Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Immunohistochemistry (IHC) is the use of antibody-based reagents for localization of specific epitopes in tissue sections. Over the past several decades, IHC has become a powerful tool to assist the surgical pathologist in many clinically critical settings. It is important to recognize that IHC has two components, each with its own strengths and weaknesses. These components may be thought of as the “hardware” (i.e., antibodies, detection systems) and the “software” (i.e., analytic processes). No matter how selective the antibodies or how powerful the detection system, the method fails if the analytic tools are inadequate. This chapter focuses on the antibody component of the hardware and some of the analytic processes in which IHC assists in the diagnosis of soft tissue neoplasms.
It cannot be overemphasized that IHC is an adjunctive diagnostic technique to traditional morphologic methods in soft tissue pathology, as in any other area of surgical pathology. It is critical to recognize that the diagnosis of many soft tissue tumors does not require IHC (e.g., osteocartilaginous tumors), and that there are no markers or combinations of markers that will reliably distinguish benign from malignant tumors (e.g., nodular fasciitis from leiomyosarcoma). Furthermore, reliable specific markers do not yet exist for certain mesenchymal cell types and their tumors, and a subset of soft tissue tumors is better defined by the tumor’s molecular rather than immunophenotypic profile, and techniques other than IHC, such as cytogenetic or molecular genetic studies, may prove more valuable in this setting. Lastly, it is important to acknowledge that a subset of soft tissue tumors defy classification, even with exhaustive IHC or genetic study.
The expression of certain antigens, or clusters of antigens, is characteristic of some tumors. Whereas thousands of monoclonal and polyclonal antibodies are available to assist in tumor diagnosis, only a small subset has proved to be of practical value in the diagnosis of soft tissue neoplasms. Tables 6.1 and 6.2 present an overview of the markers discussed in the following sections; the question marks highlight the gaps in our understanding of the cellular biology of many soft tissue tumors.
| Antibodies to: | Expressed by: |
|---|---|
| Keratins | Carcinomas, epithelioid sarcoma, synovial sarcoma, some angiosarcomas and leiomyosarcomas, mesothelioma, rhabdoid tumor |
| Vimentin | Sarcomas, melanoma, lymphoma, some carcinomas |
| Desmin | Benign and malignant smooth and skeletal muscle tumors |
| Glial fibrillary acidic protein | Gliomas, some schwannomas |
| Neurofilaments | Neural and neuroblastic tumors |
| Smooth and skeletal muscle actins (HHF35) | Benign and malignant smooth and skeletal muscle tumors; myofibroblastic tumors and pseudotumors |
| Smooth muscle actin (1A4) | Benign and malignant smooth muscle tumors; myofibroblastic tumors and pseudotumors |
| Caldesmon | Smooth muscle tumors, glomus tumors |
| Myogenic nuclear regulatory proteins (myogenin, MyoD1) | Rhabdomyosarcoma |
| S-100 protein | Melanoma, benign and malignant peripheral nerve sheath tumors, cartilaginous tumors, normal adipose tissue, Langerhans cells, many others |
| SOX10 | Melanoma, benign and malignant peripheral nerve sheath tumors, myoepithelial tumors |
| Epithelial membrane antigen | Carcinomas, epithelioid sarcoma, synovial sarcoma, perineurioma, meningioma, anaplastic large cell lymphoma |
| CD31 | Benign and malignant vascular tumors |
| Von Willebrand factor (factor VIII–related protein) | Benign and malignant vascular tumors |
| CD34 | Benign and malignant vascular tumors, solitary fibrous tumor, epithelioid sarcoma, dermatofibrosarcoma protuberans, GIST |
| CD99 | Ewing sarcoma/primitive neuroectodermal tumor, some rhabdomyosarcomas, some synovial sarcomas, lymphoblastic lymphoma, mesenchymal chondrosarcoma, small cell osteosarcoma, many others |
| MUC4 | Low-grade fibromyxoid sarcoma |
| CD45 | Non-Hodgkin lymphoma |
| CD30 | Anaplastic large cell lymphoma, embryonal carcinoma |
| CD68 and CD163 | Macrophages, fibrohistiocytic tumors, granular cell tumors, various sarcomas, melanomas, carcinomas |
| Melanosome-specific antigens (HMB-45, melan-A, tyrosinase, microphthalmia transcription factor) | Melanoma, PEComa, clear cell sarcoma, melanotic schwannoma |
| MDM2/CDK4 | Atypical lipomatous tumor and dedifferentiated liposarcoma |
| Claudin-1 | Perineurioma, synovial sarcoma, epithelioid sarcoma |
| GLUT-1 | Perineurioma, infantile hemangioma |
| SMARCB1 (INI1) | Expression lost in extrarenal rhabdoid tumor, epithelioid sarcoma, some epithelioid MPNSTs, and extraskeletal myxoid chondrosarcomas |
| CD117 and DOG1 | GIST |
| Tumor Type | Normal Cell Counterpart | Useful Markers |
