Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cardiovascular disease is the leading cause of death in industrialized countries and accounts for over 31% of deaths globally. Over the last century, a multitude of discoveries and intense research have elucidated the mechanisms of atherosclerosis, a word derived from the Greek atheros , meaning gruel and sclerosis , meaning hard. It has been well established, for over six decades now, that the major atherosclerotic risk factors include hyperlipidemia, hypertension, cigarette smoking, and a positive family history of the disease. Diabetes mellitus, obesity, lack of exercise, and poor dietary habits can also be added to the previous list ( Box 13.1 ). At the molecular level, hypercholesterolemia and inflammatory cells play a key role in the development of the atherosclerotic plaque. , Numerous large-scale studies have confirmed that lowering low-density lipoproteins (LDL) levels by 30% to 50% will significantly reduce cardiovascular mortality. Between 1980 and 2000, therapeutic cholesterol reduction has decreased mortality from atherosclerotic cardiovascular disease (ASCVD) by one third. Every few years, the American College of Cardiology (ACC) and the American Heart Association (AHA) publish guidelines and recommendations for lipid management in asymptomatic and symptomatic patients. The focus of this chapter is on the pathophysiologic role of hyperlipidemia in causing atherosclerosis, the physiology and metabolism of lipids, and the diagnosis and management of hyperlipidemia, as well as an update to the most recently published guidelines.
1910 Human and atherosclerotic plaques contain cholesterol
1913 High cholesterol diet causes atherosclerosis in rabbits
1919 Heart attacks recognized in humans
1933 Feedback inhibition of cholesterol synthesis demonstrated
1938 Familial hypercholesterolemia described
1950 Cholesterol biosynthetic pathway elucidated
1951 High-fat diets raise plasma cholesterol in humans
1953 Risk factor concept advanced
1955 LDL identified as risk factor for CHD
1973 LDL receptor discovered
1976 HMG CoA reductase inhibitors (statins) discovered
1981 Statins increase LDL receptors in vivo
1987 First statin (Mevacor) approved for human use
1994 Statins decrease heart attacks and prolong life
1997 SREBP pathway elucidated
2006 PCSK9: Destroyer of LDL receptors
The notion that dietary fat had something to do with atherosclerosis has existed for some time. In 1910, a German scientist found that human aortic plaque had 25 times more cholesterol than a “normal” aorta. Three years later, a Russian pathologist induced atherosclerosis in rabbits fed a high cholesterol diet. In 1919, an American clinician correlated electrocardiographic changes in patients suffering from angina caused by ASCVD. Subsequently, familial hypercholesterolemia was diagnosed and the cholesterol synthesis pathway described. Over the last 50 years, the LDL receptor was discovered and pharmaceutical agents, the statins which inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, were approved and prescribed to treat patients with hyperlipidemia ( Table 13.1 ). More recently, the transcription factor sterol regulatory element-binding protein-1 (SREBP-1) was identified as well as its complex interaction with intracellular and nuclear elements to regulate LDL.
| Year | Scientist(s) | Discovery |
|---|---|---|
| 1913 | Nikolai Anitschkow | Fed pure cholesterol to rabbits and demonstrated development of hypercholesterolemia and extensive aortic atherosclerosis |
| 1950 | John Gofman | Described the major classes of plasma lipoproteins using ultracentrifugation. Demonstrated direct and inverse association of LDL-C and HDL-C levels, respectively, with incidence of myocardial infarction |
| 1964 | Konrad Bloch and Feodor Lynen | Received Nobel Prize for unraveling the metabolic pathway of cholesterol synthesis |
| 1972 | Akira Endo | Discovered compactin, the forebearer to the first statin, from a blue-green mold |
| 1973 | Michael Brown and Joseph Goldstein | Received the Nobel Prize for the discovery of the LDL receptor and its feedback regulation |
Lipids are hydrophobic and are chemically insoluble in blood. They are transported as lipoproteins in which the hydrophobic lipid components are surrounded by an envelope of hydrophilic phospholipids and proteins known as apolipoproteins (apo). This creates a water-soluble spherical particle that can be carried in the blood ( Fig. 13.1 ). The principal function of lipoprotein particles is transporting lipids from the intestine and liver through the bloodstream to the cells of the body, where they can be stored, used for synthetic processes, and metabolized to yield energy. The various apolipoproteins, such as apo B, apo A, apo E, and apo C, serve as cofactors in metabolism of the contained lipid and as ligands to facilitate binding of lipoproteins to receptors on the surface of cells throughout the body. Five major lipoproteins are recognized.
