Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Liver tumors in children account for approximately 1.1% of all malignancies in children younger than 20 years of age according to the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) reports. The age-adjusted incidence rate of liver tumors is approximately 11 per million in infants younger than the age of 1 year and approximately 6 per million in children between 1 and 4 years of age. This translates to approximately 100 to 150 new cases of liver cancer annually in children in the United States.
Liver malignancies in children can be divided into three broad classifications: hepatoblastoma, which accounts for two thirds of malignant liver cancers and the predominant malignant tumor type in young children; hepatocellular carcinoma (HCC), the predominant tumor type in adolescents; and other rare hepatic malignancies of childhood. A spectrum of benign tumor histologies broadens the differential diagnosis of the infant or child with a liver mass.
In an early meta-analysis of eleven separate series totaling 1256 primary liver tumors in children reported by Weinberg and Finegold, 43% were hepatoblastoma, 23% HCC, 13% benign vascular tumors, 6% mesenchymal hamartomas, 6% sarcomas, 2% adenomas, 2% focal nodular hyperplasia, and 5% other tumors. Rare tumors of the liver also include rhabdoid tumors and hepatic germ cell tumors. An international working group recently provided guidelines regarding the histologic classification of pediatric liver tumors.
Most liver tumors in childhood are more common in boys than girls. The male-to-female ratio observed for hepatoblastoma in cooperative group trials has ranged from 1.5 to 1 to 2 to 1; a combined analysis of multiple studies suggests that the male-to-female ratio in hepatoblastoma is 1.65, consistent with SEER data.
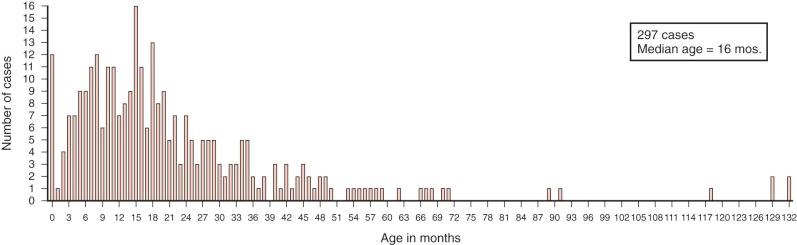
Hepatoblastomas have a unique age distribution ( Fig. 58-1 ). Two peak ages occur, one at birth or within the first month of life and a second broad peak between 16 and 20 months of age. Ninety percent of hepatoblastomas occur in children younger than 4 years of age, and 95% of hepatoblastomas occur by age 5. Although rare, hepatoblastoma has been seen in adults, as described in multiple sporadic case reports. A systematic review of hepatoblastoma in adults suggests that these tumors are associated with a particularly poor prognosis, although the time frame over which these cases were gathered spanned multiple decades. In contrast hepatoblastomas diagnosed very early in life (i.e., in the neonatal period) are associated with a favorable outcome.
Hepatoblastoma in children older than 5 years of age is often more aggressive than the typical hepatoblastoma, has characteristics of HCC, and has been termed transitional liver cell tumor. More recently pathologists prefer to designate this poorly understood entity as hepatocellular tumor, not otherwise specified, pending a much-needed and more thorough understanding of the biology of these tumors with features of both hepatoblastoma and HCC. HCCs make up the bulk of liver tumors seen after age 10 years and throughout adolescence and adulthood. As with hepatoblastoma, HCC is more common in male subjects than female subjects. The fibrolamellar variant of HCC is a notable exception, with equal occurrence in male and female subjects.
The incidence of liver tumors has increased over recent decades, perhaps more than any other type of childhood tumor. During a single decade the incidence rates of hepatic tumors in infants younger than 1 year of age increased from 4 to 8 per million. The increase in rates was especially pronounced in female infants, in whom rates of hepatic cancer increased from 2 to 12 per million in a single decade. Ross and Gurney estimated that the average annual percentage change in incidence of hepatoblastoma between 1973 and 1992 was 5.2% for males and 8.2% for females. The rise in incidence of hepatoblastoma has been estimated to be 4% per year between 1992 and 2004 and continues to rise in recent years compared with even recent SEER data. The largest contributing factor to the increased incidence is thought to be the increased survival rates of premature infants who are at markedly increased risk of hepatoblastoma, as discussed in subsequent sections of this chapter.
