exposure to these infections occurs during childhood, and the systemic and mucosal-based immune systems keep these infections controlled. However, with immunodeficiency, disease may become overt. Conversely, Candida is a commensal organism of the oropharynx and esophagus and, although usually present in small numbers, overt Candida infection can be observed even in the normal host under certain conditions, such as antibiotic use; use of inhaled or ingested corticosteroids; antacid therapy or hypochlorhydria states; diabetes mellitus; alcoholism; malnutrition; old age; radiation therapy to the head, neck, and chest; and esophageal motility disturbances. Alterations in cellular immunity lead to Candidal colonization and superficial infection, whereas humoral immunity (granulocytes) prevents invasive disease and dissemination. Under conditions of absolute granulocytopenia or severely impaired granulocyte function, commensal bacteria, particularly gram-positive organisms, including Streptococcus viridans, Staphylococci, and other bacilli, may invade mucosa that has been damaged from gastric acid reflux disease, from radiation therapy, or from chemotherapy, leading to an active local infection and potential dissemination.
GI infections may result secondarily from active disease in adjacent organs. Esophageal disease may be caused by contiguously infected mediastinal lymph nodes or pulmonary parenchymal infection and by spread of infection via a draining fistula or obstructed lymphatics, resulting in tracheoesophageal fistula. Widespread lymphohematogenous dissemination of opportunistic infections causes either diffuse or focal disease anywhere in the gut; this process is generally limited to only the most severely immunocompromised patients.
Most intestinal infections result in tissue inflammation of varying degrees. Local upregulation of cytokines plays a central role in the local immune response to the pathogen but may also cause tissue injury. CMV esophagitis is associated with high mucosal concentrations of the proinflammatory cytokine tumor necrosis factor. Toxin production, virulence factors, and the local microbiome will also determine the clinical expression and tissue damage caused by many GI infections, especially bacterial infections.
Luminal digestive tract infections occur under specific epidemiologic conditions and in the appropriate host. The tissue-based immune system is crucial for preventing opportunistic infections; when this system is absent, infection with such pathogens may be chronic and potentially life-threatening. Exposure to the pathogen is important; however, concurrent predisposing factors that were elucidated previously, such as antibiotic or chemotherapy treatment, can play a pathogenic role in both the normal and the immunosuppressed host.
Numerous factors guide the approach to a patient with suspected GI infection. Given the breadth of potential etiologic pathogens, the diagnostic approach should be based on the character and chronicity of the symptoms, the organ system(s) involved, and the findings on physical examination.
The most common cause of esophageal infection in both the normal host and the immunosuppressed patient is Candida, followed by the herpesviruses. CMV occurs more commonly in patients with AIDS, whereas HSV is more often observed in the normal host and non–HIV-infected immunosuppressed patients. Odynophagia is the characteristic symptom of esophageal infection, and infections resulting in esophageal ulceration almost uniformly cause odynophagia. Although less common, dysphagia may be observed with esophageal infections, especially Candida esophagitis , or may represent esophageal obstruction or dysmotility from the infection or its sequelae (e.g., stricture). Odynophagia is almost uniformly present and is characteristically severe with CMV esophagitis. Chest pain, weight loss, and fever may be reported. The onset of symptoms is often more subacute than the acute presentation of HSV. A prior or coexistent diagnosis of CMV infection in other organs (e.g., retinitis or colitis) is frequent. Although rare in transplant patients, retinitis may be observed in approximately 15% of AIDS patients at the time of diagnosis of GI disease. Bleeding is generally observed only when there is ulceration, and although generally mild, it can be severe if there is an associated coagulopathy. Pulmonary symptoms may predominate when there is fistula formation to the tracheobronchial tree or coexistent pulmonary involvement. Patients with AIDS often have multiple coexisting esophageal disorders, which further complicates management.
