Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In this chapter, we discuss a group of hereditary, often progressive, muscle disorders. These can be categorized as channelopathies, metabolic and mitochondrial myopathies, muscular dystrophies, and congenital myopathies ( Table 70.1 ).
| Myopathy | Type | Locus | Gene Product |
|---|---|---|---|
| Dystrophies | |||
| Myotonic | |||
| Classic distal | 1 a | AD 19q13 | Myotonin protein kinase |
| Proximal (PROMM) | 2 a | AD 3q21 | ZNF9 (zinc finger protein) |
| Limb-girdle | 1A | AD 5q22–34 | Myotilin |
| 1B | AD 1q11–21 | Lamin A/C | |
| 1C | AD 3p25 | Caveolin-3 | |
| 1D | AD 7q36 | DNAJB6 | |
| 1E | AD | Desmin | |
| 1F | AD | Transportin-3 | |
| 1G | AD 4q21 | ? | |
| 1H | ? | ? | |
| 2A | AR 15q15 | Calpain-3 | |
| 2B b | AR 2p13 | Dysferlin | |
| 2C b | AR 13q12 | γ-Sarcoglycan+ | |
| 2D b | AR 17q12 | α-Sarcoglycan+ (adhalin) | |
| 2E b | AR 4q12 | β-Sarcoglycan+ | |
| 2F b | AR 5q33 | δ-Sarcoglycan+ | |
| 2G | AR 17q11 | Telethonin | |
| 2H | AR 9q31–33 | E3 ubiquitin ligase (Trim 32) | |
| 2I | AR 19q13.3 | Fukutin-related protein | |
| 2J | AR 2q31 | Titin | |
| 2K | AR 9q34.1 | POMT1 | |
| 2L | AR | Anoctamin-5 | |
| 2M | AR | Fukutin | |
| 2N | AR | POMT2 | |
| 2O | AR | POMGnT1 | |
| 2P | AR | Alpha dystroglycan | |
| 2Q | AR | Plectin-1 | |
| 2R | AR | Desmin | |
| 2S | AR | TRAPPC11 | |
| Dystrophinopathies | |||
| Duchenne | XR Xp21 | Dystrophin | |
| Becker | |||
| Facioscapulo-humeral | b | AD 4q35 | ? |
| Scapuloperoneal | AD 12 | ? | |
| Emery-Dreifuss | 1 b | X Xq28 | Emerin |
| 2 | AD 1q11–q21 | Lamin A/C, nesprin-1 and -2 | |
| Oculopharyngeal | b | AD 14q11.2–q13 | Poly (A) binding protein 2 |
| Bethlem | AD 21q22, 2q37 | Collagen type VI | |
| Subunit α1 or α2 | |||
| AD 2q37 | Collagen type VI | ||
| Subunit α3 | |||
| Congenital Muscular Dystrophy | |||
| Classic CMD b | AR 6q22–23 | Laminin-α2 chain of merosin | |
| α7 Integrin CMD | AR 12q13 | Integrin α7 | |
| Fukuyama | AR 9q31–33 | Fukutin | |
| Walker-Warburg | AR ? | ? | |
| Muscle-eye-brain | AR 1p32–34 | O -mannose β-1,2- N -acetylglucosaminyl transferase | |
| Rigid spine | AR 1p35–36 | Selenoprotein N1 | |
| Congenital Myopathies | |||
| Central core | AD 19q13.1 | Ryanodine receptor | |
| Nemaline | AD 1q21–23 | α-Tropomyosin | |
| Nemaline | AR 2q21.2–22 | Nebulin | |
| Nemaline | AR 1q42 | α-Actin | |
| Nemaline | AD 9p13 | β-Tropomyosin | |
| Nemaline | AR 19q13 | Troponin T | |
| Centronuclear | AD 19p13.2 | Dynamin-2 | |
| Myotubular | X Xq28 | Myotubularin | |
| Myotubular | AR? | ? | |
| CFTD | ? | ? | |
| Myofibrillar | AD 11q22 | α,β-Crystallin | |
| Myofibrillar | AD 2q35 | Desmin | |
| Myofibrillar | AR? | ? | |
| Metabolic | |||
| Glycogen Storage | |||
| II–Acid maltase deficiency c | AR 17q21–23 | α1,4-Glucosidase | |
| III–Debrancher deficiency c | AR 1p21 | Amylo-1,6-glucosidase | |
| IV–Branching enzyme deficiency c | AR 3 | Branching amylo-1,4-1,6-transglucosidase | |
| V–Myophosphorylase deficiency | |||
| VII–Phosphofructokinase | |||
| Lipid storage | Carnitine deficiency | AR/AD | |
a DNA mutational analysis commercially available.
A 56-year-old man sought medical attention for recurrent attacks of stiffness and weakness. He recalls that when he was a child and attended summer camp, he had a spell of weakness after drinking grapefruit juice. For the past 10 years, he has had weakness triggered by certain foods, activities in the cold such as shoveling snow, and exercise. He reports stiffness, with difficulty relaxing his grip when he shakes hands. At the time of diagnosis, genetic testing was not available and he was diagnosed with hyperkalemic periodic paralysis (HyperKPP) by a potassium challenge test. Subsequent genetic testing showed a mutation in the voltage-gated skeletal muscle sodium channel Nav1.4 (encoded by the SCN4A gene on chromosome 17q23–25). He was treated with acetazolamide for the prevention of attacks and mexiletine for his myotonia; he has remained in good health overall, although recently he has developed fixed proximal weakness in his hands and legs. More recently he has started taking dichlorphenamide, which had been approved for this condition, instead of acetazolamide; in addition to its lower efficacy, acetazolamide had also caused kidney stones.
