Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The ventricles arise from the lumen of the forebrain, midbrain, and hindbrain. At approximately the sixth week of gestation, the lateral ventricles arise as extensions from the anterior-superior aspect of the third ventricle and communicate through the foramen of Monro. The lumen of the hindbrain expands to become the fourth ventricle. The cerebral aqueduct persists in the midbrain and allows communication between the third and fourth ventricles.
Cerebrospinal fluid (CSF) is produced in the choroid plexuses as well as the vascular ependymal cells and pia mater ( Fig. 11.1 ). The CSF circulates from the lateral ventricles → foramen of Monro → third ventricle → cerebral aqueduct → foramina of Magendie and Luschka → subarachnoid spaces along the brain and spinal cord. CSF drains through the arachnoid villi and at the spinal nerve sheaths.
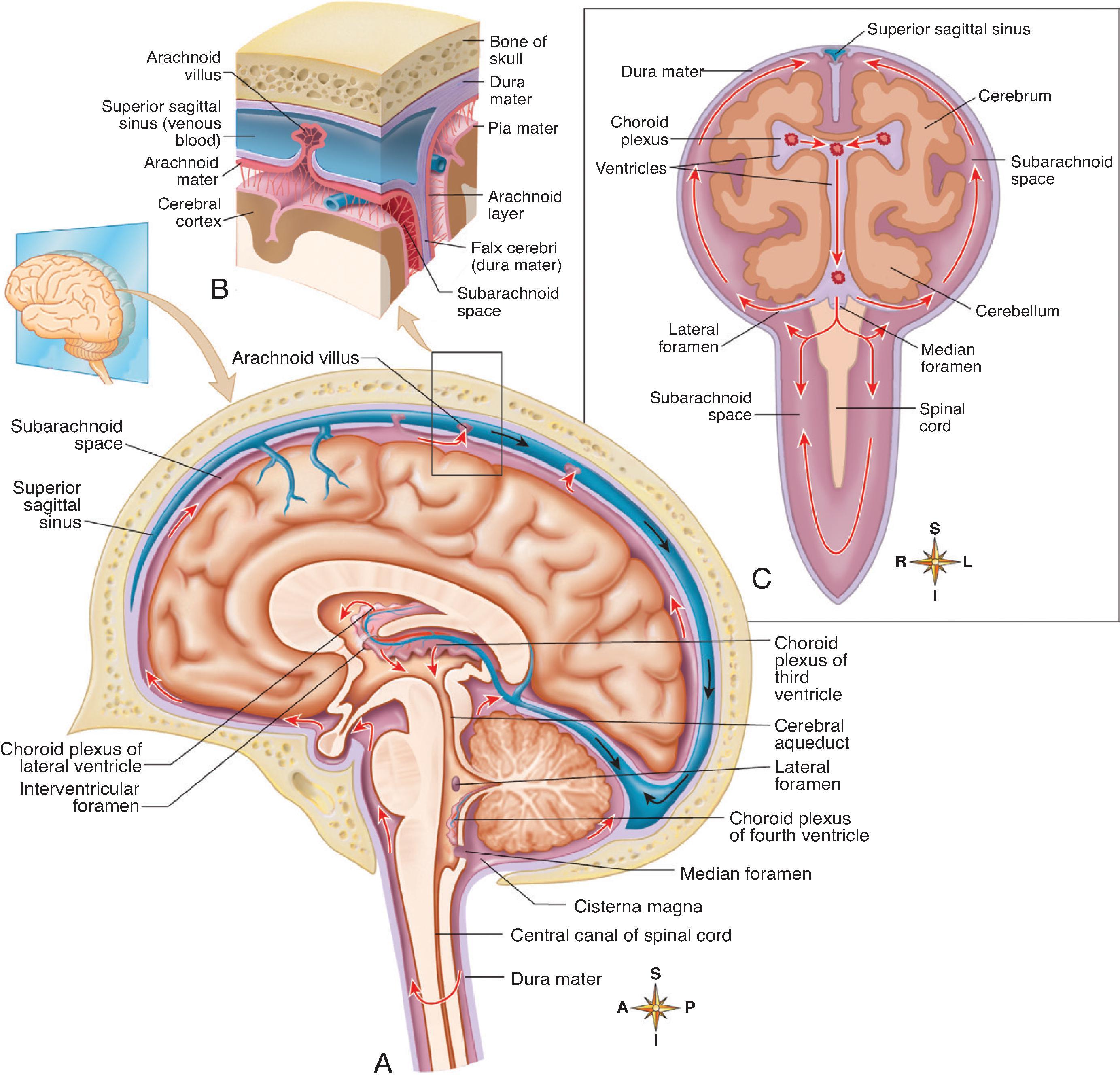

The CSF volume in the CNS is approximately 50 mL in neonates, 60 to 100 mL in children, and 130 to 150 mL in adults. CSF is produced at a rate of 0.35 mL/min, and in children the daily turnover is 10 to 15 mL/kg compared to 500 mL in adults. CSF pressure is affected by age, positioning, elasticity of the brain, skull, and meninges, and arterial and venous pressure. CSF pressure is approximately 3 to 7 mm/Hg in neonates, 6 to 15 mm Hg in infants, and 10 to 20 mm/kg in adults.
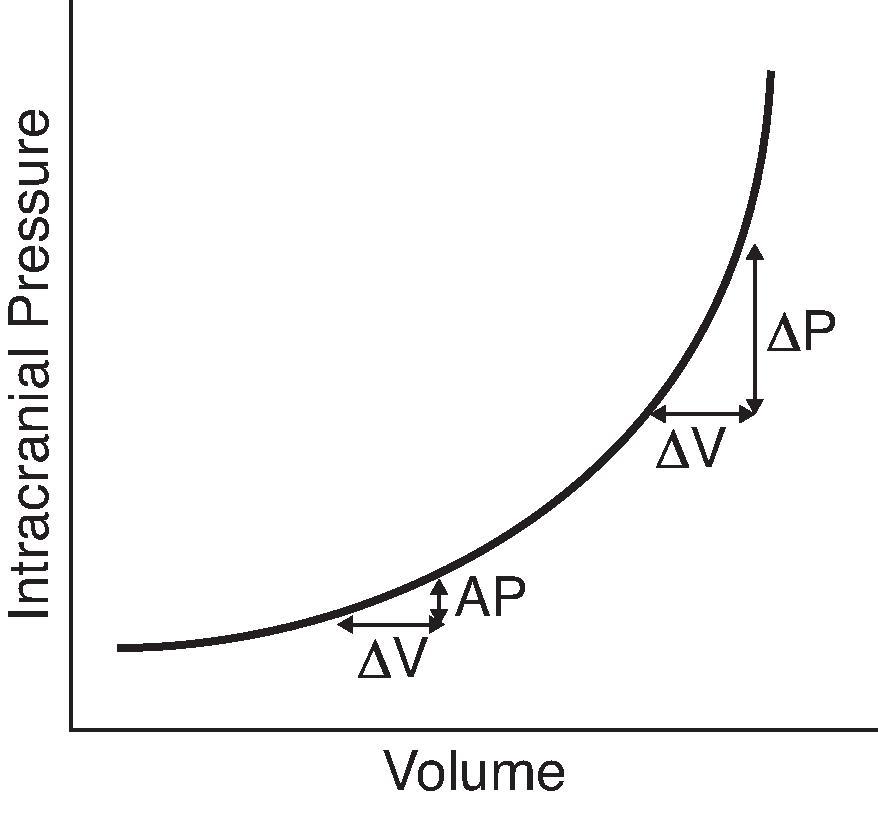
There is a monoexponential relationship between the change in CSF volume and intracranial pressure ( Fig. 11.2 ). The same added volume of fluid at a lower total CSF volume causes a smaller change in intracranial pressure versus a larger change in intracranial pressure when the total CSF volume is high. Disorders affecting CSF physiology discussed in this chapter will include hydrocephalus, idiopathic intracranial hypertension, intracranial hypotension, and benign enlargement of subarachnoid spaces.
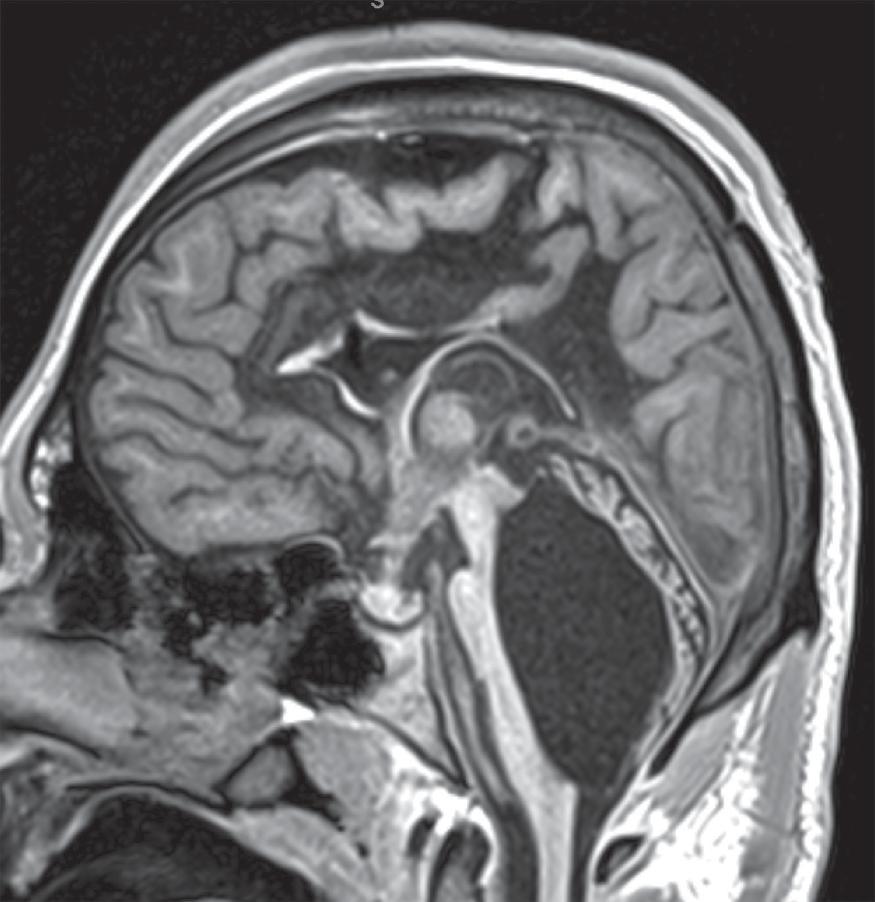
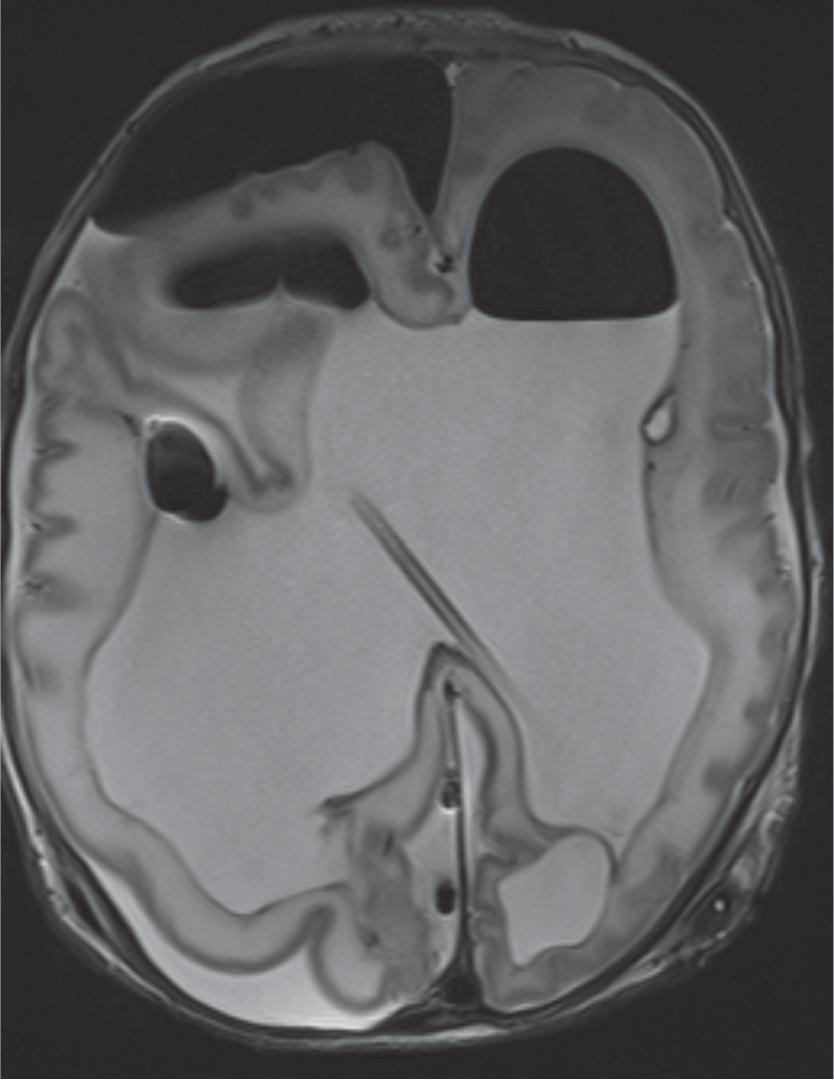
Hydrocephalus refers to increased CSF volume in the ventricles and subarachnoid spaces.
