Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The authors acknowledge the contributions of Drs. Heiner Wedemeyer, Jacqueline G. O’Leary, and Gary L. Davis to this chapter in previous editions of the book.
More than 71 million people worldwide are chronically infected with HCV. , In the USA, conservative estimates suggest that more than 1.9 million people live with HCV. Unfortunately, HCV successfully evades the host immune response in 50% to 90% of acutely infected persons, thereby leading to chronic infection in the majority of cases. The natural history of hepatitis C varies greatly; reasons for this heterogeneity remain incompletely understood but are related to viral, host, and environmental factors. Chronic HCV infection can lead to cirrhosis and HCC; HCV-related mortality increased dramatically after 1995, plateaued around 2002, and has been rapidly increasing since 2003, owing to the aging HCV population. Complications of HCV-related cirrhosis remain a leading indication for LT in the USA and Europe. With the introduction of highly effective DAA therapy, the frequency of these complications is expected to decline by 2030.
Chronic hepatitis C is the only chronic viral infection that can be cured by antiviral therapy. Importantly, successful antiviral treatment can prevent short- and long-term complications of HCV infection in many patients. Substantial progress in understanding the HCV replication cycle, along with development of the replicon system and crystallization of the HCV proteins, has enabled the development of new therapeutic agents that target discrete steps in the viral lifecycle, culminating in highly potent well-tolerated interferon (IFN)-free therapy. Combination therapy with DAAs has altered the treatment landscape dramatically, affording sustained virologic response (SVR) at 12 weeks (SVR12) rates (defined as absence of HCV RNA in serum 12 weeks after discontinuation of treatment) in excess of 95% for most patients. An SVR12 is almost always associated with an SVR at 24 weeks (SVR24, defined as absence of HCV RNA in serum 24 weeks after discontinuation of treatment, which was the prior decision point for determining cure with IFN-based therapy) and durable long-term eradication of the virus. The term SVR now implies an SVR12.
The HCV virion is an enveloped virus that is 50 nm in diameter. The 2 envelope proteins, E1 and E2, heterodimerize and assemble into tetramers, creating a smooth outer layer, which has a “fishbone” configuration with icosahedral symmetry. The envelope proteins are anchored to a host cell–derived lipid bilayer envelope membrane that surrounds the nucleocapsid. The nucleocapsid is believed to be composed of multiple copies of the core protein and forms an internal icosahedral viral coat that encapsulates the genomic RNA. HCV circulates in various forms in the serum of an infected host, including (1) virions that are bound to VLDL and LDL and appear to represent the infectious fraction; (2) virions bound to immunoglobulin (Ig); and (3) free virions.
HCV is a single-stranded positive-sense RNA virus that belongs to the Flaviviridae family and has been classified as the sole member of the genus Hepacivirus . The genome of HCV contains approximately 9600 nucleotides with an open reading frame (ORF) that encodes one large viral polypeptide precursor of about 3000 amino acids. The HCV ORF is flanked upstream by a 5′ untranslated region (UTR) that functions as an internal ribosome entry site to direct cap-independent translation (i.e., without the addition of an extra ribonucleotide to the 5′ end of the viral messenger RNA) and downstream by a 3′ UTR that is critical for initiation of new RNA strand synthesis. The 5′ and portions of the 3′ UTR are the most conserved regions of the HCV genome.
Although peripheral blood mononuclear cells, B cells, T cells, and dendritic cells have been reported to support HCV replication, hepatocytes are the major site of viral replication. , HCV entry involves the attachment of envelope proteins E1 and E2 to cell surface molecules ( Fig. 80.1 ). The expression and function of CD81, a member of the tetraspan superfamily, is essential for HCV entry into hepatocytes. In addition, human scavenger receptor class B type 1, a selective importer of cholesteryl esters from HDL into cells, has been shown to interact with E2 and is also essential for HCV entry. Whereas CD81 and scavenger receptor class B type 1 are required early in the process of viral entry, claudin-1, a tight junction component that is highly expressed on hepatocytes, and occludin are required later in the cell entry process. , Heparin sulfated proteoglycans and LDL have also been shown to be involved in HCV cell entry. , Additional cellular factors and receptors suggested to be required for viral entry include EGF and Niemann-Pick C1-Like 1, a cholesterol uptake receptor.
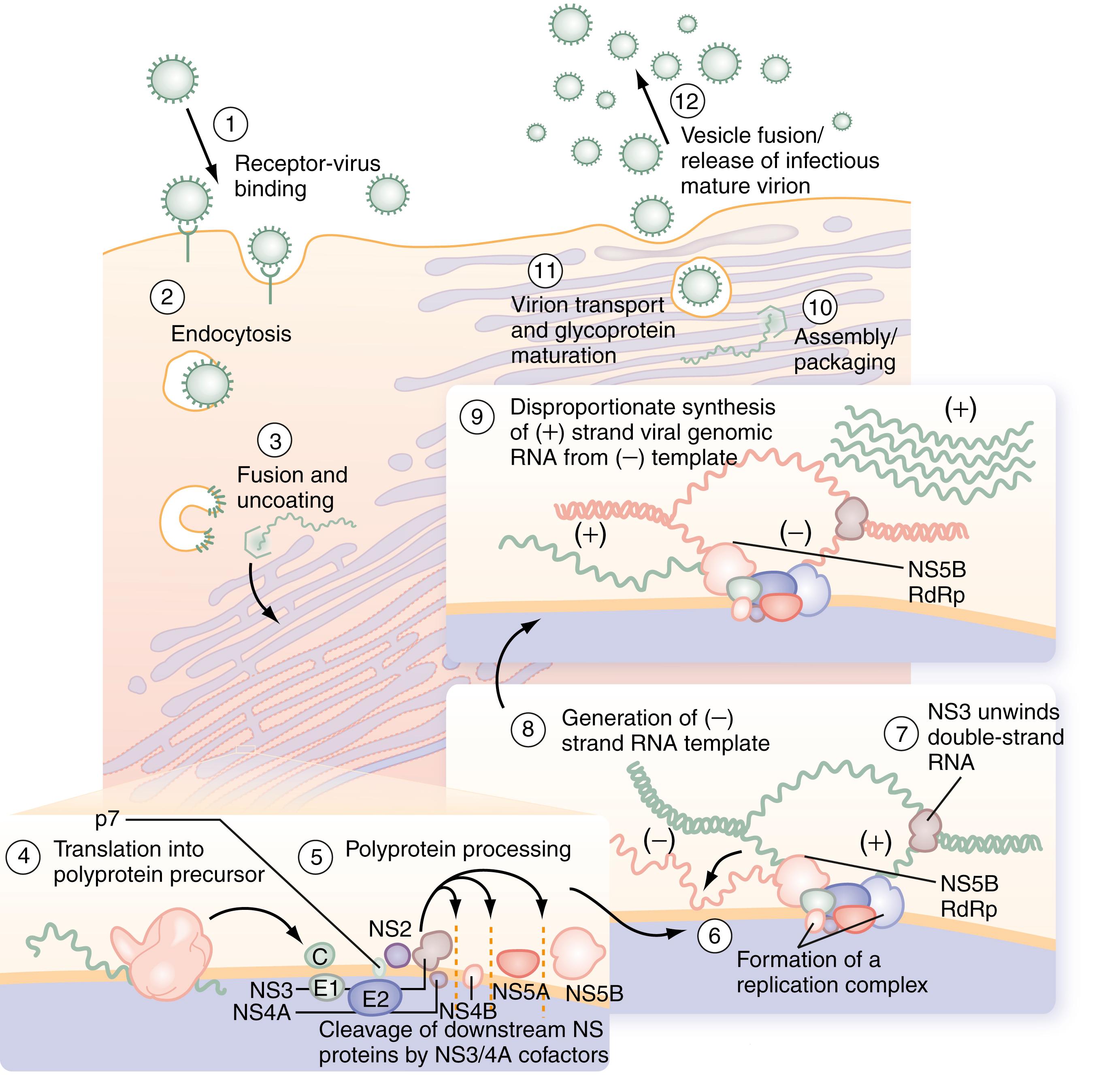
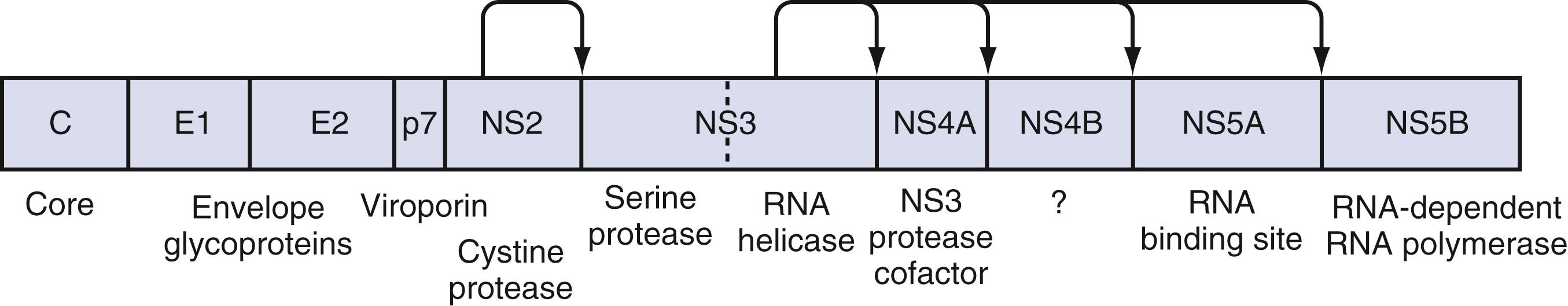
Once HCV attaches to the cell, endocytosis of the bound virion is presumed to occur, as with other flaviviruses. A pH drop in the vesicle causes conformational changes in the glycoproteins that lead to fusion of the viral and cellular membranes and release of viral RNA into the cytoplasm. In the cytosol, the 5′ UTR functions as an internal ribosome entry site, which directs the RNA to its docking site on the endoplasmic reticulum and mediates cap-independent internal initiation of HCV polyprotein translation by recruiting both cellular proteins, including eukaryotic initiation factors 2 and 3, and viral proteins. The large polyprotein is co- and post-translationally processed proteolytically into at least 11 viral proteins, including both structural (nucleocapsid [C], or p21; envelope 1 [E1], or gp31; and envelope 2 [E2], or gp70) and nonstructural (NS2, NS3, NS4A, NS4B, NS5A, and NS5B) proteins ( Fig. 80.2 ). The functions of these specific nonstructural proteins are described later in the chapter.
After polyprotein processing, NS4B expression causes the membrane alterations that are seen on electron microscopy known as a membranous web. The replication complex associates viral proteins, cellular components, and nascent RNA strands. HCV replication is catalyzed by the NS5B RNA-dependent RNA polymerase (RdRp). The positive-strand genomic RNA serves as a template for the synthesis of a negative-strand intermediate. The negative-strand RNA serves as a template for production of numerous strands of RNA of positive polarity that are used for polyprotein translation and synthesis of new intermediates of replication and that are packaged into new virus particles.
Finally, viral particle formation is initiated by the interaction of the core protein with genomic RNA in the endoplasmic reticulum. By analogy with pestiviruses, HCV packaging and release are likely to be inefficient because much of the virus remains in the cell. Following release, viral particles may infect adjacent hepatocytes or enter the circulation, where they are available for infection of another cell or host.
The large polyprotein generated by translation of the HCV genome is cleaved by cellular and viral proteases to form structural and nonstructural proteins. The structural proteins are separated from the nonstructural proteins by the short membrane peptide p7, believed to be a viroporin, a protein that plays a role in viral particle maturation and release. The crystal structures of most of the ORF proteins have been elucidated and have led to an understanding of protein interactions and functions. Although these proteins are most important for viral replication, some also interact with host proteins and may facilitate persistence of the virus by impairing the host immune response.
The core protein is first cleaved from the large polypeptide and then further processed by a host signal peptidase. In infectious HCV virions, the core protein forms the viral nucleocapsid and binds RNA. The core protein has been found attached to lipid rafts and the endoplasmic reticulum, and it translocates into the nucleus. When core protein attaches to lipid rafts, it recruits nonstructural proteins, thereby resulting in the assembly of infectious virions. The core protein can also interact with the host immune system by inactivating the RNA silencing activity of Dicer, a cellular endoribonuclease that produces small interfering RNA to bind and target HCV RNA for destruction by the cell. The core protein can also bind to Janus kinase-1 (JAK1) and JAK2 and alter the activation of signal transducer activator of transcription (STAT) proteins, leading to impairment of IFN production. Extracellularly, core protein inhibits T-cell activation and proliferation, possibly by down-regulating co-stimulatory molecules on dendritic cells. Specific polymorphisms in core protein have also been associated with intracellular lipid accumulation ; this may be the result of facilitation of phosphorylation of insulin receptor substrate-1, thereby leading to insulin resistance. Mutations in core protein have also been associated with an increased risk of HCC in patients; core protein alone can cause HCC in transgenic mice.
E1 and E2 proteins are cleaved from the polypeptide by host signal peptidase. The 2 proteins form highly glycosylated heterodimers and then tetramers that are essential for viral assembly (see earlier). They also mediate cell entry by binding to surface receptors. Subsequently, they are responsible for fusion between the host cell membrane and the viral envelope. Because E1 and E2 are expressed on the surface of the virion, they are targets of host antibodies. The first 27 amino acids of E2 form hypervariable region 1 (HVR1); alterations in HVR1 are believed to be an attempt by the virus at antibody-mediated immune evasion.