Hepatoblastoma has long been thought to have prenatal origins, and increasing evidence points to prematurity, prenatal exposures, and overgrowth in early infancy as factors contributing to hepatoblastoma. It has long been presumed that embryonal tumors derive from primitive cells and that hepatoblastoma, as an embryonal tumor of the liver in which cells morphologically resemble cells in the developing embryonal and fetal liver, derives from a primitive hepatic cell. In rodents immature oval cells that proliferate after exposure to hepatocarcinogens express both biliary and hepatocytic markers. These cells are characterized by staining with OV-1 and OV-6 markers. Ruck and colleagues demonstrated the existence of a similar cell type in hepatoblastoma on the basis of marking with OV-1 and OV-6 in a small population of cells within the tumor. These OV-1 and OV-6 staining cells account for fewer than 1% of the cells within the tumor and provided evidence to support the existence of a population of stem cells in hepatoblastoma. Fiegel and coworkers analyzed hepatoblastomas for stem cell markers including CD34, Thy1, and c-kit and demonstrated the co-occurrence of these stem cell markers with hepatobiliary markers in ductal cells phenotypically resembling stem cells, which suggests that stemlike cells play a role in the histogenesis of hepatoblastoma. Other markers of hepatic progenitor cells including epithelial cell adhesion molecule (EpCAM), cytokeratin 19 (CK19), octamer-binding transcription factor 3/4 (Oct-3/4), and delta-like 1 homologue (DLK1) have been shown to be expressed in hepatoblastoma tumor cells. NOTCH2, which peaks in the second half of fetal development and then declines toward birth, is expressed in most hepatoblastomas. CITED1, expressed in embryonic mouse liver and a marker of hepatic progenitor cells, is shown to be present in hepatoblastoma, providing another similarity of hepatoblastoma to hepatic precursor stem cells.
Most cases of childhood liver malignancy are not associated with an apparent major genetic syndrome. However numerous syndromes have been observed in association with hepatoblastoma or HCC, as well as some benign hepatic tumors. A complete summary of genetic syndromes and liver tumors in children is provided in Table 58-1 . The major genetic syndromes associated with childhood liver tumors are discussed in the subsequent sections.
| Disease | Tumor Type | Gene |
|---|---|---|
| Familial adenomatous polyposis | Hepatoblastoma, adenoma, hepatocellular carcinoma, biliary adenoma | APC |
| Beckwith-Wiedemann syndrome | Hepatoblastoma, hemangioendothelioma | Multiple candidates |
| Simpson-Golabi-Behmel syndrome | Hepatoblastoma | GPC3 |
| Trisomy 18 | Hepatoblastoma | No single gene |
| Li-Fraumeni syndrome | Hepatoblastoma, undifferentiated sarcoma | TP53 |
| Glycogen storage disease types I-IV | Hepatocellular adenoma, carcinoma hepatoblastoma | Glucose-6-phosphatase |
| Hereditary tyrosinemia | Hepatocellular carcinoma | Fumarylaceto-acetate hydrolase |
| Alagille syndrome | Hepatocellular carcinoma | JAGGED-1, NOTCH2 |
| Other familial cholestatic syndromes | Hepatocellular carcinoma | FIC1, ABCB11 |
| Neurofibromatosis | Hepatocellular carcinoma, malignant schwannoma, angiosarcoma | NF-1 |
| Ataxia—telangiectasia | Hepatocellular carcinoma | ATM |
| Fanconi anemia | Hepatocellular carcinoma, fibrolamellar cancer, adenoma | FAA, FAC, others (20%) |
| Tuberous sclerosis | Angiomyolipoma | TSC1, TSC2 |
Familial adenomatous polyposis (FAP) is caused by germline mutation of the APC gene, FAP is best characterized by the presence of colonic polyps, which without intervention are associated with the virtual certainty that adenocarcinoma of the colon will develop. Germline mutation of the APC gene is, however, also associated with a marked increased risk of hepatoblastoma. The risk of hepatoblastoma in children in FAP kindred is estimated to be 800-fold. The risk that a parent with an APC mutation will have a child with hepatoblastoma is 0.4%. Because FAP is an autosomal dominant disorder, genetic testing should demonstrate a predisposing mutation in 50% of offspring, such that the risk of hepatoblastoma in an infant who is a documented mutation carrier would be slightly less than 1%. Within a series of consecutive hepatoblastoma patients for whom family histories were obtained, approximately 8% of sequential cases of hepatoblastoma have a family history suggestive of FAP, and abnormalities of the APC gene were detectable in all cases.
