Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter will focus on those hereditary neuropathies that can present in late childhood and adolescence. Inherited neuropathies are often referred to collectively as Charcot-Marie-Tooth (CMT) disease, an eponym used in recognition of the three men who initially described the disorder. CMT is, however, an umbrella term that encompasses a wide variety of inherited sensory and/or motor neuropathies. These inherited neuropathies also include other closely related neuropathies such as hereditary neuropathy with liability to pressure palsy (HNPP); distal hereditary motor neuropathy (dHMN), also known as distal spinal muscular atrophy (dSMA); hereditary sensory and autonomic neuropathy (HSAN/HSN); and neuropathies associated with inherited metabolic disease (see Table 17.1 ). HSAN/HSN will be discussed in Chapter 18 and neuropathies associated with inherited metabolic disease will be discussed in Chapter 19 .
| CMT Subtype | Inheritance Pattern | Neurophysiology |
|---|---|---|
| CMT1 | AD | NCV <38 m/s |
| CMT2 CMTX |
AD X-linked |
NCV >38 m/s & axonal Slow/intermediate velocities |
| CMT4 CMT5 CMT6 |
AR AD AD |
Variable NCV >38 m/s & axonal NCV >38 m/s & axonal |
| CMT Intermediate | AD or AR | NCV<&>38 m/s |
| Dejerine-Sottas | AD or AR | NCV <15 m/s & axonal |
| HSAN/HSN dHMN/dSMA |
AD or AR AD or AR |
NCV >38 m/s & axonal – sensory only NCV >38 m/s & axonal – motor only |
The first step is to determine whether the patient has a genetic neuropathy. Where there is an affected parent, either an autosomal dominant (AD) or X-linked (if there is no definite male-to-male transmission) inheritance is likely. If there are multiple affected siblings and/or consanguineous parents, then autosomal recessive (AR) inheritance is likely. However, recognizing CMT can be challenging, particularly where there is no family history or if families are small. Factors pointing to an inherited neuropathy in such circumstances include presentations in infancy, slow progression, the presence of foot deformities, and the lack of positive sensory symptoms (dysesthesias, paresthesias) in the presence of clear sensory signs. Many patients may have undergone foot surgery in childhood or report difficulties with sports at school.
CMT is the most common inherited neurological condition with a prevalence of around 1:2500. A heterogeneous disorder, CMT is divided into subtypes based on the pattern of inheritance and also by neurophysiological studies. Subtypes include AD demyelinating (CMT 1), AD axonal (CMT 2), AR (CMT 4), and X-linked (CMTX). CMT 1 typically has slow nerve conduction velocities (less than 38 m/s in the upper extremities) and pathological evidence of a hypertrophic demyelinating neuropathy, whereas CMT 2 has relatively normal nerve conduction velocities with evidence of axonal degeneration.
As most forms of CMT have both motor and sensory involvement, CMT 1 and 2 are often classified as hereditary motor and sensory neuropathy (HMSN) type I or II. Neurophysiology studies have also identified another group, CMT Intermediate, with nerve conduction demonstrating intermediate velocities (less than and greater than 38 m/s). Originally severe forms of CMT with uninformative family histories were thought to be AR in inheritance and were classified as HMSN III or termed Dejerine-Sottas syndrome. Advances in molecular technology have led to a slight revision of this classification with most of the patients in the AR HMSN III group demonstrated to have spontaneous mutations in dominantly inherited genes. The term HMSN III is no longer used; however, Dejerine-Sottas syndrome is still used to describe severely affected infants with CMT and typically very slow nerve conduction velocities.
