Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Advances in surgical techniques and immunosuppressive regimens have had a pivotal role in optimizing outcomes after transplantation. The introduction of cyclosporine in the 1980s and tacrolimus a decade later heralded the era of modern immunosuppression, and transplantation advanced from being a quasiexperimental procedure to an established and accepted modality of treatment for a wide range of end-organ diseases. Table 308.1 depicts the most recent data on graft and patient survival reported by the United Network for Organ Sharing (UNOS). The best results are for living-related kidney transplantation; ≈99% of the recipients are alive, and 96% have functioning allograft 1 year after transplantation. The most significant progress may have been in liver transplantation, in which the 1-year survival rate before the use of cyclosporine was only 32% compared with 89% at present. Outcomes after lung transplantation continue to be limited by the development of chronic lung allograft dysfunction with 1-, 5-, and 10-year survival rates of 80%, 50%, and 20%, respectively in the current era. Fewer than 150 intestinal transplantations are performed annually in the United States; however, 1-year patient survival rates at high-volume experienced centers approach 90%.
| TYPE OF TRANSPLANT | NO. OF TRANSPLANTS | GRAFT SURVIVAL (%) (1 YR) |
PATIENT SURVIVAL (%) (1 YR) |
|---|---|---|---|
| Kidney (living donor) | 4348 | 98.8 | 99.1 |
| Kidney (deceased donor) | 13969 | 96.8 | 96.6 |
| Kidney pancreas | 798 | 98.7/91.6 a | 97.4 |
| Heart | 2573 | 91.7 | 92.0 |
| Liver (living donor) | 359 | 90.1 | 94.4 |
| Liver (deceased donor) | 6768 | 90.1 | 92.0 |
| Intestine | 141 | 78.0 | 83.7 |
| Lung | 1826 | 88.8 | 89.7 |
| Heart-lung | 15 | 73.3 | 73.3 |
a Graft survival for kidney was 98.7 and for pancreas was 91.6%, respectively.
Improvements in graft and patient survival have been paralleled by decline in the number of deaths associated with infections ( Fig. 308.1 ). Current data from the Organ Procurement and Transplant Network show that infection-related mortality within the past decade has continued to decline in all types of organ transplantations (see Fig. 308.1 ). Nevertheless, infections still account for ≈24% of the first-year deaths in kidney and heart transplant recipients and up to 34% in lung transplant recipients ( Table 308.2 ). In liver transplant recipients approximately 32% of the mortality within the first posttransplantation year was infection associated. Infections contribute to 10% to 13% of the deaths after 10 years in kidney, liver, and heart recipients and 25% in lung recipients ( Table 308.3 ).
| PERCENTAGE OF DEATHS DUE TO SPECIFIED CAUSES IN THE FIRST POSTTRANSPLANT YEAR | |||||
|---|---|---|---|---|---|
| TYPE OF TRANSPLANT | VASCULAR EVENTS | INFECTION | MALIGNANCY | GRAFT FAILURE | OTHER |
| Kidney | 26.4 | 24.4 | 3.1 | 0.7 | 1.6 |
| Liver | 16.7 | 32.3 | 5.1 | 12.2 | 10.6 |
| Heart | 18.2 | 23.4 | 2.1 | 26.2 | 11.3 |
| Lung | 14.0 | 34.2 | 3.3 | 16.1 | 5.22 |
| PERCENTAGE OF DEATHS IN ORGAN RECIPIENTS SURVIVING AT LEAST 10 YEARS | |||||
|---|---|---|---|---|---|
| TYPE OF TRANSPLANT | VASCULAR EVENTS a | INFECTION | MALIGNANCY | GRAFT FAILURE | OTHER b |
| Kidney | 18.7 | 11.3 | 9.3 | 1.0 | 1.3 |
| Liver | 13.0 | 13.0 | 10.0 | 6.8 | 7.2 |
| Heart | 20.8 | 10.0 | 16.7 | 5.9 | 6.7 |
| Lung | 8.7 | 25.2 | 14.6 | 19.3 | 16.3 |
a Vascular events include cardiovascular and cerebrovascular events.
b Other includes multiorgan failure in liver, kidney, and heart recipients, and respiratory complications in lung transplant recipients (respiratory failure, bronchiolitis obliterans, pulmonary hypertension, pulmonary embolism).
The clinical manifestations of infection are variable and depend on the infecting pathogen, prior immune status of the host, the type of transplantation, time elapsed since transplantation, and the intensity of pharmacologic immunosuppression ( Table 308.4 ). With this complexity in mind, it is useful to address some general principles that may facilitate the approach to diagnosis, management, and understanding of infections after transplantation.
| VARIABLE | EXAMPLES AND COMMENTS |
|---|---|
| Pretransplantation Host Factors | |
| Underlying medical conditions and chronic infections | Conditions that persist or recur (diabetes mellitus, malignancy) |
| Conditions that exacerbate (chronic bronchitis, gallbladder disease) | |
| Lack of specific immunity | Conducive to important primary infections (e.g., cytomegalovirus [CMV], Epstein-Barr virus [EBV], varicella-zoster virus [VZV], toxoplasmosis) |
| Prior colonization | Nosocomial gram-negative bacilli, Candida and Aspergillus organisms, staphylococci, vancomycin-resistant enterococci, |
| Prior latent infections | Reactivation produces clinical infection (tuberculosis, CMV, herpes simplex virus, VZV, cryptococcosis, Trypanosoma cruzi ) |
| Prior medications | Immunosuppressive agents and antibiotics influence posttransplantation susceptibility to infection. |
| Transplantation Factors | |
| Type of organ transplanted | Site of transplantation and allograft are most common sites of infection. |
| Allograft may transmit infection or be more susceptible to infection as a result of ischemic injury or allograft reactions. | |
| Trauma of surgery | Surgical stress, duration of surgery |
| Immunosuppression | |
| Immunosuppressive agents | Corticosteroids, azathioprine and other cytotoxic agents, cyclosporine, tacrolimus, rapamycin, polyclonal and monoclonal antilymphocyte serums |
| Immunosuppressive viruses | CMV, EBV, human herpesvirus 6, and chronic hepatitis C virus infection associated with higher risk of bacterial and fungal infections |
| Allograft Reactions | |
| Graft-versus-host reaction | Affects all areas of immunity and is a major factor in bacterial, viral, and fungal infection in stem cell transplantation |
| Host-versus-graft reaction | Possible cofactor in infections affecting the allograft |
Among factors contributing to the occurrence of infections in transplant recipients, an obvious and probably the most consequential is iatrogenic immunosuppression. The effects of immunosuppressive agents have become more apparent as surgical techniques have improved, and antimicrobial prophylaxis has come to be used more widely. The goal is to optimize immunosuppressive regimen such that it prevents rejection but preserves antimicrobial immunity and minimizes long-term metabolic complications and the risk of malignancy.
Although inadequate as sole agents to sustain graft survival, corticosteroids remain a key component of most immunosuppressive regimens. Corticosteroids broadly inhibit immune responses, including innate inflammatory responses, phagocytic function, cellular immunity, and, to a lesser extent, antibody formation. In an effort to obviate undesirable side effects of corticosteroid therapy, more transplantation centers practice early withdrawal and use steroid-free regimens. Meta-analyses of trials of corticosteroid-free regimens in kidney and liver transplant recipients have not shown beneficial effect of corticosteroid reduction on overall infectious risk, but analysis of specific infectious outcomes in liver transplantation suggested that corticosteroid avoidance might reduce the risk for cytomegalovirus (CMV) infection and hepatitis C virus (HCV) recurrence.
The introduction of cytotoxic drugs, such as azathioprine, was a major advance in immunosuppression that made transplantation across human leukocyte antigen (HLA) barriers feasible. All cytotoxic drugs interfere with DNA synthesis, thereby suppressing the bone marrow and reducing peripheral blood cell counts. In addition to marrow suppression, azathioprine may cause pancreatitis, a reversible hepatitis, rash, and gastrointestinal (GI) disturbances. As such, use of azathioprine has decreased in the current era.
Mycophenolate mofetil, approved in 1995, is a cytotoxic drug with an antiproliferative effect on T and B lymphocytes and has replaced azathioprine in triple-drug regimens, comprising a calcineurin inhibitor agent and corticosteroids. Its use has been associated with lower rates of biopsy-proven rejection compared with azathioprine, with similar risk of infections. The main side effects of mycophenolate mofetil are myelosuppression and diarrhea.
