Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Antigens can have a wide variety of types of chemical composition such as proteins, carbohydrates, and lipids. They can range in size from very small molecules, such as hormones, to very large ones on viral or bacterial particles.
Antibodies can be polyclonal as in an immune response with somewhat broad specificity. Monoclonal antibodies are developed from cloned hybridomas; thus, they have very high specificity for antigens.
Immunoassays are based on interactions of antigens and antibodies that are described mathematically with standard kinetic equations of immunochemistry.
Laboratory immunoassays have evolved from simple immunoprecipitation and nephelometry through the more sensitive radioimmunoassays, enzyme immunoassays, fluorescent immunoassays, and chemiluminescent immunoassays.
Instrumentation for modern immunoassays relies on highly precise and accurate specimen delivery as well as very sensitive detection techniques to avoid spectrophotometric interferences.
High-throughput instrumentation allows for rapid and low-cost operations in measurement of multiple analytes by immunoassay.
New portable testing with single-sample devices also exploits immunoassays to achieve rapid results at point-of-care settings.
Highly sensitive immunoassays have the capacity to detect even single molecules, which promises sophisticated monitoring of diseases, such as cancer recurrence.
Biological ligands based on the affinity between molecules, such as enzyme and substrate, hormone and receptor, and antigen and antibody, play an important role in living organisms. Specific recognition characteristics of immunoassays (antigen–antibody [Ag–Ab] reactions) have become widely used as analytic tools, adding to the wide range of methods available in clinical laboratory testing.
Immunoassays can be used for the detection of antigens or antibodies. For antigen detection, the corresponding specific antibody should be prepared as one of the reagents. The reverse is true for antibody detection. The sensitivity of immunoassays has been enhanced through the development of new types of signal detection systems and solid-phase technology. Immunoassays have been optimized to detect less than 0.1 pg/mL of antigen present in blood. They can be applied to the detection of haptens as small molecules; proteins and protein complexes as macromolecules; and any antibody to allergens, infectious agents, and autologous antigens.
An antigen can be defined as any substance that can represent antigenic sites (epitopes) to produce corresponding antibodies, from small molecules such as haptens and hormones to macromolecules such as proteins, glycoproteins, glycolipids, and other natural products. Artificial chemical compounds can also be antigens acting as haptens. Antigens should have at least one epitope. Epitopes that can be recognized by antibodies include amino acid sequences of peptides in proteins and high-dimensional protein structures, such as neoantigenic sites.
Immunoglobulin, an important plasma protein, refers to antibodies in the context of the biological functions of immunoglobulin specific to antigens. Antibodies, therefore, are produced in response to antigenic stimulation. Antibodies are formed of both functional and heterogeneous molecules that bind antigens via the antigen-combining site. Five classes (isotypes) of immunoglobulin have been identified: IgG, IgM, IgA, IgD, and IgE. IgG is further divided into four subclasses, and both IgA and IgM have two subgroups. All known antibody molecules have a heavy chain with a κ or a λ light chain. The molecular structure of antibodies is composed of variable regions and constant regions. The hypervariable domain (epitope-binding spot) can be assembled to interact with a wide variety of epitopes (antigen determinants). In laboratory medicine, two categories of antibodies can be distinguished: antibodies as reactants and antibodies as analytes. Antibodies as analytes are often classified by IgG, IgM, and IgA subtypes. Antibodies as reactants are prepared from antiserum obtained through animal immunization with purified antigen.
A polyclonal antibody can be obtained through immunization with an antigen, which presents various epitopes. In other words, the antibody generated is specific against each epitope. The avidity of a polyclonal antibody to a complex antigen is usually stronger than a single monoclonal antibody. Carrier proteins may be needed for the immunization of smaller molecules, such as haptens or hormones.
Monoclonal antibodies ( ) have been developed using the biotechnologies somatic cell fusion, selection of the resulting hybridoma, and limiting dilution to ensure that the clone is derived from a single cell. They are defined as uniform homogeneous antibodies directed to specific epitopes. An established cell line allows the secretion of all reactive immunoglobulin specific to single epitopes. Monoclonal antibodies have made it possible to analyze molecules on an epitope-to-epitope basis because of their narrow specificity. Yet, monoclonal antibodies, in contrast to polyclonal antibodies, do not have the ability to recognize the entire molecule. For monoclonal antibodies, different antigens with a common epitope appear to be the same antigen. CA 19-9 antibody ( ) as a tumor marker can detect different sizes and shapes of molecules that have common carbohydrate epitopes. Monoclonal antibodies enable identification of isoenzymes, subtypes, and isotypes of protein, and conformational changes in molecules because they can discern the slightest differences in molecules.
Monoclonal antibodies may be cross-reactive with different antigens. This cross-reactivity can be explained by the probable existence of the same amino acid sequences, carbohydrates, or lipids on different molecules. Monoclonal antibody technology has allowed the development of extremely useful and nearly ideal immunoassay systems for clinical laboratory testing.
The production methods and applications of monoclonal antibodies have been extensively reviewed ( ; ). Advantages of monoclonal antibodies are as follows:
Monoclonal antibodies provide a well-defined reagent.
Monoclonal antibody production can yield an unlimited quantity of homogeneous reagent with highly consistent affinity and specificity.
Monoclonal antibodies can be prepared through immunization with a nonpurified antigen.
Monoclonal antibodies have certain limitations in their use, as follows:
Insufficient reactivity is seen in precipitation or agglutination because network formation in the immunocomplex is weak or does not occur when single monoclonal antibodies are used.
Antigens with multiple heterogeneous epitopes are more difficult to characterize immunochemically with a single monoclonal antibody.
developed an expression system for the Fv fragment of variable domains of an antibody specific to phosphoryl choline using recombinant technology in Escherichia coli . This technology makes it possible to produce chimeric antibody fused to an enzyme.
The technology called phage display has emerged for the production of antibodies ( ). In this method, antibody fragments of predetermined binding specificity are constructed from a repertoire of antibody variable ( V ) genes, thereby eliminating the need for immunization and hybridoma technology. The V genes can be assembled in vitro. The phage selected from the repertoire by binding to antigen and antibody fragments is expressed in infected bacteria. Furthermore, the binding affinity of the antibodies is improved through a mutation/maturation process.
Seo et al. indicated an ex vivo method for rapid generation of monoclonal antibodies on the basis of the finding that gene conversion in DT40 cells was enhanced by treatment of the cells with a histone deacetylase inhibitor, trichostatin A ( ). This treatment involves a diversified library of DT40 cells (ADLib), in which each cell has different surface IgM specificity. Antigen-specific DT40 cells are selected from ADLib using antigen-conjugated magnetic beads; their specificity can be examined by various immunologic assays, using culture supernatant containing secreted IgM. The antibody from selected cells is expressed in mammalian cells as a recombinant antibody.
These technologies to develop monoclonal antibodies without immunization for animal and hybridoma technology will allow the use of specific antibodies with high avidity or affinity by improving the selection process or maturation technology.
Certain aspects of equilibrium or the law of mass action in chemistry can be applied to the Ag–Ab reaction. The kinetics of the reversible Ag–Ab reaction is as follows ( ):
where
Ag = free antigen
Ab = free antibody sites
AgAb = antigen–antibody complex concentration
K 1 and K 2 = association and dissociation rate constants, respectively
The rate of formation of the Ag–Ab complex is represented as follows:
and at equilibrium, the net rate is zero. Therefore,
(association equilibrium constant or affinity constant).
K a is the parameter limited to site-to-site reactions, although antigens and antibodies often have multiple binding sites on the molecule. The apparent association constant for multiple antigen and antibody reactions may be referred to as avidity instead of affinity . The K a value may be obtained from the following equations and experimental data:
where
[ Ab ] = antibody concentration at equilibrium
[ Ab ] t = total original antibody concentration
where
F = free antigen or analyte
B = bound antigen or analyte
A Scatchard plot is produced when the amount of antigen bound ( B ) is plotted on the x -axis and the bound over free ( B/F ) ratio of analytes is plotted on the y -axis. The two parameters that can be determined from the Scatchard plot are the dissociation or affinity constant from the slope of the line and the concentration of antibody-binding sites from the X-intercept ( ).
A brief classification and a list of features of various immunoassays appear in Table 45.1 . Precipitation immunoassays provide the simplest method for antigens and antibodies to react with each other without involving the detection of any labels. The resulting Ag–Ab complex in the gel or liquid phase may be observed qualitatively as a precipitant by the naked eye and quantitatively with a detector.
| Labels (Reporter Groups) | B/F Separation ∗ | Signal Detection | Sensitivity | |
|---|---|---|---|---|
| Precipitation immunoassays | Not required | Not required | Naked eye, turbidity, nephelometry | ∼10 μg/mL 1 |
| Particle immunoassays | Blood cells, artificial particles (gelatin, particles, latex, etc.) | Not required | Naked eye, pattern analyzer, spectrophotometry, particle counting | ∼5 ng/mL 2 |
| Radioimmunoassays | Radioisotopes ( 125 I, 3 H) | Required | Photon counting | ∼5 pg/mL |
| Enzyme immunoassays | Enzymes | Required | Spectrophotometry, fluorometry photon counting (CL-EIA) | ∼5 pg/mL ∼0.1 pg/mL 3 |
| Fluorescence immunoassays | Fluorophores | Required | Photon counting | ∼5 pg/mL 4 |
| Chemiluminescence immunoassays | Chemiluminescent compounds | Required | Photon counting | ∼5 pg/mL 4 |
| Electrochemiluminescence immunoassays | Required |
∗ Washing step for separation of bound labels in immunocomplex from free labels. Homogeneous assays included are not required for B/F separation.
The particle agglutination immunoassay ( ) uses inert particles as labels as opposed to direct precipitation of the Ag–Ab complexes. Antigens or antibodies attached to particles such as erythrocytes, latex, or metal sol react with the analyte in the specimen. As a result of this immune reaction, large particles show significant agglutination patterns that may be seen by the naked eye. reported on the development of a radioimmunoassay (RIA) using radioisotopes as labels. This breakthrough allowed for the quantitative detection of a trace level of analytes and contributed to the advancement of basic research and clinical medicine. Insulin, for example, was quantified by RIA, which subsequently replaced the insulin bioassay. RIA may be formatted in a solid-phase procedure for easy separation of bound and free labels. Since the development of RIAs, the search for alternative labels to hazardous radioisotopes has intensified, with the aim of developing nonisotopic immunoassays using enzymes, fluorescent labels, and other reporter groups.