Current understanding of the pathogenesis of hydrocephalus is explained by the bulk flow model and the hemodynamic model. The bulk flow model is the classic explanation, in which hydrocephalus is caused by CSF overproduction, obstruction of CSF flow, or abnormal CSF resorption at the arachnoid granulations. The hemodynamic model proposed by Greitz et al suggests that pathologic processes reduce the normal arterial pulsations → greater pressure transmitted to the brain → larger transmantle cerebral pressure → ventricular dilatation.
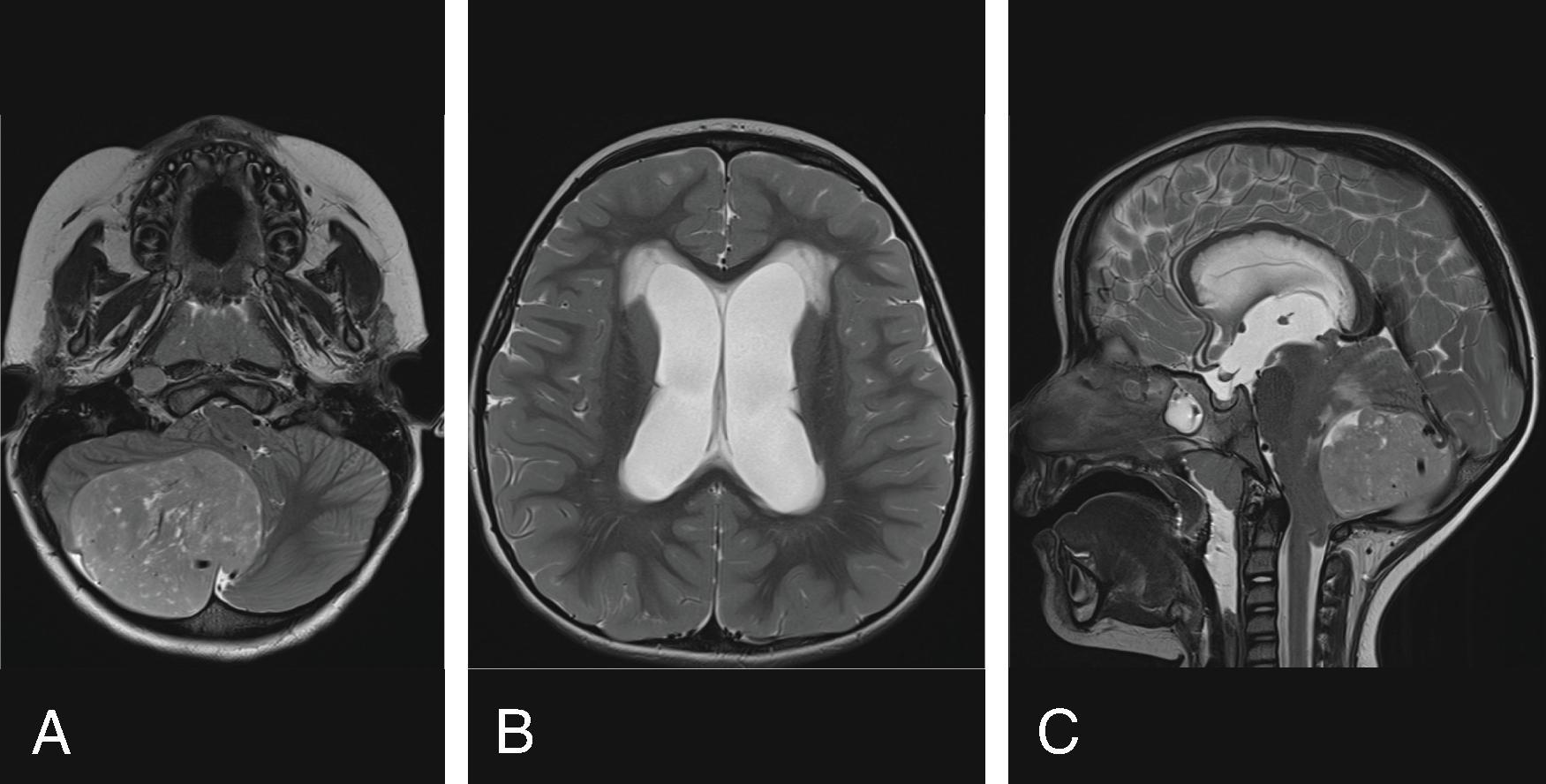
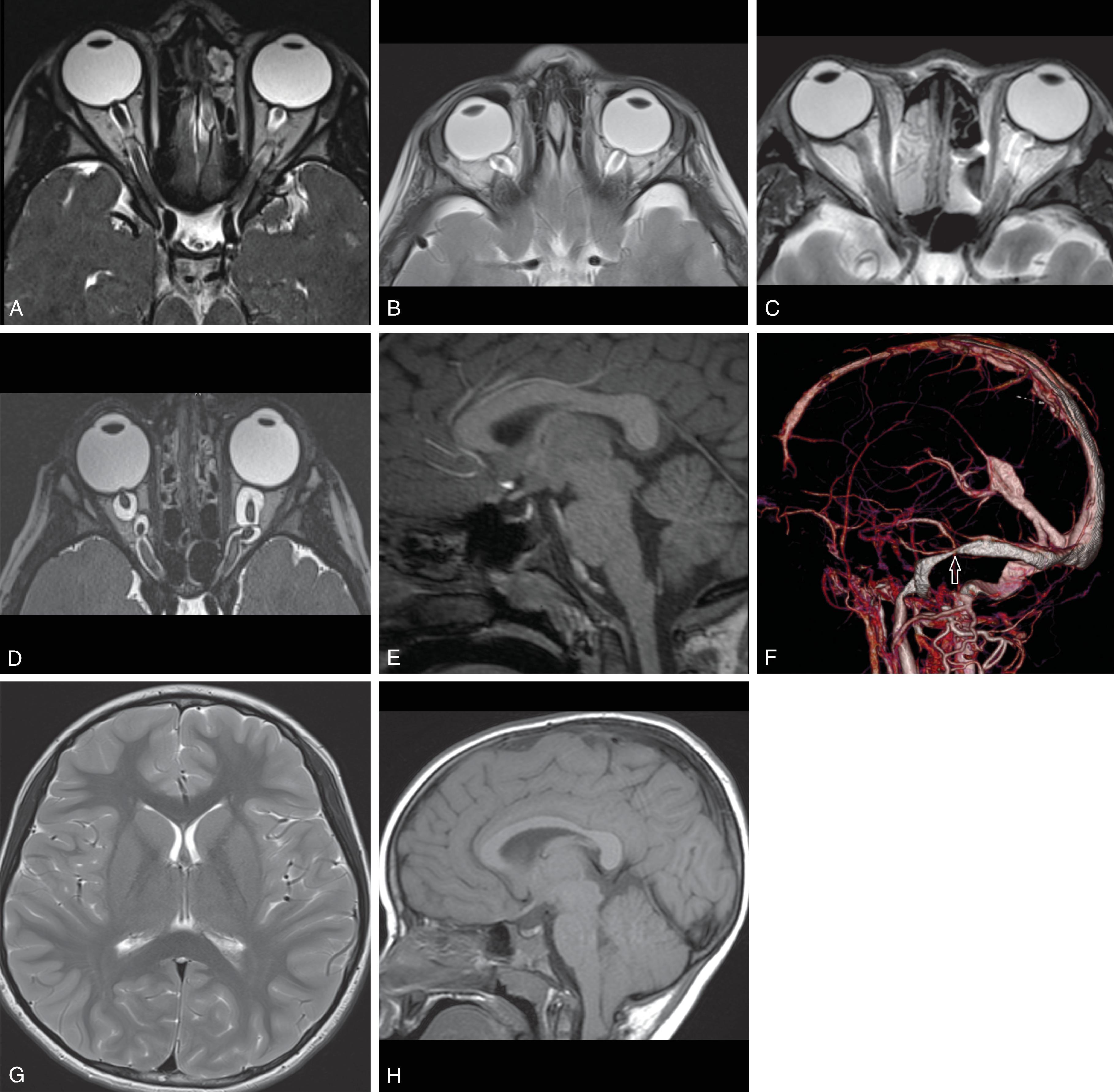
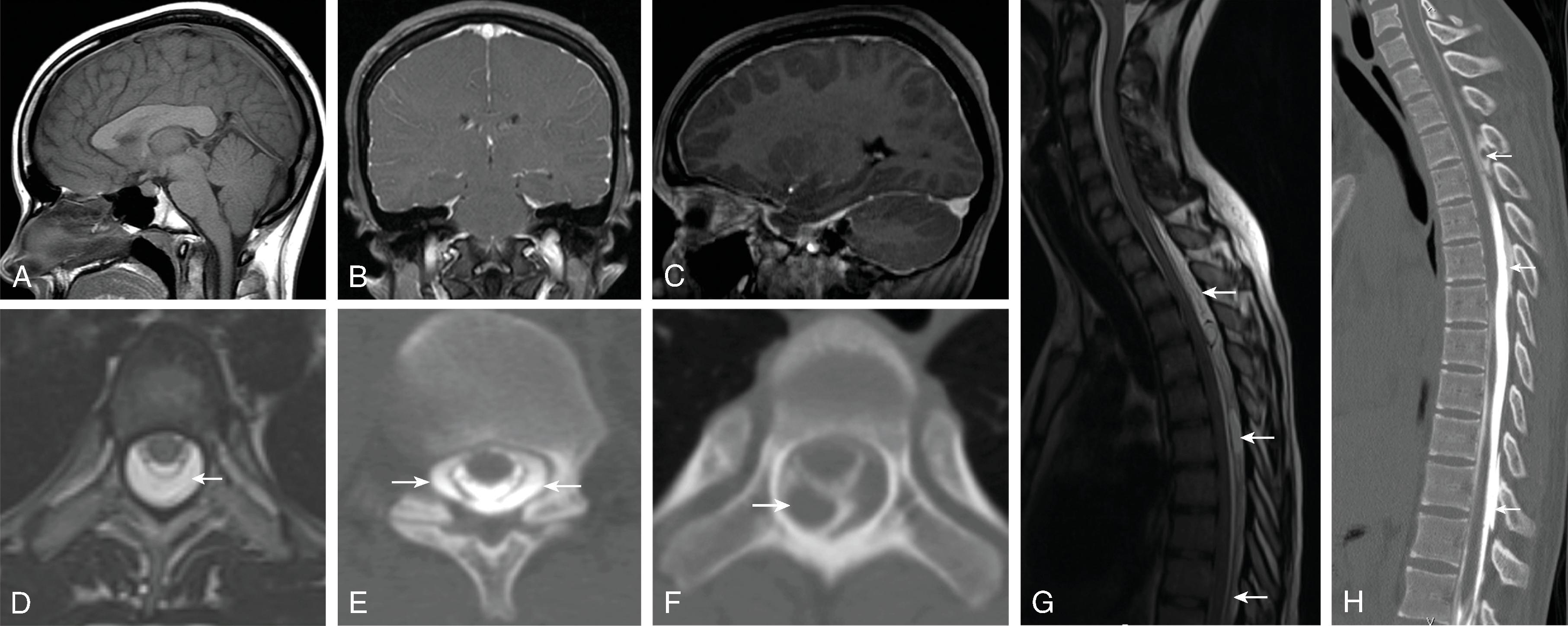
Most common etiologies of hydrocephalus include intraventricular hemorrhage, infection, myelomeningocele, aqueductal stenosis, tumor, and others. Rare causes of hydrocephalus can include CSF overproduction from a choroid plexus papilloma and hydrocephalus from a spinal tumor possibly through increased CSF protein causing high CSF viscosity, tumoral obstruction of the foramen magnum, or subarachnoid spaces in the thecal sac.
