Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Idiopathic interstitial pneumonias comprise a number of relatively uncommon clinicopathologic entities, which can be distinguished from one another and from other forms of diffuse interstitial lung disease by their clinical, radiologic, and histologic features. The basic approach to diagnosing these entities is included in American Thoracic Society/European Respiratory Society (ATS/ERS) consensus statements from 2002, 2011, and 2013. The major entities referenced include idiopathic pulmonary fibrosis (IPF), idiopathic nonspecific interstitial pneumonia (NSIP), cryptogenic organizing pneumonia (COP), acute interstitial pneumonia (AIP), respiratory bronchiolitis–associated interstitial lung disease (RB-ILD), and desquamative interstitial pneumonia (DIP). Although two of these entities are typically smoking associated (RB-ILD and DIP), these have traditionally been included in the category of idiopathic ILDs. Another probable variant of smoking-related ILD, respiratory bronchiolitis with fibrosis (RB-F) will also be discussed later. The 2013 ATS/ERS statement also includes the two rare entities of idiopathic lymphoid interstitial pneumonia (LIP) and idiopathic pleuroparenchymal fibroelastosis (PPFE) ( Table 16.1 ). Finally, a category of unclassifiable interstitial pneumonia is also recognized to acknowledge that some cases of idiopathic interstitial pneumonia defy precise classification ( Table 16.1 ).
| Major Idiopathic Interstitial Pneumonias | Histologic Pattern |
|---|---|
| Idiopathic pulmonary fibrosis | Usual interstitial pneumonia |
| Nonspecific interstitial pneumonia | Nonspecific interstitial pneumonia pattern |
| Cryptogenic organizing pneumonia | Organizing pneumonia |
| Acute interstitial pneumonia | Diffuse alveolar damage |
| Respiratory bronchiolitis-associated interstitial lung disease | Respiratory bronchiolitis |
| Desquamative interstitial pneumonia | Desquamative interstitial pneumonia pattern |
| Rare Idiopathic Interstitial Pneumonias | |
| Lymphoid interstitial pneumonia | Lymphoid interstitial pneumonia pattern |
| Idiopathic pleuropulmonary fibroelastosis | Intraalveolar fibrosis with elastosis |
| Unclassifiable Idiopathic Pulmonary Fibrosis | |
A fundamental principle of clinical ILD classification is that histopathology needs to be combined with the clinical and radiologic impression for a final clinicopathologic diagnosis. This process is a dynamic one in that findings in one sphere are reconsidered in light of findings in the other. Relevant features include the location of the biopsy taken with respect to the radiologic findings, the time course of the process, and any drug therapy provided. In recognition of this complexity, the diagnostic gold standard in this field is considered a multidisciplinary conference among radiologists, pathologists, and clinicians during which patients are discussed from the viewpoint of the various specialties. The diagnosis may also evolve as additional information becomes available. These conferences are particularly useful when multiple patterns are present or there is conflicting information. Use of such a conference is associated with markedly increased confidence and reproducibility in the final diagnosis. In the absence of such a conference, it should be made clear in any pathology report that clinical and radiologic correlation is required for a final diagnosis.
Although transbronchial lung biopsy may be used to identify some diseases associated with ILD, including sarcoidosis and certain infections, such a biopsy does not typically yield sufficient material for the diagnosis of the majority of cases of idiopathic interstitial pneumonia. There are selected exceptions to this general rule, keeping in mind that the interpretation is highly dependent on the clinical circumstance. In a smoking patient with patchy terminal airway ground-glass opacities, a transbronchial biopsy that shows pigmented macrophage accumulation in conjunction with a lavage that shows absence of lymphocytosis may be considered diagnostic of RB-ILD. However, in cases with more severe fibrosis or with a history suggestive of another process, such a finding might be considered nonspecific. If a decision is made to proceed with a surgical lung biopsy, optimal sampling needs to be discussed with the surgeon. Any surgical lung biopsy should include samples from at least two lobes and should be several centimeters in depth and width. Biopsies should avoid both areas of end-stage lung and areas that are radiologically unremarkable. Finally, it is critically important for the pathology laboratory to inflate such specimens with formalin before processing, ideally after the surgical staples have been removed. This is easily done with a syringe and a small needle filled with formalin. In the absence of such inflation, biopsies may not be interpretable.