Also reported in children from FAP kindreds, albeit less commonly, are other liver tumors, including HCCs. A benign precursor lesion, hepatocellular adenoma has been reported in a patient with a germline APC mutation and colonic polyps. Within resected liver tissue from children with germline APC mutations and hepatoblastoma, multiple hepatic nodules were consistent with precursor lesions with potential to progress to either hepatoblastoma or HCC. Presumably the timing of additional mutations or key developmental processes could determine the resulting histologic tumor type in the predisposed liver.
Significantly of nine reported cases of hepatoblastoma in siblings, at least four were in families with FAP; Of the remaining reported sibling pairs, one was affected with type 1a glycogen storage disease, and two sibling pairs had no apparent familial syndrome, and one recent report of a sibling pair with hepatoblastoma demonstrated an APC mutation in only one of the siblings. These reports suggest that whereas APC mutation accounts for a significant percentage of familial cases of hepatoblastoma, additional predisposition genetic changes may exist either as a distinct underlying cause of hepatoblastoma or as a modifying factor in infants with an existing APC mutation.
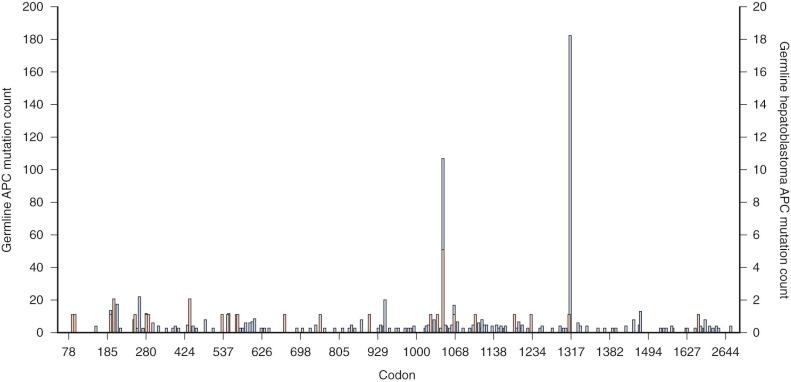
The percentage of sporadic hepatoblastoma patients (those without an obvious family history) who carry a germline mutation of the APC gene is not well defined. However it has been suggested both that children with hepatoblastoma be screened for germline APC mutations and that asymptomatic children in families with known polyposis be screened for hepatoblastoma. Within the APC gene genotype-phenotype correlations suggest regions of the gene that predict both the severity of polyposis as well as the presence of extracolonic manifestations. The literature suggests that germline mutations in FAP families with children who are affected by hepatoblastoma can occur throughout the gene ( Fig. 58-2 ) and can also include whole gene deletions or chromosome rearrangements such that the site or type of the individual APC mutation cannot be used to predict the occurrence of hepatoblastoma. This finding implies a risk to all APC mutation carriers.
