Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Standardized marker systems are used in testing panels to generate allelic phenotypes; short tandem repeats (STRs) form the basis of commercially available kits.
Forensic testing requires documentation of all steps taken during collection, extraction, and testing so results can withstand legal challenges.
Deoxyribonucleic acid (DNA) can be obtained from any sample that contains cellular material. The stability of DNA allows it to withstand harsh environmental conditions and long postmortem intervals.
Care must be taken to maintain the integrity of DNA evidence and to avoid contact or exposure of the evidence to any conditions that may contaminate and/or further degrade the original evidence.
Mitochondrial DNA can be extracted from bone, teeth, and hair after hundreds and even thousands of years because it is small and present at hundreds to thousands of copies per cell. Mitochondrial DNA is maternally inherited and can be useful for identifying remains.
DNA is used in identification of remains and can be used to link a suspect to a crime, to exculpate falsely accused suspects, to recognize serial crimes, to distinguish copycat crimes, and to aid in accident reconstruction (e.g., to determine who was driving a car by the identification of the bloodstains on the driver’s side of the windshield).
Pathology laboratories can use DNA testing to resolve specimen mixups, such as samples inadvertently switched or pathologic material floats onto a histologic or cytologic slide.
Standards and quality assurance guidelines for identity testing laboratories have been developed by the American Association of Blood Banks (for parentage testing), the US Department of Justice Federal Bureau of Investigation’s Scientific Working Group on DNA Analysis Methods, the Quality Assurance Standards for Forensic DNA Testing Laboratories, and the Quality Assurance Standards for DNA Databasing Laboratories and the National Institute of Standards and Technology’s Organization of Scientific Area Committees for Forensic Science.
The authors would like to acknowledge Herbert Polesky MD for material from chapters in previous editions.
The discovery of the ABO blood group system by Karl Landsteiner in 1900 ( ) and the recognition that these measurable characteristics follow the genetic rules described by Gregor Mendel provided objective evidence from laboratory results that could be used by courts to aid in parentage and criminal casework. In the United States, laws addressing the use of genetic markers for proving nonpaternity were enacted in 1935 ( ). In the ensuing years, knowledge about useful genetic marker systems increased dramatically. By 1976, joint guidelines developed by an American Medical Association–American Bar Association committee recommended seven systems for routine blood group investigations in cases of disputed parentage: ABO, Rh, MNSs, Kell, Duffy, Kidd, and HLA ( ). Other genetic systems such as polymorphic serum proteins and red cell enzymes were also recognized as useful. At the time, a variety of the aforementioned genetic markers were used to compare crime scene evidence with suspects or victims; however, DNA testing is used almost exclusively in the current era.
In 1980, Botstein and colleagues described DNA restriction fragment length polymorphism (RFLP) ( ). Sir Alec Jeffreys is credited with the first report in the scientific literature to suggest that DNA typing might be useful for forensic identification, and coined the term “DNA fingerprinting” in his Nature articles in 1985 ( , ). Commercial laboratories began using DNA testing for parentage and criminalistic casework in 1986, and government crime laboratories began using DNA testing in 1989 ( ). Today, hundreds of laboratories worldwide use DNA testing for the criminal justice system. In 1990, when the first edition of Standards for Parentage Testing Laboratories ( ) was published, specific requirements for RFLP testing were included alongside those for more traditional serologic methods (red blood cell surface antigens, human leukocyte antigens, red cell enzymes, and polymorphic serum proteins). DNA testing has since replaced these techniques.
The forensic DNA testing community has endured many iterations of DNA methods and technologies, and has standardized its testing with a core set of short tandem repeat (STR) loci as the current mainstay of testing to build its international offender databases. The national offender database, the Combined DNA Index System (CODIS), includes hardware and software systems that network crime laboratories at the local, state, and national levels. It links all 50 states and territories for laboratories to upload and search profiles for case-to-case matches and matches to offender samples. Additionally, CODIS houses the Missing Persons index to search for missing persons against family members’ DNA samples for kinship testing. The core loci were expanded to include 7 additional loci in 2017 for a total of 20 CODIS core loci. This expansion was necessary to reduce the number of adventitious matches, increase international capability, and increase the power of discrimination for criminal and missing persons cases ( , , ). The 20 CODIS core loci are often supplemented by other DNA testing. This supplemental data includes testing on allosomes, or sex chromosomes, which includes gender analysis (amelogenin is a protein with a single copy on the X and Y chromosomes and has been used in sex determination of samples) and X and Y chromosome markers. Other supplemental data commonly used is mitochondrial DNA (mtDNA) testing. Currently, capillary electrophoresis with fluorescence detection is the main instrument platform; however, next-generation sequencing (NGS) will likely supplant this method in the forensic laboratories of the future.
