Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cardiotoxicity is a major reason for drug attrition and is thus a core subject in preclinical and clinical safety testing of new drugs. Cardiac safety testing of new chemical entities that become lead drug candidates is a critical aspect of the drug discovery and development pipeline. A large number of cardiac side effects of cardiac and noncardiac drugs are caused by drug interaction with one or more cardiac ion channels. , Cardiac ion channels regulate cellular excitability, contractility, and overall cardiac performance and alteration of cardiac ion channel function can lead to sudden cardiac death. This contributed to the release of International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH S7B) guidelines dedicated to identifying fatal arrhythmia risk of drugs in preclinical testing. Since 2005 cardiac safety of a compound has been determined almost exclusively by its effect or potential effect on the QT interval of the electrocardiogram (ECG) or the action potential duration (APD; Fig. 57.1A–B ) and potential to lead to the life-threatening arrhythmia of torsades de pointes (TdP). QT prolongation, however, is not the ideal indicator for TdP as drugs that prolong QT interval do not always cause TdP. Despite the key role that QT prolongation studies play in preclinical drug development, it is recognized that this parameter is only a surrogate marker for proarrhythmia. Data from preclinical and clinical trials have shown that there is no fixed relationship between the magnitude of QT prolongation and the risk for development of fatal arrhythmias such as TdP. , Furthermore, a number of blockbuster drugs have been withdrawn from the market because they were found to cause cardiotoxicity in humans even when the drugs were found to be safe in preclinical animal testing. , For these reasons there is a revolution dawning in the cardiotoxicity preclinical testing field and a search for a more predictive and reliable test.
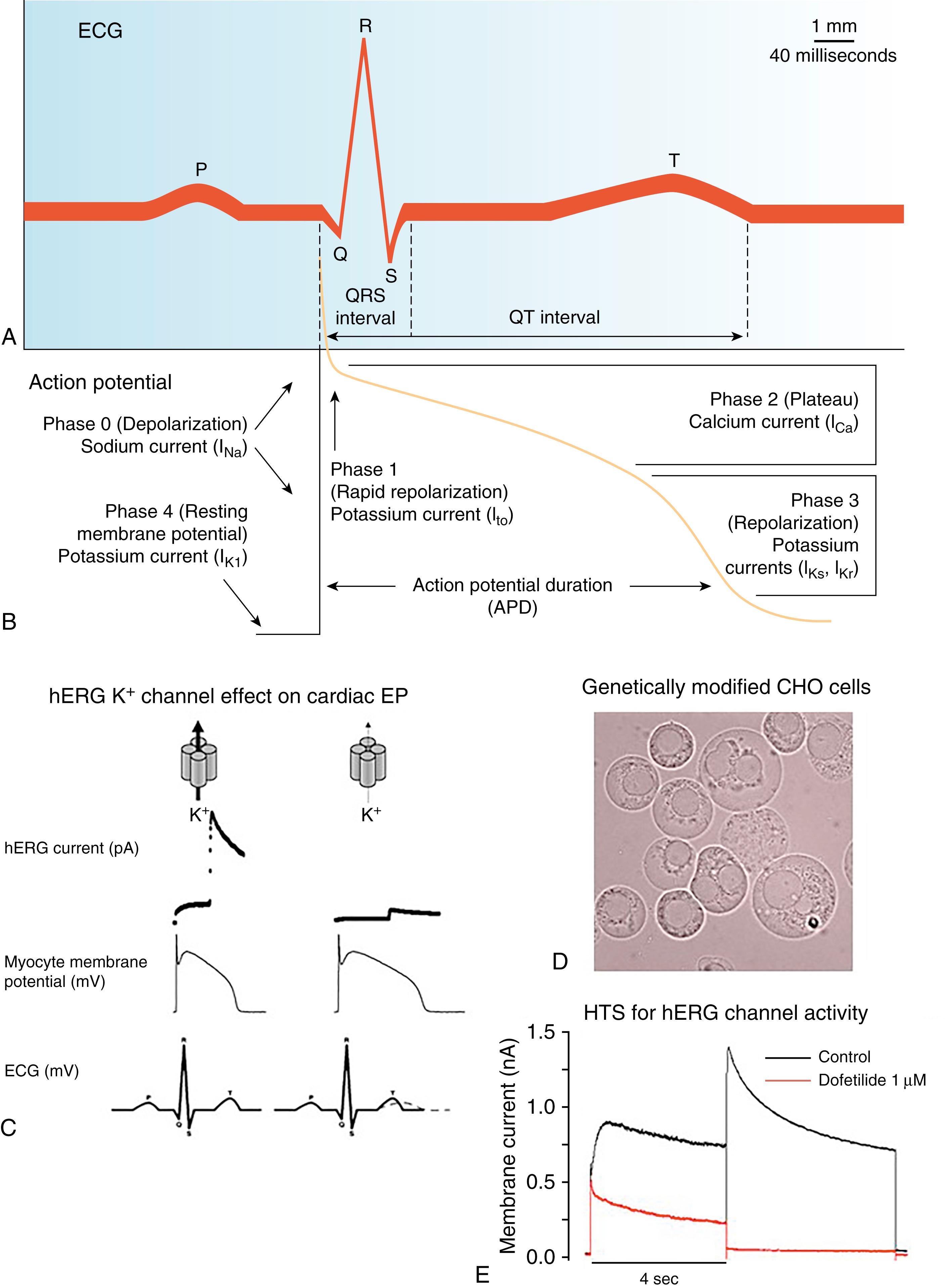