A chronic interstitial pneumonia of unknown etiology showing the histologic pattern of usual interstitial pneumonia in a surgical lung biopsy
The incidence is estimated as 10.7 cases per 100,000 males per year and 7.4 cases per 100,000 females per year. The incidence has been increasing in recent years in the United States.
Slightly more common in males
There is a dramatic age-related increase with almost all cases over 50 and incidence rising after that
Insidious onset of otherwise unexplained dyspnea on exertion and cough
Bibasilar, inspiratory crackles (dry or “Velcro” type in quality)
Abnormal pulmonary function studies that include evidence of restriction and impaired gas exchange
On HRCT, bibasilar reticular abnormalities with or without honeycombing and absence of features against the disease, including ground glass greater than the reticular abnormality, absence of extensive airtrapping, and upper or midlung predominance or areas of consolidation
The clinical course is typically one of gradual deterioration punctuated in some cases by acute declines
The median length of survival from the time of diagnosis for untreated cases is between 2.5 and 3.5 years, but prolonged survival happens in a minority of cases
Definite UIP: All of the following
Extensive fibrosis, which disrupts the architecture in a subpleural and paraseptal distribution. Fibroblast foci, which are at the border between areas of established fibrosis and uninvolved lung. Areas of normal lung. Absence of features inconsistent with the diagnosis, including more than a minor amount of organizing pneumonitis and inflammation (including granulomas), hyaline membranes, airway-centered changes, identifiable inorganic dust or other more specific features
Probable UIP: Honeycombing only or patchy fibrosis without fibroblast foci or diffuse fibrosis with fibroblast foci in absence of features inconsistent with diagnosis
Possible UIP: Patchy fibrosis but not as severe as above but with absence of features inconsistent with diagnosis
Inconsistent with UIP: Any case with features inconsistent with diagnosis
Nonspecific interstitial pneumonia, fibrosing pattern
Diffuse alveolar damage, organizing phase
Unclassifiable fibrotic interstitial lung disease
Airway centric fibrotic interstitial lung disease (eg, chronic hypersensitivity pneumonitis)
Idiopathic pulmonary fibrosis
Connective tissue diseases
Drug or radiation reaction
Hypersensitivity pneumonia
IPF, formerly sometimes known as cryptogenic fibrosing alveolitis, is the most common idiopathic interstitial pneumonia. Lung biopsies from patients with IPF show the histologic pattern of usual interstitial pneumonia (UIP). The UIP pattern shows both normal lung and established fibrosis, the latter of which significantly disrupts the lung architecture. The histologic pattern of UIP is not completely specific for IPF; a similar pattern can be seen in some patients with connective tissue diseases, some cases of chronic hypersensitivity pneumonitis, and a few other conditions. The clinicopathologic term of IPF is appropriate only if other causes of UIP are excluded, typically clinically. For instance, if a lung biopsy reveals UIP in the setting of an underlying disease (eg, rheumatoid arthritis), the final diagnosis should be UIP, and the underlying condition should be mentioned, if known (eg, UIP associated with rheumatoid arthritis).
The estimated incidence of IPF is between 6.8 and 16.3 cases per 100,000 in the general population per year, with a slight excess of males over females, and appears to be rising over time in the United States. The incidence rises with age starting roughly at the age of 50. Risk factors include cigarette smoking, a variety of environmental exposures, and gastroesophageal reflux. Although a variety of microbial agents, especially herpesvirus family members including Epstein-Barr virus, have been associated with IPF, none are currently considered causal. Patients typically present with a history of dyspnea on exertion and nonproductive cough. Bibasilar, late inspiratory, fine crackles (“Velcro” rales) are found on chest auscultation. Pulmonary function tests (PFTs) reveal restriction and impairment of gas exchange. Lung volumes are typically reduced, but may be normal in patients with superimposed emphysema. A minority of patients may present with a so-called acute exacerbation in which case the biopsy will show extensive established fibrosis, as well as superimposed acute lung injury.