The calcineurin inhibitor agent cyclosporine is a cyclic peptide derived from Tolypocladium inflatum and was approved for clinical use in 1983. It inhibits the production of cytokines, primarily interleukin-2 (IL-2), and consequently the generation of effector T cells or CD4 + T cells ( Fig. 308.2 ). Concentrations of the drug as low as 100 ng/mL effectively inhibit mixed lymphocyte reactions. Patients treated with cyclosporine alone for various autoimmune diseases show low rates of clinical infection, which demonstrates the importance of corticosteroids and other cofactors for infection in transplant recipients. Tacrolimus, approved in 1994, is a macrolide produced by Streptomyces tsukubaensis . Despite some differences, its mode of action is similar to that of cyclosporine in that it inhibits production of IL-2 and CD4 + T cells. However, it is 10 to 100 times more potent than cyclosporine. Although a major component of the immunosuppressive effects of cyclosporine and tacrolimus is accounted for by the antagonism of calcineurin activity, tacrolimus also inhibits steps distal to calcineurin activation in the T-cell activation cascade. As such, tacrolimus has the ability to reverse steroid-resistant allograft rejection, whereas cyclosporine is ineffective for the treatment of rejection. Clinical trials have demonstrated that tacrolimus-based immunosuppression results in lower rates of acute rejection and graft loss than cyclosporine-based therapy, particularly in kidney and liver transplant recipients, with no convincing evidence that it enhances the risk for infection.
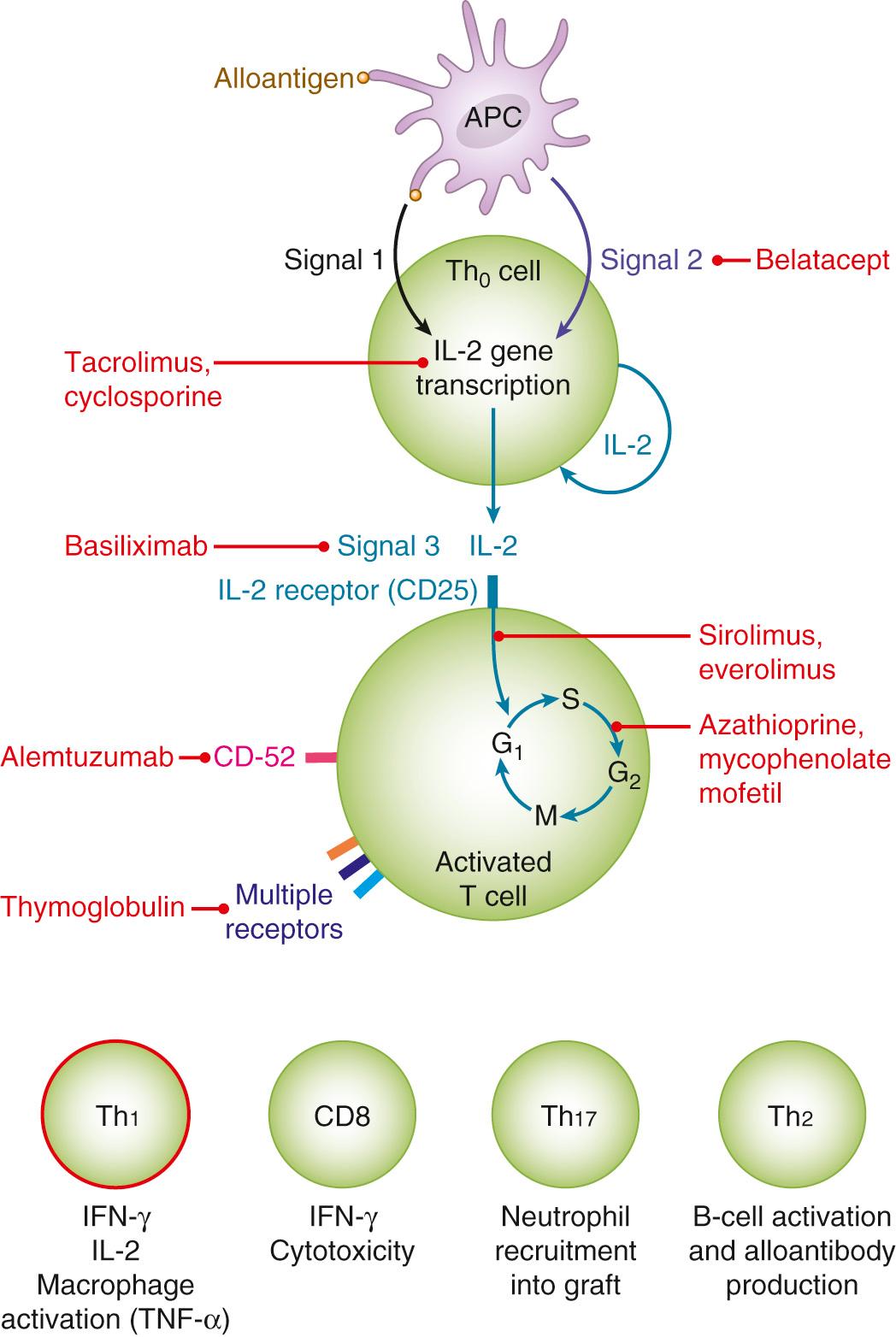
Major adverse effects of calcineurin inhibitor agents are nephrotoxicity, thrombotic thrombocytopenic purpura/hemolytic uremic syndrome, neurotoxicity, hypertension, and diabetic mellitus. In comparison with cyclosporine, tacrolimus is associated with less hypertension but higher rates of diabetes mellitus and neurotoxicity.
Rapamycin, also known as sirolimus, was released in 1999. It is a macrolide antibiotic derived from Streptomyces hygroscopicus . Rapamycin interferes with cell-cycle proliferation and blocks intracellular signaling mechanisms by inhibiting a regulatory kinase—mammalian target of rapamycin (mTOR) (see Fig. 308.2 ). Unlike cyclosporine and tacrolimus, mTOR inhibitors have no direct nephrotoxicity. They frequently cause hyperlipidemia and occasionally cause myelosuppression. They have also been linked to delayed wound healing, oral ulcerations, and a rare drug-induced interstitial pneumonitis that can mimic Pneumocystis pneumonia. Some data suggest that rapamycin use is associated with reduced rates of posttransplantation malignancy and CMV disease. Everolimus is a hydroxyethyl derivative of rapamycin that differs from sirolimus in its pharmacokinetics, but it has a similar side-effect profile and infectious risk.
Although calcineurin inhibitors have remained the mainstay of immunosuppression for more than 3 decades, long-term outcomes remain suboptimal largely due to renal dysfunction and metabolic complications from cumulative exposure to these agents. For example, chronic renal dysfunction develops in 7% to 21% of the organ transplant recipients and increases the risk of death by approximately fourfold. These concerns have spawned a growing interest in new regimens that enhance early immunologic tolerance, for instance, IL-2 receptor inhibitors (basiliximab) and T-cell–depleting antibodies (alemtuzumab or Thymoglobulin). These agents have delayed and reduced the incidence of acute rejection, but it is unclear if they have any impact on long-term outcomes. They have no role as maintenance immunosuppressants.
The biologic agents used for immunosuppression in transplant recipients are shown in Table 308.5 . These include polyclonal or monoclonal antibody preparations that are used either to treat refractory rejection or as induction therapy that is administered in the immediate posttransplantation period and aims to provide a high early level of immunosuppression while avoiding nephrotoxicity from calcineurin inhibitors. These agents can generally be divided into T-cell nondepleting and depleting drugs. The first such agent, a murine monoclonal antibody (Muromunab-CD3 [OKT3]), was associated with an unfavorable toxicity profile, including cytokine-release syndrome (fever, rigors, hypotension, or rarely, severe pulmonary edema and respiratory distress) and a substantial risk of opportunistic infections. This agent was withdrawn from the market in 2010.
| AGENT | ADVERSE EFFECTS |
|---|---|
| Polyclonal Antibodies | |
| Antithymocyte globulins a | Serum sickness, thrombocytopenia, lymphopenia (can last up to 2–3 years with Thymoglobulin), increased risk of cytomegalovirus and posttransplantation lymphoproliferative disease |
| Anti–human thymocyte immune globulin (rabbit) (Thymoglobulin) | |
| Lymphocyte immune globulin, antithymocyte (equine) (ATGAM) | |
| Monoclonal Antibodies | |
| Anti-CD25 (interleukin-2 receptor) antibodies b Basiliximab (Simulect) |
Hypersensitivity reactions, infection risk not significantly increased |
| Anti-CD20 antibody c Rituximab (Rituxan) |
Infusion reactions, hepatitis B virus reactivation |
| Anti-CD52 antibody d Alemtuzumab (Campath) |
Infusion reactions, increased risk of cytomegalovirus, Pneumocystis jirovecii pneumonia, invasive fungal infections, immunosuppression that can last up to 12 mo |
| Other Agents | |
| Anti–B7 fusion protein (costimulation ligand) e Belatacept (Nulojix) |
Increased rate of Epstein-Barr virus–associated posttransplantation lymphoproliferative disease |
a Used for induction and rejection treatment.
b Used for induction but not to treat rejection.
c Used primarily to treat humoral rejection, blood type (ABO) mismatch, and recipients with a positive crossmatch (off-label use).
d Used for induction and rejection treatment (off-label use).