Physical examination, particularly of the oropharynx, may be helpful in suggesting the diagnosis of esophageal infection. Approximately two-thirds of patients with AIDS and esophageal candidiasis have oral candidiasis (thrush). In other immunocompromised patients, oropharyngeal candidiasis is also commonly associated with esophageal candidiasis. Thrush may be absent, however, if antifungal therapy, such as nystatin, is currently administered. The presence of oropharyngeal candidiasis does not prove that Candida is the only cause of symptoms, and the absence of oropharyngeal candidiasis does not exclude Candida esophagitis . Patients with chronic mucocutaneous candidiasis may have fungal involvement of various mucous membranes, hair, nails, and skin and may have a history of adrenal or parathyroid dysfunction. Coexistent oropharyngeal ulceration is common in patients with HSV esophagitis but is infrequent in patients with CMV esophagitis or other systemic infections.
After Candida species, herpesviruses are the most frequent infectious agents that cause esophagitis. After organ transplantation, HSV and CMV occur with equal frequency as causes of esophagitis, whereas in patients with AIDS, HSV esophagitis is uncommon and far less frequent than CMV. In a study of 100 HIV-infected patients with esophageal ulcers, HSV was found in only nine (in four patients it was a copathogen with CMV). HSV esophageal infection commonly manifests with the sudden onset of severe odynophagia, heartburn, or chest pain. Autopsy studies suggest that esophageal symptoms may be absent. Herpes labialis (i.e., cold sores) and oropharyngeal ulcers may coexist, antedate, or develop during the esophageal infection, whereas skin infection is rare. Numerous systemic manifestations, including low-grade fever or upper respiratory symptoms, may precede the onset of esophageal symptoms. In untreated immunocompetent persons, spontaneous resolution of HSV esophageal infection occurs within 2 weeks of the onset of symptoms. Rarely, bleeding is the initial presentation and may be observed in the absence of esophageal complaints.
The frequency of esophageal involvement with other pathogens is rare. Bacterial esophagitis has been observed in patients with severe neutropenia, usually patients with hematologic malignancies, but occasionally it is observed after bone marrow transplantation, diabetic ketoacidosis, steroid therapy, or in AIDS. The presentation is similar to the presentation with other infectious agents. Fungi other than Candida species and parasitic diseases have rarely been reported to involve the esophagus.
The symptoms of esophageal TB depend on the degree and type of involvement. Systemic symptoms of fever and weight loss are common. Pulmonary complaints often predominate because of a fistula to the trachea, bronchus, or pleural space. Dysphagia may be prominent with the formation of long strictures or traction diverticula resulting from the fibrotic response. Upper GI hemorrhage caused by esophageal ulcers or tuberculous arterioesophageal fistulas may rarely be the primary manifestation. Other mycobacterial infections such as Mycobacterium avium complex (MAC) are rare.
Barium radiography plays a minor role in the diagnosis of esophageal infection. In any patient, the presence of severe odynophagia limits the ability to drink barium, hampering the adequacy of the examination. Although specific barium esophagram findings may be more typical for certain disorders, given the potential overlap, many of the findings are nonspecific, and endoscopy with biopsy is generally indicated. The wide spectrum of causes, coupled with the specific antimicrobial regimens that are required, necessitates a definitive diagnosis rather than empiric antimicrobial therapy except when Candida is highly expected. Nevertheless, a sinus tract or fistulous connection to the bronchial tree or mediastinum at the level of the hilum is highly suggestive of TB, although it may also be the result of malignancy. An esophageal neoplasm may be mimicked by an ulcerated tuberculous granulomatous mass or CMV ulcer. Chest radiography or computed tomography (CT) scan of the chest may support the diagnosis of TB, although a tissue diagnosis will be required.