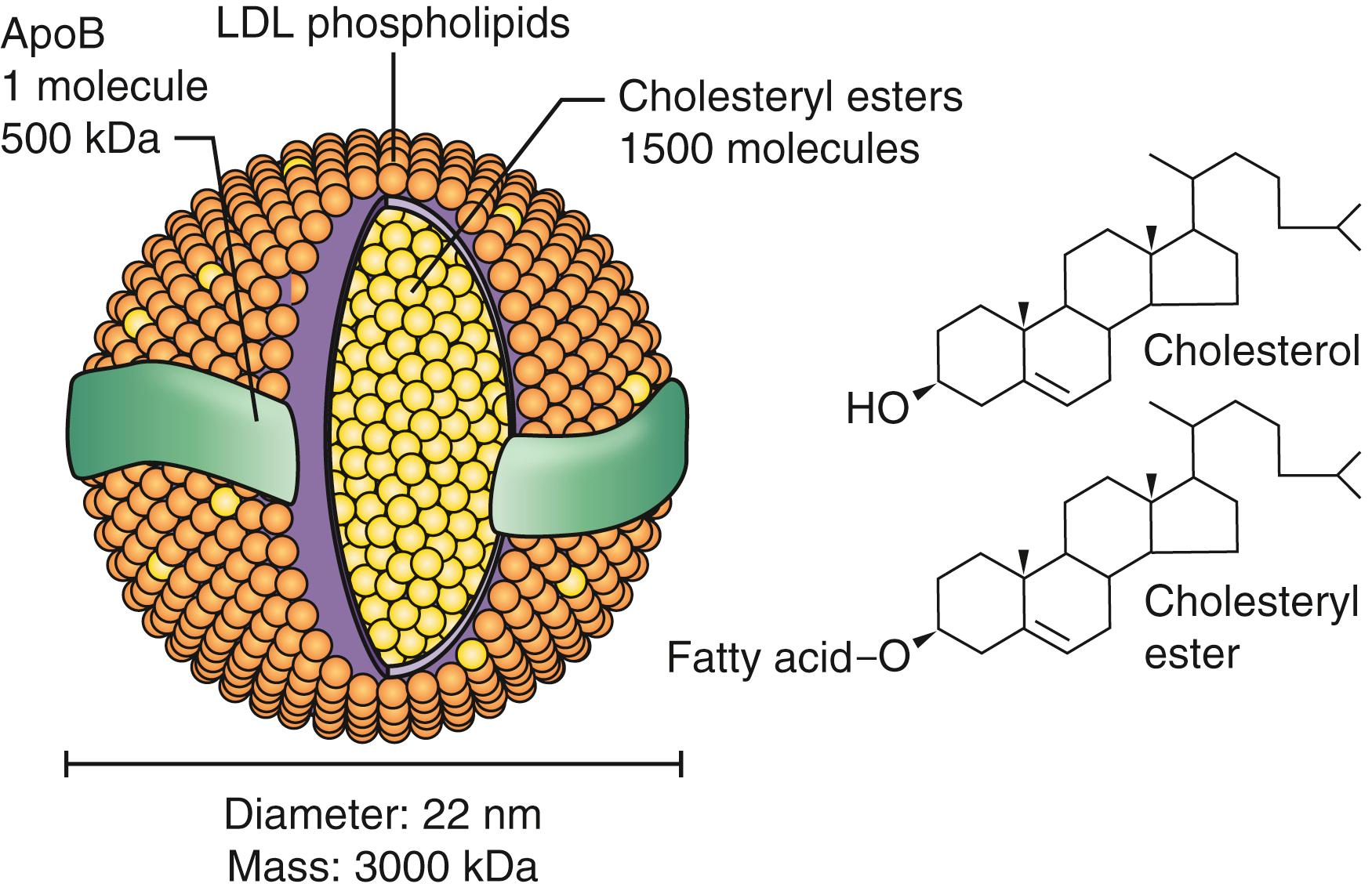
Lipoprotein metabolism can be divided into exogenous and endogenous phases. During the exogenous phase, both dietary lipids and recirculated lipids within bile are absorbed into enterocytes and packaged as very large lipoproteins called chylomicrons. Although dietary cholesterol and triglycerides are absorbed by different mechanisms within the gastrointestinal (GI) tract, they are combined in this single chylomicron particle for transport from the GI tract through the thoracic duct and into the circulation to the rest of the body. Triglycerides constitute approximately 90% of the chylomicron’s lipid content. When present in significant concentration, chylomicrons account for the turbidity or “milkiness” of plasma, known as postprandial lipemia, which is seen in some individuals with metabolic and inherited disorders of lipid metabolism ( Fig. 13.2 ). Chylomicrons are distinguished by the presence of one apo B-48 molecule in each particle. In the bloodstream, the enzyme lipoprotein lipase (LPL) hydrolyzes the triglyceride contained within the chylomicron into free-fatty acids (FFAs). FFAs are taken up by peripheral tissues and used for energy. In adipose and muscle cells, FFAs can also be re-esterified into triglycerides and stored for future energy production. The remaining triglyceride-poor chylomicron remnant contains only the absorbed dietary cholesterol, which is then transported to the liver for storage. Although chylomicrons are typically considered a transport lipoprotein, evidence suggests atherosclerotic lesions contain apo B-48 within plaque, thus implicating these chylomicron remnants as atherogenic particles. ,
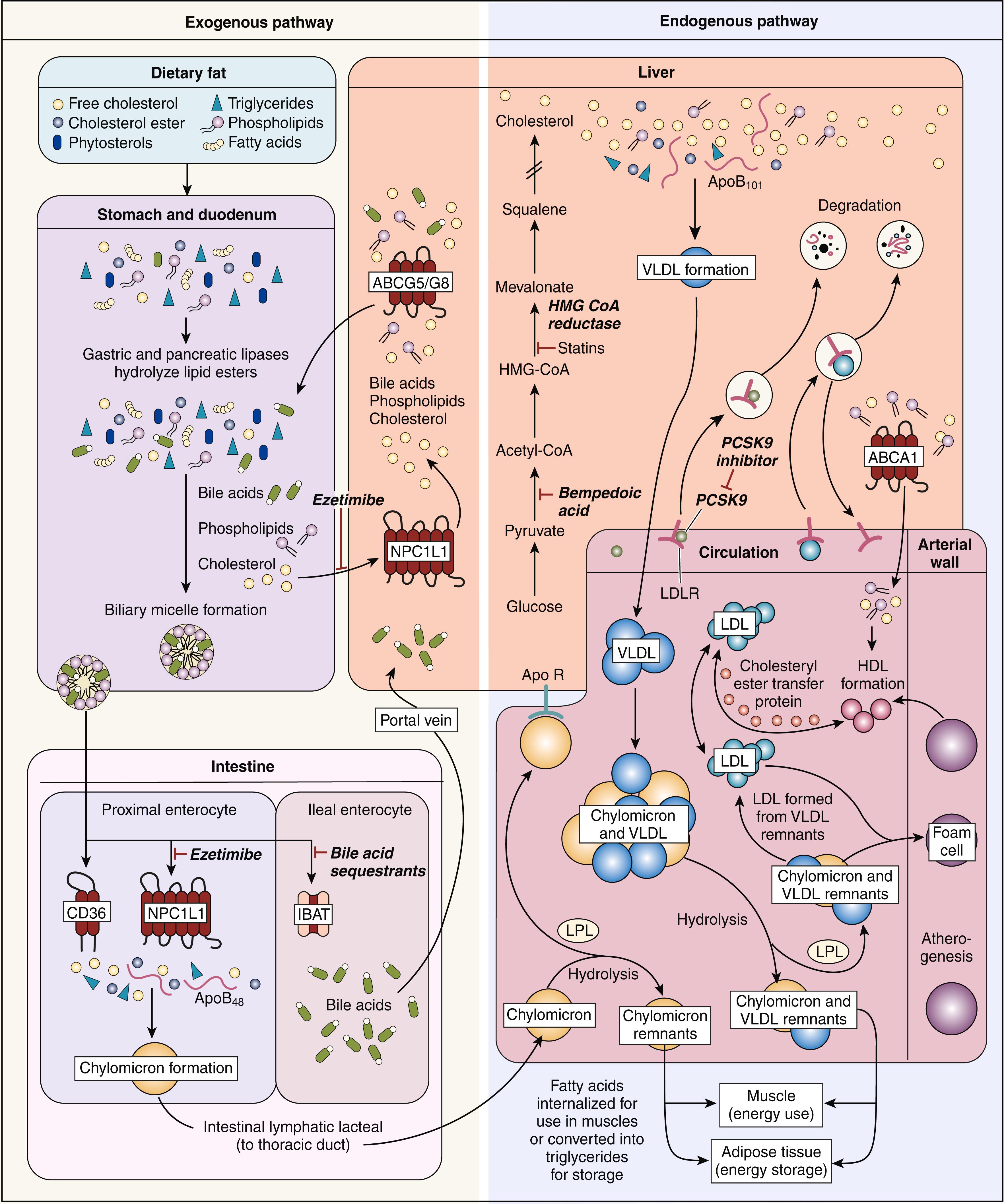