|---|---|---|
| Angiosarcoma | Endothelium | CD31, CD34, FLI-1, ERG, von Willebrand factor |
| Kaposi sarcoma | Endothelium | CD31, CD34, VEGFR3, podoplanin, HHV8 LANA |
| Epithelioid hemangioendothelioma | Endothelium | CAMTA1 |
| Epithelioid sarcoma–like (pseudomyogenic) hemangioendothelioma | Endothelium | FOSB |
| Leiomyosarcoma | Smooth muscle | Muscle (smooth) actins, desmin, caldesmon, myosin heavy chain |
| Rhabdomyosarcoma | Skeletal muscle | MyoD1, myogenin; muscle (sarcomeric) actins; desmin |
| Ewing sarcoma | ? | CD99, FLI-1, ERG, NKX2.2 |
| Ewing-like CIC -rearranged sarcoma | ? | ETV4, WT-1 |
| Ewing-like BCOR -rearranged sarcoma | ? | BCOR, CCNB3, SATB2 |
| Synovial sarcoma | ? | Keratin, EMA, TLE1 |
| Epithelioid sarcoma | ? | Keratin, CD34, SMARCB1 (INI1) loss |
| Malignant peripheral nerve sheath tumor | Nerve sheath (e.g., Schwann cell, perineurial cell) | S-100, EMA, claudin-1, GLUT-1, SOX10, H3K27me3 loss |
| Malignant melanotic schwannian tumor | ?Schwann cell | S100 protein, SOX10, melanoma markers, loss of PRKAR1A |
| Liposarcoma | Adipocyte | S-100 protein, MDM2, CDK4 |
| Chondrosarcoma | Chondrocyte | S-100 protein |
| Osteogenic sarcoma | Osteocyte | SATB2, osteocalcin |
| Myofibroblastic lesions (e.g., nodular fasciitis) | Myofibroblast | Smooth muscle actins |
| Gastrointestinal stromal tumor | Interstitial cells of Cajal | CD117a (c-kit), CD34, DOG1 |
| Solitary fibrous tumor | ? | CD34, STAT6 |
| Glomus tumors | Glomus cell | Smooth muscle actins, caldesmon, type IV collagen |
| Angiomatoid (malignant) fibrous histiocytoma | ? | Desmin, EMA, CD68 |
| Alveolar soft part sarcoma | ? | TFE3 |
| Perivascular epithelioid cell neoplasms (PEComas) | ? | Smooth muscle actins, melanocytic markers |
The intermediate filaments comprise the major component of the cytoskeleton and consist of five major subgroups—vimentin, keratins, desmin, neurofilaments, and glial fibrillary acidic protein (GFAP)—and a small number of minor subgroups (e.g., nestin, peripherin). Ultrastructurally, the intermediate filaments appear as wavy unbranched filaments that often occupy a perinuclear location in the cell. The original thinking that intermediate filament expression was restricted to specific cell types (e.g., keratins in carcinomas, vimentin in sarcomas) is now well recognized as an oversimplification. The following sections on intermediate filaments concentrate not only on the normal pattern of expression of these proteins, but also on the situations where intermediate filaments show “anomalous expression.”
Vimentin, a 57-kilodalton (kDa) intermediate filament protein, is expressed in all mesenchymal cells. Vimentin is ubiquitously expressed in all cells during early embryogenesis and is gradually replaced in many cells by type-specific intermediate filaments. In some mesenchymal tissues, vimentin is typically coexpressed along with the cell type–specific intermediate filaments (e.g., desmin and vimentin coexpression in muscle cells, vimentin and GFAP in some Schwann cells). Vimentin is expressed in virtually all mesenchymal tumors and is thus of minimal value in identifying particular tumors. Given the frequent coexpression of vimentin along with keratin in carcinomas, vimentin expression is also of little value in the immunohistochemical distinction of carcinomas from sarcomas. Vimentin immunoreactivity has been touted as a good marker of tissue preservation. However, vimentin expression, similar to that of all the intermediate filaments, is rather hardy and may remain present in tissues in which all other immunoreactivity has been lost. The absence of vimentin expression may occasionally be a clue to the diagnosis of rare vimentin-negative mesenchymal tumors, such as alveolar soft part sarcoma and perivascular epithelioid cell neoplasms. In general, there is no value in performing vimentin immunostains on any spindle cell neoplasm.
Keratins, also known as cytokeratins , the most complex members of the intermediate filament protein family, are a collection of more than 20 proteins. The keratins may be grouped by their molecular weights (40–67 kDa) into acidic and basic subfamilies or by their usual pattern of expression in simple or complex epithelium ( Fig. 6.1 ). In practice the keratins are most often seen as low-molecular-weight (LMW) keratins (generally keratins 8, 18, and 19) and high-molecular-weight (HMW) keratins (generally keratins 1, 5, 10, and 14). Keratins are highly sensitive markers for identifying carcinomas and are generally employed as markers distinguishing epithelial/mesothelial from nonepithelial tumors (i.e., lymphomas, sarcomas, melanomas) ( Fig. 6.2 ). Over the past decade it has become clear that keratin expression is not restricted to carcinomas.


