Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
For in vitro fertilization (IVF) with and without intracytoplasmic sperm injection, the delivery rate per cycle in which ova are retrieved is as high as 50%, depending on the age of the woman. The rate of pregnancy after IVF is directly related to the number of embryos placed in the uterine cavity.
Strict guidelines set forth by American Society for Reproductive Medicine (ASRM), which limit the number of embryos transferred, has reduced the rate of high-order and twin multiple pregnancies in the United States.
Preimplantation genetic testing of embryos for aneuploidy (PGT-A) or monogenetic disease before embryo transfer may help improve pregnancy rates, especially in older women. When PGT-A is performed, a single euploid embryo should be transferred at a time.
Oocyte and embryo cryopreservation gives women the possibility of maintaining their future fertility potential even when faced with the possibility of premature menopause as a result of gonadotoxic medical treatments. Though still experimental, ovarian tissue freezing is another possible method of fertility preservation. Oocyte cryopreservation is available for healthy females who are interested in elective fertility preservation, but they need to be carefully counseled about the low pregnancy rates associated with cryopreserved oocytes.
IVF technology has helped to expand research into stem cell therapy, which may further improve treatments for infertility and other medical conditions in the near future.
Because of the central importance of in vitro fertilization (IVF) in the treatment of infertility and its potential to answer fundamental questions in reproductive biology, a separate chapter has been devoted to this topic. IVF is often used interchangeably with the term assisted reproductive technology (ART), which the American Society for Reproductive Medicine (ASRM) has defined as the manipulation of sperm and egg outside the body; however, some clinicians include ovarian stimulation cycles with the use of intrauterine insemination (IUI) in the definition of ART.
In 2010, Robert Edwards was awarded the Nobel Prize for physiology and medicine for his pioneering work in making IVF a reality ( ). IVF has revolutionized the field of reproduction. Not only has it opened many avenues of research and broadened our understanding of basic human reproductive physiology, but its successful clinical use has allowed millions of couples to conceive who might otherwise have been unable to do so. IVF has provided the ability to diagnose significant genetic defects before implantation and has led to the possibility of embryonic stem cell research. IVF has also opened possibilities for fertility preservation ( ). Reproductive-age patients newly diagnosed with cancer can undergo ART to cryopreserve oocytes or embryos before embarking on their cancer therapy. Healthy reproductive-age women not yet ready to conceive but concerned about the normal age effect on their fecundity may pursue elective fertility preservation. Indications for IVF are listed in Box 41.1 .
Blocked or absent fallopian tubes
Low sperm counts or absent sperm (azoospermia requiring TESE)
Advanced reproductive age
Endometriosis
Unexplained infertility unresponsive to IUI therapy
Screening for aneuploid embryos and/or genetic disease
Fertility preservation
IVF was originally developed primary for women with tubal disease. This is still a major indication for IVF, and with the success of IVF, reconstructive tubal surgery is much less commonly performed, in favor of the more direct approach of IVF. As discussed in Chapter 40 , a significantly dilated tube (hydrosalpinx) on ultrasound is an indication for salpingectomy before IVF because implantation rates are reduced in the presence of a communicating hydrosalpinx. Endometriosis is also a major indication for IVF, likely because of its impact on tubal function and oocyte pick up.
In the past, if there were severe abnormalities in the semen analysis, such as severe oligozoospermia (low sperm concentration), the prognosis for fertility was less than that for any other cause of infertility, even with the use of IVF. Attempts to enhance fertilization rates of aspirated oocytes with the technique of subzonal insemination of sperm were unsuccessful because fertilization rates remained low at approximately 15%. After Van Steirteghem and associates developed the technique of intracytoplasmic sperm injection (ICSI) ( ), fertilization rates of oocytes injected with a single normal sperm obtained from men with severe abnormalities in their semen analysis increased to more than 50%. Pregnancy rates per embryo transfer are now similar after ICSI compared with other indications for IVF . Fertilization rates of approximately 60% of the oocytes injected may be achieved with sperm from semen samples containing no motile sperm, few motile sperm, and high numbers of motile sperm ( ). In addition, a fertilization rate of approximately 60% was attained whether the sperm were freshly obtained by masturbation or electroejaculation or were previously frozen. A fertilization rate of oocytes of almost 50% was also achieved when the sperm were aspirated directly from the epididymal fluid. In routine clinical use, a fertilization rate of greater than 80% can be achieved.
The excellent results obtained with ICSI by the group that originally described the technique have been replicated in other centers. By using this technique, the pregnancy rate of couples whose male partner has an extremely low concentration of motile sperm in semen samples (<100,000/mL) can reach a normal pregnancy rate for IVF, based on the age of the woman (discussed later). Studies of pregnancies resulting from ICSI and standard IVF have revealed a similar rate of pregnancy loss and multiple gestation . Therefore ICSI is now the treatment of choice for all causes of male infertility, as well as for couples with no known cause of infertility for whom fertilization has failed with standard IVF insemination procedures. At present, ICSI is used in 60% to 70% of all cases of IVF. The number of ICSI cases has increased because ICSI has been used even for minor sperm abnormalities and in other cases such as unexplained infertility and in older women where there is a concern about the rate of fertilization ( Fig. 41.1 ). (Also see the video of an ICSI procedure [Video 41.1].)