The endogenous phase of lipoprotein metabolism involves the hepatic formation of very-low-density lipoproteins (VLDLs) containing cholesterol and triglycerides derived from stores within the liver and adipocytes ( Table 13.2 ). Each VLDL particle contains apos C and E as well as one molecule of apo B-100 per particle. Similar to chylomicrons, the predominant lipid component of VLDL is triglyceride, which accounts for approximately 70% of its lipid content. Although not as large as chylomicrons, VLDL is large enough to cause lipemia when present in very high concentrations. VLDL is released from the liver into the bloodstream, where LPL again facilitates removal of the triglyceride component of VLDL and presents it to the muscle cell as fuel for energy production. Through this endogenous mechanism, triglycerides stored in adipocytes, hepatocytes, and muscle cells can be used for energy during fasting or starvation as a more energy-rich alternative to glucose. As the triglyceride is removed, two additional atherogenic lipoprotein particles are formed: VLDL remnants and intermediate-density lipoproteins (IDLs).
| Conventional Units (mg/dL) | SI Units (mmol/L) | |
|---|---|---|
| Low-Density Lipoprotein Cholesterol | ||
| Optimal | <100 | <2.59 |
| Near optimal | 100–129 | 2.59–3.34 |
| Borderline high | 130–159 | 3.37–4.12 |
| High | 160–189 | 4.14–4.90 |
| Very high | >190 | >4.92 |
| High-Density Lipoprotein Cholesterol | ||
| Low | <40 | <1.04 |
| High | >60 | >1.55 |
| Triglycerides | ||
| Normal | <150 | <1.70 |
| Borderline | 150–199 | 1.70–2.25 |
| High | 200–499 | 2.26–5.64 |
| Very high | >500 | >5.65 |
IDLs carry cholesterol esters and triglycerides. IDLs, similar to LDL, have been shown to increase the risk of cardiovascular disease. These triglyceride-rich lipoprotein particles are present in patients with metabolic syndrome and type 2 diabetes mellitus. The MARS study showed that IDL is associated with increased carotid artery intima-media thickness (CIMT).