Comment: This patient is a classic example of periodic paralysis; symptoms typically begin in midchildhood. The use of a carbonic anhydrase inhibitor initially protected him from frequent attacks of weakness; however, as he reached his fifth and sixth decades, he developed a fixed proximal weakness that limited his activities of daily living (ADLs).
The various genetically determined hyperkalemic or hypokalemic channelopathies are phenotypically similar disorders related to abnormal ion passage within the muscle membrane ion channels. Their clinical picture is often stereotypical, as illustrated by the previous vignette. It is often difficult to document the occurrence of transient serum hyperkalemia or hypokalemia as it is unusual to evaluate a patient during an episode when the abnormal values typically occur.
Variable mutations within genes encoding muscle membrane ion channels are responsible for the different forms of periodic paralyses as well as other myotonic disorders ( Table 70.2 ). Most of these patients have an autosomal dominant inheritance. During the episodic paralyses, the skeletal muscle membrane's excitability transiently disappears. The degree of weakness may vary from one family member to another.
| Age at Onset | Duration of Episodes | Weakness | Myotonia | Precipitants | Alleviating Factors | Gene Mutation/Inheritance and Cation | |
|---|---|---|---|---|---|---|---|
| Hyperkalemic periodic paralysis (HyperKPP) | Infancy–early childhood | Minutes–hours | Episodic, possibly permanent later in life | Possibly (between episodes of weakness) EMG (+) | Potassium loading, cold, fasting, rest, after exercise | Carbohydrate loading, exercise | CN4A 17q23: AD Sodium channel |
| Paramyotonia congenita (PMC) | Infancy | Minutes | Very uncommon | Present EMG (+) | Repeated exercise, cold, fasting | Warming | SCN4A 17q23: AD Sodium channel |
| Sodium channel myotonia | Childhood–adolescence | Variable | Very uncommon | Present (often painful) | Rest after exercise, potassium loading, fasting | — | SCN4A 17q23: AD Sodium channel |
| Hypokalemic periodic paralysis (HypoKPP) | Puberty | Hours–days | Episodic, possibly permanent later in life | Absent | Cold, rest after exercise, carbohydrate loading | Potassium loading, exercise | CACNLA3, SCN4A 17q23: AD Calcium channel |
| Myotonia congenita | Infancy–early childhood | Minutes | Uncommon | Present EMG (+) | Exercise after rest, cold | Repeated exercise | CLC-1 7q: AD (Thomsen), AR (Becker) Chloride channel |
| Anderson-Tawil syndrome | Childhood–adolescence | Variable | Episodic, can have cardiac arrythmias and distinctive skeletal abnormalities | No | Usually hypokalemia but can be normo or hyperkalemic during attacks | — | KCJN2 (ATS1) encoding for Kir2.1 |
HyperKPP and paramyotonia are sodium channel disorders, whereas hypokalemic periodic paralysis (HypoKPP) is due to voltage-gated calcium channel dysfunction. The congenital myotonias are chloride channel disorders inherited in either a dominant (Thomsen disease) or recessive (Becker disease) fashion.
Although most instances of weakness related to periodic paralysis have a symmetric distribution, occasionally a patient may have a focal or asymmetric distribution of weakness. The latter occurs when a few specific muscles are overutilized; for example, a jeweler who developed symptoms confined to his dominant hand or the patient in the clinical vignette who developed weakness in his dominant arm when shoveling snow. Hypokalemic patients may also have paralytic events precipitated by rest after exercise as well as occurring subsequent to significant carbohydrate intake, alcohol ingestion, or cold weather. Bulbar and respiratory muscles are typically not affected. However, by midlife, the periodic events usually cease and some individuals may develop a fixed weakness, as illustrated in the vignette.