These same technologies are used to identify victims of war or mass disasters ( ), whether the disaster is natural, man-made, or industrial, and in cases of wildlife forensics. DNA testing is also used for accident reconstruction, in medical cases of sample mixups, and in animal husbandry. DNA technology is here to stay; newer technologies will be adopted that offer higher powers of discrimination, lower costs, enhanced sensitivity, increased efficiency, and even information on origin of ancestry and the physical traits (e.g., eye color, hair color) ( ) of remains or biological material left at a crime scene. As these newer technologies evolve and are validated, so will the field of forensic science and the demand for their use.
DNA permits the direct identification of the source; it is individual, or unique, and manifests biological variation. The human genome is approximately 99.9% the same between individuals; it is the 0.1% difference of the human genome that forensic scientists interrogate to identify stains, recovered remains from accidents and mass disasters, and biological relatives. DNA is useful as an identity marker because it is (1) present throughout all cells of the body, (2) the same in all cells of the body, (3) the same throughout life from the time of conception, and (4) different in all individuals. There are exceptions to the previously mentioned points, in order: (1) DNA is not recovered from mature red blood cells, (2) haploid sex gametes have half of the DNA material as compared to the other cells in the body, (3) progenitor stem cell transplantation can result in the presence of the donor’s DNA rather than the recipient’s inherited DNA, and (4) identical twins have the same DNA. DNA tests have a sensitivity that is far superior to the previously mentioned traditional serological markers. With the use of polymerase chain reaction (PCR)–based testing, DNA results can be obtained from minute samples and even invisible trace DNA deposits (trace DNA). DNA is a robust molecule resistant to strong acids, alkalis, detergents, and environmental factors ( ). The typing information from DNA is found within the sequence of nucleotides. Consequently, DNA results are more successfully attained than serology tests on specimens that are older and have been exposed to greater environmental insults, and DNA results are possible to attain from many sample types.
Much of the casework for crime laboratories in the United States is from sexual assaults, most often a female victim with a male perpetrator. Hereto, a collected vaginal swab may contain female epithelial cells and bacteria as well as the male sperm. DNA from sperm can be separated from nonsperm DNA by a differential lysis procedure because of the protective capsule of spermatozoa. This procedure allows for individualization of the source of the semen without the confounding data of the nonsemen evidence. DNA targets are also generally human or primate specific, so the presence of bacterial DNA is of no consequence.
Additionally, other advantages to DNA testing include Y chromosome testing where a mixture of female and male DNA will reveal only the male’s DNA type from the male contributor’s Y chromosome. This testing technique is extremely useful in the presence of mixed DNA when predominantly female DNA is present in the sample. Mitochondrial DNA testing is advantageous for those cases where there are highly degraded remains such as burned skeletal remains or where only a single shed hair is recovered. Additionally, mtDNA testing is important when making a comparison to a close or even a distant maternal relative.
Buccal swabs and, less often, bloodstains on filter paper or fresh whole blood are used for parentage testing, reference standards, and databases. For forensic evidence recovered from a crime scene (e.g., property from a vehicle exam) or for biological samples recovered from the victim’s body in the emergency department or from an autopsy, identifying the proper sample to collect is extremely important to the success of the case. To visualize the biological fluids and stains, oftentimes alternate light sources or presumptive chemical tests are used. Blood spatter pattern analysis is also employed to help the investigator interpret and intelligently collect specimens from large pools of blood and bloodstains. When a biological fluid has been deposited on a surface or an item that cannot be collected, the fluid should be sampled with a clean sterile swab. For large items where the biological fluid is dry, the sterile swab should be moistened with sterile distilled water, the evidence wiped with the swab, and allowed to air dry prior to packaging. For collection of DNA from bitemarks on bodies and also dried biological material, a double swab technique ( ) that involves rubbing the evidentiary area with a wet swab followed by a dry swab is often used. These items (e.g., blood on clothing, collection swab) should be collected one item at a time, packaged separately, clearly labeled, and itemized.
