Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
DDX: Hepatocellular adenoma, well-differentiated hepatocellular carcinoma, regenerative nodule
Focal nodular hyperplasia (FNH) is the most common benign hepatocellular tumor and is thought to represent a localized, regenerative proliferation of hepatocytes in an area of abnormal blood flow. While it most often affects women between 20 and 50 years, FNH can occur in either sex and in all age groups. Lesions are usually solitary, ranging in size from subcentimeter masses to more than 10 cm across, and the background liver is not cirrhotic.
FNH has been associated with vascular malformations or hemangiomas in 20% to 30% of cases. Most are discovered incidentally on imaging, and FNH-like areas may also arise adjacent to other hepatic tumors due to localized vascular changes secondary to mass effect. Molecular studies have shown that the hepatocellular component is polyclonal. Consequently, FNH is termed a lesion rather than a neoplasm, and it carries no risk of malignant transformation.
Classically, FNH appears as a single well-circumscribed, tan-white, nodular mass. Most are less than 5 cm, but they can grow larger. The characteristic feature is a central stellate scar that radiates through the lesion and imparts a grossly nodular appearance.
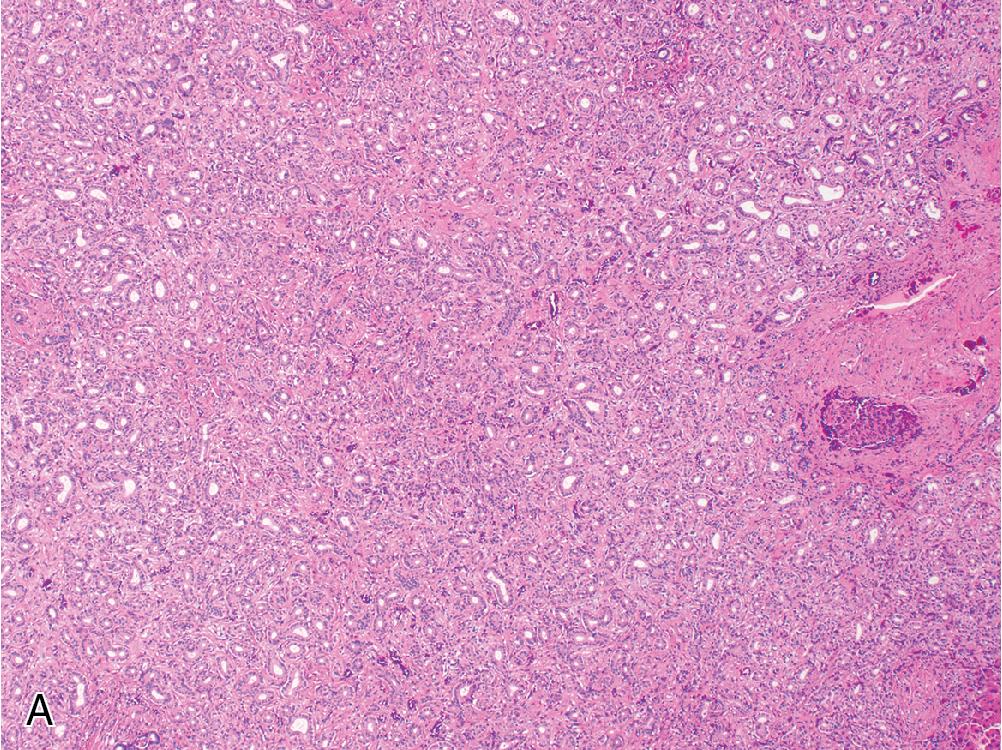
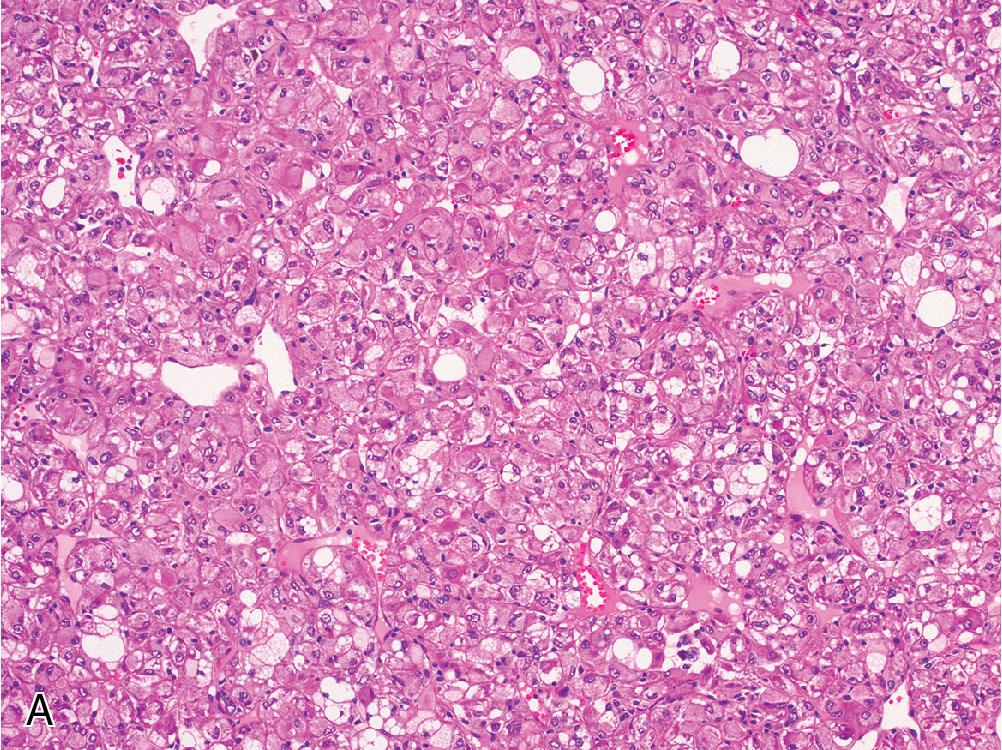
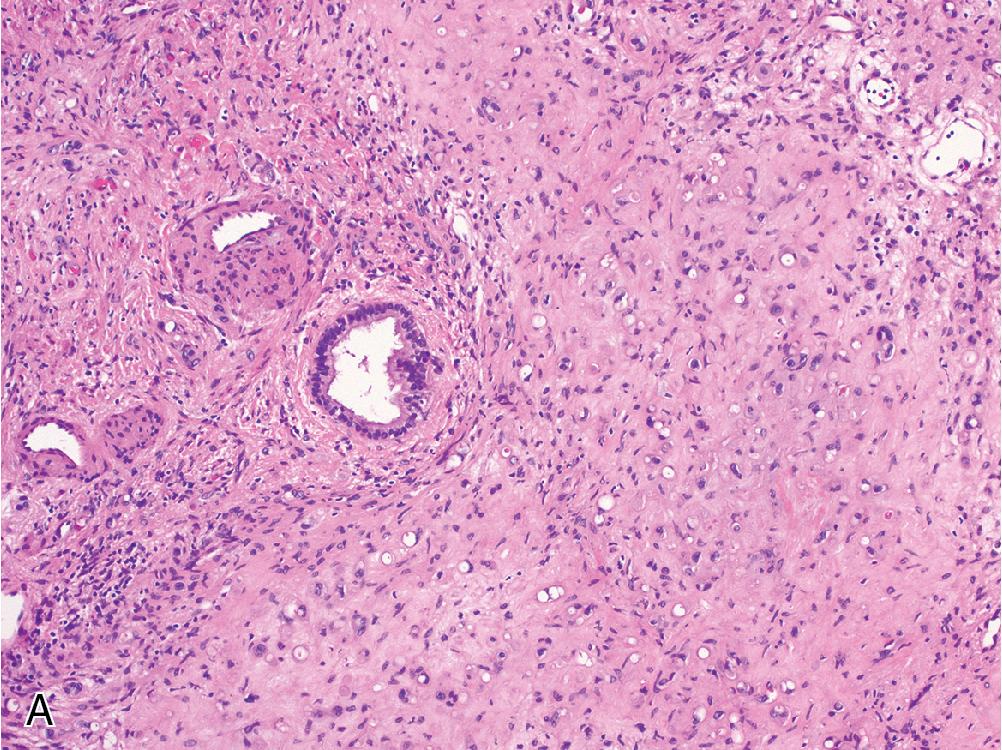
FNH contains hyperplastic nodules of benign hepatocytes separated by fibrous bands radiating from a central scar-like area ( Fig. 4.1 A). Ductular reaction spreads alongside the periphery of these fibrous septae ( Fig. 4.1 B), but the lesion itself does not contain true portal tracts. Instead, the fibrous areas may contain thick-walled dystrophic arteries with medial hypertrophy and intimal fibrosis, and variable amounts of lymphocyte-predominant inflammation are present.
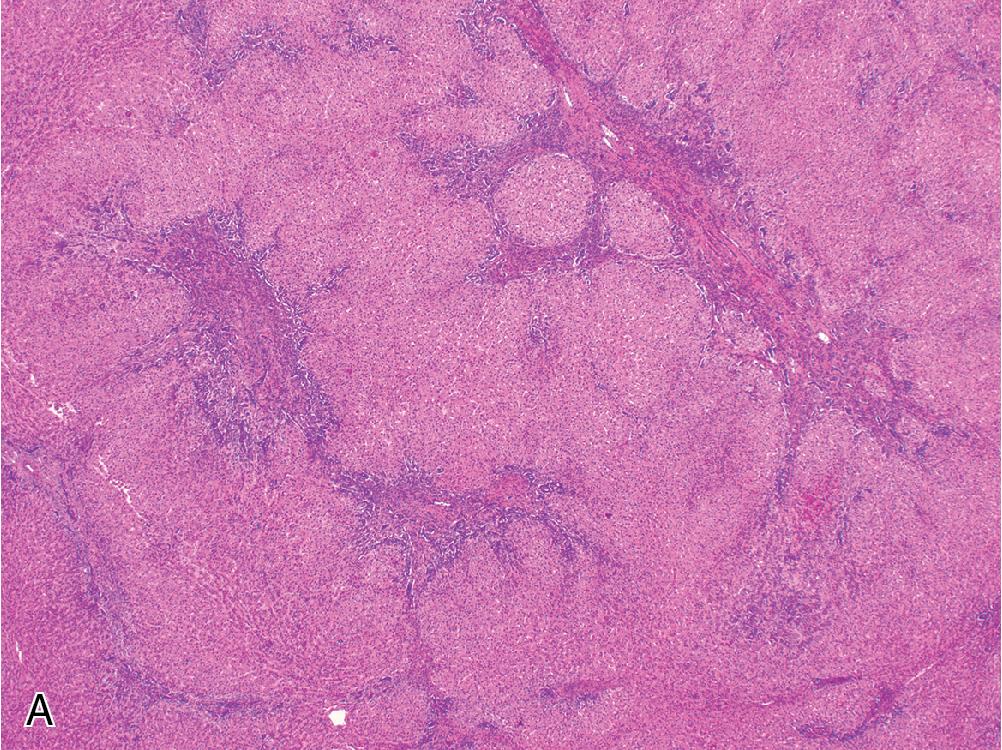
Within the hepatocellular nodules, the trabecular width is largely normal (i.e., 1 or 2 cells across), but focal areas may show mild regenerative changes manifesting as patchy plate widening or slight architectural disarray. Hepatocytes can sometimes exhibit cytoplasmic swelling or Mallory hyaline accumulation around fibrous septae.
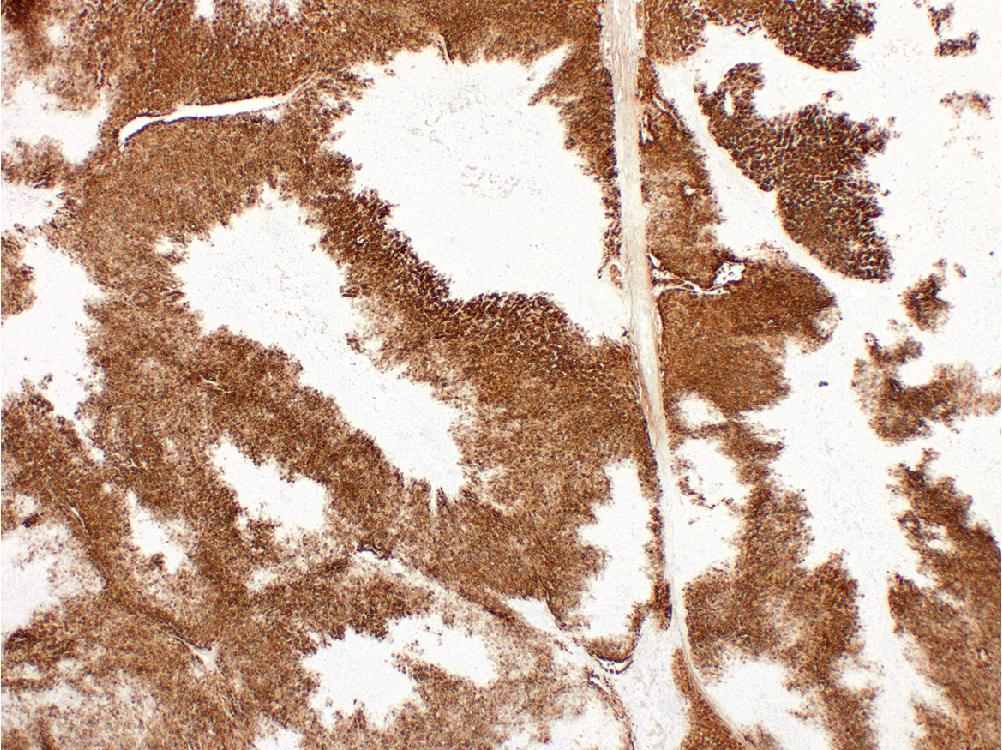
Immunohistochemistry (IHC) for glutamine synthetase (GS) highlights a characteristic “geographic” or “map-like” staining pattern within the lesion that manifests as broad, interconnecting channels of cytoplasmic labeling ( Fig. 4.2 ). This differs from the pattern of GS expression in normal liver, which is restricted to a thin rim of hepatocytes around central veins.
FNH is a benign lesion with no significant risk of malignant transformation, rupture, or hemorrhage. As such, surgery is not always indicated, and prognosis is excellent.
The primary differential diagnosis for FNH is hepatocellular adenoma (HCA), as both are mass lesions that arise in noncirrhotic livers. The presence of fibrous bands containing large dystrophic vessels and lined by prominent ductular reaction is characteristic of FNH. “Map-like” GS expression is not seen in HCA and is diagnostic of FNH. Between the characteristic morphologic features and GS expression pattern, this diagnosis is usually straightforward on resection specimens.
However, this differential is more often encountered on small biopsy samples, which may not show distinct morphologic features or a well-developed geographic staining pattern with GS. The presence of fibrosis, ductular reaction, and thick-walled vessels may suggest a diagnosis of FNH in such cases, but these features are often not well-developed, and focal scarring or ductular reaction can also be present in adenomas. For truly inconclusive biopsies, our practice is to exclude β-catenin activation by IHC (see Hepatocellular Adenoma section) and diagnose these as well-differentiated hepatocellular lesions.
FNH is not diagnosed in cirrhotic livers. However, if the presence of cirrhosis is unknown, the differential diagnosis may include regenerative nodules of cirrhosis or even a well-differentiated hepatocellular carcinoma (HCC). Regenerative nodules will contain portal tract components that are absent in FNH. If considering a diagnosis of HCC, reticulin staining in FNH will show a preserved framework and normal hepatic plate thickness, while HCC will feature abnormally widened hepatic plates, a disrupted reticulin framework, and/or increased cytologic atypia on hematoxylin and eosin (H&E) staining.