The enzyme immunoassay (EIA), using enzymes as labels, was developed in the early 1970s ( ; ) and rapidly gained wide popularity. Enzymes can amplify signals, depending on the turnover of enzyme catalytic activity. Efforts to improve substrates and to increase sensitivity have led to the introduction of chromophore, fluorophore, and later chemiluminescent compounds. Depending on the substrate chosen, the assay method can be defined as a fluorescent enzyme immunoassay or as a chemiluminescent enzyme immunoassay ( ).
Fluorescent immunoassays (FIAs) use fluorophores as labels. Fluorophores require optimal wavelength light energy for their excitation to produce detectable emission light. FIA sensitivity is likely to decrease because of the nonspecific background fluorescence present in biological specimens. Fluorophores that have a delayed fluorescence emission time of 100 ns (nanoseconds) are suitable for application on time-resolved FIA. The introduction of a new class of fluorescent compounds has resulted in improvements in FIA, such as the elimination of background noise. Sophisticated instrumentation has been introduced that can detect low concentrations (10 −15 M) of analytes using FIAs.
Chemiluminescent immunoassays use chemiluminescent compounds as labels. Chemiluminescent compounds include chemically synthesized molecules as well as natural products such as aequorin. Unlike fluorophores, most chemiluminescent compounds require chemical rather than light energy to generate emission light. The reduction–oxidation reaction is a process common to all chemiluminescent assays. Signal amplification is not expected of chemiluminescent labels because chemiluminescent molecules generate just one photon through molecular decomposition. A series of new and innovative compounds for electrochemiluminescence have proved suitable for application on immunoassays. Metal chelate with tribiphenyls emits light through a continuous reduction–oxidation reaction on the surface of electrodes ( ).
In the various types of assays mentioned earlier, the main factors affecting assay sensitivity are the association constant (affinity or avidity) of the reactant, the signal intensity of the labels, and the signal/noise ratio of the detection signal reduced by background from the signals themselves or by a nonspecific reaction.
Iodine-125 ( 125 I) requires about 7 million molecules to generate 1 photon/second, based on half-life calculations of radioisotopes ( ). Chemiluminescent substrates to enzymes can increase events by an order of magnitude six times greater than that of iodine-125. This is attributable to the catalytic amplification capacity of enzymes.
Depending on the assay chosen, the method of conjugation that couples one molecule to others, enzymes (or cofactors) to antigens (or antibodies), or antigens (or antibodies) to solid phase may vary. The coupling reaction applied should be performed under conditions that avoid the reduction of any of the biological activities of the protein. In the glutaraldehyde method ( ), glutaraldehyde, along with two (or possibly more) aldehyde groups as coupling reagents, has been used for conjugation of protein amino groups. This method is based on mixed reactions, including aldol condensation at high pH (the pKa of the NH residue is 8.6 to 10.8). With this method, shown in Figure 45.1 , the resulting conjugate has different forms because of the existence of multiple active sites for coupling. The periodate oxidation method ( ) for the conjugation of antibodies uses horseradish peroxidase, which contains carbohydrates in its molecules. The methods mentioned earlier are not suitable for regulation of site-specific reactions, such as when configurational stereospecificity of conjugates is required.
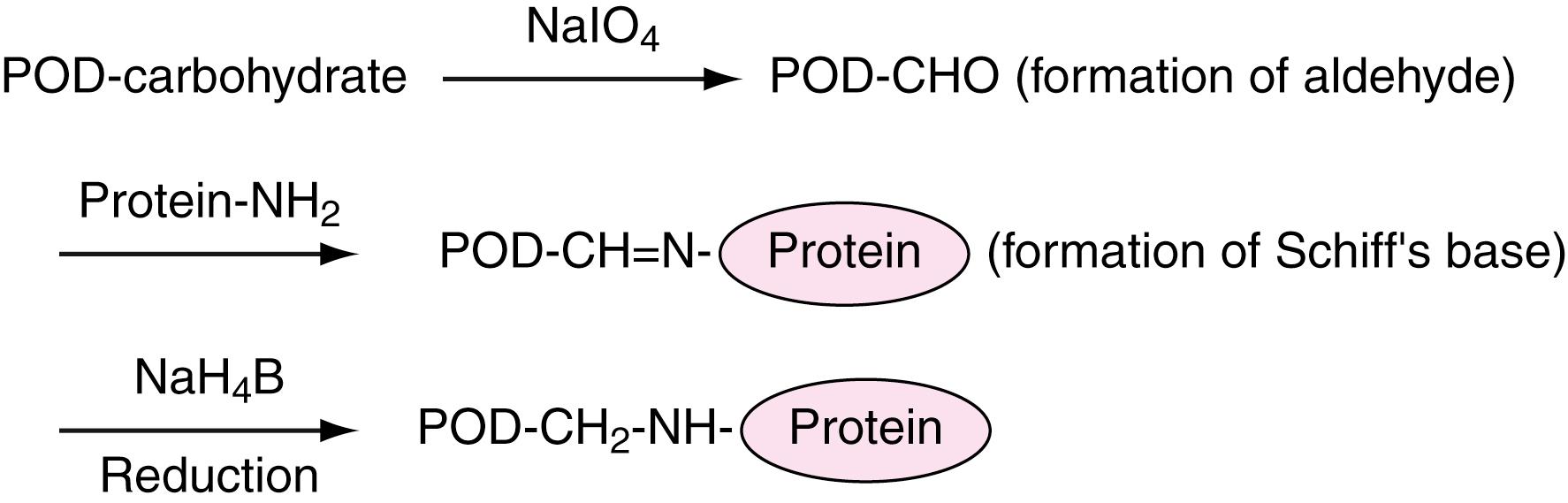
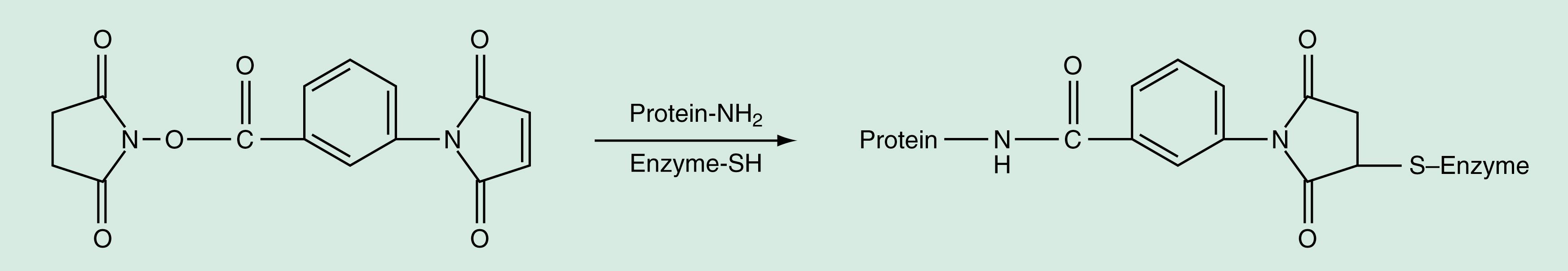
As shown in Figure 45.2 , new coupling methods ( ; ) have been developed using a sophisticated coupling reagent, m -maleimidobenzyl- N -hydroxysuccinimide ester (MBS). The MBS is a bivalent reagent consisting of an activated ester and maleimide, and it can react with an NH 2 group and an SH group, respectively. The carboxy group can also be used as a specific site for conjugating with α- or ε-NH 2 residues of proteins using N -hydroxysuccinimide (NHS) as the coupling reagent. The NHS coupling reagent extends to conjugate protein or the carboxyl group introduced on the solid phase.
All heterogeneous immunoassays using conjugate with labels, including radioisotopes, require at least one separation step to distinguish the reacted immunocomplex (bound) from unreacted materials (free). Immobilization of antigens or antibodies for solid phase is performed by covalent binding or physical adsorption through noncovalent interactions. Gel particles made of agarose, polyacrylamide, and plastic beads, or titer plates composed of polystyrene, have been used as the solid phase, as well as particles coated with iron oxide that can be separated by a magnetic field.
The inner wall of a tube or a microtiter well is commonly used as a solid phase. With these relatively large solid phases, shaking of the reaction mixture may be needed to shorten the time required for the immune reaction to take place. The prozone phenomenon, or hook effect, caused by high concentrations of the analyte is likely to be observed in a one-step immunoassay when limited quantities of solid-phase antibody or labeled antibody are employed. Microtiter or strip-type plates may cause an “edge effect.” This effect can be explained by the different kinetics of the immune reaction or enzyme activity with variations in temperature. A difference in temperature may be present between the wells located at the edge of the microtiter plate and the center of the plate. A difference of about 2°C may be observed with an infrared thermometer between the edge and the center of wells at ambient temperature. The size and shape of the solid phase are critical factors affecting the immunoreaction kinetics and the capturing capacity of solid-phase antibody or antigen. Small spherical magnetic particles or latex particles with 3000 Å diameters provide a larger total surface area for immunoabsorbency than is usually obtained with other solid phases. The larger total surface area helps shorten the immunologic reaction time ( ).
The particles used for the particle agglutination assay are listed in Table 45.2 . The phenomenon of particle agglutination (direct agglutination) caused by an immune reaction was first observed in tests after incubation of bacterial pathogens with infected patient serum. Agglutination of erythrocytes after incubation with serum led to the discovery of ABO blood types. Particle immunoassays are based on the agglutination principle and use the reactant of an antibody or an antigen attached to the inert particle as a label, as opposed to direct precipitation of an Ag–Ab immunocomplex. As a label, the particle can significantly increase the immunoassay sensitivity regardless of whether the resulting agglutination is detected by the naked eye or with spectrophotometric instruments for quantification.
| Assay Method | Supply | |
|---|---|---|
| Human erythrocyte | Direct hemagglutination (Landsteiner) | ABO blood type |
| Erythrocyte antibody hemagglutination: titer plate/slide | Human immunodeficiency virus antibody | |
| Avian erythrocyte | Direct hemagglutination | Human influenza virus (HIV) antibody |
| Fixed animal erythrocyte | Passive hemagglutination: titer plate | Treponema pallidum antibody |
| Reverse passive hemagglutination: titer plate | Hepatitis B surface antigen (HBsAg) | |
| Latex | Reverse passive agglutination: slide | Chorionic gonadotropin |
| Reverse passive agglutination: turbidimetry | Immunoglobulin (Ig) E | |
| Reverse passive agglutination | Ferritin | |
| Latex (color) | Immunochromatography | Human chorionic gonadotropin (hCG) |
| Microcapsule | Passive agglutination: titer plate | T. pallidum antibody |
| Gelatin particle | Passive agglutination: titer plate | HIV, T. pallidum antibody |
| Reverse passive agglutination: charge-coupled device (CCD) camera | Human hemoglobin (hHb) | |
| Polypeptide particle | Passive and reverse agglutination | T. pallidum antibody, HBs antibody |
| Silicate particle | Passive agglutination: titer plate/CCD camera | T. pallidum antibody |
| Gold particle | Reverse agglutination enhancement photometry | Total estrogen |
| Metal sols | Reverse agglutination | hCG, hHb |
Erythrocytes, gelatin particles, liposomes, metal sols, and various kinds of latex particles, including latex modified with iron oxide or dyes, are all suitable solid phases. The diameter of these particles used for agglutination reactions varies from 0.01 to 7 μm. No single theory can explain the kinetics of agglutination ( ) because of the wide variety of particle sizes used as labels. Brownian motion is not significant at room temperature for dispersed particles larger than 3 to 5 μm. However, the theory of potential energy of interaction between particles, or the theory of colloidal coagulation reaction, can apply to small particles, such as latex microparticles. The IgM antibody, being multivalent, is estimated to be 750 times more efficient than the bivalent IgG antibody in an agglutination reaction. The distance between particles in flocculation should be less than or equal to 120 Å because of the molecular length of the antibody. In summary, important factors to consider in an immune reaction are the surface properties of particles, such as charge and hydrophobicity, and the stability of dispersion.