The discovery of causal genes has led to the modification of the classification of CMT further to include the gene. The groups (e.g. CMT1, CMT, CMTX, as outlined in Table 17.1 ) remain but letters have been added to incorporate the specific gene that causes the disorder. Each type of CMT is now subdivided according to the specific genetic cause of the neuropathy (see Table 17.2 ). For example, the most common form of CMT1, termed CMT1A, is caused by a duplication of a fragment of chromosome 17 containing the peripheral myelin protein 22-kDa ( PMP22 ) gene, while CMT1B is caused by mutations in the myelin protein zero ( MPZ ) gene (see later). Currently, mutations in more than 70 genes have been identified as causes of inherited neuropathies.
| Type | Gene/Locus | Specific Phenotype |
|---|---|---|
| Autosomal dominant | ||
| CMT1 (AD CMT1) CMT1A | Dup 17p ( PMP22 ) | Classic CMT1 |
| CMT1B | MPZ | CMT1/DSN/CHN/intermediate/CMT2 |
| CMT1C | LITAF | Classic CMT1 |
| CMT1D | EGR2 | Classic CMT1/DSN/CHN |
| CMT1E | PMP22 (point mutation) | Classic CMT1/DSN/CHN |
| CMT1F | NEFL | CMT2 but can have slow MCVs in CMT1 range +/− early onset severe disease Classic CMT1/DSN/CHN |
| Hereditary neuropathy with liability to pressure palsies (HNPP) | ||
| HNPP | Del 17p ( PMP-22 ) | Typical HNPP |
| PMP-22 (point mutation) | Typical HNPP | |
| X linked CMT1 (CMT1X) | ||
| CMT1X | GJB1 | Intermediate +/− patchy MCVs/male MCVs, female MCVs |
| Autosomal recessive demyelinating (CMT4) | ||
| CMT4A | GDAP1 | CMT1 or CMT2 usually early onset and severe/vocal cord and diaphragm paralysis described/rare AD CMT2 families described |
| CMT4B1 | MTMR2 | Severe CMT1/facial/bulbar/focally folded myelin |
| CMT4B2 | MTMR13 | Severe CMT1/glaucoma/focally folded myelin |
| CMT4C | KIAA1985 ( SH3TC2 ) | Severe CMT1/scoliosis/cytoplasmic expansions |
| CMT4D (HMSNL) | NDRG1 | Severe CMT1/gypsy/deafness/tongue atrophy |
| CMT4E | EGR2 | Classic CMT1/DSN/CHN |
| CMT4F | PRX | CMT1/more sensory/focally folded myelin |
| CMT4H | FGD4 | CMT1 |
| CMT4J | FIG4 | CMT1 |
| CCFDN | CTDP1 | CMT1/gypsy/cataracts/dysmorphic features |
| HMSN Russe | 10q22–q23 | CMT1 |
| CMT1 | PMP22 (point mutation) | Classic CMT1/DSN/CHN/HNPP |
| CMT1 | MPZ | CMT1/DSN/CHN/intermediate/CMT2 |
| Autosomal dominant CMT2 (AD CMT 2) | ||
| CMT2A | KIF1Bb | Classic CMT2 |
| CMT2A | MFN 2 | CMT2/usually severe/optic atrophy |
| CMT2B | RAB7 | CMT2 with predominant sensory involvement and sensory complications |
| CMT2C | TRPV4 | CMT2 with vocal cord and respiratory involvement |
| CMT2D | GARS | CMT2 with predominant hand wasting/weakness or dHMN-V |
| CMT2E | NEFL | CMT2 but can have slow MCVs in CMT1 range +/2 early onset severe disease |
| CMT2F | HSP27 ( HSPB1 ) | Classic CMT2 or dHMN-II |
| CMT2G | 12q12-q13.3 | Classic CMT2 |
| CMT2L | HSP22 ( HSPB8 ) | Classic CMT2 or dHMN-II |
| CMT2M | DNM2 | CMT2 with infantile onset |
| CMTN | AARS | CMT2/dHMN |
| CMTO | DYNC1H1 | CMT2 with proximal involvement |
| CMT2 | MPZ | CMT1/DSN/CHN/intermediate/CMT2 |
| CMT2 (HMSNP) | 3q13.1 | CMT2 with proximal involvement |
| Autosomal recessive CMT2 (also called CMT4) | ||
| AR CMT2A | LMNA | CMT2 proximal involvement and rapid progression described; also causes muscular dystrophy/cardiomyopathy/lipodystrophy |
| AR CMT2B | MED25 | Typical CMT2 |
| AR CMT2 | GDAP1 | CMT1 or CMT2 usually early onset and severe/vocal cord and diaphragm paralysis described/rare AD CMT2 families described |
| Intermediate CMT (DI-CMT) | ||
| DI-CMTA | 10q24.1-25.1 | Typical CMT |
| DI-CMTB | DNM2 | Typical CMT |
| DI-CMTC | YARS | Typical CMT |
| CMT RIA | GDAP1 | Onset in early childhood |
| CMT RIB | KARS | ± CNS features |
| CMT RIC | PLEKHG5 | Typical CMT |
| Maternal inheritance | ||
| Mitochondrial | MT-ATP6 | Typical CMT2 or dHMN |
| Hereditary neuralgic amyotrophy (HNA) | ||
| HNA | SEPT9 | Recurrent neuralgic amyotrophy |
The prevalence of CMT is about 1 in 2500, without ethnic predisposition. The 17p11.2 duplication causing CMT1A accounts for 60% to 70% of CMT1 patients, CMT1X for approximately 20% of CMT1 cases, and CMT1B for less than 10% of CMT1 cases. Only around 25% of patients with CMT2 receive a genetic diagnosis with the majority accounted for by CMT2A. The prevalence of HNPP is not well known, but this subtype probably represents around 6% of the total CMT population.