P7 is cleaved by the endoplasmic reticulum signal peptidase and forms an ion channel. This viroporin protein is essential for efficient assembly and release of infectious virions but not for cell entry.
NS2 complexes with NS3 and zinc to form a cysteine protease, with 2 composite active sites, that autocatalytically cleaves NS2 from NS3. No other function of NS2 has been discovered to date. NS3 has several functions in addition to complexing with NS2 for autocatalytic cleavage of the NS2-NS3 site. Its function as a serine protease is markedly enhanced by its association with NS4A. The enzyme results in cleavage of the polyprotein at the NS3-NS4A, NS4A-NS4B, NS4B-NS5A, and NS5A-NS5B sites. , The NS3 protease also cleaves and thereby destroys the function of Cardif and TRIF (Toll/interleukin receptor domain-containing adapter-inducing IFN-β), which are intermediates in 2 separate pathways of host-cell IFN secretion in response to viral infection. This property may have a significant effect in impairing the host response to HCV infection. Finally, a portion of the NS3 protein functions as a helicase that unwinds viral RNA as well as host DNA. The helicase function is dependent on ATP, may require dimerization of NS3, and progresses in discrete steps like an inchworm. NS4A complexes with NS3 and functions to stabilize the protease and helicase activities and anchor the complex to the endoplasmic reticulum membrane. , It also regulates hyperphosphorylation of NS5A. The only known function of NS4B is to induce the formation of the membranous web on which HCV transcription occurs. NS5A binds zinc and forms homodimers that are bound to the endoplasmic reticulum membrane. NS5A is essential for viral replication and is believed to provide an RNA-binding site within the replication complex. In addition, NS5A inhibits apoptosis in infected cells, , and some mutations confer improved sensitivity to IFN therapy. NS5B is the viral RdRp. The crystal structure elucidates the tunnel of the enzyme that directs single-stranded RNA into the active site. It can synthesize both negative-strand HCV RNA templates and positive-strand HCV RNA genomes.
HCV has an inherently high mutational rate that results in considerable heterogeneity throughout the genome. This high mutational rate is in part a consequence of the RdRp of HCV, which lacks 3′- to 5′-exonuclease proofreading ability that ordinarily would remove mismatched nucleotides incorporated during replication. An average of one error occurs for every 10 4 to 10 5 nucleotides copied. This phenomenon is favored by a high viral turnover rate; 10 10 to 10 12 virions are produced per day. The estimated half-life of HCV in serum is only about 45 minutes. A substantial proportion of newly synthesized viral genomes have alterations. Because of the functional differences in HCV proteins, genetic variation in some parts of the genome confers advantages by evading or inhibiting the host immune system, whereas other mutations may be lethal to the virus if they lead to defective replication machinery. Therefore, genetic variation is distributed irregularly along the genome. Each new genetic variant is produced in a single cell and may or may not spread through the liver and into the serum. The result is not only genetic diversity in the serum, but also compartmentalization of variant virions in different parts of the liver and perhaps in extrahepatic sites.
Because of the vast genetic variation, a classification scheme was devised whereby viral sequences are given a genotype and subtype. The first division used to describe the genetic heterogeneity of HCV is the viral genotype , which refers to genetically distinct groups of HCV isolates that have arisen during the evolution of the virus. Nucleotide sequencing has shown variation of up to 34% between genotypes. The most conserved region (5′ UTR) has a maximum nucleotide sequence divergence of 9% between genotypes, whereas the highly variable regions that encode the envelope proteins (E1 and E2) exhibit a nucleotide sequence divergence of 35% to 44% between genotypes. The sequences cluster into 7 major genotypes (designated by numbers), with sequence similarities of 60% to 70%, and more than 67 subtypes (designated by a lower-case letter) within these major genotypes, with sequence similarities of 77% to 80%. In this scheme, the first variant, which was cloned by Choo and colleagues, is designated type 1a. The HCV genotype is an intrinsic characteristic of the infecting HCV strain and does not change over time; therefore, the genotype only needs to be determined once in an infected person. Mixed-genotype infections may be seen and reflect either coinfection with more than one HCV virus or methodologic problems in genotype testing. In addition, intergenotypic HCV recombinants have been described ; these are thought to arise because of recombination between different genotypes in patients with repeated exposure. The recombination events have been reported to occur in or between NS2 and NS3.
Global geographic differences exist in the distribution of HCV genotypes, as well as in the mode of acquisition. In the USA, genotype 1a is the most prevalent, accounting for approximately 46% of HCV infections, followed by genotype 1b in 26%, genotype 2 in 11%, genotype 3 in 9%, and genotype 4, 5, 6, or mixed/other in less than 8%. Racial differences are seen in the prevalence of genotypes; approximately 90% of African Americans are infected with HCV genotype 1, whereas only 70% of whites and 71% of Hispanics are infected with genotype 1. In Europe, the most prevalent genotype is 3 (41%), followed by 1b (40%), 1a (13%), and 1c/other (18%). Genotype 4 is found mainly in Egypt, the Middle East, and Central Africa. , In Egypt, approximately 6.5 to 7% of the population is infected with HCV, and more than 90% have HCV genotype 4. , Genotype 5, although originally isolated in South Africa, is also seen in specific regions of Europe (France and Belgium) and the Middle East (Lebanon and Syria). , Genotype 6 is found predominantly in Asia. The distribution of genotypes is ever changing with immigration and alterations in the primary modes of viral transmission. Therefore, the frequencies of viral genotypes change over time.
In the era of IFN-based therapy, HCV genotype was an important predictor of response to treatment. Although HCV genotype as a predictor of treatment outcome is less relevant with DAA-based therapy, HCV genotype is still important, because some DAAs only have activity against specific HCV genotypes (see later). HCV genotype may also play a role in disease progression and complications of chronic HCV infection. Specifically, HCV genotype 3 has been associated with faster liver fibrosis progression, as well as with an increased risk of cirrhosis and HCC.
The second component of genetic heterogeneity is quasispecies generation. Quasispecies are closely related, yet heterogeneous, sequences of HCV RNA within a single infected person that result from mutations that occur during viral replication. The rate of nucleotide changes varies significantly among the different regions of the viral genome. The highest proportion of mutations are found in the E1 and E2 regions, particularly in HVR1. Even though this region represents only a minor part of the E2 region, it accounts for approximately 50% of the nucleotide changes and 60% of the amino acid substitutions within the envelope region.
The development of quasispecies may be one mechanism by which the virus escapes the host’s immune response and establishes persistent infection. During acute infection or during treatment, lack of diversity in the quasispecies is associated with viral clearance, and the development of numerous quasispecies is associated with viral persistence. In acute disease, patients in whom genetic variation in the HVR1 region develops after antibody seroconversion progress to chronic disease, whereas those in whom such genetic variation does not develop are more likely to achieve viral clearance. Genetic variation before seroconversion does not correlate with outcome, indicating that quasispecies formation results from antibody-mediated immune pressure. Interestingly, no intrinsically IFN-resistant variants of HCV have been defined, indicating that both viral and host factors play important roles in determining whether the virus persists or is cleared. An increased number of quasispecies has also been associated with more rapid progression to cirrhosis and the development of HCC.
The worldwide prevalence of HCV infection, based on detection of HCV RNA in serum, is estimated to be 1%, with more than 71 million people infected chronically. The overall worldwide prevalence increased from 1990 to 2010. Marked geographic variation exists, with infection rates ranging from 0.1% in the Netherlands, Fiji, and Samoa, to 0.9% in the USA, 6.3% in Egypt, and 7% in Gabon. In 2002, between 3.2 and 5 million persons were infected with HCV in the USA ; however, these estimates were based on HCV seroprevalence (presence of anti-HCV antibody only). More recent studies estimate the viremic prevalence to be 2.9 million, which has been stable since 2006, although this is likely to change with the advent of highly potent IFN-free DAA therapy. , The highest seroprevalence in different age groups shifted from 35 to 44 years (2.5%) to 55 to 64 years in 2005 (2.7%). It had therefore been recommended that all persons born between 1945 and 1965 be tested for anti-HCV. In 2020, the Centers for Disease Control and Prevention (CDC) recommended that all adults over age 18 and all pregnant women be tested for anti-HCV unless they are in a setting in which the prevalence of HCV infection is less than 0.1% .The prevalence is higher in males (2.1%) than in females (1.1%), and in African Americans (3%) than in whites (1.5%). Other risk factors for HCV infection are injection drug use, blood transfusion before the implementation of routine blood product screening in many countries in 1992, more than 50 lifetime sexual partners, family income below the poverty level, occupational exposure, and being born in an endemic country. The prevalence of HCV infection in the USA may be underestimated because the National Health and Nutrition Examination Survey data did not evaluate persons who are homeless, incarcerated, or in the military. Trends in the epidemiology of HCV infection suggest an increase in prevalence in young drug-using persons.
Worldwide, 3 different epidemiologic patterns of HCV infection have emerged: (1) previous exposure through health care with a peak prevalence in older persons; (2) exposure through injection drug use, the major risk factor since data first became available in about 1960, with a peak prevalence among middle-aged persons; and (3) ongoing high levels of infection in areas where high rates of infection occur in all age groups.
Given the factors that influence viral diversity (see earlier), estimating the site of origin and age of HCV by phylogenetic analysis is difficult. The best estimate is that HCV originated in western and sub-Saharan Africa. Subsequent global spread probably occurred coincident with trade and human migration. Evolution of the virus led to a geographic distribution of genotypes, so that genotypes 1, 2, and 3 are most common in North America and Europe, genotype 4 is most common in the Middle East, and genotype 6 is most common in Southeast Asia. In Japan, HCV transmission transitioned from constant to exponential growth in the 1920s, and the prevalence of HCV infection is highest in older persons. In Japan, and later in southern and eastern Europe, health care–related procedures—particularly reuse of contaminated syringes—played a major role in viral spread. In the USA, Australia, and other developed countries, peak prevalence is in persons 40 to 49 years of age, and analysis of risk factors suggests that most HCV transmission occurred between the mid-1980s and the mid-1990s, through injection drug use. In Egypt, the spread of HCV increased exponentially from the 1930s to the 1980s because of mass vaccination campaigns with reuse of medical equipment. In Egypt and other developing countries, high rates of infection are observed in all age groups, suggesting that an ongoing risk of HCV acquisition exists.
In the USA, there have been distinct changes in the epidemiology of acute HCV infection. The incidence of acute HCV peaked in 1989, gradually declining over the following 15 years, before stabilizing from 2006 until 2010. The peak incidence was estimated to be 180,000 cases per year in the mid-1980s, but the rate declined to less than 20,000 cases. Many factors have contributed to the falling incidence of acute hepatitis C. In the 1980s, when blood was purchased from donors, 2% to 10% of blood units were infected with HCV, leading to a high rate of transfusion-acquired HCV infection. The institution of volunteer blood donation, creation of recombinant clotting factors, and implementation of HCV blood testing (between 1990 and 1992) dramatically decreased transfusion-acquired HCV infection. However, since 2011, the incidence of acute hepatitis C has increased 4-fold, particularly in the 18- to 39-year-old age group (a 400% increase in 18- to 29-year-olds and 325% increase in 30- to 39-year-olds), attributable to the opioid injection drug use epidemic.
An important mechanism of transmission worldwide has been the lack of sterilization of medical instruments such as syringes. Although the incidence of HCV transmission by medical instruments has also decreased markedly, the risk has not been eliminated, even in the USA. New HCV infections in the USA and other developed countries occur primarily as a result of injection drug use.
Modes of transmission of HCV can be divided into percutaneous (blood transfusion and needlestick inoculation) and nonpercutaneous (sexual contact and perinatal exposure). Patients are often unwilling to disclose percutaneous risk factors, and therefore apparent nonpercutaneous transmission may represent occult percutaneous exposure.
Blood transfusion (before the introduction of screening) and injection drug use are the most clearly documented risk factors for HCV infection. Following the introduction of anti-HCV screening of blood donors between 1990 and 1992, the number of transfusion-related cases of HCV infection declined sharply to the point that less than 1 case occurs per 2,000,000 units transfused, virtually eliminating transmission of HCV by blood transfusion. In many countries, blood products are assayed directly for HCV RNA by “mini-pool” testing, although not all developing countries implement blood product screening; therefore, post-transfusion associated HCV infection remains a risk in these regions.
Injection drug use has always been the major route of HCV acquisition in the USA and accounts for the majority of newly acquired HCV cases. , The frequency of HCV infection in persons who inject drugs (PWID) ranges from 57% to 90%. , Although risk factors for HBV and HIV infection overlap with those for HCV infection, the prevalence of HCV infection in this population is the highest among the 3 viruses. The majority of PWID become anti-HCV positive within 6 months of initiating injection drug use with shared paraphernalia.
Chronic hemodialysis is also associated with increased rates of HCV infection. The frequency of anti-HCV in patients on hemodialysis ranges from less than 10% in the USA to 55% to 85% in Jordan, Saudi Arabia, and Iran. Serologic assays for anti-HCV may underestimate the frequency of HCV infection in this relatively immunocompromised population, and HCV RNA testing may be necessary for accurate diagnosis.