The precipitate that forms when large complexes of antigen and antibody combine to form an insoluble lattice has been widely used to identify and quantify immunoprecipitin reactions. The modes of application of precipitin techniques have the advantages of sensitivity, specificity, and simplicity. The sensitivity limitation of these assays is a major consideration. Even under the best conditions of enhanced sensitivity afforded by newer light-scattering techniques, the lower limit of sensitivity of immunoprecipitin assays remains in the range of 0.1 to 0.5 mg/dL. This lower limit of sensitivity appears to be sufficient for the quantification of many major serum proteins. The precipitin reaction forms the basis for many quantitative and qualitative immunochemical techniques now used in the clinical laboratory ( ).
Factors affecting the precipitin reaction were extensively investigated by Heidelberg in 1935, who found that the relative proportions of reactants; conditions of temperature, pH, and ionic strength of the medium; and antibody characteristics of avidity and affinity were all important in the formation of the immune precipitate ( ). On the pattern of precipitin formation, it can be noted that there is a point at which precipitation is maximum or optimal, which is designated as the point of equivalence . Continued addition of antigen once the point of equivalence has been reached produces a solubilizing effect on the precipitate. The dynamic range suitable for the determination of analytes should be up to the zone of equivalence. Optimization of the antibody concentration as a reactant is necessary in addition to optimization of buffer solutions. Typical precipitant reaction methods are as follows:
Qualitative Precipitant Assay Methods
Single immunodiffusion ( )
Double immunodiffusion ( )
Double immunodiffusion in two dimensions ( )
Electroimmunodiffusion reaction ( )
Immunoelectrophoresis ( )
Semiquantitative Precipitant Assay Methods
Single radial immunodiffusion ( ; )
Single-dimension electroimmunodiffusion (“rocket” electrophoresis) ( )
A number of techniques for immunoprecipitin analysis have been developed that use light-scattering devices ( ). The occurrence of immune complex formation has been related to the amount of such light scattering and has been used as a basis for antigen quantitation. Sophisticated instruments have been designed to rapidly measure light scattering. Measurements of scattered light are generally referred to as turbidimetry or nephelometry.
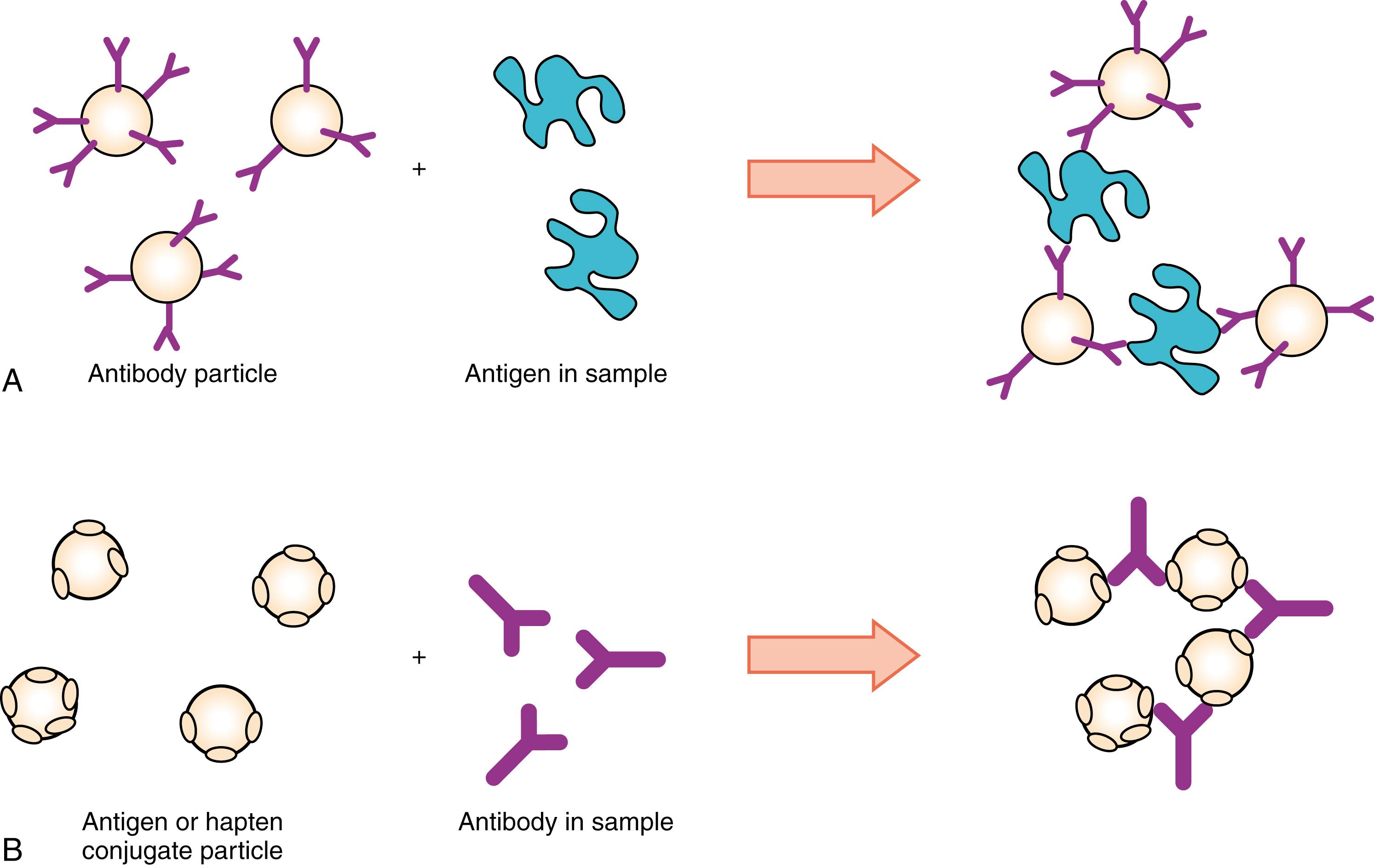
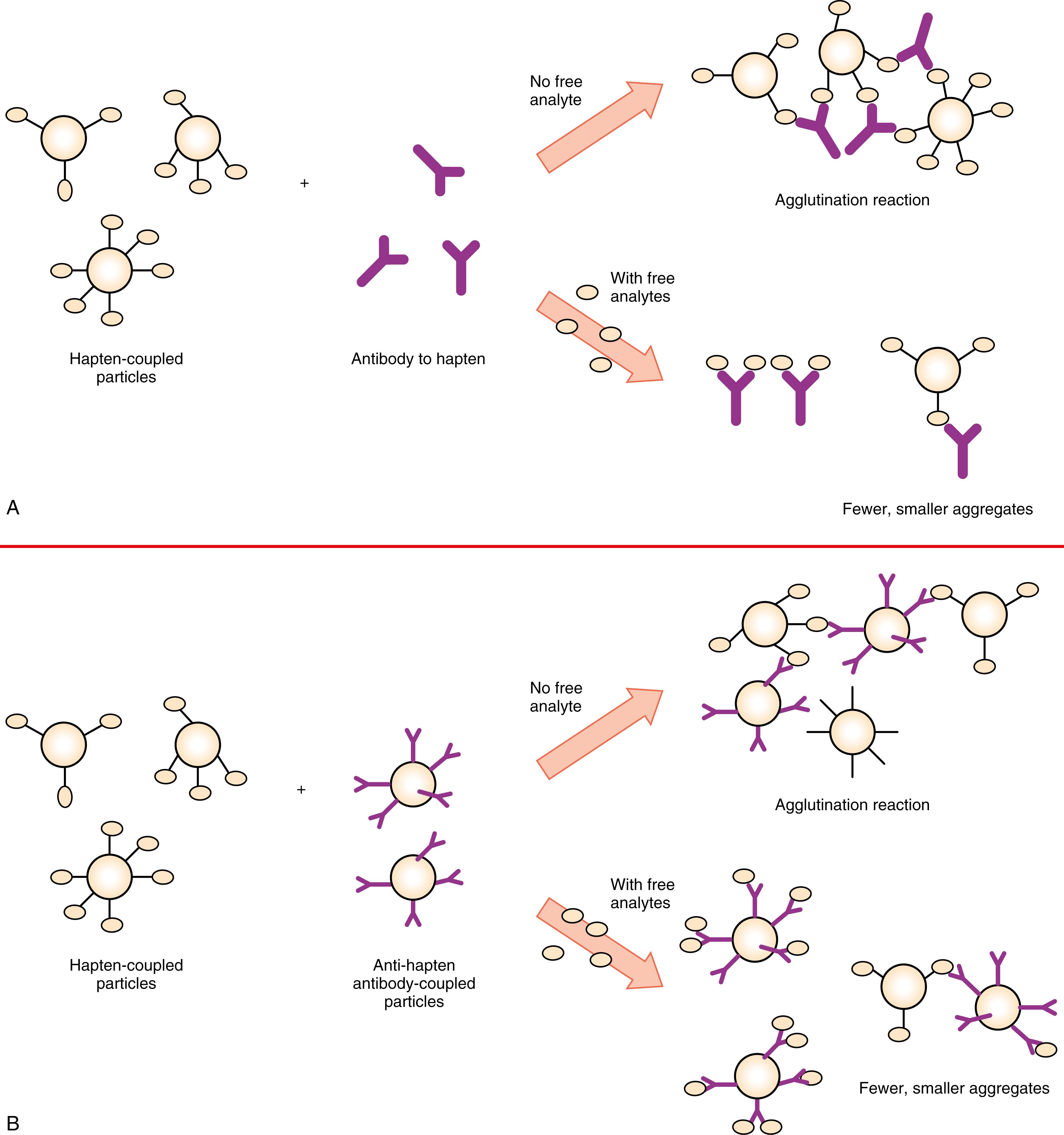
The agglutination reaction may be used to detect antibodies in specimens with specific antigens sensitized to a particle (passive or direct agglutination; Fig. 45.3B ). Reverse agglutination using a corresponding antibody sensitized to particles can be employed to detect soluble antigens in the specimen ( Fig. 45.3A ). A hapten unit single-binding site (for drugs, hormones, or small particles) does not form a cross-linking structure; thus, it cannot become agglutinated unless it is immobilized on the solid phase. As shown in Figure 45.4 , when a particle or carrier immobilized with a hapten is used as a reactant, an agglutination inhibition reaction occurs that allows detection of the hapten. This assay is based on a competitive-type principle in which agglutination of hapten particles with a limited amount of antibody, whether free or sensitized on particles, is inhibited by the hapten present in the specimen. Also, labeled particles can react with reactants fixed on the solid phase of the membrane. After immunoreaction, particles within the immunocomplex can be developed to show color on part of the membrane. This type of assay has been popular as a simple device for the qualitative detection of human chorionic gonadotropin (hCG) and other analytes.