Basilixmab is a nondepleting agent approved by the US Food and Drug Administration (FDA) for prophylaxis of rejection in kidney transplantation. An anti-CD25 monoclonal antibody, it binds to the IL-2 receptor and prevents activation of T cells by circulating IL-2. It is relatively well tolerated and is not associated with cytopenias or serum sickness. However, it cannot be used for the treatment of acute rejection as it causes no T-cell depletion.
Antithymocyte globulins, such as Thymoglobulin, are polyclonal antibodies prepared in rabbits or horses by immunization with human thymocytes. As such, they are foreign proteins that may cause serum sickness that typically begins about 10 days after administration. They result in profound immunosuppression due to T-cell depletion, which lasts for 3 to 6 months. Antithymocyte globulin increases the risk of CMV disease and posttransplantation lymphoproliferative disease (PTLD).
Alemtuzumab is a monoclonal antibody that is approved for the treatment of B-cell chronic lymphocytic leukemia. This agent targets a cell surface molecule (CD52) common to many immune cells and causes significant reduction in CD4 + and CD8 + T cells, natural killer (NK) cells, and CD19 + B cells. This effect may last 12 months or longer after administration. It is increasingly used with transplant recipients for either induction therapy or for the treatment of acute rejection unresponsive to corticosteroids. Receipt of alemtuzumab for induction therapy confers a higher risk of CMV viremia and more severe and disseminated fungal infections. In contrast to agents that affect only T cells, its use has not been associated with an increased rate of PTLD, probably because its action against B cells that suppresses Epstein-Barr virus (EBV). The infectious risk of alemtuzumab is significantly higher when used as salvage treatment for acute rejection than when used as induction therapy.
Rituximab is another chimeric mouse/human monoclonal antibody that targets the CD20 antigen expressed on B lymphocytes. Rituximab is approved to treat a variety of B-cell malignancies and autoimmune diseases. In organ transplantation it has been primarily used to treat antibody-mediated rejection. Rituximab also does not appear to increase the general risk for infections but has been associated with reactivation of HBV infection and progressive multifocal leukoencephalopathy. Patients receiving therapy with rituximab may also mount poor antibody responses to immunization.
Belatacept is a novel fusion protein that avidly attaches to receptors on antigen-presenting dendritic cells. It selectively inhibits costimulation signaling, thereby interfering with effective T-cell activation. Belatacept requires intravenous (IV) administration on a defined schedule that tapers off to every-4-weeks infusions by 2 to 3 months after transplantation. It is approved as an antirejection agent in kidney recipients, based on trials that showed significantly better creatinine clearance and similar graft survival when it replaced cyclosporine. The overall infectious risk of belatacept does not appear to be greater than cyclosporine-based immunosuppression. However, development of progressive multifocal leukoencephalopathy and PTLD, particularly involving the central nervous system (CNS) has been documented. For this reason, belatacept use is contraindicated in EBV-seronegative recipients.
Evaluation of the patient for active and latent infection will allow optimization of pretransplantation and posttransplantation management. The UNOS has issued a list of required infectious disease screening tests for all transplantation candidates. The initial transplantation evaluation should consist of a thorough history and physical examination, with a particular emphasis on demographics, country of origin, occupation, travel history, and exposure history, including exposure to animals. Current medications should be reviewed, specifically immunosuppressants such as corticosteroids, which may predispose the patient to opportunistic infections before transplantation. All relevant microbiology and hospital medical records should be examined for evidence of latent or active infection and colonization with drug-resistant bacteria. The pretransplantation setting is also an important opportunity to update the potential recipient's immunization as vaccinations are more effective when given before immunosuppression (discussed later in the immunizations section).
Pretransplantation serologic screening is pivotal in mitigating the risk of infection posttransplantation, as outlined in Table 308.5 . Knowledge of the recipient's herpesvirus serostatus, especially that of CMV, is used in conjunction with the donor's CMV serostatus to risk stratify the recipient and determine the optimal posttransplantation preventive strategy. Persons who are EBV mismatched (EBV-seropositive donor/EBV-seronegative recipient [D + /R − ]) are at an increased risk of PTLD and are more closely monitored for EBV. Routine pretransplantation evaluation should also include testing for human immunodeficiency virus (HIV), latent tuberculosis (TB), Toxoplasma gondii (particularly in heart transplant candidates), hepatitis A and B (for which vaccination should be offered, if seronegative), hepatitis C, and syphilis.
Other than patients with cystic fibrosis (CF), the natural history of transplant candidates colonized with multidrug-resistant (MDR) organisms, particularly MDR gram-negative bacteria, has not been fully described. A few observational studies have demonstrated that pre–liver transplant rectal colonization with extended-spectrum β-lactamase-producing or carbapenem-resistant Enterobacteriaceae is a risk factor for posttransplant colonization and infection. However, while mortality rates approached 50% for persons with posttransplant CRE infection, pretransplant CRE colonization was not a risk factor for death.
Additional testing for human T-cell lymphotropic virus 1 (HTLV-1), Trypanosoma cruzi, malaria, Strongyloides stercoralis , Brucella, or human herpesvirus 8 (HHV-8) may be required in patients with residence or extended travel in regions where these infections are prevalent. Strongyloides stercoralis is also endemic within the southeastern United States, where screening should be considered. Serologic screening for Leishmania before transplantation may be considered for candidates with a history of potential exposure to this protozoan parasite in tropical or subtropical regions and in the Mediterranean countries, although conclusive data are lacking. Screening for Coccidioides antibodies should be routinely performed in recipients from areas of endemicity of this fungus. HTLV-1 is endemic to Southeast Asia, the Pacific Islands, Western Africa, parts of South America, and the Caribbean. The effect of immunosuppression on the natural history of HTLV-1 is not well defined, and routine HTLV-1 serologic testing of donors is no longer routinely used because of concerns about specificity. Transplant candidates seropositive for HTLV-1 can be considered for transplantation but should be informed about potentially increased risk of developing acute T-cell leukemia and HTLV-1–associated myelopathy/tropical spastic paraparesis ( Table 308.6 ).
| ANTIMICROBIALS | EFFECT ON CYP3A4 |
IMMUNOSUPPRESSANT LEVEL | DOSAGE ADJUSTMENT a | |||
|---|---|---|---|---|---|---|
| CALCINEURIN INHIBITOR | mTOR INHIBITOR | CALCINEURIN INHIBITOR | mTOR INHIBITOR | |||
| Azoles | Fluconazole | Moderate-strong inhibitor at doses > 400 mg | ↑↑↑ | ↑↑ | Reduce FK and CsA by |
Monitor levels/reduce doses |
| Voriconazole | Strong inhibitor | ↑↑ | ↑↑ | Reduce FK by |
Technically “contraindicated,” but usually reduce mTOR dose | |
| Posaconazole | Strong inhibitor | ↑↑ | ↑↑ | Reduce FK by |
||
| Isavuconazole | Weak inhibitor | ↑ | ↑ | Monitor levels; currently no preemptive adjustment recommended | ||
| Rifamycins (CYP3A4 induction: rifampin > rifapentine > rifabutin) |
Rifampin | Strong inducer | ↓↓↓ | ↓↓↓ | Avoid rifampin | |
| Rifabutin | Moderate inducer | ↓↓ | ↓↓ | Increase calcineurin inhibitor /mTOR dose by 50%; frequent monitoring |
||
| Macrolides | Azithromycin | — | — | — | None | |
| Clarithromycin | Moderate to strong inhibitor | ↑↑ | ↑↑ | Reduce calcineurin-inhibitor mTOR dose by |
||
| ARV (ritonavir/cobicistat) | Strong inhibitors | ↑↑↑ | ↑↑↑ | Major interaction; calcineurin inhibitors have been dosed once weekly/q2 weeks. In the current era, switch to ART to avoid boosted agents (e.g., use integrase inhibitors) | ||
| Nafcillin (not oxacillin) | Weak inducer | ↓ | ↓ | Monitor levels; no empiric adjustment | ||
a Dose adjustments per prescribing information; actual practices may vary.
The frequency, types of infections, and specific pathogens encountered after transplantation generally follow a predictable time to onset. However, evolving medical practices and preventive strategies have led to modifications in the risk and timeline of several infections, as discussed later. Nevertheless, infections in transplant recipients must be evaluated in the context of time elapsed since transplant.