Categorization: Hydrocephalus is divided into noncommunicating (obstructive) and communicating hydrocephalus.
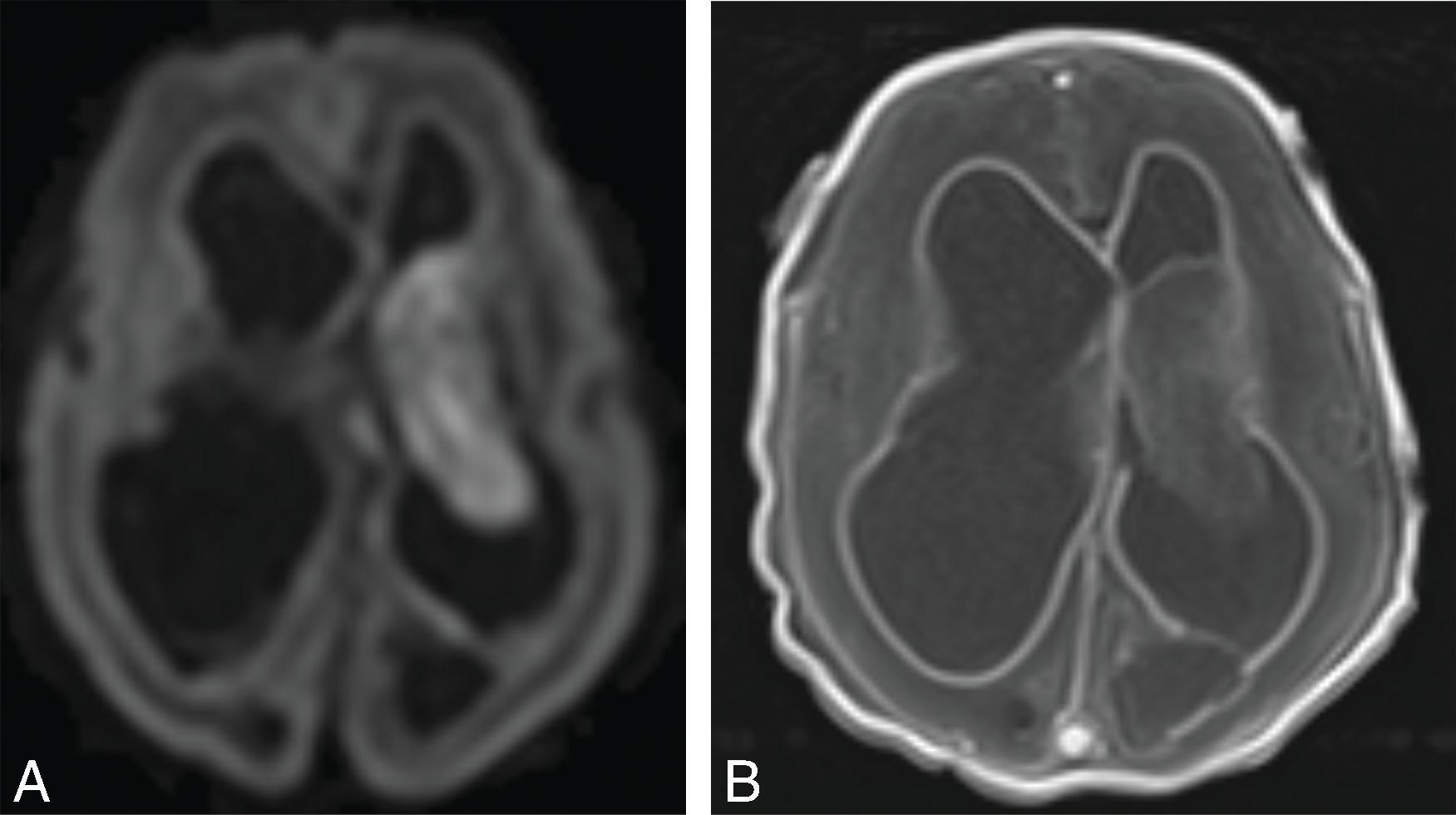
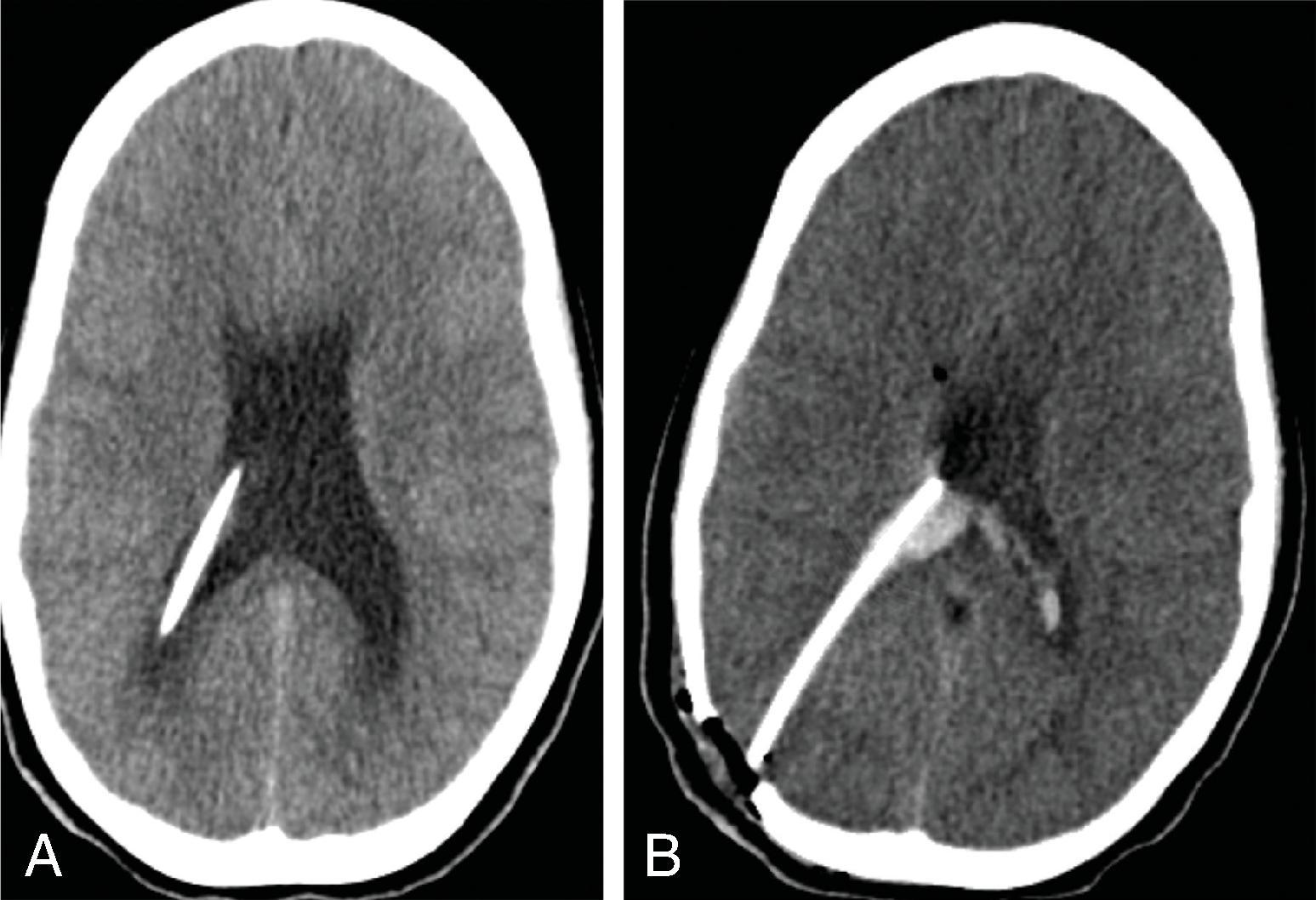
Obstructive hydrocephalus occurs when there is obstruction of the CSF pathways. The CSF pathways proximal to the obstruction become distended and the elevated intracranial pressure leads to periventricular interstitial edema. Etiology can be due to tumors, cysts, adhesions from infection or hemorrhage, and malformations.