A common feature of most genes mutated in CMT is the role they play in maintaining the structure or function of cellular components of the peripheral nervous system, Schwann cells, and the axons of the peripheral nerve ( Figure 17.1 ).
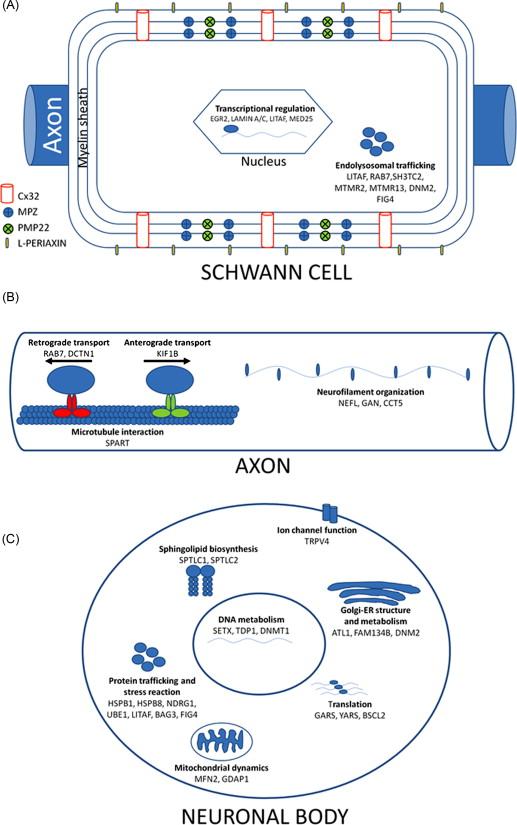
The first genes identified to cause CMT express proteins that are essential for compact myelin (peripheral myelin protein 22 [ PMP22 ] and myelin protein zero [ MPZ ]) and noncompact myelin (gap junction protein beta 1 [ GJB1 ]), and their altered expressions cause demyelination or dysmyelination. A novel concept derived from the identification of PMP22 duplication as the basic pathomechanism in CMT 1A is that of gene-protein dosage. It has become clear that correct stoichiometry of PMP22 is necessary to maintain compact myelin integrity. Too much PMP22 (i.e. with a duplication) causes CMT1A; too little (as seen with a deletion) causes hereditary neuropathy with liability to pressure palsies. Abnormal expression of MPZ also causes demyelination, although in this case it is usually due to point mutations in the MPZ gene.