Non-neoplastic proliferation of hepatocytes due to alterations in hepatic blood flow
More common in women
Occurs in noncirrhotic livers
Often found incidentally
Most often between 20 and 50 years of age
Usually solitary, but can be multiple in up to 20% of cases
FNH-like areas can appear adjacent to other mass lesions due to locally altered blood flow
Typically asymptomatic, but rarely can present with abdominal pain
Prominent central scar
Hypodense lesion on CT with arterial enhancement
Observation if asymptomatic
Resection if symptomatic
No risk of malignant transformation or significant hemorrhage
Well-circumscribed, often solitary, nodular lesion, typically under 5 cm
Most characteristic feature is a central stellate scar
Bands of irregular fibrosis with ductular reaction impart a nodular appearance
Large vessels present with thick walls and intimal fibrosis within central scar
Hepatocytes appear normal
Map-like staining pattern with GS
Hepatocellular adenoma
Regenerative nodule
Well-differentiated hepatocellular carcinoma
DDX: focal nodular hyperplasia, well-differentiated hepatocellular carcinoma, normal liver
HCA is a clonal proliferation of benign hepatocytes that arises in noncirrhotic livers and accounts for approximately 10% of benign hepatocellular tumors. Three major subtypes are recognized, each with specific clinical, molecular, and morphologic characteristics: (1) inflammatory adenoma, (2) hepatocyte nuclear factor 1α (HNF1α)–inactivated adenoma, and (3) β-catenin–activated adenoma. The overwhelming majority of cases are seen in women of reproductive age (20–40 years), and the association between adenomas and oral contraceptive use, as well as observations of rapid tumor growth during pregnancy, suggest that sex hormones promote HCA proliferation. HCAs have also been reported in association with exogenous androgen administration, and this particular risk factor has shown an increased association with the β-catenin–activated subtype. Any adenoma greater than 5 cm is at risk of hemorrhage, but inflammatory adenomas have an increased bleeding risk at any size.
Inflammatory adenomas are defined by stimulation of signal transducer and activator of transcription 3 (STAT3), a major inflammatory response pathway component. STAT3 promotion is caused by an activating mutation in one of several pathway constituents ( IL6ST, FRK, STAT3, GNAS, JAK1 ) and these tumors are characterized by overexpression of STAT3 targets such as serum amyloid A (SAA) and C-reactive protein (CRP).
HNF1α-inactivated adenomas are characterized by loss of HNF1α function, a transcription factor involved in hepatocellular differentiation. Consequently, these lesions lack expression for downstream HNF1α targets, such as liver fatty acid binding protein (LFABP).
β-catenin–activated adenomas are defined by activation of the Wnt/β-catenin pathway, either by mutation of CTNNB1 directly or a β-catenin suppressor gene such as APC . While malignant transformation can be seen in any adenoma, it is far more common in β-catenin–activated adenomas harboring mutations in exon 3 of CTNNB1 .
HCA typically appears as a well-circumscribed tan-brown mass in a non-cirrhotic liver, usually lacking a fibrous capsule. HCA is most often a solitary lesion, although multiple can occur synchronously, and the presence of 10 or more adenomas is referred to as adenomatosis . The prototypical HCA measures between 5 and 15 cm in greatest dimension and may show foci of gross discoloration due to bile excess, hemorrhage, or necrosis.
HCAs are well-differentiated hepatocellular neoplasms characterized by normal-appearing hepatocytes with minimal cytologic atypia, arranged in trabeculae of normal thickness (1–2 cells wide). Adenomas may either blend seamlessly with the background parenchyma or compress the adjacent liver and produce a thin fibrous rim. They are identified by their lack of normal portal structures and scattered small arterioles without paired bile ducts ( Fig. 4.3 ).
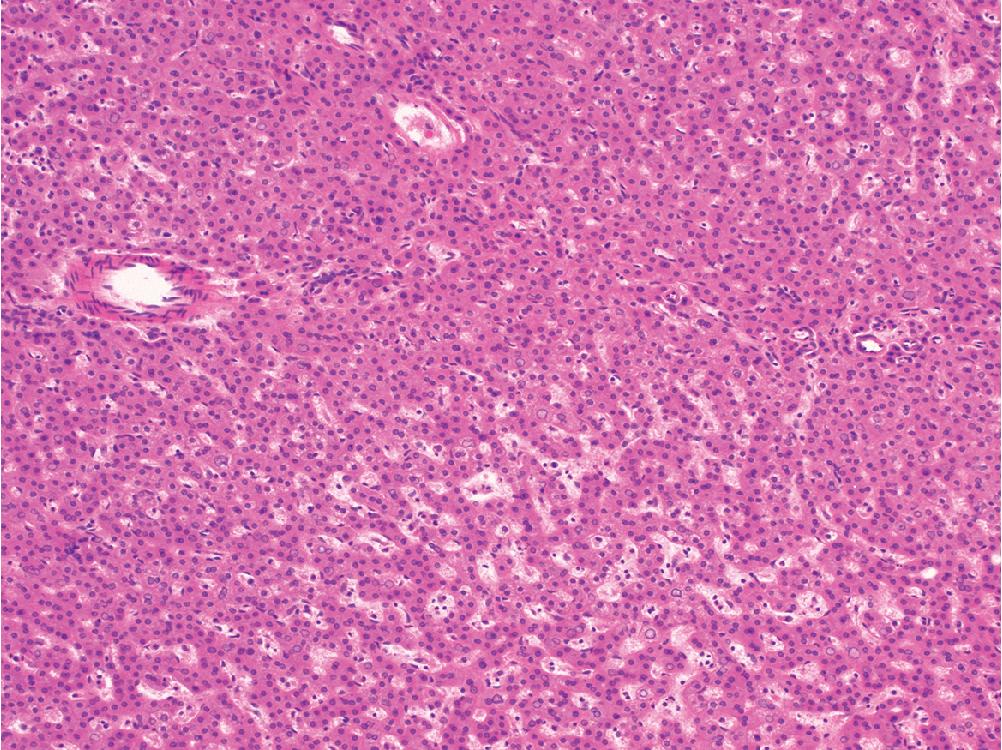
The hepatocytes in HCAs harbor minimal cytologic atypia, with relatively monomorphic nuclei, eosinophilic cytoplasm, and a nucleus-to-cytoplasm (N/C) ratio comparable with the background liver. Focally, HCAs can show mildly increased cytologic atypia as well as regenerative architectural changes that manifest as occasionally thickened or disordered cell plates. However, these changes are mild and localized. Adenomas may contain a variable amount of steatosis and sinusoidal changes, but certain morphologic features are more commonly seen in certain HCA subtypes.
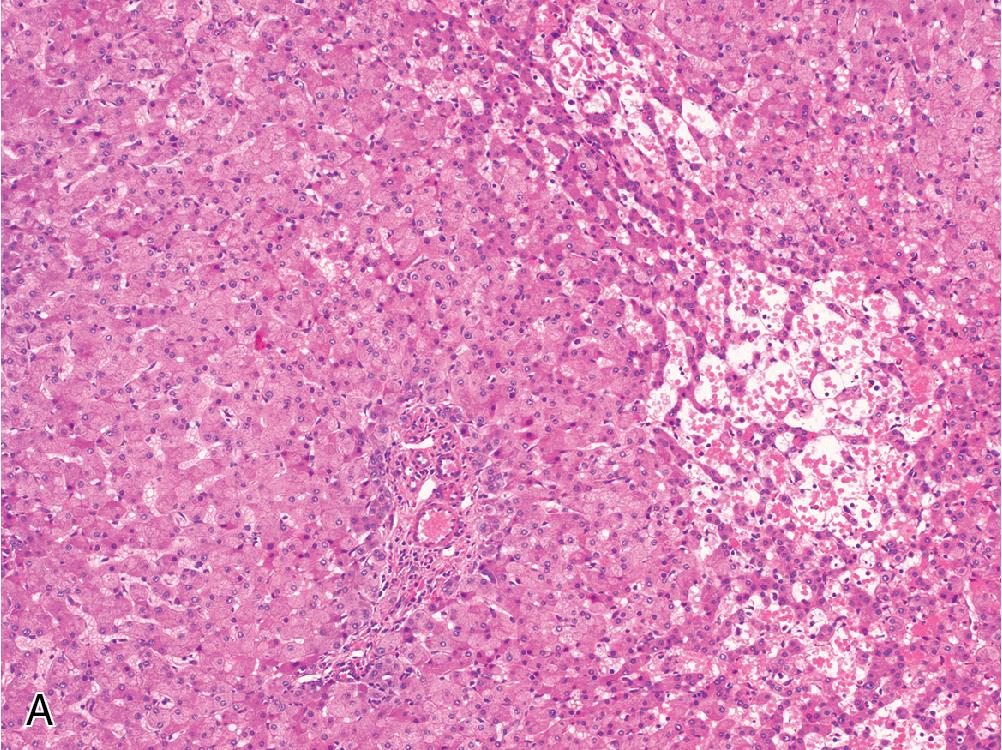
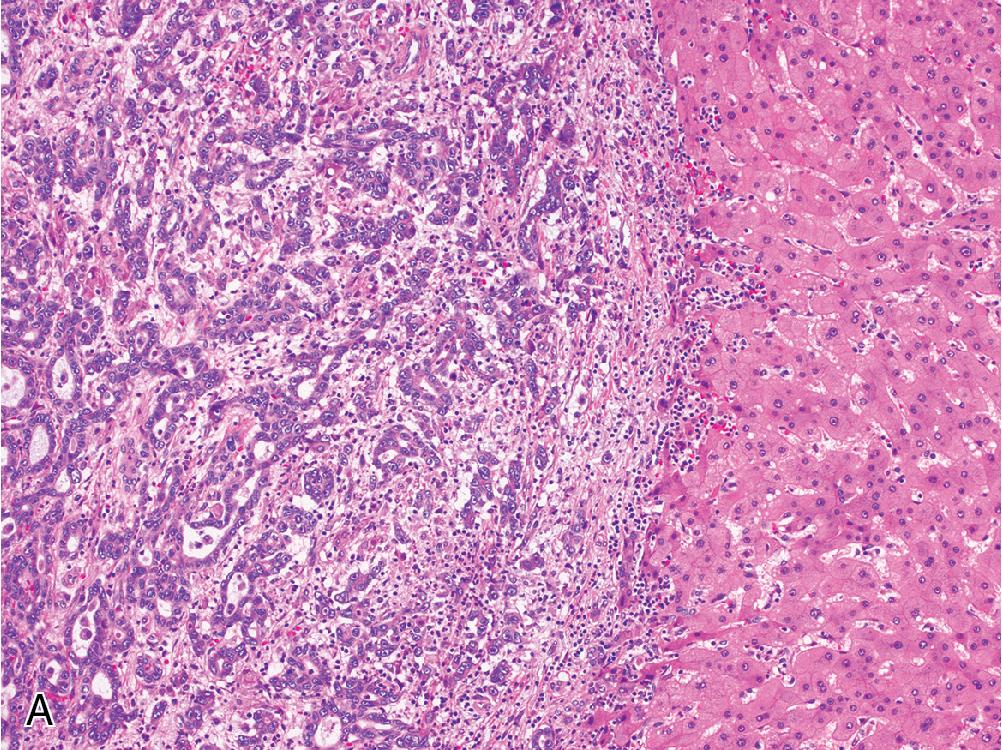
Inflammatory adenomas often contain foci of lymphocytic inflammation and areas of dilated sinusoidal spaces, which are often prominent and filled with blood ( Fig. 4.4 A), explaining their increased association with spontaneous hemorrhage if left untreated. Focal ductular reaction can be seen more often in this subtype.
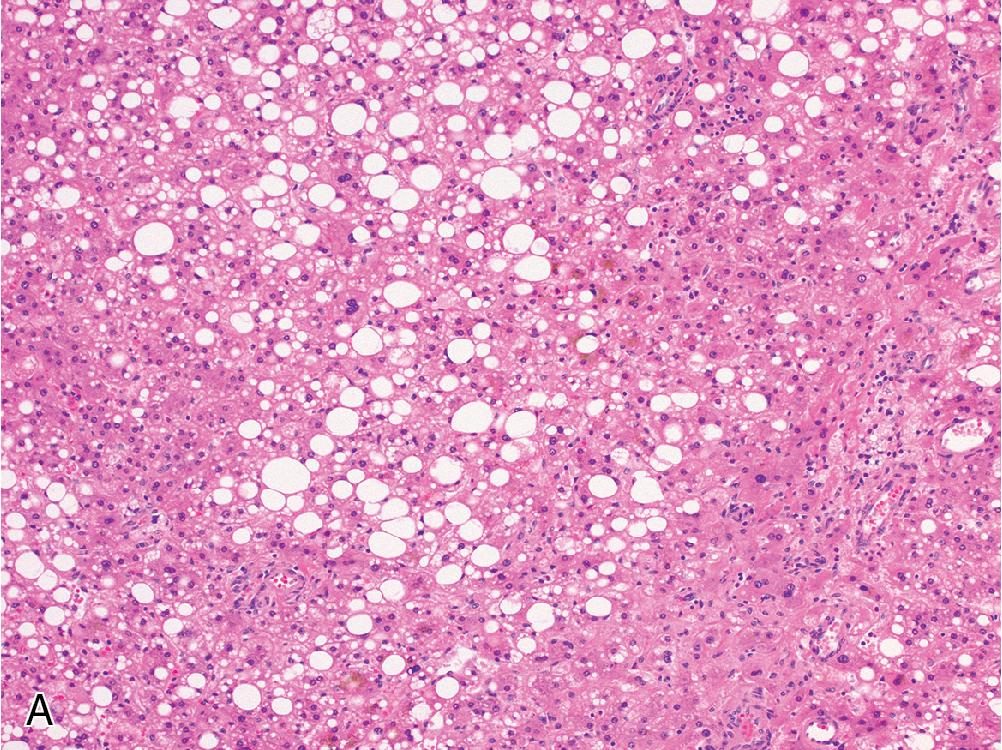
HNF1α-inactivated adenomas are classically associated with bland steatosis ( Fig. 4.5 A), which may or may not be present in the background liver. Notably, HNF1α-inactivated adenomas do not contain features of steatohepatitis such as Mallory hyaline or pericellular fibrosis.
β-catenin–activated adenomas lack any characteristic morphologic features on H&E, and hepatocytes vary from cytoarchitecturally unremarkable to mildly atypical. This diagnosis is principally made by IHC or molecular testing, and β-catenin activation can overlap with other phenotypically distinct adenoma subtypes.
Rarely, some adenomas may show features that are suspicious for, but not diagnostic of, well-differentiated HCC, such as very focal reticulin loss or increased cytologic atypia. These lesions have been called atypical adenomas and definitive diagnosis may only be possible on resection specimens.
Despite certain characteristic morphologic features, the adenoma subtypes show considerable histologic overlap and are best distinguished using either IHC or molecular testing. Adenoma markers are typically ordered as a panel, and consist of β-catenin, GS, LFABP, CRP, and SAA. The characteristic staining pattern of each subtype is summarized below.
| HCA Subtype | β-Catenin | Glutamine Synthetase | LFABP | CRP | SAA |
|---|---|---|---|---|---|
| β-Catenin activated | Nuclear localization | Diffuse expression | +/– | +/– | +/– |
| Inflammatory | – | Variable | + | + | + |
| HNF1α inactivated | – | Variable | – | +/– | +/– |
In clinical practice, the presence of β-catenin activation is critical to identify because this confers an increased risk of malignant transformation. Normal liver typically shows membranous expression of β-catenin on IHC, but CTNNB1 mutation in adenomas manifests as focal nuclear staining. While this pattern is diagnostic of β-catenin activation, it has a low sensitivity in practice due to focality of staining.