Even though mature red blood cells are devoid of DNA (both nuclear and mitochondrial) from blood for postmortem processing, ample DNA is present from the circulating white blood cells in the blood for DNA testing. The appropriate blood sample collection tubes with anticoagulants are listed in Box 74.1 . Further, sources of DNA other than blood are preferred if there is significant decomposition. Virtually any tissue may be successfully used for DNA typing purposes. Some tissues that may be intuitively thought to be better sources of DNA because they are more densely cellular and would contain higher concentrations of DNA are, in fact, less optimal because of higher rates of postmortem degradation ( ). Among soft tissues, the DNA recovered from liver tissue degrades quickly due to the presence of autolytic enzymes, whereas brain tissue is a relatively good source in the intermediate postmortem period. Bone, teeth, and nails (fingernails and toenails) ( ) are stable sources of postmortem tissue DNA. Informative DNA is routinely obtained from skeletal remains that are decades old. Generally, the greater the body decomposition and the longer the postmortem period, the greater the degradation of the DNA from cadaveric tissues.
Ethylenediaminetetraacetic acid (EDTA; “purple top tubes”) and citrate phosphate dextrose (CPD; “yellow top tubes”) are often the anticoagulants used and requested for DNA testing. Heparin-anticoagulated blood (“green top tubes”) is not recommended for DNA testing. Tubes with no anticoagulants are not recommended.
Great care should be taken to prevent contamination of potentially significant specimens and stains by other sources of DNA. Examiners should collect evidence and specimens using single-use gloves, clean instruments, and masks; these methods are to protect the evidentiary item from first responders, the examiner, and cross-contamination of other evidence. When possible, nonexposed tissue should be collected by an incisional biopsy technique. Proper storage of specimens can be critical for the outcome of the DNA results. Many samples can be stored at room temperature while others are best preserved either at 4°C or -20°C ( ). Tissues in formalin are not optimal; however, results can usually be obtained with PCR-based testing techniques (see Chapter 69 ). No tissues or biological fluids and stains should be discarded as inadequate without first attempting DNA testing.
DNA in most specimens will undergo progressive random fragmentation or degradation, reducing the high molecular weight DNA to lower molecular weight DNA ( ). As time progresses, high molecular weight DNA can become degraded even after a short time from fresh tissues and fluids. Residual smaller DNA fragments may persist despite considerable degradation. In contrast, high molecular weight DNA may be obtained for years after a fresh sample has been frozen or desiccated. Because of its high copy number (hundreds to tens of thousands of copies per cell), mtDNA often survives when no nuclear DNA is recovered. Consequently, mtDNA testing is routinely used to identify older skeletal remains ( ; ).
Rapid DNA degradation or damage may occur under certain conditions ( ). Chemical hydrolysis of DNA is normally quite slow. Tests have shown that mixing DNA with detergents, oil, gasoline, and other adulterants does not alter the DNA result. However, metal ions may catalyze oxidative hydrolysis (Gaydosh Combs et al., 2015). Ultraviolet radiation is known to cause thymidine dimerization; fragmentation of ancient DNA sequences is primarily due to depurination that creates abasic sites leading to DNA breaks.
Results from testing evidence are often used in legal proceedings. Thus all aspects of the specimen collection procedures should be well documented (i.e., chain of custody) since they may be subject to legal challenges. It is important to ensure clear documentation of the identification of the tested individuals, the collection of the evidence, and the labeling of samples. Although many parentage disputes are civil actions, some jurisdictions require documents showing the chain of custody of samples similar to those used in criminal matters and other required information. Likewise, the forensic sample must be properly collected, packaged, sealed, and stored and demonstrate evidence of proper chain-of-custody documentation. Typical requirements are summarized in Box 74.2 .
AABB-accredited laboratories are required to obtain a history of any recent transfusion (in the preceding 3 months) or a hematopoietic stem cell transplant. A photographic identification and appropriate informed consent must also accompany each sample. The consent for a minor should be from an individual with legal rights to the custody of the child.
The first and most critical step for DNA testing of evidence is the extraction, or purification, of the DNA from blood, biological stains, or other biological sources. DNA from a bloodstain, a vaginal swab, or other sources must be isolated from other cell components and environmental contaminants. Numerous DNA extraction methods are used in both parentage and forensic laboratories. Solid-phase column extraction methods and magnetic resin particles are most often used for DNA extraction since these methods are highly amenable to automated techniques (see Chapter 67 ). The column extraction and magnetic particle methods yield DNA that is highly purified for forensic samples. Contaminants such as those found in cigarette butts and certain dyes may be removed. Silicon beads are another excellent method to purify DNA when inhibitors need to be removed. Many laboratories eliminate the extraction step altogether, for reference and databasing samples, by performing direct PCR from a punch or small sample of a buccal collector or bloodstain.