An important biologic feature common to both neurons and Schwann cells is their highly specialized and polarized cellular architecture. Although the polarization of neurons is a well-recognized feature of these cells, with their axons extending more than 1 m in humans, Schwann cells are also very polarized because their membranes have to expand while they concentrically wrap around axons. To overcome the long distances between the cell nucleus and the more distal segments of the membrane, Schwann cells have areas of noncompact myelin rich in gap junctions that provide a radial pathway directly to the layers of myelin sheath. Connexin 32 (Cx32), the protein expressed by the GJB1 gene, is the main component of gap junctions in the myelin of Schwann cells and this may explain, at least in part, why GJB1 mutations cause CMT1X. The high polarization of neurons and Schwann cells may also explain why mutations in ubiquitously expressed genes, such as mitofusin 2 ( MFN2 ), ganglioside-induced differentiation-associated protein 1 ( GDAP1 ), or glycyl-tRNA synthetase ( GARS ), cause preferential dysfunction of the peripheral nervous system. The length-dependent neuropathy commonly found in patients with CMT seems to support this hypothesis that distal peripheral axons are especially susceptible to disruptions in organelle and metabolite axonal transport.
Schwann cells and axons interact at multiple points along the peripheral nerve, including the adaxonal (opposing the axon) membrane, paranodal myelin loops, microvilli, and juxtaparanodal basal lamina. These interactions are mutually beneficial, providing trophic support to the axon and myelinating cues to the Schwann cell. An example of this important interaction is the occurrence of secondary axonal degeneration in all forms of demyelinating CMT. This axonal degeneration is deemed to occur as a consequence of ineffective Schwann cell support to the axon and is actually more directly related to the clinical functional impairment than the demyelination itself.
Several recent studies have demonstrated a susceptibility of Schwann cells to mutations yielding misfolded proteins, as seen in certain PMP22 and MPZ point mutations. Misfolded proteins may accumulate in the endoplasmic reticulum (ER) of Schwann cells inducing a transitory unfolded protein response (UPR), a series of cellular events that helps the ER to cope with the increased metabolic demand caused by retention of the misfolded protein. This, in turn, causes downregulation of the myelination program genes and dedifferentiation of Schwann cells, a toxic gain of function that worsens with the demyelination and is potentially amenable to therapeutic intervention.
At a pathological level, dysmyelination, demyelination, remyelination, and axonal loss are characteristic features of the various demyelinating forms of CMT1. In Dejerine-Sottas neuropathy, myelin may never have formed normally, which is referred to as dysmyelination. In CMT1, onion bulbs of concentric Schwann cell lamellae are usually present on nerve biopsies, with loss of both small- and large-diameter myelinated fibers and sometimes axons. Focal, sausage-like thickenings of the myelin sheath (tomacula) are characteristic of HNPP but may also be found in other forms of CMT1, particularly CMT1B. In CMT1, as aforementioned, disability typically correlates better with secondary axonal degeneration than with demyelination itself, thereby demonstrating the importance of Schwann cell–axonal interactions in demyelinating disease.
Despite phenotypic variability, the typical clinical course of CMT1 and CMT2 patients includes normal development before weakness and sensory loss appearing gradually within the first two decades of life. This is often referred to as the “classical phenotype.” Affected children are often slow runners and have difficulty with activities that require balance (e.g. skating, walking along a log across a stream). Ankle-foot orthotics (AFOs) are frequently required by the third decade. Fine movements of the hands for activities such as turning a key or using buttons and zippers may be impaired, but the hands are rarely as affected as the feet. Most patients remain ambulatory throughout life and have a normal lifespan.
A minority of CMT patients have a more severe phenotype with delayed motor milestones and onset in infancy, termed “Dejerine-Sottas neuropathy.” Especially severe cases are classified as congenital hypomyelination if myelination appears to be disrupted during embryologic development. Many patients have de novo autosomal dominant disorders, and the term Dejerine-Sottas neuropathy is currently used primarily to denote severe early-onset clinical phenotypes regardless of the inheritance pattern.
Patients with distal hereditary motor neuropathies (dHMN) sometimes have mild sensory abnormalities, and patients with hereditary sensory and autonomic neuropathies (HSAN/HSN) usually have some weakness. Indeed, the same mutation in the same gene can cause both CMT and dHMN within the same family; for example, glycyl-tRNA synthetase or GARS mutations can cause both CMT2D and hereditary motor neuropathy type V.