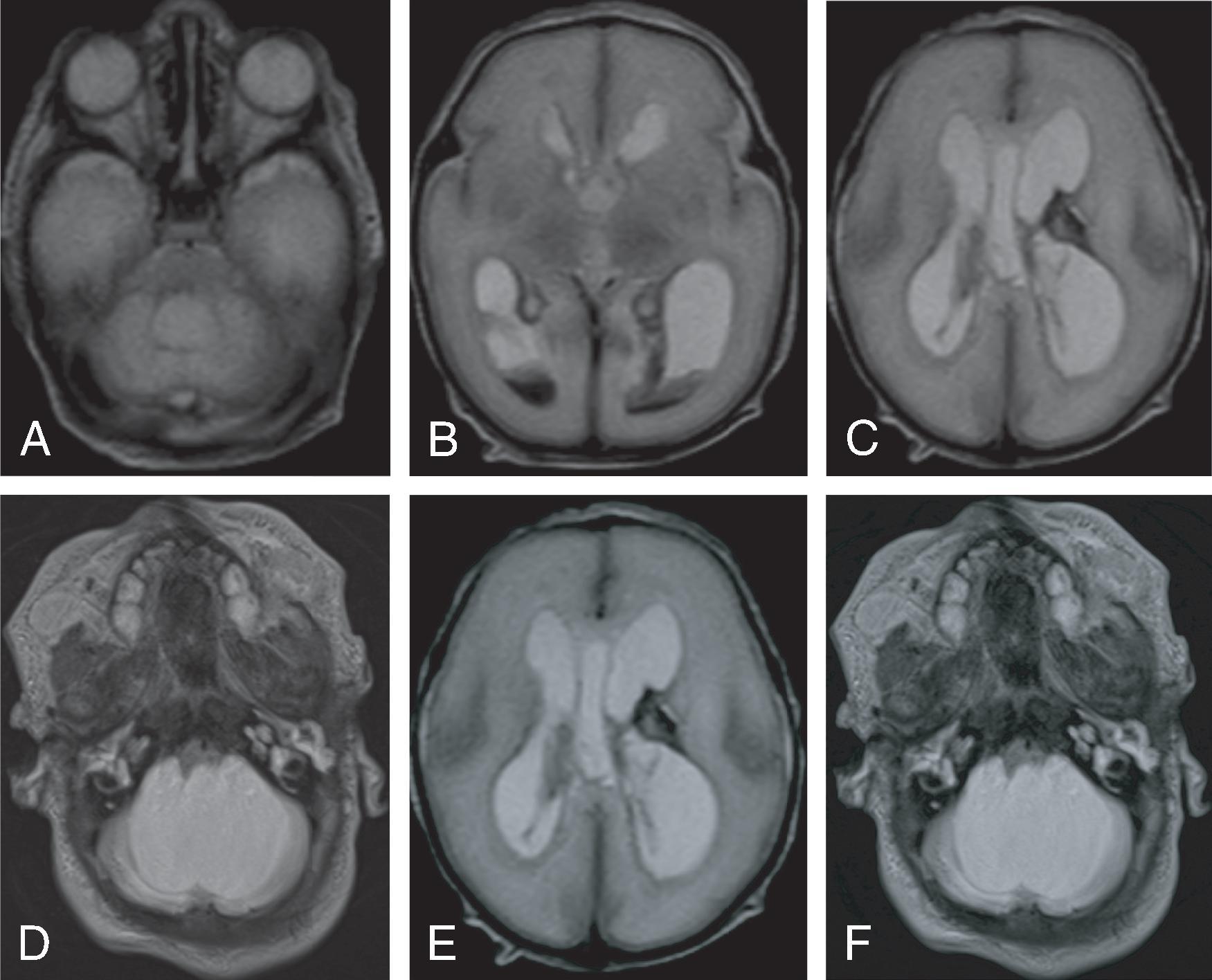
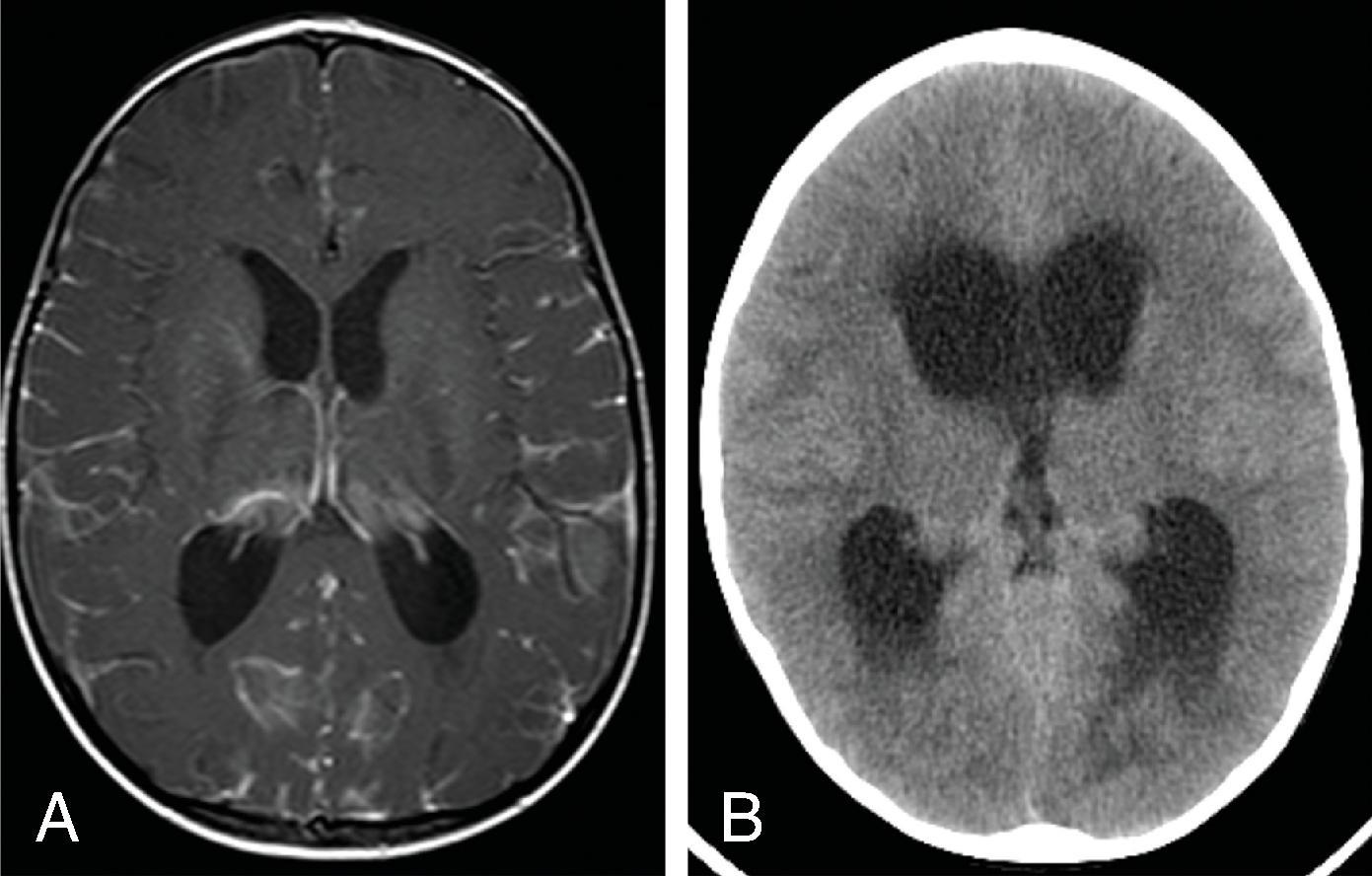
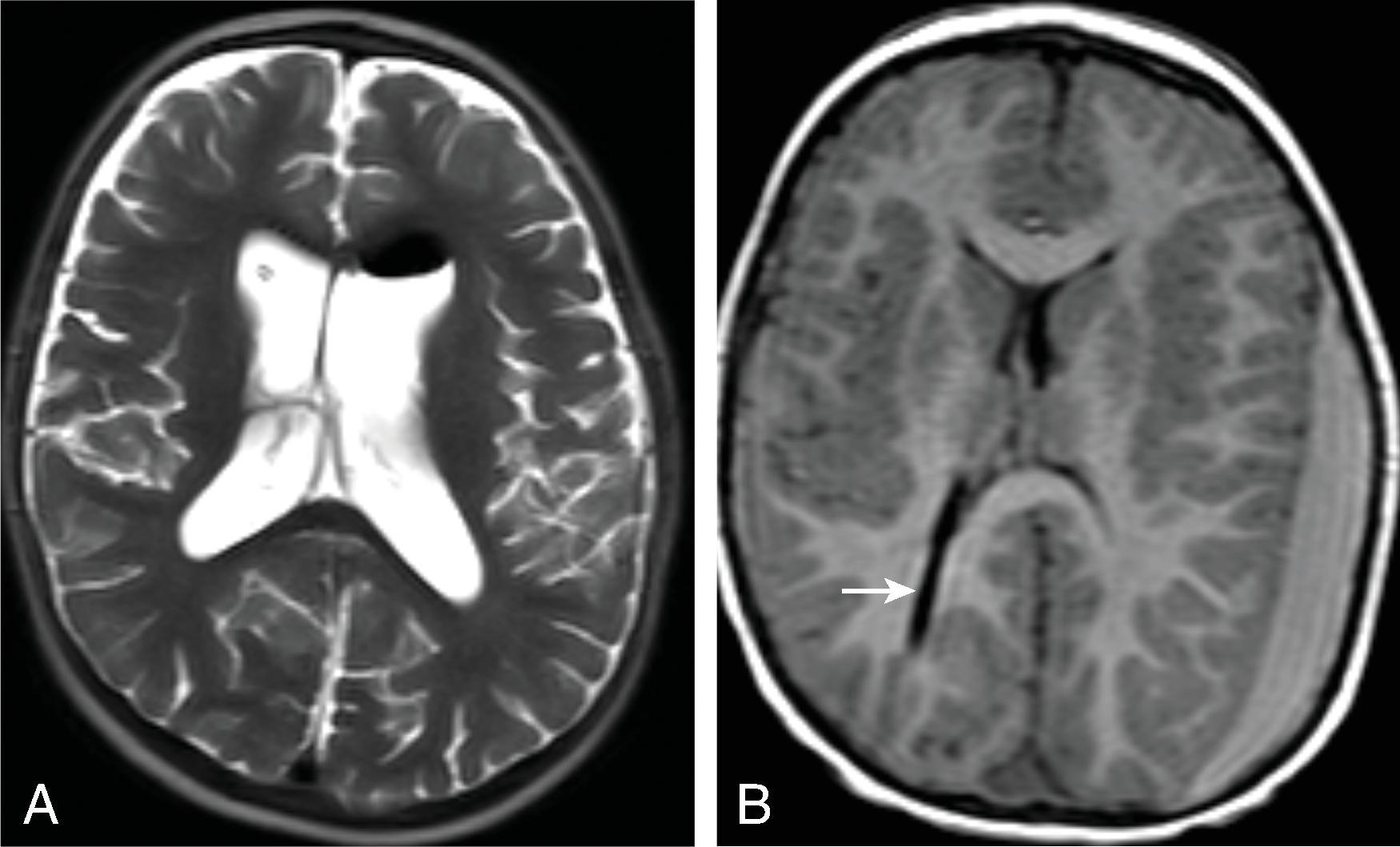
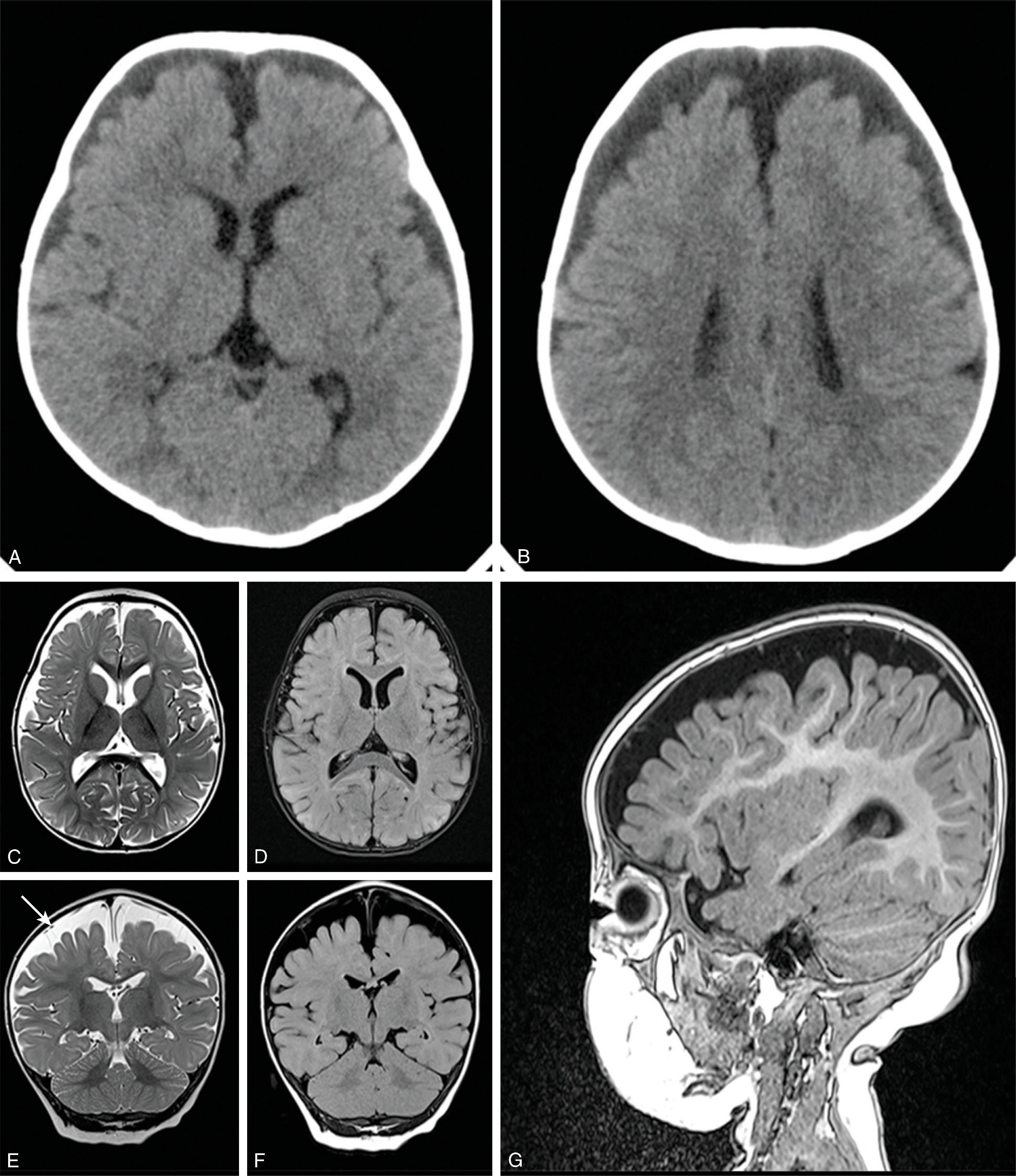
Communicating hydrocephalus occurs when there is no visible obstruction of the CSF pathways and is presumed to be caused by a failure of CSF absorption typically at the arachnoid granulations. Etiologies include meningitis, hemorrhage, leptomeningeal carcinomatosis, and venous hypertension. Communicating hydrocephalus demonstrates enlargement of the lateral, third, and fourth ventricles, although the fourth ventricle may only be mildly enlarged.