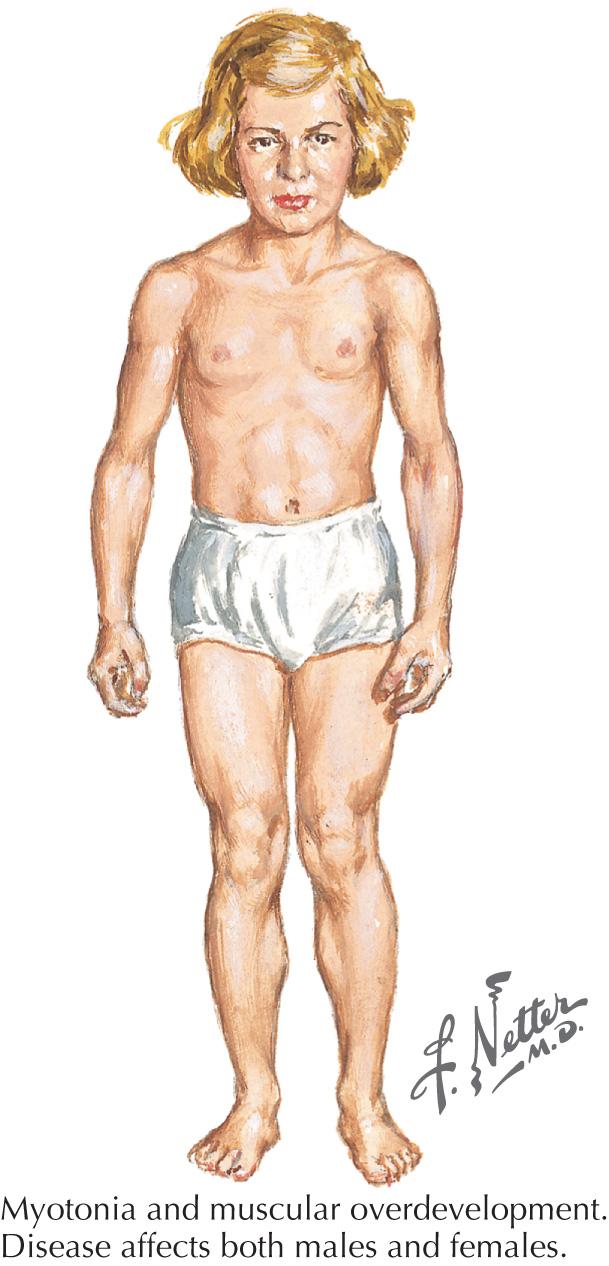
HyperKPP, unlike HypoKPP, can be associated with clinical or electrical myotonia (impaired muscle relaxation) or with paramyotonia. Clinical myotonia can be seen on exam in the eyelids, finger extensors, or thenar eminence by percussing the muscle or by activity. We find that the finger extensors are particularly helpful in eliciting percussion myotonia. Myotonia congenita (MC), a chloride channelopathy, is especially aggravated by immobility and ameliorated by exercise and warming; in MC, the myotonia is generally elicitable on examination. Fixed weakness is not usually present in dominant MC (Thomsen disease). A rather unique characteristic of Thomsen disease is the pseudo-hypertrophy of the skeletal muscles, providing the patient with a pseudo-herculean habitus ( Fig. 70.1 ). It may be so profound that athletic coaches enthusiastically encourage these individuals to participate in sports activities. Unfortunately some of these individuals may develop mild progressive weakness. Transient episodes of true weakness precipitated by sudden movements after rest that are relieved by exercise are characteristic of MC. Interesting examples include a baseball player who cannot run after hitting the ball or a subway rider who wishes to get off when the train stops but is frozen in place or falls when he arises to leave the train.
Paramyotonia congenita (PMC) is an even more uncommon hyperkalemic disorder often associated with periodic paralysis. Similar to MC, cold weather exacerbates muscle stiffness in paramyotonia. In contrast to MC, where rest promotes weakness, exercise exacerbates the stiffness in PMC patients.
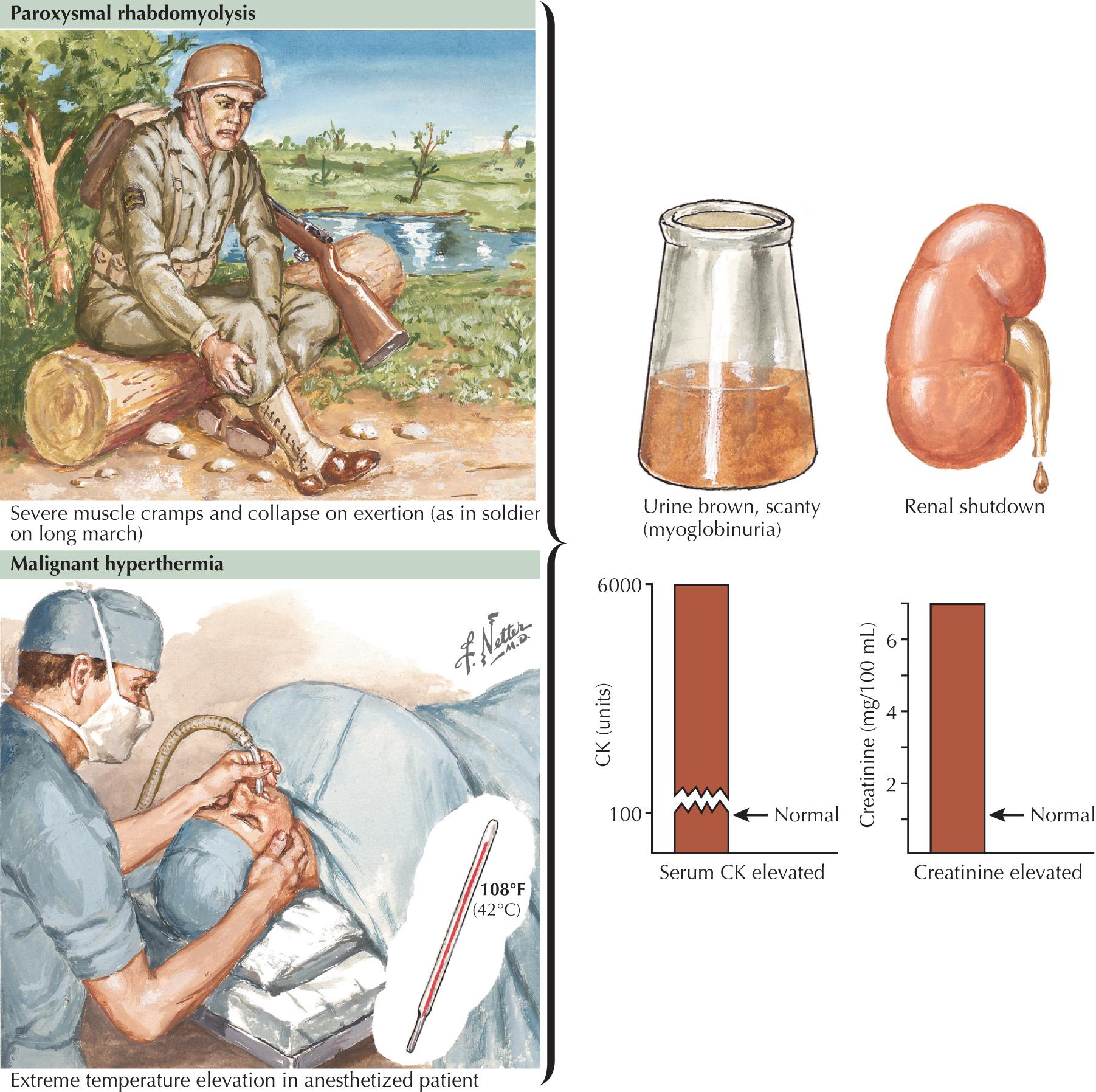
Malignant hyperthermia (MH) is a heterogeneous syndrome mentioned with the channelopathies in that it may be caused by mutations in the ryanodine receptor (RYR1) gene located on chromosome 19q13.1, leading to abnormalities in the release of calcium from the sarcoplasmic reticulum. In MH (see Fig. 70.3 , bottom), patients can have severe life-threatening episodes of hyperthermia following inhaled anesthetics and muscle relaxants; such episodes are characterized by stiffness, myoglobulinuria, cardiac complications, and even death if there is a delay in diagnosis and treatment.
The differential diagnosis of a patient with periodic paralysis includes disorders of neuromuscular transmission, notably myasthenia gravis (MG). MG classically has fluctuating symptoms, often with diurnal variation, predominantly confined to ocular bulbar muscles and typically proximal extremity musculature. Acquired myopathies can occasionally present with stiffness and myalgias as well as weakness (e.g., dermatomyositis).