Among the sarcomas are two patterns of keratin expression. A small subset of sarcomas display true epithelial differentiation, as defined by usual expression of keratin and other epithelial proteins, such as the desmoplakins and occludin (e.g., synovial sarcomas, epithelioid sarcomas). A larger group of tumors occasionally display “anomalous” keratin expression (i.e., keratin expression by cells and tumors without true epithelial differentiation). Synovial sarcomas and epithelioid sarcomas are the best, if not the only, examples of sarcomas manifesting true epithelial differentiation ( Fig. 6.3 ). Expression of both LMW and HMW keratin isoforms is seen in both synovial sarcoma and epithelioid sarcoma, confirming the presence of true epithelial differentiation. Antibodies to specific keratins, such as keratins 7 and 19 for synovial sarcoma and keratins 5/6 in epithelioid sarcoma, may also be diagnostically useful in select cases.

Anomalous keratin expression is typically characterized by immunostaining (even under optimal technical conditions) in only a subset of the target cell population. In these cells, keratin is present in only a portion of the cytoplasm, often yielding a “perinuclear” or “dotlike” pattern of immunostaining. This dotlike pattern is not always an indication of anomalous keratin, however, because it is typically seen in some neuroendocrine carcinomas (e.g., small cell carcinomas, Merkel cell tumors) and extrarenal rhabdoid tumors ( Fig. 6.4 ). In addition, it is rare to find keratins other than those corresponding to the Moll catalog 8 and 18 (corresponding to positivity with antibodies CAM5.2 or 35βH11) in tumors manifesting anomalous keratin expression.

Contrary to earlier suggestions, anomalous keratin expression is not a universal feature of sarcomas. It is instead a feature of a limited subset of nonepithelial tumors, particularly smooth muscle tumors, melanomas, and endothelial cell tumors; as such, it may serve as a clue to the diagnosis of these tumors. Interestingly, the normal cell counterparts of some of these tumors (i.e., smooth muscle cells and endothelial cells) have been found to express keratins in nonmammalian species, and in our experience these are frequently keratin positive in routinely processed sections, particularly with newer, more sensitive antibodies, such as the OSCAR wide-spectrum keratin antibody.
Frozen sections of the smooth muscle cell–rich myometrium of the uterus (along with myocardial cells) were first reported to “react with” various antikeratin antibodies. Brown et al. and Norton et al. verified these findings using slightly different techniques, although Norton failed to find corroborative biochemical evidence of keratin expression by these smooth muscle cells. Gown et al. first presented biochemical documentation of true “anomalous” keratin expression of keratins 8 and 18, in which immunostaining was corroborated by Western blots; it was further documented by two-color immunofluorescence studies of myometrial smooth muscle cells grown in vitro. Subsequent studies have shown that at least 30% of leiomyosarcomas manifest keratin.
Despite that many studies completed during the mid-1980s concluded that melanomas were vimentin-positive, keratin-negative tumors, Zarbo et al. first confirmed the keratin positivity of many melanomas and demonstrated the positive immunostaining as a function of tissue preparation and fixation, with 21% of cases positive in frozen sections but far fewer in formalin-fixed, paraffin-embedded (FFPE) sections. They also performed one- and two-dimensional gel electrophoresis with immunoblotting, confirming that keratin 8 was expressed by the tumor cell population. More recently, Romano et al. documented keratin expression, using the OSCAR and AE1/AE3 antibodies, in up to 40% of epithelioid melanomas and in a smaller percentage of spindle cell melanomas. Although anomalous keratin expression was previously thought to be more common in metastatic than in primary melanomas, this does not seem to be the case.
Early reports suggested that vascular tumors manifesting epithelioid histologic features (e.g., epithelioid hemangioendothelioma, epithelioid angiosarcoma) express keratin in most cases ( Fig. 6.5 ). The largest published series of angiosarcomas of deep soft tissue documented keratin expression in about one-third of cases. Aberrant expression of keratins is essentially always present in epithelioid sarcoma–like (pseudomyogenic) hemangioendotheliomas.

A surprising number of tumors in the category of “small, blue round cell tumors” of childhood typically coexpress keratin in a pattern similar to that of anomalous keratin expression. These tumors include Ewing sarcoma, rhabdomyosarcoma, Wilms tumor, and desmoplastic small round cell tumor of childhood. Expression of LMW keratin isoforms may be seen in almost 25% of Ewing sarcomas, usually confined to less than 20% of the neoplastic cells. Expression of HMW keratins is much less common and is restricted to the rare “adamantinoma-like” variant.
The literature is replete with reports of keratin expression in other sarcomas, including undifferentiated pleomorphic sarcoma, chondrosarcoma, osteosarcoma, and malignant peripheral nerve sheath tumors. Nonetheless, in our experience, keratin expression in these tumors is exceedingly rare. Aberrant expression of keratins is typically seen in the recently described superficial CD34-positive fibroblastic tumor, where it is an important clue to the correct diagnosis.
It is important to remember that immunoreactivity and true antigen expression are not necessarily synonymous. Several factors can theoretically account for positive keratin immunostaining in tumors without true keratin expression. This includes the use of antibodies at inappropriately high concentrations, potentially altered specificities following the use of heat-induced epitope retrieval techniques (“antigen retrieval”), and cross-reactivity of antikeratin antibodies with other proteins, such as GFAP in gliomas and some schwannomas. Punctate keratin staining from exogenous keratin (dandruff) is an additional pitfall ( Fig. 6.6 ), as is the presence of reactive submesothelial fibroblasts in or around many tumor types ( Fig. 6.7 ). By employing an approach to IHC that includes a panel of antibodies, the pathologist can generally avoid misinterpretation that might result from “misbehavior” of one antibody.