Beckwith-Wiedemann syndrome (BWS) is an overgrowth disorder classically characterized by large birth weight, macroglossia, omphalocele, and visceromegaly. It is associated with a markedly increased risk of all embryonal tumors, including Wilms tumors, hepatoblastoma, neuroblastoma, and adrenal cortical adenoma. A registry of children with BWS followed at the National Cancer Institute determined that the relative risk of hepatoblastoma in children with BWS was 2280, higher than the relative risk of any other type of cancer in BWS. Unlike hepatoblastoma and FAP, hepatoblastoma has not been seen in siblings with BWS, despite the extraordinarily high relative individual risk in a patient with BWS. Presumably, this is because of the low incidence of familial occurrence of BWS.
BWS maps to chromosome 11p15, a region known to show loss of alleles in hepatoblastoma as well as parental-specific expression of genes (i.e., imprinting), including IGF2 and H19 . Molecular subtyping demonstrated that genetic alterations underlying BWS include mutations, epigenetic events involving imprinted genes, or uniparental disomy and that imprinting defects are of two types—those with imprinting defect at the imprinting control element 1 (IC1) at distal 11p15.5 and involving IGF2 and H19 hypermethylation and those with a defect at the imprinting control element (IC2), which is more proximal and involved imprinting changes of KvDMR1 and KCN11OT1. One series demonstrated that significantly more neoplasia is noted with either uniparental disomy or imprinting defects at IC1, although hepatoblastoma has been observed in BWS patients with mutations at the IC2 locus. Although differences may exist specific to the type of underlying genetic alteration, it is not possible at this time to tailor screening recommendations to genetic subtype. Present screening recommendations involve periodic ultrasonography of the abdomen and measurement of serum alpha-fetoprotein (AFP). In a series of tumors detected in children with BWS who underwent such surveillance, all tumors were small and resectable at diagnosis. Because hepatoblastoma has been noted to occur in the early neonatal period in BWS, some experts recommend screening at an early age. Even though AFP levels are normally very high in the neonatal period, screening with AFP should confirm a downward trend in the absence of tumor.
Simpson-Golabi-Behmel syndrome (SGBS) is an X-linked syndrome characterized by overgrowth with clinical features reminiscent of BWS, including an increased risk of embryonal tumors. Individual cases of SGBS with hepatoblastoma have been reported. The underlying gene for SGBS, GPC3, is highly expressed in hepatoblastoma compared with normal liver, which is consistent with a potential role in the tumorigenesis of hepatoblastoma. Although no longitudinal tumor surveillance studies have been carried out for SGBS, the overlap in tumor types observed suggests that surveillance similar to that used for BWS may be appropriate.
Sotos syndrome, an overgrowth syndrome that has clinical features overlapping with BWS and is associated with a predilection for tumors, has been reported in a child with hepatoblastoma. No large series have been reported, however, and the benefit of screening is unclear at this time.
Li-Fraumeni syndrome predisposes affected individuals to multiple tumor types in childhood and early adulthood. Although liver tumors are not typically included in Li-Fraumeni syndrome, hepatoblastoma has been reported in two Asian Li-Fraumeni kindreds. A kindred has also been reported in which a child developed undifferentiated (embryonal) sarcoma of the liver. Acquired mutations of the TP53 gene in hepatoblastoma tumor specimens, however, are rare or absent but have been reported in the undifferentiated embryonal sarcoma of the liver.
Both hepatoblastoma and HCC have occurred in children with primary biliary atresia. However it is unclear whether liver tumor development in children with biliary atresia is a complication resulting from liver disease or whether a common predisposition to both biliary atresia and tumor development exist. Significantly the same immature cells thought to share similarities to stem cells seen in hepatoblastoma are also seen in biliary atresia. Multiple cases of hepatoblastoma and trisomy 18 have been reported. This is somewhat notable in that trisomy of chromosome 18 is not seen as an acquired event despite the observation of multiple other recurring trisomies.