Hemagglutination tests ( ) are simple to perform and do not require special equipment. For this reason, both advanced and developing countries have adopted a variety of hemagglutination tests. A popular worldwide hemagglutination test is used for the detection of antibody to Treponema pallidum , marketed as SERODIA-TP, by Fujirebio, Inc. (Tokyo, Japan). In the United States, the hemagglutination test for T. pallidum was approved in 1981 by the Centers for Disease Control and Prevention. It was also recommended by the World Health Organization because of its superiority over other tests in terms of specificity and sensitivity ( ).
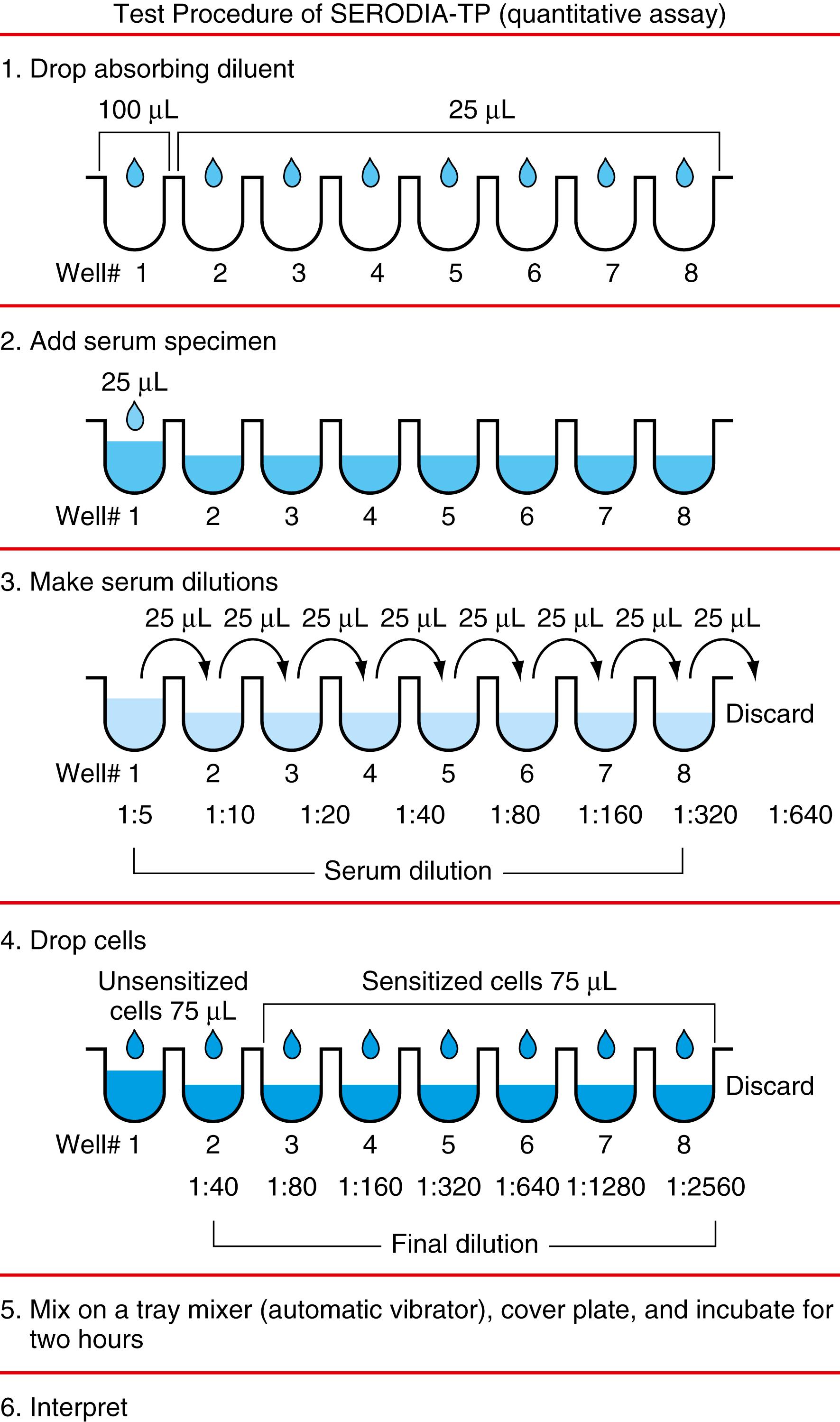
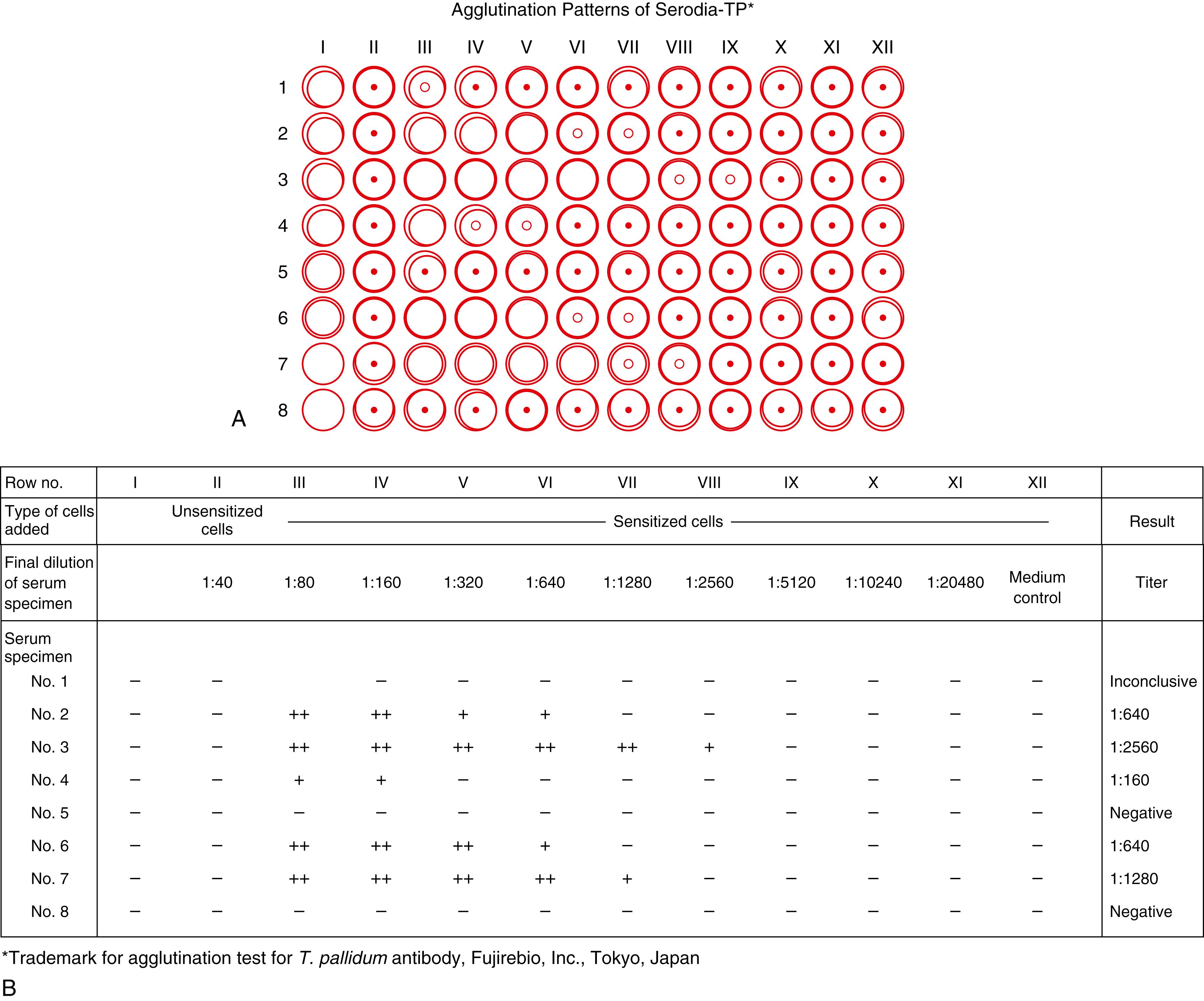
In the T. pallidum agglutination test, the reagent consists of sensitized and unsensitized sheep red cells as well as a serum diluent solution for the reconstitution of lyophilized sensitized cells as a positive control. Both qualitative and semiquantitative tests can be carried out using the following serum dilution protocol and a titer plate as a reaction container as follows: using a 25-μL pipette dropper, place 4 drops 100 μL of serum diluent in well 1 ( Fig. 45.5) and 1 drop in wells 2 to 4 for the qualitative assay (wells 2–8 are used for the quantitative assay). The resulting agglutination patterns are shown in Figure 45.6 . Negative patterns, indicating that immunoreaction has not taken place, show condensed flocculation particles with a cross-packing structure at the bottom of the microtiter well. On the other hand, positive patterns, indicating that immunoreaction has occurred, show an expanded agglutination pattern of particles. Agglutinated particles cannot be sedimented any further to obtain condensation as negative patterns because their global shape is lost on account of the agglutination of particles. In the first and second rows, serum and unsensitized cells (negative control) were used, respectively. Specimens 1 and 2 show negative results. Specimens 3 to 8 show negative or positive results, depending on the specimen dilution. A positive result is observed for specimens 7 and 8 up to 1:2560 (rows 3–8).