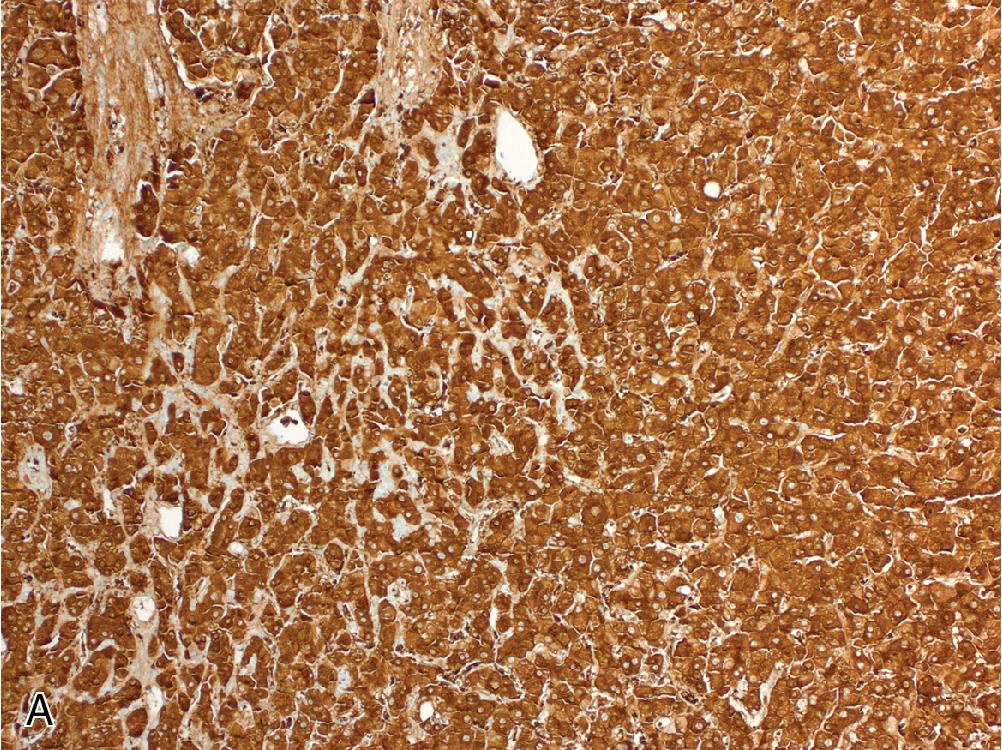
GS is an extremely useful surrogate marker, as studies have correlated diffuse expression of GS with β-catenin mutation status. Diffuse GS expression is defined as moderate-to-strong staining in more than half of tumor cells without a map-like distribution, and this is further subdivided into two groups. A pattern of diffuse, homogenous GS expression is defined as staining in more than 90% of tumor cells ( Fig. 4.6 A) and is highly correlated with β-catenin activation, often involving deletions in exon 3 of CTNNB1 . The diffuse, heterogenous pattern is defined as expression in 50% to 90% of tumor cells ( Fig. 4.6 B), and this immunophenotype shows a weaker correlation with β-catenin activation. However, either pattern of diffuse GS labeling is considered a high-risk feature. In small biopsies, however, the pattern of expression may appear abnormal yet cannot be reliably characterized. In these cases, molecular testing may be prudent.
Inflammatory adenomas are characterized by increased CRP and/or SAA expression ( Fig. 4.4 B and C). Both markers are nonspecific acute-phase reactants that may be variably expressed in the liver depending on physical state, and diagnosis is most reliable when expression patterns in the adenoma can be compared to the background liver. Diagnostic confidence increases when both CRP and SAA are positive, although SAA often demonstrates a weak cytoplasmic staining profile that is less sensitive (but more specific) than CRP.
HNF1α-inactivated adenomas will show loss of LFABP ( Fig. 4.5 B and C), a marker normally expressed at variable levels in all hepatocytes.
In some cases, especially biopsy specimens, the diagnosis of adenoma will be clear, but tumor cells are negative for β-catenin, GS, CRP, SAA, and they retain LFABP expression. These lesions are termed unclassified HCAs.
The most common complication of HCA is massive hemorrhage into the peritoneal cavity. Inflammatory adenomas have an increased association with bleeding, but any adenoma greater than 5 cm is at heightened risk of hemorrhage and is usually resected for this reason. HCAs are premalignant lesions, and the published rate of transformation ranges from 3% to 12%.
The diagnosis of a well-differentiated hepatocellular tumor requires two steps. The first step is identifying lesional tissue by the absence of normal portal structures. This is usually straightforward but can be challenging in biopsies that only contain minimal tissue and/or heavy fragmentation. IHC can be helpful in this situation, as lesional areas of the liver often exhibit abnormal endothelialization of the sinusoidal spaces that manifests by expanded CD34 expression. Normal liver sinusoids should be negative for CD34 apart from focal periportal expression, but hepatocellular lesions such as adenomas, FNH, and HCC typically show more diffuse CD34 staining that can be helpful in identifying lesional tissue in small or disrupted biopsies.
The second step is classifying the tumor, and the differential diagnosis changes depending on the status of the background liver. In a non-cirrhotic liver, the most common hepatocellular lesions are HCA and FNH. Well-differentiated HCC can also arise in non-cirrhotic livers and may recapitulate the bland cytomorphology and largely intact architecture seen in HCA.
Both HCA and FNH are composed of bland hepatocytes without cytologic atypia. The hepatic plate architecture may show focal regenerative changes in either lesion, but the reticulin framework remains intact. FNH characteristically shows a central scar with radiating bands of fibrosis and accompanying ductular reaction that yields a nodular structure. Conversely, the typical HCA lacks a central scar, nodular fibrosis, and ductular reaction. Instead, HCA contains small, thin-walled, unpaired arterioles in the lobule without corresponding veins and ducts. These morphologic features can show some degree of overlap, but the diagnosis is usually clear on large resection specimens.
In practice, however, this diagnosis is usually rendered on needle core biopsies, where features may not be well-developed and the diagnosis can be challenging. In these cases, IHC for GS is essential. GS expression in normal liver highlights only a thin rim of perivenular hepatocytes in zone 3, but this pattern changes in lesional tissue. FNH demonstrates a characteristic “map-like” staining pattern. In contrast, HCA can exhibit multiple staining patterns: entirely negative, diffusely positive with either a homogenous or heterogenous distribution, or indeterminate expression that is neither map-like nor indicative of definite β-catenin mutation. The combination of morphologic features and GS expression pattern is usually sufficient to separate FNH from HCA.
The diagnosis of HCC primarily relies on H&E features and reticulin integrity, and all adenoma subtypes harbor risk of malignant transformation ( Fig. 4.7 ). In this differential, the status of the reticulin framework is essential to diagnosis. Adenomas exhibit an intact reticulin network, while well-differentiated HCC will show at least focal disruption. However, the presence of fat can complicate reticulin evaluation, as macrosteatosis disrupts the framework and causes reticulin fragmentation even in non-neoplastic tissue. Given that steatosis can be seen in adenomas, especially the HNF1α-inactivated subtype, the reticulin framework may not always be easily evaluated. In problematic cases, glypian-3 IHC may help distinguish these entities. Adenomas are negative for this marker, but HCC may be positive (see Hepatocellular Carcinoma section). In addition, features of steatohepatitis within a tumor, such as ballooning degeneration, Mallory hyaline, and pericellular fibrosis, are indicative of the steatohepatitic variant of HCC and should not be mistaken for an adenoma, which only contain bland steatosis.
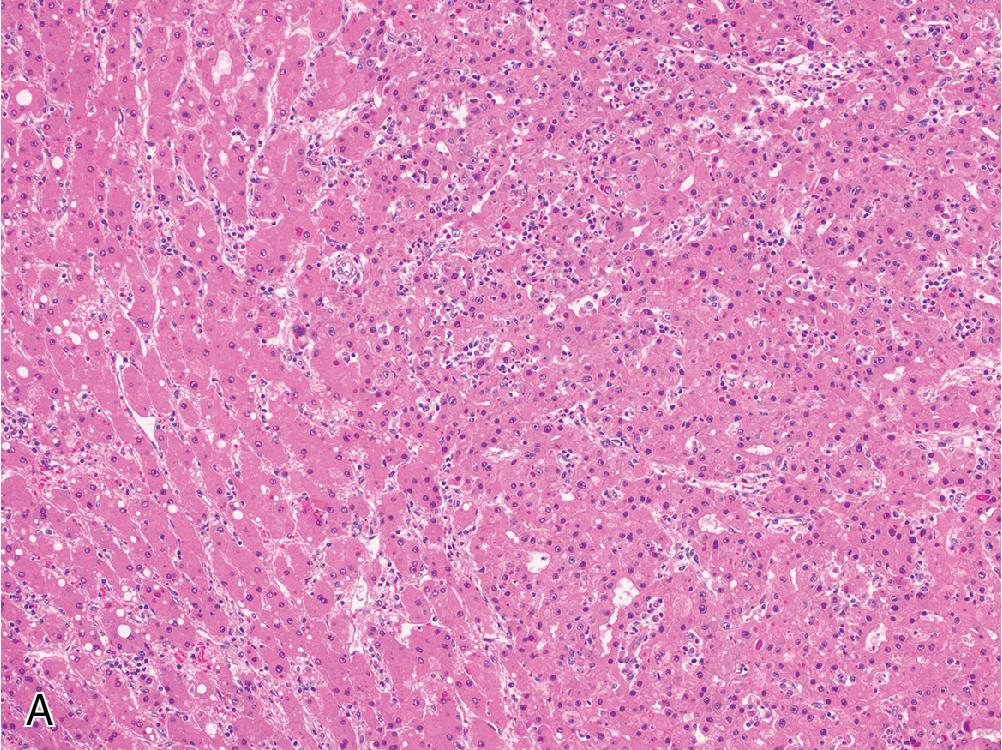
Importantly, adenomas should only be subtyped after a diagnosis of HCA is rendered, as the stains involved in subtyping are not diagnostically helpful and provide no additional insight without a clear diagnosis of HCA. For instance, while LFABP expression is lost in HNF1α-inactivated adenomas, its expression may also be absent in HCC.
Clonal proliferation of benign hepatocytes
Account for 10% of benign hepatocellular tumors
Mostly occur in women, rare in men
Can be seen in children with glycogen storage disease
20–40 years
Most patients have history of oral contraceptive use
Also associated with exogenous androgen use (β-catenin mutant subtype)
Presents incidentally or with right upper quadrant pain
Usually solitary, but can be multiple (adenomatosis if > 10 lesions)
Vascular lesion with irregular enhancement on CT
Well-defined mass on MRI, low signal on T1-weighted images
Overall malignant transformation rate ranges from 3% to 12%
β-catenin–activated subtype has increased risk of malignant transformation
Large size (>5 cm) and inflammatory subtype are risk factors for clinically significant hemorrhage
Circumscribed, usually non-encapsulated tumors
Arise in non-cirrhotic livers
May be hemorrhagic
Identified by lack of portal tracts and thin-walled arterioles within tumor
Benign hepatocytes without appreciable atypia
No thickened hepatic plates or loss of reticulin
Divided into four subtypes:
Inflammatory subtype: scattered lymphocytic inflammation, dilated sinusoidal spaces with or without congestion, may have focal ductular reaction. Positive for CRP and SAA.
HNF1α-inactivated subtype: usually contains bland steatosis. Shows loss of LFABP.
β-catenin–activated subtype: no distinct morphology. Shows nuclear β-catenin staining and/or diffuse GS expression.
Unclassified subtype: does not fit any specific morphologic pattern or immunophenotype
Focal nodular hyperplasia
Well-differentiated hepatocellular carcinoma
Normal liver
DDX: hepatocellular adenoma, regenerative nodule, cholangiocarcinoma, neuroendocrine tumor
HCC is the most common primary liver cancer in adults, accounting for more than 75% of malignant hepatic tumors. It represents a complex and heterogenous cancer linked to behavioral factors, environmental exposures, genetic disorders, and chronic liver disease. Approximately 60% to 90% of HCC arises in cirrhotic livers, and the presenting symptoms typically reflect the patient’s underlying liver disease: upper abdominal pain, weight loss, jaundice, ascites, and hemorrhage from esophageal varices, among others.
Cirrhosis of any cause is a major risk factor for HCC. As the primary agent of chronic hepatitis worldwide, hepatitis B and hepatitis C infections can be attributed to most cases of HCC globally. The mechanism of carcinogenesis is believed to be a combination of direct genomic changes due to viral insertion, oncogenic viral proteins, and a continuous cycle of injury and regeneration over many years. In addition to viral infection, exposure to aflatoxin, a fungal toxin produced by the Aspergillus family, is another significant risk factor due to relatively high rates of food contamination in certain parts of the world. Iron accumulation has been associated with HCC as well, and this seems to hold true whether iron overload is secondary to hereditary hemochromatosis or merely a consequence of cirrhosis.
Several other risk factors are not directly carcinogenic but do represent leading causes of cirrhosis and are therefore indirectly associated with HCC development. Alcohol consumption and metabolic syndrome (diabetes, obesity, etc.) represent some of the most common risk factors for cirrhosis in the United States and Europe, and the annual risk of developing HCC once cirrhotic is estimated at 3%. In addition to hereditary hemochromatosis, other inherited metabolic disorders, such as α1-antitrypsin deficiency, show a stronger association with HCC development beyond the risk conferred by cirrhosis alone.
Rates of incidence and mortality show a striking geographic diversity. While HCC represents the third leading cause of cancer mortality worldwide, HCC is far more common in Southeastern Asia and sub-Saharan Africa than in the United States and Europe. This is believed to largely reflect the geographic distribution of viral hepatitis and aflatoxin contamination, both of which are significant risk factors.
In areas with a lower incidence of HCC, such as the United States and Europe, most tumors occur between 60 and 70 years of age. In higher-incidence regions like sub-Saharan Africa, peak incidence is seen in the 20s and 30s. Regardless of the geographic region, HCC arises more often in men with a ratio between 2:1 and 5:1.
Most HCC is diagnosed radiographically by CT or MRI. Criteria proposed by the American Association for the Study of Liver Diseases state that tumors larger than 1 cm in a cirrhotic background do not require biopsy confirmation, so long as characteristic imaging features are present. A standardized system of reporting HCC risk has been developed, termed Liver Imaging Reporting and Data Systems (LI-RADS), which summarizes imaging findings into five categories ranging from definitely benign (LR-1) to definitely malignant (LR-5).
In addition to imaging modalities, serum alpha fetoprotein (AFP) is a helpful biomarker used to track the development of HCC. Although its sensitivity is somewhat limited, measuring the serum AFP level can be a useful screen in patients at high risk for developing HCC, and for monitoring recurrence in patients who have undergone treatment.
Macroscopically, HCC can present as a single mass (with or without adjacent satellite nodules), multiple distinct masses, or rarely as the so-called “cirrhosis-like” pattern with diffuse and innumerable tumor nodules that mimic cirrhosis. HCC within a cirrhotic background is usually well-demarcated and wreathed by a dense fibrous capsule due to atrophy and fibrosis of the surrounding parenchyma, secondary to mass effect. HCC arising in a non-cirrhotic liver may instead appear diffusely infiltrative, with ill-defined borders. Most HCC are soft masses that bulge outward from their cut surface. Exceptions to this include fibrolamellar or scirrhous variants, which appear firm and densely fibrous. Tumor size is quite variable, ranging from around 1 cm to larger than 30 cm. HCC is typically tan, yellow, or green if bile-stained. Necrosis can be seen, either from a large tumor outgrowing its blood supply or secondary to preoperative treatment. Involvement of the portal or hepatic vein is not uncommon in advanced disease.