Epithelial membrane antigen (EMA) is an incompletely characterized antigen that is present in a group of carbohydrate-rich, protein-poor, HMW molecules present on the surface of many normal types of epithelium, including those in the pancreas, stomach, intestine, salivary gland, respiratory tract, urinary tract, and breast. Among normal mesenchymal cells, EMA expression is limited to perineurial cells and meningeal cells. Uses for EMA are limited in sarcoma diagnosis. EMA expression is a more sensitive, but less specific, marker of poorly differentiated synovial sarcomas; it may be helpful in cases with only focal (or absent) keratin expression. Perineuriomas and malignant peripheral nerve sheath tumors with perineurial differentiation are characterized by a sometimes subtle expression of EMA along cell processes, as well as claudin-1, GLUT-1, and type IV collagen expression ( Fig. 6.8 ). Ectopic meningiomas, as with their meningeal counterparts, are characterized by EMA and vimentin expression in the absence of keratin expression. Patchy expression of EMA (along with desmin and CD68) is seen in approximately 50% of angiomatoid fibrous histiocytomas. EMA expression has been documented in a significant subset of genetically confirmed low-grade fibromyxoid sarcomas, a potentially serious pitfall, given the morphologic similarities between these tumors and perineuriomas. In our experience, patchy EMA immunoreactivity may be seen in a fairly broad range of mesenchymal tumors, emphasizing the need to employ antibodies to EMA as part of a panel of immunostains.

There are three types of muscle differentiation. The first is skeletal muscle differentiation, as recapitulated in rhabdomyoma and rhabdomyosarcoma. The second is “true” smooth muscle differentiation, reflected in leiomyoma and leiomyosarcoma. The third is “partial” smooth muscle differentiation, as seen in the myofibroblasts that constitute a significant population of cells in healing wounds and the stromal reaction to tumors. There is also a subset of soft tissue tumors (e.g., nodular fasciitis, myofibroblastoma), the phenotype of which greatly resembles myofibroblasts rather than true smooth muscle cells. The principal markers of muscle differentiation are the intermediate filament desmin, the various actin isoforms, caldesmon, and the myogenic regulatory proteins.
Desmin is the intermediate filament protein associated with both smooth and skeletal muscle differentiation; it is rarely expressed by myofibroblasts and their corresponding tumors. In skeletal muscle, desmin is localized to the Z zone between the myofibrils, where it presumably serves as binding material for the contractile apparatus. In smooth muscle, desmin is associated with cytoplasmic dense bodies and subplasmalemmal dense plaques. Desmin may also be expressed by nonmuscle cells, including the fibroblastic reticulum cell of the lymph node, the submesothelial fibroblast, and endometrial stromal cells. Desmin is among the earliest muscle structural genes expressed in the myotome of embryos, and some regard it as the best single marker for the diagnosis of poorly differentiated rhabdomyosarcoma. Although the early literature on desmin questioned its sensitivity in formalin-fixed, deparaffinized tissue sections, more recent studies have confirmed its excellent sensitivity. In our experience, with the use of heat-induced epitope retrieval techniques and modern antibodies such as D33, desmin is the most sensitive marker of skeletal and smooth muscle differentiation in terms of both the fraction of tumors so identified and the fraction of tumor cells in given tumors that are positive. Desmin expression is present in almost 100% of rhabdomyosarcomas of all subtypes, including very poorly differentiated ones ( Fig. 6.9 ).

Desmin expression is apparently not as specific for muscle tumors as originally thought, because it has also been described in Ewing sarcoma, desmoplastic small round cell tumors, neuroblastoma, mesothelial cells and tumors, the blastemal component of Wilms tumor, giant cell tumors of the tendon sheath, and ossifying fibromyxoid tumors of soft parts; in none of these contexts is there thought to be true muscle differentiation ( Fig. 6.10 ). Desmin expression, along with more limited expression of MyoD1 and myogenin, is a surprisingly common finding in mesenchymal chondrosarcoma. Expression of desmin along with EMA and CD68, in the absence of other muscle markers, is highly characteristic of angiomatoid fibrous histiocytomas ( Fig. 6.11 ). Desmin expression may be seen in up to 24% of melanomas and may rarely be seen in schwannomas. Definitive identification of tumors manifesting skeletal muscle differentiation therefore may require the additional use of antibodies to myogenic transcription factors such as myogenin and MyoD1 (see later).