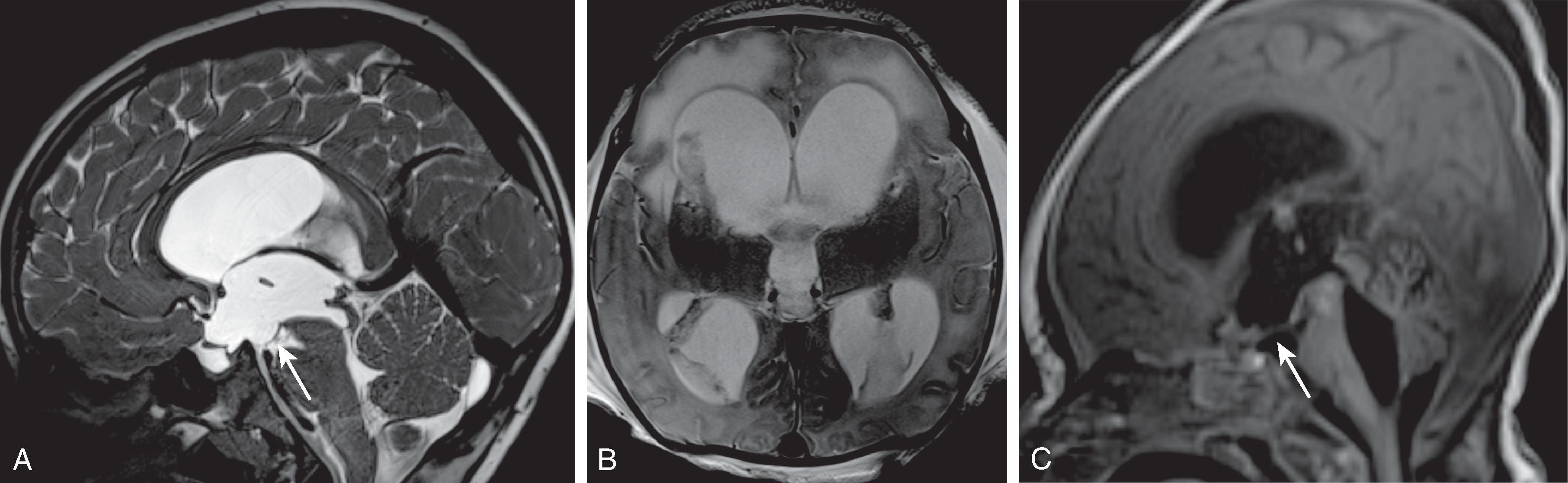
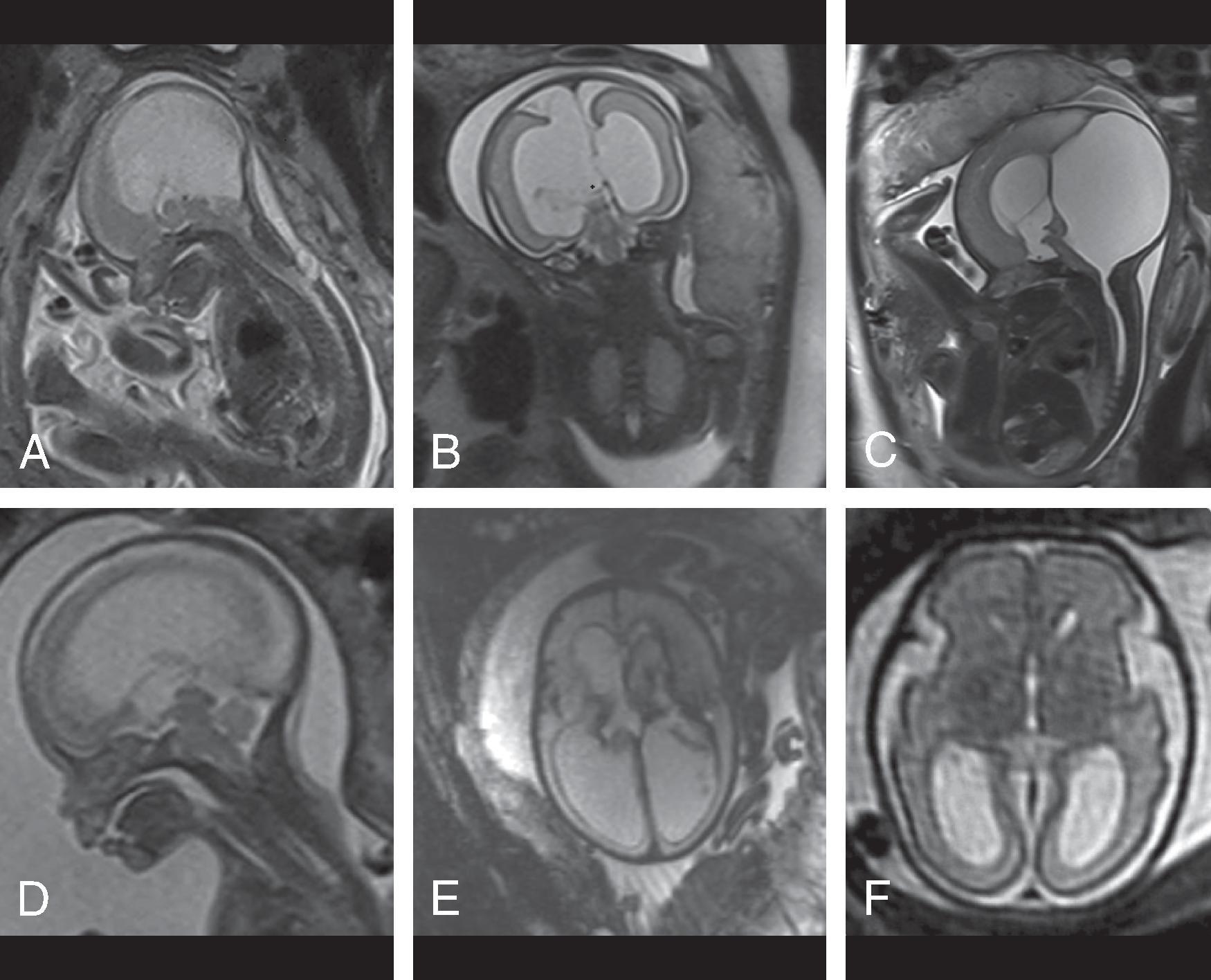
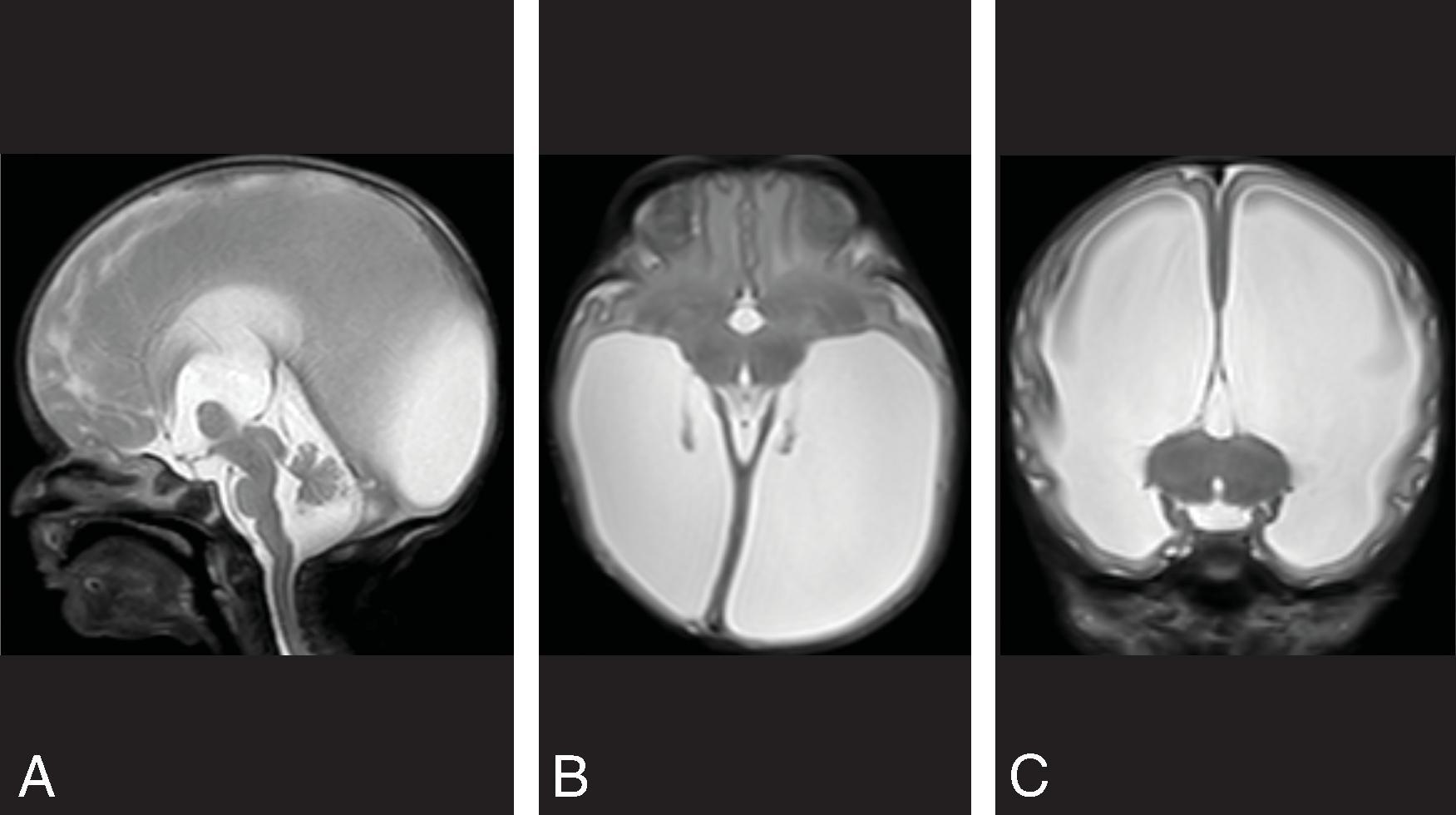
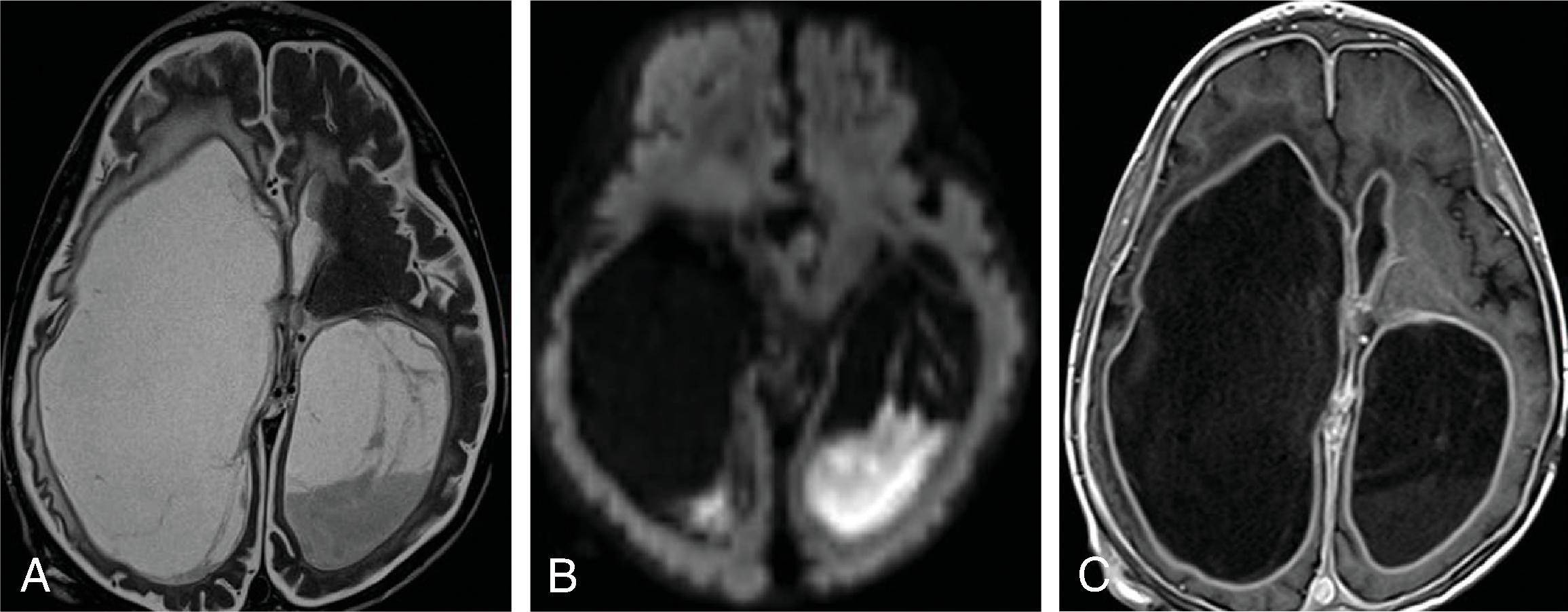
Enlarged ventricles in a fetus may not be associated with macrocephaly and instead are termed ventriculomegaly. Causes of fetal ventriculomegaly include malformations (most commonly myelomeningoceles, aqueductal stenosis, callosal anomalies), hemorrhage, and isolated ventriculomegaly when no etiology is found. Ventriculomegaly in a fetus is defined as measuring >10 mm at the level of the atrium of the lateral ventricles. Between a 10- and 12-mm diameter is defined as mild ventriculomegaly, and the outcome is normal in 93%. Between 12 and 15 mm is defined as moderate ventriculomegaly and has a 21% to 25% probability of resulting in developmental delay. More than 15 mm is defined as severe ventriculomegaly and has a 40% probability of developmental delay.
For infants younger than age 2, hydrocephalus is associated with macrocephaly, abnormal rate of increase in head circumference, and abnormal eye movement. Children older than age 2 present with morning headache, vomiting, and papilledema.
Hydrocephalus can ultimately lead to histopathologic changes of choroid plexus degeneration and sclerosis, ependymal cell loss and microlacerations, subependymal fibrosis, white matter gliosis, and axonal degeneration. With shunting, axons can regenerate and lead to improved white matter volume after shunting.
Prior to the 1950s, before shunting was performed, the prognosis of hydrocephalus was poor: 49% of patients died by the end of a 20-year observation period, and only 38% of survivors had an IQ greater than 85.