As mentioned earlier, vaginal swabs require special conditions for extraction using a differential lysis procedure because of the need to separate the male DNA in sperm from the female DNA in epithelial cells ( ). The female fraction (the vaginal epithelial cells) is separated by standard extraction procedures. The male fraction (spermatozoa) is then extracted by centrifugation and breaking the disulfide bridges of the protective capsule of spermatozoa with a dithiothreitol or other reducing agent solution. Sensitive PCR-based systems often exhibit female source DNA in the male fraction, producing mixed DNA profiles.
Once the samples are extracted, quantification is performed. The forensic standards require quantification of forensic evidence for STR testing. When performing quantification and knowing the amount of DNA in a sample, amplification is usually more successful. Too little or too much DNA in a PCR-based assay results in nonoptimal amplification, which can produce poor results. In addition, having a priori information on the total human male DNA and a qualitative assessment of the total human DNA is extremely useful in determining which assays to perform in the downstream analysis. Today, real-time quantitative PCR is multiplexed to simultaneously obtain quantitative and qualitative assessment of total human and human male DNA in a single assay. Further, many laboratories have adopted a “stop-at-quant” procedure; since the quantification methods are sensitive enough to detect low levels of DNA, samples are oftentimes stopped at quantification if insufficient DNA is present. Furthermore, sexual assault cases, as described previously with a female victim and a male perpetrator, may stop at quantification if no male or insignificant amounts of male DNA are present.
Multiple genetic systems are used for parentage and identity testing. Ideally, the system should have multiple alleles distributed in the population so that there is a high power of exclusion and the least common phenotype has a frequency that can be determined reliably. All markers in the system should be expressed (i.e., with few to no null alleles) as codominant, mutation rates should be known, and phenotypes should be stable under usual storage conditions. Methods for detecting the markers should be reliable, reproducible, and feasible for a large number of laboratories. The genetics of the system must be known and follow established inheritance patterns (Mendelian laws). The system should be independent of other markers routinely tested. If the system is intended for calculating estimates of paternity or identity, the gene frequencies in various populations must be established ( ).
The DNA from every human (except identical twins) is unique because of the presence of polymorphisms, which are differences in the DNA between individuals. However, the vast preponderance of the DNA sequence is identical between individuals. On average, only 1 in 1300 nucleotide bases differs between individuals. Nonetheless, this amounts to an average of 3 million bases that differ between any two individuals and accounts for the tremendous genetic variation among individuals. Although the diversity in the coding regions of DNA (genes) is significant, the noncoding regions of DNA also provide for a great deal of diversity.
Length-based polymorphisms are found in repetitive DNA. More than 90% of the human genome is composed of noncoding, or “junk,” DNA, of which approximately 20% to 30% is composed of repetitive regions. Many of the repetitive regions vary in the number of core repeats between individuals—so-called variable number of tandem repeat (VNTR) loci. DNA fragments containing VNTRs vary in length and are thus useful for analysis. Dinucleotide repeats are most common, but larger core repeats (i.e., tri-, tetra-, penta-, sextanucleotide repeats) are more useful for forensic purposes since interpretation is much easier than the dinucleotide repeats that exhibit an extremely high amount of reproducible artifacts from amplification (see Chapter 69 ). RFLP, amplified DNA fragment length polymorphisms, and STRs are examples of fragment length-based analytic techniques. Sequence polymorphisms exist within the DNA sequence of similarly sized DNA fragments. Sequence polymorphisms consist of differences in one or more bases in the DNA sequence at a particular location in the genome. Sequence variations can manifest as regions of alternative alleles or base substitutions, additions, or deletions. Most sequence polymorphisms are mere point mutations within repetitive and nonrepetitive regions and are known as single nucleotide polymorphisms (SNPs). SNPs, sequence-specific oligonucleotides, and mtDNA tests are examples of sequence-based tests as well as the introduction of NGS for forensic genomics. Identity testing has not yet widely adopted the power of the more than 2 million SNPs in the human genome, but the use of this technology is clearly on the horizon for forensic DNA testing.