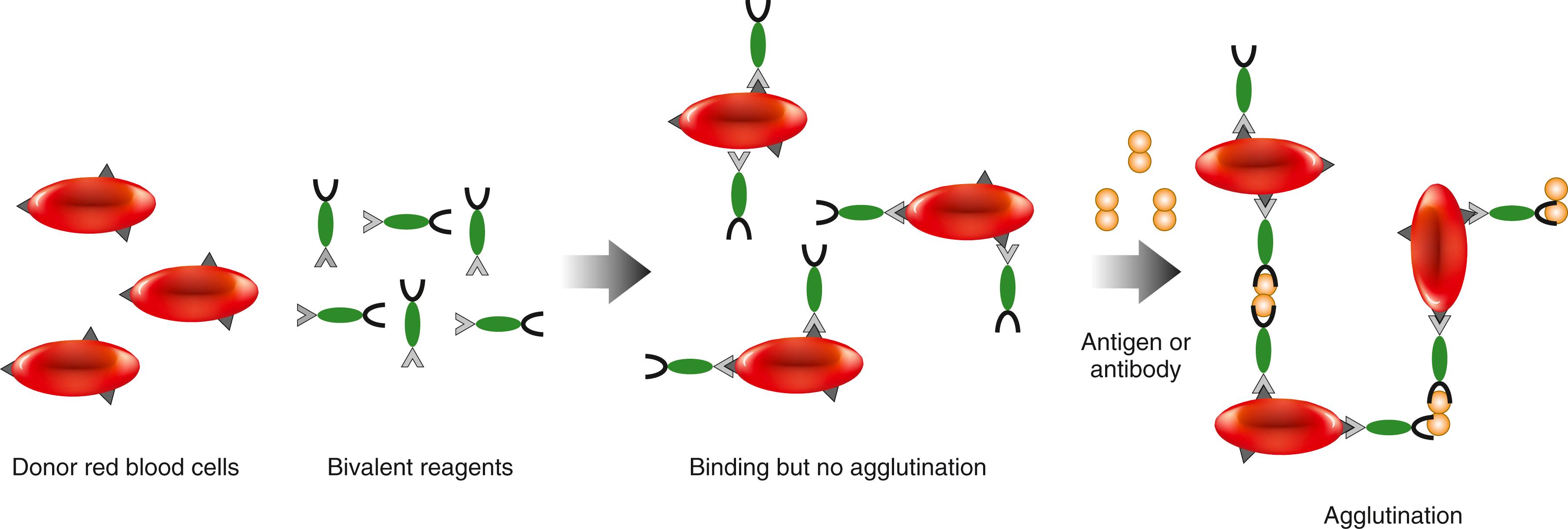
Hemagglutination kits are now available for the detection of antibodies to hepatitis type B virus (HBV), hepatitis type C (HCV), human immunodeficiency virus (HIV1/2), thyroglobulin, thyroid microsome, and other substances. Reverse hemagglutination tests are used for the detection of HBV surface antigen, α-fetoprotein (AFP), human hemoglobin in stool specimens, and so forth. The sensitivity of hemagglutination tests is approximately 50 ng/mL for antigen (analyte) detection. Kemp and colleagues (1988) developed a hemagglutination assay that uses a mouse monoclonal antibody specific to the surface antigen of human erythrocytes. The antibody can recognize an epitope common to different types of red cells or to abnormal cells, such as those found in sickle cell anemia. As shown schematically in Figure 45.7 , blood cells in the specimens are used as solid-phase particles; the resulting agglutination can be observed by the naked eye. Bivalent antibodies are conjugated chemically; thus, one antibody specifically reacts with the surface epitopes of the blood cell and the other is specific to the target antigen or analyte. The assay is applied for the detection of antibody to HIV and for the detection of various antigens. Unlike conventional agglutination formats, the separation of plasma or serum is not required. This assay is simple, saves time, and offers safety advantages because it eliminates the need for serum separation for hazardous HIV- or HBV-positive specimens.
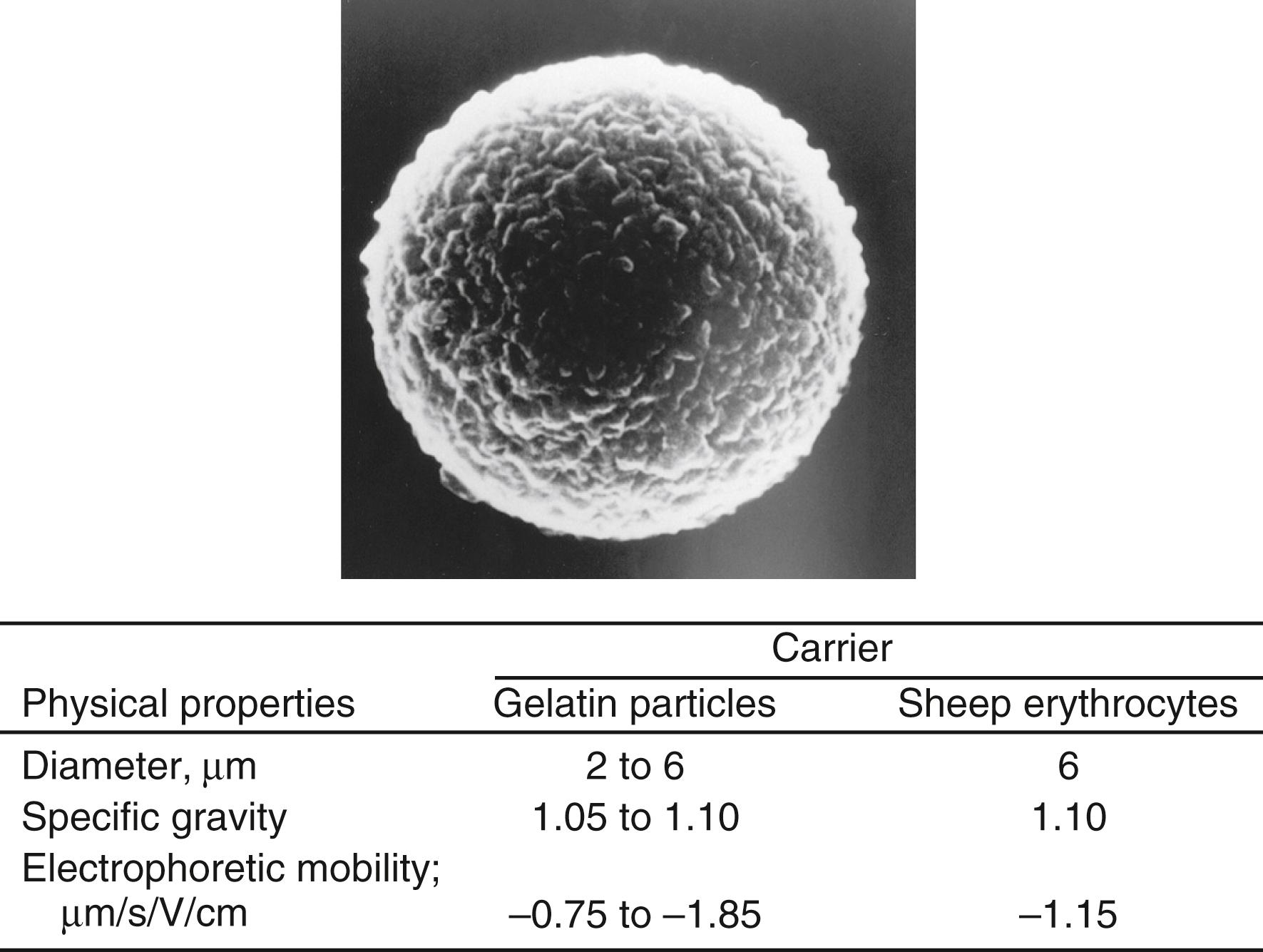
The development of a special gelatin particle with a highly hydrophilic surface that is able to prevent nonspecific binding of materials present in a specimen has provided an alternative to erythrocytes ( ). This particle is made by phase separation and three-dimensional cross-linkage at 40°C and optimum pH. The resulting particle is fixed with formaldehyde or glutaraldehyde, and its diameter is about 3 μm. The physical properties of gelatin particles in comparison with those of erythrocytes are shown in Figure 45.8 . A gelatin particle has no antigenicity; therefore, it is free from problems associated with heterophilic antibodies when erythrocytes are used as particles. This type of artificial particle requires much less serum dilution to avoid nonspecific binding and guarantees a more sensitive detection than with blood cells. Other synthetic particles ( ) made from block copolymer composed of l -glutamic acid and derivatives have been developed by Hirayama and colleagues (1991) as alternatives to gel particles. These synthetic particles can be stained with dye of any color because the particles themselves are colorless, as are gelatin particles. The gelatin particle agglutination test was initially applied to the detection of antibodies to human T-cell lymphotropic virus, which was first discovered as a retrovirus in humans. This agglutination test ( ) soon became very popular for blood screening for HIV, HBV, and HCV because of its high sensitivity and specificity, its simplicity, and the fact that strict temperature control is not required to perform the tests. Gelatin particle agglutination can replace any assay based on hemagglutination with the exception of assays that use RBCs in specimens as particles.
Latex agglutination ( ), using latex as particles, has been employed for the detection of various analytes, such as hCG for qualitative pregnancy tests, and for the quantitative detection of other plasma proteins with or without instrumentation. The format of this qualitative assay is simple. For example, one might have to mix only a couple of drops of latex sensitized with reactant and specimen using a stick on a black slide. Two to three minutes later, phase inversion agglutination resulting from the immune reaction can be observed by the naked eye. Latex agglutination was adapted for quantitative assays using light detection methods based on turbidimetry (light absorption) or nephelometry (light scattering). With these techniques, latex agglutination achieves an enhanced sensitivity of subnanograms per milliliter, while the sensitivity of the assay measuring intact precipitation of the Ag–Ab complex remains below 0.5 μg/mL.
Light adsorption (light loss by scattering on the surface of a particle) is proportional to the diameter of the particle and depends on the wavelength of light used. Most latex reagents commercially available use latex with a diameter of less than 1 μm and are applied in automated chemistry analyzers using photometric measuring principles. To further improve sensitivity, efforts were made to upgrade reagents and instrumentation and to optimize particle size, selection of appropriate wavelength, and improvements in computer software for integration of data. Automated systems now use latex particles that can perform about 200 tests per hour at a subnanogram-per-milliliter sensitivity.