Occupational transmission may occur from infected patients to health care workers. Anti-HCV seroconversion rates are approximately 0.3% to 4% in longitudinal studies of health care workers after percutaneous inoculation from anti-HCV–positive sources, although the risk is dependent on the type of needle (hollow vs. solid, infusion vs. withdrawal), volume of inoculum, depth of injury, time the body fluid has spent ex vivo, level of viremia (viral load), and HIV status of the inoculating body fluid. Although less common, transmission of HCV may also occur from health care workers to patients. Because acute HCV infection is often subclinical, nosocomial transmission may occur with greater frequency than has been recognized previously. Strict adherence to universal precautions to protect health care workers and patients is critically important. No treatment has been proved effective for post-exposure prophylaxis, and no data support such treatment even if it were available.
Nonpercutaneous modes of HCV transmission include sexual practices and childbirth. Available evidence indicates that transmission by nonpercutaneous routes occurs but is inefficient. From 10% to 20% of patients with HCV infection report that their only risk factor is sexual exposure to a partner with HCV infection. Most seroepidemiologic studies, however, have demonstrated anti-HCV in only a small proportion of sexual contacts of HCV-infected persons. In a large prospective study of monogamous seronegative partners of HCV-infected patients who denied anal intercourse and intercourse during menstruation, no instances of HCV transmission of a virus with the same gene sequence occurred over a 10-year period of time. Similarly, a study in which 500 anti-HCV–positive persons and their long-term heterosexual partners were followed identified only 3 couples (0.6%) with concordant viral strains. The calculated maximum HCV transmission rate was 1 per 190,000 sexual contacts (0.07% per year). Therefore, many of the cases presumed to be the result of sexual transmission are likely the result of other, perhaps unreported or unrecognized, exposures. If the index sexual partner is infected with HIV or the partners engage in high-risk sexual practices (e.g., anal intercourse), however, the transmissibility of HCV is increased.
The incidence of acute hepatitis C has been reported to have increased in HIV-infected men having sex with men in the 2000s in different regions of the world, including the USA, Australia, and Europe. Permucosal risk factors including specific sexual practices and mucosally administered drugs have been suggested to be responsible for the increase in incidence of HCV transmission.
Compared with the high efficiency of perinatal transmission of HBV infection (see Chapter 79 ), the risk of perinatal transmission of HCV infection is low, averaging 5.1% to 6.7% for HCV-monoinfected patients and 2 to 3 times higher for HCV-HIV coinfected patients. , Mothers with a high serum level of HCV RNA (high viral load) are more likely to transmit HCV to their infants, a finding that may explain why infants born to mothers with HCV-HIV coinfection are at higher risk of HCV infection. The use of antiretroviral therapy (ART) in HCV-HIV coinfected mothers may decrease the risk of perinatal transmission of both HIV and HCV. Data regarding the risk associated with vaginal delivery as opposed to cesarean delivery are uncontrolled, but evidence for a higher risk of HCV transmission with vaginal delivery is unconvincing. This issue remains controversial, and some authorities recommend elective cesarean section before membrane rupture.
Although little data exist, the risk of HCV transmission from breastfeeding is negligible to small. The CDC and international societies have concluded that breastfeeding by HCV-infected mothers is generally safe. , Because anti-HCV can be acquired passively by the infant, testing for HCV RNA is required if the diagnosis of HCV infection is suspected. Infants of infected mothers should not undergo serologic testing for anti-HCV before 18 months of age, because maternal antibodies may persist in the infant’s serum and lead to diagnostic confusion.
The source of transmission is unknown in up to one third of cases of HCV infection. Such sporadic HCV infection probably results from an undisclosed or unrecognized percutaneous route of infection. This presumption is supported by the observation that intranasal cocaine use is not considered a major risk factor for HCV transmission (although it was considered a risk factor in the past). HCV infection can be acquired from noncommercial tattooing and body piercing when equipment is reused, shared, or improperly sterilized. Commercial tattooing is now well controlled and likely conveys little risk of HCV infection. Iatrogenic transmission of HCV is well documented in a variety of circumstances, most notably via contaminated multi-use vials and inadequately sterilized multi-use instruments and syringes, as seen with schistosomal treatment campaigns in Egypt.
Determinants of persistence of HCV include (1) the evasion of immune responses through several viral mechanisms; (2) inadequate induction of the innate immune response; (3) insufficient induction or maintenance of an adaptive immune response; (4) the production of viral quasispecies; and (5) the induction of immunologic tolerance or exhaustion. , Chronic hepatitis develops in 50% to 90% of persons with acute HCV infection. In the minority of patients in whom acute HCV resolves spontaneously, an early and multispecific T-cell response occurs. This response can be detected up to 20 years after resolution of infection and may contribute to protection in the case of subsequent exposures to HCV. Although the immune response is essential in preventing viral persistence, in those without viral clearance the immune response mediates hepatic cell destruction and fibrosis.
In chronically infected patients, the pathogenesis of liver damage is largely immune mediated. In a small subset of immunocompromised HCV-infected patients among both HIV-infected patients and organ transplant recipients, however, a syndrome termed fibrosing cholestatic hepatitis develops (see Chapter 97 ). , Such cases are thought to result from direct viral hepatotoxicity of infected cells, because viral levels are typically greater than 30 million copies/mL and hepatocytes contain enormous concentrations of virus and viral proteins. Survival in such patients has been poor.
The majority of patients with HCV infection have a variable immune response that, although inadequate to eradicate acute infection, appears to regulate the vigor of persistent infection and avoid the development of fibrosing cholestatic hepatitis. The immune response to HCV is incompletely understood because animal models that recapitulate human disease and immunology are not readily available, and therefore most studies in humans rely on observations in peripheral blood rather than the hepatic immune environment.
HCV infection elicits an immune response in the host that involves both an initial innate response and a subsequent adaptive response. The innate response is the first line of defense against the virus and includes several arms such as natural killer (NK) cell activation and cellular antiviral mechanisms triggered by pathogen-associated molecular patterns recognized by the cell (see Chapter 2 ). These processes can lead to apoptosis of infected cells within the first few hours of infection. NK cells, as the effector cells of the innate immune system, also produce TNF-β and IFN-α, cytokines that are critical for dendritic cell maturation and subsequent induction of adaptive immunity. NK cells can also attack virus-infected cells directly, as do other immune cells by different effector molecules. Subsequently, however, the virus initiates a number of mechanisms that undermine the ability of the host to control the infection.
Virus-related disruption of the innate, and later adaptive, immune response occurs at several levels. NK cell function is slowed possibly because NK cell-mediated cytotoxicity and production of cytokines are interrupted when the HCV E2 protein binds its cellular receptor CD81. Expression of TNF-related apoptosis-inducing ligand on NK cells correlates with disease activity in both acute and chronic hepatitis C, thereby suggesting that NK cells have a direct role in the immunopathogenesis of hepatitis C. Pathogen-associated molecular patterns activate several cellular processes, including the JAK-STAT (Janus kinase‒signal transducer and activator of transcription) proteins pathway and Toll-like receptor-3, activation of both of which ultimately results in production of cellular IFNs, IFN-stimulated genes (ISGs), and IFN-regulated factors that convey antiviral properties to the cell. NS3/4 protease degrades TRIF, an essential intermediate in this pathway, and cleaves IFN promoter stimulator-1, an intermediate in the signaling cascade, to block activation of IFN when retinoic inducible gene-1 binds viral intermediates. In addition, HCV core protein promotes STAT-1 degradation, inhibits STAT-1 phosphorylation, promotes suppressor of cytokine signaling induction (an inhibitor of JAK-STAT signaling), and impairs ISG factor-3 (ISGF3), a heterotrimer of STAT-1, STAT-2, and IFN-β promoter stimulator (IRF-9) from binding to the promoter regions of IFN-stimulated response elements, thereby inhibiting transcription of IFN response genes. Even when IFN response genes are activated, NS5A and E2 both can disrupt protein kinase R function to suppress translation, thereby allowing viral replication to continue. In addition, NS5A inhibits 2′-5′-oligoadenylate synthetase, which is expressed in response to HCV infection and leads to HCV RNA degradation. Taken together, HCV is able to disrupt the innate immune response at several levels, and these strategies appear to be pivotal in establishing the chronicity of infection.
The ability of HCV to impair the innate immune response prevents development of a vigorous adaptive immune response to the infection. NK cells do not adequately activate dendritic cells, and as a result, the priming of CD8 + and CD4 + T cells in HCV-infected patients is inadequate. Even if an adequate T-cell response is created, HCV-infected patients have a large number of regulatory T cells in their portal tracts ; intrahepatic immune regulation by these cells has not been demonstrated but is presumed.
HCV-specific T cells are enriched at the site of viral replication, with an increased number in the liver when compared with the peripheral blood. CD8 + lymphocytes predominate, suggesting that cytotoxic T lymphocytes are the main perpetrators of hepatocellular injury. The T-cell immune response in the liver may result in direct lysis of infected cells and inhibition of viral replication by secreted antiviral cytokines.
Whereas the cellular immune response plays a pivotal role in the pathogenesis of HCV infection, the importance of the humoral immune response is less clear. Antibodies to viral proteins are produced and do not appear to correlate with the stage of infection or immune reactivity. Furthermore, administration of high-titer HCV-enriched or HCV-specific Ig has little effect on viral levels or persistence in humans.
In summary, viral products play an integral role in the immune regulation that leads to chronic infection instead of viral clearance. Both the virus and the immune response probably play a role in the development of hepatocellular injury. The mechanisms by which hepatocellular injury leads to hepatic fibrosis are discussed in Chapter 74 .
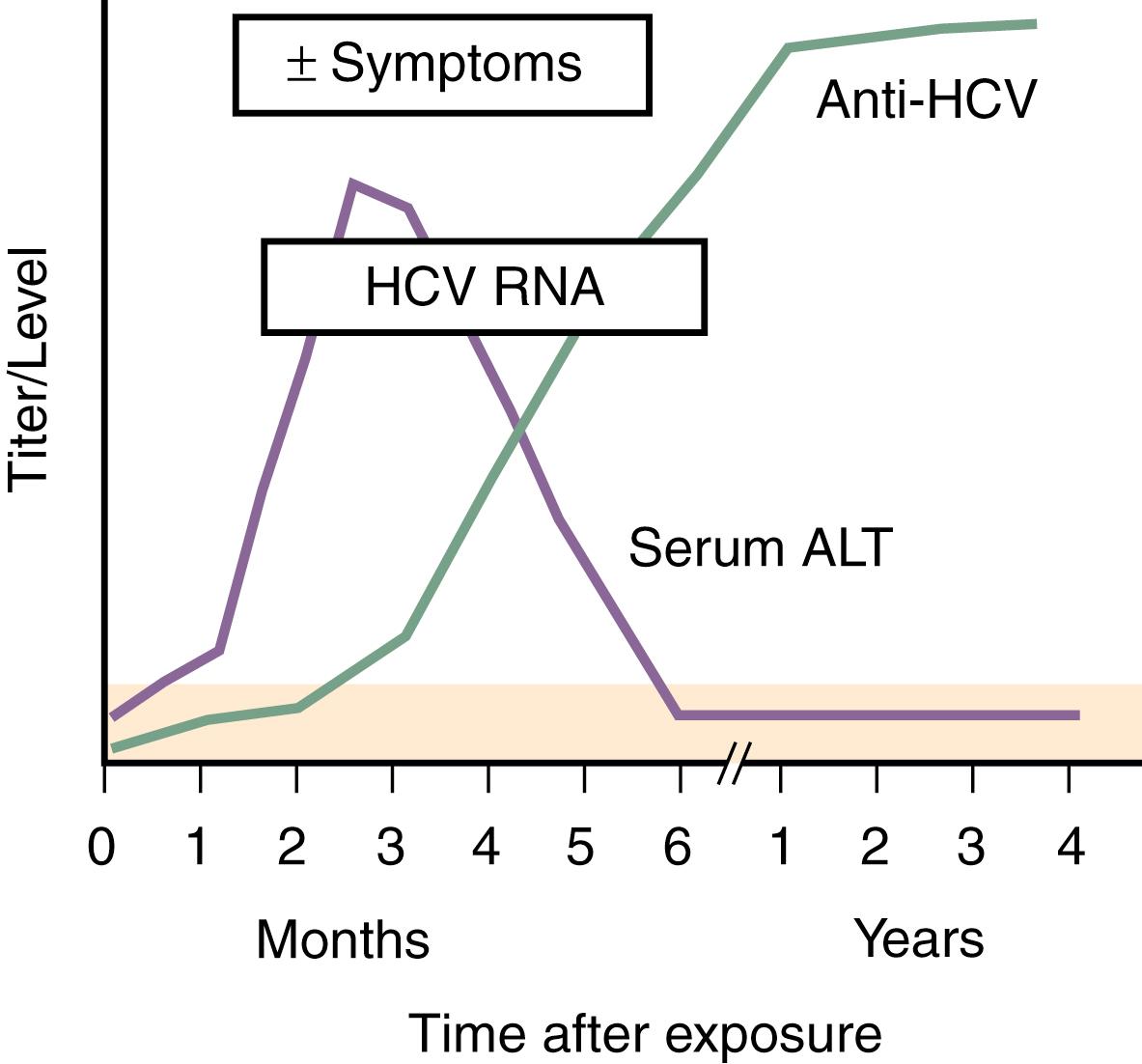
HCV accounts for an estimated 20% of cases of acute hepatitis. Acute hepatitis C is rarely seen in clinical practice, however, because nearly all cases are asymptomatic. Within 7 to 21 days after viral transmission, HCV RNA becomes detectable in serum. Longer incubation periods can occur, especially in cases in which only a small amount of virus has been transmitted. These data suggest that the duration of the incubation period may vary among different transmission routes. HCV RNA levels rise rapidly in serum after infection, followed by a delayed increase in serum ALT levels 4 to 12 weeks after infection, indicative of hepatic injury. Serum ALT levels frequently reach values more than 10 times the upper limit of normal, with concomitant rises in the serum bilirubin level in some inidividuals ( Fig. 80.3 ). Some patients also develop clinical symptoms 2 to 12 weeks after viral transmission, but the majority of patients remain asymptomatic during the acute phase and most infected persons do not become aware of their disease. Therefore, it is not easy to study the early phase of HCV infection. Several studies have investigated patients recruited during the acute symptomatic phase of HCV infection, and 80% of the patients have presented with diverse symptoms. Even in symptomatic patients, however, most of the clinical symptoms are nonspecific. Commonly reported symptoms include fatigue, nausea, abdominal pain, loss of appetite, mild fever, itching, and myalgia. Jaundice, which is the most specific liver-related symptom, develops in 50% to 84% of patients with clinically overt acute HCV infection. ALF caused by HCV has been reported in only single cases, in contrast to infections with other hepatotropic viruses (see Chapter 95 ). The presentation may be more apparent and the clinical course more severe when acute HCV infection occurs in patients who drink large amounts of alcohol or have coinfection with HBV or HIV.