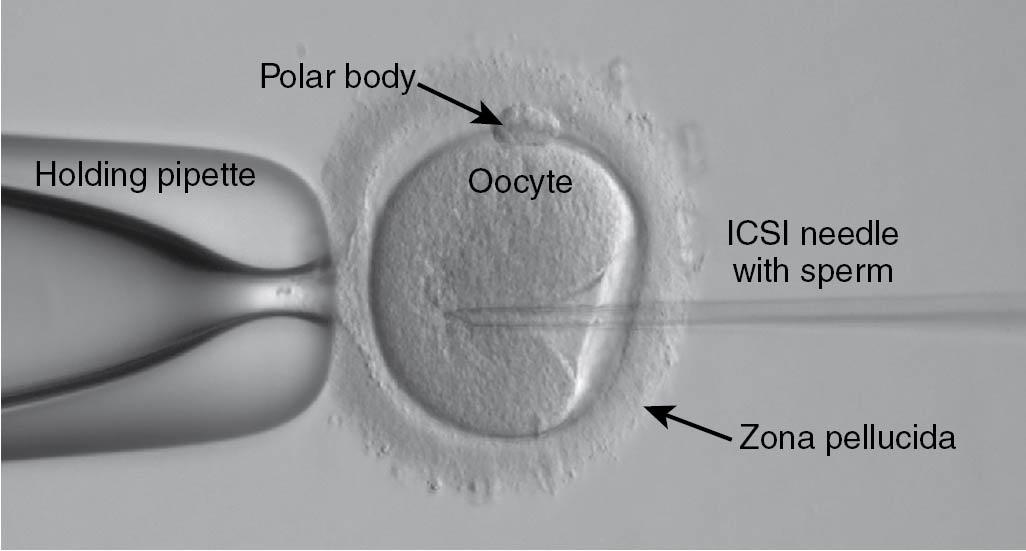
The clinical pregnancy rate is higher when testicular or epididymal sperm is retrieved from men with obstruction of the vas deferens (obstructive azoospermia) than when testicular sperm is retrieved from men with azoospermia without reproductive tract obstruction (nonobstructive azoospermia) ( ). Even if the sperm retrieved from the testes remain immotile, the pregnancy rate after ICSI is acceptable at 15% or higher. The likelihood of retrieval of spermatozoa from testicular tissue of men with azoospermia and normal follicle-stimulating hormone (FSH) levels is almost 100%. Even if the man’s FSH levels are markedly elevated, there is at least a 50% likelihood that spermatozoa can be retrieved from the testes and used to perform an ICSI procedure ( ). Thus the presence of a combination of azoospermia and an elevated FSH level is not a contraindication for performing a testicular sperm extraction (TESE) procedure, although it is advantageous to carry out a diagnostic TESE before an IVF cycle. Diligence is required with the aid of microscopy to select the best sperm for use in ICSI, and several groups have advocated a surgical microdissection of the testicular tubules to obtain viable sperm ( ). Microdeletions in the azoospermia factor (AZF) region of the Y chromosome have been linked to nonobstructive azoospermia. Sperm has been successfully retrieved from men with AZFb and c microdeletions but not with the AZFa microdeletion, and extraction is not recommended in these cases.
Patients with unexplained infertility may consider IVF treatment particularly if they fail to conceive after a few treatments with controlled ovarian stimulation and IUI. In fact, women of advanced reproductive age and infertility may be advised to consider moving directly to IVF, bypassing IUI therapy altogether ( ).
A large prospective trial was carried out by Reindollar and colleagues to assess whether it was reasonable to skip gonadotropin and timed IUI therapy and proceed to IVF after three cycles of clomiphene-IUI (called “fast track”) in women with unexplained infertility ( ). The logical next step after clomiphene or gonadotropin-IUI therapy is still IVF . One of the concerns with unexplained infertility is that there may be failure to fertilize, even if there are normal ovulation and semen characteristics. IVF also has a higher cycle fecundity rate than IUI. In the prospective trial, which also included a cost analysis, conventional therapy included 3 months of clomiphene-IUI followed by three cycles of gonadotropin-IUI and then up to six cycles of IVF. The other arm of this randomized trial omitted the gonadotropin-IUI step and proceeded directly to IVF after clomiphene-IUI ( Fig. 41.2 ).