The in vitro surrogate for predicting potential QT prolongation effects of a drug compound involves measurement of single potassium current (I Kr ). The human ether-a-go-go related gene ( hERG or KCNH2 ) encodes the pore-forming α-subunit of a voltage-gated potassium channel in the heart. hERG (or K V 11.1) (I Kr ), which is involved in cardiac repolarization, has become the centerpiece for preclinical in vitro cardiac safety pharmacology. Inhibition of the hERG current is thought to cause cardiac APD prolongation and by inference QT interval prolongation resulting in potentially fatal ventricular tachyarrhythmia (TdP; see Fig. 57.1 ). In vitro optical mapping of neonatal rat ventricular cardiomyocytes has revealed that spatial gradients of repolarization caused by hERG activity can lead to arrhythmia phenotypes. Thus hERG channel function and spatial dispersion is critical for normal cardiac function.
The relationship between the ventricular action potential, hERG channel function, and the ECG is depicted in Fig. 57.1C . hERG channel function contributes to repolarization of the action potential during phases 2 to 4 of the cardiac action potential. Reduction of the hERG current can cause ventricular cardiomyocyte APD prolongation and is expected to result in QT prolongation in vivo . Fig. 57.1 demonstrates the simple scenario that became apparent for the drug discovery process to screen drug compounds early in the development phase to determine potential proarrhythmia risk. The dogma followed today is that under normal circumstances potassium outward current via hERG is a dominant factor in the repolarization phase of the ventricular action potential and thus is a key determinant of the APD. Any inhibitions of this hERG channel activity are presumed to slow repolarization, prolong APD, and by inference must also prolong the QT interval. This dogma is a gross oversimplification of a complex biologic process and does not take into account the potential contributions of other ion channels to compensate for the blockade of the hERG channel. Nevertheless this paradigm has become the critical element to enable an early in vitro preclinical approach focused on a quantifiable molecular marker (hERG) to predict a potentially fatal adverse drug reaction (TdP).