Chest radiographs in patients with IPF typically show a bilateral reticular pattern, involving mainly the lower lung zones with volume loss. Less than 10% of the patients have a normal chest x-ray at presentation.
Early in the course of disease, high-resolution computerized tomography (HRCT) typically shows lower lobe peripheral reticular markings in the absence of other features. Later, there is volume loss, traction bronchiectasis, and honeycombing. A number of features are considered radiographically atypical for IPF/UIP, including ground-glass opacities more extensive than areas of reticulation, areas of consolidation, upper lobe predominance, peribronchovascular predominance, extensive airtrapping, and/or presence of cysts and nodules. The current guidelines for evaluation of HRCT for IPF recognizes cases as definite UIP, possible UIP, and inconsistent with UIP. Definite UIP shows the presence of lower lobe and peripheral reticular opacities with honeycombing and the absence of findings that are considered atypical for the diagnosis. About half of patients with IPF will have such a scan, which is considered sufficiently specific as to make a diagnosis in absence of a surgical biopsy. Possible UIP is similar to definite UIP but there is no definite evidence for honeycombing. So-called inconsistent scans have at least some of the atypical features described earlier. The term inconsistent is unfortunate because, depending on the result of the biopsy, some of these patients will, in fact, have IPF (see discussion of integration of radiology with pathology later).
The lungs tend to be small, and the visceral pleura shows retractions along the interlobular septa. The cut surfaces reveal patchy, dense, established fibrosis, more severe in the lower lobes ( Fig. 16.1 ). Central and upper portions of the lung may be relatively preserved. The fibrosis typically produces grossly observable fibrotic cysts. Because these fibrotic cysts tend to be back to back with little intervening normal lung, they resemble a honeycomb—hence the term honeycombing.
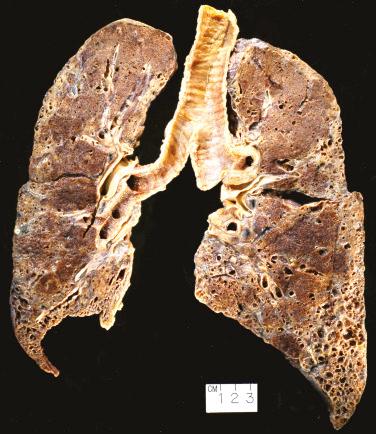
Microscopically, UIP is characterized by its variegated appearance; areas of fibrosis alternate with areas of normal lung ( Fig. 16.2 , [CR] ). The fibrosis is subpleural and paraseptal in distribution and significantly disrupts the normal alveolar architecture. At the interface of the established scar and normal lung are areas of active fibrogenesis, termed fibroblast foci, composed of myofibroblasts and loose connective tissue underneath a degenerating and desquamating epithelium. Fibroblast foci need to be distinguished from areas of organizing pneumonia, which are more exuberant and fill airspaces and alveolar ducts, rather than line up along walls of alveoli. There should only be minimal inflammation in the lung in areas away from scar. No more than very rare granulomas are acceptable, and even then, they raise the possibility of an alternative diagnosis.