The particle-counting immunoassay (PACIA) ( ) uses optical cell counting to assess the decrease in the number of unagglutinated particles after an immune reaction. In the PACIA format, the rate assay—that is, the rate of decrease in the number of unagglutinated particles—or the endpoint of the assay reaction can be measured. The endpoint assay can be used to obtain a sensitivity at the nanogram-per-milliliter level. However, a longer incubation time is required for an endpoint immunologic reaction.
Quasielastic scattering immunoassays were developed using a measurement of the change in response to particle size distribution ( ). This technique uses a laser light beam to measure the reduction in the mean diffusion coefficient of particles as a result of immune reaction. Other methods measure the change in angular anisotropy of scattered light with the increasing average size of the particle. Particles with diameters about equal to the size of a wavelength achieve a size-dependent angular variation of scattered light.
Indirect hemagglutination and gelatin particle agglutination tests using microtiter plates are popular procedures for qualitative and semiquantitative determination of various analytes. These tests do not require additional instrumentation or strict temperature control. Most important, they guarantee a sensitivity equal to or greater than conventional enzyme immunoassays when applied to antibody detection of infectious agents. Latex as a solid phase provides great kinetic advantages, such as a shorter immune reaction time. For this reason, it was possible to adapt latex for use in existing or sophisticated instruments by applying different principles, resulting in an assay sensitivity of about three orders of magnitude greater than standard immunoprecipitation assays. Latex is susceptible to interferences from unknown factors present in specimens. To eliminate this problem, various absorbents have to be used in both the reagent and the incubation medium. The great advantage of particle immunoassay is its simplicity because it does not require separation of bound and free reactants.
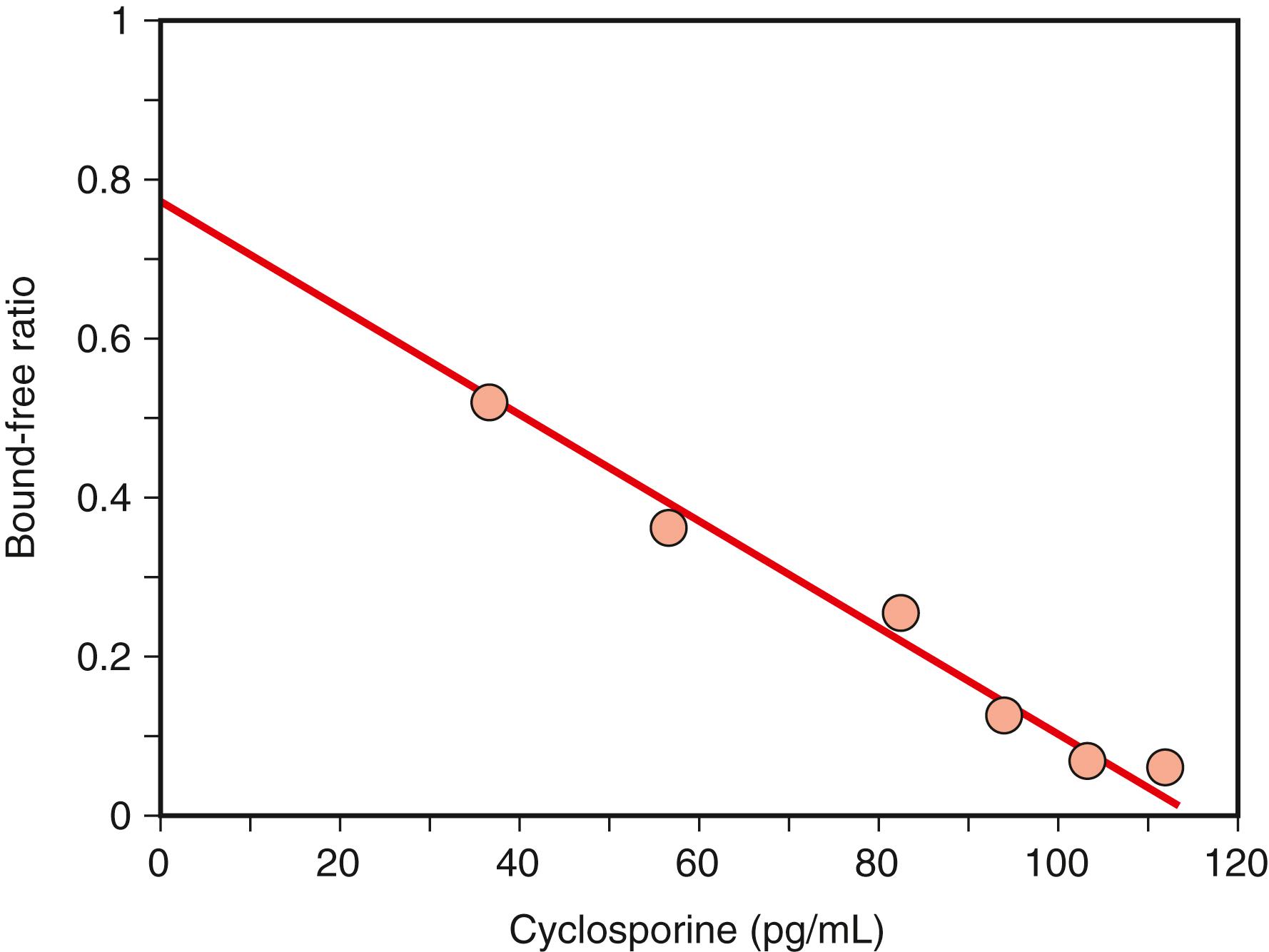
Since RIA technology using radioisotopes as labels was first developed by , it has been improved dramatically in sensitivity and precision. Numerous variations in the method have been introduced into the clinical laboratory. Two main RIA techniques, competitive and noncompetitive heterogeneous formats, require washing steps to separate bound and free labels (conjugates). The competitive assay follows the law of mass action, which specifies the reaction between analytes and binding proteins, receptors, and antibody. The key factor in assay optimization is the binding affinity of the antibody. The Scatchard plot of the ratio of bound to free antibody to analyte concentration is commonly used to evaluate antibody performance. Figure 45.9 shows the Scatchard plot for cyclosporine determination. As described earlier in the Kinetics of Antigen–Antibody Reaction section, from the following equation:
the y -axis is the B/F ratio that is proportional to free [ Ab ] from [ Ab ] t − B , equal to free [ Ag ]. Therefore, K a represents the slope of the plot. In Figure 45.9 , the affinity constant K a is 8.1 × 10 9 L/M for the antibody specific to cyclosporine. Radioactive emissions, such as γ-rays of iodine-125 labels, can be measured in terms of counts per minute (CPM) using a γ-scintillation counter. Typical radioisotopes used as labels and their properties are shown in Table 45.3 . The choice of label affects the assay protocol considerably. For example, the most popular label, iodine-125, requires a rather short time for signal counting but has a limited shelf life because of its short half-life. On the other hand, the tritium ( 3 H) label requires a longer time for counting, thereby increasing the total assay time. Most RIAs now use iodine-125 as labels to expedite the conjugation process and to retain the biological activity of the reactants. A commonly used method for conjugation of iodine-125 with proteins is the chloramine-T method ( ). Tyrosine, in particular, has greater reactivity with iodate because of its hydroxy group at the para-position on the aromatic ring. The signal can be measured in terms of CPM as γ-ray emissions. Unlike enzymes, isotopes as labels with a small Stokes radius are not likely to disrupt antigenic activity because of the lack of steric hindrance when isotopes are conjugated with small antigens (haptens).
| Isotope | Half-Life | Type of Decay | Specific Activity (mCi/mmol) |
|---|---|---|---|
| 125 I | 60 days | γ | 2200 |
| 131 I | 8.1 days | β – , γ | 16,100 |
| 3 H | 12.3 years | β – | 29 |
| 14 C | 5760 years | β – | 6062 |
| 32 P | 14.3 days | β – | 9120 |
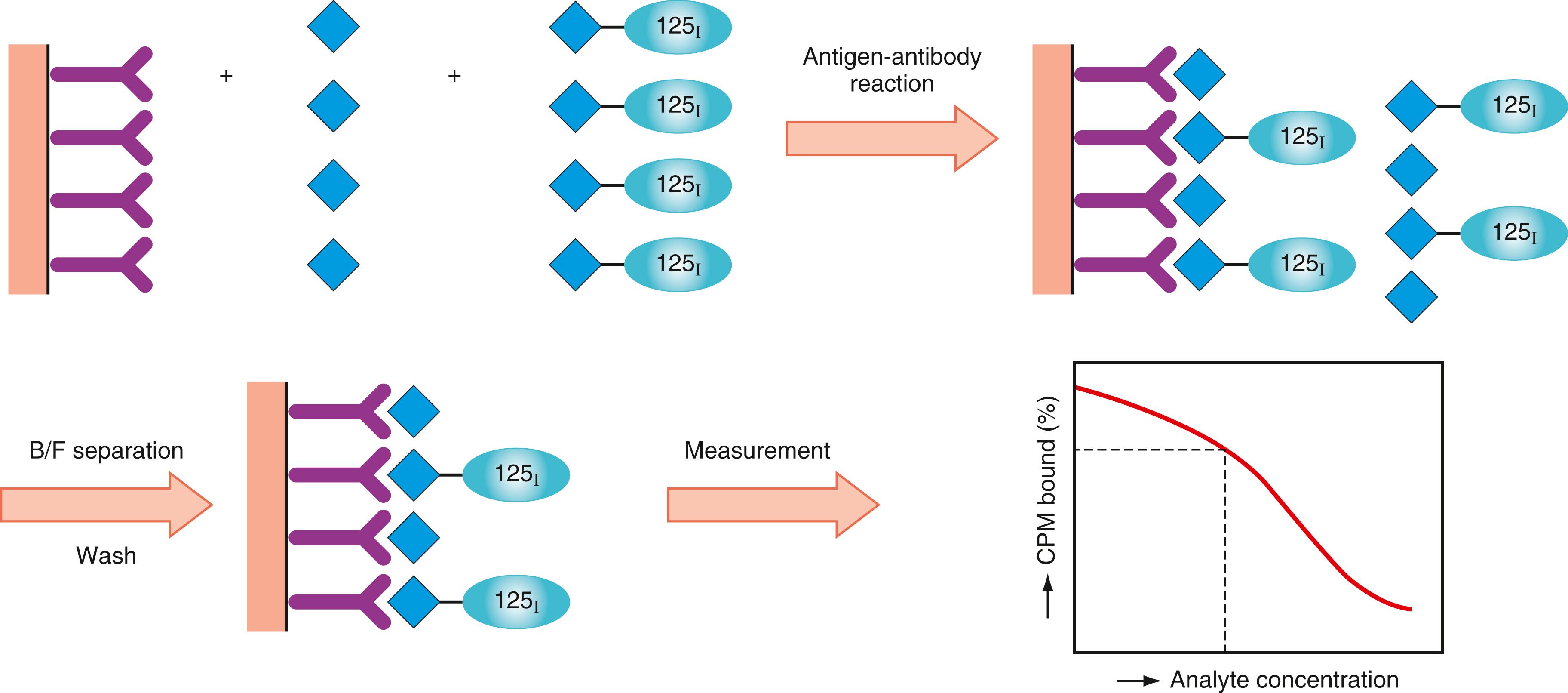
Various methods (antigen excess) based on the competitive binding reaction ( ) to antibodies between labeled antigens and nonlabeled antigens (analytes present in the specimen) have been developed for a wide variety of analytes. A conventional competitive method is shown in Figure 45.10 . At first, known amounts of labeled antigen and antigen in the specimen are mixed and reacted competitively with a constant amount of antibody coated on a solid phase, such as sepharose beads or the inner wall of plastic tubes. After the immune reaction reaches its equilibrium, the mixture is washed to remove unreacted conjugates and antigens, and the immune complex trapped on the solid phase is separated. The washing step is referred to as B/F separation. Applying the competitive principle, the plot of antigen-bound percentage against logarithmic concentration of analyte provides the standard curve shown in Figure 45.10 . The CPM plot on the standard curve gives the concentration of analytes.