Hepatoblastoma has been reported in a female subject with monosomy 7 myelodysplasia, a predisposition syndrome not previously seen with hepatoblastoma. Hepatoblastoma has also been reported in neurofibromatosis type 1, although it is not characteristic of this disorder.
A recent report has noted a possible association of hepatoblastoma with prune-belly syndrome, a congenital disorder of lax abdominal musculature and urogenital abnormalities. A review of data from the Children's Oncology Group (COG) demonstrated a significant association with kidney and bladder congenital abnormalities.
HCC, in contrast to hepatoblastoma, is seen in genetic syndromes that are characterized by hepatic cirrhosis. Progressive familial intrahepatic cholestasis, a condition marked by neonatal hepatic cirrhosis, is associated with liver tumor development in children. Both HCC and cholangiocarcinoma are known to occur in this disorder. The disorder, also known as Byler disease, is a heterogeneous syndrome. The underlying genetic etiology is a germline mutation of the ABCB11 gene, which encodes a bile salt export pump. In a series of 11 cases of HCC in children younger than 5 years with neonatal hepatitis with clinical characteristics of familial intrahepatic cholestasis, 10 had immunohistochemical evidence of a deficiency in bile acid transporter bile salt export pump. In all patients in whom genotyping was possible, a germline mutation of the ABCB11 gene encoding bile salt export pump was detected.
Alagille syndrome is an autosomal-dominant disorder characterized by a characteristic facies, congenital heart disease, intrahepatic cholestasis with neonatal jaundice, and a paucity of hepatic bile ducts resulting in neonatal jaundice. The liver pathology may progress to frank cirrhosis. HCC has been reported in children with Alagille syndrome. The vast majority of these Alagille syndrome–associated HCCs occur in boys. In one family three children were reported with HCC in the absence of any other type of liver disease other than the Alagille syndrome. Alagille syndrome is associated with germline mutations in the JAGGED1 gene, the endogenous ligand for NOTCH2, a developmental gene involved in controlling maturation of the heart and liver. In a minority of cases of Alagille syndrome, in which the JAGGED1 gene is not found to be mutated, the NOTCH2 gene itself is mutated. It has been shown that the mechanism of liver disease in Alagille syndrome may relate to an increased expression in hepatocyte growth factor in cells that carry JAGGED1 mutations.
The family of glycogen storage disorders has been associated with liver tumorigenesis. Glycogen storage disease type I has been reported in association with benign adenomas, HCC, and hepatoblastoma, although the latter has been reported only in adolescents. Similarly glycogen storage disorder type III has been associated with HCC, and type IV has been associated with hepatocellular adenoma. Because HCC is known to occur in diseased liver, with long-term follow-up HCC may be considered a complication of increased survival of patients with glycogen storage disease.
Hereditary tyrosinemia type 1, characterized by the deficiency of fumarylacetoacetate hydrolase is likewise associated with HCC in the setting of severe liver disease.
Fanconi anemia is associated with liver tumors, although the association has been almost always reported in association with the use of androgenic steroid treatment. In a review of 1301 reported cases of Fanconi anemia, 2.8% of subjects were reported to have a liver tumor. The most common type of liver tumor reported in Fanconi anemia is HCC, but adenomas are also seen in these patients. The incidence appears to increase with age and does not appear to plateau. In multiple instances liver malignancy was seen in patients with Fanconi anemia who also had a diagnosis of leukemia. The extent of the associated androgen treatment with development of hepatic malignancy versus the effect of the underlying genetic defects in DNA repair as manifested by chromosome breakage in Fanconi anemia is unclear, but a review of the association of androgens and liver tumors suggests that patients with Fanconi anemia develop liver tumors after smaller and briefer androgen exposure than do individuals without Fanconi anemia. It is also unclear whether there is a difference among the genetic subtypes of Fanconi anemia with regard to the predisposition to hepatic and other malignancy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here