The rate of viral persistence after acute infection ranges from 45% to more than 90%. Age and gender clearly influence the risk of chronicity, with younger and female patients having the lowest rates of chronicity. Other factors that may play a role include the source of infection and size of inoculum (chronicity may be less common in PWID than in those who acquire HCV infection by blood transfusion), immune status of the host (chronicity rates are higher in persons with immunodeficiency states such as agammaglobulinemia and HIV infection), and the patient’s race (rates of viral persistence are higher in African Americans than in whites and Hispanic Americans in the USA). Finally, the rate of spontaneous clearance is higher in symptomatic patients in whom jaundice develops during acute infection than in those who remain asymptomatic.
Single nucleotide polymorphisms (SNPs) close to the IFN lambda-3, or interleukin 28B, gene ( IFN-λ3, IL28B ) have been found to be associated with the outcome of acute hepatitis C. The IFN-λ3 gene is located on chromosome 19 and encodes IFN- λ 3. Ge and colleagues reported a significant role for a specific SNP in the IFN-λ3 gene region (rs12979860 CC) in the response to pegylated IFN-α–based therapy for chronic hepatitis C in 2009. Shortly thereafter, Thomas and colleagues identified a major contribution for the same SNP in spontaneous clearance of acute HCV infection. Subsequently, these findings were confirmed by other investigators in different cohorts. , An association between clearance of acute HCV infection and the IFN-λ3 genotype may differ, however, between symptomatic and asymptomatic patients, because the CC IFN-λ3 polymorphism was associated with spontaneous recovery only in nonicteric patients in a cohort of East German women exposed to HCV in a single-source outbreak in the late 1970s.
Serum ALT levels are usually elevated in patients with chronic HCV infection. Because levels commonly fluctuate, however, as many as half of patients may have a normal ALT level at any given time. The ALT level may remain normal for prolonged periods of time in about 20% of cases, although transient elevations occur even in these cases. Persistently normal ALT levels are more common in women, and such cases typically are associated with lower serum HCV RNA levels and less inflammation and fibrosis on liver biopsy specimens.
Most patients with chronic hepatitis C are asymptomatic before the onset of advanced hepatic fibrosis. Patients who have been diagnosed with chronic infection, however, often complain of nonspecific symptoms such as fatigue, vague abdominal pain, or depression, and they consistently score lower than HCV-negative persons in all aspects of health-related quality of life (HRQOL). Whether the decrease in HRQOL is related to viral factors, social factors (e.g., injection drug use), social stigmatization, or worry related to the diagnosis itself is unclear. Nevertheless, HRQOL scores improve if the patient achieves a sustained response to antiviral therapy. Less common symptoms may include arthralgias, paresthesias, myalgias, sicca syndrome, nausea, anorexia, and difficulty with concentration. The severity of these symptoms may be, but is not necessarily, related to the severity of the underlying liver disease.
Patients with HCV infection may present with extrahepatic conditions, or these manifestations may occur in patients known to have chronic HCV infection. Classification of the extrahepatic manifestations of HCV is shown in Box 80.1 and is based on the strength of available data to prove a correlation. Types 2 and 3 cryoglobulinemia, characterized by polyclonal IgG plus monoclonal IgM and polyclonal IgG plus polyclonal IgM, respectively, can both be caused by HCV infection. Among HCV-infected patients, 19% to 50% have cryoglobulins in serum, but clinical manifestations of cryoglobulinemia are reported in only 5% to 10% of these patients and are more common in patients with cirrhosis. Symptoms and signs include fatigue, arthralgias, arthritis, purpura, Raynaud phenomenon, vasculitis, peripheral neuropathy, and nephropathy. The diagnosis is clear when a rheumatoid factor is detected, cryoglobulins are present, and complement levels are low in serum; however, the reliability of cryoglobulin measurements is dependent on proper handling and processing of the sample.
Autoimmune thyroiditis
B-cell non-Hodgkin lymphoma
Lichen planus
Mixed cryoglobulinemia
Monoclonal gammopathies
Porphyria cutanea tarda
Chronic polyarthritis
Diabetes mellitus
Idiopathic pulmonary fibrosis
Noncryoglobulinemic nephropathies
Sicca syndrome
Thyroid cancer
Renal cell carcinoma
Vitiligo
Glomerular disease generally manifests as cryoglobulinemic nephropathy, membranoproliferative glomerulonephritis, and membranous nephropathy. Cryoglobulinemic nephropathy manifests as hematuria, proteinuria, edema, and renal insufficiency of varying degrees and features of membranoproliferative glomerulonephritis on renal biopsy specimens. At diagnosis, 20% of patients with type 2 cryoglobulinemia have renal involvement, and renal involvement develops in another 35% to 60% over time. In about 15% of patients, cryoglobulinemic nephropathy progresses to end-stage kidney disease requiring dialysis.
As HCV infection drives these extrahepatic manifestations, treatment of the underlying HCV infection should be considered in patients with symptomatic cryoglobulinemia. There are limited data regarding the use of IFN-free DAA regimens to treat extrahepatic manifestations of HCV, the majority of which involved treatment with the first-generation protease inhibitors combined with PegIFN/RBV, which are no longer recommended. However, given the high efficacy of the newer IFN-free DAA regimens, consensus guidelines recommend treatment of extrahepatic manifestations with IFN-free DAA regimens as per standard HCV infection treatment guidelines. In addition to antiviral therapy for HCV infection, monoclonal antibody therapy targeting B cells (anti-CD20 therapy with rituximab) has been shown to be useful for HCV-related cryoglobulinemia, particularly in patients with severe renal disease, because rituximab reduces B-cell clones that are responsible for producing cyroglobulins. , Although this approach has been shown to be effective in randomized controlled studies when used alone or in combination with IFN plus RBV, rituximab is not licensed for treatment of extrahepatic manifestations of HCV. Prednisone, cyclophosphamide, other chemotherapeutic agents, and plasmapheresis have been used with variable success; however, these approaches do not treat the underlying HCV infection.
Patients with vasculitis due to HCV infection may benefit from low-dose interleukin-2 therapy. This cytokine may promote the survival of immunosuppressive regulatory T cells.
HCV infection is associated with the development of B-cell non-Hodgkin lymphoma and monoclonal gammopathy of uncertain significance. The relative risk of lymphoma is small (1.28) in the USA. The most prevalent forms of lymphoma found in patients infected with HCV are follicular lymphoma, chronic lymphocytic lymphoma, lymphoplasmacytic lymphoma, and marginal zone lymphoma. Type 2 cryoglobulinemia evolves into lymphoma over time in 8% to 10% of patients. Despite the known association of HCV infection with lymphoma, HCV RNA does not integrate into the host genome and therefore HCV cannot be considered a typical oncogenic virus. Rather, HCV shows lymphotropism and may facilitate the development and selection of abnormal B-cell clones by chronic stimulation of the immune system. In addition, genetic rearrangements in B cells, specifically the Bcl2/J H rearrangement and the t(14;18) translocation, have been found in HCV-infected patients in some, but not all, studies.
Other extrahepatic manifestations of HCV infection include porphyria cutanea tarda, lichen planus, and sicca syndrome. In addition, insulin resistance and diabetes mellitus have been thought to be associated with HCV infection, although the association has been questioned. The SVR to antiviral therapy for HCV infection is reduced in insulin-resistant patients; however, if HCV can be eradicated, insulin resistance often improves, an observation that further supports the relationship between HCV infection and insulin resistance. Although associations between HCV infection and both thyroid cancer and idiopathic pulmonary fibrosis have been described, data about the effect of HCV eradication on disease progression are lacking. A myriad of other conditions has been observed in association with HCV infection, but a true link has not been firmly established for these disorders (see Box 80.1 ).
Although not associated with disease, seropositivity for autoantibodies (e.g., ANA with a titer greater than 1:40 in 9%, smooth muscle antibodies with a titer greater than 1:40 in 20%, anti‒liver-kidney microsomal antibodies in 6%) is found in many HCV-infected persons. Therefore, the diagnosis of an autoimmune condition in a patient with HCV infection can never be based on serology alone.
The spectrum of extrahepatic manifestations may adversely impact the overall survival of HCV-infected persons. The prospective Taiwanese population-based R.E.V.E.A.L.-HCV (Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer) study, in which almost 24,000 adults 30 to 65 years of age were followed, demonstrated that HCV-infected persons had not only increased liver-related mortality, but also higher mortality from extrahepatic diseases compared with anti-HCV–negative persons.
Several immunologic and molecular assays are used to detect and monitor HCV infection. The presence of anti-HCV in high titer in serum (generally an enzyme immunoassay [EIA] ratio > 9) indicates exposure to the virus but does not differentiate among acute, chronic, and resolved infection. Anti-HCV usually persists for many years in patients after spontaneous resolution of infection or an SVR following antiviral therapy. Anti-HCV titers may decline over time, however, and can become undetectable 5 to 20 years after HCV clearance. , Serologic assays are used initially for diagnosis, whereas virologic assays are required for confirming infection, monitoring response to treatment, and evaluating immunocompromised patients.
EIAs detect antibodies against different HCV antigens. The time course of the development of symptoms, detection of anti-HCV, and appearance of HCV RNA after acute infection is shown in Fig. 80.3 . Three generations of EIAs have been developed. The third-generation EIAs detect antibodies against HCV core, NS3, NS4, and NS5 antigens as early as 7 to 8 weeks after infection, with sensitivity and specificity rates of 99%. Despite ongoing viral replication, serologic test results can be negative in patients who are on hemodialysis or are immunocompromised. Because the performance characteristics of third-generation EIAs are so good, confirmation with a recombinant immunoblot assay is no longer required. Instead, patients who are anti-HCV positive should undergo HCV RNA testing to determine if they have active viremia or have cleared the infection.
Quantitative, highly sensitive, “real-time” HCV RNA tests represent the state of the art for determining HCV viremia in anti-HCV–positive persons. The lower limit of detection of most assays varies from 10 to 15 international units (IU)/mL. These assays have a linear dynamic range of 1 to 7 log 10 IU/mL and are the preferred testing method in practice. Transcription-mediated amplification is also extremely sensitive, but available assays are not quantitative in the lower dynamic range of the test. The advantages of these very sensitive tests include positivity within 1 to 3 weeks after acute infection and detection of low-level residual infection during antiviral therapy.
A disadvantage of all quantitative tests is the lack of comparability among different assays. Although conversion to a standard IU/mL concentration attempted to resolve such discrepancies, results are still variable. Reported conversion factors vary from 0.9 copies/mL to 5.2 copies/mL per IU/mL. For this reason, the same laboratory and assay are recommended during antiviral treatment monitoring.
A cheaper and faster alternative to nucleic acid testing for HCV RNA to confirm HCV viremia is the HCV core antigen assay. Fully automated immunoassays have been developed that detect the HCV core antigen, and the assays have proved to be robust across HCV genotypes and in different patient populations, , but with major limitations in sensitivity. Therefore, the assay cannot be used to monitor response to antiviral therapy and make decisions regarding therapy. If viremia needs just to be confirmed, however, HCV core antigen testing is a reasonable alternative to HCV RNA testing.
Identifying the genotype and subtype of HCV is important because some DAA regimens are only recommended for certain HCV genotypes and subtypes. HCV genotyping can be accomplished by several methods. The most accurate approach uses PCR methodology and direct sequencing of the NS5B or E1 region; however, this approach is not practical in clinical practice. HCV genotyping can be performed by evaluating type-specific antibodies and has a 90% concordance in immunocompetent patients when results are compared with sequence analysis of the HCV genome. Testing can also be accomplished with reverse hybridization to genotype-specific probes, restriction fragment length polymorphism analysis, or PCR amplification of the 5′ noncoding region of the HCV genome. These tests have 92% to 96% concordance with the correct genotype; genotype 1 is identified with the highest accuracy. Because of mutations in the regions studied, errors in subtype identification occur in 10% to 25% of cases regardless of the technique used. A line-probe assay (INNO-LiPA) using genotype-specific probes for reverse transcription of the 5′ portion of the HCV genome is the most popular commercial assay for HCV genotyping.
