Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Risk factors for infection in older adults are numerous and often coexist in complex relationships. Seemingly similar patients by conventional criteria may have very different risk due to measures not often used in young adults. These include comorbid illness, polypharmacy, functional status (physical, cognitive, sensory), place of residence, and individual variations in physiologic changes that accompany age (e.g., declining glomerular filtration rate, reduced gag/cough reflexes).
The most important cofactor for infection in older adults is the accumulation of comorbid disease with age. Many infectious diseases are a direct or indirect result of chronic comorbid conditions (e.g., diabetes mellitus, renal failure, chronic pulmonary disease, edema, immobility). These comorbidities most often result in reduced local innate immunity. For example, chronic obstructive pulmonary disease (COPD) is associated with impaired mucociliary clearance, alveolar macrophage dysfunction, and suppressed cough mechanism, substantially increasing the risk for lower respiratory tract infection in older adults with COPD.
Comorbid diseases in older adults with infection can also be important predictors for worse outcomes—more important than age itself. In patients with community-acquired pneumonia (CAP), multiple comorbid conditions and advanced age greatly increase the risk of mortality. Age itself dominates many CAP prognostic scoring algorithms, but age alone is not a strong predictor of mortality once patients exceed 75 years of age; in patients at the extreme of older age, comorbidity dominates, not age itself. Furthermore, cognitive decline and other barriers that delay diagnosis or reduce adherence to medical regimens often necessitate hospitalization of older adults in circumstances where their younger counterparts can be treated as outpatients, further increasing costs and enhancing the rate of complications.
Although comorbidities substantially predispose older adults to infection, there is an underlying waning of immune responses that accompany old age even in the absence of comorbidity; this is called immune senescence. Immune senescence is not merely a global state of reduced immunity but a dysregulation of immune responses at multiple levels. A complete review of immune senescence is beyond the scope of this chapter, but both innate and adaptive responses are significantly dysregulated.
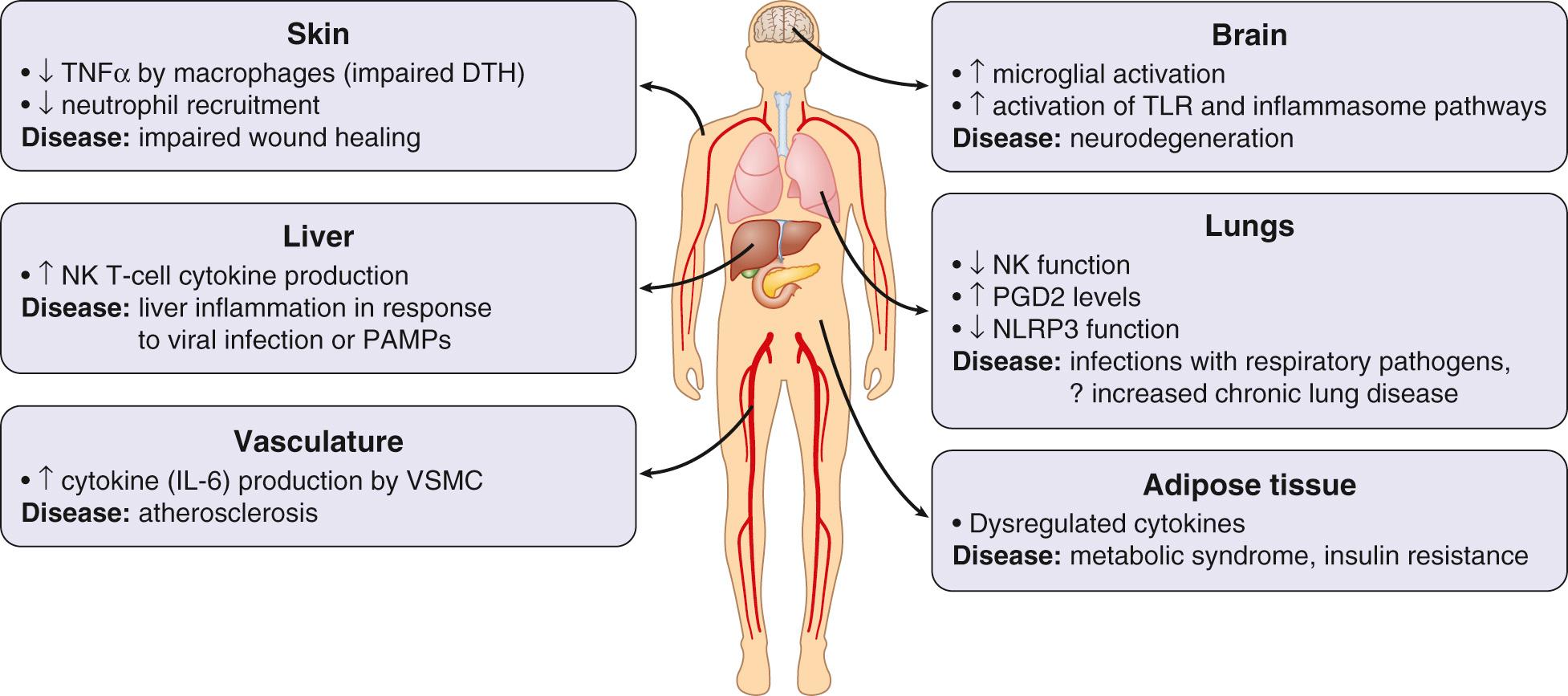