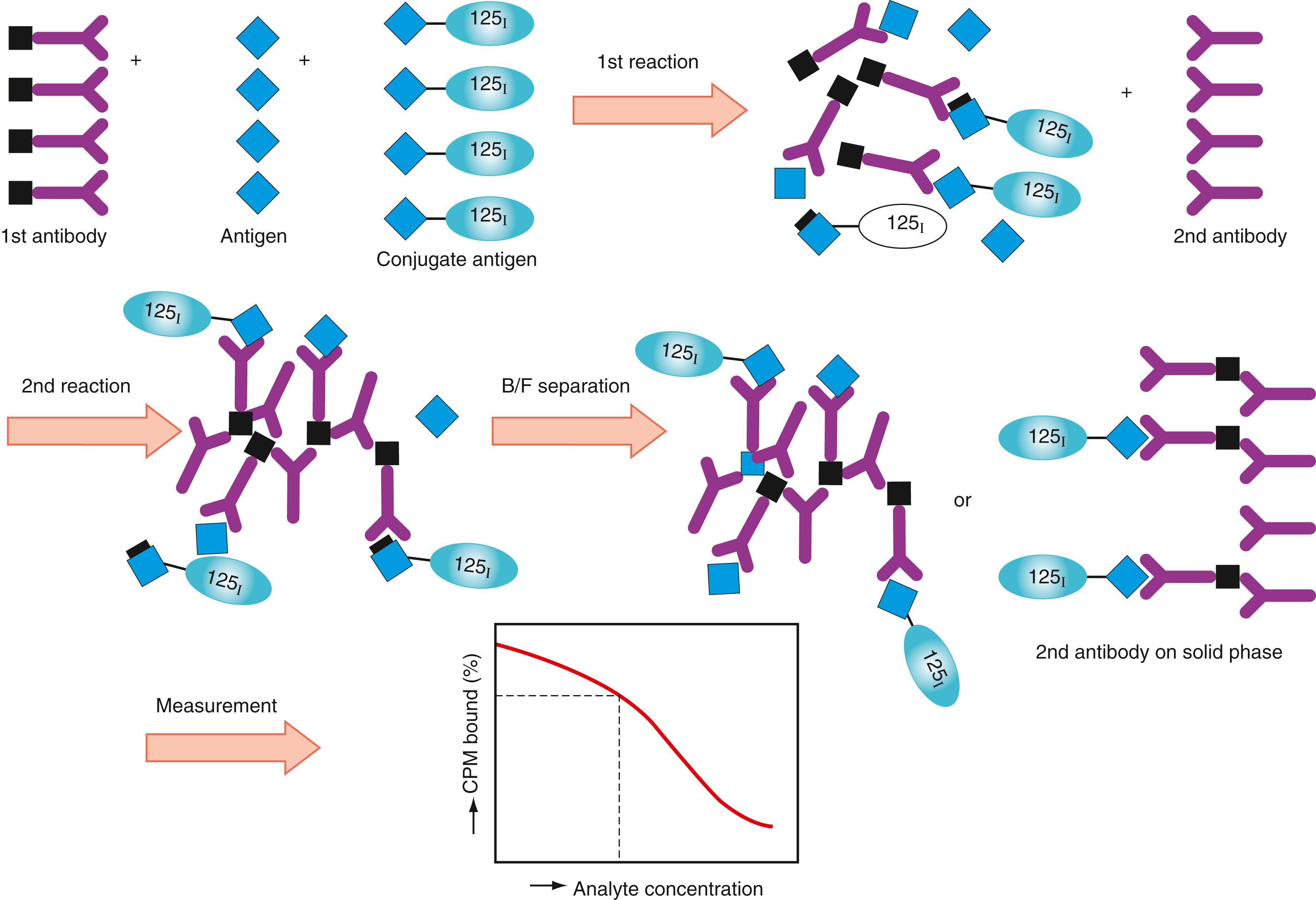
A second antibody method is shown in Figure 45.11 . The first antibody specific to a particular antigen (analyte) reacts competitively with both the conjugate and the antigen. Then, the immune complex is captured by the second antibody specific to the first antibody, on the solid phase. When the second antibody is coated on a fine solid phase, the immune complex on the second reaction can be separated as a precipitant from the unreacted molecules. For antibody determination, labeled antibody and antibody in the specimen react competitively with the antigen (analyte) fixed on the solid phase. The steeper slope of the standard curve provides more precise data. Competitive methods require lesser amounts of antibody or antigen (analyte) than the sandwich-type assays described later. Noncompetitive assays, originally demonstrated by , have recently gained greater popularity.
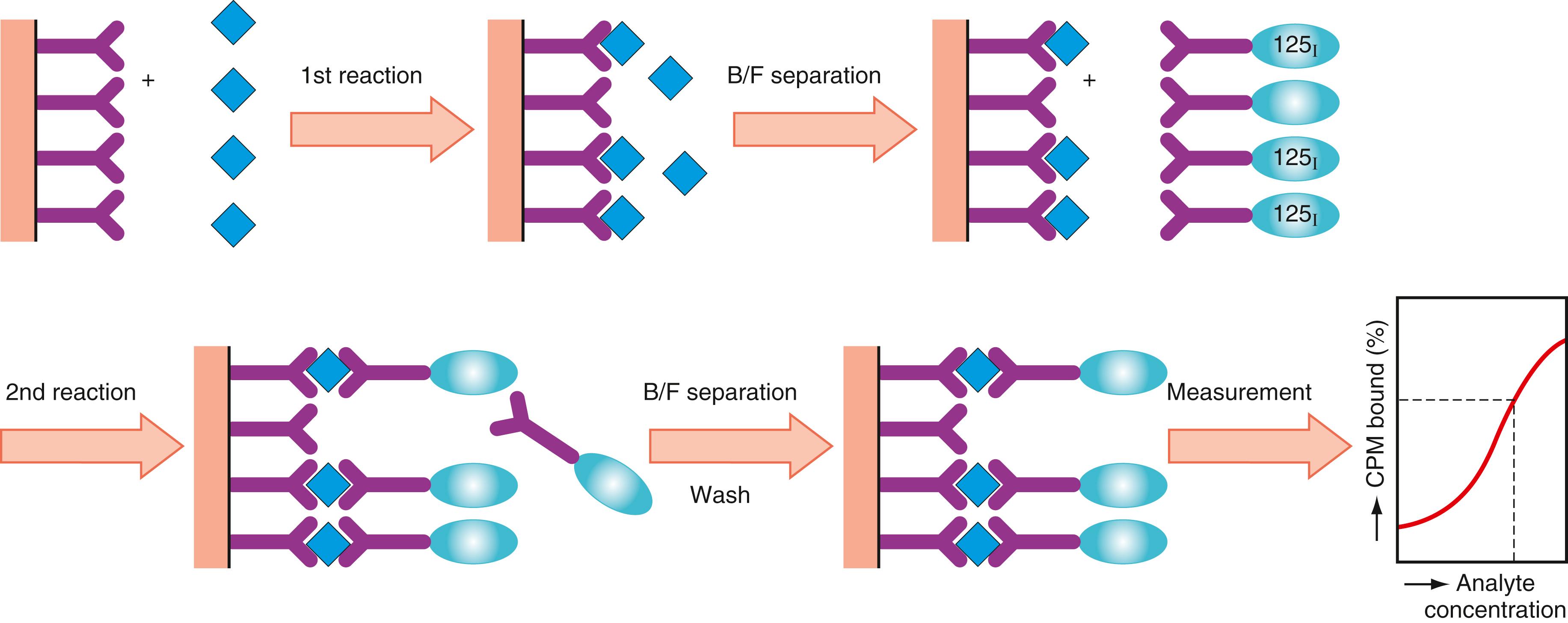
Immunoradiometric assays and sandwich assays ( Fig. 45.12 ) have alternative relationships between analyte and antibody. The classical competitive assay achieves an immunologic response with a minimum amount of antibody; the sandwich assay uses a large amount of antibody on the solid phase. Monoclonal antibody technology has made it possible to manufacture large quantities of specific antibodies at moderate costs, thereby allowing the sandwich assay to be exploited. The sandwich assay, which uses excess antibody, is more sensitive than the competitive assay. When background noise is completely omitted, the ultimate theoretical sensitivity of the sandwich assay is one molecule of analyte, which is possible when the amount of antibody used in the assay system approaches infinity. As shown in Figure 45.12 , the antibody on the solid phase first captures the antigen (analyte) in the specimen. Following B/F separation, the conjugate reacts with the antigen (analyte) fixed on the solid phase. The signal can then be counted after the elimination of free conjugates through a washing step. This assay requires antigens with more than two antigenic sites. When two different antibodies (i.e., a solid-phase antibody specific to one antigenic site and a conjugate antibody specific to another antigenic site) are used, the assay protocol can be simplified by performing a one-step sandwich. Thus, the solid phase can mix together with the antigen in the specimen and conjugate simultaneously. Interference does not occur because the antibodies on both the solid phase and conjugate are capable of recognizing different antigenic sites. In this assay, the signal generated is proportional to the analyte concentration present in the specimen, as in the two-step sandwich assay. This assay method can be applied to antibody detection with an assay format using antigen as the solid phase or labeled antigen. For a two-step format, antigen as the solid phase or labeled antibody specific to the target antibody can be used. Using the sandwich format, assay sensitivity ( ) is high. An example is seen when an immunoradiometric assay is used for determination of the peptide hormone thyroid-stimulating hormone (TSH). The level of assay sensitivity is below 0.07 μU/mL of TSH as compared with about 0.7 μU/mL with conventional competitive antigen-labeled assay.