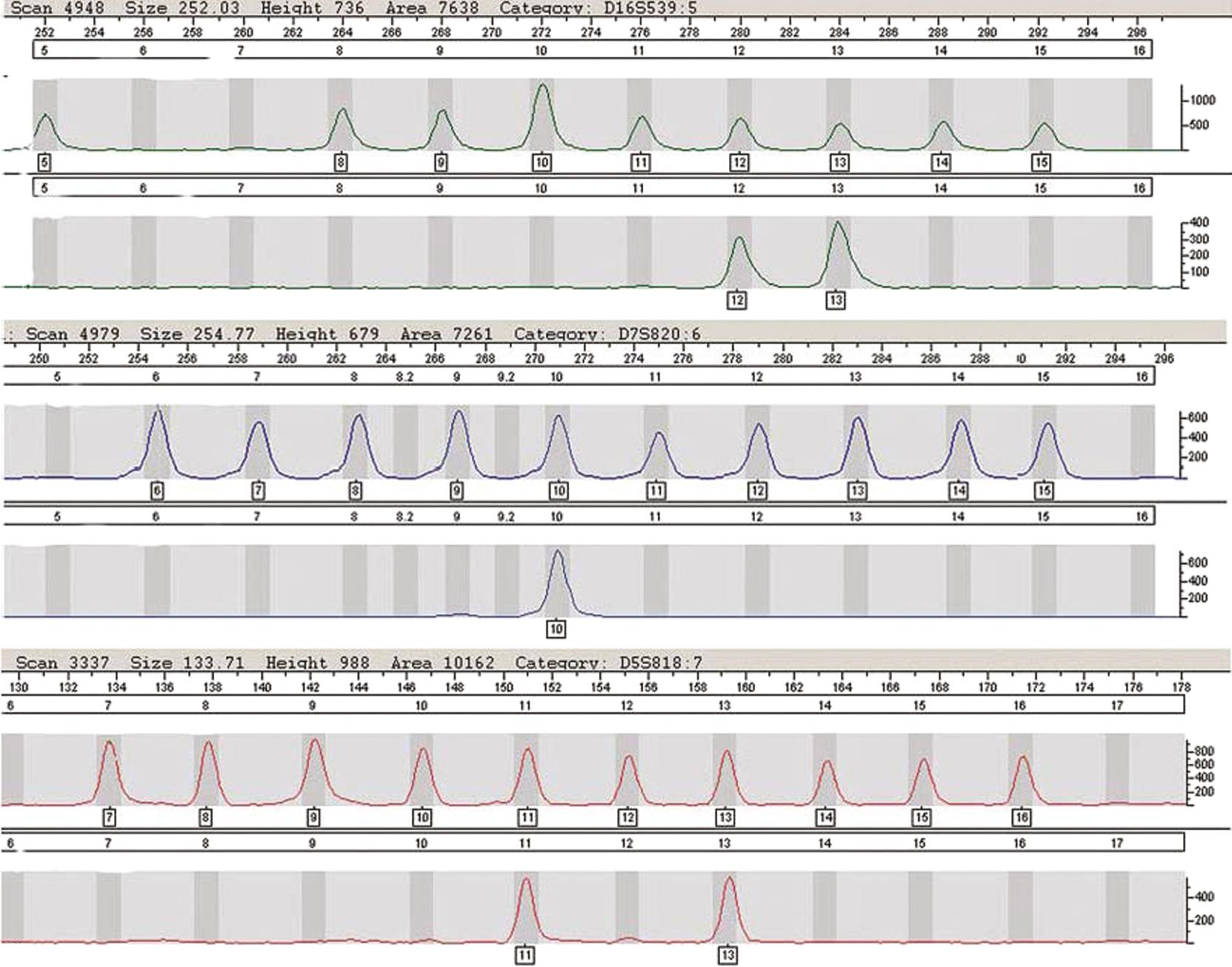
Loci typically used in commercial assays consist of repeat units of three to seven nucleotides; these short tandem repeats occur throughout the genome ( ). Alleles at these STR loci are defined by the number of repeat units in the PCR-amplified product (see Chapter 69 ). Extracted sample DNA is added to a PCR reaction mix containing Thermus aquaticus (Taq) polymerase, or another enzyme, with specific forward and reverse primers for each locus tested, deoxynucleoside triphosphates, magnesium, a buffer solution, and typically bovine serum albumin. The PCR reaction mixture is amplified in a thermal cycler using carefully defined parameters to maximize detection of the alleles. Primers with different fluorescent labels are used in a multiplex reaction to detect alleles at many loci in a single amplification. The PCR products, which vary in length, are separated by capillary electrophoresis. (See Chapter 24 for a discussion of this technique.) Detection is performed using a laser to excite the labeled fluorophore that has been incorporated in the primer. The loci in the multiplex reaction differ by size and color ( Fig. 74.1 ). Sizing is accomplished by including a size standard with each sample injected in the capillary and using sizing software algorithms; phenotyping of each peak is achieved by comparing the peaks in the sample to an allelic ladder accounting for most alleles for each locus and using sophisticated genotyping software algorithms for allele calls that adjust for minor variations in electrophoresis for each injection ( Fig. 74.2 ).