In addition to LPL, hepatic lipase participates in the conversion of IDL to LDL, the most atherogenic of all lipoprotein particles. Although a number of other apolipoproteins are attached to the original VLDL particle, only one, apo B-100, is present in each LDL particle (see Fig. 13.1 ). LDL binds to specific LDL receptors on the surface of each cell, facilitating transfer of the remaining cholesterol to these cells, where it can be stored and used as cell membranes, steroid hormones, and bile acids. The number of exposed LDL receptors is regulated by the intracellular concentration of cholesterol within each cell. When excess plasma LDL is present, atherosclerosis can possibly increase in proportion to the concentration of circulating LDL.
Nabel and Braunwald received the Nobel Prize in 1985 for discovering the LDL receptor. , They were able to demonstrate that the circulating LDL concentration in plasma is determined by the number of LDL receptors on the various cells of the body, with the liver accounting for more than 70% of this total receptor number. When the intracellular cholesterol content of the cells is low, LDL receptor synthesis is upregulated, receptor numbers increase, and the LDL plasma concentration decreases. In contrast, when intracellular cholesterol is increased, LDL receptor synthesis is downregulated, receptor numbers diminish, and circulating LDL rises. Humans are born with a maximum number of LDL receptors and a very low circulating LDL level of 25 to 30 mg/dL (0.65 to 0.78 mmol/L). Over a lifetime, the Western lifestyle of excessive calories, cholesterol, and saturated fat intake, along with inactivity, results in increasing intracellular cholesterol levels, causing secondary downregulation of LDL receptors. Therefore observed increases in excess plasma LDL-C levels coincide with the epidemic of atherosclerosis seen worldwide.