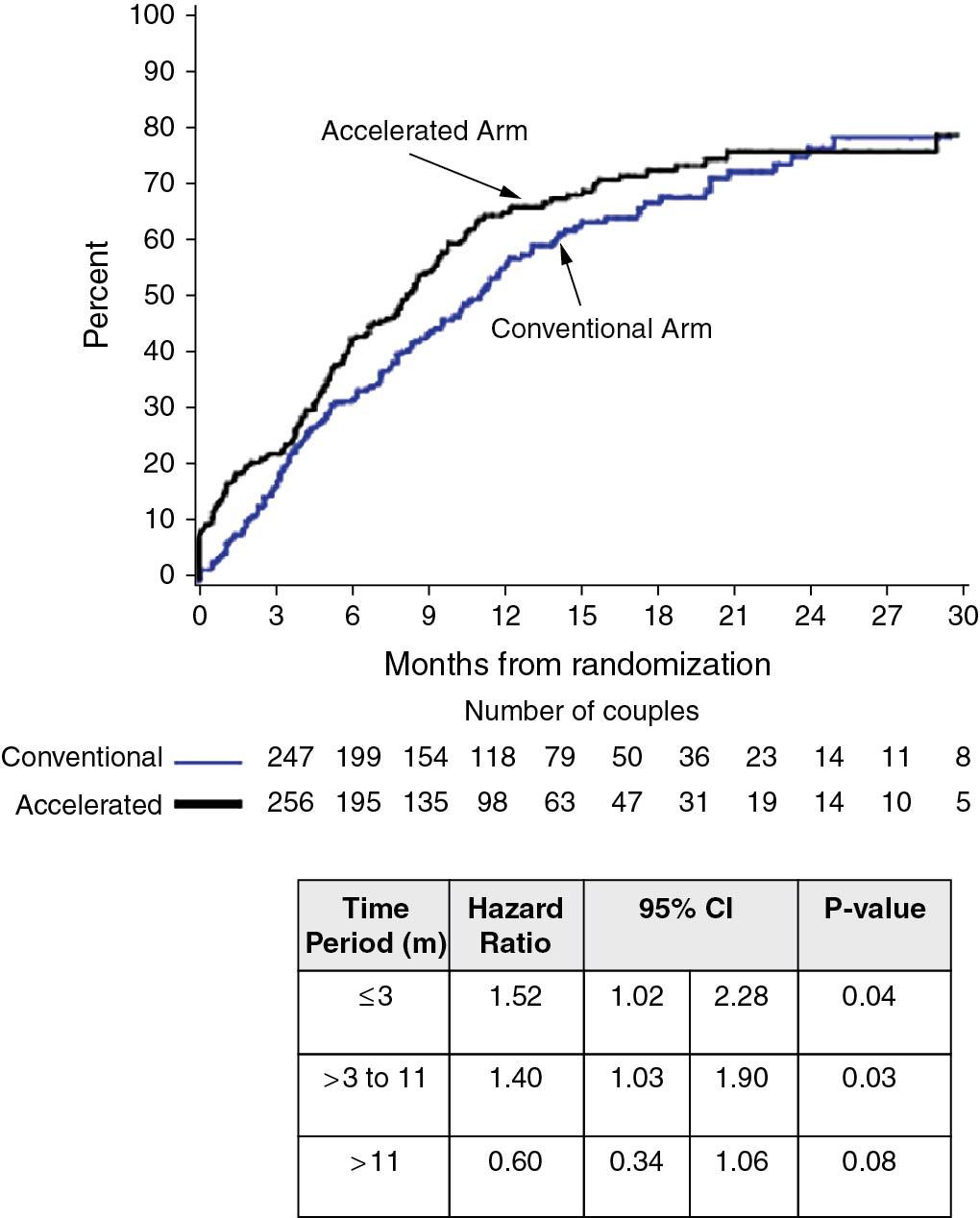
As shown in Fig. 41.2 , an increased pregnancy rate was reported in the accelerated arm (100% to 156%; hazard ratio, 1.25). The median time to pregnancy was also shorter, 8 versus 11 months, and average charges per delivery were $9800 lower. Individual per cycle pregnancy rates for clomiphene-IUI, gonadotropin-IUI, and IVF were 7.6%, 9.8%, and 30%, respectively. The authors concluded that the gonadotropin-IUI step in the usual algorithm for unexplained infertility may be omitted; however, this remains an area of controversy and may not be applicable to couples who do not have insurance coverage for IVF, unlike in Massachusetts (where the study was carried out) where IVF coverage is mandated.
The extension of this study (the Forty and Over Treatment Trial [FORT-T]) in older women, 38 to 42 years, strongly suggested that infertile women in this age category should consider going directly to IVF ( ). In this study, patients who proceeded directly to IVF achieved significantly higher clinical pregnancy rates compared with patients who tried two cycles of IUI treatment (with either clomiphene citrate or gonadotropin therapy). Additionally, the ratio of treatment cycles to live birth rates was significantly lower in patients who went straight to IVF versus patients who started with conservative treatment ( Table 41.1 ).
| First Two Treatment Cycles | Duration of Study | ||||
|---|---|---|---|---|---|
| Randomized Treatment Arm | No. of Couples (%) | No. of Clinical Pregnancies * , † (%, 97.5% CI) | No. of Live Births ‡ (%, 97.5% CI) | No. of Clinical Pregnancies § (%, 97.5% CI) | No. of Live Births § (%, 97.5% CI) |
| CC/IUI | 51 (33.1) | 11 (21.6, 10.2-37.3) | 8 (15.7, 6.2-30.5) | 38 (74.5, 58.4-86.9) | 25 (49.0, 32.9-65.2) |
| Gonadotropin (FSH)/IUI | 52 (33.8) | 9 (17.3, 7.3-32.2) | 7 (13.5, 4.9-27.6) | 34 (65.4, 49.0-79.5) | 22 (42.3, 27.1-58.7) |
| Immediate IVF | 51 (33.1) | 25 (49.0, 32.9-65.2) | 16 (31.4, 17.7-47.9) | 38 (74.5, 58.4-86.9) | 24 (47.1, 31.2-63.4) |
| Total ¶ | 154 | 45 (29.2, 21.3-38.2) | 31 (20.1, 13.4-28.4) | 110 (71.4, 62.5-79.3) | 71 (46.1, 37.0-55.4) |
* Number of clinical pregnancies includes all ultrasound confirmed pregnancies, including pregnancy losses.