Addison disease requires urgent consideration during the evaluation of any acute, generally weak patient presenting with hyperkalemia. Thyrotoxicosis can be associated with periodic paralysis in some patients, particularly young Asian men; therefore such patients must also be screened for clinical signs and laboratory findings of thyroid dysfunction.
The clinical history is the best means to diagnose a channelopathy. This may be relatively simple in patients with a positive family history examined while symptomatic and documented to have an abnormal serum potassium level or found to have demonstrable myotonia clinically or electrodiagnostically. In sporadic cases, diagnosis may be more difficult, especially when clinical examination results are normal and provocative testing does not demonstrate any biochemical or neurophysiologic abnormalities.
Regardless of whether the underlying channelopathy leads to hyperkalemia or hypokalemia, the serum potassium is normal between attacks. The clinician rarely has the opportunity to obtain a serum specimen during an event. However, during episodes of HyperKPP, the serum potassium is elevated. It is between events that the cold-induced and electromyography (EMG)-defined myotonia occurs. Similarly, the HypoKPP patient has low serum potassium findings, also confined to the precise time of paralysis. Measurement of the serum potassium level is indicated in any patient observed during spontaneous episodes of weakness or, if recurrent, an attack of periodic paralysis.
Genetic testing has greatly enhanced the specificity of the diagnostic evaluation of a patient with a question of periodic paralysis. These include DNA testing for the skeletal muscle sodium channel as seen in HyperKPP and the skeletal muscle calcium channel specific for HypoKPP. The previously used provocative studies, such as carbohydrate loading to make the patient hypokalemic, are therefore no longer used today because of the availability of genetic testing.
Nerve conduction studies sometimes demonstrate decreased compound motor action potential amplitudes in the rare instance when a clinician has the opportunity to examine a patient during an episode of periodic paralysis; otherwise these are normal. In the EMG laboratory, having the potential periodic paralysis patient exercise for prolonged periods may lead to progressive diminution in compound motor action potential amplitudes. Much more rarely, when a patient is being evaluated during individual episodes of periodic paralysis, needle EMG will demonstrate that affected muscles are electrically inactive when they are fully depolarized. Myotonic discharges occur on EMG in the sodium channelopathy HyperKPP as well as in the chloride channelopathies. This electrophysiologic finding is particularly useful in the differential diagnosis of hyperkalemic and paramyotonic varieties from the nonmyotonic hypokalemic variety. Clinical myotonia is often not evident in HyperKPP, although it may be so in paramyotonia when the patient is exposed to the cold.