Actin, a ubiquitous protein, is expressed by all cell types; high concentrations of actins and unique isoforms, however, help make actin a marker of muscle differentiation. In general, actins can be grouped into muscle and nonmuscle isoforms, which differ by only a few amino acids in a protein with a molecular weight of 43,000 kDa. It has nevertheless been possible to generate antibodies specific to muscle actins versus nonmuscle actins and to specific actin isotypes with respect to the various muscle types (e.g., smooth muscle vs. skeletal muscle). Although early literature cites the “specificity” of antiactin polyclonal antibodies for muscle cells, in most of these studies it is a quantitative rather than a qualitative phenomenon; that is, muscle cells have much more actin than many other cells, and demonstration of positivity is determined on this basis alone. Whereas there are monoclonal antibodies that can identify all actin isoforms (i.e., the C4 clone ), given the sensitive immunohistochemical techniques available, this antibody cannot be used to distinguish muscle from nonmuscle actins. The antibody HHF35, which has been widely used to identify muscle cells and tumors, displays specificity for all muscle (vs. nonmuscle) actins. Antibody 1A4 is a monoclonal antibody that specifically identifies smooth muscle actin isoforms and thus can distinguish smooth from skeletal muscle cells and tumors.
Smooth muscle actin isoforms are also expressed by myofibroblasts, and the characteristic pattern of actin expression in these cells may help distinguish them from true smooth muscle cells. In general, myofibroblasts show expression of smooth muscle actin only at the periphery of their cytoplasm (“tram-track” pattern), in contrast to the uniform cytoplasmic expression in smooth muscle ( Fig. 6.12A ). On occasion, this tram-track pattern is a clue that a myofibroblastic process is present (e.g., fasciitis) rather than a leiomyosarcoma. For unknown reasons, the 1A4 smooth muscle actin antibody occasionally shows aberrant nuclear immunoreactivity, which should not be interpreted as evidence of smooth muscle actin expression in a tumor ( Fig. 6.12B ). Antibody asr-1 is monoclonal and specifically identifies sarcomeric actins (skeletal, cardiac); it identifies rhabdomyosarcoma but not leiomyosarcoma. One should be aware that some rhabdomyosarcomas, particularly the paratesticular spindle cell type, can express low levels of smooth muscle actins.

Heavy caldesmon (h-caldesmon) is a calcium-binding protein involved in the regulation of smooth muscle contractility. The sensitivity of h-caldesmon for smooth muscle tumors is lower than that of antibodies to smooth muscle actin, but h-caldesmon is not expressed by myofibroblastic tumors. H-caldesmon expression is frequently present in glomus tumors and in myopericytic neoplasms of various types ( Fig. 6.13 ) . h-Caldesmon is generally best used for the distinction of poorly differentiated smooth muscle tumors from myofibroblastic tumors, rather than as a stand-alone marker of smooth muscle cells.

Myogenic regulatory proteins (i.e., transcription factors of the MyoD [myogenic determination] family) play a critical role in the commitment and differentiation of mesenchymal progenitor cells to the myogenic lineage and subsequent maintenance of the skeletal muscle phenotype. MyoD1 and myogenin are members of the basic helix-loop-helix family of DNA-binding myogenic nuclear regulatory proteins; the other members include Myf5 and MRF4. These genes encode transcription factors, whose introduction into nonmuscle cells in culture can initiate muscle-specific gene expression and muscle differentiation. In addition, such regulatory factors are expressed much earlier in the normal skeletal muscle differentiation program than structural proteins such as desmin, actin, and myosin; indeed, expression of these myogenic regulatory proteins leads to activation of the latter. Antibodies to both MyoD1 and myogenin, but not the other myogenic nuclear regulatory proteins, have been studied in terms of diagnosing rhabdomyosarcoma. Both MyoD1 and myogenin are expressed in more than 90% of rhabdomyosarcomas of all subtypes. Antibodies to both MyoD1 and myogenin show excellent specificity (see Fig. 6.9C ). There is only a single report of nuclear immunoreactivity for MyoD1 in FFPE sections in a pleomorphic liposarcoma. Four alveolar soft part sarcomas have been demonstrated to express MyoD1 by IHC on frozen sections and by Western blot. Cytoplasmic immunoreactivity for MyoD1 has been reported in a small number of nonrhabdomyosarcomas, including primitive neuroectodermal tumor, Wilms tumor, and undifferentiated sarcoma. Only nuclear immunoreactivity for MyoD1 should be taken as evidence of skeletal muscle differentiation, because the epitope recognized by the most commonly used antibody to MyoD1, 5.8A, includes amino acid sequences with close homology to the class 1 major histocompatibility antigen and transcription factors E2A and ITF-1, 123 suggesting that cytoplasmic immunoreactivity may represent a cross-reaction rather than true MyoD1 expression.
As previously noted, both MyoD1 and myogenin are expressed by more than 95% of embryonal rhabdomyosarcomas (ERMS) and alveolar rhabdomyosarcomas (ARMS), including the well-differentiated spindle cell variant of ERMS and the solid variant of ARMS. In general, ARMS express very high levels of myogenin and comparatively less MyoD1, whereas ERMS show the opposite pattern, or equal levels of expression. The recently described spindle cell/sclerosing variant of RMS typically shows very strong expression of MyoD1, but much more limited myogenin expression. Pleomorphic rhabdomyosarcoma are less frequently MyoD1 or myogenin positive, with a recent large series documenting expression in only 53% and 56% of cases, respectively. In addition, pleomorphic rhabdomyosarcoma may show only a small percentage of tumor cells that are positive.
Although the available evidence appears to strongly support the view that MyoD1 and myogenin expression are highly specific for rhabdomyoblastic differentiation, it is important to realize that their expression does not obligate a diagnosis of rhabdomyosarcoma. In our experience and that of others, expression of MyoD1 and/or myogenin may be seen in a variety of rare tumors with rhabdomyoblastic differentiation, including Wilms tumors with myogenous differentiation, neuroendocrine carcinoma (including Merkel cell carcinoma) with rhabdomyoblastic differentiation, malignant glial tumors with myoblastic differentiation, malignant peripheral nerve sheath tumors with rhabdomyoblastic differentiation (malignant Triton tumor), and teratomas with rhabdomyoblastic differentiation. Expression of both MyoD1 and myogenin has recently been demonstrated in mesenchymal chondrosarcomas, likely indicative of true skeletal muscle differentiation in this tumor secondary to the HEY1-NCOA2 fusion ( Fig. 6.14 ). It is also important not to mistake myogenin and MyoD1 expression in degenerating and regenerating skeletal muscle for tumor cell expression, particularly in the setting of diffuse skeletal muscle infiltration by a nonmyogenous “small blue round cell tumor,” such as lymphoma.