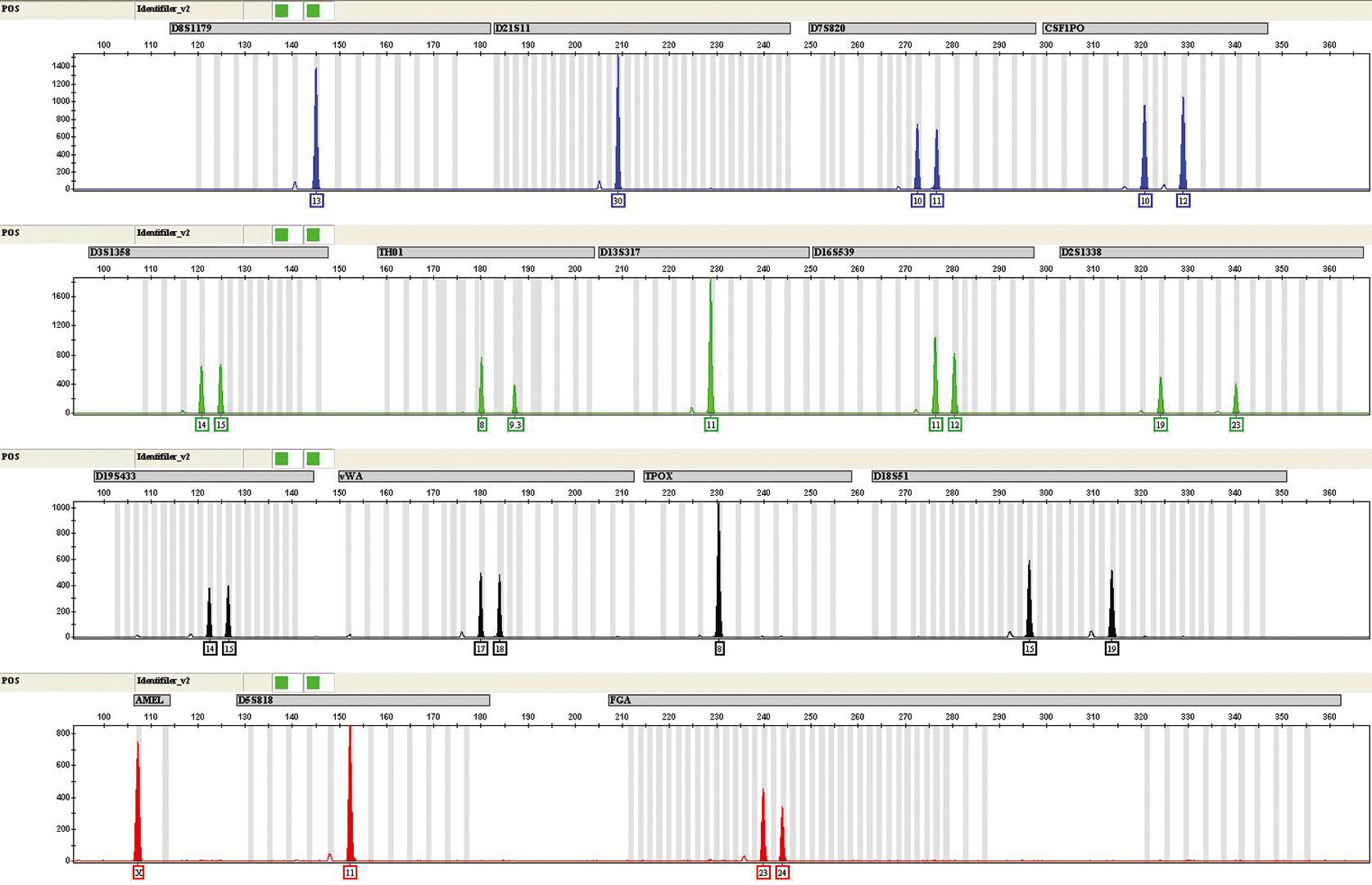
The most commonly used markers in forensic and parentage laboratories in the United States are the 20 core STR loci for CODIS ( Table 74.1 ) that have been standardized for use in obtaining genetic profiles of individuals who have been convicted of various criminal offenses ( ). The STR marker systems have discrete alleles. An STR locus may have three or four common alleles (frequencies of 0.15–0.25) and several rare alleles (frequencies of <0.01). Thus, to obtain a high cumulative probability of exclusion (CPE) and combined paternity index (CPI), it is usually necessary to test at least six or more STR loci. In a series of 50 paternity cases tested in parallel by both RFLP and nine STR loci, reported that both methods excluded the same 13 alleged fathers (based on two or more loci). The likelihood of paternity for the 37 nonexcluded men based on the STR systems was more than 99% in 36 cases. When 13 CODIS STR loci are obtained, the average random match probability is rarer than 1 in 1 trillion among unrelated individuals ( ). With 20 CODSI STR loci, it is not uncommon to obtain a random match probability rarer than 1 in 1 nonillion among unrelated individuals.