Nerve conduction velocity testing will distinguish between demyelinating and axonal neuropathies. In clinical practice, around 60% of CMT patients have demyelinating CMT (CMT1) and around 20% have axonal CMT (CMT2). Neurophysiology is also useful to detect sensory involvement that often is unreported by patients. It can also be helpful with the further classification of axonal inherited neuropathies into mixed motor and sensory axonal neuropathy (CMT2), pure motor axonal neuropathy (dHMN), and pure sensory axonal neuropathy (HSAN/HSN).
Most CMT1 patients, particularly those with CMT1A, have a uniformly slow nerve conduction velocity of about 20 m/s. However, asymmetrical slowing, which is characteristic of HNPP, may be found in patients with missense mutations in PMP22, MPZ, EGR2 , and GJB1 . With GJB1 mutations, the asymmetry is particularly common in women with the disorder. It is important to try to recognize these cases because if the disorder is in fact acquired, it raises the possibility of a superimposed inflammatory neuropathy, which can benefit from immunosuppressive therapy. Virtually all forms of CMT1 have axonal loss as well as demyelination. In cases where the motor and sensory nerve responses are absent, it is worthwhile to look at proximal nerve conduction (e.g. axillary nerve latency) to fully investigate the possibility of a severe demyelinating rather than an axonal pathology such as is found in CMT2.
Molecular testing, performed after the family history, neurologic examination, and neurophysiologic testing, have suggested the probable candidate genes (GeneClinics—available at www.geneclinics.org ), is the “gold standard” for the diagnosis of inherited neuropathies. Strategies for focused genetic testing based on inheritance and clinical phenotype have been in place for over 10 years. The choice of genes to be tested should reflect the specific population tested. For example, in Northern European and Northern American populations, most patients have dominant inheritance even if the cases are sporadic. AR CMT is observed in less than 10% of these cases. However, in populations with high rates of consanguinity, AR CMT is likely to be much more common, even reaching levels of 40%.
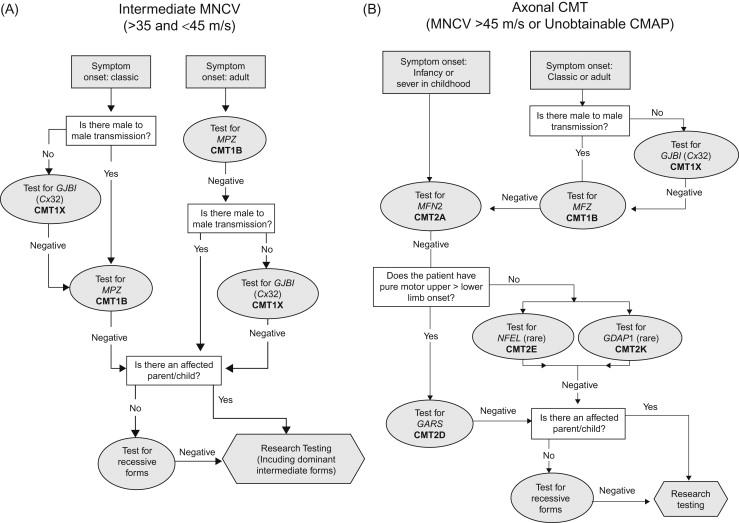
An algorithm exists based on the North American population that utilizes clinical information, neurophysiological data, and inheritance patterns to guide the clinician on which genes to consider testing. Flow charts incorporating the phenotype, illustrated in Figures 17.2 and 17.3 , guide the clinician on appropriate testing strategies. Using such tools, most CMT clinics reach a genetic diagnosis in around two-thirds of their total CMT population. Importantly, around 90% of patients found to have a genetic diagnosis are found to have changes in one of only four genes: PMP22, GJβ1, MPZ , and MFN2 . Therefore the flow charts emphasize the need for testing these particular genes first.