For patients at low risk for HCV infection, a negative result of an EIA for anti-HCV is sufficient to exclude HCV infection. HCV RNA testing should be performed to confirm active infection if a positive anti-HCV test is returned. In high-risk patients, such as those with an elevated serum ALT level who have a known risk factor for HCV, have experienced recent exposure, or are either immunocompromised or on dialysis, a positive anti-HCV result is sufficient to confirm HCV infection; however, HCV RNA testing should also be performed to confirm that the infection is active. If the anti-HCV result is negative, then HCV RNA testing should be performed in patients with a recent exposure in case the anti-HCV result is falsely negative because of insufficient time for anti-HCV to develop or immunocompromise in the host with failure to produce sufficient anti-HCV.
The risk of progressive hepatic injury from HCV infection varies considerably, with some patients showing little or no progression after decades of infection and others progressing rapidly to cirrhosis. The presence or absence of cirrhosis also influences the choice and duration of treatment; therefore, an assessment of the degree of liver injury is recommended in all patients with HCV infection. For many years, this assessment was performed by percutaneous liver biopsy ( Box 80.2 ), but noninvasive methods are now used for initial assessment of liver fibrosis stage, with liver biopsy reserved when noninvasive markers are indeterminate, noninvasive test results are incongruent with other noninvasive liver fibrosis markers or with the clinical picture, or other causes of liver disease need to be excluded.
Assessment of the need for surveillance for HCC
Evaluation for concomitant liver diseases
Guidance for decisions regarding treatment of hepatitis C
Staging of fibrosis, including when noninvasive fibrosis markers are incongruent with other methods of noninvasive fibrosis assessment or with the clinical picture
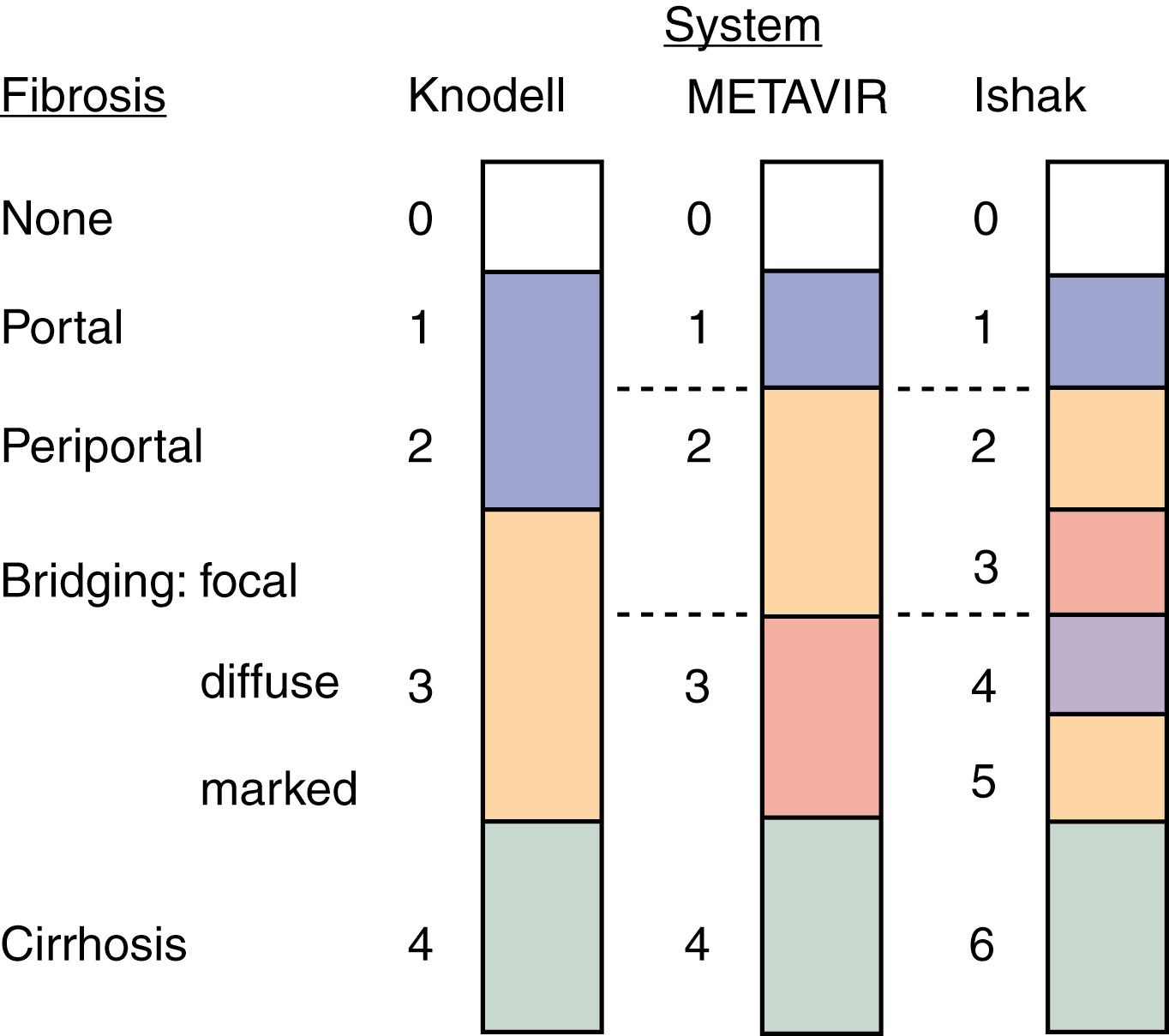
Several histologic scoring systems have been used to quantify hepatic injury into discrete grades of inflammation and stages of fibrosis ( Fig. 80.4 ) (see also Chapter 73 and Chapter 74 ). The first system used was the Histology Activity Index described by Knodell and colleagues. The components of this system include periportal inflammation and necrosis (graded as 0 to 10), lobular inflammation and necrosis (0 to 4), portal inflammation (0 to 4), and fibrosis (0 to 4). This scoring system combines inflammation and fibrosis into one score. Scheuer created a simplified scoring system that separates grade of inflammation from stage of fibrosis: portal inflammation and interface hepatitis (0 to 4), lobular activity (0 to 4), and fibrosis stage (0 to 4). The Ishak system is a modification of Knodell’s system but separates histologic grade from fibrosis stage. Ishak’s fibrosis scores range from 0 to 6 (1 or 2, portal fibrotic expansion; 3 or 4, bridging fibrosis; 5 or 6, cirrhosis) (see Fig. 80.4 ). The higher number of stages of fibrosis has made the Ishak system popular for scoring progression of fibrosis in clinical trials. The METAVIR scoring system is the most popular in practice; it is simpler than all the aforementioned systems. Inflammation is graded from 0 to 4 (none, mild, moderate, and severe), and fibrosis is staged from 0 to 4 (1, portal fibrotic expansion; 2, portal fibrosis with septa formation; 3, bridging fibrosis; 4, cirrhosis) (see Fig. 80.4 ).
Although examination of liver biopsy specimens is still considered the gold standard for establishing the grade of inflammation and stage of fibrosis, limitations of liver biopsy include (1) associated morbidity (pain occurs in as many as 30% in some series, and hemorrhage or bile leak occurs in 0.3% of patients) and mortality (0.03%); (2) cost; (3) poor patient acceptance; (4) intraobserver and interobserver variability in the interpretation of findings (with current scoring systems, intraobserver and interobserver concordance for staging fibrosis among hepatopathologists is ≈90% and 85%, respectively); (5) inaccuracy in the interpretation of findings, particularly for the diagnosis of cirrhosis (with a false-negative rate of 15%); and (6) sampling error (a 33% difference in 1 stage of fibrosis and 2.4% difference in 2 stages of fibrosis is seen in simultaneously obtained biopsy specimens from the right and left hepatic lobes). , Interobserver and intraobserver variability is increased when inexperienced pathologists use a complicated scoring system to evaluate liver tissue. Sampling error is especially common when small biopsy specimens are obtained. A biopsy should be done with at least a 16-gauge needle, be 15 to 20 mm or more in length, and contain at least 6 portal triads, although 11 or greater is considered optimal. ,
Because of the limitations of liver biopsy, several noninvasive tests to estimate fibrosis have been developed ( Table 80.1 ) (see also Chapter 74 ). FibroSure (or FibroTest) is a noninvasive measure of fibrosis that creates a composite score, adjusted for gender and age, derived from the serum levels of α 2 -macroglobulin, haptoglobin, apolipoprotein A-1, GGTP, and total bilirubin. The test accurately categorizes patients with stage 0 and 1 fibrosis and those with cirrhosis; however, it is less useful in patients with intermediate scores. The AST-to-platelet ratio index (APRI) is used primarily to diagnose or exclude cirrhosis. In an initial evaluation, 81% of cirrhotic patients were accurately excluded with an APRI score of 0.5 or less; however, the index does not discriminate among lower levels of fibrosis.
| Test | Number of Patients Studied | Fibrosis Staging System | Histologic Fibrosis (F) Stages Compared | Sensitivity (%) ∗ | Specificity (%) ∗ | PPV for Fibrosis- Cirrhosis (%) | Test Accuracy (%) † |
|---|---|---|---|---|---|---|---|
| APRI | 270 | Ishak | F0-2 vs. F3-6 | 41 | 95 | 88 | 70 |
| F0-4 vs. F5-6 | 89 | 75 | 57 | 77 | |||
| FibroSure | 339 | METAVIR | F0-1 vs. F2-4 | 100 | 22 | 50 | 57 |
| F0-2 vs. F3-4 | 70 | 95 | 91 | 84 | |||
| Transient elastography (FibroScan) | 327 | METAVIR | F0-1 vs. F2-4 | 56 | 91 | 88 | 68 |
| F0-3 vs. F4 | 86 | 96 | 78 | 94 |
∗ Sensitivity and specificity for distinguishing higher stages of fibrosis from lower stages of fibrosis.
† Accuracy = (sensitivity) (prevalence) + (specificity) (1 − prevalence).
Additional techniques and instruments (e.g., transient elastography, acoustic radiation force impulse imaging, magnetic resonance elastography) are now available to determine liver stiffness (see Chapter 74 ). The most frequently used system is transient elastography (FibroScan) to assess liver stiffness, which correlates with the amount of hepatic fibrosis. In a meta-analysis, the area under the receiver operating curve (an estimate of accuracy) of FibroScan for predicting cirrhosis was 0.94. Combining transient elastography with serum markers increases the accuracy of predicting fibrosis and cirrhosis and may avoid liver biopsy in many patients. , Although noninvasive testing has improved dramatically, all available tests have limitations. Most importantly, the degree of hepatic inflammation is not assessed by these tests, and inflammation may significantly alter noninvasive test results. Moreover, although cirrhosis is accurately predicted by several noninvasive tests, the finer discrimination of the fibrosis score is not as reliable as examination of liver biopsy specimens.
Regardless of the degree of serum aminotransferase elevations, a determination of the stage of liver fibrosis, either by liver biopsy or noninvasive methods, is recommended in patients undergoing initial assessment of chronic hepatitis C. Liver biopsy is not required when cirrhosis is already suggested by clinical findings (e.g., ascites, splenomegaly, spider telangiectasias, low platelet count, prolonged prothrombin time) or imaging (e.g., nodularity of the liver, evidence of portal hypertension). It is also not indicated following successful antiviral therapy, although histology generally improves significantly over time following eradication of HCV (see later) Surveillance for HCC and varices is recommended for all patients with cirrhosis, including patients who achieve an SVR12 with antiviral therapy, because these patients remain at increased risk (see Chapter 92 and Chapter 96 ).
Once chronic HCV infection is established, spontaneous HCV clearance rarely occurs. Chronic hepatitis C can cause continuous liver damage, resulting in liver cirrhosis and subsequently HCC ( Fig. 80.5 ). The individual course of liver disease is highly variable. Patients may report symptoms such as RUQ discomfort, nausea, fatigue, myalgia, arthralgias, or weight loss. All of these clinical features are nonspecific, however, and are not associated with the severity of liver injury. Most liver-related symptoms are restricted to patients with advanced cirrhosis.