Innate immune responses are increased in some respects, but decreased in others. Critical innate immune components that decline with age include physical barriers such as skin integrity, cough/gag reflex, mucociliary clearance, and gastric acid. Innate immune responses are also dysregulated at the cellular level, with impaired polymorphonuclear neutrophil function and dysregulation of inflammatory responses triggered by pathogen-associated molecular patterns via Toll-like receptors ( Fig. 310.1 ). Despite impaired stimulus-triggered responses, there is often a chronic, low-level inflammation present in older adults. The cause(s) of this low-level inflammation are not well elucidated, but it likely plays a role in chronic disease development (e.g., vascular inflammation) and inhibitory mechanisms engaged to keep this low-level inflammation in check may be a cause, in part, of the slower innate response to infection seen in older adults.
In the adaptive immune system there are decreases in naïve T-cell subsets, with accumulation of memory T cells, substantially reduced diversity of the overall T-cell pool, and impaired responses to specific antigen ( Fig. 310.2 ).
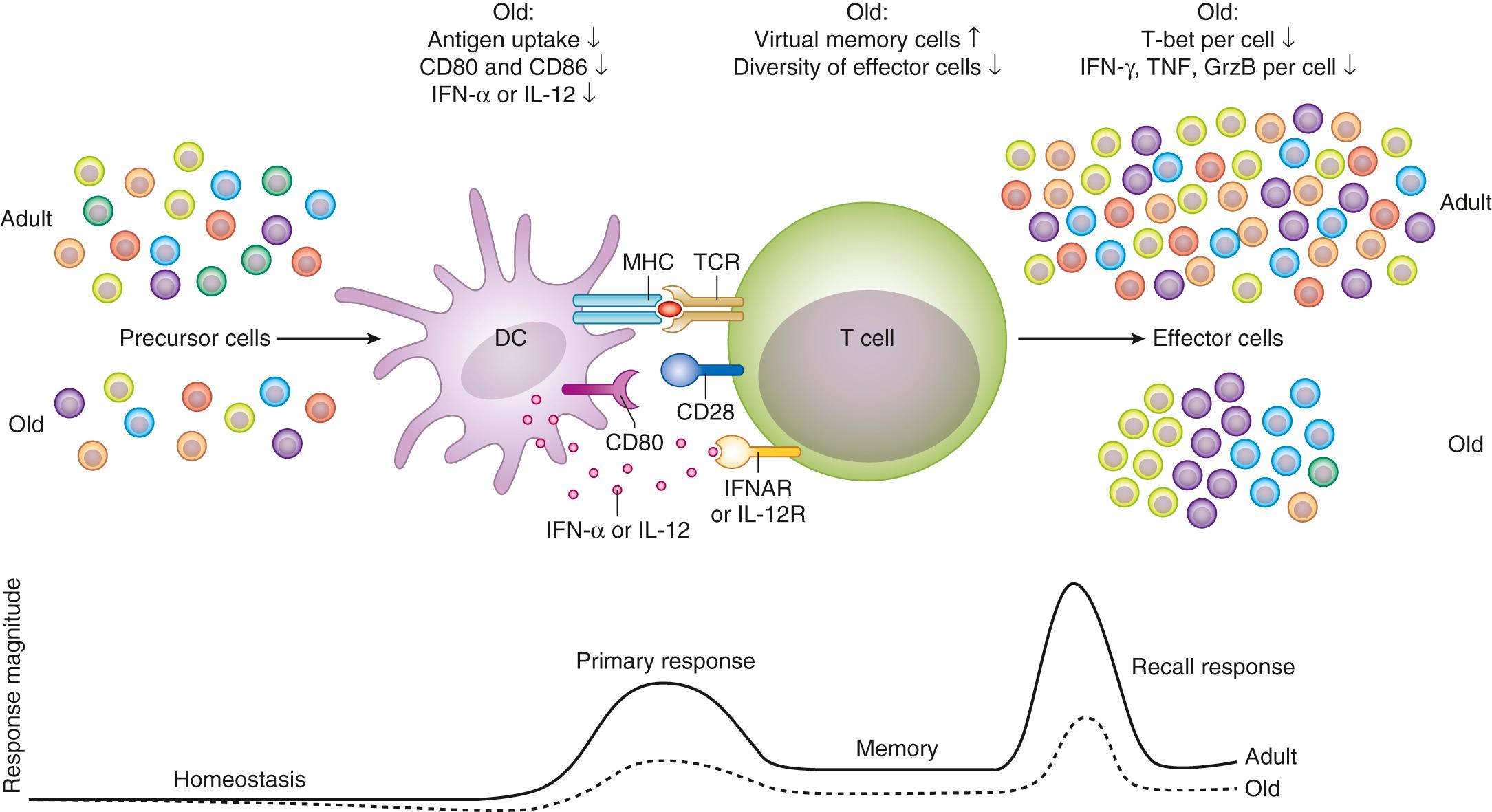
Although there is little doubt that immune senescence exists, the clinical role of this phenomenon in the predisposition of older adults to clinical infection is poorly defined. Immune senescence markers have been extensively studied in vaccine responses measured most often by antibody titers and other surrogate markers, but only a few studies have been powered to assess clinical outcomes. It is quite clear that age itself is associated with reduced responses to vaccines as early as the third or fourth decade (human papilloma virus, hepatitis B), but the deficit grows throughout adulthood such that efficacy of influenza and pneumococcal vaccines is questionable by the eighth and ninth decades. Specific vaccine formulations, very different from those used to boost early childhood responses, have been developed to try to overcome immune senescence; these include greater antigen concentration (e.g., high-dose influenza vaccine) or a mixed-action adjuvants (e.g., zoster subunit vaccine) and appear to substantially mitigate immune senescence, not only enhancing postvaccine markers of immunity but reducing clinical disease risk.
Protein-energy malnutrition (PEM) is common in older adults; 30% to 60% of subjects ≥65 years of age admitted to the hospital have PEM, which is linked to delayed wound healing, pressure ulcer formation, risk of CAP and nosocomial infection, longer hospital stays, and increased mortality. PEM often goes unrecognized outside the hospital, but even mild PEM (i.e., seniors with a serum albumin of 3.0–3.5 g/dL) demonstrate reduced vaccine responses. Despite this, the role of high-protein/high-calorie nutritional supplements for preventing infection or boosting immune responses remains controversial and largely an open question except for those with specific indications (e.g., wound healing).