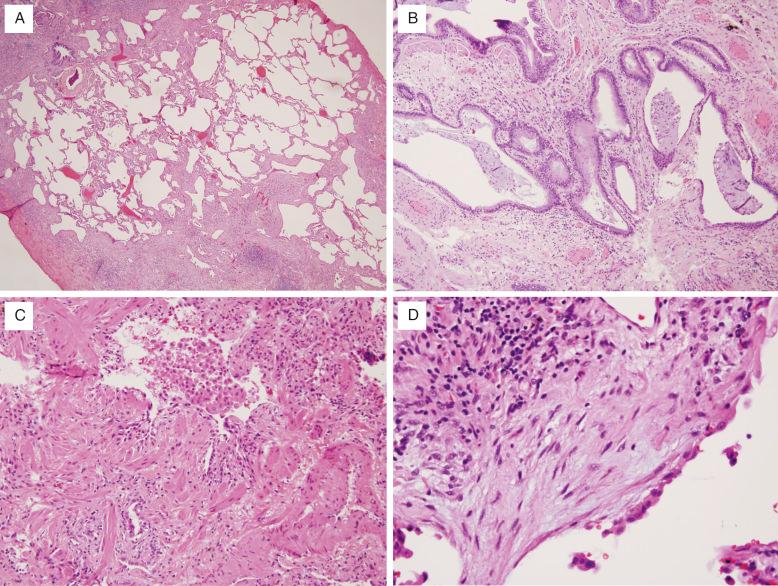
The lung is thus described as having both spatial (alternating areas of normal and fibrotic lung) and temporal (presence of normal, fibrosing, and fibrotic lung) heterogeneity. Scarring results in remodeling of the pulmonary architecture with formation of cystic spaces (so-called microscopic honeycombing ). The cysts may contain mucus and/or inflammatory cells. Inflammation and fibroblast foci found entirely within areas of honeycombing are considered nonspecific. Smooth muscle metaplasia may accompany scarring, thus resulting in the historic phrase “muscular cirrhosis of the lung” for this disease. Secondary vascular changes are common and consist of medial thickening and intimal fibrosis. These latter findings do not necessarily correlate with clinical pulmonary hypertension.
Similarly to the radiologic findings, biopsies should be categorized as definite UIP, probable UIP, possible UIP, and inconsistent with UIP. Biopsies are considered definite UIP if they show all of the findings of UIP as described earlier. Biopsies are considered probable UIP if they have honeycomb change only or absence of spatial or temporal heterogeneity. Biopsies are considered possible UIP if they show relatively mild fibrosis but with no other distinguishing feature. Biopsies with extensive organizing pneumonitis, marked inflammation including eosinophils or granulomas, or other inconsistent features (as described later) are considered inconsistent with UIP.
The histopathologic categories are combined with the radiology either to make final diagnoses or to form the basis of discussion at a multidisciplinary conference as described previously. For instance, given a definite or possible UIP radiologic diagnosis, most pathologic categories lead to a diagnosis of probable or definite IPF, whereas an inconsistent with UIP pathology pattern would refute the diagnosis. Radiology that is inconsistent with IPF, on the other hand, leads to a diagnosis other than IPF, regardless of histopathology, other than in those patients with a definite UIP histology pattern. Even then, these latter patients need to be reviewed at a multidisciplinary conference discussion before making the diagnosis. Furthermore, one caveat to the exclusion of hyaline membranes and organizing pneumonia in IPF/UIP is that biopsies from patients with the so-called accelerated decline or acute exacerbation of IPF may show a combination of UIP and diffuse alveolar damage (DAD) and/or organizing pneumonitis ( Fig. 16.3 ).
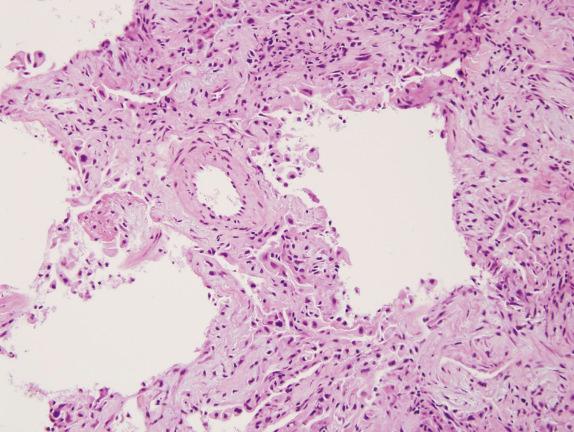
Differentiating UIP/IPF from other entities involves histologic and etiologic considerations. The histologic differential diagnoses include the fibrosing pattern of NSIP, organizing DAD, and diseases with airway-centered inflammation and fibrosis (eg, chronic hypersensitivity pneumonitis and chronic aspiration). In separating UIP from NSIP and DAD, the presence or absence of temporal heterogeneity is the most helpful histologic finding. The fibrosing pattern of NSIP is characterized by relatively uniform interstitial fibrosis, which may be accompanied by mild-to-moderate chronic inflammation. The hallmark of organizing DAD is diffuse interstitial fibroblast proliferation, which incorporates remnants of hyaline membranes into the alveolar septa. In contrast to NSIP and DAD, UIP has a variegated appearance with dense scars, scattered fibroblast foci, and areas of normal lung.