† For clinical pregnancy rate after first two treatment cycles. P = .0067 for comparison between CC/IUI and immediate IVF; P = .0007 for comparison between FSH/IUI and immediate IVF.
‡ For live-birth rate after first two treatment cycles. P = .101 for comparison between CC/IUI and immediate IVF; P = .035 for comparison between FSH/IUI and immediate IVF.
§ For clinical pregnancy and live-birth rates after all treatment, there are no statistically significant differences, reflecting subsequent IVF, treatment in all arms.
¶ Of these, there were 5, 2, and 4 clinical pregnancies and 5, 1, and 3 live births in the CC/IUI, FSH/IUI and immediate IVF arms, respectively, that occurred before treatment was initiated or between treatment cycles one and two. Over the duration of the study there were 11, 3, and 9 clinical pregnancies and 7, 1, and 6 live births in the CC/IUI, FSH, IUI, and immediate IVF arms, respectively, that occurred outside of treatment cycles.
Table 41.2 shows the results of a large retrospective study of the success of clomiphene-IUI ( ). Because of the age-related decline in clomiphene-IUI pregnancy rates (after age 42, cumulative pregnancy rates over 3 to 9 months are approximately 1.8%), couples in which the female partner is older should consider going directly to IVF. This recommendation is particularly suited for women age 40 or older, those with a borderline elevated day 2 to day 3 FSH level, and women with decreased antral follicle counts and antimüllerian hormone levels.
| Age of Woman (years) | Pregnancy Rate per Cycle (%) |
| <35 | ≈10-11.5 |
| 35-37 | ≈ 8.2-9.2 |
| 38-40 | ≈ 6.5-7.3 |
| 40-41 | ≈ 3.6-4.3 |
| >42 † | ≈ 0.8-1.0 |
* Large cohort of 4100 cycles of CC IUI.
† In women older than 42 years, cumulative pregnancy rates = 1.8% (1 in 55).
Oocyte and embryo cryopreservation has been growing as indication for pursuing ART procedures. Not only is it an important method to preserve fertility in young women with cancer about to undergo chemotherapy , but increasingly it is being used in healthy young women who electively wish to delay childbearing with the knowledge of the detrimental effects of aging on reproductive capacity . When thawing previously frozen oocytes, ICSI is recommended because of premature hardening of the zona pellucida. If the woman has a male partner and either is married or is in a stable relationship, use of sperm to fertilize the oocytes and then freeze embryos rather than oocytes is more successful in terms of ultimate pregnancy rates and therefore remains an option . This topic is discussed in more detail later in the chapter.
Another indication for IVF is to screen embryos for aneuploidy or genetic disease (see Preimplantation Genetic Testing later in the chapter). Couples suffering recurrent aneuploid pregnancy loss (as a result of a balanced translocation in a partner, for example) may consider IVF with preimplantation genetic testing for aneuploidy (PGT-A) as a means of screening their embryos before conceiving again and potentially suffering another miscarriage; however, the studies have not been able to demonstrate a statistical benefit, in part because of a relatively high rate of success with natural conception after two to three losses ( ). The cost efficacy of this approach has also been questions. This approach has also been increasingly used for couples with prior IVF failure or advanced reproductive age and to optimize selection for elective single embryo transfer .
Couples who are found to carry mutations in life-threatening diseases (e.g., cystic fibrosis, Tay-Sachs disease) may opt to do IVF with preimplantation genetic testing for monogenic diseases (PGT-M) to eliminate the transmission of these diseases by excluding affected embryos. Specific probes are developed for each couple to improve accuracy by using linked markers on the chromosome with the mutation. Although controversial for ethical reasons, couples may also opt to undergo IVF/PGT for sex selection or “family balancing.”
The goal of a successful IVF treatment is the generation of good-quality embryos capable of implanting and resulting in a live birth. IVF can be carried out in a natural cycle, which was the original approach used by Steptoe and Edwards that led to the birth of Louise Brown; however, in this case usually only one oocyte and one embryo can be expected, and the success rate is far lower than with conventional IVF, in which gonadotropins are administered to increase the number of oocytes retrieved. Although unstimulated IVF remains an option on an individual basis, its use is limited because of the lower pregnancy rates and the expectations of couples to become pregnant as efficiently as possible. Improvement in embryo cryopreservation has provided more opportunities for pregnancy from supernumerary frozen embryos.
With conventional IVF, gonadotropin stimulation with recombinant FSH and/or purified human menopausal gonadotropin (HMG) is administered, usually subcutaneously, for approximately 8 to 10 days. The starting daily dose typically ranges from 150 to 450 IU of FSH and/or HMG. HMG preparations contain both 75 IU of FSH and 75 IU of luteinizing hormone (LH) in the form of human chorionic gonadotropin (HCG). The starting dose is chiefly determined by the patient’s ovarian reserve and age, with higher doses used for older patients with decreased ovarian reserve and lower doses for patients with a more robust reserve and for women with polycystic ovary syndrome (PCOS). The traditional long cycle protocol begins with downregulation using a gonadotropin-releasing hormone (GnRH) agonist for 2 weeks before gonadotropin administration. Once downregulation is evidenced with estradiol levels less than 50 pg/mL, the patient is started on gonadotropin stimulation while maintaining ovulation suppression with the daily use of the GnRH agonist. Increasingly, however, a GnRH antagonist is used to block spontaneous ovulation from occurring once follicular development occurs. With this “short protocol,” gonadotropins are begun on cycle day 2 or 3, without prior downregulation, and the antagonist is added around day 6 of stimulation. Data support an equal efficacy of the two approaches ( ).