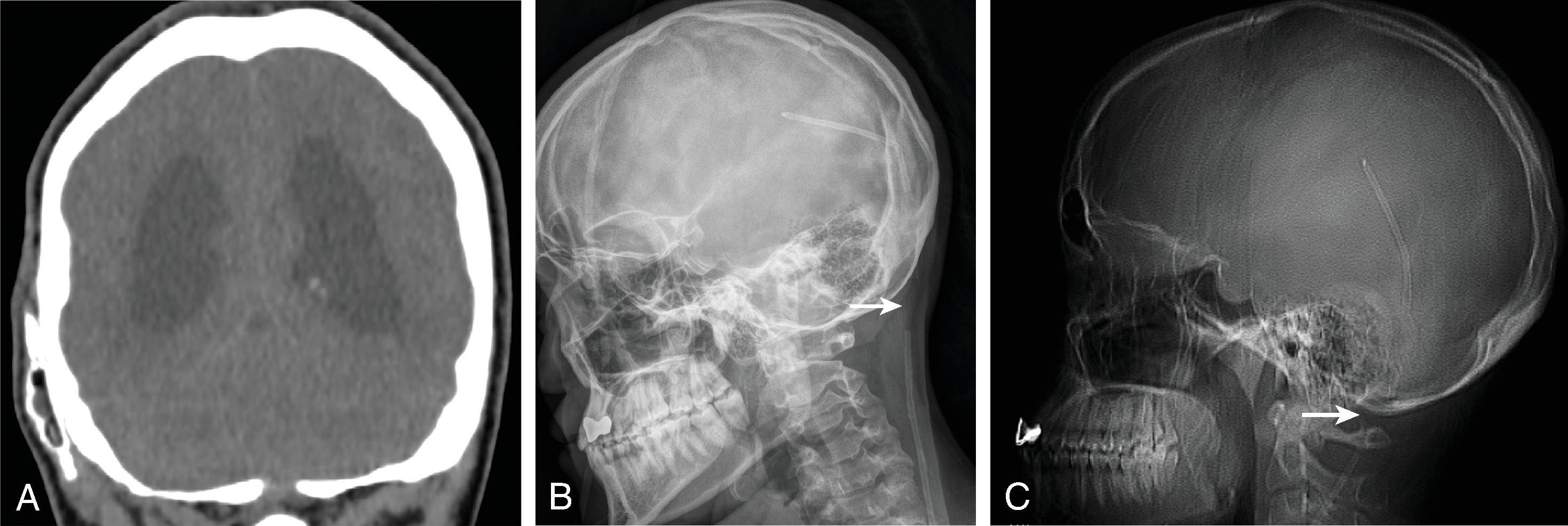
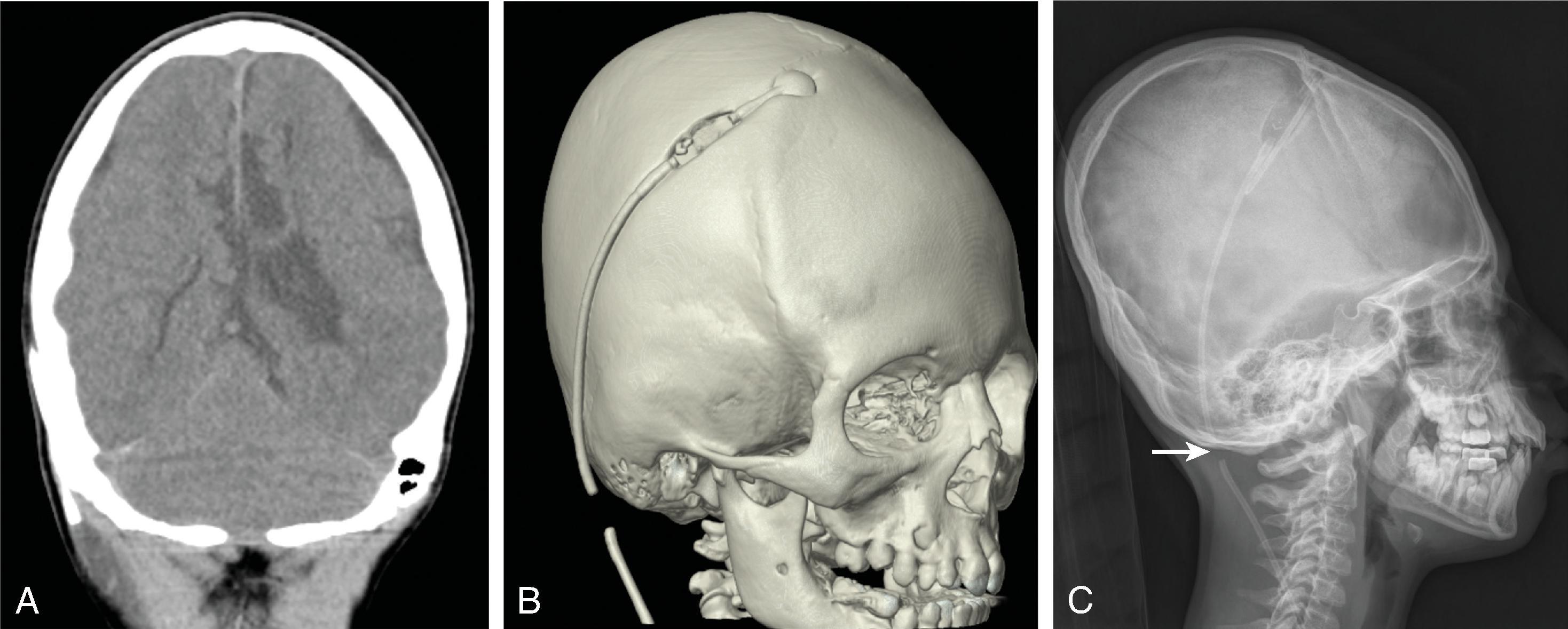
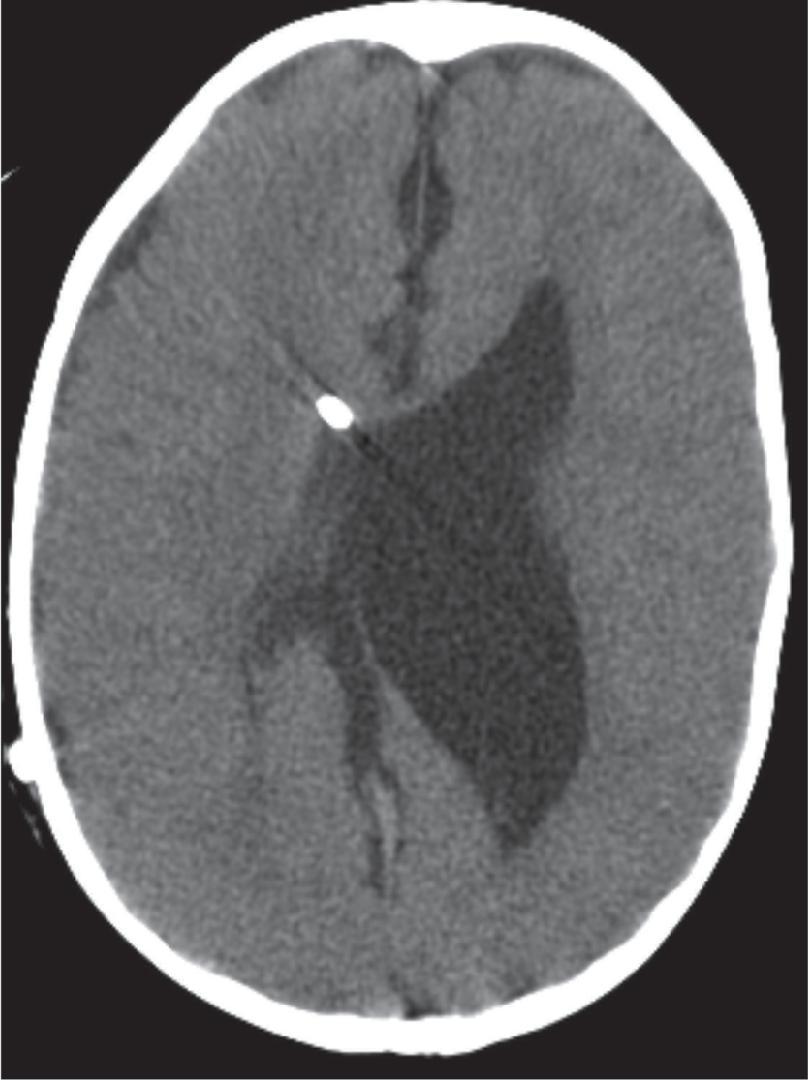
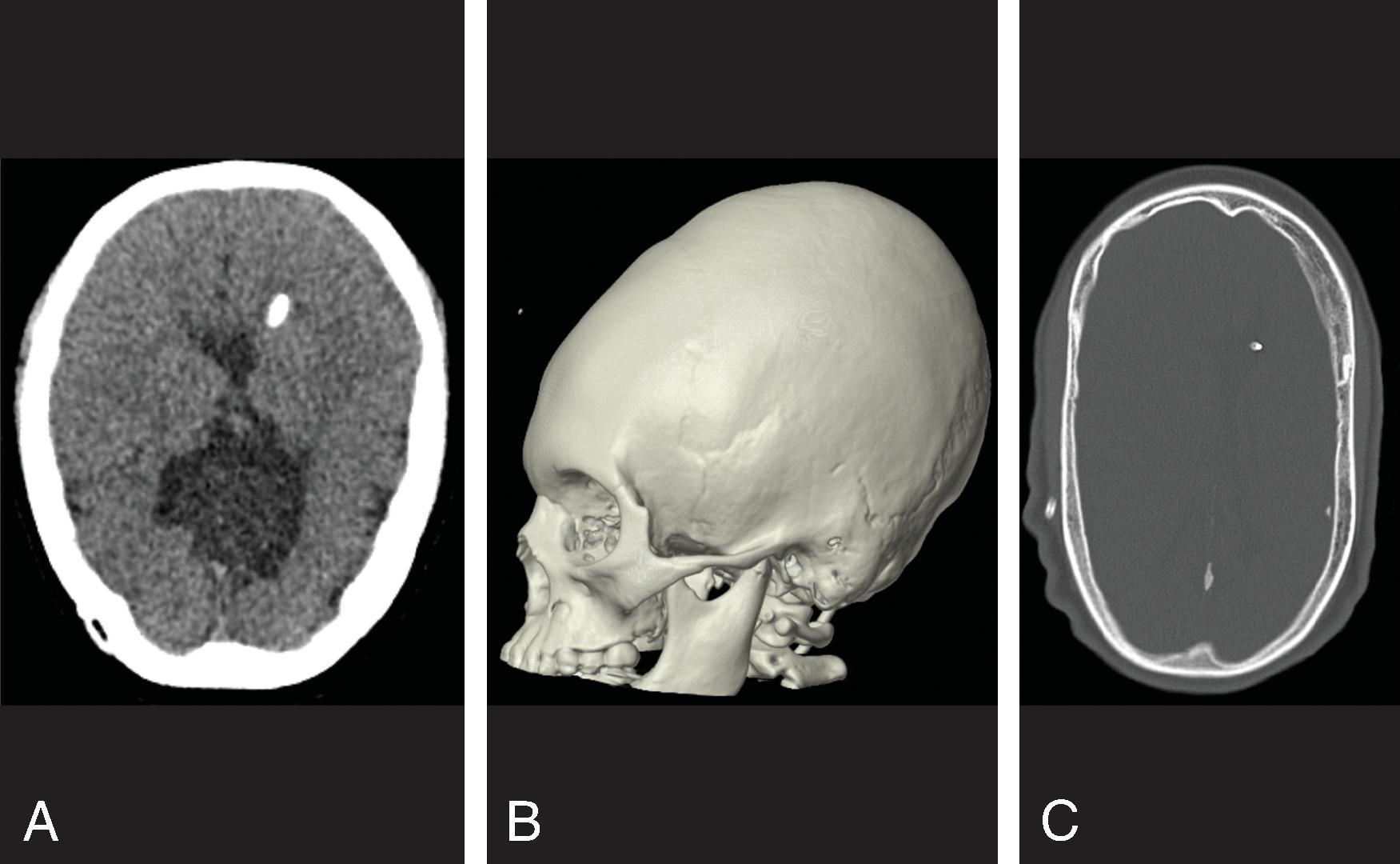
Treatment includes addressing the cause of ventricular obstruction and potential placement of a temporary or permanent ventricular catheter(s), depending on the long-term requirement for CSF shunting. Shunts result in reduced morbidity and mortality.
Long-term shunting typically drains CSF distally into the peritoneal cavity or less commonly the right atrium of the heart or pleura.
Third ventriculostomy is an alternate treatment of hydrocephalus when there is obstruction at the cerebral aqueduct but is less effective in children younger than 2 years of age due to immature arachnoid granulations.
There is no consensus regarding the management of obstructive hydrocephalus in children with posterior fossa tumors before, during, or after surgery. Approximately 30% of children with posterior fossa tumors will require shunting. Children with symptom duration less than 3 months, larger Evan's index (>0.33), and larger frontal occipital horn ratio (>0.46) were found to correlate with need for postoperative shunting. Children with midline tumors, medulloblastomas, and ependymomas were more likely to require shunting.
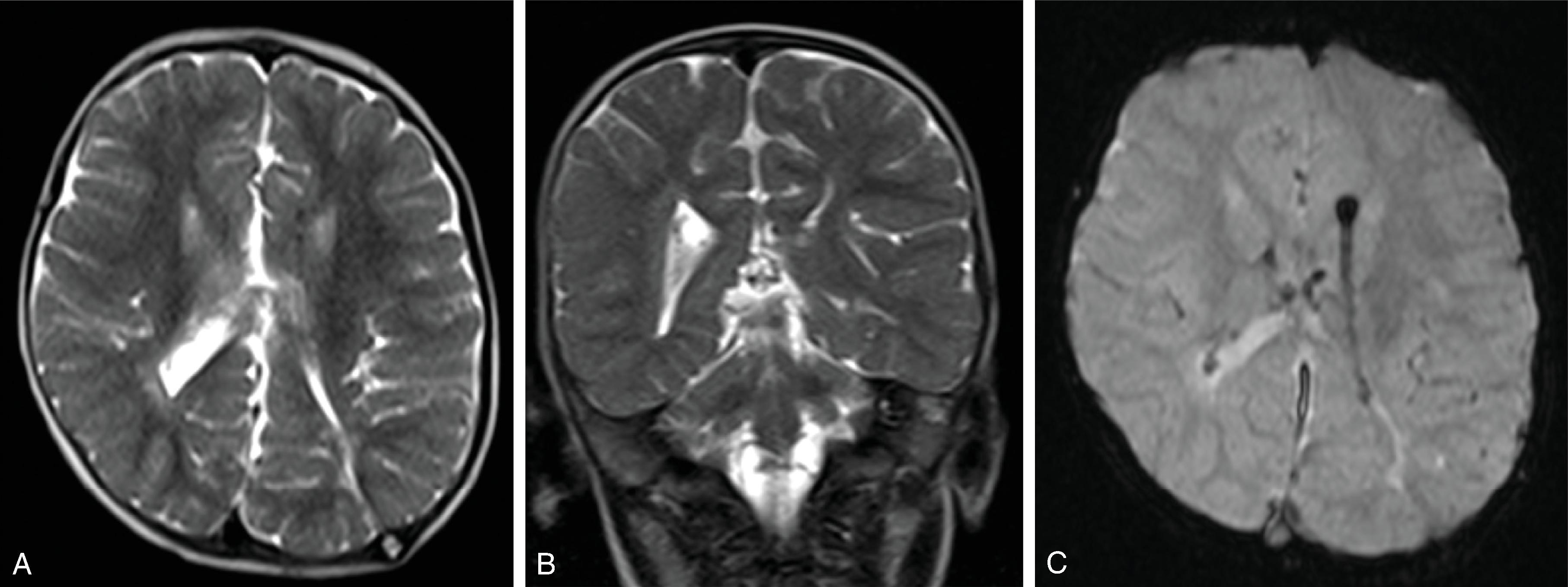
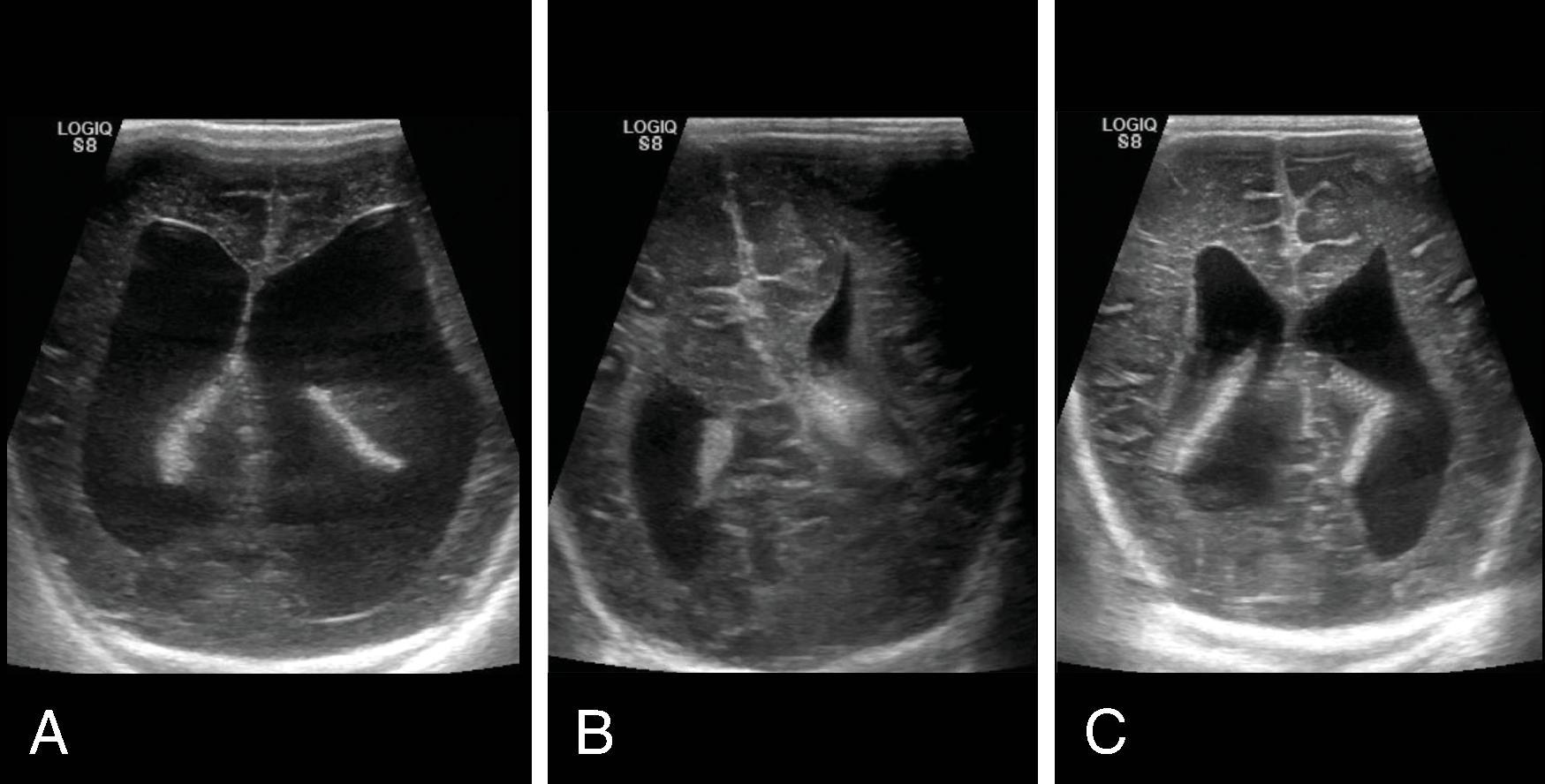
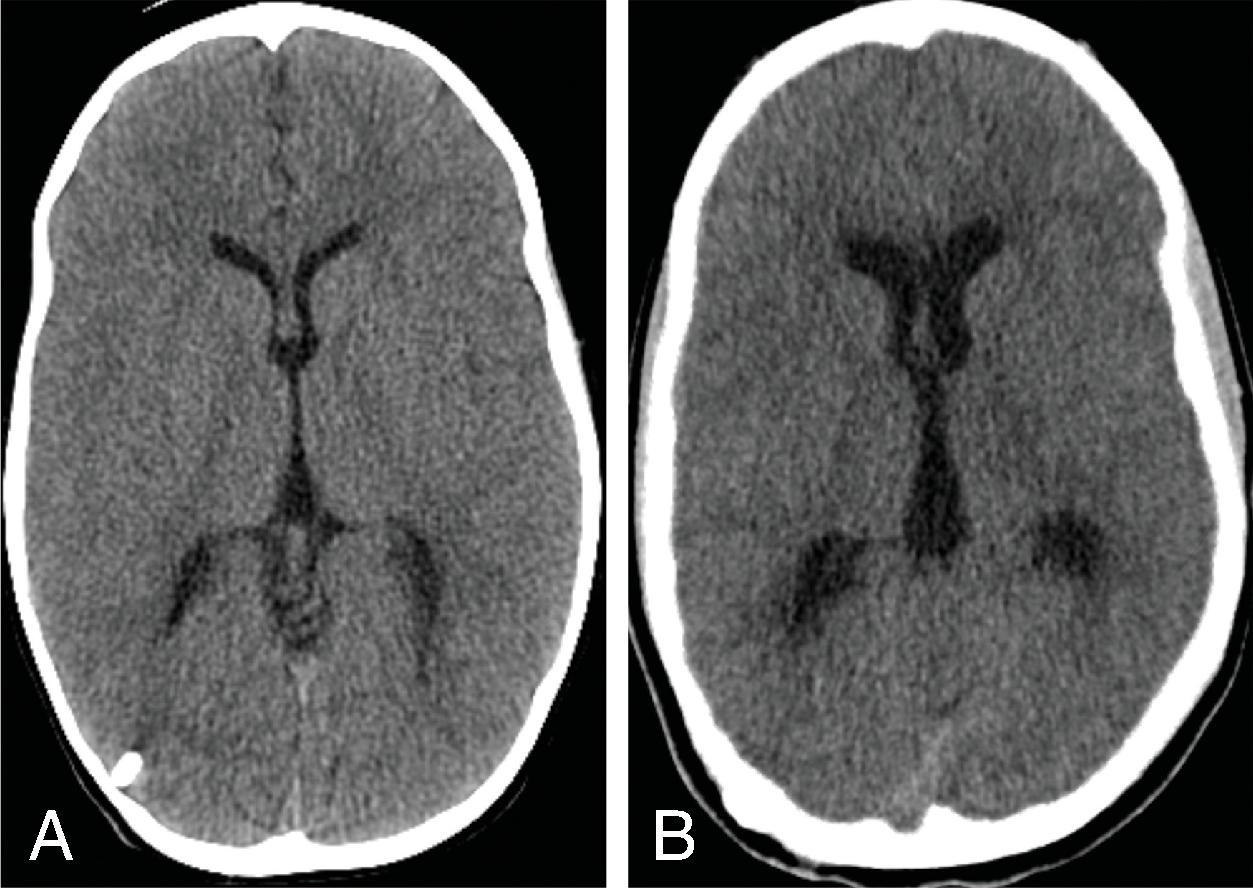
Imaging of hydrocephalus can be performed with ultrasound, CT, and MRI.