| Locus Designation | Repeat Sequence 5’ → 3’ | Chromosome Location |
|---|---|---|
| Amelogenin | NA | Xp22.1–22.3 and Yp11.2 |
| CSF1PO | AGAT | 5q33.1 (149.436Mb) |
| D1S1656 | TAGA Complex | 1q42 (228.972Mb) |
| D2S1338 | TGCC/TTCC | 2q35 (218.705Mb) |
| D2S441 | TCTA | 2p14 (68.214Mb) |
| D3S1358 | TCTA Complex | 3p21.31 (45.557Mb) |
| D5S818 | AGAT | 5q23.2 (123.139Mb) |
| D7S820 | GATA | 7q21.11 (83.433Mb) |
| D8S1179 | TCTA Complex | 8q24.13 (125.976Mb) |
| D10S1248 | GGAA | 10q26.3 (130.567Mb) |
| D12S391 | AGAT/AGAC Complex | 12p12 (12.341Mb) |
| D13S317 | TATC | 13q31.1 (81.62Mb) |
| D16S539 | GATA | 16q24.1 (84.944Mb) |
| D18S51 | AGAA | 18q21.33 (59.1Mb) |
| D19S433 | AAGG Complex | 19q12 (35.109Mb) |
| D21S11 | TCTA Complex | 21q21.1 (19.476Mb) |
| D22S1045 | ATT | 22q12.3 (35.779Mb) |
| FGA | TTTC Complex | 4q28 (155.866Mb) |
| TH01 | AATG | 11p15.5 (2.149Mb) |
| TPOX | AATG | 2p25.3 (1.472Mb) |
| vWA | TCTA Complex | 12p13.31 (5.963Mb) |
Sex determination is commonly performed using the amelogenin locus (see Fig. 74.1 ). The primer for this locus amplifies an X-specific band (Xp22.1 to 22.3) and a Y-specific band (Yp11.2). This locus is coamplified and coanalyzed with most multiplexed STR systems designed for human identity testing. Sex determination marks a departure from all other routine identity markers, in that it provides specific phenotypic information about the source individual (i.e., male or female). It may also be important in the investigation of potential suspects as well as in the categorization of specimens as originating from a victim or suspect.
Although polymorphic Y chromosome markers ( Fig. 74.3 ) are not used for sex determination, they are extremely useful in the typing of casework when there is a mixture of a male and female and a mixture of males. For example, an excellent use of Y-STRs is sexual assault evidence that displays a mixture of both the female epithelial cell DNA and male sperm DNA. When the quantity of male DNA is much less than the female DNA, Y-STRs are excellent in obtaining a profile of the male subject with no interference from the female subject since Y-STR results are only obtained from males. They also are used to characterize the paternal lineage in human remains identifications ( Box 74.3 ). A haplotype is generated from Y-STRs versus a phenotype in autosomal STRs; haplotypes do not afford the same level of discrimination as autosomal STR profiles.