When criteria for progression to oocyte retrieval are reached—for example, at least two mature follicles on ultrasound (18 mm) with an appropriate rise of serum estradiol (E 2 ), a bolus of HCG is administered (5000 to 10,000 IU). Vaginal ultrasound aspiration of the follicles is typically performed 34 to 36 hours later ( Fig. 41.3 ). This procedure is usually performed with the patient sedated, although it can be performed with local anesthetic use. Complications include infection, torsion, and internal bleeding, though rates are low (<0.25%) ( ).
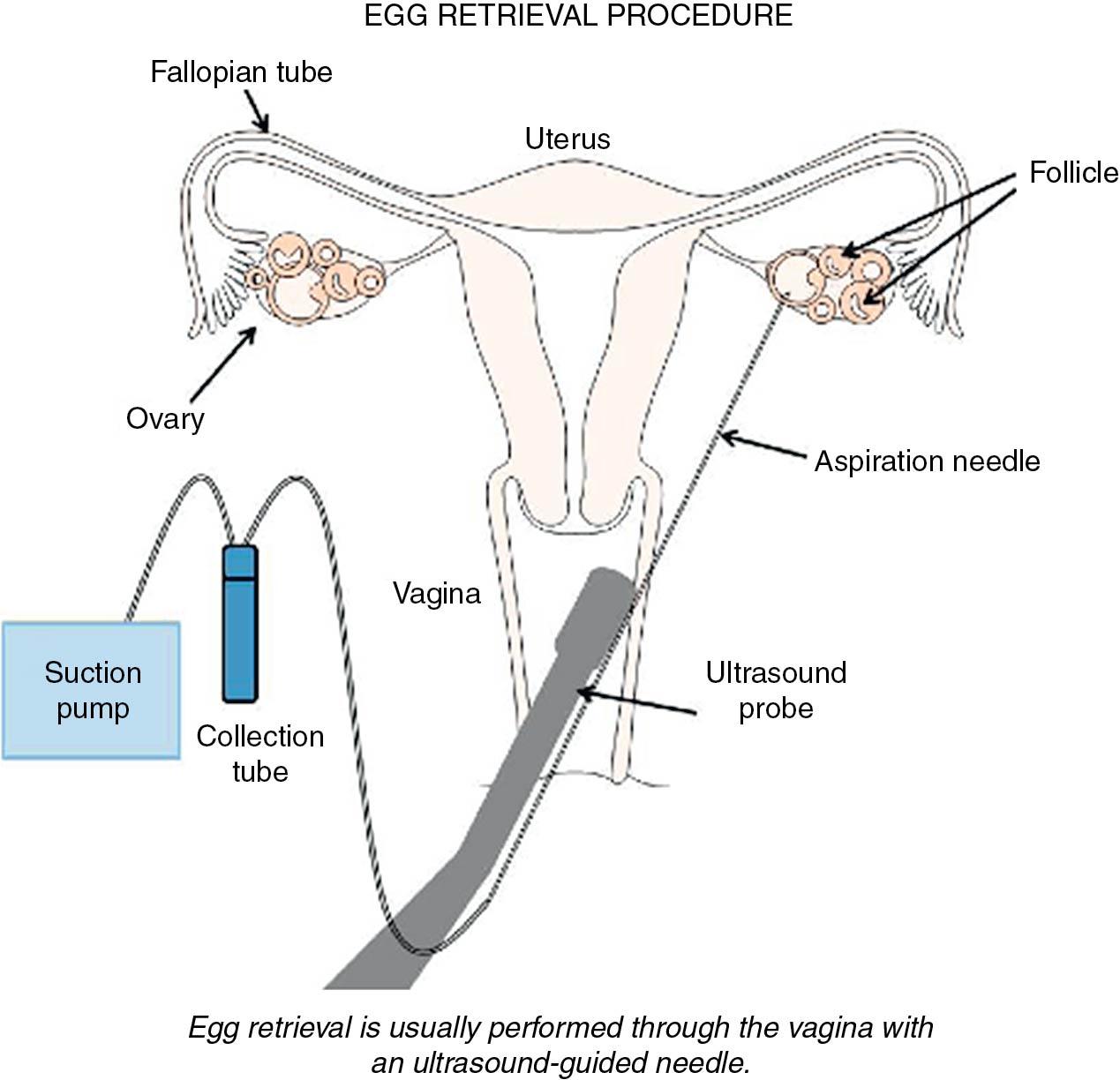
The harvested oocytes are then combined with a sperm sample from a male partner or from donor sperm. One of two methods is used to fertilize the oocytes: insemination (where tens of thousands of highly motile sperm are placed in a microdroplet with one or two oocytes) or ICSI. On average, about 60% to 70% of the mature oocytes should fertilize with insemination and normal-functioning sperm and oocytes. With ICSI, embryologists can select individual sperm and microinject a sperm into each mature, metaphase II (MII) oocyte. Approximately 12 to 18 hours later the oocytes are inspected for evidence of fertilization ( Fig. 41.4 ). ICSI does not yield better fertilization rates than in the normal situation; rather, it helps to produce normal fertilization rates in situations where fertilization may not occur or will occur at a low rate.