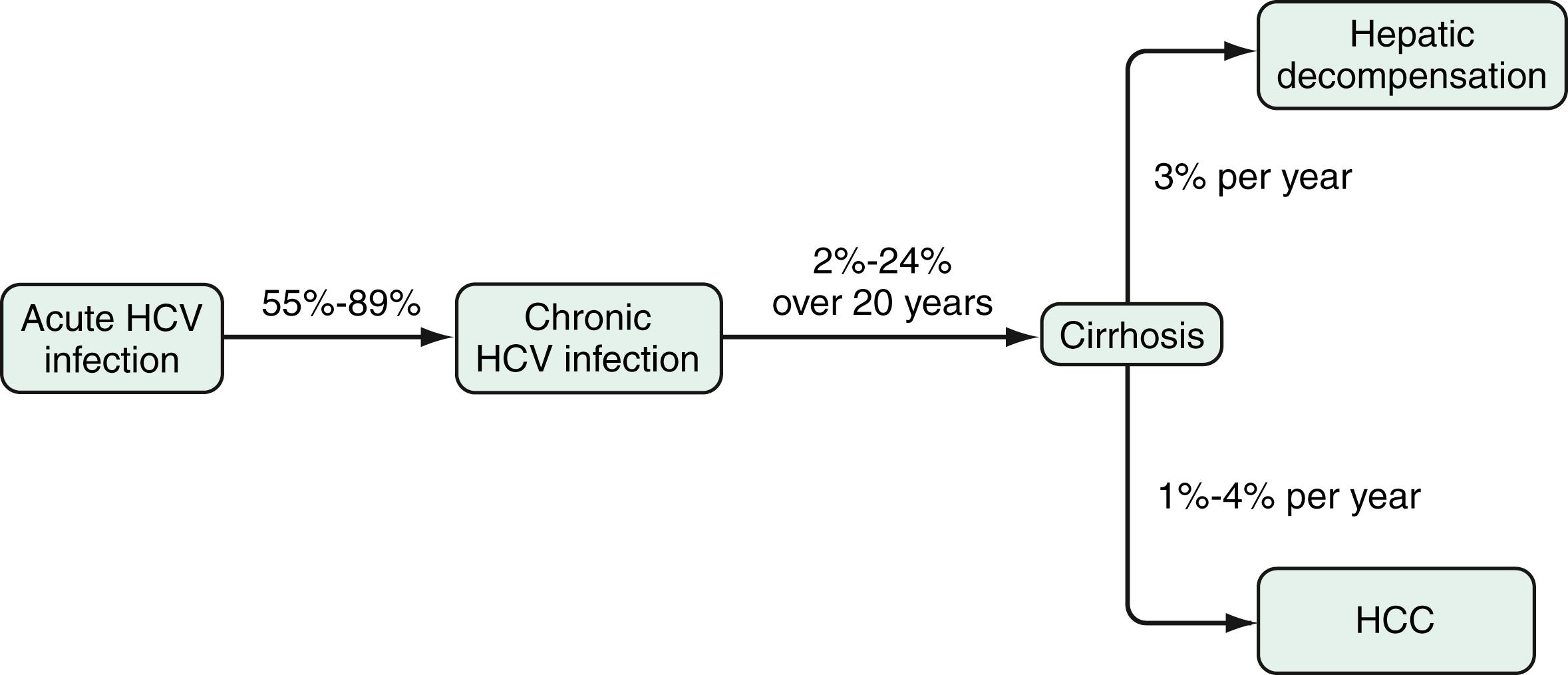
The most feared complication of chronic HCV infection is liver-related mortality due to decompensated liver cirrhosis (see Chapter 74 and Chapter 92, Chapter 93, Chapter 94 ) or development of HCC (see Chapter 96 ). Studies published since the 1990s have shown remarkably different frequency rates of cirrhosis. Whereas very low rates of cirrhosis were reported in some cohorts like young women infected in the late 1970s through receipt of contaminated anti-D immune globulin, cirrhosis has been described in up to 69% of patients in hospital-based settings. In a meta-analysis, Thein and colleagues calculated that in a large number of studies that have been published, cirrhosis developed on average in 16% of patients within 20 years after the onset of HCV infection. Cirrhosis was attributable to HCV infection in 27% of cases, with a wide range among studies (14% to 62%) that can be explained by regional differences and the presence of cofactors. ,
A key challenge in clinical practice is to identify persons at high risk for disease progression who may require more immediate antiviral therapy. Several factors reported to influence the liver-related outcomes of chronic hepatitis C remain controversial ( Table 80.2 ; see next section). Still, some of these factors may help estimate the risk of cirrhosis and identify groups of patients who require immediate antiviral treatment.
| Established | Possible | Not Associated |
|---|---|---|
|
|
|
Age is one of the most important risk factors for fibrosis progression in chronic HCV infection (see Table 80.2 ). A longer duration of infection has also been associated with a higher stage of liver fibrosis, but HCV infection acquired during childhood seems to follow a milder course. Overall, the development of HCV-related cirrhosis seems to be a dynamic process that accelerates exponentially with increasing age. The mechanisms by which progression of fibrosis accelerates with aging are not well defined. Changes in the regenerative capacity of the liver, alterations in the immune system, and telomere shortening may play roles. A higher risk for fibrosis progression in patients older than 40 years has been described in patients with various causes of liver disease. Some studies, but not others, have suggested that older age in general, and more specifically older age at the time of infection, is a risk factor for progression of fibrosis. Overall, cirrhosis has been predicted to develop in most patients with hepatitis C by about 65 years of age, irrespective of the age at infection.
Some studies have suggested that the mode of viral transmission may influence the degree of liver damage; however, the role of the route of transmission in fibrosis progression remains controversial. By contrast, female gender seems to be protective, and fibrosis progression is much faster in HCV-infected men, thereby suggesting that hormonal factors may be important in the regulation of liver fibrosis. Genetic factors also play a role in the development of cirrhosis. Histologic activity and the frequency of cirrhosis are lower in African Americans than in Caucasians. Several specific genes have been suggested to be involved in fibrosis progression; these include certain variants of the HLA class I and II antigens. A cirrhosis risk score based on polymorphisms in 7 genes has been proposed for patients with HCV infection ; this score was able to predict fibrosis progression in patients with initially mild chronic hepatitis C.
Elevated serum aminotransferase levels are used widely as a surrogate for ongoing intrahepatic inflammation, and elevated serum ALT levels during chronic hepatitis C are associated with an increased risk of liver fibrosis progression. Lower progression rates of fibrosis are reported in patients with normal serum ALT levels, but normal levels do not exclude the possibility of fibrosis progression.
Differences in the natural history of hepatitis C have been reported for different HCV genotypes. Several studies have described accelerated disease progression in patients infected with HCV genotype 3, which is consistent with higher reported mortality rates in patients infected with HCV genotype 3. Flares of hepatitis seem to occur more frequently in HCV genotype 2 infection and may result in a more severe course of liver disease. By contrast, viral load is not related to the degree of liver damage or fibrosis.
Hepatic steatosis is a histologic hallmark of chronic hepatitis C. Several studies have shown that steatosis is linked to the stage of liver fibrosis in patients with chronic HCV infection. Some studies suggest that HCV infection itself can trigger hepatic steatosis as well as NASH, and HCV infection may cause insulin resistance. There is also evidence for a direct association between HCV infection and hepatic steatosis. The strongest association exists between HCV genotype 3 infection and steatosis, and a direct molecular effect has been shown in a mouse model in which HCV genotype 3 is expressed.
Mild to moderately increased hepatic iron stores are associated with more advanced liver fibrosis. A consistent relationship between C282Y or H63D heterozygosity (see Chapter 75 ) and increased progression of fibrosis in patients with HCV infection has not been established, however. A reduction in hepatic iron concentrations does not reduce the risk of progression of fibrosis or improve the response to antiviral treatment.
Excessive alcohol consumption is clearly an independent major cause of cirrhosis, and chronic alcohol intake of more than 50 g/day is associated with a remarkable increase in the risk of cirrhosis in HCV-infected patients. On the other hand, coffee consumption has been reported to have a beneficial effect on overall mortality from HCV infection in population-based studies, and drinking coffee has been associated with a more favorable course of liver disease in general. Freedman and colleagues have also shown that greater coffee consumption correlates with a lower stage of liver fibrosis, reduced fibrosis progression, less steatosis and insulin resistance, and lower serum ALT levels; the best outcomes occur in persons who drink 3 or more cups per day. ,
The incidence of HCC has been rising rapidly in the industrial countries since the 1980s (see Chapter 96 ). In the USA, the incidence of HCC is 3 times higher than in 1975, and the global HCV epidemic has contributed to the rising incidence of HCC worldwide. Overall, chronic hepatitis C is responsible for approximately 25% of cases of HCC worldwide, with particularly high prevalence rates in East Asia. Because the development of HCC in HCV-infected patients is an indolent and age-dependent process, the peak incidence of HCV-related HCC has not been reached yet in Europe and the USA, where the majority of infections occurred in the 1970s and 1980s. In some European countries such as Italy, however, the peak rate of HCC-related mortality may have been reached. In contrast to chronic hepatitis B, HCC due to HCV usually does not develop in noncirrhotic livers, although HCC may be detected in some patients in whom cirrhosis has not yet developed. Lok and colleagues reported an HCC frequency rate of 0.8% per year in noncirrhotic patients with chronic hepatitis C who had advanced liver fibrosis ; however, the risk is much higher in patients with cirrhosis, with a rate of 1.4% to 4.9% per year. The overall 5-year risk of HCC has been reported to be as high as 7% to 30% in patients with HCV-related cirrhosis. , The appearance of HCC is frequently the first clinical complication of HCV-related cirrhosis and often occurs before hepatic decompensation becomes evident.
Risk factors for the development of HCC in patients with chronic HCV infection are similar to those associated with the development of cirrhosis. For example, older age is related to a higher frequency of HCC, and male gender and substantial alcohol consumption are well-established risk factors. Moreover, type 2 diabetes mellitus has been identified as an important independent risk factor. , Coinfection with HBV increases the risk of HCC. Importantly, the various risk factors act synergistically to enhance the overall risk of HCC. Genetic factors also contribute to the development of HCC. Kumar and colleagues performed a genome-wide association study in 721 persons with HCV-related HCC and showed that a SNP (rs2596542) at the gene encoding MICA (major histocompatibility class I polypeptide-related sequence A) was strongly associated with the development of HCC in HCV-infected persons. Conversely, coffee consumption has been associated with a reduced risk of HCC.
IFN-α monotherapy was approved for the treatment of chronic hepatitis C, then known as non-A, non-B hepatitis, before HCV was even identified. Substantial advances have been made since then with the introduction of prolonged treatment periods, longer-acting pegylated formulations of IFN, the oral guanosine analog RBV, and, most recently, the DAAs. The development of the replicon system and crystallization of the HCV nonstructural proteins (see earlier) paved the way for characterization of the HCV life cycle, generation of high throughput models for drug development, and, ultimately, the development of DAAs. In 2011, the first DAAs, telaprevir and boceprevir, were approved for the treatment of chronic HCV genotype 1 infection, and in 2013, simeprevir (another protease inhibitor) and sofosbuvir (a first-in-class nucleotide polymerase inhibitor) were approved, all initially used in combination with PegIFN and RBV. The development of highly potent, well-tolerated IFN-free DAA regimens, many of which have been approved by the FDA, has resulted in a complete shift in the treatment paradigm of hepatitis C. As treatment has been evolving, the choice of agents will likely change from being increasingly highly individualized, based on the availability of DAAs, HCV genotype, and stage of liver disease, to a single regimen that is applicable to all patients.
