Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In many epidemiologic studies, hidradenitis suppurativa (HS) has been found to disproportionately affect women of childbearing age. Nearly three-quarters of new diagnoses are in women, with the highest incidence in female patients aged 30 to 39. Furthermore, the reported average annual incidence in the United States is 12.1 per 100,000 for women, which is more than twice that of men (5.1 per 100,000). Women may also have an earlier onset of disease coincident with the earlier onset of puberty in girls. In women, HS can be found in the inframammary folds, breasts, and vulva, in addition to the axilla, inguinal, and buttock regions, as well as other anatomic sites. A hormonal influence on HS in women is strongly suggested given changes observed in HS disease status peri-menstruation and during pregnancy. Female sex hormones present in high levels, such as estrogen and progesterone, have been thought to play a role in the sexual dichotomy of HS prevalence. Women face unique challenges in managing their HS related to menses, pregnancy, lactation, and menopause. Special consideration should be taken to provide comprehensive care to women with HS and improve their quality of life.
The range of peri-menstrual HS flares has been reported to be between 44% and 63%. The ovarian cycle enters the luteal phase after ovulation, where the remaining granulosa cells undergo luteinization and combine with theca-lutein cells to form the corpus luteum. The corpus luteum functions as an endocrine organ and primarily secretes progesterone to prepare the endometrium for possible embryo implantation. Typically, eight or nine days after ovulation, during the mid-luteal phase, serum levels of progesterone and estradiol peak. If human chorionic gonadotropin (hCG) is not produced as