Serum creatine kinase levels are usually normal or minimally increased in the periodic paralyses and myotonic disorders. Muscle biopsy is normal early in the course of periodic paralyses. However, after patients develop persistent weakness, biopsy may demonstrate vacuolar myopathy with tubular aggregates. Muscle biopsy is, however, rarely necessary for diagnosis.
The treatment of choice for a patient having an acute attack of periodic paralysis is by the correction of abnormal potassium levels. Severe hyperkalemia necessitates emergency treatment with intravenous (IV) glucose and insulin. Inhaled β-adrenergic agents or ingestion of carbohydrates is fine for less severe episodes. With any patient experiencing his or her first episode of hyperkalemia-associated paralysis, Addison disease is always a diagnostic possibility; therefore the administration of IV corticosteroids is indicated after a serum cortisol level is obtained. IV potassium or, in the milder case, oral supplementation is the best means of caring for the patient with a hypokalemic episode. Such episodes are prevented by avoiding dietary carbohydrate loads.
Maintenance therapy with acetazolamide, hydrochlorothiazide, or dichlorphenamide is usually indicated to prevent attacks. When the treatment of myotonia is also required, therapy with mexiletine or other membrane stabilizers is usually effective.
Generally a diminution in the frequency and severity of periodic paralysis attacks occurs in middle age. However, in some patients with periodic paralysis, as in the initial vignette of this chapter, fixed, progressive, typically proximal weakness develops over time and independent of attacks.
A 14-year-old female high school student was seen in the emergency room for evaluation of muscle pain and dark urine occurring subsequent to track-and-field practice. Her creatine kinase (CK) level was found to be 50 times normal. Myoglobin was demonstrated in her urine. The patient was admitted to the hospital and treated with vigorous IV hydration. Her symptoms resolved within several days.
The exercise forearm test failed to demonstrate the normally expected postexercise increase in lactate but did show significant and normal elevations of venous ammonia levels. The latter demonstrated that the patient had successfully stressed her muscle metabolism. The combination of no change in lactate and appropriate rise in ammonia levels was classic for the presence of a glycogen storage disease. On muscle biopsy, subsarcolemmal blebs seen on a periodic acid-Schiff–stained muscle biopsy specimen was consistent with glycogen excess. Biochemical analysis demonstrated decreased levels of myophosphorylase, confirming the diagnosis of McArdle disease.