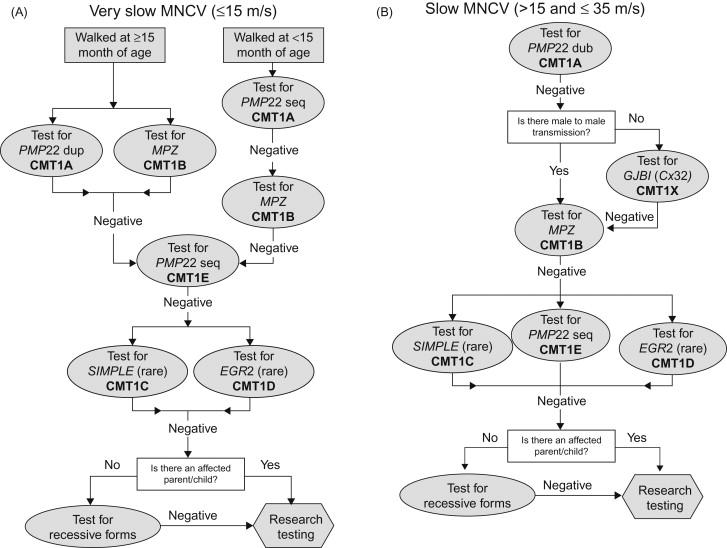
Many patients in this group had an onset in infancy with delayed walking. However, most patients with very slow MNCV and who walked by 15 months of age have CMT1A; therefore, in this scenario, testing for PMP22 duplications is warranted in the child or adolescent. If CMT1A testing is negative in this group, the next most common cause of CMT is CMT1B. If this is also negative, then testing for more rare forms of CMT may be indicated.
This represents the largest group seen in a typical CMT clinic with most patients giving a history of walking on time with onset of symptoms in the first or second decade. Approximately 89% of this group had CMT1A, so again testing should begin with analysis for PMP22 duplication. This test should be performed even in the absence of a family history as 10% of cases present with apparently de novo mutations. CMT1X is the next most common form of CMT in this group but should only be investigated if there is no male-to-male transmission. CMT1X is much more likely if the patient has intermediately slowed NCV (see the following). CMT1B is a much less likely possibility in this scenario, with testing only warranted if CMT1A or CMT1X is negative or if there is evidence of male-to-male transmission.
Intermediate conductions in a classical CMT clinical picture are typically caused by CMT1X or later onset forms of CMT1B. The first step is to identify an accurate family history. Where there is no male-to-male transmission, test for CMT1X as this represented almost 80% of such cases in one large CMT clinic. If this testing is negative or if there is male-to-male transmission, then CMTIB should be investigated.
With a classical CMT picture with onset by the second decade, the two major genetic considerations are CMT1X (if there is no male-to-male transmission) and CMT1B. The most common cause of axonal CMT, CMT2A, will have presented clinically in infancy or early childhood. If these tests are negative, clinical findings can help guide the clinician to the diagnostic genetic test (e.g. severe involvement of upper extremities should prompt the consideration of CMT2D).
While a detailed review of the pros and cons for genetic testing is beyond the scope of this chapter, we think it is reasonable to provide some information about how to pursue genetic testing. Clearly, not every patient with a genetic neuropathy wants or needs testing to identify the genetic cause of their disease. The ultimate decision rests with the patient and their parents (when the patient is under the age of 18 years). Reasons that patients and their families give for obtaining testing include identifying the inheritance pattern of their CMT, making family planning decisions, and obtaining knowledge about the cause and natural history of their form of CMT. As yet, natural history data is only available for CMT1A and CMT1X, which can provide guidance for prognosis recognizing that there can be phenotypic variability within these subtypes. Patients with other forms of CMT frequently choose to undergo genetic testing to contribute to the natural history data collection for other patients with the same subtype. Some decline testing for reasons including the high cost of commercial testing and fears of discrimination in school or the workplace or in obtaining health insurance. Because there are currently no medications to reverse any form of CMT, some decide against testing. We maintain that it is always the patient’s (or parents'/guardian's) decision whether or not to pursue genetic testing.
Once genetic testing has been done in a patient, other family members usually do not need genetic testing but can be identified by clinical evaluation with neurophysiology. We do not typically test patients for multiple genetic causes of CMT simultaneously, although our group has identified 11 patients out of nearly 1000 patients with multiple genetic causes of CMT. It is our current policy to only consider genetic testing in clinically affected family members if their phenotype is atypical for the type of CMT in the family. In addition, we do not test asymptomatic minors with a family history of CMT, either electrophysiologically or by genetic testing, owing to the chance for increased psychological harm to the child. We do routinely perform limited nerve conduction studies (NCSs), though not needle electromyogram (EMG), on symptomatic children with CMT. Because changes such as slowing are uniform, testing of a single nerve is often adequate to guide genetic testing or determine whether a symptomatic child is affected in a family with CMT.