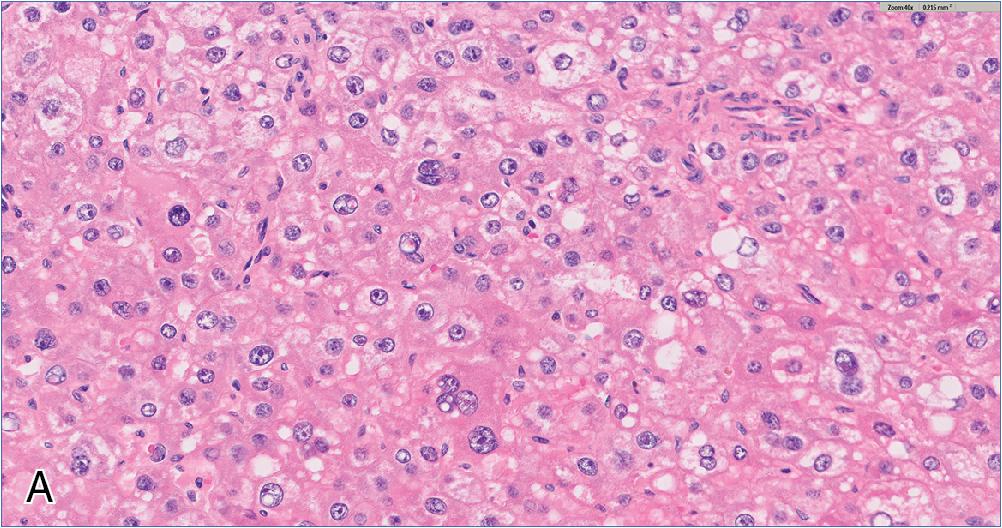
The microscopic appearance of HCC is quite variable, and any given tumor may contain multiple clonal populations exhibiting different morphologic phenotypes. Four primary architectural patterns have been described and correlated with tumor behavior. The most common growth pattern is trabecular , which recapitulates the normal arrangement of hepatic plates, but with expansion to more than 3 cells in width ( Fig. 4.8 A). These trabeculae remain separated by modified sinusoidal spaces and do not contain intervening stroma. Often, the bile canaliculi can dilate to form vague acinar or pseudoglandular structures, which may contain bile plugs or central debris ( Fig. 4.8 B). The solid growth pattern is associated with more aggressive HCC and is characterized by obliteration of the normal sinusoidal channels to form a sheet-like tumor mass ( Fig. 4.9 A). Macrotrabecular growth is another architectural pattern associated with more aggressive tumor behavior and is defined as widely expanded trabecular plates greater than 6 cells thick ( Fig. 4.9 B). In general, the trabecular and pseudoglandular patterns are the most common tumor architecture, while solid and macrotrabecular patterns are considered evidence of poorer differentiation and have been associated with more aggressive tumor behavior. Other specialized growth patterns include fibrolamellar, steatohepatitic, scirrhous, sarcomatoid, and lymphoepithelioma-like, which are described later.
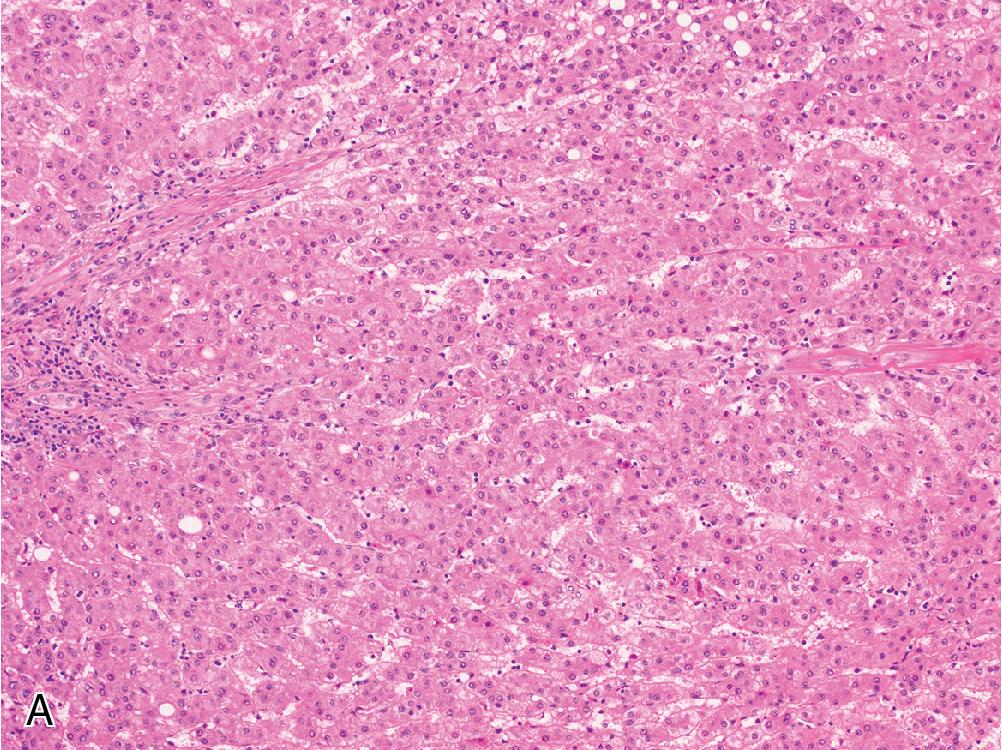
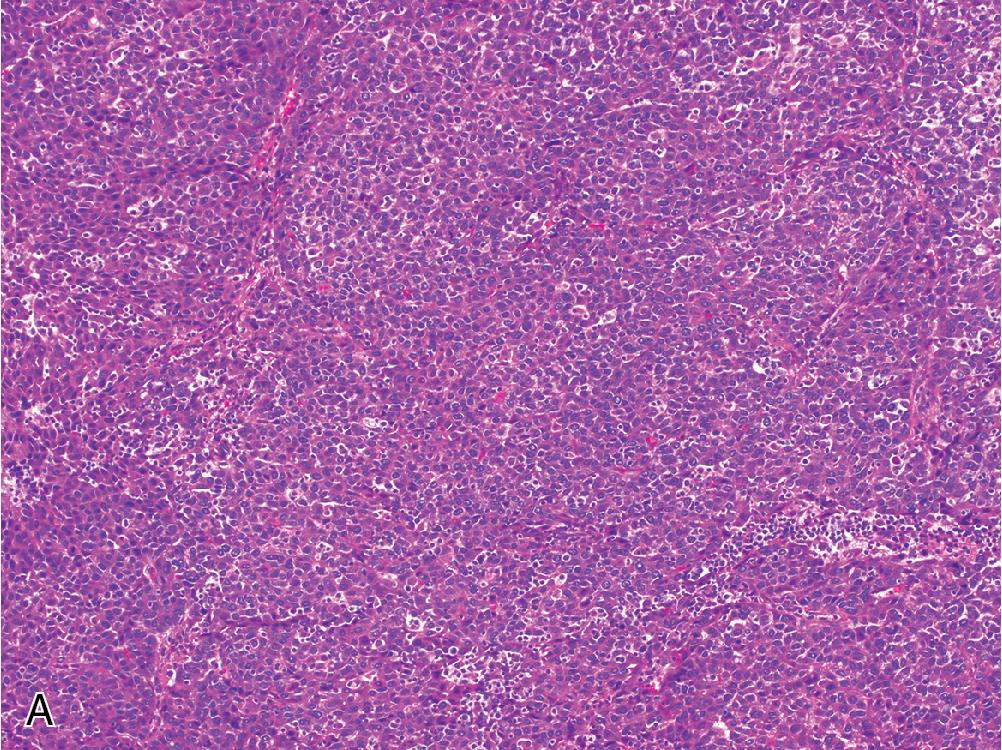
Cytologically, a well-differentiated HCC resembles non-neoplastic hepatocytes, with abundant granular, eosinophilic cytoplasm and round-to-ovoid nuclei without significant pleomorphism. There may be a slightly increased N/C ratio compared with the background liver. Macronucleoli, mitotic activity, and nuclear hyperchromasia are variably present. As HCC becomes more poorly differentiated, its overall appearance becomes less hepatoid. This manifests as a shift from eosinophilic to more amphophilic or basophilic cytoplasm, an increase in the N/C ratio toward and beyond a 1:1 proportion, and prominent nuclear pleomorphism. Additional non-specific histologic changes can be seen occasionally, including clear cell features and multinucleated giant cells. The presence of bile or Mallory hyaline can be helpful evidence of hepatocellular derivation. While a few studies have delineated specific cytoarchitectural criteria for assigning HCC tumor grade, current WHO guidelines advocate for a synthesis of cytologic and architectural features along the aforementioned spectrum.
Special staining for reticulin is a tremendously helpful way to assess the hepatic plate architecture, and it may be diagnostically critical in well-differentiated tumors. In most HCC, the reticulin framework is at least focally fragmented or lost, and this architectural disruption may not always be apparent on H&E sections. Some very well-differentiated HCC will retain an intact reticulin framework, but the stain can also help confirm diffuse expansion of the hepatic plates, bizarre architecture, and in some tumors, the reticulin framework may show an abnormal encasement of single cells or small clusters of cells in a way that is not typical of benign lesions. In fatty lesions, however, reticulin staining becomes unreliable, as disruption of the normal framework typically occurs secondary to steatosis.
Several distinct histologic variants of HCC are described below, and awareness of these variants is important to prevent misdiagnosis.
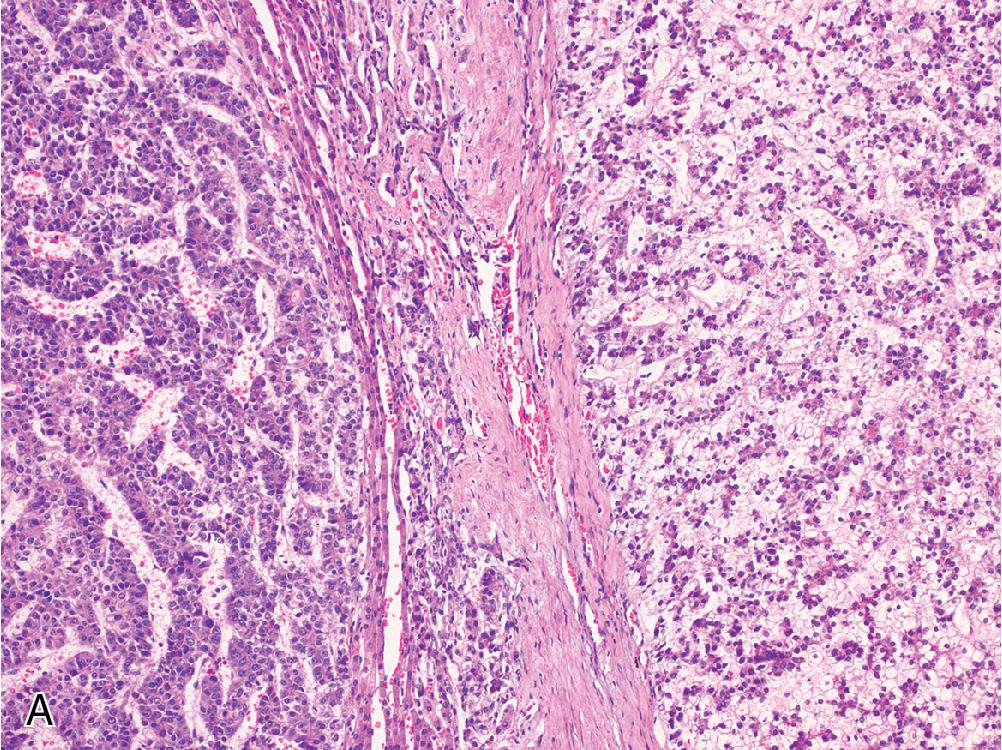
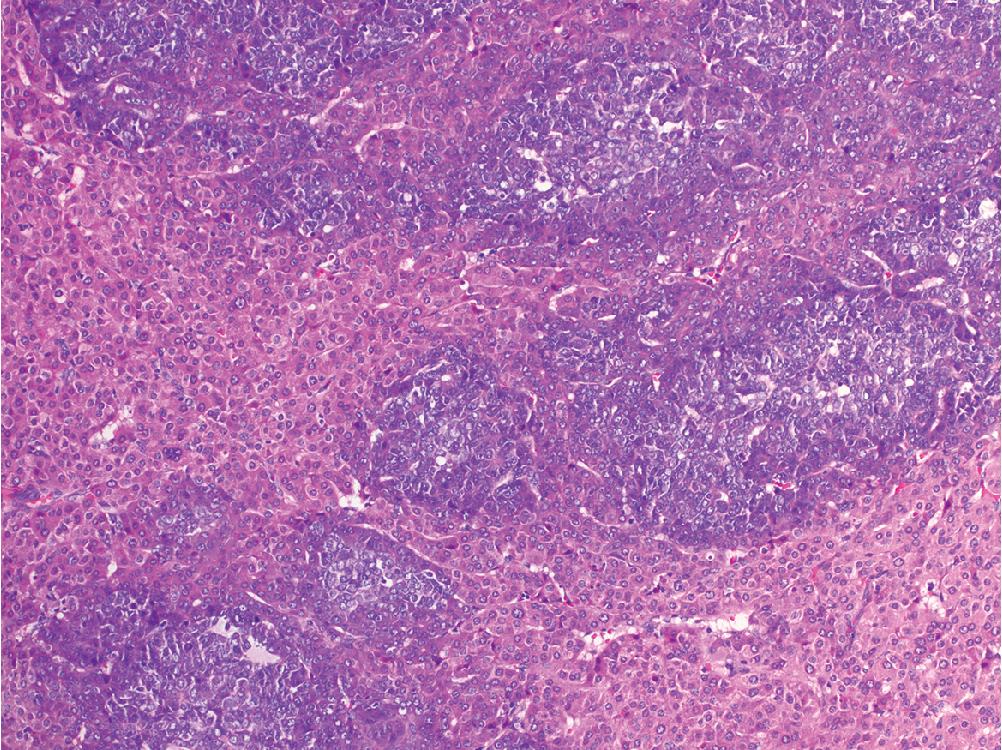
Scirrhous hepatocellular carcinoma describes a subset of HCC that contains nests of tumor set within prominent fibrotic stroma that comprises a significant proportion of the total tumor mass ( Fig. 4.10 ). Scirrhous HCC tends to occur in subcapsular areas, and some reports indicate a higher rate of K7 and K19 expression compared with typical HCC, although most will also express arginase and glyican-3. The scirrhous variant is associated with worse outcome than traditional HCC.
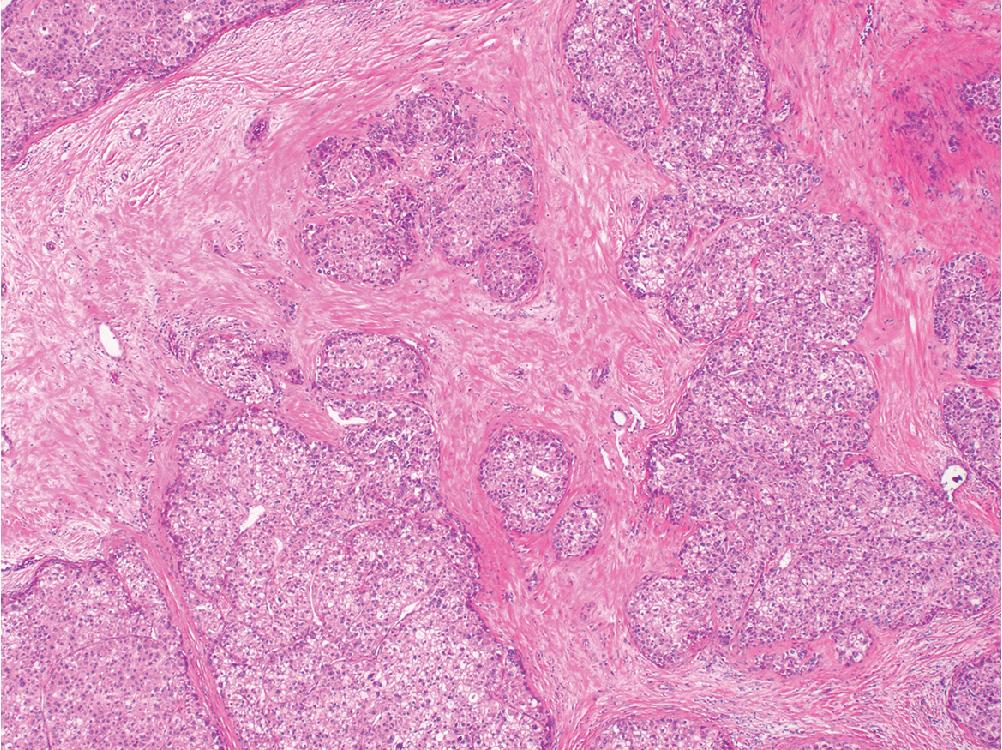
Fibrolamellar hepatocellular carcinoma is a rare tumor distinct from other forms of HCC. It occurs in young adults without cirrhosis and is characterized by a somatic deletion that yields a characteristic DNAJB1-PRKACA fusion transcript, which can be detected by RT-PCR and FISH assays. Histologically, the fibrolamellar variant shows parallel bands of collagen-rich fibrosis infiltrating and separating tumor nests ( Fig. 4.11 A). The tumor cells are large and polygonal, with abundant eosinophilic cytoplasm that often contain subtle cytoplasmic inclusions known as “pale bodies.” Tumor nuclei are rounded and contain a single prominent macronucleoli. Hepatocellular markers are positive, but this HCC variant differs in that it is nearly always positive for K7 and CD68 as well ( Fig. 4.11 B and C). While early studies suggested a favorable outcome compared with traditional HCC, recent evidence has shown that outcomes are in fact similar, and that fibrolamellar HCC is an aggressive tumor with a greater propensity for early nodal spread.