a result of pregnancy, the corpus luteum will undergo luteolysis, leading to a decline in progesterone production and resulting in the menstrual period. Estradiol also decreases during the luteal phase of the ovarian cycle, though to a lesser degree. Premenstrual HS flares are hypothesized to be caused by the change in steroid sex hormone levels immediately preceding the onset of menstruation ( Fig. 31.1 ), which leads to immunologic changes; however, the definitive relationship between progesterone, estrogen, and HS disease status has yet to be established.
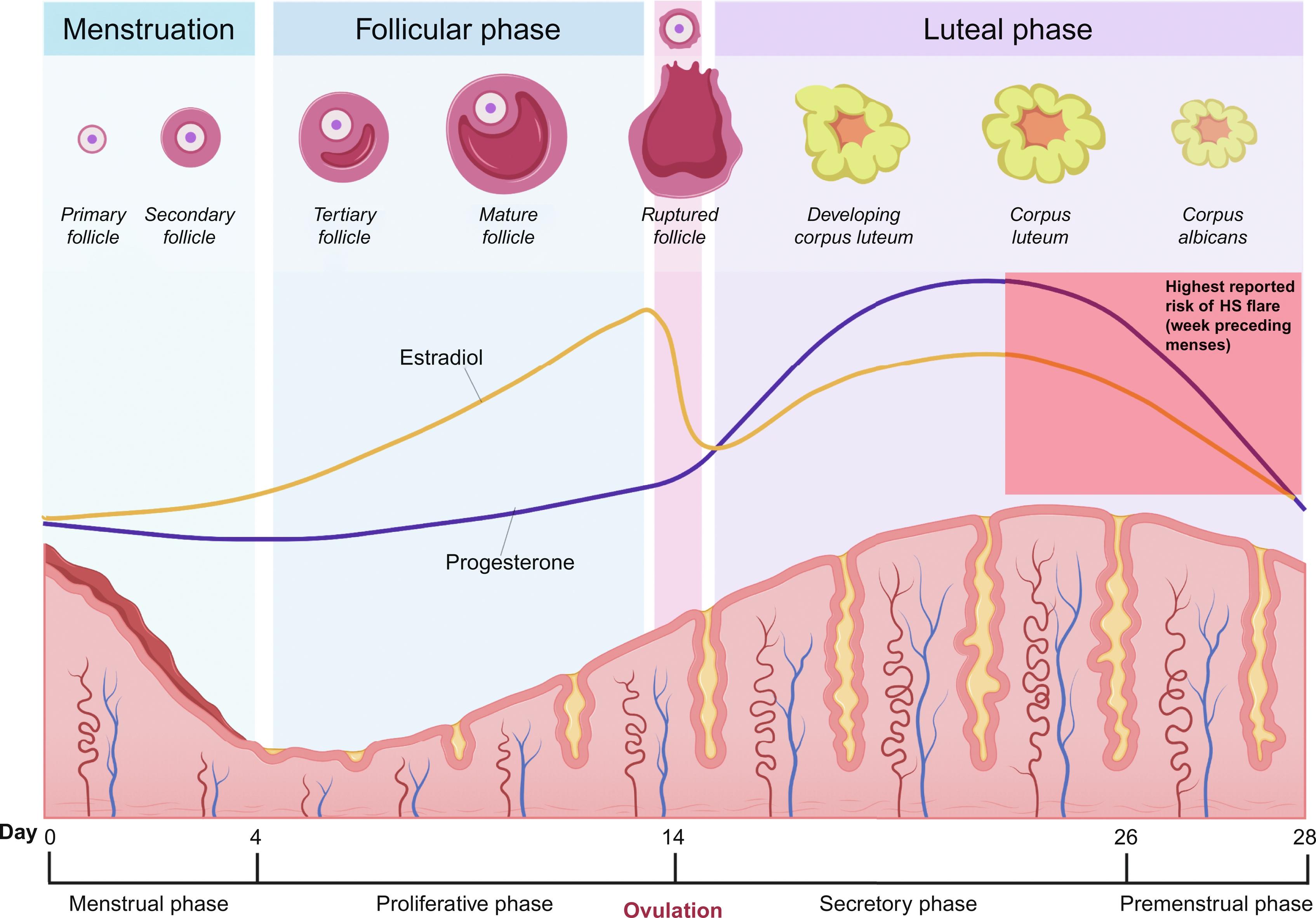
Immune dysregulation has been implicated in HS pathogenesis, with studies reporting elevation in several pro-inflammatory (and some anti-inflammatory) cytokines, including TNF-α, interleukin (IL)-1β, IL-10, IL-17, IL-12, IL-23, IL-22, and IL-20 in lesional HS skin compared to healthy control skin.
Estrogen and progesterone have been shown to differentially modulate immune tolerance through varying physiological mechanisms ( Fig. 31.2 ). Estrogen stimulates the production of interferon alpha (IFN-α) by plasmacytoid dendritic cells. In mice, this has been shown to occur via estrogen receptor alpha (ER-α)-mediated upregulation of the transcription of type 1 interferon (IFN) producing genes. Type 1 IFNs (including IFN-α, β, ɛ, τ, and δ) then further increase estrogen signaling within immune cells by inducing expression of more ER-α, resulting in a “feed-forward” loop. Estrogen can also enhance the release of inflammatory cytokines including IL-1 and TNF-α from human monocytes and macrophages and pro- and anti-inflammatory cytokine IL-6. In addition, in vivo mouse studies suggest that estrogen induces ER-α mediated differentiation of naïve CD4 + T cells into T helper (Th) 1 cells, which secrete pro-inflammatory IFN-γ.