In patients with acute respiratory failure, the differential may include AIP vs. IPF/UIP with an acute exacerbation. Early in the disease, AIP will not show established fibrosis, whereas the presence of honeycombing with a superimposed pattern of organizing-phase DAD suggests IPF/UIP with an exacerbation. Later in the disease, there may be some degree of established fibrosis in both, at which time the distinction requires careful clinical correlation with previous clinical findings. If those are not available, the distinction may not be possible.
Sometimes, in a patient with multiple biopsies, UIP is seen in one lobe and an NSIP pattern is seen in another lobe. In such cases, the overall histologic diagnosis should be UIP. If the distinction between the two patterns cannot be made with certainty, the term unclassifiable fibrotic interstitial pneumonia can be used. If there are areas of honeycombing, however, the biopsy most likely represents UIP/IPF.
The fibrosis in UIP is centered under the pleura and along septa. A critical distinction is between this pattern and that where the fibrosis is centered on airways, so-called airway centric fibrosis (ACF). When there is a moderate amount of inflammation such as in subacute chronic hypersensitivity pneumonitis, this distinction is relatively easy. In the absence of inflammation, this distinction can be difficult. ACF will typically be centered in the lung parenchyma, radiating away from airways and can extend to the pleura, in a wedge shape. Mucus can accumulate in these fibrotic airways but this differs from the mucus accumulation of honeycomb lung.
By current classification, ACF is considered inconsistent with UIP/IPF and suggests an airway-based disease, such as chronic hypersensitivity pneumonitis or chronic aspiration. While some cases of bronchocentric fibrosis have been reported as a novel form of idiopathic ILD, it is difficult if not impossible to exclude occult antigen exposure or aspiration in these cases. Aspirated foreign material should be carefully sought. Scattered granulomas also suggest either chronic hypersensitivity pneumonitis or aspiration. As this process becomes more extensive, there will be overlap with honeycomb lung, and it may not be possible to recognize the initial pattern. In the past, this problem was not of major clinical significance given the limited therapeutic options available. However, given the recent availability of medications that are currently only approved for IPF (see below) and not for other entities, this has now become a problem area with no simple resolution.
Connective tissue diseases, many drug and radiation reactions, and familial fibrotic ILD with a UIP pattern usually cannot be separated from UIP/IPF without clinicopathologic correlation. However, some soft clues may be present. Although asbestosis (interstitial fibrosis due to asbestos) virtually always shows identifiable ferruginous bodies, pleural plaques also are associated with asbestosis, and their presence should prompt a search for asbestos bodies and an inquiry into asbestos exposure. The presence of prominent lymphoid aggregates, lymphoid follicles, and especially chronic pleuritis should raise the possibility of a connective tissue disease. The very rare Hermansky-Pudlak syndrome is an autosomal-recessive condition that is characterized by defects in platelet aggregation, oculocutaneous albinism, and accumulation of ceroid-filled histiocytes in many organs. Pulmonary involvement manifests as fibrotic ILD, typically with a nonspecific pattern of fibrosis, with foamy hyperplastic type II cells due to accumulation of phagolysosomes (see also Chapter 35 ).
For untreated patients with IPF, the course of disease progression is variable. Some patients have stable disease, others have slow or rapid progression, and still others have relatively stable disease punctuated by acute exacerbations. This latter condition has a particularly grim prognosis. The mean length of survival from the time of diagnosis is between 2.5 and 3.5 years. Despite this statistic, some patients with IPF can survive for many years, prompting an interest in prognostic features, including pathologic features (multiple fibroblast foci), physiologic features (even slight falls in PFTs are ominous harbingers), and biomarkers, for example, serum levels of matrix metalloproteinase 7. Respiratory failure is the most frequent cause of death, although there is also an increase in other diseases, especially lung cancer. Aggressive immunosuppressive and cytotoxic therapy, which formerly was commonly used in this disease, is now considered contraindicated. Two novel antifibrotic agents, pirfenidone and nintedanib, have recently been approved for this disease by the U.S. Food and Drug Administration. These agents have similar efficacy in reducing rate of decline in forced vital capacity per year by roughly 50% among patients with moderately advanced disease. There is a suggestion of a survival benefit as well. It is critical therefore to distinguish between diseases in which immunosuppression has a role (eg, hypersensitivity pneumonitis or, COP—see later) versus those in which it is not only contraindicated but in which other therapies are available.