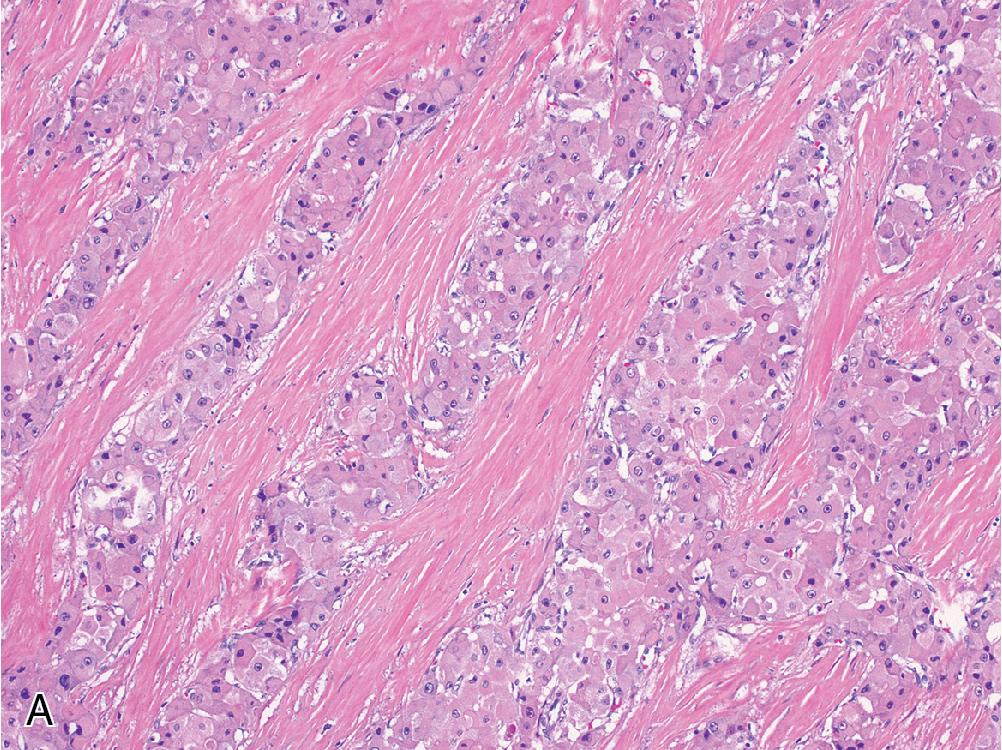
Steatohepatitic hepatocellular carcinoma is a relatively common variant of HCC that recapitulates the prototypical features of steatohepatitis within HCC, including steatosis, ballooning degeneration, prominent Mallory hyaline inclusion, and pericellular fibrosis ( Fig. 4.12 ). This form of HCC can occur even in the absence of background steatohepatitis or any associated risk factors. Outcome is similar to typical HCC.
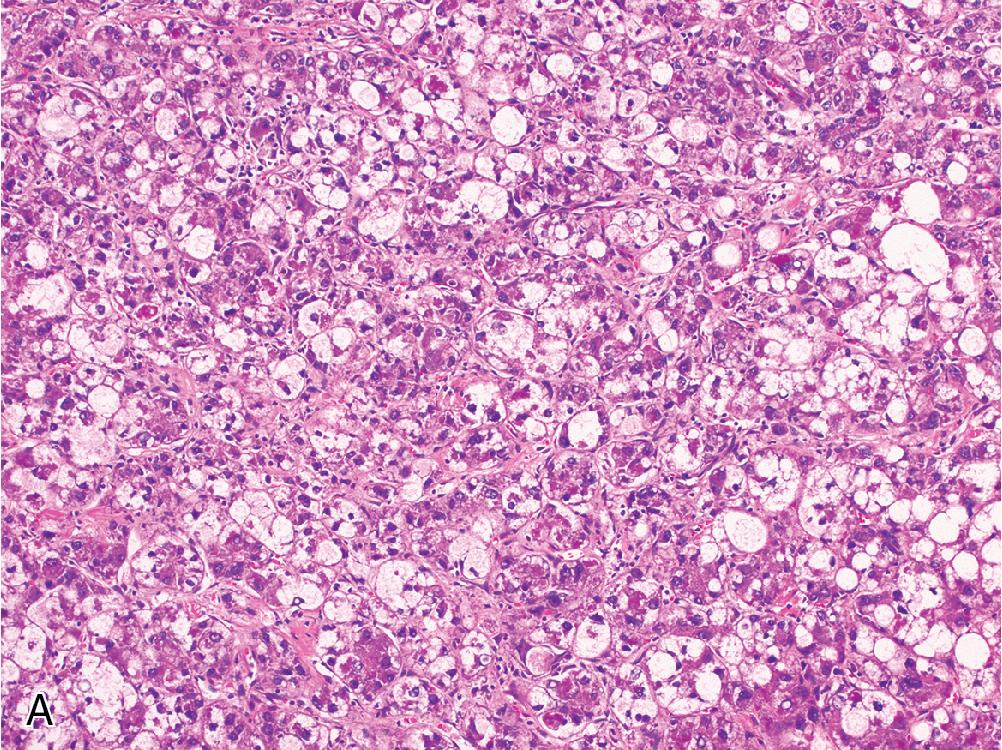
Lymphocyte-rich hepatocellular carcinoma is a rare HCC variant characterized by a prominent lymphoid infiltrate ( Fig. 4.13 ). Some cases can superficially resemble a nasopharyngeal carcinoma, but Epstein-Barr virus testing is nearly always negative. The hepatocellular component is usually well-to-moderately differentiated. Tumors with a more poorly differentiated hepatocellular component alongside a dense lymphoinflammatory infiltrate have been called lymphoepithelioma-like hepatocellular carcinoma .
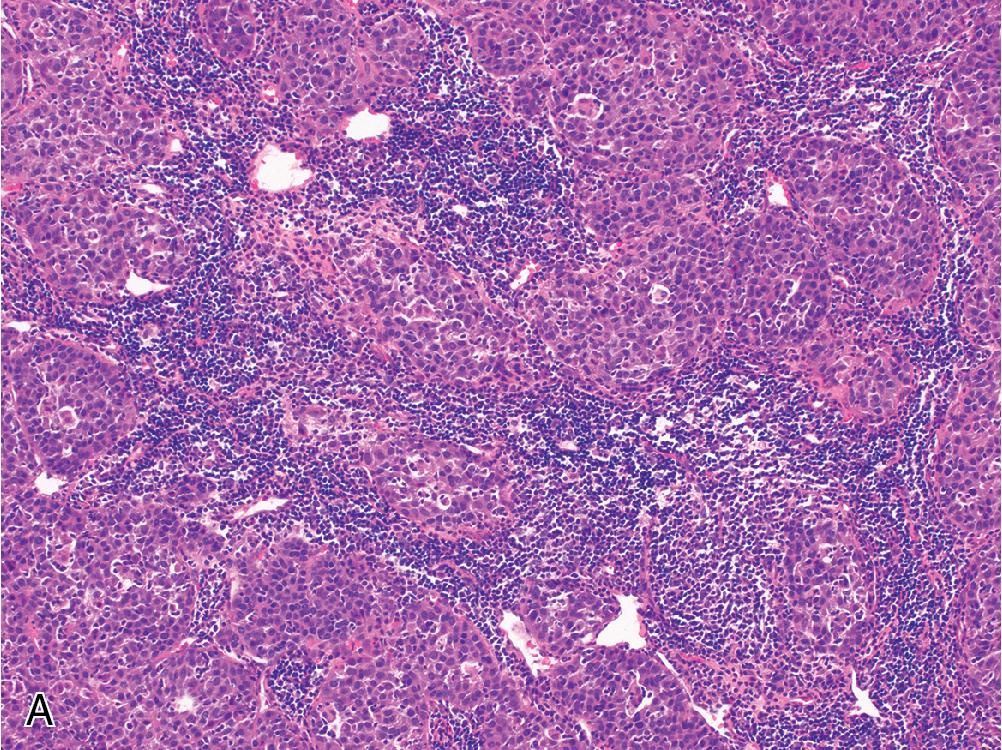
Sarcomatoid hepatocellular carcinoma describes a very rare HCC subtype that features sarcoma-like foci, often alongside more classical HCC. The sarcomatous component consists of malignant spindle and epithelioid cells that often express keratins, but rarely stain for hepatocellular markers. Heterologous elements may be present. Sarcomatoid changes can be seen following chemotherapy or embolization. In the absence of a recognizable HCC component, this diagnosis can be extremely difficult, and these tumors have a worse prognosis than typical forms of HCC.
Combined hepatocellular-cholangiocarcinoma (CHC) is defined as a tumor containing unequivocal and mixed elements of both HCC and cholangiocarcinoma. This entity is discussed in the cholangiocarcinoma section.
Several immunohistochemical markers are available to confirm hepatocellular origin, although most do not distinguish benign from malignant hepatocytes. Arginase and HepPar1 are both highly sensitive and specific hepatocyte markers. Both tend to be quite useful in confirming hepatocellular lineage in well-to-moderately differentiated HCC, but they tend to exhibit less reliable staining in poorly differentiated HCC. Glypican-3 shows the opposite pattern, with increased utility in poorly differentiated HCC and less reliable staining in more well-differentiated tumors ( Fig. 4.14 ). Glypican-3 also differs from the other two markers in that it is not specific to the liver. However, it is also not expressed in benign hepatocellular lesions like FNH and HCA. Albumin in situ hybridization is a new and fairly specific marker of hepatic origin that is positive in liver tumors of either hepatocytic or cholangiocytic derivation, but this marker is not widely available at present.
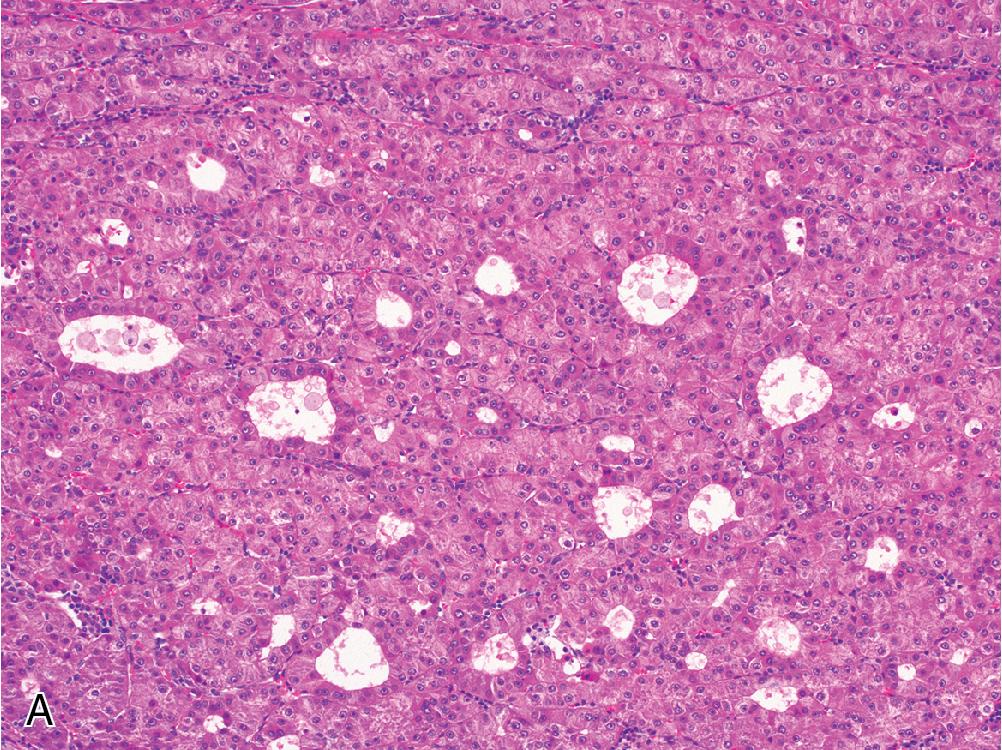
Less useful markers include polyclonal carcinoembryonic antigen (CEA), which demonstrates a characteristic canalicular staining pattern in normal and neoplastic hepatocytes. However, this stain is unreliable in more poorly differentiated HCC, and the distinction between canalicular and membranous staining can sometimes be challenging to interpret. CD10 and villin are similar stains with similar drawbacks. AFP is an oncofetal protein expressed in hepatocellular and germ cell tumors, but its use as a marker of HCC is limited by poor sensitivity.
In general, keratin expression profiles are not useful in HCC. Most will stain with K8, K18, and CAM5.2. A minority of tumors will also express K7 and/or K19, which some research has associated with a less favorable prognosis.
Overall, the median survival after diagnosis lies between 10 and 18 months, and the primary prognostic determinant is tumor resectability. Surgical resection is the only cure, and liver transplantation may be offered to patients who meet certain radiographic criteria regarding tumor size and extent (e.g., Milan criteria and others). Successful surgical resection or transplantation has a 5-year survival up to 80%, although more than half of patients do experience tumor recurrence. Imaging-guided ablation and transarterial embolization are other common treatment modalities, used as definitive therapy for small lesions or as bridging therapy in patients awaiting resection or transplantation.
Favorable prognostic factors include well-to-moderate tumor differentiation, low serum AFP, absence of vascular invasion, patient age less than 50 years, female sex, and lack of background cirrhosis.
The differential diagnosis of HCC is broad and varies with morphology. In most cases of well-differentiated HCC, the tumor will retain a clearly hepatoid appearance and the main considerations will include other hepatocellular lesions, notably HCA. If the background liver is cirrhotic, a diagnosis of HCA is highly unlikely, but HCC also arises de novo in non-cirrhotic livers and through malignant transformation of an existing adenoma. In these situations, the distinction is primarily based on H&E evaluation. Both lesions lack portal structures and may contain unpaired arterioles, but the hepatocytes of HCA are generally bland, without notable cytologic atypia or mitotic activity. Well-differentiated HCC can pose a diagnostic challenge, as cytologic features may be similarly bland, so demonstration of reticulin framework disruption/loss is often critical to making this diagnosis. IHC is of marginal utility. Markers of hepatocellular differentiation (arginase, HepPar1) will be positive in both. However, glypican-3 is not expressed in adenomas and can potentially help distinguish these two entities. In practice, however, glypican-3 is less frequently positive in well-differentiated HCC, so morphologic features and reticulin integrity are often more diagnostically useful.