Comment: This is a classic example of myophosphorylase deficiency (McArdle disease) ( Fig. 70.2 ), the most common GSD.
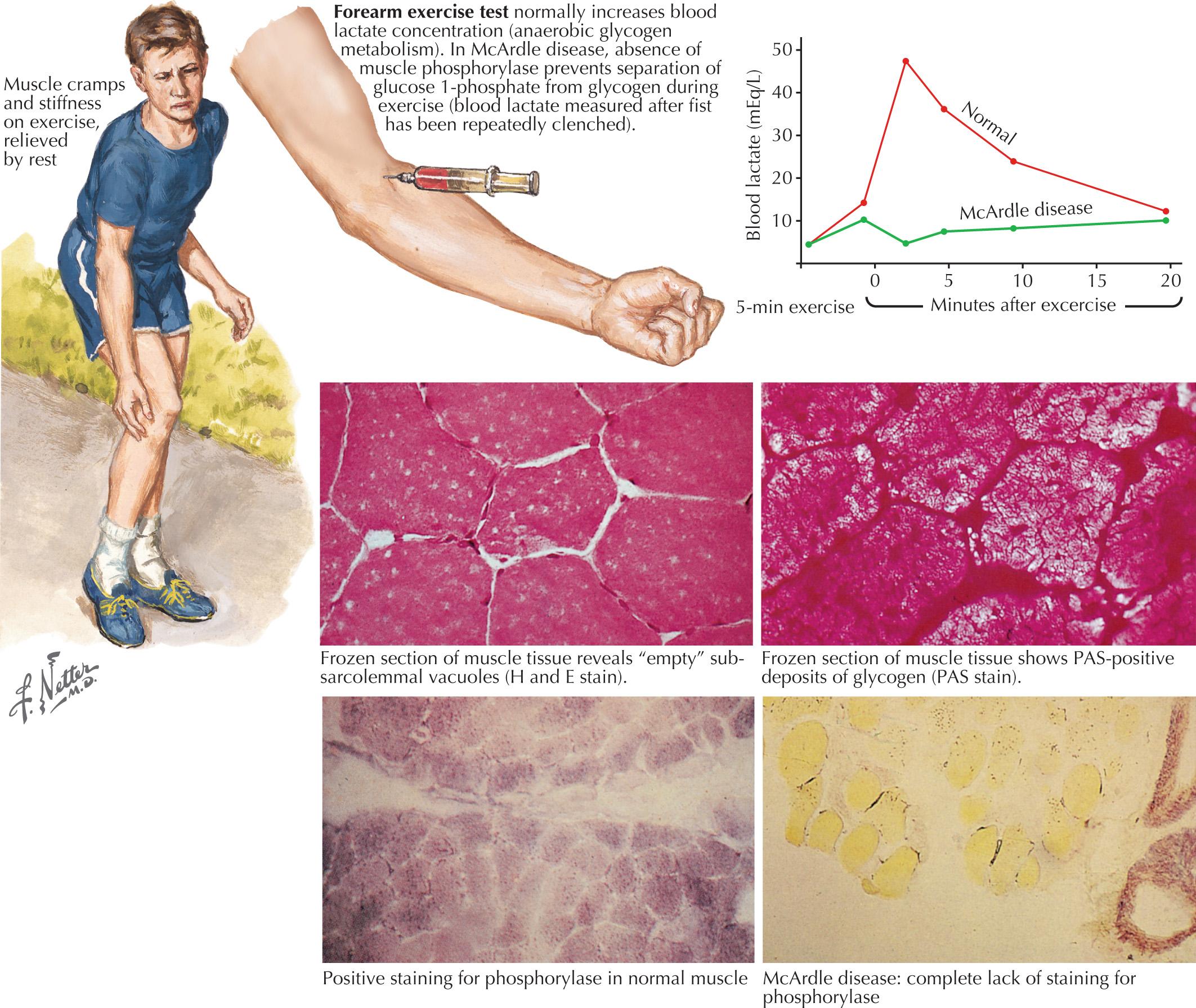
GSDs are relatively uncommon clinical entities. Myophosphorylase deficiency is the most common of them. The classic picture of the GSDs is one of exercise-induced painful muscle cramps associated with myoglobinuria. However, not all patients will have myoglobinuria and not all attacks are related to exertion. Generally GSDs may demonstrate dynamic symptoms (e.g., exertional cramps, myalgias, and myoglobinuria) and/or static symptoms (fixed progressive weakness). Very rarely, prolonged use of one extremity in isolation will uncover the presence of a previously unsuspected GSD. The concomitant laboratory documentation of profoundly elevated serum CK levels and myoglobinuria suggests either a carbohydrate or lipid enzymatic deficiency of inborn metabolism ( Fig. 70.3 and Table 70.3 ).
| Glycogenoses | Respiratory Chain Defects | Lipid Metabolism Disorders |
|---|---|---|
| Myophosphorylase deficiency (McArdle disease)—Type V | Complex 1 deficiency | Carnitine deficiency |
| Phosphofructokinase deficiency (Tauri disease)—Type VII | Coenzyme Q 10 deficiency | Carnitine palmitoyltransferase deficiency |
| Phosphorylase B kinase deficiency—Type VIII | Complex III deficiency | Very long chain, long-chain, medium-chain, or short-chain acyl-CoA dehydrogenase deficiency |
| Phosphoglycerate kinase deficiency—Type IX | Complex IV deficiency | 3-Hydroxy acyl-CoA dehydrogenase deficiency protein deficiency |
| Phosphoglycerate mutase deficiency—Type X | Complex V deficiency | Glutaric aciduria type II (electron-transferring flavoprotein and CoQ oxidoreductase deficiencies) |
| Lactate dehydrogenase deficiency—Type XI | Combination of I–V | Neutral lipid storage disease with myopathy; neutral lipid storage disease with ichthyosis |
| Beta enolase deficiency—Type XII |
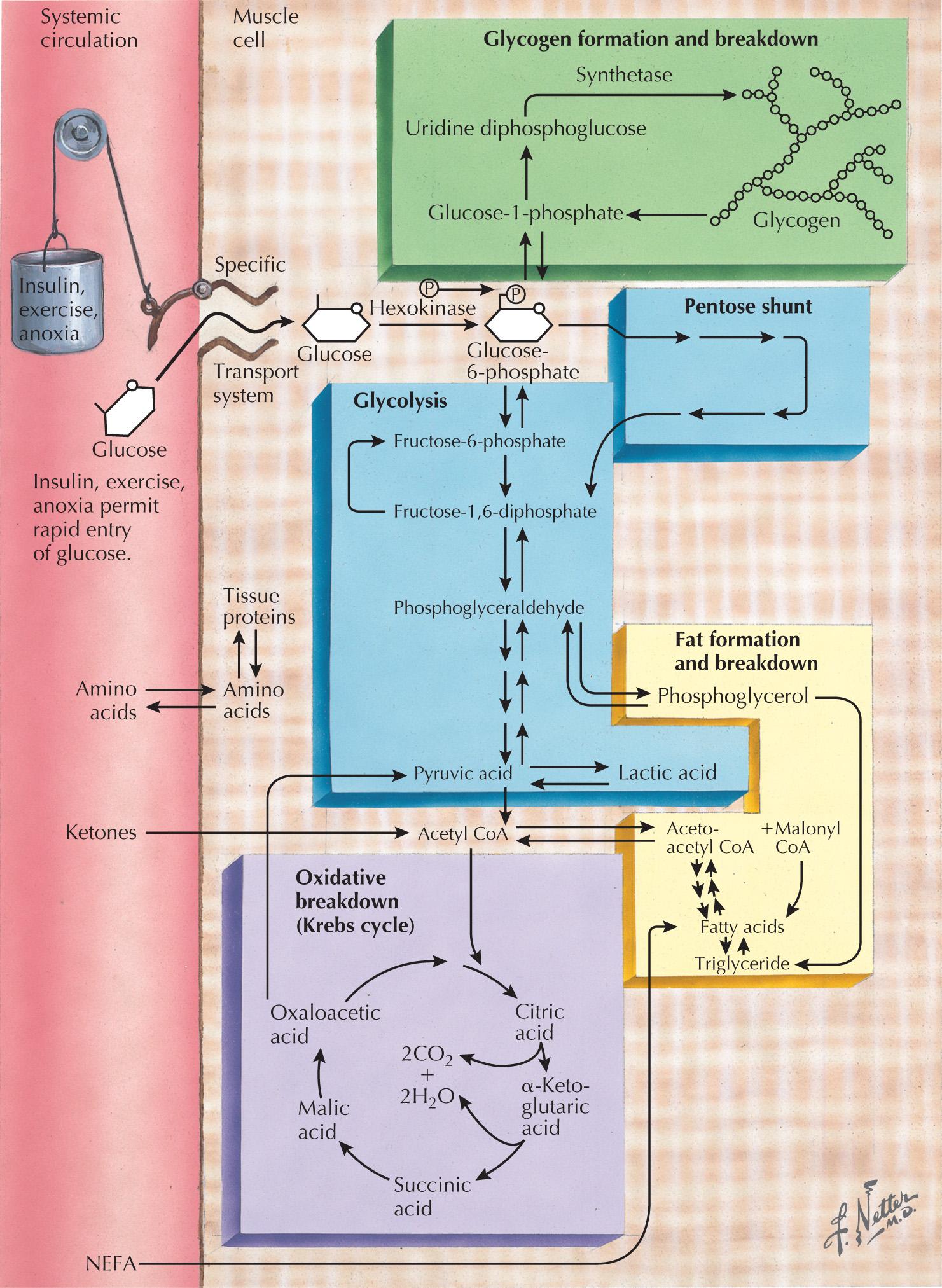
Skeletal muscle function is energy-dependent. Normal muscle metabolism requires the presence of both circulating glucose and free fatty acids ( Figs. 70.4 and 70.5 ). At rest, muscles use fatty acids for basal metabolic demands. When one first begins to exercise vigorously, usually within the first 10 minutes, the glycolysis of glycogen, already stored within muscle tissues, is the primary energy source. Its breakdown produces glucose, but only for a relatively short time. However, when the