PAX7 is a paired-box transcription factor essential for the developmental specification of skeletal muscle satellite cells ( Fig. 6.15A and B ). Recently, antibodies to PAX7 have been shown to be sensitive markers of embryonal, spindle cell/sclerosing, and pleomorphic rhabdomyosarcomas, present in more than 70% of cases ( Fig. 6.15C and D ). Interestingly, PAX7 expression is much less common in alveolar rhabdomyosarcoma (19%) and is confined to tumors harboring the PAX3-FOXO1A fusion. PAX7 expression has also been demonstrated to be present in all Ewing sarcoma, but not in other nonrhabdomyosarcoma small round cell tumors.

Antibodies to myoglobin, an oxygen-binding heme protein found in skeletal and cardiac muscle but not smooth muscle, were the first markers used in the immunohistochemical diagnosis of rhabdomyosarcoma. Unfortunately, myoglobin is present in demonstrable amounts in fewer than 50% of rhabdomyosarcomas; it may be identified in nonmyogenous tumor cells that are infiltrating skeletal muscle and phagocytosing myoglobin. Commercially available myoglobin antibodies have a high level of nonspecific, “background” staining, which may be difficult to distinguish from true myoglobin expression. This is in distinct contrast to desmin and the myogenic regulatory protein, which do not diffuse. We do not use antibodies to myoglobin in our routine practice. Other muscle markers that have been used for diagnosing rhabdomyosarcoma include antibodies to myosin, creatine kinase subunit M, and titin. In general, these alternative markers suffer from a lack of sensitivity and/or specificity, and their use cannot be recommended.
In summary, for identifying skeletal muscle differentiation, the myogenic regulatory proteins myogenin and MyoD1 are the most specific; antibodies to desmin and muscle actins (i.e., HHF35) are of high sensitivity but are not skeletal muscle specific. For identification of smooth muscle differentiation (e.g., in leiomyosarcomas), antibodies to desmin and muscle actins (i.e., antibody HHF35) or smooth muscle α-actin (e.g., antibody 1A4) are the best markers of smooth muscle differentiation. For identifying myofibroblasts (e.g., the type of differentiation present in lesions such as nodular fasciitis), antibodies to desmin are useful only for distinguishing myofibroblasts from true smooth muscle cells, because the former (in contrast to the latter) generally do not express desmin. Both cell types express smooth muscle actins, however, although myofibroblasts generally express the latter in a characteristic wispy or tram-track pattern of immunostaining that, on higher resolution, can be demonstrated to correspond to the peripheral bundles of actin filaments, which are the hallmark of this cell type. Myofibroblasts also manifest little or no expression of the smooth muscle and myoepithelial cell-associated proteins caldesmon and smooth muscle myosin heavy chain, and these markers may help to distinguish myofibroblastic and true smooth muscle proliferations.
The S-100 protein is a 20-kDa acidic calcium-binding protein, so named for its solubility in 100% ammonium sulfate. The protein is composed of two subunits, α and β, which combine to form three isotypes. The α-α isotype is normally found in myocardium, skeletal muscle, and neurons; the α-β isotype is present in melanocytes, glia, chondrocytes, and skin adnexa; and the β-β isotype is seen in Langerhans and Schwann cells.
Immunohistochemically, S-100 protein can be demonstrated in a large number of normal tissues, including some neurons and glia; Schwann cells; melanocytes; Langerhans cells; interdigitating reticulum cells of lymph nodes; chondrocytes; myoepithelial cells and ducts of sweat glands, salivary glands, and the breast; serous glands of the lung; fetal neuroblasts; and sustentacular cells of the adrenal medulla and paraganglia ( Figs. 6.16 and 6.17 ). In the immunohistochemical diagnosis of soft tissue neoplasms, S-100 protein is of most value as a marker of benign and malignant nerve sheath tumors and melanoma. S-100 protein is strongly and uniformly expressed in essentially all schwannomas. The finding of uniform S-100 immunoreactivity may be a valuable clue to the diagnosis of cellular schwannoma, because malignant peripheral nerve sheath tumors usually show only patchy, weak expression of S-100, 60,153,156,157 and fibrosarcomas would not be expected to be S-100 positive ( Fig. 6.18 ). S-100 protein expression is much more variable in neurofibromas than in schwannomas. S-100 protein expression is seen in 40% to 80% of malignant peripheral nerve sheath tumors.