Infections during this period are primarily surgical or technical complications related to transplantation or are health care–associated ( Table 308.7 ). Bacterial infections, including those due to antimicrobial resistant pathogens, are by far the most frequently occurring infections; vascular-catheter infections, health care–associated pneumonia, Clostridioides difficile (formerly Clostridium difficile ) colitis, and surgical site infections being most common. Invasive fungal infections are uncommon in the first month, except for invasive candidiasis, which generally manifests as candidemia or surgical site infections. In the absence of mold-active prophylaxis, invasive aspergillosis can occur in lung transplant recipients with prior mold colonization. Viral infections such as herpes simplex virus (HSV) and HHV-6 may be encountered very early posttransplantation. Certain donor-transmitted infections (discussed later) may also manifest during this period.
| MAJOR PREDISPOSING FACTORS | |||
|---|---|---|---|
| DAY 0–30 | DAY 30–180 | DAY 180 AND BEYOND | |
| Pathogens | Surgical complications, nosocomial infections, pretransplant colonizers, donor-derived infections; low opportunistic infection risk | Effects of iatrogenic immunosuppression, high risk of opportunistic pathogens, residual postsurgical sequelae | Community acquired pathogens; risk of opportunistic pathogens lifelong and determined by overall state of immunosuppression such as treatment of rejection, intensity of maintenance regimen |
| Bacteria | Gram-negative and gram-positive bacteria (including multidrug-resistant strains), Clostridioides difficile , pretransplant colonizers (e.g., Burkholderia cepacia ) | Nocardia , tuberculosis and nontuberculous mycobacteria, nosocomial gram-negative/gram-positive bacteria, | Streptococci , Legionella, Listeria ; nosocomial bacteria if hospitalized; risk of opportunistic bacteria never resolves |
| Fungi | Candida spp.; Aspergillus uncommon, except for patients with cystic fibrosis | Molds ( Aspergillus , mucormycosis), Candida and Pneumocystis less common in the current era | Cryptococcus and endemic mycoses; ongoing risk for invasive mold disease; Pneumocystis and toxoplasmosis if off prophylaxis |
| Viruses | Respiratory viruses; herpesviruses uncommon in the current era of routine antiviral prophylaxis |
Herpesviruses (cytomegalovirus [CMV]) if off prophylaxis; BK virus, respiratory viruses | Reactivation of chronic viral infections (herpes zoster, CMV), Epstein-Barr virus (posttransplantation lymphoproliferative disorder), respiratory viruses, norovirus, JC virus |
| Other | Donor-derived bacteria/fungi, unrecognized donor-derived viruses, opportunistic fungi such as Cryptococcus and endemic mycoses | Wide geographic and institutional variability; should be evaluated in the context of the prophylactic regimen and endemic pathogens (leishmaniasis, Chagas disease, gastrointestinal parasites) | Timeline is “reset” and opportunistic infection risk increases markedly with the treatment of rejection, particularly with T-cell–depleting agents (alemtuzumab, Thymoglobulin) |
Although nosocomial infections continue to pose a threat in patients requiring prolonged hospitalization, rates of surgical infections typically decline after 1 month, and opportunistic infections associated with immunosuppression emerge. These include Pneumocystis jirovecii, fungi, T. gondii, Nocardia, and most important, cytomegalovirus (CMV). Historically, CMV infection and disease have occurred between 4 and 6 weeks posttransplantation. However, in the era of routine use of antiviral prophylaxis, typically given for 3 to 6 moths posttransplantation, most CMV disease now occurs later, after antiviral prophylaxis has been discontinued (“late-onset” CMV disease). Pneumocystis pneumonia and, to a large extent toxoplasmosis, are seen infrequently today because of the effectiveness of trimethoprim-sulfamethoxazole (TMP-SMX) prophylaxis. Invasive aspergillosis has long been regarded as an early-occurring infection. However, most cases now occur between 1 and 6 months posttransplantation. Notably, in lung transplant recipients Aspergillus infections occur after 12 months; the median time to onset posttransplantation was 357 days in one report and 33 ± 19.6 months in another. Widespread use of mold-active antifungal prophylaxis and chronic rejection and/or its treatment likely account for these trends.
Infections more than 6 months after transplantation are mostly community acquired and similar to those seen in the general population. However, contemporary data from the Spanish consortium for the study of infections in transplant recipients show that although the rate of infections declined from 3.5 per 1000 transplant-days in the first 6 months to 0.4 per 1000 transplant-days in the late period, the etiology of infections and infection-related mortality was similar in the two periods. Patients with chronic graft dysfunction and graft-related reoperations are at higher risk for late infections. Heart, kidney, and liver transplant recipients have lower rates (≈0.3/1000 transplant-days), whereas pancreas and lung transplant recipients have the highest incidence rates of late infections (0.76 and 1.4/1000 transplant-days, respectively).
Other late manifestations may represent reactivated or chronic viral infections. Herpes zoster may occur at any time after transplantation. A number of tumors related to viral infection may also occur late, the most frequent being cutaneous malignancies. Also, some lymphomas and lymphoproliferative syndromes related to EBV occur after 1 year. Infections due to mycoses, such as cryptococcosis or histoplasmosis, often manifest late and without an apparent inciting event or change in immunosuppression. Finally, although the risk for classic opportunistic infections declines, it never disappears completely. In this regard, treatment of rejection, particularly with T-cell–depleting agents resets the timeline and renders the patient susceptible to opportunistic pathogens noted earlier, the risk of which declines in the late posttransplantation period (see Table 308.7 ).
Table 308.8 presents the type, severity, and typical sites of infections in kidney, heart, heart-lung, and liver transplant recipients observed during the first year after transplantation. In addition, certain risks are unique for specific types of transplantation. Kidney transplant recipients have the lowest number of episodes of infection per patient, whereas liver and heart transplant recipients have intermediate rates. Heart-lung or lung recipients, by contrast, have more than three times the number of infections. Liver transplant recipients have almost twice the rate of bacteremia of the renal group. Invasive fungal infections are frequent in liver and heart-lung or lung recipients, intermediate in heart recipients, and rare in renal recipients. Table 308.3 also shows that the most common sites of infection are closely related to the site of surgery.
| TYPE OF TRANSPLANT | INFECTION EPISODES PER PATIENT | BACTEREMIA (%) | FUNGAL INFECTIONS (%) | MOST COMMON SOURCE OF INFECTION |
|---|---|---|---|---|
| Kidney | 0.98 | 5–10 | 0.7 | Urinary tract |
| Heart | 1.36 | 8–11 | 8 | Lung |
| Heart-lung | 3.19 | 8–25 | 23 | Lung |
| Liver | 1.86 | 10–23 | 16 | Abdomen and biliary tract |
Most infections, including bacteremia in these patients, arise from the urinary tract. By 3 years after transplantation, more than half of all renal recipients would have been diagnosed with upper or lower urinary tract infection (UTI). Although most infections were uncomplicated cystitis, graft pyelonephritis occurred in 13% of 1387 renal recipients over a 13-year period. In this study, pyelonephritis occurring in the first 3 months was associated with reduced graft survival, but later infections were not. However, a larger, multivariate analysis of more than 28,000 kidney recipients in the Medicare database showed that UTIs after 6 months were associated with worse long-term graft function and survival. Abnormalities such as ureteral reflux, strictures at the ureterovesical junction, or neurogenic bladder should be sought in patients with recurrent infections. Administering an extended course of antibiotics (≥4 weeks) and consideration of secondary antibiotic prophylaxis are reasonable in patients who have noncorrectable urologic abnormalities and severe graft pyelonephritis or recurrent infections. Although asymptomatic bacteriuria is common in kidney transplant recipients, a randomized, controlled trial in kidney transplant recipients showed no benefit of routine screening and treatment for asymptomatic bacteriuria.
The clinician also should be alert to the occurrence of uncommon urinary pathogens. For example, urinary tract TB may arise from a focus in the native kidney. Mycoplasma hominis may cause a breakdown of the ureterovesical anastomosis with subsequent graft loss. Presence of struvite stones, encrusted cystitis, alkaline urine, and apparent sterile pyuria is suggestive of Corynebacterium urealyticum . This bacterium may be overlooked in routine cultures in standard media as it requires incubation for more than 48 to 72 hours or requires special media for isolation. Histoplasmosis may involve the transplanted kidney and cause renal failure. Adenovirus may cause hemorrhagic cystitis and/or nephritis. BK virus is an important cause of renal allograft infection and is not typically associated with systemic clinical signs or symptoms.
Historically, pneumonia occurred in 25% to 30% of kidney transplant recipients and was the most common infectious cause of death. In the past, transplantation-associated pneumonia was often due to CMV and pathogens such as fungi, Nocardia , and Pneumocystis. Currently, however, these opportunistic agents have come under better control, and conventional bacterial pathogens have become far more common in transplant populations. Wound infections are infrequent (4%–5%) but may be a serious problem, particularly if they involve the perinephric space. Body mass index ≥30 kg/m 2 , urinary leak, reoperation through the transplant incision, mycophenolate mofetil use, and diabetes mellitus are risk factors for infection. Some renal transplant recipients continue to have frequent problems with infection even after the first 6 months. These patients have often received excessive immunosuppression or have chronic dysfunction of the allograft or other major organs.