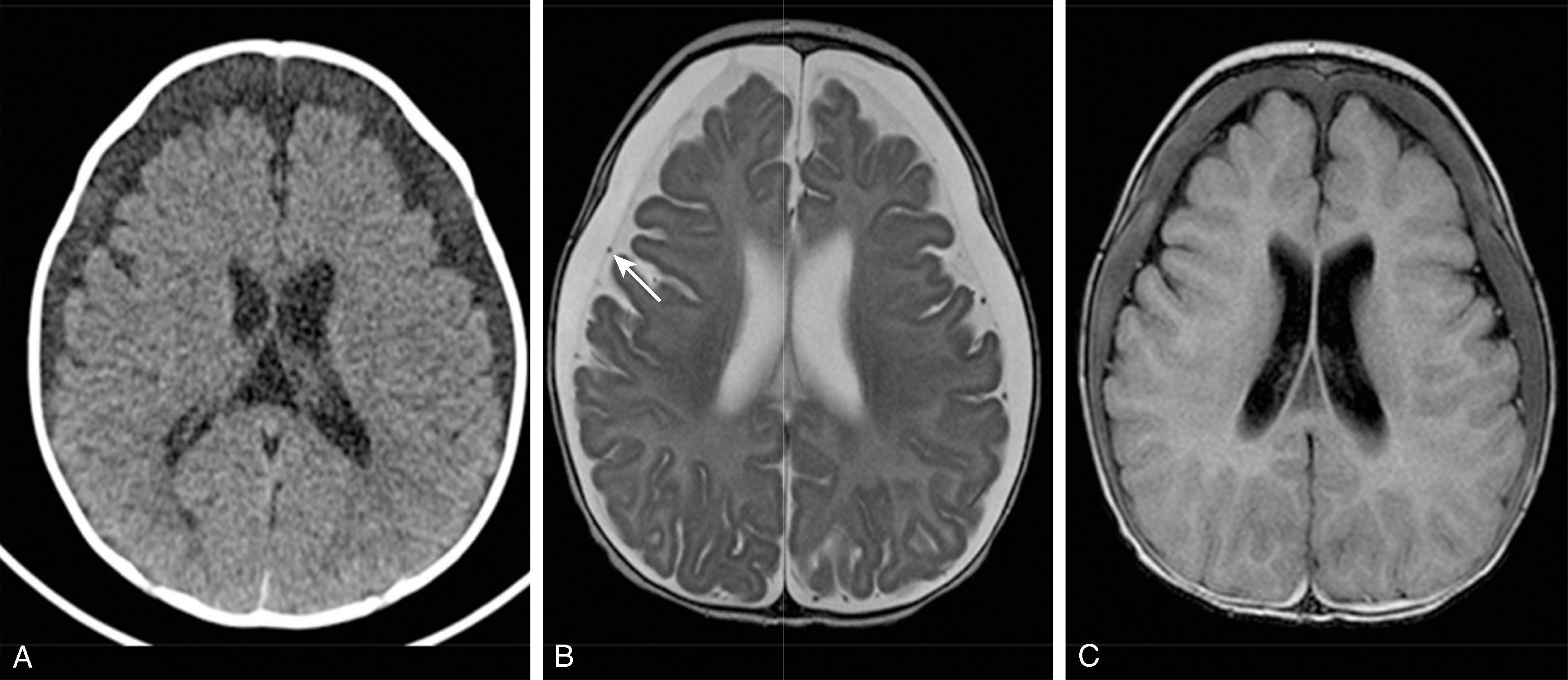
Imaging findings indicative of hydrocephalus include disproportionate enlargement of the ventricles relative to the sulci, inferior bowing of the third ventricle, enlarged anterior or posterior recess of the third ventricle, and periventricular interstitial edema/transependymal edema.
Imaging is also helpful in conjunction with the clinical history for determining the cause of hydrocephalus through identification of additional findings such as evidence of hemorrhage, malformation, tumor, or infection.
Ultrasound of the head is the modality of choice for following ventricular size in neonates as it is fast, widely available, does not require sedation, and does not have ionizing radiation. Ultrasound can also measure the resistive index, which has shown value in hydrocephalus:
The resistive index (RI) = (peak systolic velocity – end diastolic velocity)/peak systolic velocity and is increased with increased intracranial pressure (>0.8 in neonates and >0.65 in infants). The change in RI between compression and noncompression at the anterior fontanelle (ΔRI = 100 × (compression RI – baseline RI) / baseline RI) showed the best correlation with elevated intracranial pressure (r = 0.8), and infants with a ΔRI > 45% required ventricular drainage.
MRI is often performed for better assessment of the etiology of the hydrocephalus, including infections, malformations, and tumors. Specific MRI additions to help diagnosis include the following:
Thin section (1 mm) 3D T2W is useful for assessing stenosis or web at the cerebral aqueduct and patency of a third ventriculostomy.