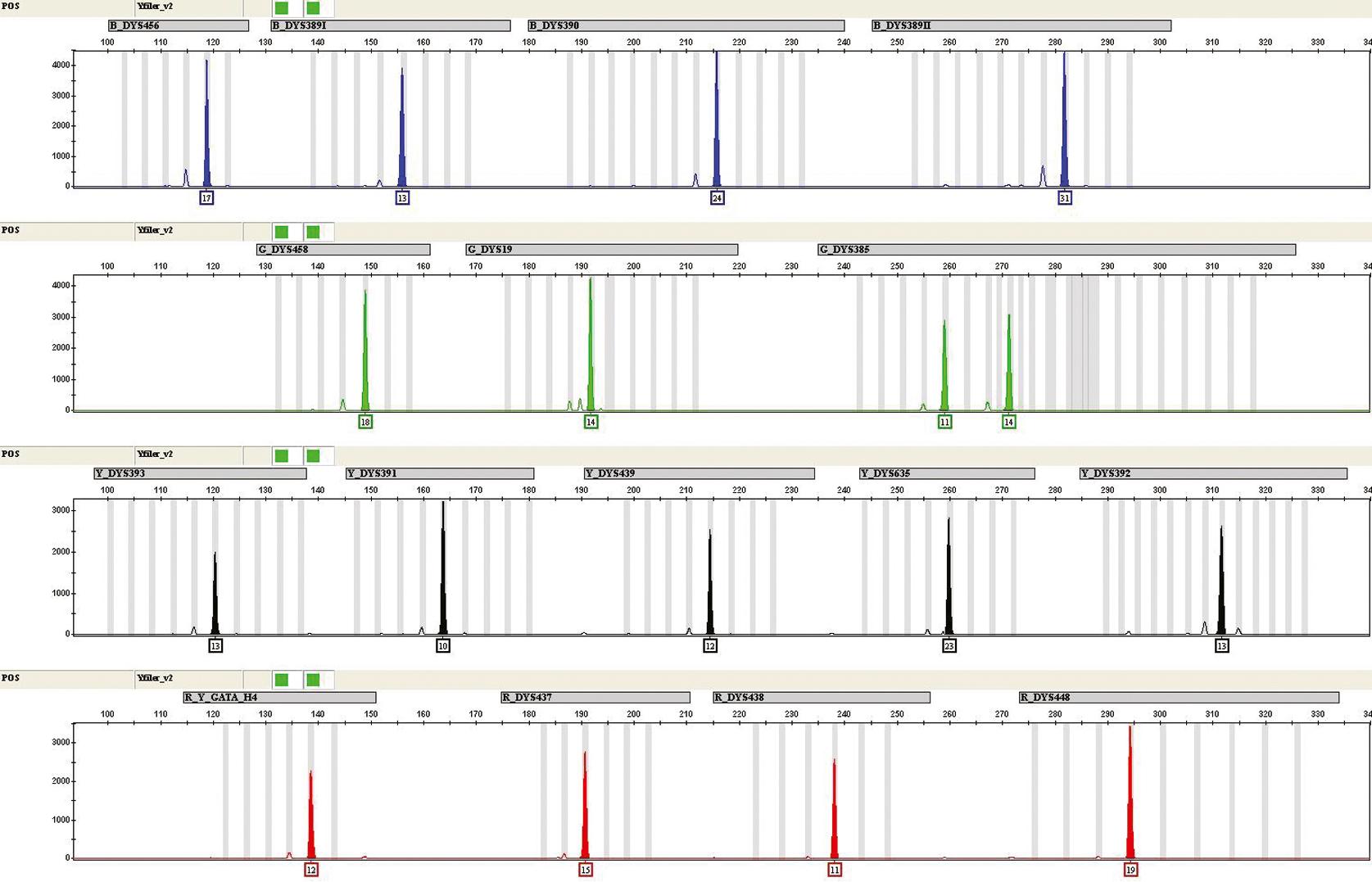
The characterization of the Thomas Jefferson family lineage was accomplished using Y chromosome markers ( ), an example of an historical and genealogic study. Data from extensive Y-STR typings have demonstrated that certain haplotypes are indicative of ethnic origin and can be used for human migration and evolutionary studies.
Mitochondrial DNA sequencing is most applicable when extremely small quantities of sample DNA are present or when the DNA is extremely degraded, such as in shed hairs (which have virtually no detectable nuclear DNA) and skeletal remains ( ; ). Hundreds to thousands of copies of mtDNA are present within a single cell with only one copy of nuclear DNA. Accordingly, an mtDNA profile can often be obtained when a nuclear DNA profile cannot. Further, mtDNA testing is used when references from distant relatives are available to compare to an evidentiary sample. A single distant relative in the maternal line may be used as a reference when reference samples are limited because nuclear DNA testing generally requires multiple close relatives.
Mitochondrial DNA is composed of a circular piece of DNA 16,569 base pairs (bp) in length. The region of mtDNA that is analyzed for human identification is the noncoding displacement loop (D-loop), also known as the control region. This locus spans approximately 1200 bp and contains two hypervariable regions. Mitochondria are maternally inherited; hence, mtDNA has no paternal contribution. Unlike nuclear DNA, which is present in pairs of chromosomes, generally only a single mtDNA sequence is present in the cell (homoplasmic) and consequently no genetic recombination occurs. An exact mtDNA sequence match can be traced through the maternal lineage of a family for many generations. However, the discriminatory power of mtDNA sequencing is limited—on the order of one in a few thousand. This testing is expensive and time consuming. NGS may prove to eliminate some of the costs and time for mtDNA testing.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here