NSIP is an idiopathic interstitial pneumonia characterized by temporally uniform interstitial chronic inflammation and/or fibrosis
Less frequent than idiopathic pulmonary fibrosis, but more common than other idiopathic interstitial pneumonias
Female predominance
Median age of patients is between 40 and 50 years at onset
Breathlessness, cough, fatigue, and weight loss are common
Abnormal pulmonary function studies that include evidence of restriction and impaired gas exchange
On HRCT, ground-glass attenuation is the predominant finding in the majority of cases
The prognosis of NSIP is more variable than that of idiopathic pulmonary fibrosis
Considered steroid and other immunosuppressant responsive
5-year survival with cellular NSIP is 100%
With fibrosing NSIP, 5- and 10-year survival rates are 90% and 35%, respectively
Mild-to-moderate interstitial chronic inflammation
Temporally homogeneous interstitial fibrosis
Chronic inflammation may or may not be present
Lymphoid interstitial pneumonia pattern
Organizing pneumonia
Usual interstitial pneumonia
Diffuse alveolar damage, organizing phase
Idiopathic NSIP
Hypersensitivity pneumonia
Connective tissue diseases
Drug toxicity
Infection
Immunodeficiency
NSIP is both a histologic pattern and a clinicopathologic entity. The NSIP pattern is characterized by mild-to-moderate interstitial chronic inflammation and/or temporally uniform interstitial fibrosis. Additionally, the NSIP pattern lacks specific histologic features of other interstitial lung diseases (eg, hyaline membranes of DAD). Although NSIP can be idiopathic, other systemic or associated conditions need to be excluded, including connective tissue diseases, drug toxicity, extrinsic hypersensitivity pneumonitis, or resolution phase of DAD.
NSIP is less common than UIP, but occurs more frequently than the remaining chronic idiopathic interstitial pneumonias. The average age at presentation is between 40 and 50 years, with a female predominance. A histologic pattern of NSIP may occur in children, but it typically has different associations, including cases of surfactant protein and surfactant transport protein mutations. Clinical symptoms of NSIP are similar to those of IPF; patients often present with dyspnea on exertion and cough. Crackles are common findings on auscultation. PFTs usually reveal a restrictive defect with lowered diffusing capacity of the lung for carbon monoxide (DLCO). Exercise-associated gas exchange abnormalities are commonly present.
NSIP has no specific radiographic findings, but ground-glass opacities and areas of consolidation are common.
On HRCT, areas of ground-glass attenuation are seen in virtually all cases. Although a reticular pattern is common, it is rarely the predominant finding. There is sometimes subpleural sparing of peripheral lung. This contrasts with the subpleural honeycombing seen in cases of IPF. Honeycombing is rarely present on presentation but may develop during follow-up.
Histologically, NSIP is divided into cellular and fibrosing patterns. The rarer cellular NSIP pattern is characterized by mild-to-moderate interstitial chronic inflammation with a mixture of lymphocytes and plasma cells ( Fig. 16.4 , [CR] ). Lymphoid aggregates are a common finding. Sometimes an organizing pneumonia pattern is also present, especially when seen in a patient with underlying collagen vascular disease ( [CR] ).