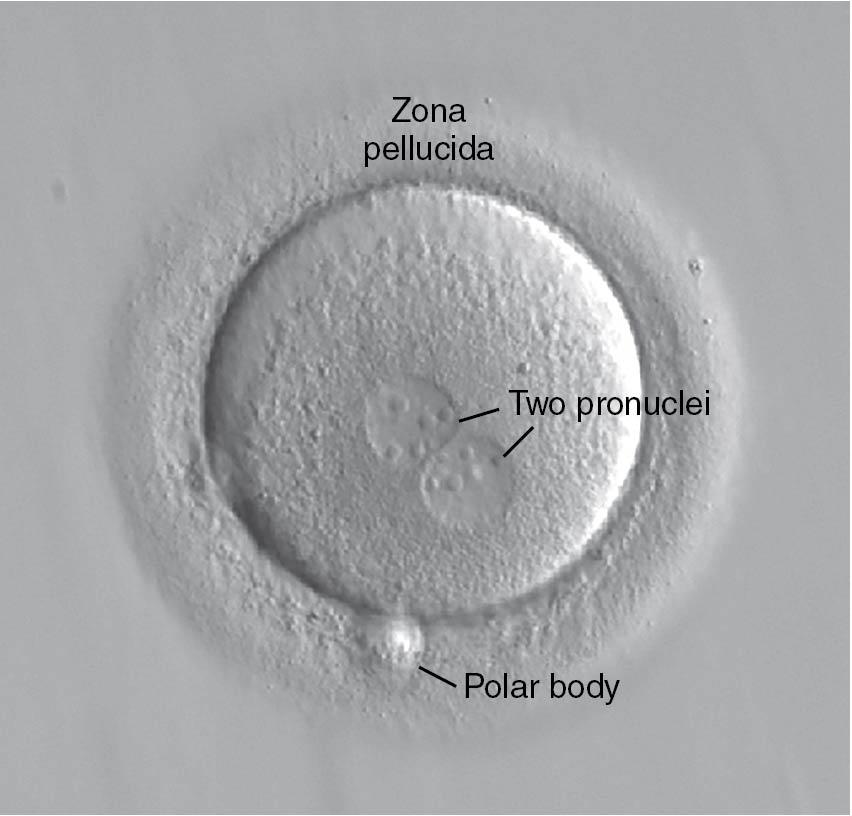
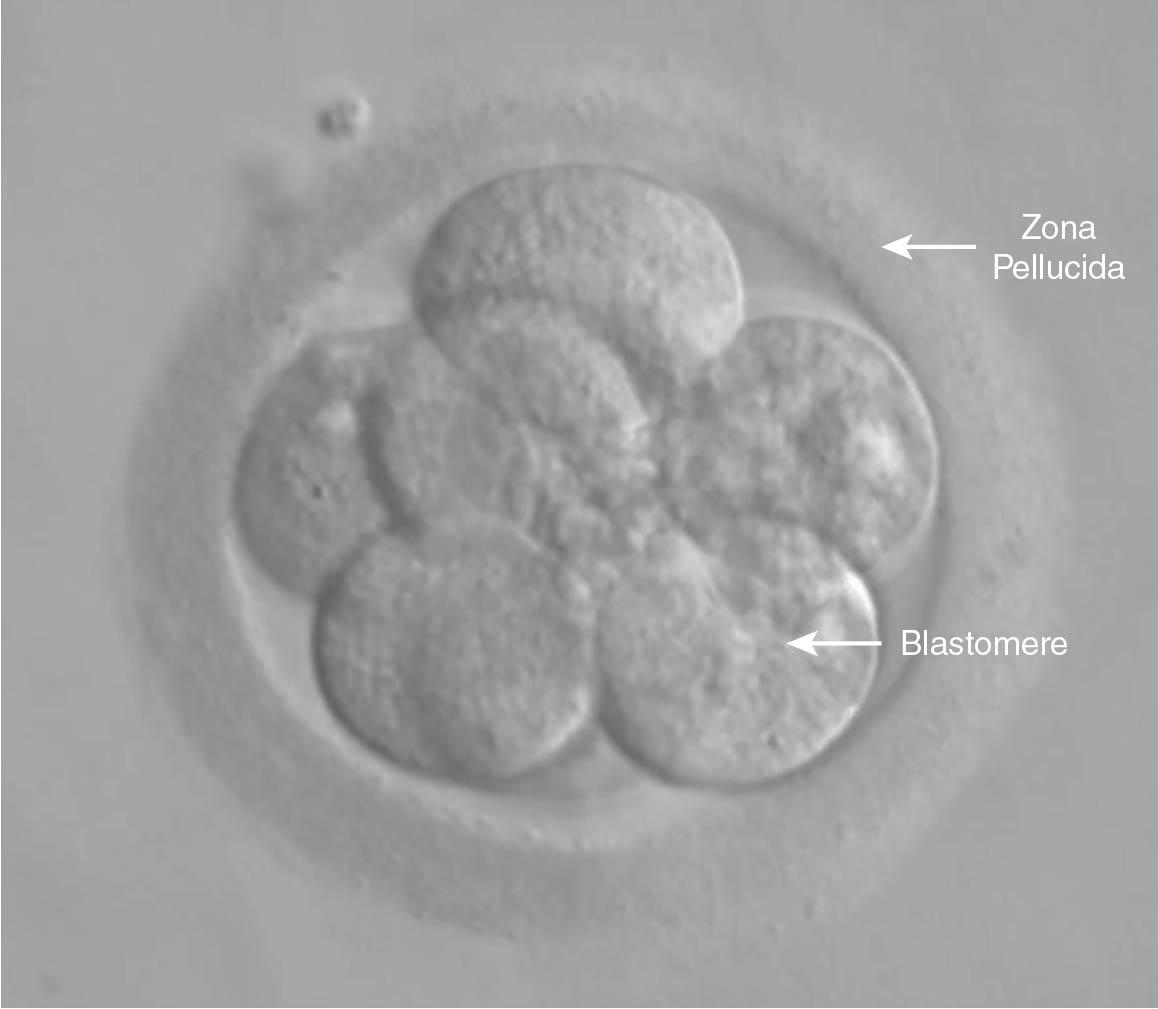
The embryology laboratory environment is the key factor in the success of IVF . Typically, 3 days after oocyte aspiration, cleavage stage embryos with six to eight cells are obtained. Three-day-old embryos can be assessed for potential transfer using criteria assessing the blastomere morphology and symmetry as well as the degree of embryo fragmentation ( Fig. 41.5 ). Fragmentation is felt to be the extracellular debris created by the dividing embryonic cells, and embryos with a high degree of fragmentation (i.e., >10% of the embryo) are associated with lower implantation and pregnancy rates ( ).
At the time of a day 3 transfer, the embryologist may opt to use assisted hatching (AH) . Using acid or a laser, the embryologist