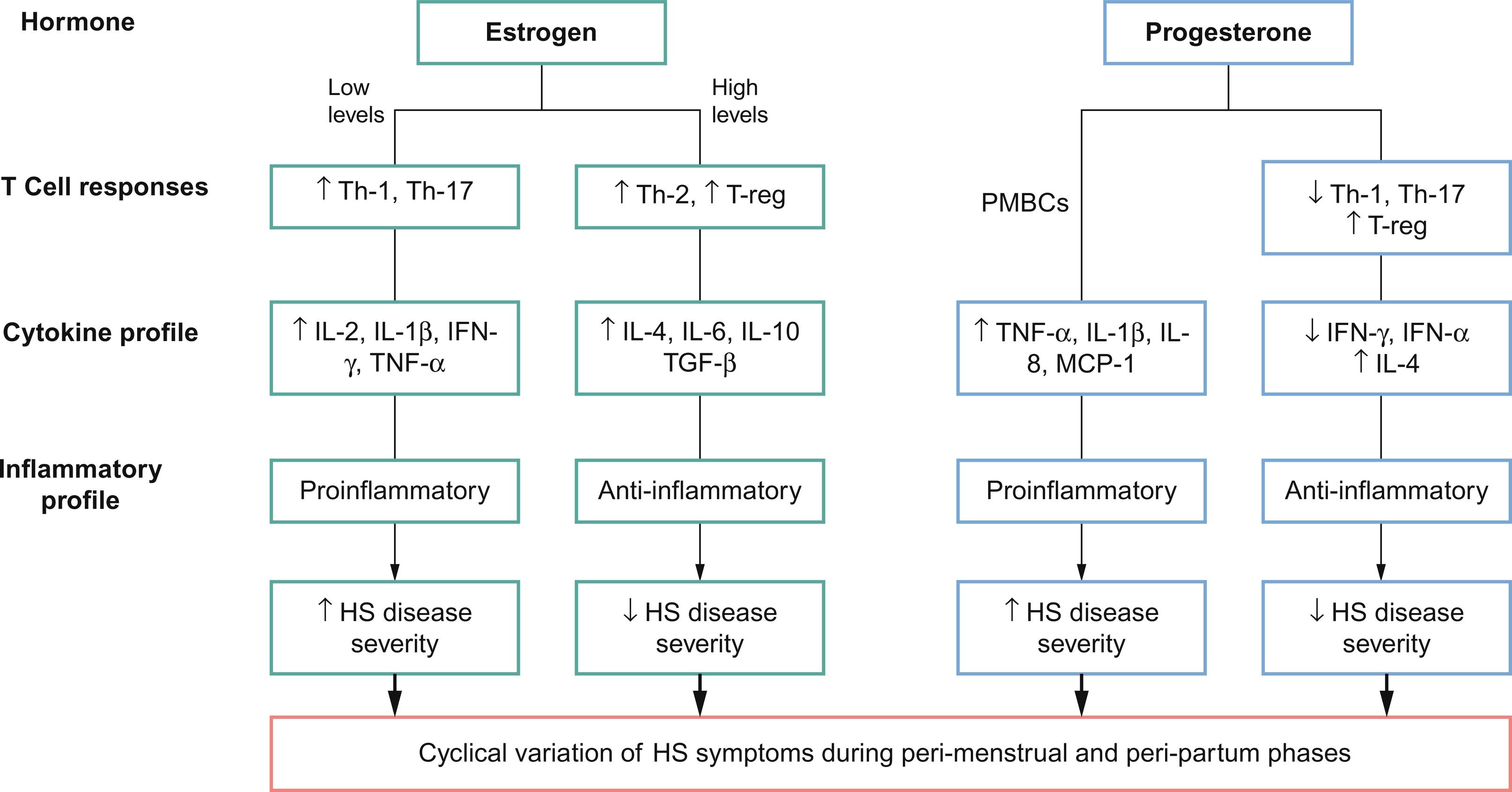
Though these results suggest that estrogen plays a predominantly pro-inflammatory role, discordant mouse studies have demonstrated inhibition of CD4 + T cell differentiation into Th1 and Th17 cells via ER-α signaling in T lymphocytes. Other in vivo mice studies have suggested that the effects of estrogen on cell-mediated immunity vary according to dose, with low doses of estrogen promoting enhanced pro-inflammatory Th1 responses and high doses leading to anti-inflammatory Th2 responses. Estrogens have also been shown to have a biphasic effect on TNF-α in human T cell clones, with enhancement at low concentrations and inhibition at high concentrations.