However, as may be inferred from the long list of normal tissues that express this protein, significant S-100 protein expression may be seen in a subset of nonneural tumors included in the differential diagnosis of malignant peripheral nerve sheath tumors: synovial sarcoma, rhabdomyosarcoma, leiomyosarcoma, and myoepithelioma. Other tumors that may express S-100 protein include adipocytic tumors, chondrocytic tumors, ossifying fibromyxoid tumors, and chordoma. Malignant melanomas of all types, including the desmoplastic and sarcomatoid variants, are almost always strongly positive for S-100 protein. Uniform, strong S-100 protein expression may be a valuable clue that a melanoma is present rather than a malignant peripheral nerve sheath tumor of skin or soft tissue because, as noted, S-100 protein expression in malignant peripheral nerve sheath tumors tends to be weaker and more patchy. Our experience is that approximately 2% to 3% of melanomas (more often in the metastatic setting) are negative for S-100 protein; additional immunostaining for a melanosome-specific marker such as gp100 protein (identified by antibody HMB-45 166 or melan-A ) is essential for arriving at the correct diagnoses in these patients.
SOX10 is a transcription factor involved in neural crest development and differentiation of neural crest cells into melanocytic and schwannian lineages. Nonaka et al. first demonstrated SOX10 expression by IHC in melanocytic and schwannian neoplasms, noting expression in greater than 85% of melanomas of all subtypes, 60% of clear cell sarcomas, more than 93% of neurofibromas of all subtypes, 100% of schwannomas, and 30% of malignant peripheral nerve sheath tumors ( Fig. 6.19 ). They also noted SOX10 expression in sustentacular cells of various neuroendocrine tumors, but in no other epithelial or mesenchymal tumor studied. Karamchandani et al. reported similar findings. In general, expression of SOX10 parallels that of S100 protein in melanomas and malignant peripheral nerve sheath tumors. SOX10 expression is also common in myoepithelial tumors. In general, other S100 protein-positive tumors, such as ossifying fibromyxoid tumor and chordoma, are SOX10 negative.

The claudins are a family of approximately 20 homologous proteins that help determine tight junction structure and permeability. Claudins appear to be differentially expressed in tissues, with claudin-1 expression relatively widespread among epithelia and claudin-3 expression confined to lung and liver epithelia. Claudins are integral transmembrane proteins that complex with other transmembrane proteins such as junctional adhesion molecule (JAM) and occludin and interact with scaffolding proteins such as ZO-1, ZO-2, and ZO-3. Among normal mesenchymal tissues, claudin-1 expression appears to be limited to perineurial cells. In the appropriate histologic context, claudin-1 is a useful marker of perineuriomas, present in 20% to 90% of perineuriomas, but not in other tumors in this differential diagnosis, such as neurofibromas, schwannomas, low-grade fibromyxoid sarcoma, desmoplastic fibroblastoma, dermatofibrosarcoma protuberans, or fibromatosis (see Fig. 6.8 ). Aberrant, nonpolarized expression of claudin-1 and other tight junction–related proteins is seen in a significant number of synovial sarcomas and Ewing sarcoma/primitive neuroectodermal tumor (ES/PNET).
GLUT-1 is the erythrocyte-type glucose transporter protein, which plays a particular role in transporting glucose across epithelial and endothelial barrier tissues. Expression of GLUT-1 protein has been shown to be a consistent feature of normal perineurial cells and benign and malignant perineurial tumors. However, in soft tissue and bone tumors, GLUT-1 expression is frequently identified adjacent to foci of necrosis, presumably representing upregulation of this protein within hypoxic zones, secondary to upstream activation of proteins such as hypoxia-inducible factor 1α, and thus GLUT-1 is a highly nonspecific marker of perineurial differentiation in malignant-appearing lesions. Among vascular tumors, expression of GLUT-1 protein is seen in essentially all juvenile capillary hemangiomas, but not in other pediatric vascular tumors, such as vascular malformations and kaposiform hemangioendothelioma ( Fig. 6.20 ). Its expression in vascular tumors appears unrelated to the proliferative activity of the lesions.