vigorous exercise is prolonged past these first few minutes, the body shifts to anaerobic glycolysis. This is manifested clinically by the second-wind phenomenon. Here lipid stores in the form of free fatty acids are mobilized as the primary source of energy. Effective glycolysis is blocked in the various muscle glycogenoses. This essentially deprives muscle of the initial need for glucose; consequently an accumulation of underutilized glycogen occurs within muscle. These muscles are inappropriately stressed by what is considered normal exercise in healthy individuals.
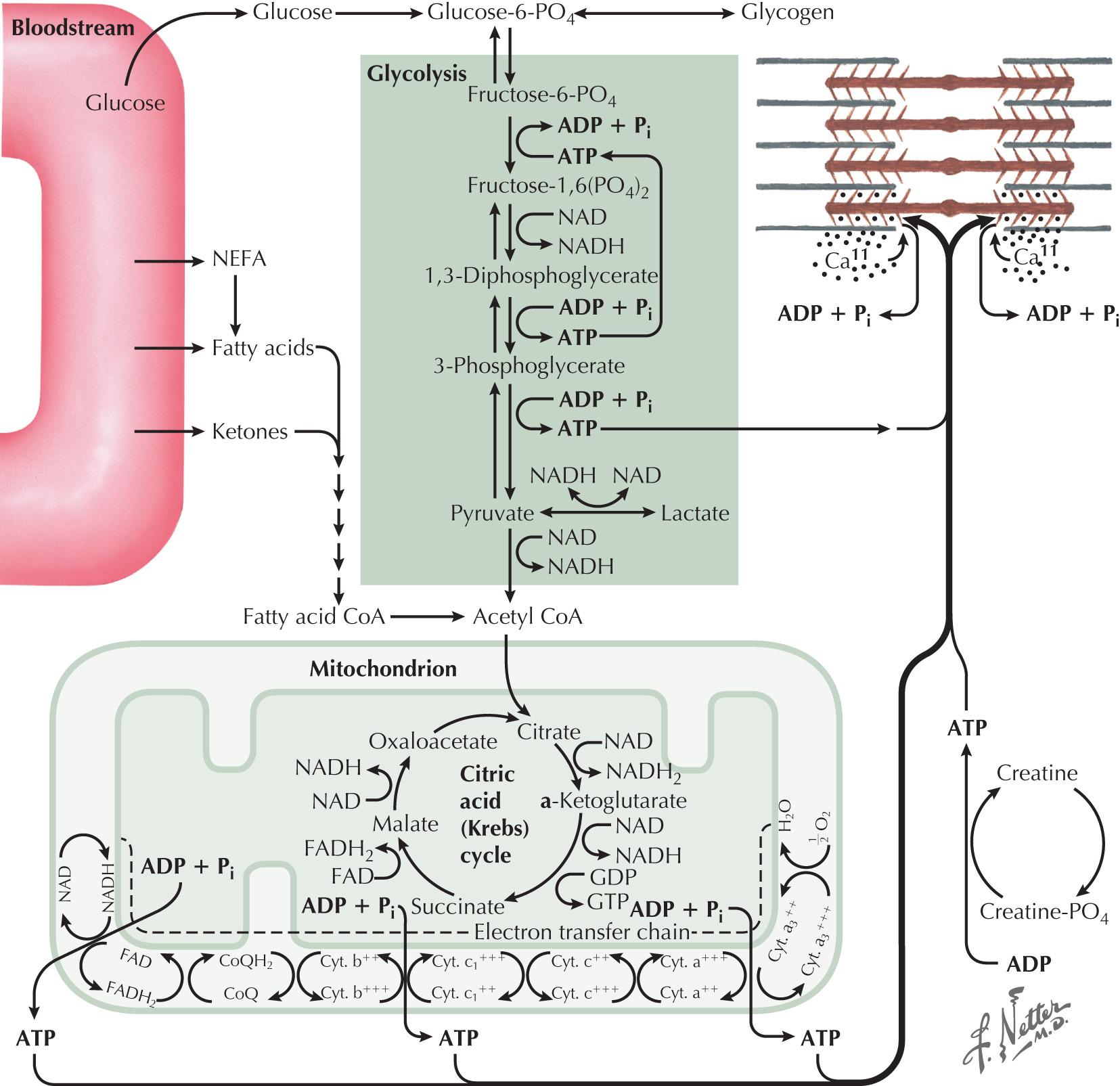
Myophosphorylase deficiency is an autosomal recessive disorder caused by the glycogen phosphorylase, muscle associated (PYGM) gene, which encodes myophosphorylase. In contrast, the much more rare phosphoglycerate kinase deficiency is usually X-linked. The remaining other glycogenoses, as well as the various disorders of lipid metabolism, the respiratory chain defects, and muscle adenylate deaminase deficiency are usually recessively inherited. As based on the specific enzyme deficiency, there are 15 different types (0-XV) of GSDs. Type V, McArdle disease, is the most common, presenting with classic exercise-induced painful symptomatology (see Fig. 70.2 ). Similar phenotypes occur with types VII, IX, X, and XI GSDs.
Severe painful muscle cramping and stiffness occurring after exertion are the hallmark of an enzymatic deficiency glycogen or lipid storage disorder. There may be a characteristic second-wind phenomenon, where brief periods of rest at the onset of myalgia alleviate symptoms and enable prolonged exercise. Symptomatic relief may come with rest (see Fig. 70.3 ). Recurrent myoglobinuria is common, and permanent weakness may develop in older patients. Patients with myalgias and exercise intolerance with or without hypercalemia are commonly evaluated by neuromuscular specialists. The exercise-induced symptomatology with the subsequent myalgia (in contrast to joint or soft tissues), muscle stiffness, and myoglobinuria provides the primary diagnostic criteria for investigating the possibility of a metabolic myopathy. Specific defects of muscle energy metabolism, as described in this chapter, are sometimes precisely defined; however, more commonly and very frustratingly, specific enzymatic defects cannot be identified in this setting.
A few patients with a GSD, particularly acid maltase deficiency in the adult, present with fixed, often progressive weakness. These individuals have no typical history of episodic symptomatology.