High-density lipoprotein (HDL) is synthesized by the liver and intestine as apo A-I, which is released into the bloodstream as a lipid-poor discoid particle. Stored cholesterol released from peripheral cells through the action of a specific transporter known as adenosine triphosphate-binding cassette (ABC) transporter A1 is absorbed by the discoid apo A-I and converted to cholesterol ester under the influence of lecithin-cholesterol acyltransferase (LCAT). During this process, HDL becomes a spherical particle. Additional cellular cholesterol is then added by another cassette transporter, ABCG1, and through the action of the scavenger receptor B1 (SR-B1). The HDL particle can then return to the liver, where it binds to hepatic SR-B1 and releases its cholesterol, or it can exchange a portion of its cholesterol content for triglyceride from VLDL through the chemical action of the cholesterol ester transfer protein (CETP). This removes triglyceride from VLDL, converting it into LDL, which is then removed from circulation through the hepatic LDL receptor. This process is known as “reverse cholesterol transport” and plays an important role in the antiatherogenic properties of the HDL particle. ,
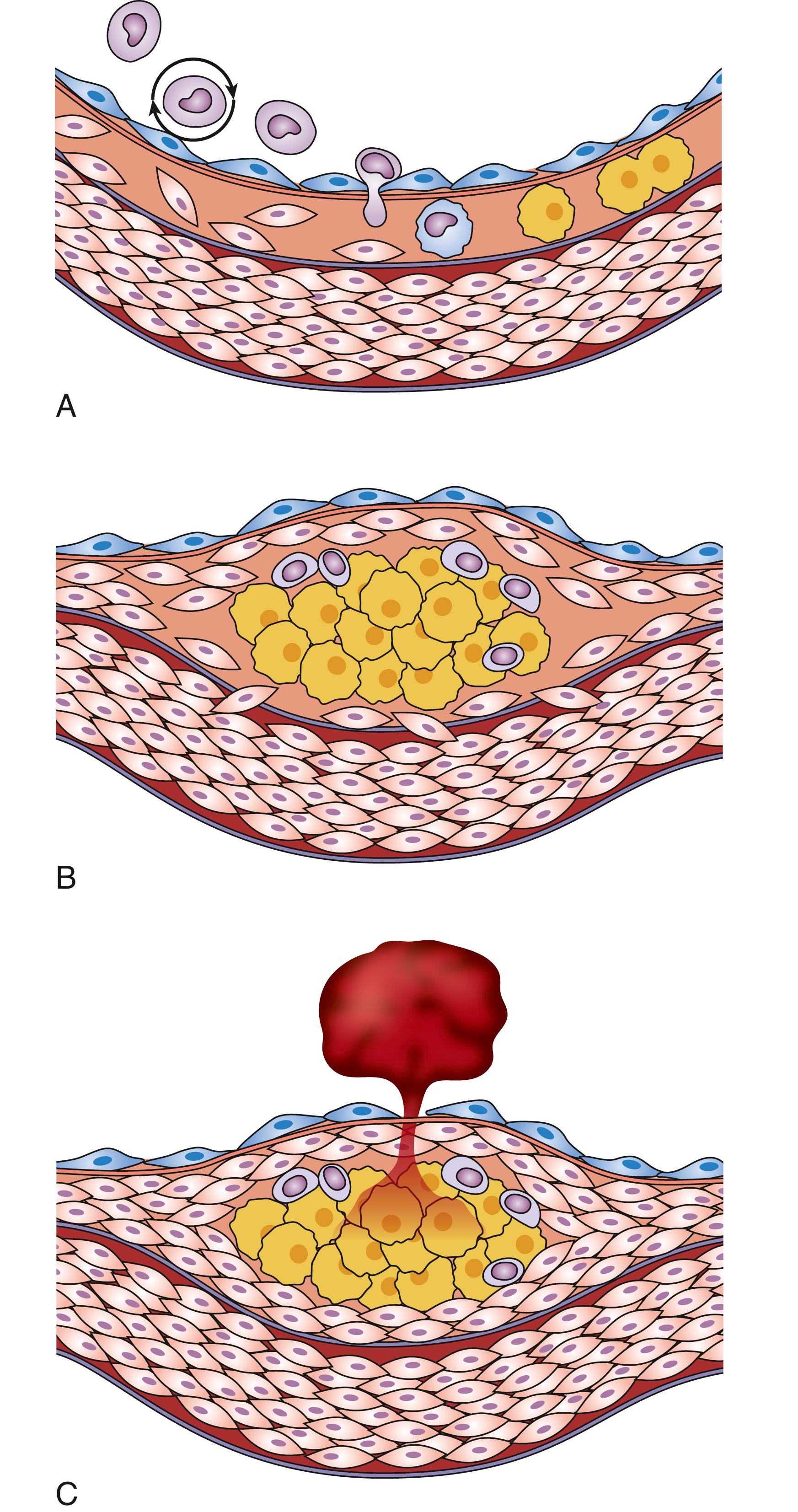