The most common infections after heart transplantation are bacterial pneumonias, urinary infections, herpesvirus infections, and invasive fungal infections. Pneumonia in heart transplant recipients is mostly caused by common pathogenic bacteria ( Table 308.9 ). Although the incidence of pneumonia is highest during the first few months after transplantation, bacterial pneumonias occur sporadically in the late posttransplantation period, after the patient has recovered from the immediate effects of surgery. Heart transplant recipients requiring renal replacement therapy and thoracic reoperation are at an increased risk for invasive pulmonary aspergillosis and should receive voriconazole prophylaxis.
| EARLY PNEUMONIA (≤30 DAYS) | LATE PNEUMONIA (>30 DAYS) |
|---|---|
| Common Causes | |
|
|
| Less Common Causes | |
|
|
a These include influenza virus, parainfluenza virus, respiratory syncytial virus, metapneumovirus, coronavirus, rhinovirus, or adenovirus.
Mediastinitis and sternal wound infections are postoperative complications unique to heart and heart-lung transplant recipients and occur in ≈2.5% of patients. The pathogens seen are similar to those observed in other patients undergoing cardiothoracic surgery, with staphylococci predominating. One must also be alert to the possibility of unusual pathogens. Mediastinitis and sternal wound infections in heart recipients have been caused by M. hominis, Legionella, Aspergillus , Mucormycetes, and Nocardia. Factors that predispose to mediastinitis in this population are repeat operations for hemorrhage, use of antirejection therapy, and diabetes mellitus. Surgical drainage is a crucial component of treatment of mediastinitis in the transplant patient.
Left ventricular assist devices (LVADs) are now widely used as a bridge to transplantation. Infections of these devices are common and fall into distinct types: driveline infections, which are often limited to the exit site; deep infections in the pocket surrounding the device; and internal infection of the device, which is often associated with bloodstream infection. Management of these infections is challenging; however, in many cases the infection can be controlled well enough to permit transplantation. Available experience suggests that the use of antimicrobial therapy before, during, and after transplantation is associated with fewer relapses than short-course therapy. Although pretransplantation LVAD infection is associated with a higher rate of periheart transplantation complications, long-term outcomes appear to be reasonable, and LVAD infection is not considered a contraindication to heart transplantation.
A number of other infections occur more commonly in heart recipients than in patients receiving other types of transplants. These include toxoplasmosis, nocardiosis, and Chagas disease. Toxoplasma -seronegative heart recipients are at risk for toxoplasmosis because the infection can be acquired from organisms encysted in the heart muscle of Toxoplasma -seropositive donors. Clinical toxoplasmosis usually occurs a few weeks to a few months after transplantation and is manifested by necrotizing pneumonitis, myocarditis, and encephalitis. Before the use of preventive therapy, the rates of toxoplasmosis were 60% to 80% in donor Toxoplasma -seropositive/recipient Toxoplasma -seronegative patients and ≈5% to 10% in Toxoplasma -seropositive recipients. Clinical toxoplasmosis is now uncommon in heart recipients, likely because of the use of TMP-SMX for Pneumocystis prophylaxis. In heart transplant recipients who are Toxoplasma mismatches but are intolerant of TMP-SMX, the prophylactic regimen must cover both Toxoplasma and Pneumocystis .
In endemic regions such as Latin America, symptomatic reactivation of Trypanosoma cruzi may be seen in immunocompromised patients, including transplant recipients. About one-fourth of patients who undergo heart transplantation for cardiomyopathy caused by chronic T. cruzi infection have relapses of acute Chagas disease, with clinical manifestations of fever, myocarditis, and skin lesions. The disease can usually be controlled with chemotherapy. Detection of parasitemia by microscopy or polymerase chain reaction (PCR) assay in blood samples is useful to monitor for reactivation and guide chemotherapy after transplantation.
Nocardia infections have also been more frequently reported in heart and lung transplant recipients than in recipients of kidney or liver transplants, but the biologic reason for this increased rate of nocardiosis is unknown. The doses of TMP-SMX used for Pneumocystis prophylaxis might not provide reliable protection against Nocardia infection. However, resistance to TMP-SMX remains uncommon, even among breakthrough Nocardia infections.
Heart transplant recipients frequently incur trauma to the tricuspid valve and right ventricular endocardium from repeated endomyocardial biopsies, a common posttransplantation practice. Patients with cardiac valvulopathy are at high risk for adverse outcomes from endocarditis, and prophylaxis with dental procedures is appropriate in this group.
The heightened vulnerability of the transplanted lung to infection is multifactorial. In addition to mechanical factors related to decreased mucociliary clearance, diminished lymphatic drainage, and ablation of the cough reflex appear to play an important, if poorly understood, role. The types of infections seen in lung transplant recipients are similar to those in heart-lung recipients, although the overall survival rate is better. Heart-lung transplantation is now usually reserved for patients who have Eisenmenger syndrome and whose cardiac abnormalities cannot be surgically repaired. Single- and double-lung procedures leave the donor heart available for another patient with end-stage heart disease. A unique aspect of single-lung transplantation is the occurrence of infections in the native lung that may be predisposed to infection because of defects of ventilation or perfusion caused by the underlying lung disease.
Lung and heart-lung transplant recipients experience a high rate of bacterial lung infections, especially during the first few weeks after transplantation. These patients also have higher rates of mediastinitis, invasive fungal infections, and CMV pneumonia than heart recipients. Some patients undergoing lung transplantation are chronically colonized with multidrug-resistant bacteria (e.g., CF, non-CF bronchiectasis) and are therefore at high risk for postoperative infection at the time of transplant surgery. Multidrug-resistant gram-negative bacteria in patients with CF, such as Stenotrophomonas maltophilia and Pseudomonas aeruginosa, do not preclude transplantation; instead, the posttransplant prophylactic regimen must target these organisms.
Pretransplant Burkholderia cepacia complex infections in patients with CF pose a unique challenge. Burkholderia multivorans infections do not portend poor outcomes, in contrast with Burkholderia cenocepacia . For this reason, patients with CF who are known to be colonized with B. cenocepacia are often denied listing for lung transplantation at many centers. However, selected persons with B. cenocepacia can undergo lung transplantation at referral centers with experience in managing these infections, which often includes prolonged posttransplant antibiotics. Patients and their families should be counseled about the risk of poor outcomes. Lung transplant candidates with CF may also be colonized with molds, such as Scedosporium complex and Lomentospora (Scedosporium) prolificans, and voriconazole is commonly used as prophylaxis in patients with fungal colonization. However, institutional practices on routine screening and management vary widely.
In the late posttransplant period, up to two-thirds of patients eventually develop chronic lung allograft dysfunction (CLAD), thought to represent chronic allograft rejection, and manifests as two major phenotypes: bronchiolitis obliterans syndrome or a more recently described restrictive allograft syndrome. CLAD has been associated with recurrent pulmonary infections and is one of the major causes of death in lung transplant patients. The lung allograft is also particularly susceptible to viral infections, as has been documented for CMV, HSV, and community-acquired respiratory virus infections (influenza virus, parainfluenza [PIV], respiratory syncytial virus [RSV], metapneumovirus (MPV), coronavirus, rhinovirus, and adenovirus) that are a significant cause of morbidity and have been associated with both short-term and long-term impairment of allograft infection.
Pulmonary nontuberculous mycobacterial infections are relatively common in lung transplant recipients and may arise from pretransplant colonizing strains or exogenous acquisition. Mycobacterium abscessus warrants special mention, as it can lead to devastating consequences posttransplantation, including aggressive and destructive surgical site infections and disseminated disease and mortality. Because of drug resistance and poor treatment response, pretransplantation colonization or disease is considered by some centers to be a contraindication to lung transplantation. However, some centers have reported acceptable outcomes in patients with pretransplantation M. abscessus disease, with the understanding that posttransplantation complications can be expected and should be managed with aggressive and prolonged therapy. Every effort should be made to control the infection before transplantation, and antibiotics should generally be continued in the posttransplantation setting.
Acute development of hyperammonemia after lung transplantation is a rare but an often fatal complication after lung transplantation, the etiology of which is poorly understood but includes unmasking of urea cycle disorders, effects of immunosuppressive agents, or infections with urea-splitting organisms, such as Mycoplasma or Ureaplasma . A multimodal treatment approach is required, which may include renal replacement therapy, bowel decontamination, amino-acid supplementation, nitrogen scavenger therapy for urea-splitting organisms, and antibacterial therapy (quinolones, tetracyclines, or macrolides) for microbiologically proven cases.
Prospective donors for lung transplantation are intubated in intensive care units (ICUs). Therefore the airways of these donors are often colonized with microorganisms, and occult parenchymal infection may be present. Before implantation of the lungs, it is customary to obtain cultures and Gram stains of the donor airways to guide antibiotic therapy in the recipient. Initial antibiotic prophylaxis should be aimed at common nosocomial pathogens encountered in the ICU, including methicillin-resistant S. aureus (MRSA) and enteric and nosocomial gram-negative bacilli; the regimen can be narrowed to target the donor's pathogens. Another problem unique to lung transplantation has been dehiscence of the airway anastomosis. This occurs during the first few weeks after transplantation. It is frequently associated with bacterial or fungal infection at the anastomotic site. The incidence of this complication appears to be declining.