The in vitro hERG channel assay is most routinely performed in contract research organizations (CROs) that perform toxicity studies for pharmaceutical companies. The vast majority of hERG channel assays use genetically modified animal cells overexpressing just the hERG channel (see Fig. 57.1D–E ). In the hERG inhibition assay Chinese hamster ovary cells transfected with the hERG gene (CHO-hERG) are plated into a high-throughput single-cell planar patch-clamp system. The high-throughput nature of the hERG assay performed in 384 well plates allows recordings of hERG current in up to 384 individual cells within minutes. For this reason the hERG assay, performed in heterologous cell systems, is currently positioned in lead optimization to eliminate compounds and/or chemically reengineer the compounds early in drug discovery that may have the potential for TdP risk. , Using this simplified approach to a complex biologic process may erroneously label compounds as proarrhythmic and eliminate potentially useful drugs from development and commercialization.
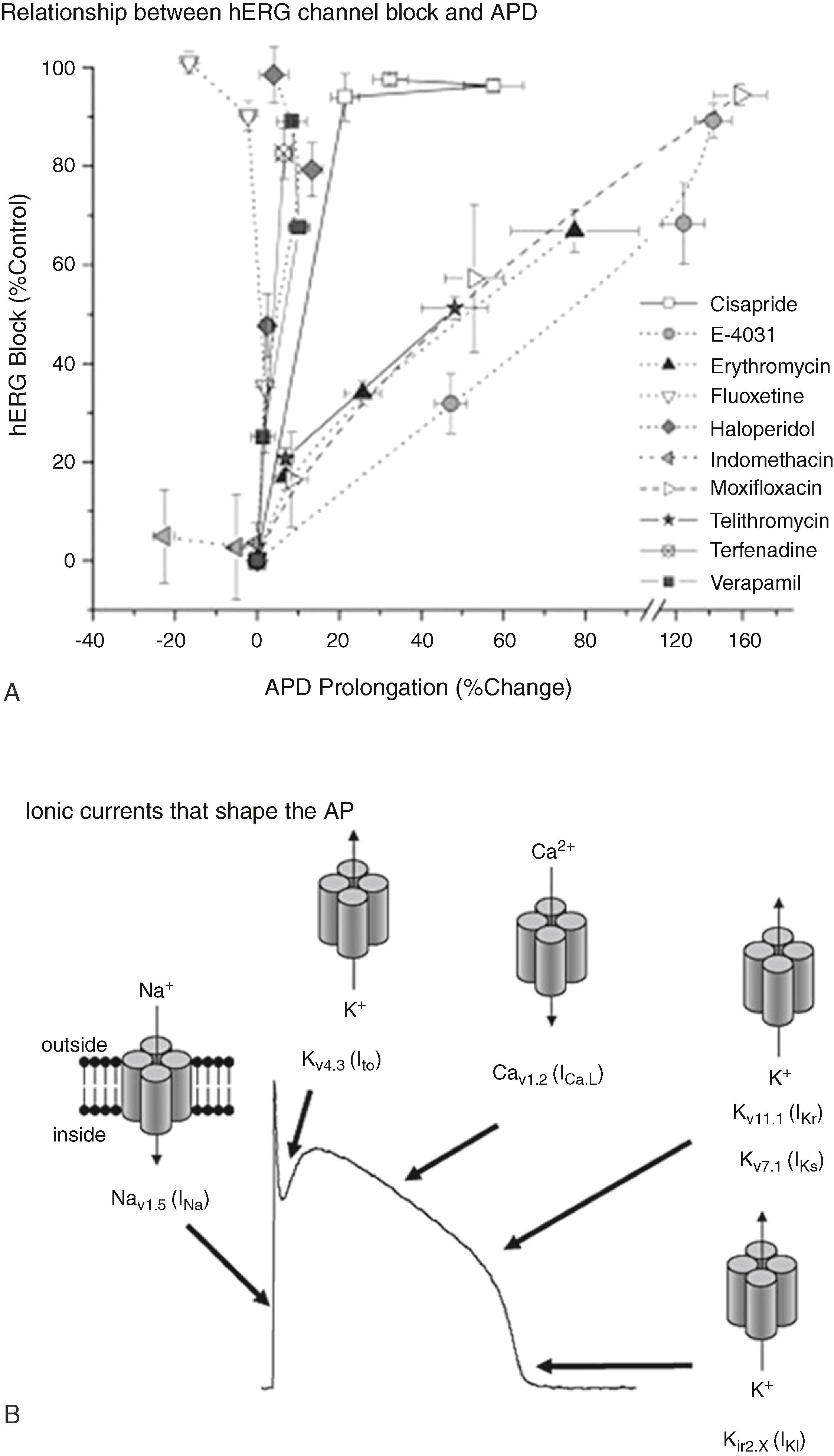
In fact, evidence shows that hERG channel inhibition does not always correlate to APD prolongation, QT prolongation, and risk for proarrhythmia (TdP). For example, Fig. 57.2A demonstrates that not all drugs that block the hERG channel in vitro also cause APD prolongation in cardiomyocytes. Fig. 57.2A compares the effects of 10 different drug compounds on hERG current block in the hERG assay with prolongation of the canine Purkinje fiber APD. Drugs that block hERG channel activity and clearly increase APD are referred to as overt hERG blockers (e.g., E-4031 and moxifloxacin). On the other hand, there are drugs that block hERG with high potency but have no effect on APD of cardiomyocytes (e.g., haloperidol and fluoxetine). These drugs are called covert hERG blockers. Verapamil is a widely prescribed calcium channel blocker that also blocks the hERG channel in preclinical tests but has not been associated with either QT prolongation or TdP. In fact verapamil reportedly shortens the QT interval. This effect of verapamil is attributed to concomitant block of L-type calcium current that mitigates effects of hERG channel block. Also, clinically safe drugs such as fluoxetine (Prozac, antidepressant) and citalopram (Celexa, antidepressant) are examples of hERG blocking compounds that do not affect APD (see Fig. 57.2A ) or QT duration and do not lead to clinical TdP. Under the current ICH cardiotoxicity guidelines the popular and revolutionary antidepressant drug Prozac (world-wide exposure of over 54 million patients), which has a low incidence of arrhythmogenic liability, would likely not have been considered