Atherosclerosis is a pathologic process that begins in the arterial endothelial layer. Normal endothelium is smooth and repels circulating blood elements. On the other hand, damaged endothelium attracts various cellular elements. After the initiation of an atherogenic diet, patches of endothelial cells express selective adhesion molecules that bind to various classes of leucocytes. Vascular cell adhesion molecule-1 (VCAM-1) binds to monocytes and T-lymphocytes detected in human atheromas. In the setting of elevated LDL and low HDL, peripheral blood mononuclear cells become cholesterol enriched ( Fig. 13.3 ). Intracellular droplets of cholesterol ester accumulate in these engorged cells, and give them a characteristic appearance, “foam cells.” Foam cells are more apt to adhere to damaged endothelium and then migrate into the intima layer and become macrophages. In response to this, VSMC proliferate and cause thickening of the intima. As the process is repeated, plaque thickness increases and a fibrous cap is formed ( Fig. 13.4 ). This pathologic process is the same in the peripheral and cerebrovascular circulation as it is in the coronary arteries. To varying degrees, the traditional risk factors are also similar, regardless of the location of atherosclerotic disease, including hyperlipidemia.
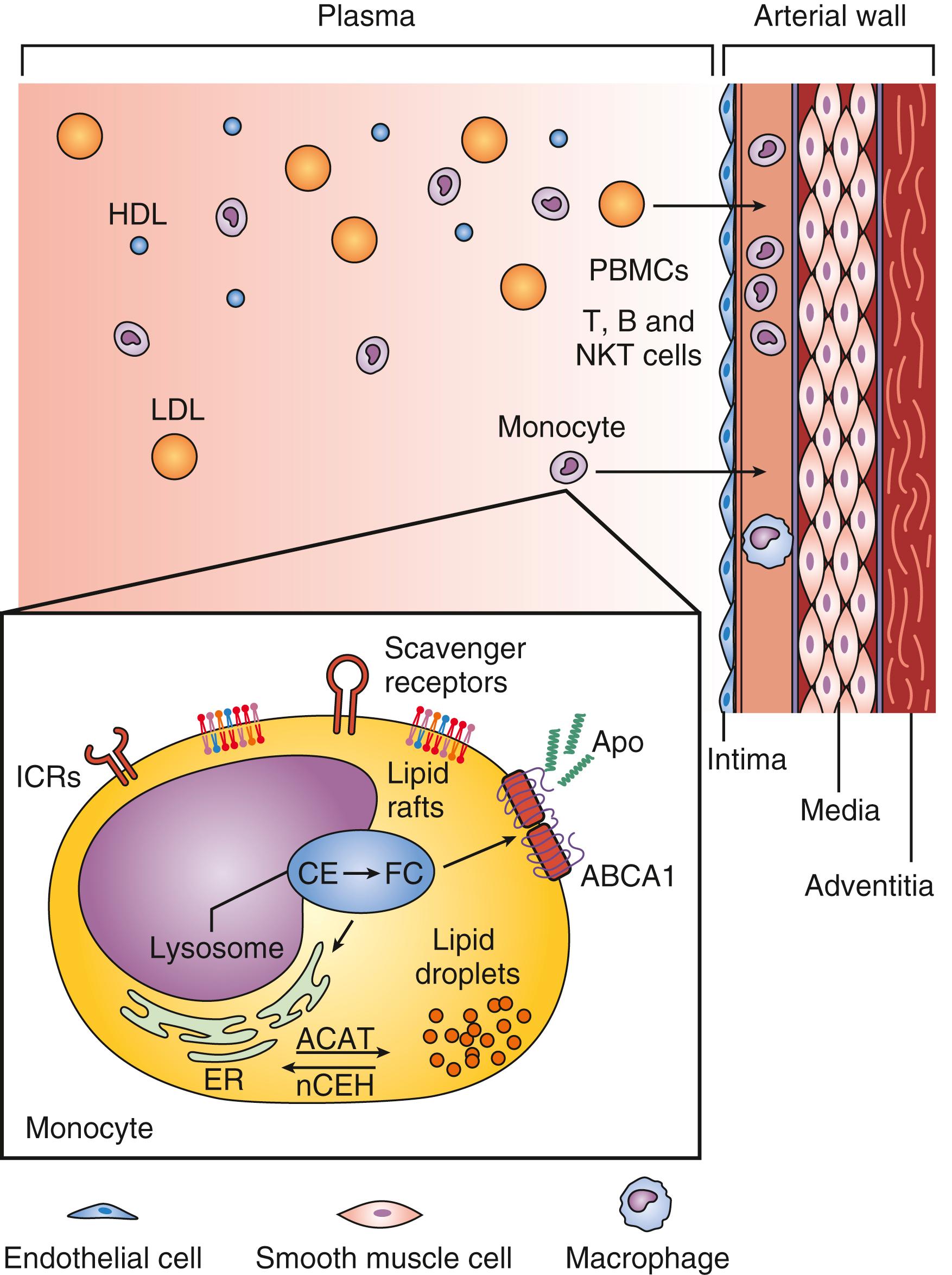
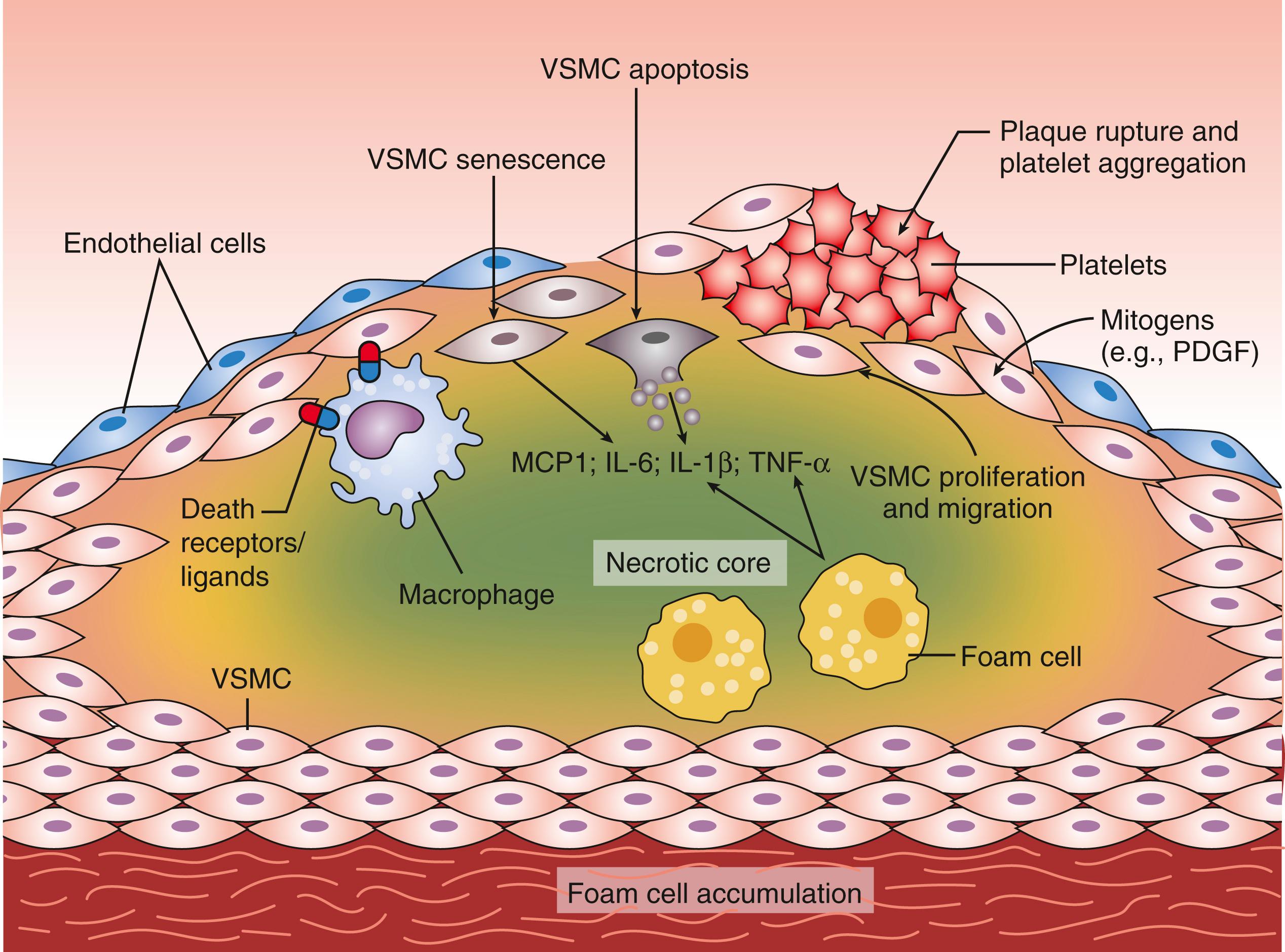
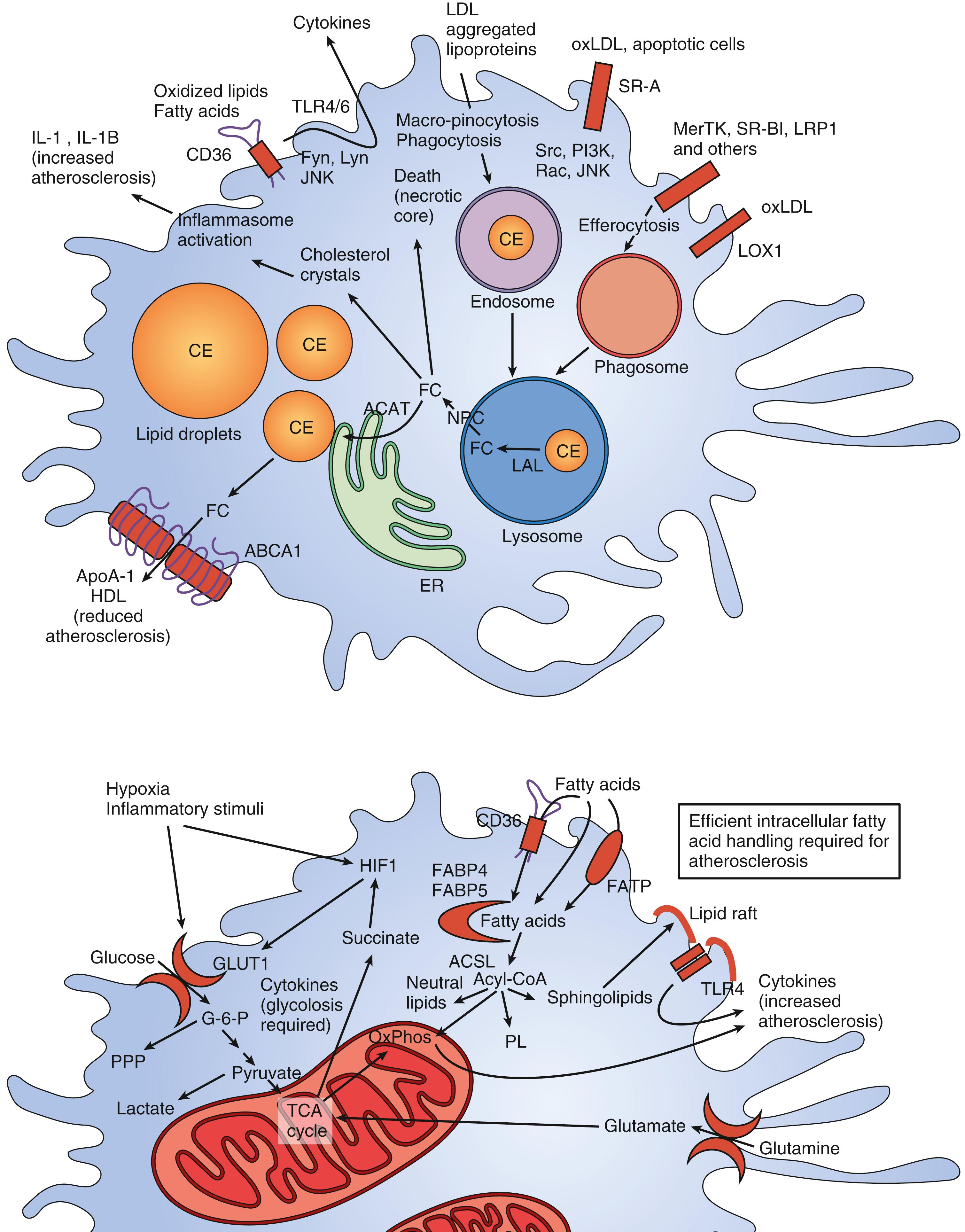
The damaged endothelium also becomes permeable to LDL and other circulating atherogenic lipoproteins (VLDL remnants, IDL, and chylomicron remnants). Once inside the vessel wall, LDL is oxidized by free radicals, furthering the inflammatory immune response and initiating the pathologic process of atherosclerosis. Oxidized LDL is subsequently absorbed through specific scavenger receptors into fixed tissue