The primary goal of therapy for HCV infection is eradication of the virus. A consequence of achieving this goal is prevention of liver-related deaths associated with the development of decompensated cirrhosis and HCC. SVR—the absence of detectable virus in blood 12 weeks after completion of therapy—is an excellent surrogate marker for the resolution of HCV infection. Late relapses are rare. Long-term follow-up studies confirm sustained responses in more than 99% of cases if the patient is HCV RNA negative in serum 12 weeks after completion of DAA therapy. SVR is also associated with a reduction in hepatic inflammation and regression of fibrosis during IFN plus RBV therapy ( Fig. 80.6 ). Moreover, an improvement in HRQOL has been documented in patients successfully treated with IFN plus RBV. Data regarding these endpoints during DAA therapy are limited owing to the more recent implementation of these regimens and thus limited long-term follow-up; however, elastography fibrosis scores improve following successful DAA therapy, although it is unclear if this represents true regression of fibrosis or resolution of hepatic inflammation. ,
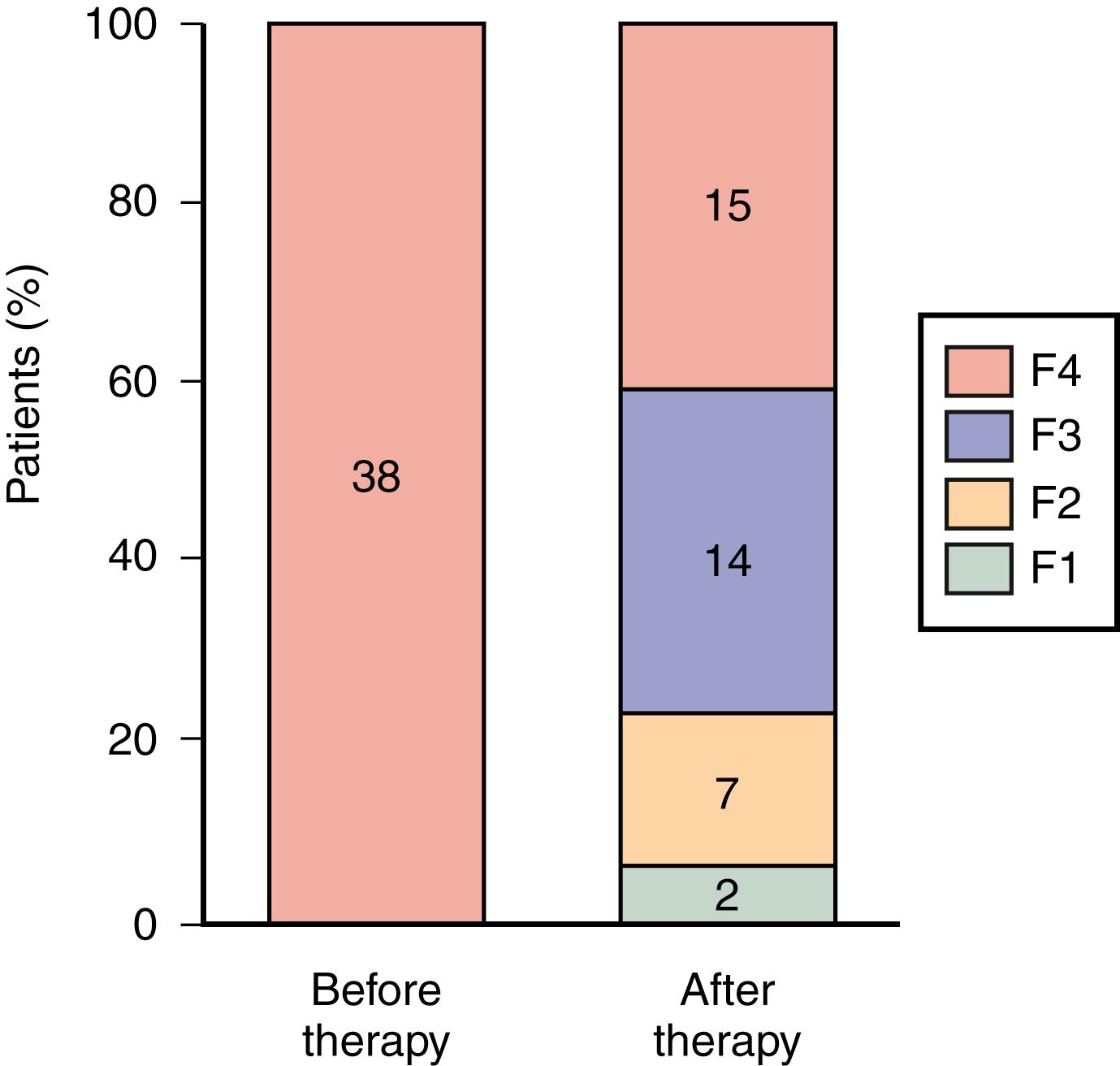
Antiviral treatment prevents the development of clinical endpoints. A significant reduction in liver-related deaths and hepatic decompensation can be observed following SVR with DAAs even in patients who already have advanced liver fibrosis. A reduction in the frequency of HCC has also been documented in patients who respond during antiviral therapy. , An SVR is also associated with improved overall survival in patients who had advanced fibrosis or liver cirrhosis at the time of therapy. , In particular, an SVR following PegIFN/RBV has been associated with a lower frequency of end-stage renal failure, cardiovascular disease, , and cerebrovascular disease. ,
The development of highly efficacious and well-tolerated IFN-free DAA regimens, together with the clear benefit of HCV eradication on all-cause and liver-related mortality and quality of life, means that antiviral therapy should be considered in all patients with chronic hepatitis C. Furthermore, with the increasingly broad range of IFN-free and many RBV-free DAA regimens with different pharmacokinetic and drug-drug interaction (DDI) properties available, there are very few clinical scenarios for which treatment is contraindicated, with the exceptions being early acute HCV infection, decompensated cirrhosis with a high MELD score in a patient waitlisted for LT (see later and Chapter 97 ), and pregnancy.
Because RBV is a teratogen, unwillingness of the patient and his or her partner to practice adequate contraception and avoid pregnancy during treatment and for 6 months after the discontinuation of therapy is an absolute contraindication to starting or continuing a DAA regimen that includes RBV. There are insufficient data regarding the safety of the new DAAs in pregnancy, and, therefore, they are not recommended during pregnancy.
During IFN-based therapy, the rate of HCV clearance from the circulation was an important predictor of subsequent SVR24. Predictors of an SVR included a rapid virologic response (RVR, negative HCV RNA level at treatment week 4) and a complete early virologic response (negative HCV RNA level at treatment week 12). However, with the extremely potent DAAs, the majority of patients achieve an RVR during DAA therapy. Furthermore, failure to attain an RVR does not preclude subsequent SVR12 with DAA-based therapy. , The AASLD/Infectious Diseases Society of America (IDSA) Guidance recommends HCV RNA testing at treatment week 4, not to determine treatment stopping rules, but to ensure treatment adherence.
IFN-based regimens became the cornerstone of antiviral therapy for HCV infection in the late 1980s. IFNs are naturally occurring glycoproteins that exert a wide array of antiviral, antiproliferative, and immunomodulatory effects. Pegylated IFNs consist of IFN bound to a molecule of polyethylene glycol (PEG) of varying length. The large size of the molecule increases the half-life of the IFN, thereby allowing once-weekly dosage. Two pegylated IFNs are licensed for use in the USA and elsewhere. The first is 40-kd peginterferon alfa-2a, used in a fixed dose of 180 μg per week. The second is 12-kd peginterferon alfa-2b, prescribed according to the patient’s body weight in a dose of 1.5 μg/kg per week. Pegylated IFNs replaced standard IFN, used in the past, and resulted in a significant increase in the SVR. The use of IFN has been succeeded by IFN-free DAA regimens.
RBV is an oral guanosine analog with activity against DNA and RNA viruses. When RBV is used in combination with IFN, the end-of-treatment response improves and the relapse rate decreases. Several mechanisms to explain the synergistic effect of RBV when administered in combination with IFN have been proposed, including (1) alterations of the cytokine milieu leading to a change from a type 2 T-helper cell (Th2) to a Th1 immune response; (2) depletion of intracellular guanosine triphosphate through inhibition of the host enzyme inosine monophosphate dehydrogenase; (3) inhibition of the action of the HCV RdRp; (4) induction of lethal mutagenesis during HCV RNA replication; and (5) increasing responsiveness to type I IFNs. RBV generally is well tolerated, although it results in a dose-dependent hemolytic anemia. The dose administered is based on the patient’s weight, and the patient’s Hgb level must be monitored during treatment. Furthermore, in patients with a history of cardiopulmonary disease who cannot tolerate a sudden fall in the Hgb level, RBV must be used with caution, if at all. In addition, RBV is teratogenic; patients taking RBV and their partners are required to avoid pregnancy during therapy and for 6 months after cessation of the drug. RBV has a long cumulative half-life in serum and is excreted by the kidneys; as a result, it can lead to severe side effects, particularly hemolysis, in patients with kidney disease. The dose of RBV must be adjusted for renal function, and the drug should be administered with extreme caution to patients with a creatinine clearance less than 50 mL/min. RBV is not removed by hemodialysis. RBV is still used in some IFN-free DAA regimens in more difficult to treat patient populations, such as genotype 3 HCV infection, cirrhosis, and prior treatment failure.
Novel DAAs against HCV include compounds that target the HCV NS3/NS4A protease, the HCV NS5A protein, and the HCV NS5B polymerase. These drugs inhibit HCV replication by interfering with the respective steps in the HCV life cycle. An ideal DAA regimen should have activity against all HCV genotypes; have high antiviral potency and good oral bioavailability, allowing once daily dosing; possess few drug-drug interactions (DDIs); be well tolerated with minimal toxicity; and have a high barrier to resistance. In contrast to IFN plus RBV, resistance to DAAs, either baseline resistance or resistance selected during DAA therapy, is a well-known consequence of DAA therapy that has implications for the success of certain treatment regimens and for retreatment following DAA failure. There are 2 factors to consider regarding the barrier to resistance: the genetic barrier, which is the number of amino acid substitutions required to confer resistance, and the fitness of the resistance-associated substitution (RAS), which is the ability of the RAS to sustain replication and be selected within the quasispecies ( Box 80.3 ).
NS5A RAS testing is recommended for all HCV genotype 1a patients considered for elbasvir/grazoprevir. If resistance is present, the duration of therapy should be extended (see text) or an alternative regimen should be considered.
NS5A RAS testing is recommended for HCV genotype 1a patients who are treatment-experienced and are being considered for sofosbuvir/ledipasvir. If an NS5A RAS is detected that confers greater than 100-fold reduced activity to ledipasvir, an alternative regimen should be considered.
NS5A RAS testing is recommended for HCV genotype 3 patients who have underlying cirrhosis or are treatment-experienced if sofosbuvir/velpatasvir is being considered. If the Y93H NS5A RAS is present, ribavirin should be added or an alternative regimen should be considered.
NS5A RAS testing is recommended for HCV genotype 3 patients who have underlying cirrhosis or are treatment-experienced if sofosbuvir plus daclatasvir is being considered. If the Y93H NS5A RAS is present, ribavirin should be added or an alternative regimen should be considered.
Many of these DAAs are now available as coformulated fixed-dose combinations (FDCs), allowing for once-daily dosing of a single pill for several of the approved IFN-free DAA regimens. The properties of each DAA class and approved DAAs are listed in Table 80.3 .
| Genotype Coverage | Potency | Barrier to Resistance | Drug-Drug Interactions | Metabolism | Approved Drugs ∗ | |
|---|---|---|---|---|---|---|
| NS3/4A Protease Inhibitors (-previrs) | + to ++ | ++ to +++ | + to ++ | ++ to +++ | Hepatic | Simeprevir Grazoprevir Paritaprevir Glecaprevir Voxilaprevir |
| NS5A inhibitors (-asvirs) | + to +++ | +++ | + to ++ | + to ++ | Hepatic | Daclatasvir Ombitasvir Elbasvir Ledipasvir Velpatasvir Pibrentasvir |
| Nucleoside NS5B inhibitors (-uvirs) | +++ | +++ | +++ | + | Renal | Sofosbuvir |
| Non-nucleoside NS5B inhibitors (-uvirs) | + | + to ++ | + | ++ | Hepatic | Dasabuvir |
HCV NS3/4A protease inhibitors generally have high antiviral potency but differ in respect to the development of resistance. Most of the compounds show better response rates in HCV genotype 1b than in genotype 1a infection. Boceprevir and telaprevir were the first 2 DAAs approved by the FDA in 2011; however, these agents were associated with significant toxicity and limited HCV genotype antiviral activity and required twice-daily or 3 times daily dosing with a large pill burden. Subsequently, the second wave of first-generation protease inhibitors, simeprevir and faldaprevir, were developed with more favorable safety profiles and dosing schedules; simeprevir was approved by the FDA in 2013. These protease inhibitors were used in combination with PegIFN and RBV and have been superseded by second-generation protease inhibitors in combination with other DAAs that have the added advantage of extended HCV genotype activity. FDA-approved protease inhibitors are simeprevir, paritaprevir (boosted with ritonavir), grazoprevir, glecaprevir, and voxilaprevir; these agents are all used in combination with DAAs from other classes.
NS5A inhibitors are characterized by very high antiviral potency at picomolar doses. The cross-genotype efficacy of these agents varies, with greater genotypic coverage with the second-generation than first-generation NS5A inhibitors. Approved and second-generation NS5A inhibitors include daclatasvir, ledipasvir, ombitasvir, and elbasvir, velpatasvir, and pibrentasvir.
HCV NS5B polymerase inhibitors are categorized as nucleoside or nucleotide analog and non-nucleoside polymerase inhibitors. Non-nucleoside polymerase inhibitors are the weakest class of compounds against HCV because of a low barrier to resistance. Most drugs in this class are active mainly against HCV genotype 1b and to a lesser extent against HCV genotype 1a. Different domains in the polymerase protein can be targeted by non-nucleoside polymerase inhibitors, and theoretically, use of a combination of different non-nucleoside polymerase inhibitors is possible. Importantly, there is also no cross-resistance between drugs targeting different polymerase domains. The only FDA-approved non-nucleoside inhibitor is dasabuvir. In contrast, the nucleos(t)ide analogs are active across all HCV genotypes and have a high barrier to resistance. Nucleos(t)ide analog RAS may emerge but have very low fitness and do not expand rapidly, because they cause a chain termination and thereby block HCV replication. A triphosphorylated agent is usually required for activity. The first compound tested in a large number of patients was mericitabine. , Unfortunately, the compound had only modest antiviral activity, and relapses after treatment and breakthroughs during treatment were observed frequently in phase 2 trials when mericitabine was used in combination with protease inhibitors. The first approved nucleotide NS5B polymerase inhibitor was sofosbuvir, which has pangenotypic activity and a very high barrier to resistance.
Sofosbuvir is a pangenotypic NS5B nucleotide inhibitor that is administered as a single 400-mg tablet or as part of an FDC with other DAAs (discussed later), with or without food. Sofosbuvir is predominantly excreted renally (80%), mostly as an active metabolite. Therefore, dose reduction is recommended in patients with an estimated glomerular filtration rate (eGFR) less than 30 mL/min/1.73m 2 . No dose adjustment is required with severe hepatic impairment, thereby allowing sofosbuvir to be administered in patients with decompensated cirrhosis. Sofosbuvir is well tolerated, and when used in combination with RBV, the most common adverse events were fatigue and headache. Although sofosbuvir is not metabolized extensively by the liver, it is transported by P glycoprotein (P-gp); therefore, sofosbuvir should not be co-administered with strong inducers of P-gp, such as rifampin, carbamazepine, phenytoin, or St. John’s Wort. Hepatic decompensation with lactic acidosis has been reported in patients with advanced fibrosis (compensated and decompensated cirrhosis) treated with sofosbuvir plus RBV. This is thought to be due to mitochondrial toxicity caused by RBV, because sofosbuvir has not been convincingly linked to mitochondrial toxicity, or to lactic acidosis, which is known to occur in the context of hepatic decompensation.