can make a small breech in the zona pellucida of the embryo. A meta-analysis suggested improved implantation and clinical pregnancy rates with AH use but concluded that live birth rates were not improved ( ). The authors concluded that AH may be most useful in older women (i.e., >37 years old) and in women with previously unexplained IVF failures. One downside to the use of AH is the slightly increased potential for monozygotic twinning (1% to 2%) ( ).
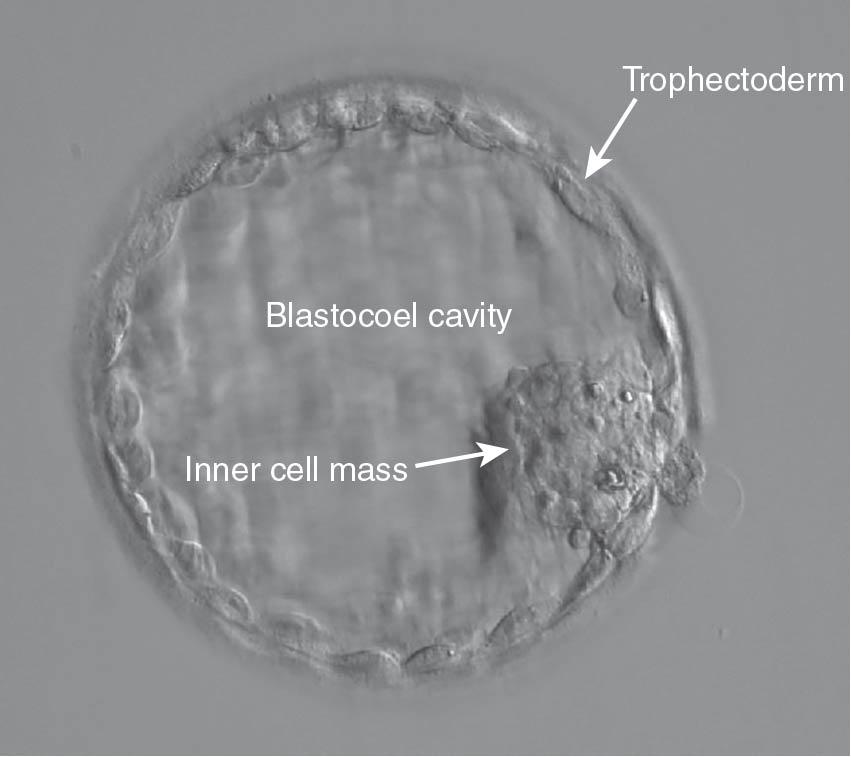
Increasingly, with improved quality of sequential culture media, embryo culture is continued to days 5 to 6, when a blastocyst has developed ( Fig. 41.6 ). Fertilized oocytes that can be cultured on day 5 or 6 are usually of better quality and afford a higher pregnancy rate compared with day 3 embryos ( ).