macrophages, thereby transforming them into lipid-filled foam cells ( Fig. 13.5 ). Groups of foam cells accumulate underneath the endothelium and become the initial lesion of atherosclerosis (the fatty streak). As this process continues, the foam cells undergo apoptosis (programmed cell death) allowing the lipid contained in them to spill out to form the lipid core of an atherosclerotic plaque. The initial response of the arterial wall is to expand, a process known as positive remodeling. As the plaque increases in thickness, it begins to encroach on the arterial lumen, thereby reducing the blood flow to points distal. The concept of stable and unstable plaque has been developed, with stable plaques possessing a thick fibrous cap and solid lipid core. Plaques accumulating a large lipid core trigger an intense local inflammatory reaction as this lipid is oxidized resulting in the infiltration of additional macrophages and inflammatory cells. The vulnerable or unstable plaque usually remains small and does not critically compromise the luminal diameter ( Fig. 13.6 ). However, the thin fibrous cap is prone to ulceration or rupture. When this occurs, a platelet-rich clot rapidly forms on top of the plaque and produces complete obstruction of the involved artery and subsequent acute clinical infarction or ischemia. The relationship between LDL level, plaque formation and ASCVD has been extensively studied. At low physiologic LDL levels (20–40 mg/dL), the probability of LDL particle retention and atherosclerosis development is quite low. As LDL-C levels increase, there is a dose-dependent progressive formation of atherosclerotic plaque.