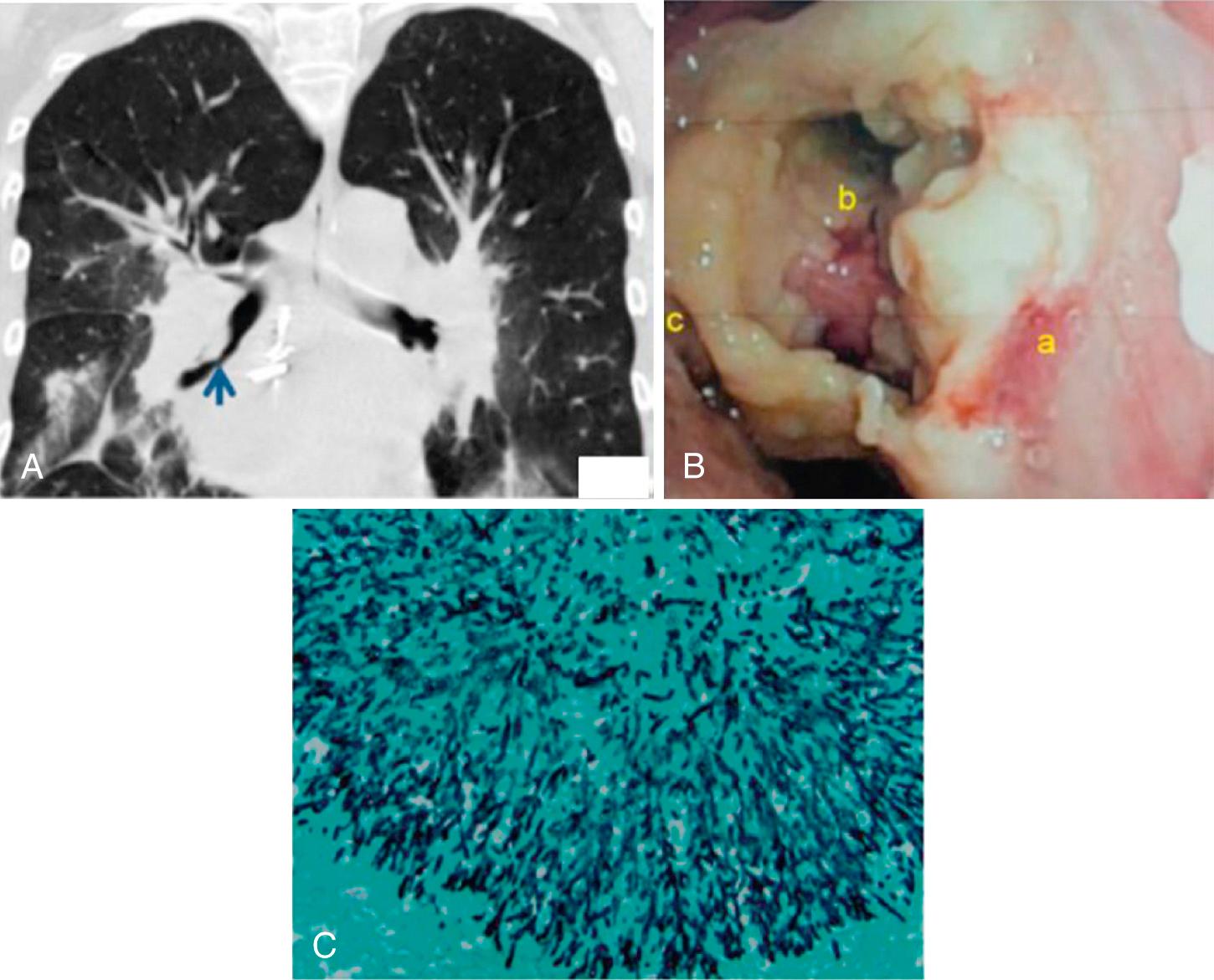
Heart-lung and lung transplant recipients develop invasive pulmonary aspergillosis more frequently than patients with other types of organ transplants. Risk factors for invasive aspergillosis in addition to renal dysfunction are relative ischemia at the anastomotic site, receipt of a single-lung transplant, hypogammaglobulinemia, CMV infection, precolonization and/or postcolonization of the airways with Aspergillus, and augmented immunosuppression for allograft rejection. A unique form of aspergillosis involving the airway mucosa or bronchial anastomotic sites, called tracheobronchial aspergillosis, is observed almost exclusively in lung and heart-lung recipients ( Fig. 308.3 ). The airway lesions of this disease can be directly visualized during bronchoscopy. This form of aspergillosis has a better prognosis than aspergillosis invading the lung parenchyma and is commonly treated with systemic and local (i.e., nebulized) antifungal therapy combined with surgical débridement, as feasible.
Continuous exposure of the organ to the environment and posttransplantation Aspergillus colonization poses a risk for subsequent invasive disease in lung transplant recipients. As such, many lung transplantation centers routinely use antifungal preventive strategies. Direct administration by means of inhalation of the polyene agents (amphotericin B formulations) allows deposition of the drug directly into the distal bronchial tree, thereby obviating the systemic side effects and drug interactions. Inhaled amphotericin B deoxycholate and the lipid formulations (lipid complex and liposomal) are both generally well tolerated; however, aerosolized lipid formulations are associated with fewer side effects. A potential limitation of aerosolized amphotericin B is the fact that deposition in single-lung transplant recipients occurs preferentially in the allograft, with erratic distribution in the native lung, which could be a potential source of infection. In addition, aerosolized amphotericin B may not prevent systemic fungal infections because candidemia has been reported in lung transplant recipients with such an approach. Finally, long-term use of these agents has also been associated with the emergence of Aspergillus spp., such as A. flavus, A. terreus, and A. alliaceus, with reduced susceptibility to amphotericin.
Triazoles, including itraconazole and voriconazole, have led to a decrease in the incidence of invasive aspergillosis in lung transplant recipients. The potential for hepatotoxicity is a concern, particularly with voriconazole. Association of prolonged voriconazole use with skin cancers and periostitis can be treatment-limiting toxicities in lung transplant recipients. Sparse data exists for posaconazole prophylaxis in lung transplant recipients. The risk of hepatotoxicity may be lower with this drug; however, achieving therapeutic levels in the perioperative period is challenging, particularly with the suspension formulation. Although serum posaconazole concentrations correlated with levels within the bronchoalveolar fluid in lung transplant recipients, steady-state levels were rarely reached in the newly transplanted lungs. Extended-released and IV formulations of posaconazole were approved in 2013 and 2014, respectively, and have been shown to have much more favorable pharmacokinetics parameters, although data on their use in lung transplant prophylaxis are limited. Thus the role of posaconazole as primary prophylaxis in the perioperative period is uncertain. There are no data on the use of isavuconazole for prophylaxis after lung transplantation. Immunosuppressant levels should be monitored and dosages adjusted accordingly in patients receiving azole antifungal agents ( Table 308.10 ).
| ETIOLOGY | |
|---|---|
| INFECTIOUS | NONINFECTIOUS |
Bacterial
Fungal
Viral |
Malignancies
Nonmalignant Lesions |
Therapeutic drug monitoring, although not necessary for fluconazole, should be performed for azole antifungal agents in transplant recipients if the use of these agents is anticipated for at least a week (see Table 308.6 ). Adequate plasma trough levels for the triazole antifungal agents have correlated with successful outcomes, both when used as prophylaxis and treatment of invasive fungal infections after transplantation. In addition, monitoring trough plasma levels avoids overexposure for example, voriconazole concentrations >5.5 mg/L are thought by some to predispose to visual and neurologic adverse events in transplant recipients.
Liver transplant recipients have higher rates of infection than renal or heart transplant recipients, and most deaths are associated with infection, either as a primary or a secondary cause (see Table 308.2 ). Bacterial infections occur in 59% to 68% of the patients after liver transplantation, and ≈50% of these infections occur within 2 weeks after transplantation. The most important sites of infection are the abdomen and biliary tract, the surgical wound, the lungs, and the bloodstream, with or without associated catheter infection.