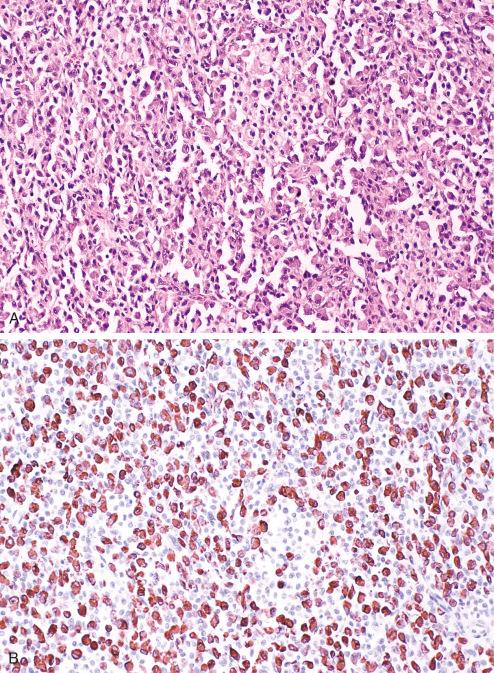
The product of the pseudoautosomal MIC2 gene, CD99 is a transmembrane glycoprotein of 30 to 32 kDa (p30/32). Its exact function is unknown, although it appears to play a role in cellular adhesion and regulation of cellular proliferation. The MIC2 gene is expressed and the CD99 antigen produced in almost all human tissues, although the level of expression varies significantly. Normal tissues that usually display strong CD99 expression include cortical thymocytes and Hassall corpuscles, granulosa and Sertoli cells, endothelium, pancreatic islets, adenohypophysis, ependyma, and some epithelium, including urothelium, squamous epithelium, and columnar epithelium.
The most important use of antibodies to CD99 is for immunohistochemical diagnosis of Ewing sarcoma (ES). Many studies show that more than 90% of ES express CD99, with a characteristic membranous pattern ( Fig. 6.21 ). Despite early claims that CD99 expression was also specific for ES, this clearly is not true. It is particularly important to recognize that a significant subset of other small blue round cell tumors considered in the differential diagnosis of ES may express this antigen. CD99 expression is seen in more than 90% of lymphoblastic lymphomas, 20% to 25% of primitive rhabdomyosarcomas, more than 75% of poorly differentiated synovial sarcomas, approximately 50% of mesenchymal chondrosarcomas, and in rare cases of small cell osteosarcomas and intraabdominal desmoplastic round cell tumor. CD99 expression is not a feature of neuroblastomas or esthesioneuroblastomas. In general, “Ewing-like” primitive sarcomas of the type showing CIC or BCOR rearrangements typically show only patchy and weak expression of CD99, a finding that may be an important clue to the diagnosis of these rare sarcomas.

IHC analysis of CD99 expression plays a limited role in the diagnosis of pleomorphic or spindle cell soft tissue neoplasms. As noted, many synovial sarcomas express CD99, which may be helpful in discriminating them from malignant peripheral nerve sheath tumors and fibrosarcomas. Expression of CD99 may also be seen in solitary fibrous tumors, mesotheliomas, leiomyosarcomas, and undifferentiated pleomorphic sarcomas. In general, use of CD99 in the diagnosis of spindle cell neoplasms has been superseded by the development of much more specific immunohistochemical (e.g., STAT6 in solitary fibrous tumor) or molecular (e.g., SS18 FISH in synovial sarcoma) markers.
The 140-kDa isoform of the neural cell adhesion molecule, CD56, is an integral membrane glycoprotein that mediates calcium-independent homophilic cell-cell binding. CD56 is expressed by many normal cells and tissues, including neurons, astrocytes, and glia of the cerebral cortex and cerebellum, adrenal cortex and medulla, renal proximal tubules, and follicular epithelium of thyroid; gastric parietal cells; cardiac muscle; regenerating and fetal skeletal muscle; pancreatic islet cells; and peripheral nerve. CD56 is also ubiquitously expressed on human natural killer (NK) cells and on a subset of T lymphocytes.
As might be expected from this long list of CD56-positive normal tissues, CD56 expression is widespread among sarcomas. Soft tissue tumors that often express CD56 include synovial sarcoma, malignant peripheral nerve sheath tumor, schwannoma, rhabdomyosarcoma, leiomyosarcoma, leiomyoma, chondrosarcoma, and osteosarcoma. For this reason, examination of CD56 expression is not helpful when evaluating spindle cell soft tissue tumors. However, CD56 expression may be useful for evaluating primitive small blue round cell tumors, particularly in combination with CD99. CD56 expression is seen in only 10% to 25% of ES and in rare lymphoblastic lymphomas, compared with almost 100% of neuroblastomas, poorly differentiated synovial sarcomas, alveolar and primitive embryonal rhabdomyosarcomas, small cell carcinomas, Wilms tumors, and mesenchymal chondrosarcomas. The absence of CD56 expression may be a clue to the diagnosis of ES in cases where results with more specific positive markers such as CD99, CD45, keratin, and desmin are equivocal. Demonstration of CD56 expression may also be of some value in the diagnosis of ossifying fibromyxoid tumor, particularly when S-100 protein expression is weak or absent.
A transmembrane glycoprotein found in presynaptic vesicles, synaptophysin plays an important role in the packaging, storage, and release of neurotransmitters and functions as a membrane channel protein. It is routinely expressed by neural, endocrine, and neuroendocrine cells, as well as adrenal cortical cells and tumors. Synaptophysin expression is typically confined to neural and neuroendocrine tumors, including neuroendocrine carcinomas of various grades, Merkel cell carcinomas, paragangliomas, neuroblastomas, and esthesioneuroblastomas. Synaptophysin expression is relatively uncommon in “neuroectodermal” tumors, such as ES. Aberrant synaptophysin expression may also be seen in alveolar rhabdomyosarcomas, a minority of extraskeletal myxoid chondrosarcomas, and rare melanomas. Aberrant expression of synaptophysin and CD56 is one of the defining features of the recently described composite hemangioendothelioma with neuroendocrine marker expression ( Fig. 6.22 ).

A calcium-binding protein member of the granin family, chromogranin A is stored in the dense core granules of neural and neuroendocrine cells. Chromogranin A expression in normal tissues is generally similar to that of synaptophysin, although it is not expressed by adrenocortical cells. Aberrant expression of chromogranin A is much less common than that of synaptophysin and when present tends to be confined to very few cells.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here