Moderately differentiated HCC is not typically confused with benign hepatocellular tumors due to more obvious cytoarchitectural atypia, but poorly differentiated HCC may not appear overtly hepatoid and can resemble other cancers. HCC with clear cell features can mimic clear cell renal cell carcinoma (RCC) or adrenocortical carcinoma. IHC for hepatocellular markers, PAX8, inhibin, and calretinin will distinguish these entities. Epithelioid-predominant angiomyolipoma (AML) can appear hepatoid, but these lack expression of hepatocellular markers and instead label with HMB-45 and smooth muscle actin (SMA). Epithelioid gastrointestinal stromal tumors can also mimic HCC, but these tumors show diffuse expression of CD117 and/or DOG-1 without labeling by hepatocellular markers. Neuroendocrine tumors commonly metastasize to the liver and can resemble a poorly differentiated HCC with a high N/C ratio; some even exhibit clear cell features, which can complicate the H&E interpretation. However, these strongly express neuroendocrine markers (synaptophysin, chromogranin, insulinoma-associated protein 1 [INSM1]) and are negative for arginase and HepPar1.
Cholangiocarcinoma can sometimes appear as hepatoid tumor nests, and the pseudoglandular structures of HCC may mimic an adenocarcinoma. The presence of intraluminal mucin supports a diagnosis of cholangiocarcinoma (or metastatic adenocarcinoma), and strong K7, K19, and MOC-31 expression is characteristic of cholangiocarcinoma. While some HCCs will also express these keratin markers, MOC-31 staining is not typically seen in HCC, and cholangiocarcinoma lacks arginase and HepPar1 labeling. Importantly, the diagnosis of CHC can only be made in the context of two separate morphologic patterns and not solely on the basis of a biphasic immunophenotype (see Cholangiocarcinoma section).
Finally, tumor growth patterns typically differ between HCC, cholangiocarcinoma, and metastatic tumors. While HCC often exhibits a pushing border, cholangiocarcinoma and metastatic lesions are generally more infiltrative and accompanied by a densely fibrous or desmoplastic stroma. However, certain forms of HCC are also embedded within dense background fibrosis, such as the fibrolamellar and scirrhous variants, and immunophenotype can be helpful if the morphology is unclear.
Most common malignant primary liver tumor
Carcinoma derived from hepatocytes
Incidence varies widely with the geographic distribution of viral hepatitis and aflatoxin
Southeast Asia and sub-Saharan Africa have highest incidence and mortality
Age of incidence varies geographically
In the United States and Europe, mean age is 60–70 years
In Southeast Asia and sub-Saharan Africa, mean age is 20–40 years
Most arise in cirrhotic livers (70%–90%)
Cirrhosis of any cause is a risk factor and confers worse prognosis
Additional risk factors include HBV, HCV, aflatoxin, metabolic disorders
LI-RADS criteria used to confer risk of malignancy
In cirrhotic livers with imaging characteristics of HCC, no biopsy is indicated
Only cure is surgical resection or transplantation
Survival measured in months if left untreated, but 5-year survival up to 80% if successfully resected
Embolization or ablation therapies used to treat small tumors or as bridging therapy in larger tumors before surgery
Soft tumor of varying size, may contain hemorrhage or necrosis
May be encapsulated or have infiltrative edges
Rare “cirrhosis-like” pattern shows diffuse small nodules of HCC
Characterized by expanded hepatic plates, fragmented or abnormal reticulin staining
Bile sometimes seen in well-differentiated HCC
Well-differentiated features include: trabecular or pseudoglandular architecture, eosinophilic cytoplasm, low N/C ratio, minimal nuclear pleomorphism
Poorly differentiated features include: solid or macrotrabecular architecture, amphophilic/basophilic cytoplasm, increased N/C ratio, prominent nuclear pleomorphism
Notable variants: fibrolamellar, scirrhous, steatohepatitic, sarcomatoid, lymphocyte-rich
Hepatocellular adenoma
Regenerative nodule
Cholangiocarcinoma
Neuroendocrine tumor
Renal cell carcinoma
Adrenocortical carcinoma
DDX: hepatocellular carcinoma, pediatric small round blue cell tumors, germ cell tumor, normal liver
Hepatoblastoma is the most common liver tumor in children. Most occur before age 5, and the majority of patients are male. Presenting symptoms typically include abdominal swelling with occasional pain, weight loss, nausea, vomiting, and/or jaundice. Low birth weight confers a higher risk of developing hepatoblastoma, although the mechanism behind this is unclear. Most patients will have a markedly elevated serum AFP level.
Approximately 5% of patients also report concurrent congenital anomalies, and hepatoblastoma is associated with various disorders such as familial adenomatous polyposis, Li-Fraumeni syndrome, Beckwith-Wiedemann syndrome, trisomy 18, and others. Studies have shown mutations in the Wnt/β-catenin signal transduction pathway in more than 80% of hepatoblastomas, and CTNNB1 is mutated in more than half.
Most hepatoblastomas present as a single, well-circumscribed mass that can grow over 20 cm, but they are occasionally multifocal. The background liver parenchyma is normal. The tumor’s macroscopic appearance depends on its histologic makeup, but a purely epithelial hepatoblastoma will appear as a soft, tan-white mass. In tumors with a prominent mesenchymal component, cystic degeneration with possible necrosis, hemorrhage, and/or calcification can be seen.
Hepatoblastoma mimics the histologic appearance of the embryonal liver. Broadly, these tumors are categorized as either purely epithelial (70% of all hepatoblastomas) or mixed epithelial and mesenchymal patterns (30%). The epithelial pattern is divided into several histologic subtypes.
The fetal pattern is the most common epithelial subtype, seen alone or in combination with other patterns in the vast majority of hepatoblastomas. Fetal morphology is characterized by thin trabeculae or sheets of polygonal cells that resemble immature hepatocytes, with round nuclei, prominent nucleoli, and variably distributed eosinophilic and clear cytoplasm that imparts a distinctive alternating “light and dark” appearance at low magnification ( Fig. 4.15 ). Extramedullary hematopoiesis is often seen. In most cases, the fetal pattern exhibits a low proliferative rate, but occasional examples of high mitotic activity are seen (defined as >2 mitoses [MF] per 10 high-power fields [HPFs]).
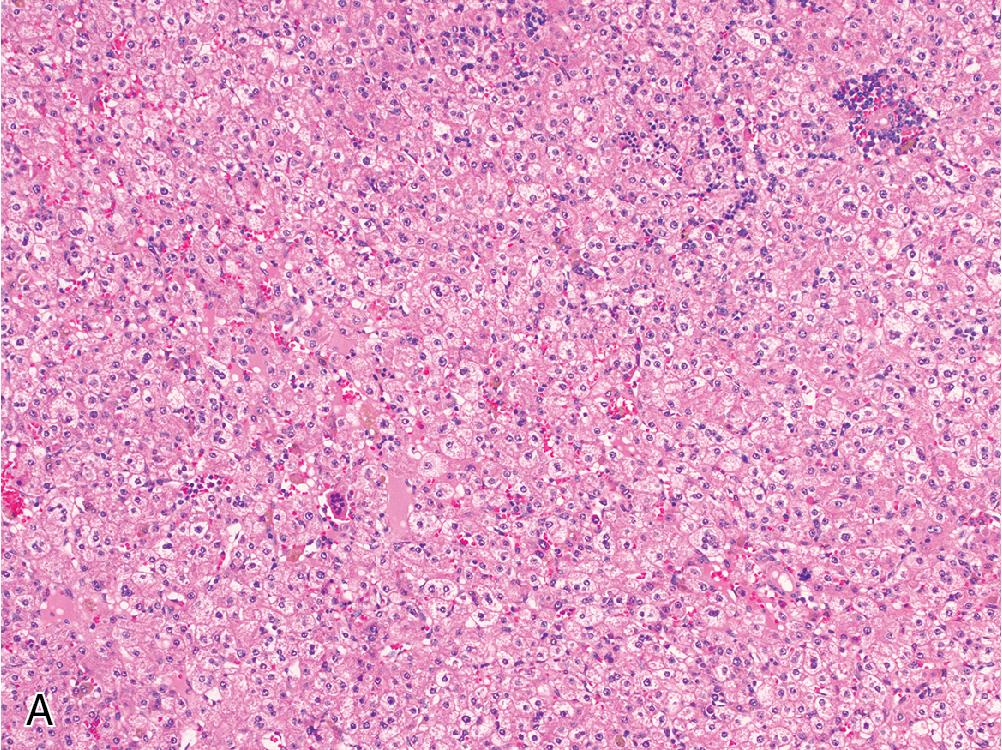
The embryonal pattern is seen in about 30% of tumors and is nearly always admixed with fetal morphology. This pattern is defined by sheets or clusters of small, hyperchromatic cells with a high N/C ratio, prominent nucleoli, and more brisk mitotic activity than the fetal form ( Fig. 4.16 ). Extramedullary hematopoiesis is typically more prominent than in the fetal pattern.
The pleomorphic pattern is uncommon and often resembles adult HCC more so than the primitive morphology seen in other hepatoblastomas subtypes ( Fig. 4.17 A). This pattern exhibits more pronounced nuclear atypia than the previous growth patterns.
The macrotrabecular pattern is a rare architectural variant defined by the presence of prominently widened trabeculae (>10 cells in thickness) and can be composed of cells showing either fetal or embryonal morphology ( Fig. 4.17 B). This appearance can also be mistaken for HCC but is usually mixed with other, more common hepatoblastoma patterns.
Another uncommon morphology is the cholangioblastic pattern , which contains scattered duct-like structures within loose stroma that superficially resembles ductular reaction. However, the degree of cytologic atypia is usually more pronounced than in reactive ductules, and while ductular reaction might be expected at the periphery of the tumor, this morphologic variant is more often seen toward the central portion of the mass.
The small cell undifferentiated pattern is rarely seen and describes a tumor with clusters or sheets of “small round blue cells” that share a non-specific and undifferentiated morphology with a number of other pediatric tumors ( Fig. 4.18 ). Mitotic activity can be brisk or inconspicuous. Occasional rhabdoid morphology can be seen, characterized by eccentric nuclei and eosinophilic globules within the cytoplasm.
Approximately 30% of hepatoblastomas are classified as epithelial-mesenchymal pattern and contain a mixture of the aforementioned epithelial subtypes with various mesenchymal components such as osteoid, fibrous tissue, and/or hyaline cartilage ( Fig. 4.19 ). These mesenchymal areas are often positive for keratin markers and are therefore believed to represent metaplastic changes. Some mixed hepatoblastomas also exhibit teratoid features and include heterologous elements such as neuroectodermal, squamous, and intestinal structures.
The immunophenotype varies based on histologic pattern, but nuclear and/or cytoplasmic β-catenin expression is typical of most hepatoblastomas, excepting small cell undifferentiated and well-differentiated fetal patterns, which can show variable β-catenin patterns. In very well-differentiated fetal patterns, membranous expression is more common.
In addition, the fetal patten of hepatoblastoma shows keratin positivity, labeling by hepatocellular markers (arginase, HepPar1), glypican-3 staining, and diffuse expression of GS. The embryonal immunophenotype is similar, but its expression of hepatocellular markers and GS is less reliable.
The small cell undifferentiated pattern exhibits variable nuclear and cytoplasmic expression of β-catenin. but is typically negative for hepatocellular markers and GS. Glypican-3 and keratin expression may be focally present. A subset of small cell undifferentiated hepatoblastomas show loss of INI1 and behave similar to malignant rhabdoid tumors.
Surgery is the primary treatment for hepatoblastoma, and complete resection is the only cure. The most important prognostic factor is the tumor stage at the time of resection. Approximately 20% are metastatic at diagnosis. In most unresectable disease, however, chemotherapy can reduce the tumor burden prior to definitive surgery, and liver transplantation can be offered to many patients who remain unresectable after chemotherapy. The overall survival rate is 80%, with early-stage disease approaching 100% and falling to 40% once metastatic. Macrotrabecular and small cell undifferentiated patterns are associated with worse outcomes.
The differential diagnosis of hepatoblastoma depends on the tumor’s predominant morphologic subtype. Hepatoblastoma with a purely fetal pattern can resemble the normal developing liver with extramedullary hematopoiesis. However, the low-power appearance of alternating light and dark zones is not typical of developing liver, and hepatoblastoma will lack both normal portal zones and central veins. IHC can also help distinguish these entities, as normal liver will not express glypican-3 or show diffuse GS, and it exhibits only membranous expression of β-catenin.
Pleomorphic and macrotrabecular areas can be mistaken for HCC. Although HCC rarely affects this age group, the cytologic features, immunophenotype, and serum AFP levels do exhibit considerable overlap and may prove a diagnostic challenge in some cases. The presence of other hepatoblastoma components, such as fetal, embryonal, or mesenchymal patterns, can be extremely helpful in excluding HCC. In addition, the presence or absence of chronic disease in the background liver may be diagnostically useful, as the former would strongly favor HCC.
The small cell undifferentiated pattern resembles any number of morphologically similar pediatric tumors such as Wilms tumor, neuroblastoma, rhabdomyosarcoma, Ewing sarcoma, and lymphoma. As before, the presence of other hepatoblastoma patterns is diagnostically helpful, but these are not always seen. In their absence, clinicoradiographic correlation and immunophenotype are critical, and the lack of a renal or adrenal mass argues against both Wilms tumor and neuroblastoma. In addition, the small cell undifferentiated pattern of hepatoblastoma will often show nuclear β-catenin localization while lacking expression for WT-1, neuroendocrine or lymphoid markers, desmin, and CD99.
Germ cell tumors enter the differential if a teratoid component is present. These are often focal, and the hepatocellular component of a teratoma usually resembles more mature liver as opposed to the fetal or embryonal patterns seen in hepatoblastoma. Glypican-3 is not diagnostically useful in this scenario, but the presence of nuclear β-catenin supports hepatoblastoma.
Most common malignant liver tumor in children
Composed of fetal and/or embryonal liver
Rare disease (approximately 1 per 1 million children)
Slight male predominance
Peak incidence in first 2 years, almost never seen beyond age 5
Associated with very low birth weight
Most often presents with abdominal swelling noticed by parents
5% of patients also have congenital anomalies
Associated with congenital disorders such as familial adenomatous polyposis, Li-Fraumeni syndrome, Beckwith-Wiedemann syndrome, trisomy 18, and others
Usually a solid mass
Calcifications present in more than 50% of cases
Prognosis determined by tumor stage at resection
Surgical excision is only cure, preoperative chemotherapy often used to reduce tumor stage
Small cell undifferentiated and macrotrabecular patterns have worse outcome
Typically a single, well-defined mass in a normal liver
Epithelial tumors are usually tan-white and solid
Mixed tumors have heterogenous appearance
Divided into distinct subtypes:
Fetal pattern: sheets of uniform cells resembling hepatocytes, have characteristic “light and dark” appearance on low power
Embryonal pattern: cells are smaller and show higher N/C ratio
Pleomorphic pattern: fetal or embryonal morphology with prominent cytologic atypia
Macrotrabecular pattern: fetal or embryonal cells arranged in expanded sheets at least 10 cells wide
Cholangioblastic pattern: resembles ductular reaction, but cells are more atypical and located within lesion
Small cell undifferentiated pattern: sheets of small round blue cells resembling several other childhood tumors
Mixed epithelial-mesenchymal pattern: contains fetal and/or embryonal components admixed with primitive mesenchymal elements like osteoid, fibrous tissue, cartilage
Teratoid pattern: a mixed epithelial-mesenchymal pattern that also contains heterologous elements such as neuroectodermal and intestinal derivatives
Hepatocellular carcinoma
Pediatric small round blue cell tumors
Germ cell tumor
Normal liver
DDX: bile duct adenoma, well-differentiated cholangiocarcinoma
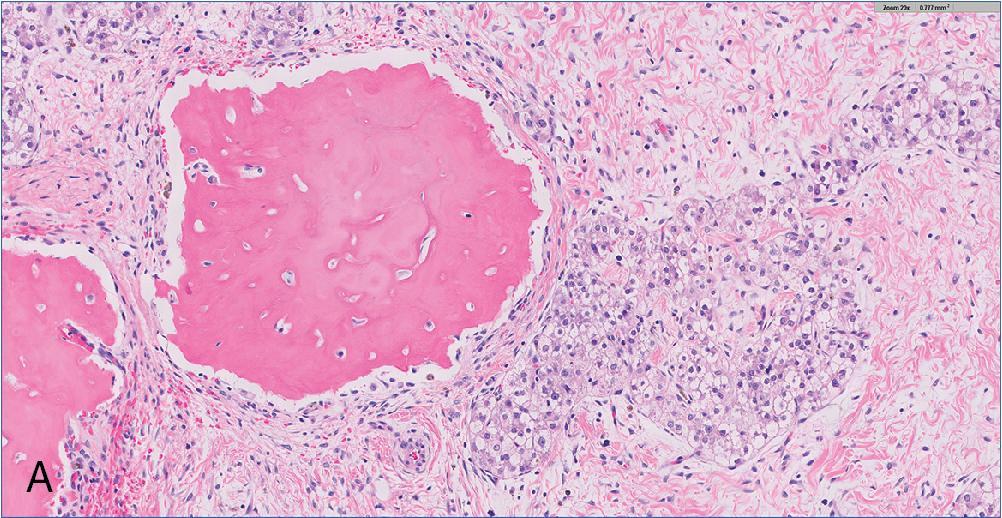
Bile duct hamartoma (also known as von Meyenburg complex) is an asymptomatic, subcentimeter liver lesion considered a type of ductal plate malformation. While they often arise sporadically, bile duct hamartomas have a strong association with adult polycystic disease, congenital hepatic fibrosis, and other entities on the ductal plate malformation spectrum. These lesions are found near portal areas and are frequently multiple. Bile duct hamartomas are benign and carry no risk of malignant transformation.