Transplantation of the liver differs from other transplantation operations in the length and difficulty of the surgery and the frequency of bleeding. In addition, many liver transplant recipients have poor nutrition and severe metabolic difficulties. Surgical site infections account for most infections in the early period after liver transplantation, and in general, short-course prophylactic antibiotic regimens targeting skin and intestinal flora are favored, with national guidelines recommending perioperative administration of piperacillin-tazobactam or ampicillin plus cefotaxime for 24 hours or less. Decisions about the optimal prophylactic regimen become more complicated in individuals colonized with drug-resistant bacteria, particularly vancomycin-resistant Enterococcus (VRE). In a study, 32% of patients colonized with VRE before liver transplantation received tigecycline prophylaxis to target their VRE. Compared with VRE-colonized patients who received ampicillin-sulbactam prophylaxis, there was no difference in the frequency of microbiologically confirmed deep surgical site infections, including those caused by VRE. As such, perioperative prophylaxis based on history of VRE colonization or infection may not be routinely necessary.
The Achilles heel of liver transplantation surgery is the function of the biliary and vascular anastomoses. In consequence, most abscesses in the transplanted liver result either from liver ischemia caused by hepatic artery thrombosis or from obstruction to bile flow from biliary strictures. The organisms responsible are gram-negative enteric bacilli, enterococci, and anaerobes. The diagnosis is made by ultrasonography or computed tomography (CT). Treatment with drainage and IV antibiotics is usually successful, provided the source is biliary infection and any structural abnormalities can be corrected. If hepatic artery thrombosis is the predisposing factor, the infectious symptoms can usually be controlled with antibiotics, but retransplantation may be necessary.
Cholangitis after liver transplantation also results from technical problems. The most common predisposing problem is biliary strictures. Patients with strictures may have periodic bouts of cholangitis. Some patients improve after dilatation procedures or stent placement, but in others operative repair is necessary. It may not be easy to establish a firm diagnosis of cholangitis because many patients do not manifest the classic Charcot triad of fever, abdominal pain, and jaundice. The clinical presentation may mimic allograft rejection. The diagnosis is more reliable if bacteremia is present or if a liver biopsy indicates pericholangitis. Empirical treatment for cholangitis should include antibiotics to cover gram-negative enteric bacilli, enterococci, and anaerobes. Procedures such as liver biopsy, T-tube cholangiography, or endoscopic retrograde cholangiopancreatography may be followed by cholangitis and occasionally by bacteremia.
Peritonitis can accompany other intraabdominal infections and frequently complicates biliary leaks or disruption of an abdominal viscus. Bile peritonitis may occur after extraction of a T-tube. It is often well tolerated and may resolve by itself, but on occasion the leak persists and the chemical peritonitis becomes secondarily infected. The most common organisms involved in peritonitis are enterococci and aerobic enteric gram-negative rods, but staphylococcal and candidal infections are not infrequent. Treatment of established peritonitis requires antibiotic therapy, together with drainage of associated abscesses and repair of technical problems such as biliary leaks.
Abdominal abscesses are usually found in patients who have had frequent or lengthy abdominal operations. Nearly one-third of the abdominal abscesses are associated with bacteremia. The location is frequently subhepatic, but splenic, pericolic, and pelvic abscesses are also seen. Most patients with abscesses have undergone an abdominal operation within the preceding 30 days. Although common enteric organisms cause most abscesses, staphylococci and Candida are also seen. Imaging studies usually define the location of the abscess. As with any other abscesses, the appropriate treatment is a combination of drainage and antibiotics.
Liver transplant recipients have a high rate of fungal infections, ranging from 15% to 42% with case-fatality rate of 25% to 82%. Risk factors for invasive fungal infections include renal dysfunction, fulminant hepatic failure, longer operative time, retransplantation, hepatic iron overload, and colonization with Candida at the time of transplantation. In the era of Model for End-stage Liver Disease (MELD) scores as the basis for prioritizing liver transplantation, a MELD score ≥30 is the most influential factor for invasive fungal infections, with fungal infections occurring in 24% to 28% of these patients.
Candida is the predominant fungal pathogen, causing 62% to 88% of the invasive fungal infections in this population. Others include Aspergillus, Cryptococcus, and molds as agents of mucormycosis. Nearly one-third of candidal infections in liver transplant recipients are caused by non- albicans species. Fluconazole prophylaxis was found to be a risk factor for the emergence of non- albicans Candida spp. as pathogens in liver transplant recipients. Invasive candidiasis contributes significantly to mortality after liver transplantation. In a case-controlled study, liver transplant recipients with invasive candidiasis had a 36% mortality rate, compared with 2% in the control patients. Unfortunately, infections due to Candida can be difficult to diagnose, and the β- d -glucan assay has limited accuracy in diagnosis invasive candidiasis in liver transplant recipients.
Invasive aspergillosis occurs in 1% to 8% of liver transplant recipients with unique predisposition to disseminated aspergillosis. Historically, outcomes in transplant recipients with invasive aspergillosis have been notably poor. with mortality rates ranging from 65% to 92%. However, judicious use of immunosuppression, early recognition, and overall advances in management have led to steady improvement in outcomes, with the currently reported mortality rate of ≈22% in liver transplant recipients with invasive aspergillosis. Well-defined risk factors exist for invasive aspergillosis in most patients. These include retransplantation, fulminant hepatic failure as an indication for transplantation, renal dysfunction and requirement of any form of renal replacement therapy, and poor allograft function. Most disease now occurs more than 90 days after transplantation, a possible consequence of improved early management and delayed occurrence of risk factors, such as CMV infection.
Antifungal prophylaxis targeted toward high-risk patients is a rational approach toward the prevention of invasive fungal infections. Practice guidelines recommend fluconazole for these patients. Potential limitations of fluconazole, however, include lack of activity against Aspergillus , emergence of fluconazole-resistant Candida spp. as pathogens, and metabolic interactions with the immunosuppressive agents. Routine use of use of triazoles is limited by the risk of hepatotoxicity and erratic pharmacokinetics profile in the posttransplantation period. The echinocandins have activity for both Aspergillus and Candida spp. and low likelihood for drug interactions. Echinocandin-resistant Candida infections have been documented, albeit less frequently. Prophylaxis with these agents may be beneficial in patients who are at high risk for invasive Aspergillus or have received fluconazole before transplantation. Both micafungin and anidulafungin are well tolerated in liver transplant recipients. It is reasonable to continue prophylaxis for 14 to 21 days or until the risk factor, such as requirement for renal replacement therapy, abates.
About 1300 pancreas transplant operations are performed in the United States annually, or approximately 1 for every 10 kidney transplantations. Current patient and graft survival rates are similar to those for kidney transplantation alone, but infection-related morbidity is higher. Some studies have shown more wound complications and CMV disease among the patients receiving combined kidney-pancreas than kidney transplantation alone.
The postoperative infection rate and the causative pathogens depend primarily on the technique used for drainage of exocrine secretions of the pancreas. In recent years the practice of draining these secretions into the small bowel (enteric drainage) has gained precedence over the previous practice of drainage into the bladder. Enteric drainage has been associated with lower rates of UTI. However, intraabdominal infections remain a significant complication of enteric drainage procedures, and aerobic and anaerobic enteric bacteria predominate in abscesses associated with enteric drainage. The microorganisms in infections in which the viscus has not been opened are usually from the skin flora. Candida , however, is a common pathogen in all types of surgical site infections, including those that use bladder drainage.
Small bowel transplantation is the preferred therapy for intestinal failure and total parenteral nutrition-related complications. Small bowel transplantation is unique as the gut not only harbors a large burden of lymphoid tissue, but these cells are the least tolerogenic of any organ. In consequence, small bowel transplant recipients are particularly susceptible to graft-versus-host disease (GVHD), and managing the risk of rejection, infection, and GVHD has proven challenging. Small bowel transplantation is often combined with a liver graft due concurrent existence of intestinal failure–associated liver disease. Of note, inclusion of liver graft with intestinal graft may be immunologically protective. However, isolated intestinal transplantation is becoming increasingly more frequent, likely due to improved management of intestinal failure–associated liver dysfunction. Despite improvements in posttransplantation management, outcomes continue to be worse than those of other organ transplants.
Greater than 90% of the small bowel transplant recipients develop significant infections, and the rates of infection are higher than in other transplant groups. Intraabdominal pyogenic infections and bloodstream infections predominate, but the transplanted intestine, like the lung allograft, is very susceptible to CMV infection, including a tendency to relapse after successful antiviral treatment. Multivisceral transplant recipients and patients undergoing colonic segment transplantation with small bowel transplantation are more likely to develop infections. The susceptibility of small bowel transplant recipients to infections continues in the late posttransplantation period, that is, more than 6 months after operation. The incidence of EBV-associated lymphoproliferative disorders approaches 10% at 5 years, and the tumors often involve the gut.
Infections of the skin are common after transplantation but are rarely life threatening. All organ transplant recipients are at risk for wound infections. The most common bacterial pathogen is S. aureus , but infections with enterococci, gram-negative bacteria, Candida, and M. hominis may also be seen. Rarely, mucormycosis can cause surgical site infection in transplant recipients. Subcutaneous infections caused by Alternaria, Exophiala , and other darkly pigmented or dematiaceous fungi are encountered occasionally. Biopsy with fungal culture is required for a specific diagnosis. Cellulitis or necrotizing infections can also be due to cryptococcosis. Unlike stem cell transplant recipients, organ transplant recipients rarely develop fusariosis.