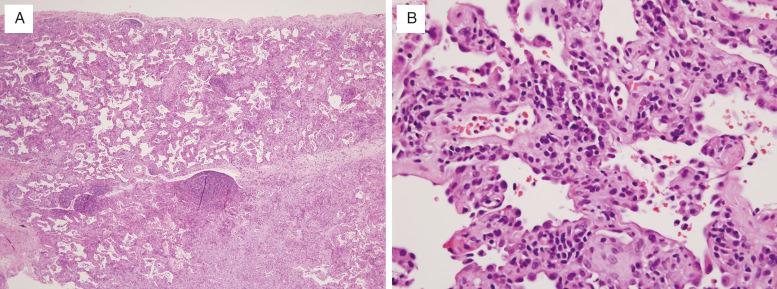
The hallmark of the more common fibrosing NSIP pattern is the presence of diffuse, temporally uniform interstitial fibrosis ( Fig. 16.5 , [CR] ). This may or may not be accompanied by mild-to-moderate chronic inflammation. The fibrous connective tissue may be dense or loose in character, but has the same appearance throughout the lung. Honeycomb change and organizing pneumonia are either absent or inconspicuous in pure forms Fig. 16.6 ).
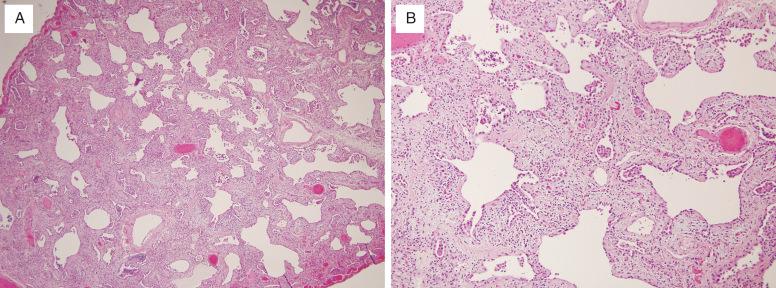
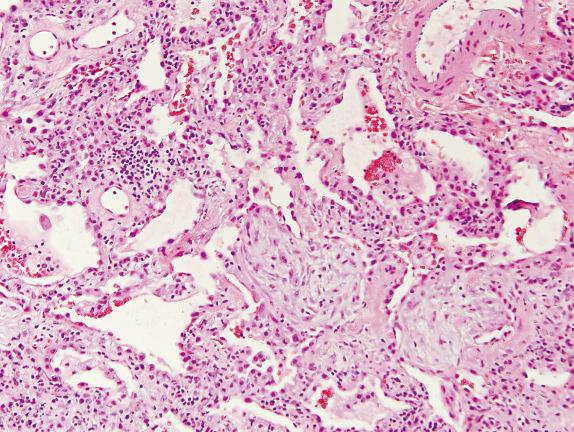
Histologic differential diagnoses for the cellular NSIP pattern include the LIP pattern and organizing pneumonia. Although both cellular NSIP and LIP are characterized by the presence of chronic inflammatory cells in the alveolar septa, the extent of expansion is dramatically greater in LIP. Organizing pneumonia can be a minor component of cellular NSIP. In NSIP, the interstitial chronic inflammation is diffuse, whereas it is limited to areas of fibroblast proliferation in COP.
Histologic differential diagnoses of the fibrosing NSIP pattern include UIP and organizing DAD. Separation of fibrosing NSIP and UIP has been discussed earlier. Fibrosing NSIP and organizing DAD are similar in that they are both characterized by uniform interstitial thickening, but the septa in organizing DAD shows proliferating fibroplasia, whereas there is dense scarring in fibrosing NSIP. Organizing DAD can be distinguished by the presence of residual hyaline membranes and the clinical history.
Etiologic differential diagnoses that must be considered in patients with NSIP include hypersensitivity pneumonitis, connective tissue diseases, drug toxicity, infection, and immunodeficiency. Poorly formed granulomas associated with the NSIP pattern should raise the level of concern to exclude these possibilities.
NSIP is treated with corticosteroids and/or other immunosuppressants, which results in improvement or recovery in many patients. However, there is a dramatic difference between purely cellular NSIP, in which the long-term survival is nearly 100%, versus fibrotic NSIP, in which the 5- and 10-year survival rates are typically cited as 90% and 35%, respectively.
Organizing pneumonia is a histologic pattern characterized by airspace-filling fibroblast plugs
COP is a clinicopathologic entity of unknown etiology with the underlying histopathologic pattern of organizing pneumonia
Equal gender distribution
Mean age of patients at onset is 55 years
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here