When seen, bile duct hamartomas present as white nodules less than 5 mm in diameter dispersed throughout the liver parenchyma. Small hamartomas may not be grossly visible.
These lesions are found directly adjacent to portal areas and consist of dilated, often branching and irregularly shaped bile ductules embedded in a dense fibrous stroma ( Fig. 4.20 ). These dilated spaces are lined by a layer of monomorphic cuboidal epithelium without appreciable atypia, and lumen frequently contain inspissated bile or proteinaceous debris. They do not contain mucin.
The primary differential diagnosis for bile duct hamartomas includes other benign biliary proliferations such as a bile duct adenoma or, occasionally, even well-differentiated cholangiocarcinoma. Bile duct adenomas are generally located in subcapsular areas of the liver, while hamartomas are dispersed throughout the hepatic parenchyma. The glands of a bile duct adenoma are smaller and more uniform in size compared with a bile duct hamartoma, and they lack intraluminal bile.
Well-differentiated cholangiocarcinoma is typically much larger than a bile duct hamartoma and is unlikely to be mistaken for one in most scenarios, although biopsy specimens can mask the overall size, and radiographic correlation is not always immediately available. Features that favor cholangiocarcinoma include significant variation in gland shape and spacing, desmoplastic stromal response, cribriform gland architecture, and notable cytologic atypia that manifests as nuclear pleomorphism, irregular membrane contours, and/or an increased N/C ratio. If needed, IHC for Ki-67 and p53 can often help separate benign from malignant biliary lesions. Benign lesions such as bile duct hamartoma will not show a Ki-67 proliferation index greater than 10%, and p53 will exhibit a wild-type expression pattern.
Common ductal plate malformation seen near portal tracts
Often sampled incidentally at surgery or found at autopsy
No geographic predilection
Seen in all ages
Asymptomatic
If numerous, may suggest genetic ductal plate disease
Benign lesion with excellent prognosis
No treatment indicated
Often inapparent
Small, subcentimeter white nodule
May be single or multiple, spread throughout liver
Located immediately adjacent to portal tracts
Consist of dilated, branching ductules set in dense fibrous stroma
Dilated ductules often contain bile or pink proteinaceous material without mucin
Low Ki-67 proliferation rate and wild-type p53 pattern
Bile duct adenoma
Well-differentiated cholangiocarcinoma
DDX: bile duct hamartoma (von Meyenburg complex), cholangiocarcinoma, metastatic adenocarcinoma
Bile duct adenomas were once thought to arise as a proliferative response to localized trauma or injury, but molecular studies now suggest that at least a subset represent true neoplasms. They are asymptomatic, often discovered incidentally, and typically located in subcapsular areas. Their distribution makes them grossly visible during abdominal surgery, where these lesions may be biopsied over concern for metastatic cancer. Bile duct adenomas may be found in both cirrhotic and non-cirrhotic livers.
Bile duct adenomas are usually well-demarcated, firm, tan-white lesions located beneath the capsular surface. Most are less than 1 cm, and they can be multiple.
Lesions are well-circumscribed, composed of small tubules with relatively uniform features and spacing, and are lined by a single layer of monomorphic cuboidal cells ( Fig. 4.21 ). Tubular dilatation is not typical, and intraluminal bile is not seen. The background stroma is variable, ranging from loosely fibrous to prominently hyalinized. Accompanying inflammation is occasionally present. The glandular epithelium typically lacks mitotic activity or atypia. Normal portal zones can be seen within a bile duct adenoma, often located near the periphery.
The differential diagnosis contains both benign and malignant glandular lesions. Bile duct adenomas do not feature the cystically dilated tubules with inspissated bile that characterize bile duct hamartomas, and they are not associated with polycystic diseases of the liver or kidney.
Clinically, bile duct adenomas are often sampled over concern for a primary or metastatic cancer. The circumscription of the lesion and presence of portal structures within bile duct adenomas can help distinguish these lesions from invasive adenocarcinoma. The cytomorphology also differs. Bile duct adenomas typically exhibit more uniform tubular size and spacing than a typical adenocarcinoma, with smooth rather than angular gland contours. There should not be significant mitotic activity or nuclear pleomorphism, although some well-differentiated pancreatobiliary adenocarcinomas can closely mimic this bland appearance and complicate diagnosis, especially on small biopsy specimens.
For difficult cases, IHC may be helpful. Benign biliary lesions such as a bile duct adenoma or hamartoma will exhibit a low Ki-67 proliferation index (less than 10%) and a wild-type pattern of p53 expression, whereas adenocarcinoma typically shows a higher proliferation rate and/or aberrant p53 expression ( Fig. 4.22 ).
Benign lesion composed of uniform tubular structures
Typically located immediately beneath liver capsule
All ages
Incidentally discovered, often during abdominal surgery or autopsy
Excellent prognosis, no treatment indicated
Small subcapsular lesion, usually less than 1 cm
Usually solitary, but can be multiple
Small, uniform tubules within variably fibrous background
Lacks notable mitotic activity or atypia
Low Ki-67 proliferation rate and wild-type p53 pattern
Bile duct hamartoma (von Meyenburg complex)
Cholangiocarcinoma
Metastatic adenocarcinoma
DDX: benign biliary proliferations/neoplasms, metastatic adenocarcinoma, hepatocellular carcinoma
Cholangiocarcinoma is a form of adenocarcinoma arising from bile duct epithelium, and it is further subclassified based on location. Intrahepatic cholangiocarcinoma (ICC) arises from the biliary system within the liver. Extrahepatic cholangiocarcinoma arises from biliary epithelium outside the liver and is divided into perihilar and distal bile duct cholangiocarcinoma, depending on whether the tumor origin is proximal or distal to the insertion of the cystic duct. While histologically similar, the molecular features of these subtypes cluster into somewhat distinct groups.
The majority of this discussion will focus on ICC, which accounts for approximately 15% of primary liver cancers. ICC exhibits tremendous geographic variability, as it is strongly associated with parasitic biliary infections endemic to certain parts of Southeast Asia (e.g., liver flukes such as Clonorchis , Opisthorchis , and Schistosoma ). In the United States, roughly 40% of cholangiocarcinoma is intrahepatic, 10% is hilar, and the remaining half arises in the distal biliary tree. These tumors show a slight male preponderance, and mostly affect people between 50 and 70 years of age. Patients with extrahepatic cholangiocarcinoma may present early with symptoms of biliary obstruction, but the majority of patients with ICC are asymptomatic until advanced disease.
Cholangiocarcinoma is classically associated with primary sclerosing cholangitis (PSC) and has been linked to several other conditions that also induce a chronic inflammatory state within the biliary tract, such as the aforementioned biliary parasites and hepatolithiasis. Congenital biliary malformations such as Caroli disease have been associated with an increased risk of ICC, and cirrhosis also acts as an independent risk factor.
Cholangiocarcinoma typically appears as a firm, tan-white mass. Although cirrhosis is a risk factor, ICC most often arises in non-cirrhotic livers. Hilar and extrahepatic cholangiocarcinoma show a similar gross appearance, but both are often accompanied by global discoloration of the liver secondary to obstructive jaundice.
The macroscopic growth behavior of ICC has been separated into three basic patterns. Mass-forming tumors are solid lesions within the liver parenchyma. Periductal tumors infiltrate along the portal zones and lead to bile duct strictures. Lastly, intraductal tumors exhibit papillary or polypoid growth within a dilated bile duct. These macroscopic growth patterns are significant when determining resectability.
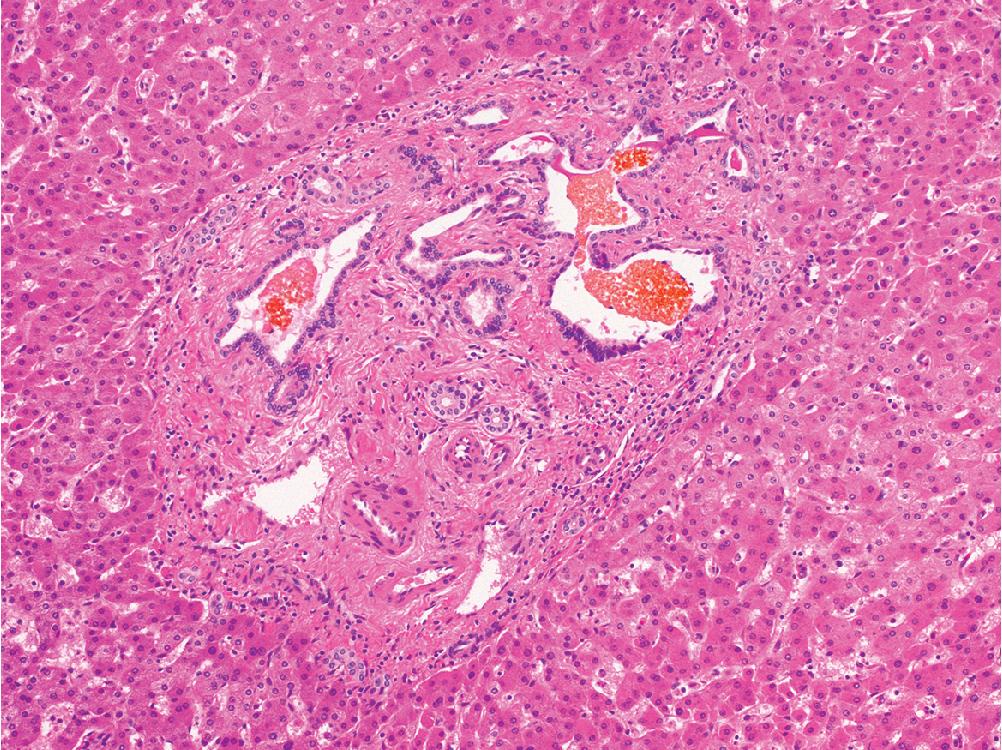
Cholangiocarcinoma usually forms invasive glands, but solid nests, cords, and papillary structures are not uncommon architectural patterns. The tumor is often set in a backdrop of abundant fibrous or desmoplastic stroma, containing at least focal mucin within glandular elements. Perineural invasion is common, and ICC has a tendency for intravascular spread within the liver and to regional lymph nodes.
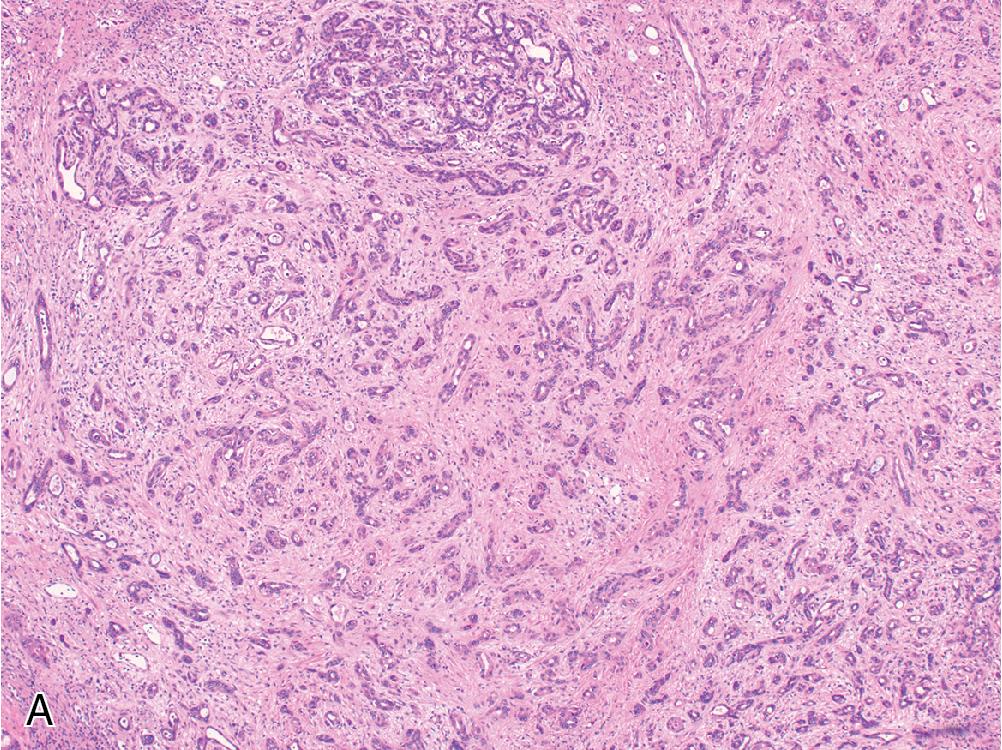
ICC has been subdivided into small duct and large duct variants, with the small duct pattern usually showing mass-forming growth at the periphery of the liver, and the large duct variant typically located near the hilum and exhibiting an infiltrative periductal growth. Small duct ICC resembles benign bile ducts, lined by cuboidal cells with ovoid nuclei and scant cytoplasm, growing in well-defined tubules or small anastomosing glands ( Fig. 4.23 A and B). In contrast, large duct ICC is characterized by large infiltrating glands lined by columnar epithelium ( Fig. 4.23 C and D). Uncommon and distinct morphologic subtypes of cholangiocarcinoma include mucinous, clear cell, signet ring, adenosquamous, mucoepidermoid, lymphoepithelioma-like, and sarcomatoid variants.
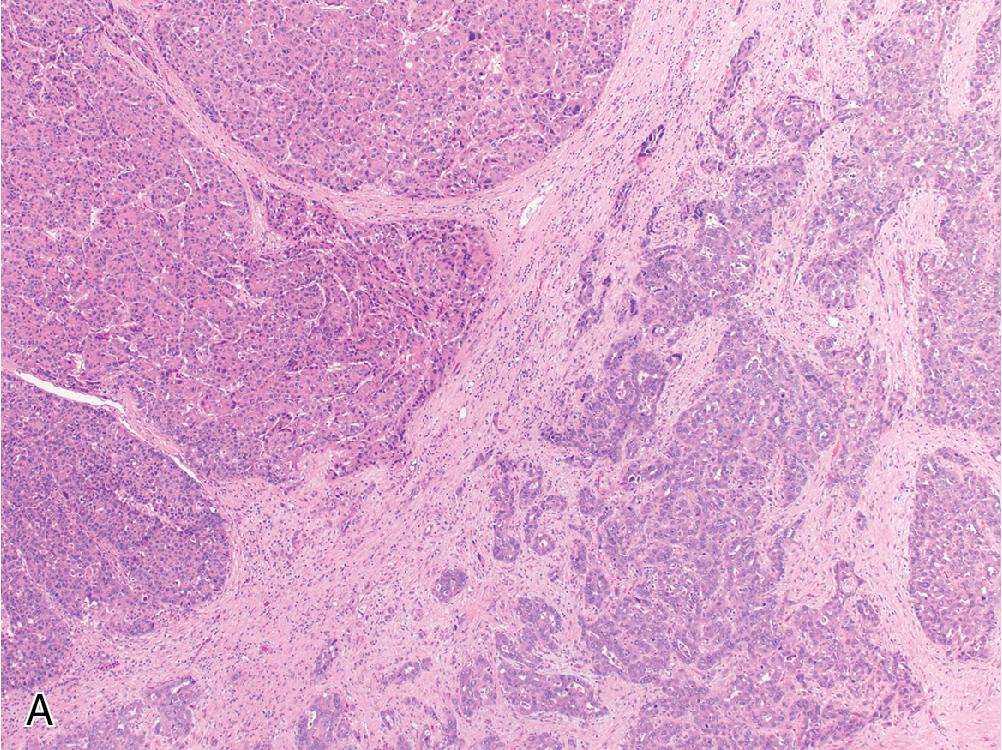
A rare subset of primary liver cancer exhibits a biphasic morphology that merges hepatocellular and cholangiocytic elements, due in part to its origin from progenitor cells in the bile ductules that contain the potential for differentiation along either lineage. This CHC contains visually distinct patterns of hepatocellular and cholangiocytic differentiation on H&E, which also demonstrate a corresponding biphasic immunophenotype ( Fig. 4.24 A).