The landscape of genetic testing is rapidly changing with the introduction of next generation sequencing (NGS) into clinical practice in some areas, which enables sequencing of either the entire genome or exome (only the protein coding sequences) in days. These new testing methods are not without fault, however; for example, the X-chromosome and many GC-rich regions are poorly covered by whole genome sequencing (WGS) and whole exome sequencing (WES). That said, even Sanger sequencing, the so-called gold standard of molecular techniques, can miss small indels (insertions or deletions of DNA) that have been picked up by NGS. Disease specific panels where multiple genes are tested via NGS are a future viable option and are in development. Here, only the coding regions of known CMT genes are tested. However, panels with a high number of genes being tested will require careful interpretation. Many genes such as periaxin and MFN 2 have a high number of polymorphisms that are not pathogenic. In addition, as more genes are being discovered in the pathogenesis of CMT, this will require modification of existing panels that will likely contribute to cost.
All things considered, it is likely that in the near future, the sequential Sanger sequencing of genes according to phenotypes as described above will no longer be the most cost-effective way of performing genetic testing. Currently, genetic testing is in a transition phase with both traditional Sanger sequencing and NGS technology being used by most dedicated CMT clinics, and whole exome sequencing is not far behind. It is our current practice to perform whole exome sequencing on patients for whom the above algorithm (using traditional techniques such as Sanger sequencing or MLPA for PMP22 duplication) has failed to identify a causal mutation. A review of these techniques is beyond the scope of this chapter, but a reference is provided. Ensuring that genetic testing is focused and cost effective is important for patients and their families.
We will mention the phrase “classical phenotype” throughout the next section. This term is based on the original description by Harding and Thomas. Affected patients with the classical phenotype begin walking on time, usually between 12 and 15 months, and develop weakness or sensory loss in the first two decades of life. Impairment slowly increases thereafter. The most common phenotypes seen in this population in particular (i.e. late childhood and adolescence) are CMT1A and CMT1X (especially in males). We will next discuss the various specific forms of CMT with emphasis on those that present in late childhood/adolescence.
CMT1 is the most common subtype of CMT and is caused primarily by mutations in four genes. These genes are essential to formation of the myelin sheath and also Schwann cell function. Two forms in particular, CMT1A and CMT1C, will present with a classical phenotype.
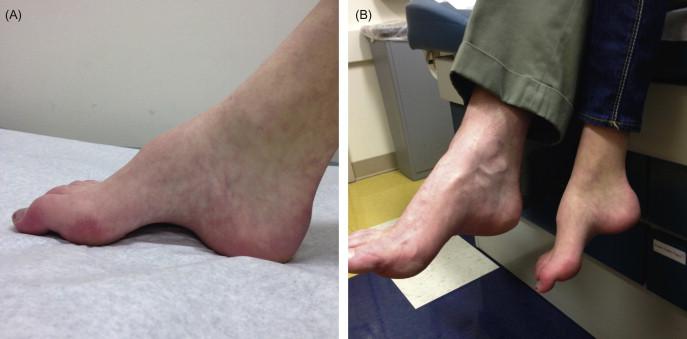
This is the most common form of CMT in most populations, affecting 55% of genetically determined CMT and 36% of all CMT. Accordingly, it is the most common form of CMT1, accounting for 80 to 90% of CMT1 cases, and will be the most commonly observed CMT neuropathy in late childhood and adolescence. Patients usually present with the “classical CMT phenotype,” which includes lower limb symptoms (difficulty walking/foot deformity) with onset in the first two decades of life accompanied by distal weakness, atrophy, sensory loss, hyporeflexia, and foot deformities such as high arches (pes cavus) and hammertoes ( Figure 17.4 ). Patients with the “classical phenotype” usually will walk on time. Lifespan is not affected. While patients frequently require ankle-foot orthotics, they rarely require wheelchairs for ambulation. Only very rarely will CMT1A patients present with a Dejerine-Sottas phenotype. Conduction velocities from the median and ulnar nerves are below 38 m/s and are typically in the 20 s range. The sensory action potentials are either reduced or absent. Nerve biopsy is not required, but, if performed, reveals demyelination and onion bulb formation. CMT1A is an autosomal dominant condition, so most patients will report a family history, but there is a de novo mutation rate of 10%. Therefore, children and adolescents without a family history with ulnar MNCV under 35 m/s should first be screened for CMT1A before proceeding with other genetic testing. CMT1A is caused by a 1.4 Mb duplication on chromosome 17p11.2 in the region that carries the PMP22 gene. It is the extra copy of PMP22 that causes the neuropathy.