Specific micronutrient deficiencies in older adults have also been linked to poor immune function (e.g., vitamin B 12 deficiency and inadequate pneumococcal vaccine responses) and risk of infection (e.g., vitamin D deficiency linked to risk of tuberculosis [TB] and Clostridioides difficile [formerly Clostridium difficile ] infection). Nutritional supplements have been studied as a means to reduce infection risk and boost immunity in older adults, and all experts agree that if true deficiency exists it should be corrected. However, in otherwise healthy adults with normal or “insufficient” (but not deficient) vitamin levels the answer is less clear. Although some data support the use of multivitamins, zinc, selenium, vitamin E (in low-modest doses), and vitamin D, inconsistent results have been achieved. More work is required to define the role of vitamin or mineral supplementation for augmenting immune response in this population.
Additional “social determinants of health” combine to influence infection risk in seniors. Population-based studies reveal that lower income is associated with higher rates of CAP and invasive pneumococcal infections among older adults. Lower socioeconomic status (SES) may predispose to infection due reduced access to care, which is well documented in seniors, but low SES is also associated with increased exposure to infectious agents (e.g., grandparents raising young children more often), poor nutrition, and increased risk of comorbid disease (e.g., asthma and exacerbations due to pollution/tobacco exposure).
Environment plays a distinct role in long-term care (i.e., nursing home) residents, who have a particularly high incidence of respiratory, genitourinary (GU), gastrointestinal (GI), and skin infections, and unique infection control challenges exist in the long-term care setting. Close contact between residents and staff plays a key role in the spread of respiratory infections (e.g., influenza, respiratory syncytial virus [RSV]), or infections transmitted by contact (e.g., Streptococcus pyogenes, C. difficile ). Many frail older adults in a confined setting can lead to severe outbreaks with high mortality rates; proximity of residents, poor adherence to basic infection control measures, and the intense use of antibiotics also increase the risk of antibiotic-resistant bacteria in this setting.