The anti-inflammatory effects of progesterone have been well-documented. Progesterone has been found to suppress type 1 IFN production as well as production of IFN-γ from Th1 cells in mouse studies. Studies have shown that medroxyprogesterone acetate at doses sufficient for contraceptive efficacy can suppress IFN-α production by plasmacytoid dendritic cells in mice in vivo and human plasmacytoid dendritic cells in vitro. Moreover, progesterone suppresses proliferation of CD4 + T cells and Th17 cells, which are involved with chronic inflammation seen in HS. Evidence from mouse models also supports the role of progesterone in facilitating regulatory T cells (Treg) differentiation involved in preventing autoimmunity and mitigating the damaging inflammation from naïve CD4 + T cells via progesterone receptor signaling.
Few studies suggest that progesterone may also have pro-inflammatory effects. Yuan et al. (2008) studied the effects of progesterone on the spontaneous production of TNF-α, IL-1β, IL-8, and macrophage chemotactic protein by unstimulated peripheral blood mononuclear cells (PMBCs) from patients with chronic hepatitis C. They discovered that the levels of each cytokine increased significantly in PBMCs treated with progesterone.
Premenstrual HS exacerbation has been suggested to result from a reduction in anti-inflammatory mediators due to lower progesterone levels prior to menses. However, the timing of HS symptom onset needs further elucidation; it is unclear if HS symptoms are triggered by high levels of progesterone and estrogen in the mid-luteal phase, or by the declining levels of progesterone and estrogen right before menses. One study found that, compared to patients with premenstrual HS flares, women without premenstrual flare had lower progesterone levels and higher testosterone and androstenedione levels. Additional research is needed to fully elucidate the role of steroid sex hormone in HS immunology.
Evidence is mixed regarding the use of oral contraceptive pills (OCPs) in the management of HS flares. Early cases have suggested a temporal relationship between HS onset and initiation of OCPs, with complete resolution occurring with either discontinuation of OCPs or switching to a formulation with a higher estrogen:progestogen ratio. Other studies have shown no differences in HS symptoms between OCP and non-OCP groups, or have shown substantial disease improvement with either ethinyloestradiol 50 μg/cyproterone acetate (CPA) 50 mg EKC or ethinyloestradiol 50 μg/norgestrel 500 μg. The literature also contains anecdotal reports of OCPs with high estrogen to progesterone ratio and low androgenic progestogens leading to HS disease amelioration. Current evidence does not allow for concrete recommendations on which specific OCP to use for the management of HS. However, if OCPs are being considered, choosing one with a less androgenic progestogen, such as norgestimate, desogestrel, gestodene, or drospirenone, may be beneficial. Though further investigation is needed, some studies suggest that progesterone-only pills, progesterone implants, or depot progesterone can cause HS exacerbation.
Spironolactone, a potassium-sparing diuretic with anti-androgenic properties, has demonstrated varying efficacy in HS management. Studies have shown HS clinical improvement with spironolactone at 3 months follow-up. One study found no differences in improvement between patients who received less than 75 EKC mg of spironolactone daily and those who received over 100 mg daily EKC. The 2019 North American HS management guidelines recommend the use of hormonal therapies, such as spironolactone and OCPs, in female patients with mild-to-moderate HS or as adjunctive agents in severe disease, and suggest that those with perimenstrual flares may be most likely to benefit.
Mechanical friction has been suggested as a pathogenic mechanism in the development of HS, supported by cases of HS lesions arising in areas of amputated limbs with prostheses-related friction and the development of new HS lesions at sites exposed to excess friction. Women may potentially experience discomfort and exacerbation of vulvar or perineal lesions during menstruation due to mechanical friction imposed by sanitary napkins. Therefore, the use of tampons in lieu of menstrual pads can help reduce friction and moisture in the area. For women who prefer menstrual pads, choosing non-fragranced pads made from gentle materials such as cotton or bamboo may be beneficial. For painful or heavily draining wounds in the groin region, women may benefit from the use of a hydrofiber dressing with silver, calcium alginates, or foams. Appropriately fitting biker shorts can be worn to help hold dressings in place.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here