This is the fourth most common type of CMT and clinically is associated with two distinct phenotypes: (1) an early infantile onset severe phenotype with delayed walking and NCV <10 m/s, often referred to as Dejerine-Sottas disease; or (2) a much later, milder phenotype with onset at around age 40 and NCV of around 40 m/s. CMT1B can, however, cause the “classical CMT phenotype,” doing so in about 15% of total CMT1B cases. CMT1B is caused by point mutations in the myelin protein zero ( MPZ ) gene on chromosome 1q22-23. MPZ is the major component of peripheral nerve myelin, comprising at least 50% of the protein. See Case Example 17.1 .
A 25-year-old man with no family history of neuropathy had been weak since infancy. He was able to stand independently by 3 years of age but was never able to run normally and always had an abnormal gait. He is currently only able to walk if wearing ankle-foot orthotics. He also has pronounced weakness with fine movements of his fingers and is unable to button his clothes, cut his own food or perform activities such as turning a key in his front door. His neurological function has been relatively stable since his teenage years. Nerve conduction studies showed markedly slowed NCVs (<10 m/s) in his upper extremities; NCVs in his legs were unobtainable at routine recording sites. Compound muscle action potentials were significantly reduced in the arms and absent in the legs. Sensory nerve action potentials were absent in the arms and legs. Genetic testing revealed an Arg98Cys mutation in MPZ (myelin protein zero) leading to a diagnosis of severe CMT1B.
In North America, if one has a genetically diagnosable form of CMT, it is likely that the causal mutation is in 1 of 4 genes ( PMP22, MPZ, GJB1 , or MFN2 ) unless the family history strongly suggests an autosomal recessive inheritance pattern (multiple affected siblings with no parents affected). CMT1A, the most common form of CMT, typically has an NCV of around 20 m/s in the arms and a classical CMT phenotype with normal early milestones and gradual weakness developing in the first two decades of life. Delayed early milestones and NCV <10 m/s are suggestive of an early onset form of CMT1B. GJB1 mutations causing CMT1X typically have intermediately slowed NCV (35–45 m/s) with an X-linked inheritance. MFN2 mutations cause the most common form of CMT2. Another group of patients with CMT1B often present symptoms in adulthood, with intermediate to normal NCV.
CMT1C is clinically indistinguishable from CMT1A, presenting in the second decade with the “classical phenotype” of distal wasting and weakness, sensory loss, reduced reflexes, and NCVs ranging from 17 to 26 m/s. It is a rare cause of CMT, accounting for <1% of the CMT population. CMT1C is caused by mutations in the lipopolysaccharide-induced tumor necrosis factor (TNF)-α factor ( LITAF ) gene, also known as SIMPLE.
The CMT1D phenotype is severe: patients typically present in infancy and may have hypomyelination (hypotonia, delayed motor milestones, MNCV<10 m/s). In addition, scoliosis and cranial nerve involvement may be features. This is a rare phenotype of CMT and is caused by mutations in EGR2 , a member of the early growth response family. EGR2 is a Schwann cell transcription factor that binds DNA along three zinc finger domains and is thought to regulate the expression of myelin genes such as MPZ and MBP. Autosomal recessive inheritance has also been described with EGR2 mutations (known as CMT4E) causing a very similar clinical phenotype.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here