The characteristics of the esophageal lesions provide very important diagnostic clues. The location, size, and appearance of all endoscopic abnormalities should be documented because these features form the basis of the differential diagnosis and are useful for comparison on follow-up endoscopic examinations. The differential diagnosis of the lesion dictates how lesions should be sampled and what recommendations for diagnostic testing should be made on the biopsy or cytologic specimens (discussed later). Serologic testing plays no significant role in the diagnosis of acute infectious esophagitis. Endoscopic examination of the esophagus is the most sensitive and specific method for diagnosing esophageal candidiasis ( Table 41.1 ).
| Finding | Esophagus | Stomach | Small Bowel | Colon |
|---|---|---|---|---|
| Plaque | Candida | Cryptococcus | MAC | Clostridium difficile |
| HSV | MAC | Cryptococcus | CMV | |
| Inflammation * | HSV | CMV | Cryptosporidium | Bacteria |
| CMV | Cryptococcus | CMV | ||
| Cryptosporidium | ||||
| Erosion or ulcer | Any infection | CMV | CMV | Bacteria |
| TB | Cryptosporidium and Cryptococcus | CMV | ||
| Syphilis | Histoplasma | |||
| Amebae | ||||
| Strongyloides | ||||
| Stricture | CMV | Cryptosporidium | CMV | CMV |
| TB | TB | TB | TB | |
| Histoplasma | ||||
| Blastomyces | ||||
| Mass | CMV | CMV | CMV | CMV |
| TB | Histoplasma |
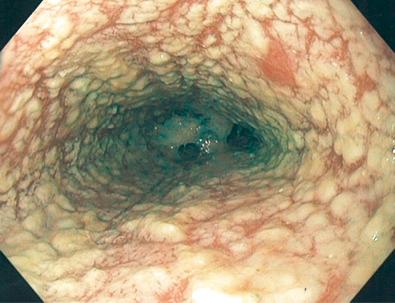
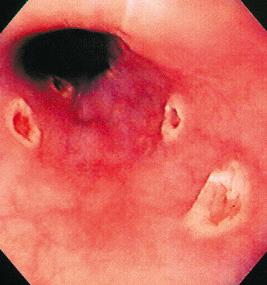
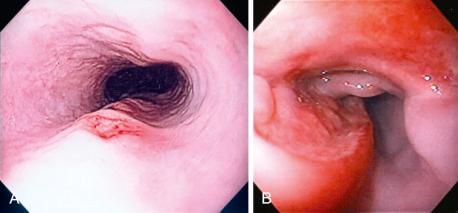
The gross endoscopic appearance of Candida esophagitis is pathognomonic ( Fig. 41.1 ) and may be graded according to published criteria. A large, well-circumscribed ulceration should not be attributed to Candida . The endoscopic characteristics of HSV esophagitis reflect the pathologic changes. HSV esophagitis appears as discrete, usually small (< 1 cm), well-circumscribed, shallow ulcers; a diffuse erosive esophagitis; or, rarely, vesicles ( Fig. 41.2 ). Small, scattered lesions covered with exudate mimic esophageal candidiasis. Deep ulcers, as seen with CMV, are very rare. CMV esophagitis is characteristically associated with one or more ulcerations that can be quite striking in patients with AIDS. Nevertheless, as with other infections, variability has been reported with appearances ranging from multiple shallow ulcers, to solitary giant ulcers, to a diffuse superficial esophagitis ( Fig. 41.3 ). Although serologic testing is not helpful because of the high rate of prior exposure to CMV, the absence of CMV DNA or antigenemia in the blood would suggest an alternative diagnosis. Esophageal TB can manifest with a fistula to the tracheobronchial tree that is easily visualized endoscopically and rarely as an ulcer or mass lesion resembling a neoplasm. In normal hosts from endemic areas in South America, Trypanosoma cruzi may involve the myenteric plexus of the esophagus, resulting in Chagas' disease and an appearance that is indistinguishable clinically, radiographically, manometrically, and endoscopically from idiopathic achalasia. This diagnosis may be established by antibody testing. The endoscopic appearances of other rare infections have been described in case reports and resemble other infections.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here