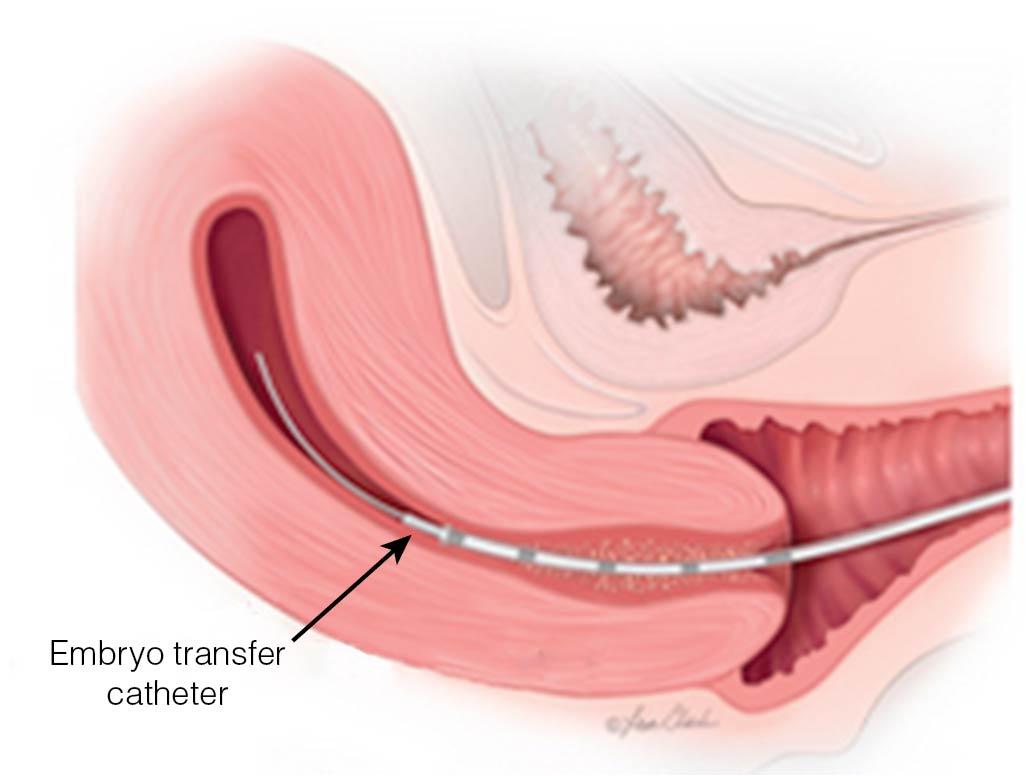
Typically, patients are awake for the embryo transfer, which is carried out with one of several specialized catheters (similar to those used for IUI) under ultrasound guidance ( Fig. 41.7 ). A major initiative by the ASRM has begun, teaching the techniques of successful embryo transfer using simulated models, with certification offered for successful completion of the course.
The decision regarding the number of embryos to transfer is key to optimizing success and reducing the chance of multiple pregnancies. ASRM has published firm guidelines to assist in this decision-making process ( ) ( Table 41.3 ). Implementation of these guidelines has proved to be successful in reducing the rate of high-order multiple pregnancies, with far less than 1% of live births after IVF being triplets or higher. The rate of twins has begun to decrease substantially with the increased use of single embryo transfer. In 2013, the Society for Assisted Reproductive Technology (SART) reported a nearly 30% twin rate in patients younger than 35; in 2016, that rate declined to 16%. In women with a good overall prognosis for pregnancy—such as women younger than 35 or women with a history of a prior live pregnancy—elective single embryo transfer (eSET) can still lead to an acceptable pregnancy rate while minimizing the risk of multiples ( ). Single embryo transfer does not totally eliminate the risk for multiples because there is a nearly 2% risk for monozygotic twinning after blastocyst transfer ( ). Transferring embryos on day 5 when the embryos are more developed and proportionately more “normal” is becoming the trend for all IVF cycles. This means, however, that some women who have poor embryo quality may not have any good-quality embryos for transfer.
| Age (years) | ||||
| Prognosis | <35 | 35-37 | 38-40 | 41-42 |
| C leavage -S tage E mbryos * | ||||
| Favorable † | 1-2 | 2 | 3 | 5 |
| All others | 2 | 3 | 4 | 5 |
| B lastocysts * | ||||
| Favorable † | 1 | 2 | 2 | 3 |
| All others | 2 | 2 | 3 | 3 |
Several methods have been suggested to help in the selection of a normal embryo for transfer. This includes an analysis of the culture media for metabolic parameters and secreted proteins, as well as an analysis of time-lapse videos of the cultured embryos for normal developmental parameters ( ; ); however, none of these methods is currently the standard of practice in IVF.
Excess embryos of good quality that are not transferred may be cryopreserved . Vitrification is a method of ultrarapid freezing using high concentrations of cryoprotectants has largely replaced slow cooling as the preferred cryopreservation technique for oocytes and embryos. The pregnancy rates from thawed, good-quality embryos may exceed the rate of fresh cycles in some instances. A large randomized trial performed in China showed that women with PCOS had a higher chance of live birth after frozen embryo transfer versus fresh (49% vs. 42%) ( ). Another trial performed in China showed that ovulatory women planning single embryo transfer also had a higher live birth rate after frozen embryo transfer than fresh (50% vs. 40%) ( ). No known increased fetal risks are associated with embryo cryopreservation; in fact, there may be a reduced risk of preterm delivery and a fetus that is small for gestational age.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here