The Adult Treatment Panel III (ATP III) of the National Cholesterol Education Program (NCEP) recommended that all adults older than 20 years be screened for hyperlipidemia every 5 years with a fasting lipoprotein profile. , Blood levels should include total cholesterol (TC), triglycerides, HDL cholesterol (HDL-C), and LDL-C. It is recommended that patients be in their usual state of health and fast for 12 hours before testing. Blood should be drawn after sitting comfortably for 5 minutes and with less than 1 minute of tourniquet time. If TC is greater than 200 mg/dL (5.18 mmol/L) or HDL is less than 40 mg/dL (1.04 mmol/L), a fasting lipoprotein profile should be obtained to better quantify the patient’s atherosclerotic risk.
ATP III recommended new guidelines for the evaluation of a fasting lipid profile (see Table 13.2 ). TC is not a good risk predictor because it can be increased by elevated HDL, which is usually a lower risk lipid profile, or decreased by low HDL, which is a higher risk lipid profile. The presence of very high triglyceride levels can also elevate TC and suggest a higher risk lipid profile than actually may be present.
LDL-C has been shown to be the most predictive lipoprotein fraction for determining atherosclerosis risk in both epidemiologic and interventional studies, and the associated risk is directly proportional to the LDL-C concentration over a wide range of values. , HDL-C is equally predictive in epidemiologic studies, with an inverse relationship between the HDL-C concentration and risk for atherosclerosis. The risk associated with each of these lipoprotein fractions is additive in individuals with increased LDL-C and decreased HDL-C. The role of elevated triglycerides in risk assessment is tougher to discern. When very sophisticated analyses of triglyceride-rich lipoproteins are performed, it is evident that large chylomicrons and VLDLs are not atherogenic, but the smaller remnant forms of both these lipoproteins and the presence of IDL definitely confer increased atherogenicity.
Clinically, elevated triglycerides in the presence of type 2 diabetes, metabolic syndrome, or familial combined dyslipidemia should indicate an increase in the risk for ASCVD. Very high levels of triglycerides (>1000 mg/dL [11.3 mmol/L]) are also associated with an increased risk for pancreatitis. Atherogenic dyslipidemia is a characteristic lipoprotein pattern frequently observed in patients with type 2 diabetes mellitus and the metabolic syndrome. In this condition, the LDL-C is often unimpressive or frankly low, but it is associated with elevated triglycerides with a decreased HDL-C concentration. More important, however, is the change in the number and composition of LDL particles associated with the metabolic syndrome and type 2 diabetes, rendering them more atherogenic. This LDL is described as “small, dense LDL,” which is an increased number of particles that contain less cholesterol per particle. Studies have shown that it is the number of LDL particles (LDL-P) that truly reflects the risk related to this profile. LDL-P can be determined by lipid analysis using nuclear magnetic resonance (NMR) studies. Because each LDL-P contains one apo B-100 molecule, another method of more accurately assessing the risk conferred by an individual’s LDL profile is to measure the apo B-100 level. Complete analysis may provide a better risk assessment because the individual risks associated with each lipoprotein abnormality in type 2 diabetes mellitus and metabolic syndrome are additive.
Non-HDL-C has been recognized in epidemiologic studies as one of the better predictors of atherosclerotic risk. Non-HDL-C is easily calculated by subtracting HDL-C from TC. ATP III recommended the use of non-HDL-C ( Box 13.2 ) as a way to more accurately assess risk in patients with increased triglycerides and made this a secondary target of therapy after the primary therapeutic goal for LDL-C is attained (vide infra). Non-HDL-C essentially represents the cholesterol content in all of the apo B–containing atherogenic lipoproteins: non-HDL-C = LDL-C + IDL cholesterol + VLDL-C + lipoprotein-(a) (Lp[a]). However, these more specialized studies of cholesterol fractions are significantly more expensive than a routine lipid profile. Therefore it is recommended that the initial risk evaluation and lipid management be performed with the standard LDL-C, HDL-C, and non-HDL-C measurements and that specialized testing be reserved for unusual situations or for fine tuning of therapy.
Known predictor of CHD in epidemiology
Equivalent to total apo B-100 and TC/HDL
Represents the sum of LDL, Lp(a), IDL, and VLDL: all atherogenic apo B-containing lipoproteins
Lipid equivalent of hemoglobin A 1c
Non-HDL cholesterol = TC – HDL cholesterol.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here