for development. Early in the current development pipeline Prozac would fail under the current guidelines because it is a potent hERG blocker and would be assumed to prolong APD and QT interval. Because of the ubiquitous and nearly exclusive use of the hERG single-cell/single-channel assay early in the drug development process it is likely that potentially useful drugs have been eliminated wrongfully from development and potential therapies are not being delivered to patients. A better in vitro testing platform would use a cell culture system made up of electrically excitable human cardiomyocytes that contain the full complement of ion channels that make up the cardiac action potential (see Fig. 57.2B ).
The use of human cardiomyocytes that mimic the phenotype of adult cardiomyocytes presents the ideal platform for early preclinical screening of drug cardiotoxicity. Single cardiomyocytes isolated from human hearts have been used to study electrophysiology, intracellular calcium dynamics, and contraction, but the viability and yield of live cells from these hearts is very low and studies can only be performed on very small numbers of cells. Availability of usable hearts is limited and logistics of obtaining explanted human hearts are complicated and require a team of people on standby to be ready to accept the heart and isolate single cardiomyocytes at any hour. Another limitation of using cardiomyocytes isolated from adult human hearts is the technical challenge of maintaining the cells in culture long term, , because of rapid dedifferentiation of the structural and functional phenotype of adult cardiomyocytes in culture. Finally, the low-throughput nature of using human cardiomyocytes obtained from explanted hearts precludes the use of this type of assay for early phases of drug development where high-throughput screens are required.
For study of cardiac electrical impulse propagation, human heart optical mapping is limited to unused donor hearts or explanted diseased hearts available for research at the time of cardiac transplantation. Although in vitro human heart optical mapping has been reported , and mechanistic insights have been published, the health and availability of the hearts used and the conclusions that can be drawn based on data collected from clinically unsuitable human hearts must be made cautiously. Here again, the low throughput of this approach precludes utility for drug screening tests and the unreliable nature of the preparation makes it unattractive for the drug development process. The development of novel approaches for the supply of human cardiomyocytes useful for the drug discovery industry is required.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here