Myoadenylate deaminase (MAD) deficiency is a controversial “disorder” because it is not clear whether this is a discrete biochemical disorder. These patients also experience exertional muscle cramping, stiffness, weakness, and pain; the relation between the MAD deficiency and the symptoms is not clear. However, in contrast to a GSD, such individuals do not demonstrate an appropriate increase in serum ammonia levels after forearm exercise but do have the normal increase in serum lactate, indicating normal glycogen metabolism. Myoglobinuria can occur, although it is relatively rare in muscle adenylate deaminase deficiency, which may represent a disorder of defective purine metabolism. Neuromuscular examination is typically normal.
Carnitine palmitoyltransferase II deficiency is the most common disorder of lipid metabolism. Dynamic symptoms include myalgia without muscle cramping. Most commonly, young men present with recurrent myoglobinuria after prolonged but not necessarily strenuous exercise. Brief periods of exercise are usually well tolerated. Episodes may also be triggered by fasting, cold, or stress. Unlike the GSDs, no second-wind phenomenon is seen, fixed weakness does not develop, and serum CK values may normalize between episodes.
Precise diagnosis of a GSD requires biochemical findings manifested by an abnormal exercise forearm test. Baseline measurements of plasma lactate, pyruvate, and ammonia are obtained. The patients then vigorously exercise their hands for 1–2 minutes (see Fig. 70.5 ). Subsequently serial lactate and ammonia determinations are made immediately after exercise and 1, 3, 6, and 10 minutes thereafter. Normally there is a fivefold rise in serum lactate and a tenfold rise in the serum ammonia level. Glycogen metabolism storage disorders are suspected where there is failure to achieve the normal increase of serum lactate. MAD deficiency is entertained when there is a lack of the expected increase in plasma ammonia after exercise and the normal increase in serum lactate. The test's sensitivity is dependent on patient effort.
Muscle tissue histochemical analysis may also be important in the workup of metabolic myopathies. A muscle biopsy may be obtained and then specific stains looking for the presence or absence of the enzyme myophosphorylase are performed. When this is normal, then other GSDs or, even more rarely, inborn errors in muscle lipid metabolism must also be assessed. Genetic testing for CPT2 is available, so biopsy can be avoided if clinical suspicion is high and the exercise forearm test is normal.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here