Phase contrast CSF flow imaging is useful for demonstration of flow across the cerebral aqueduct and third ventriculostomy.
Ultrafast MRI using T2W and T2* sequences can allow for imaging of the ventricles and shunt catheter in approximately 30 seconds per sequence. This allows for reduction in CT imaging and may reduce the need for sedation.
New areas of MRI research into hydrocephalus include measuring cerebral blood flow with arterial spin label perfusion (ASL), assessment of white matter with diffusion tensor imaging (DTI), and measurement of the venous sinus diameter. Hydrocephalus decreases the cerebral blood flow, and ASL has shown that CBF increases after alleviation of obstructive hydrocephalus. DTI has shown axonal degeneration with hydrocephalus and changes in fractional anisotropy and perpendicular and parallel diffusion within white matter lateral to the ventricles.
CT of the head has been a mainstay for imaging hydrocephalus because it is fast, widely available, and generally free from artifacts. Because of the ionizing radiation involved, reducing radiation dose for head CTs evaluating shunts is recommended.
Several measurements for hydrocephalus that can be performed, although most assessments for hydrocephalus can be done qualitatively. The frontal occipital horn ratio (FOHR) is one measurement that can be used to assess ventricular enlargement. FOHR = (frontal horn distance + atrium distance)/(2 × biparietal distance). The normal FOHR value is 0.37 and is independent of age.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here