Compared with other immunoassays, RIAs are advantageous for a number of reasons, including precision and high sensitivity, ease of isotope conjugation, signal detection without optimization, and stability against interference from the assay environment, among others. Disadvantages of RIAs are the short shelf-life of the reagents and the need to protect against hazardous radioactivity. Furthermore, as discussed later, RIAs may not be applied to homogeneous immunoassays because signals from isotopes cannot modulate Ag–Ab reactions.
Quantitative immunoassays using enzymes as labels were developed as alternatives to radioisotopes ( ; ; ). The most widely used are the enzyme-linked immunosorbent assay (ELISA), the EIA, and the enzyme-multiplied immunoassay technique (EMIT), which is a registered trade name of SYVA Co. (Dade Behring Inc., Cupertino, CA) ( ). Essentially, heterogeneous EIAs are similar to RIAs, except that they use enzymes as labels. Enzymes make it possible to develop homogeneous EIAs, eliminating the otherwise necessary washing steps for B/F separation. Table 45.4 compares the features of heterogeneous and homogeneous EIAs versus RIAs. Improvements in EIAs have provided many innovative formats with different degrees of speed, sensitivity, simplicity, and precision. Advantages and disadvantages of EIAs are listed in Box 45.1 ( ).
| Assay | Immunologic Reaction Steps | Enzyme Reaction Steps | Signal Detection Steps |
|---|---|---|---|
| Radioimmunoassay Sample + (labeled analyte or Ab) − 125 I |
Immunoreaction with washing steps for separation | Not required | Radioactive decay (γ-ray) |
| Heterogeneous enzyme immunoassay Sample + (labeled analyte or Ab) − enzyme |
Immunoreaction with washing steps for separation | Enzyme reaction with additional reagent | Optical density Fluorescence Luminescence |
| Homogeneous enzyme immunoassay Sample + (labeled analyte or Ab) − enzyme or cofactors |
Immunoreaction and/or systemic reaction is carried out in one solution, which includes reagent for signal development of enzyme. | Immunoreaction and/or systemic reaction is carried out in one solution, which includes reagent for signal development of enzyme. | Optical density Fluorescence Luminescence |
Advantages
Sensitive assays can be developed by the amplification effect of enzymes.
Reagents are relatively cheap and can have a long shelf life.
Multiple simultaneous assays can be developed.
A wide variety of assay configurations can be developed.
Equipment can be inexpensive and is widely available.
No radiation hazards occur during labeling or disposal of wastes.
Disadvantages
Measurement of enzyme activity can be more complex than measurement of the activity of some types of radioisotopes.
Enzyme activity may be affected by plasma constituents.
Homogeneous assays at the present time have the sensitivity of 10 −9 M and are not as sensitive as radioimmunoassays.
Homogeneous EIAs for large protein molecules have been developed but require complex immunochemical reagents.
The assay principle of heterogeneous EIAs is similar to that of RIAs, except that enzyme activity, not radioactivity, is measured. EIAs require a secondary process to obtain signals through the catalytic reaction of enzymes. Microtiter plate wells, plastic beads, plastic tubes, magnetic particles, and latex with filters, among others, can be used as the solid phase for the separation of bound and free conjugates. The use of small magnetic particles and latex allows shortening of the immunoreaction time, thereby reducing the total assay time. The development of substrates to be cleaved by enzymes was marked by the introduction of colorimetric and fluorometric substrates, and later chemiluminescent substrates, which increased the signal sensitivity. The enzymes commonly used in various heterogeneous EIAs are horseradish peroxidase, alkaline phosphatase, β-galactosidase, glucose oxidase, urease, and catalase. The most widely used enzymes and their characteristics are listed in Table 45.5 . Assay sensitivity using enzymes may be determined by the turnover rate of each enzyme and by selection of signal measurement in which chemiluminometry is the most sensitive method.
| Enzyme Characteristic | Peroxidase (EC1.11.1.7) | β-Galactosidase (EC3.2.1.23) | Alkaline Phosphatase (EC3.1.3.1) |
|---|---|---|---|
| Source | Horseradish | Escherichia coli | Bovine intestine |
| Molecular weight, daltons | 40,000 | 530,000 | 100,000 |
| Specific activity | 250 U/mg | 600 U/mg | 2500–5000 U/mg |
| Turnover rate ∗ | 10,000 | 318,000 | 250,000 |
| Measurement of enzyme | Colorimetry, fluorometry, luminometry | Colorimetry, fluorometry, luminometry | Colorimetry, fluorometry, luminometry |
| Highly sensitive measurement | Luminometry | Fluorometry | Luminometry |
| Method for enzyme labeling | Periodate oxidation (Nakane method) | Dimaleimide method, cross-linking reagent † | Glutaraldehyde method, cross-linking reagent † |
∗ Number of substrate molecules produced by a molecule of enzyme for 1-minute reaction; molecule number/minute.
† The reagent contains chemically reactive groups, such as maleimide and succinimide.
The assay format of heterogeneous EIAs, similar to RIAs, can be divided into competitive and noncompetitive assays ( Fig. 45.13 ). Competitive assays (analyte excess) use antigen–enzyme conjugates ( Fig. 45.13A ). Noncompetitive assays (reactant excess) include two-site immunometric sandwich assays ( Fig. 45.13B1 ) and indirect assays to measure antibodies ( Fig. 45.13B2 ). Immunometric sandwich assays have gained much popularity for the determination of antigens such as tumor markers, plasma proteins, and infectious agents. Indirect assays for antibody measurement ( Fig. 45.13B2 ) have been adopted for the detection of antibodies to infectious agents (e.g., HIV, HBV, HCV) and to autoantibodies.
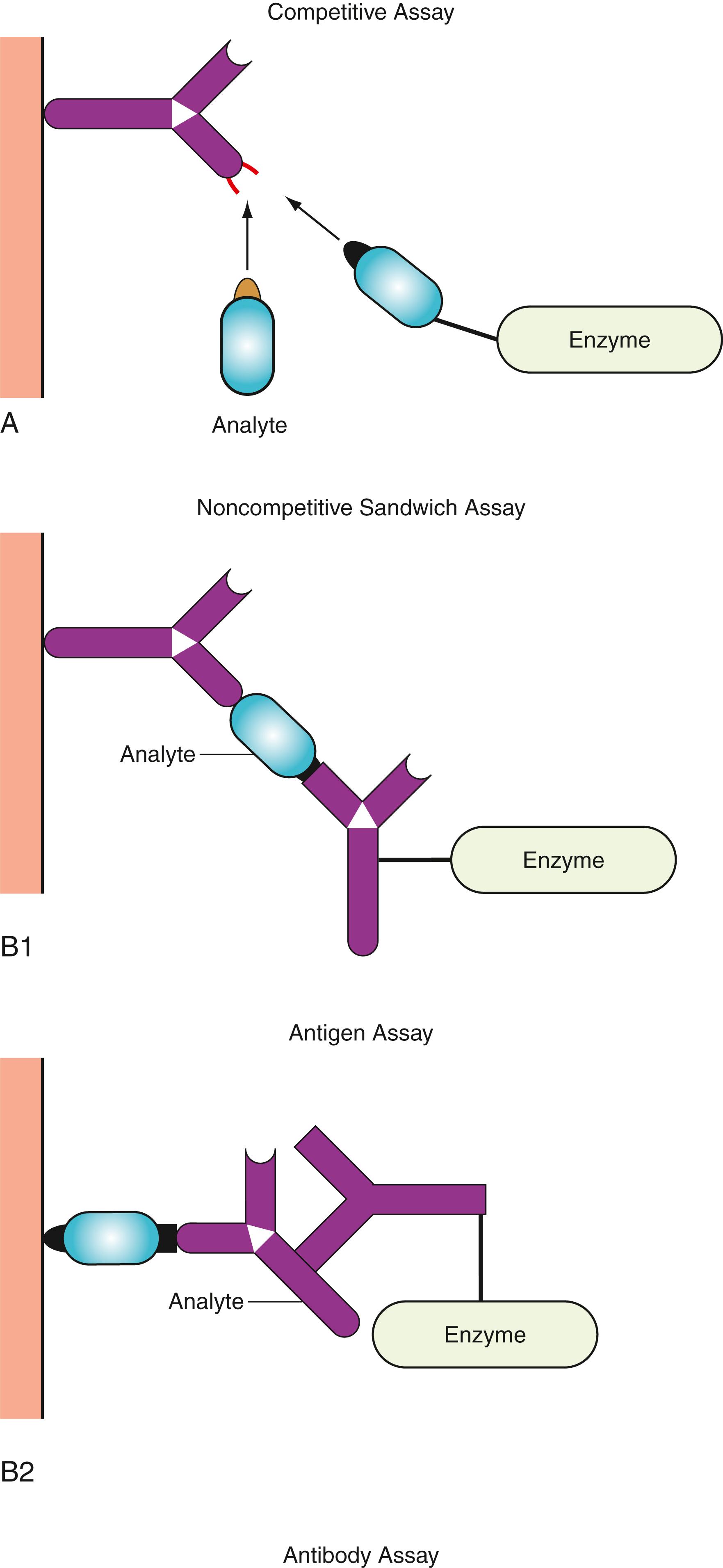
Heterogeneous EIA (see Fig. 45.13 ) consists of the following steps:
The solid phase with the attached reactants is mixed with analyte, regardless of whether the assay is based on the competitive or noncompetitive format.
Following the addition of conjugate and incubation, washing steps are performed with a buffer solution containing a detergent, one or two steps after the immune reaction. The immune reaction should reach certain yields to obtain a stable and precise assay.
The solid phase, with the immunocomplex containing enzyme-labeled antigen or antibody, is incubated at constant temperature with the enzyme–substrate solution.
The enzyme reaction is stopped (stopping is not needed in rate assay), and the substrate reaction product is measured with various detectors, depending on the substrate used.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here