Infection, even serious life-threatening infection, frequently presents with atypical features in older adults. Serious infections may be signaled by seemingly trivial, nonspecific declines in function or mentation and underlying illness (e.g., congestive heart failure or diabetes mellitus) may be exacerbated by infection, leading older adult patients to seek medical attention for symptoms related to comorbidity rather than infection. The most fundamental sign of infection, fever, is absent in up to one-third of older adults with serious infection. Several studies show that frail older adults have lower mean baseline body temperatures than the currently accepted normal of 98.6°F (37°C). Further, temperature increases in response to pyrogens are diminished with advanced age. The decline in basal temperature and blunted response to pyrogens make it more likely that an older adult will have a body temperature within the “normal” range despite infection, and a normal temperature with significant infection often leads to delayed diagnosis and treatment.
Cognitive impairment may also contribute to the difficulty of diagnosing infection in older adults, with patients unable to communicate symptoms. This can lead to overdiagnosis, as well when colonization (e.g., asymptomatic bacteriuria) is often assumed to be the cause of nonspecific symptoms. Clinicians often have a lower threshold to pursue objective assessments (e.g., laboratory and radiologic evaluations) for infection in cognitively impaired older adults, given the difficulty interpreting subtle function changes and the absence of classic signs of infection noted above. Although a high index of suspicion is warranted, clinicians should be cautioned not to overevaluate and to interpret results carefully. The poor usefulness of culture data in some situations (e.g., swab cultures of skin surface wounds or urine cultures when catheters are present) in which positive cultures are a certainty can lead to overdiagnosis and over treatment (see later).
Changes in anatomy and physiology due to age and/or comorbidity may confound interpretation of diagnostic evaluations as well. For example, there is diminished sensitivity of transthoracic echocardiography (TTE) for detecting valvular vegetations in endocarditis in older adults due to calcification and other anatomic changes associated with age (summarized in reference ). Studies suggest the sensitivity of TTE is 85% to 90% in adults age ≤55 years but is reduced to <50% for those older than 70 years. More frequent use of transesophageal echocardiogram (TEE) is therefore needed in seniors, where sensitivity returns to 85% to 90% and specificity is not reduced.
Age and comorbidity change drug distribution, metabolism, excretion, and interactions. Antibiotic dose reductions are occasionally required in the older adults due to reduced renal function or predisposition to specific side effects, but the most prevalent complications include drug interactions, which are more frequent because older adults more commonly take multiple medications. Digoxin, warfarin, oral hypoglycemic agents, theophylline, antacids, and H2-receptor antagonists are commonly administered and have significant interactions with commonly prescribed antibiotics.
The changes in physiology with age often lead clinicians to the dictum of “start low, go slow” when administering new medications in older adults. However, for antibiotics this is not an appropriate strategy. Data suggest higher antibiotic levels are particularly important for efficacy in older adults—much more important than in young adults who often have more robust immune responses and greater physiologic reserve. In older adults, outcomes of serious infection are more dependent on reaching adequate drug levels earlier than in young adults. This is especially true for antibiotics with a concentration-dependent mechanism of action (e.g., fluoroquinolones). Further, slowed gastric motility, decreased absorption, increased adipose tissue, and coadministration of other drugs can decrease blood levels of antimicrobials in older adults, and of course antibiotics reach tissues via blood flow, so poor perfusion to the site of infection, particularly in skin and soft tissue infections of the lower extremities, may reduce efficacy. Adherence may be limited by poor cognitive function, inadequate understanding of the drug regimen, impaired hearing or vision, and polypharmacy, and studies suggest that any regimen requiring greater than twice-daily dosing is associated with very poor adherence rates.
Outpatient parenteral antibiotic therapy (OPAT) is commonly used for syndromes that occur more frequently in older adults (e.g., endocarditis or osteomyelitis) but has been underused in older adults, often due to a lack of Medicare coverage in the past, and older adults were often admitted to nursing homes just to obtain appropriate therapy. With the institution of Medicare Part D, OPAT is now covered, although navigating the system to obtain reimbursement for both the antibiotic and the necessary supplies for administration takes considerable skill. OPAT has been shown to be safe and effective therapy in seniors that have adequate support and monitoring.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here