IHC is of limited utility for cholangiocarcinoma. Most will stain for K7, K19, MOC-31, and show variable K20 expression, an immunoprofile that is frequently shared by pancreatic and upper gastrointestinal cancers as well. ICC is negative for hepatocellular markers (HepPar1 and arginase) but can express the colorectal tissue marker CDX2 in up to 50% of cases.
As mentioned earlier, CHC exhibits a biphasic morphology and immunophenotype, with areas of hepatoid differentiation that stain for arginase and/or HepPar1 alongside an adenocarcinoma showing an immunoprofile consistent with cholangiocarcinoma ( Fig. 4.24 B and C). Importantly, a differential immunophenotype alone is insufficient to diagnose CHC; visualizing two distinct morphologic elements is necessary.
The overall prognosis for cholangiocarcinoma is heavily tied to the extent of disease at the time of resection. As many patients present with advanced, unresectable tumors, the usual prognosis for cholangiocarcinoma is poor, with a median survival of 6 months. For resectable tumors, the most important prognostic factors are margin status and nodal involvement. The 5-year survival for ICC with lymph node metastasis is less than 10%, but node-negative disease with clear surgical margins increases the 5-year survival to approximately 40%.
The two primary considerations in the differential diagnosis of ICC are benign biliary proliferations and other primary or metastatic cancers. Small duct ICC can mimic the features of benign tumors such as a bile duct adenoma. Resection specimens permit assessment of the lesion edge, which can be quite helpful—bile duct adenomas have a well-circumscribed, smooth border, while cholangiocarcinomas often exhibit an irregular and infiltrative margin. However, small biopsy specimens can be problematic to interpret, since the presence or absence of an infiltrative border often cannot be assessed. Further complicating this differential, both benign and malignant bile duct lesions may contain a densely fibrous or hyalinized background.
In difficult biopsies, borders and gland cytoarchitecture are the most helpful features. Small duct ICC and bile duct adenomas are both comprised of small glands or tubules, but the invasive glands of ICC more often show angular contours and complex, anastomosing growth, whereas the glands comprising adenomas are smooth and exhibit more separation. Bile duct adenomas should not contain more than rare mitotic figures, and the cuboidal lining cells should not harbor any significant atypia. Features such as nuclear pleomorphism and irregular nuclear contours favor ICC. If the diagnosis remains unclear, IHC can help separate benign from malignant biliary lesions. Benign lesions do not show a Ki-67 proliferation index greater than 10% or aberrant p53 expression. However, not all malignant tumors will show the opposite immunophenotype, so correlation with morphology is needed.
The other challenge lies in distinguishing ICC from metastatic adenocarcinoma or other primary liver tumors. Where appropriate, common tumors that metastasize to the liver can be excluded with an immunohistochemical panel that includes markers of colorectal, pulmonary, breast, gynecologic, and/or prostatic origin. Metastatic adenocarcinomas from the upper gastrointestinal tract and pancreas represent a particular challenge, as these tumors have overlapping immunophenotypes with strong K7 positivity and variable expression of CDX2. Albumin in situ hybridization is a useful marker of hepatic origin (labeling both HCC and ICC) and therefore has utility in separating ICC from metastatic adenocarcinomas. In cases of a non-specific immunophenotype, however, correlation with clinical and imaging findings is critical.
Epithelioid hemangioendothelioma (EHE) contains a prominent desmoplastic stroma that can mimic poorly differentiated ICC at low power, and the presence of intracellular lumen can be confused with vague gland formation. However, EHE lacks mucin and stains readily with vascular markers (ERG, CD31, CD34) as well as the fusion-specific CAMTA1.
In addition, ICC with a solid-predominant growth pattern and minimal/absent gland formation could be confused with HCC exhibiting compact architecture. Likewise, HCC with abundant pseudoglandular architecture may mimic a CHC. In both cases, immunohistochemical distinction is usually straightforward, as hepatocellular markers (HepPar1, arginase) will be negative in ICC and MOC-31 is almost never expressed in HCC.
Adenocarcinoma arising from bile duct epithelium
Second most common malignant liver tumor
Higher incidence in Southeast Asia compared with the United States, but rising globally
Slight male predominance (3:2)
Typically affects patients 60–70 years of age
Associated with longstanding bile duct inflammatory states (PSC, biliary parasites, stones)
Intrahepatic cholangiocarcinoma often presents late and with non-specific features
Hilar and distal bile duct cholangiocarcinoma often present with obstructive jaundice
Homogenous mass with irregular margins on CT scan
Intratumoral calcifications can be seen
Most patients have unresectable disease with median survival of 6 months
5-year survival up to 40% for resected tumors with negative margins and no lymph node involvement
Three macroscopic growth patterns:
Mass-forming: solid lesion within the liver parenchyma
Periductal: infiltrative growth along portal zones
Intraductal: papillary/polypoid growth within a dilated bile duct
Glandular morphology predominates, split into small and large duct variants
Background fibrous or desmoplastic stroma
Focal mucin production is usually seen
K7, K19, and MOC-31 positive, negative for hepatocellular markers
Benign biliary proliferations/neoplasms
Metastatic adenocarcinoma
Hepatocellular carcinoma
DDX:
Myomatous AML: hepatocellular adenoma, hepatocellular carcinoma, melanoma
Lipomatous AML: focal fatty change, steatotic liver neoplasm (such as HNF1α-inactivated subtype of hepatocellular adenoma)
Angiomatous AML: hemangioma, vascular malformation
AMLs are rare tumors of the liver that arise from the perivascular epithelioid cell and have historically been grouped among the “PEComa” family. Most are found in non-cirrhotic livers. The majority of hepatic angiolipomas are sporadic and only 5% to 10% occur in patients with tuberous sclerosis (unlike AMLs arising in the kidney). Most cases are clinically silent and only detected incidentally when the patient is imaged for other reasons, but large tumors can present with abdominal pain.
AMLs are typically solitary, circumscribed, non-encapsulated lesions. Their size shows tremendous variance, ranging from a few millimeters to well over 30 cm. Their gross appearance depends on the microscopic composition of the tumor. Lipomatous areas will be yellow and soft, while the angiomatous component is grossly hemorrhagic, and myomatous regions will be solid, firm, and tan-white.
AML is a triphasic tumor composed of three elements: epithelioid or spindled myoid cells, blood vessels, and adipose tissue. They have a variable histologic appearance due to the relative proportion of their component elements, and tumors featuring a single primary component have been termed angiomatous, myomatous, or lipomatous AMLs. The smooth muscle element of these tumors originates from vascular walls and can appear spindled or epithelioid with varying degrees of pleomorphism and cellularity. The vascular component consists of tortuous, thick-walled arterial spaces and/or thin-walled venous channels. The fatty elements are generally composed of mature adipose tissue, either scattered as individual cells throughout the tumor or coalesced into large sheets. Scattered lipoblasts may also be present.
Mixed AMLs will demonstrate all three components clearly, and these tumors are typically not a diagnostic challenge. However, hepatic AMLs frequently present with a single predominant element that can complicate diagnosis. In particular, hepatic AMLs often feature a prominent epithelioid myoid cell element with only scattered blood vessels and a minimal fat ( Fig. 4.25 A). These epithelioid cells are rounded or polygonal, with ovoid nuclei, prominent nucleoli, and eosinophilic cytoplasm that sometimes condenses around the nucleus after fixation, which may mimic swollen hepatocytes. Although mitotic activity is not pronounced, these cells can show a surprising degree of cytologic atypia. A myomatous AML with a prominent spindle cell component will be variably cellular, and myoid cells will also contain ovoid nuclei with eosinophilic cytoplasm, although their spindled histologic appearance is more obviously mesenchymal.
The different elements of AML stain with various markers, but all components are negative for keratins. The epithelioid or spindled myoid component stains with HMB-45, Melan-A, SMA, and caldesmon. Any vascular component will express endothelial markers (CD31, CD34, ERG), mature adipocytes labeled with S-100, and scattered lipoblasts stain with either S-100 or HMB-45.
Hepatic AMLs are benign lesions that can be managed by observation or, if needed, surgical resection. Malignant angiomyolipomas in the liver have been reported but are extremely rare.
AMLs with a single prominent component can be diagnostically challenging. Most problematic are epithelioid myomatous tumors, which can exhibit a remarkably hepatoid morphology, with polygonal cells containing abundant eosinophilic cytoplasm and well-demarcated cell borders. These kinds of AML can easily mimic HCAs or, if even mild cytologic atypia is present, well-differentiated HCC. Furthermore, reticulin staining on AML will highlight a poorly developed or obliterated framework, which may reinforce a mistaken diagnosis of HCC. A hepatoid neoplasm in a non-cirrhotic liver with a vaguely mesenchymal appearance and/or reticulin loss out of proportion to the degree of cytologic atypia should raise suspicion for AML, and staining for hepatocellular derivation may be prudent. Arginase and HepPar1 will be negative in AML ( Fig. 4.25 B), while myomelanocytic markers like HMB-45 and SMA will be diffusely positive ( Fig. 4.25 C). This distinction can be especially challenging in biopsy specimens, where IHC is often crucial to avoiding misdiagnosis.
Myomatous AML with a mixture of epithelioid and spindled cells can also mimic metastatic melanoma, further complicated by their overlapping immunophenotype. However, melanoma typically shows a greater degree of cytologic atypia. While melanocytic markers like HMB-45 are positive in both entities, melanoma will not co-express smooth muscle markers, and SOX10 is negative in AML.
If the tumor exhibits a vascular predominance, it may be mistaken for a hemangioma or vascular malformation. If the dominant element is fatty, these can be misinterpreted as focal fatty change or a hepatocellular lesion with prominent steatosis (such as the HNF1α-inactivated subtype of HCA). In either case, immunohistochemical staining should label associated myoid cells and confirm the diagnosis of AML.
Benign tumor composed of myoid cells, blood vessels, and fat
Rare tumors with no geographic predilection
More common in females
Average age between 30 and 50 years
Most AMLs are asymptomatic and detected incidentally
Larger lesions may present with abdominal pain
Heterogenous and circumscribed mass on imaging
Radiographic differential frequently includes hepatocellular lesions if tumor fat content is low
Excellent prognosis
Patients are either observed or undergo surgical resection
Well-circumscribed lesion, size can vary tremendously
Some combination of fatty, hemorrhagic, and tan-white solid areas, depending on tumor composition
Non-cirrhotic background liver
Triphasic tumor with a varying proportion of three cell types: epithelioid/spindled myoid cells, blood vessels, and adipose tissue
Myomatous AML is common in the liver
Fatty component is often inconspicuous in hepatic AML
Myomatous AML: hepatocellular adenoma, hepatocellular carcinoma, melanoma
Lipomatous AML: focal fatty change, steatotic liver neoplasm (HNF1α-inactivated subtype of hepatocellular adenoma)
Angiomatous AML: hemangioma, vascular malformation
DDX: adenocarcinoma (including cholangiocarcinoma), angiosarcoma, bland fibrosis
EHE is a rare, malignant vascular tumor that can arise in multiple sites, including the liver. It affects adults and is seen more often in women than in men. Hepatic EHE tends to cause venous occlusion, and involvement of the hepatic vein can result in sinusoidal obstruction or Budd-Chiari syndrome. Although this is a vascular tumor, its dense stroma protects from rupture and hemorrhage, so risk of significant bleeding is minimal.
A distinct chromosomal translocation has been identified in more than 90% of EHEs, which results in a WWTR1-CAMTA1 fusion. A small subset (fewer than 5%) also contains the YAP-TFE3 gene fusion.
EHE is typically multifocal at the time of resection, usually involves both lobes, and individual masses vary in size from a few millimeters to more than 10 cm. The cut surface is firm, tan-white, and sometimes gritty due to dystrophic calcifications.
These tumors typically exhibit prominent fibromyxoid stroma and relative paucicellularity at the center of the lesion. Cellularity increases toward the periphery of the tumor, imparting a zonal appearance at low power. At the leading front, EHE grows through sinusoidal spaces and characteristically entraps normal hepatic structures such as portal zones and hepatocyte clusters ( Fig. 4.26 A). Within the tumor’s fibromyxoid stroma are a mixture of spindled dendritic cells and rounded epithelioid cells, the latter containing vacuoles that represent intracellular capillary-type lumina (“blister cells”). These can mimic signet ring cells, but the intracellular channels occasionally contain red blood cells and lack mucin ( Fig. 4.26 B). Toward the center of the lesion, the tumor appears more solid, stroma-rich, and sometimes calcified. Obliteration of portal and central veins is a common finding.
All cases of EHE are positive for at least one endothelial marker (CD31, CD34, ERG). If available, IHC for CAMTA1 is essentially diagnostic for EHE ( Fig. 4.26 B and C), and molecular testing can be performed to detect the characteristic fusion products. Notably, EHE can sometimes show anomalous keratin expression.
EHE tends to behave in a low-grade manner compared with other malignant vascular tumors such as angiosarcoma. It grows relatively slowly, and the 5-year survival is approximately 50%.
The primary differential diagnosis for hepatic EHE includes adenocarcinoma, angiosarcoma, or a benign sclerosing process. The prominent myxoid-type stroma and presence of epithelioid elements can resemble the desmoplastic response of adenocarcinoma, and the blister cell morphology can especially resemble signet ring cells or abortive gland structures. However, the intracellular vacuoles of EHE blister cells will occasionally contain erythrocytes and lack mucin. EHE will also express vascular endothelial markers and the vast majority will also label with CAMTA1 IHC, both of which will be negative in adenocarcinoma. Be wary of focal keratin staining, as EHE can occasionally be positive, and atrophic hepatic structures may be entrapped within the tumor.
Angiosarcoma shows an overlapping immunophenotype, with variable keratin expression and strong labeling by vascular markers such as ERG, but the morphologic distinction is straightforward. Angiosarcomas tend to be larger and more hemorrhagic than EHE. They demonstrate a significantly higher degree of nuclear atypia and show prominent mitotic activity. The characteristic fibromyxoid stroma and calcifications of EHE are not shared by angiosarcoma, and the latter tumor has a notably more destructive growth pattern, without entrapment and preservation of hepatic structures.
In small biopsies, it can be challenging to distinguish EHE from areas of bland fibrosis, such as a sclerosed hemangioma or parenchymal extinction adjacent to an unsampled mass lesion. These areas of bland fibrosis tend to be more hypocellular than EHE. They may contain small, normal capillaries within the fibrous background, which will label with endothelial markers. However, the characteristic “blister cells” of EHE will be absent, and no atypical dendritic cells are seen. If available, IHC for CAMTA1 can be very helpful in these situations.
Rare, low-grade malignant endothelial neoplasm
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here