The most common cutaneous viral infections are those caused by HSV and varicella-zoster virus (VZV). Warts are a common problem, particularly more than 5 years posttransplantation.
The skin is also a target organ for many systemic infections, and bacterial, fungal, nocardial, mycobacterial, and CMV infections may include skin manifestations. Mycobacterium chelonae causes nodular lesions, often on the extremities. As a rule, one should aggressively investigate any new and/or unusual skin lesion with a biopsy.
UTIs are one of the most common causes of fever in kidney transplantation, especially in the first 6 months after transplantation. Patients with retained urinary stents are at a higher risk, resulting in a general recommendation to remove these stents within 4 weeks. These patients may not manifest “classic” features of UTIs or pyelonephritis, as normal hosts would. In addition, both allograft pyelonephritis and rejection can present with fevers, tenderness over the graft, and acute kidney injury. Allograft pyelonephritis, especially in the first 3 months, has been linked to long-term kidney graft dysfunction but does not affect graft survival at 5 years.
In patients with signs of severe infection, the choice of empirical antibiotics should take into account the history of resistant organisms as well as local epidemiologic data. Oral step-down regimens are reasonable, as long as the patient is improving. Imaging with renal ultrasound or noncontrast CT scanning should be considered to assess for complications, such as obstruction and abscesses. It is also important to consider infections caused by unusual pathogens, such as M. hominis , M. tuberculosis, or BK virus. However, BK virus may cause allograft infection but not usually fever or systemic manifestations.
Bacteremia occurs in 5% to 10% of kidney and heart transplant recipients, with higher rates (10%–25%) in liver and lung transplant recipients (see Table 308.8 ). Besides catheter-related infections, pneumonia in heart and heart-lung transplant recipients, UTIs and perinephric sources in renal transplant recipients, abdominal and biliary infections in liver transplant recipients, and surgical site and UTIs in pancreatic transplant recipients are the most commonly identifiable sources of bacteremia. Predominant gram-negative bacteria are Escherichia coli, Klebsiella spp. , and others, including extended-spectrum β-lactamase–producing and carbapenem-resistant organisms, Acinetobacter baumanii, and P. aeruginosa . The highest incidence of gram-negative bloodstream infections has been observed within the first month after transplantation (210.3/1000 person-years), with a sharp decline to 25.7 per 1000 person-years from 2 to 12 months posttransplantation. Kidney recipients are more likely to develop gram-negative bacteremia 12 months after transplantation.
In kidney transplant recipients the source of bacteremia is usually the urinary tract, due to disruption of the urinary tract during surgery, the lack of a valve between the bladder and transplanted ureter, and the presence of an indwelling stent in the early posttransplantation period. Bloodstream infections are common after liver transplantation due to the immunosuppression of pretransplant cirrhosis and the prolonged operation time. Risk factors after liver transplantation include diabetes mellitus, urinary and vascular catheterization, posttransplantation renal replacement therapy, massive blood loss and transfusions, reoperation, and bile duct complications. In a study of 704 liver transplant recipients over a 10-year period, 39.4% of bloodstream infections occurred in the immediate postoperative period (<10 days). The most frequent pathogens were Enterobacteriaceae (41%), Staphylococcus aureus (19.8%), enterococci (13.1%), P. aeruginosa (8.8%), and Candida (7.1%). Bloodstream infections occurring early after transplantation were associated with increased 1-year mortality.
In lung transplant recipients with CF the colonizing bacteria are important causes of bloodstream infections, in particular B. cepacia , which can lead to the cepacia syndrome, with progressive necrotizing pneumonia, persistent bacteremia, and death. Bloodstream infections in heart, pancreas, and intestinal transplant recipients also occur and have been linked to intravascular catheters, abdominal leaks, and UTIs.
Bacteremia due to S. aureus occurs with estimated rates of 15% to 38% in various organ transplant recipients. The rates of MRSA have increased over time. Among organ transplant recipients, lung recipients have the highest incidence and attack rate. Most common sources are vascular catheters, pneumonia, or wound infections. Nearly one-half of all S. aureus bacteremias occur within 90 days of transplantation; early-onset bacteremias are more likely to occur after liver transplantation, likely due to the large size of the incision or colonization during candidacy. The unadjusted 28-day all-cause mortality after gram-negative bloodstream infections in transplant recipients was 4.9%. Kidney recipients had lower mortality than liver transplant recipients.
The basic approach to bacteremia is the same whether or not the patient has undergone transplantation and includes ascertaining the source of the bacteremia and likely pathogen. The inability to discern a source is relatively common, especially in liver transplant recipients; up to 21% of the bacteremias in these patients may have had no documented source, but most of these probably originated in the abdomen. The trend toward increasing antimicrobial resistance among gram-negative bloodstream isolates has an impact on local decisions regarding empirical antibiotic therapy of transplant patients who present with possible bloodstream infections.
The usual microbial causes of pneumonia in the transplant recipient are listed in Table 308.9 . Pneumonia accounts for up to 80% of infections in solid-organ transplant (SOT) recipients and can occur at any time in the posttransplantation period. The etiology varies by transplant type and time of onset after transplant, with nosocomial pathogens predominating in the early posttransplantation period and opportunistic and community-acquired pathogens occurring later. Guidelines for the treatment of community-acquired and health care–associated pneumonia have been published elsewhere. Knowledge of pretransplant colonizers, particularly for lung transplant recipients with CF; pretransplantation serologies, for example CMV, recent exposures; hospitalizations; and prior antimicrobial courses should be part of the evaluation. Community-acquired respiratory viruses, such as influenza virus, PIV, RSV, MPV, coronavirus, rhinovirus, or adenovirus, may cause pneumonia in transplant recipients.
Opportunistic causes of pneumonia can occur at any time after the first month, although the net state of immunosuppression determines the ongoing risk of opportunistic pathogens. For instance, transplant recipients with stable doses of immunosuppression many years after transplantation would be considered at low risk for pulmonary infections due opportunistic pathogens, whereas those whose immunosuppression has been augmented for the treatment of rejection would be considered at higher risk. In the latter group a thorough evaluation for fungal infections (endemic mycoses in cases of compatible epidemiology, invasive mold disease, cryptococcosis), nocardiosis, mycobacterial, P. jirovecii, Rhodococcus equii , and other pathogens should be undertaken. In cases where opportunistic infections are suspected, the appearance of the radiographic abnormalities may assist in informing both diagnostic and therapeutic decisions, with the caveat that there is substantial overlap between different entities. Nodular infiltrates have a broad range of infectious etiologies (see Table 308.10 ), and certain clinical and radiographic features may be helpful in discerning a specific etiology. In one study, for example, pulmonary nodules were found to have diverse etiologies and were infectious in 56% of patients (bacterial, 22%; fungal, 33%; viral, 2%) and noninfectious in 44% (PTLD, 25%; carcinoma, 18%).
Acute rejection in lung transplant recipients can mimic pulmonary infections. Thus early bronchoscopy, with appropriate microbiologic testing and culture and nonculture diagnostics of the blood, are crucial in establishing the diagnosis when the patient fails to respond to presumably appropriate antimicrobial therapy or when the suspicion for opportunistic pathogens is high. Whether empirical therapy directed against opportunistic pathogens is warranted should be considered on a case-by-case basis; indeed, it is often difficult to rationalize or justify a regimen that will treat all possible etiologies, underscoring the importance of a thorough microbiologic investigation.
Empirical therapy to cover common typical and atypical bacterial pathogens can be initiated while culture results are awaited. Patients who have an illness lasting longer than 7 days, who have a nonproductive cough, or who have diffuse infiltrates or nodular lesions on chest imaging are more likely to have an unusual or opportunistic pathogen and should undergo invasive diagnostic procedures, including bronchoscopy, in a timely manner to establish the diagnosis.
M. tuberculosis is a major pulmonary pathogen, particularly in geographic areas where TB is endemic. The frequency of TB in transplant recipients ranges from 1.2% to 15%, with higher rates in endemic areas. Most cases of posttransplantation TB are due to reactivation of latent infection in low endemicity areas. Prior exposure to M. tuberculosis (positive tuberculin skin test [TST], positive interferon-γ [IFN-γ] release assay [IGRA], and/or residual TB lesions in pretransplantation chest imaging), dialysis, and T-cell–depleting antilymphocyte antibodies confer a higher risk for TB. IL-2 receptor antibodies (basiliximab), on the other hand, do not appear to increase this risk. Although most transplant recipients develop TB in the first year after transplantation, up to one-third of the patients, particularly kidney transplant recipients may develop TB later in the posttransplant period. Extrapulmonary or disseminated forms of disease are more frequent in the first 6 months after transplantation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here