Although the precise mechanism is still not fully known, sofosbuvir is contraindicated in patients receiving amiodarone, because severe and life-threatening bradycardia within hours to weeks of co-administration has been reported. If a sofosbuvir-containing DAA regimen is planned for a patient on amiodarone and the amiodarone can be safely discontinued, the DAAs should be delayed for at least 3 months after cessation of amiodarone, due to its long half-life. Caution is recommended with other antiarrhythmics.
The combination of sofosbuvir (see earlier) plus the NS5A inhibitor ledipasvir is administered as a 2-drug FDC of sofosbuvir 400 mg and ledipasvir 90 mg as a single, once-daily tablet, with or without food. In contrast to sofosbuvir, ledipasvir is metabolized predominantly in the liver and excreted unchanged in bile. However, ledipasvir can be given safely to patients with severe hepatic impairment with no significant effect on plasma ledipasvir levels or pharmacokinetics. Because sofosbuvir is renally excreted, this regimen is not recommended for patients with severe renal impairment (eGFR <30 mL/min/1.73 m 2 ). The most frequently reported adverse events were fatigue and headache. Both ledipasvir and sofosbuvir are transported by P-gp and breast cancer resistance protein (BCRP), and therefore, potent P-gp/BCRP inducers will lower sofosbuvir and ledipasvir levels. Because this regimen contains sofosbuvir, the same DDIs that exist with sofosbuvir alone apply to this FDC. Rosuvastatin co-administration is not recommended because of inhibition of OATP by ledipasvir, and careful monitoring for statin-related adverse events is required with use of other HMG-CoA reductase inhibitors. Medications that alter gastric pH such as antacids, H2RAs, and PPIs may also affect ledipasvir levels, because ledipasvir becomes less soluble as gastric pH increases. In real-world experiences with this regimen, SVR rates were slightly lower in patients receiving high-dose PPIs. Therefore, H2RAa and PPIs should be dosed 12 hours apart from sofosbuvir/ledipasvir, with a maximal daily dose equivalent of 40 mg for famotidine or 20 mg for omeprazole. Sofosbuvir/ledipasvir can be administered with antiretrovirals with the exception of tenofovir and ritonavir/cobicistat-containing regimens due to an increase in tenofovir levels, which require monitoring.
The combination of the NS5A inhibitor elbasvir 50 mg and the NS3/4A protease inhibitor grazoprevir 100 mg is available as a single FDC tablet administered once daily, with or without food. Both grazoprevir and elbasvir are partially metabolized by CYP3A4 and predominantly excreted in bile and feces. Both are extensively bound to plasma proteins. Grazoprevir exposure is increased greatly with severe hepatic impairment, and therefore, like all protease inhibitors, the drug is contraindicated in patients with Child-Pugh class B or C cirrhosis. This regimen is safe in patients with severe renal failure. The most common adverse events are headache and fatigue. Relevant DDIs to consider are inducers of CYP3A and P-gp, including some antiretrovirals (see Table 80.4 ).
Sofosbuvir 400 mg and the next-generation NS5A inhibitor velpatasvir 100 mg are coformulated in a single FDC tablet given once daily, with or without food. The combination has pangenotypic coverage. Velpatasvir undergoes hepatic metabolism by CYP2B6, CYP2C8, and CYP3A4 and is also transported by P-gp, BCRP, and, to a small extent, OATP1B1. Velpatasvir can be administered to patients with severe hepatic impairment without alterations in velpatasvir plasma concentration or pharmacokinetics. The major adverse events reported with this FDC were headache, fatigue, and nausea, and their frequencies were similar to those reported in the placebo arm. Due to CYP metabolism and P-gp/BCRP binding of velpatasvir, potent CYP and P-gp inducers are contraindicated with this regimen (see Table 80.4 ). As with ledipasvir, the solubility of velpatasvir decreases with increasing gastric pH levels; therefore, recommendations regarding use of antacids, H2RAs, and PPIs as outlined for sofosbuvir/ledipasvir therapy should also be followed with sofosbuvir/velpatasvir.
Sofosbuvir 400 mg, velpatasvir 100 mg, and the NS3/4A protease inhibitor voxilaprevir 100 mg coformulated as a single 3-drug FDC tablet is administered once daily with food. This FDC has pangenotypic activity. Voxilaprevir is metabolized extensively in the liver by CYP3A4 and, like velpatasvir, is an inhibitor of P-gp, BCRP, OATP1B1, and OATP1B3. As with all protease inhibitors, voxilaprevir exposure is significantly higher with moderate and severe hepatic impairment, and therefore, the drug is not recommended in patients with Child-Pugh class B cirrhosis and contraindicated in those with Child-Pugh class C cirrhosis. The most frequently reported adverse events with this triple regimen were headache, diarrhea, and nausea, suggesting increased additional GI toxicity with voxilaprevir. Because these agents are metabolized by CYP3A4 and are inhibitors of transporter proteins, DDIs occur with substrates of these transporters and potent inducers of CYP3A4, including antiretrovirals (see Table 80.4 ). Rosuvastatin, in particular, is contraindicated because co-administration was associated with 19-fold higher plasma levels of rosuvastatin. Sofosbuvir/velpatasvir/voxilaprevir is contraindicated in women taking ethinylestradiol-based contraception due to the risk of elevations in serum ALT levels. Because this regimen also contains velpatasvir, DDIs with agents that increase gastric pH are also relevant.
The combination of the NS3/4A protease inhibitor glecaprevir 100 mg and the NS5A inhibitor pibrentasvir 40 mg are coformulated as a 2-drug FDC tablet given as 3 tablets once daily with food. Both drugs are excreted predominantly in the bile and are inhibitors of the transporter proteins P-gp, BCRP, OATP1B1, and OATP1B3. Because of significantly higher glecaprevir exposure in patients with moderate and severe hepatic impairment, this combination is contraindicated in those with Child-Pugh class B and C cirrhosis. The regimen is safe in patients with severe renal impairment, including patients with end-stage renal disease (ESRD) with and without dialysis. As with many other DAAs, DDIs include strong inducers of P-gp and CYP3A4, including antiretrovirals (see Table 80.4 ). The solubility of glecaprevir decreases as gastric pH increases; however, dose modification is not required for doses of PPIs equivalent to omeprazole 40 mg per day; higher doses have not been formally studied.
Although postexposure prophylaxis against HCV is not effective, treatment of acute HCV infection is effective. However, early treatment of acute HCV was more important in the IFN era when various cohort studies demonstrated higher (>85%) response rates if acute hepatitis C was treated with IFN early after acquisition than when HCV infection was chronic and response rates were only 54% to 56% overall. Because a substantial proportion of patients may clear the acute infection spontaneously without any antiviral intervention, different strategies have been proposed to avoid unnecessary treatment, particularly with IFN. , The patient’s IFN-λ3 genotype can provide an indication as to whether a patient is more or less likely to recover spontaneously , ; however, the positive predictive value of IFN-λ3 testing is below 90%, and treatment decisions cannot be based solely on the patient’s IFN-λ3 genotype. A prospective randomized trial compared immediate treatment of acute hepatitis C with PegIFN alone and a delayed approach of starting combination therapy with PegIFN and RBV after 12 weeks in those patients who did not become HCV RNA negative spontaneously. Delayed therapy resulted in a lower SVR rate on an intention-to-treat analysis but gave similar response rates on an “adherence-to-therapy” analysis. Therefore, delayed therapy is effective, but early treatment has advantages because fewer patients are lost to follow up. Data are emerging on the efficacy of IFN-free DAA regimens for both acute HCV monoinfection and acute HCV infection in HIV-positive individuals , ; however, these studies are small, and consensus on the optimal treatment regimen, timing of initiation of therapy, treatment duration, and cost-effectiveness has not been established.
A reasonable approach for managing acute hepatitis C is to monitor the serum HCV RNA for at least 12 to 16 weeks before DAA treatment is considered to allow adequate time for spontaneous clearance. Treatment of acute hepatitis C in the context of HIV coinfection is similar to that for patients with HCV monoinfection. , , Given the high efficacy and favorable safety profiles of the DAA regimens, the same regimens used for chronic HCV infection are recommended in acute HCV infection (see later). , IFN-based regimens are no longer recommended.
In contrast to most other chronic viral infections, cure of HCV infection is possible. HCV has an entirely cytoplasmic life cycle; therefore, suppression of viral replication in the absence of resistance can cure HCV-infected cells.
Until 2011, the standard of care for the treatment of HCV infection was the combination of a PegIFN plus RBV. However, IFN-based therapy was associated with poor overall cure rates (54% to 56%), , , with significant toxicity leading to discontinuation of therapy in 14% of patients. In addition, many patients were either ineligible for or intolerant of IFN, thereby limiting treatment options in these patients.
The first DAAs (telaprevir, boceprevir, simeprevir, and sofosbuvir) were developed as “triple” therapy to be used in combination with PegIFN plus RBV. Although SVR rates were higher, limitations included restricted HCV genotypic activity, additional toxicity beyond PegIFN/RBV, poor response in cirrhotic patients and prior null responders, and RAS considerations, as well as a requirement for PegIFN/RBV eligibility. Subsequently, combination IFN-free DAA regimens were developed (see earlier). IFN-based regimens are no longer recommended for treatment of HCV infection.
Combinations of DAAs that target distinct and complementary steps in the HCV life cycle (see Fig. 80.1 ) have revolutionized the treatment of HCV infection, yielding SVR rates in excess of 95% with minimal toxicity. The first proof-of-concept study showing an SVR in 4 patients with chronic hepatitis C with an IFN-free DAA combination therapy was published in 2012. The compounds investigated were the HCV NS5A inhibitor daclatasvir and the HCV NS3 protease inhibitor asunaprevir. SVR rates were only 22% (2/9) in HCV genotype 1a patients; however, SVR rates were considerably higher in HCV genotype 1b patients (90% for treatment-naïve patients and 82% for patients who were prior nonresponders to or ineligible for IFN), providing proof-of-concept that IFN-free therapy can cure HCV, but that the efficacy of all-oral combination regimens may differ among HCV genotypes and subtypes. Since this study, many DAAs have undergone clinical development, 13 of which have been approved by the FDA as of early 2020 (5 protease inhibitors, 6 NS5A inhibitors, and 2 NS5B inhibitors). The oldest IFN-free DAA regimens are HCV genotype specific, whereas the more recently approved IFN-free regimens have pangenotypic coverage. IFN-free DAA regimens are discussed in the context of each HCV genotype/subtype, presence or absence of cirrhosis, and treatment history, because these factors are important considerations when choosing an IFN-free DAA regimen. Special populations, including persons with decompensated cirrhosis, HCV recurrence following LT, and renal impairment are discussed later.
The 13 DAAs approved by the FDA for the treatment of chronic hepatitis C infection include the NS3/4A protease inhibitors simeprevir, paritaprevir (ritonavir boosted), grazoprevir, glecaprevir, and voxilaprevir; the NS5A inhibitors daclatasvir, ombitasvir, ledipasvir, elbasvir, velpatasvir, and pibrentasvir; and the NS5B inhibitors sofosbuvir and dasabuvir (see Table 80.3 ). Pangenotypic drugs or FDCs include sofosbuvir, sofosbuvir/velpatasvir, sofosbuvir/velpatasvir/voxilaprevir, and glecaprevir/pibrentasvir. Genotype-specific drugs or FDCs include sofosbuvir/ledipasvir, and elbasvir/grazoprevir. RBV can be added to some IFN-free DAA regimens in more difficult-to-treat populations, such as HCV genotype 3 cirrhotic patients.
Several FDA-approved IFN-free DAA regimens are recommended for HCV genotype 1 patients, based on HCV subtype, treatment history, presence or absence of cirrhosis, and, for some regimens, the presence or absence of baseline NS5A RAS.
As of early 2020, there were 4 recommended IFN-free DAA regimens for HCV genotype 1a patients without and with compensated cirrhosis that all have similar efficacy ( Table 80.5 ).
| Regimen | Duration (Weeks) | Fibrosis Stage | SVR12 (%) | Study |
|---|---|---|---|---|
| Treatment-Naïve | ||||
| Genotype 1a Recommended | ||||
| Elbasvir (50 mg)/grazoprevir (100 mg) | 12 ∗ | F0-3, F4 | 92 | C-EDGE |
| Glecaprevir (300 mg)/pibrentasvir (120 mg) | 8 | F0-3 | 97-99 | SURVEYOR-I/II , ENDURANCE-1 |
| Glecaprevir (300 mg)/pibrentasvir (120 mg) | 12 | F4 | 99 | EXPEDITION-1 |
| Sofosbuvir (400 mg)/ledipasvir (90 mg) | 8 ∗ | F0-3 | 94 | ION-3 |
| Sofosbuvir (400 mg)/ledipasvir (90 mg) | 12 | F0-3, F4 | 99 | ION-1 |
| Sofosbuvir (400 mg)/velpatasvir (100 mg) | 12 | F0-3, F4 | 98 | ASTRAL-1 |
| Genotype 1a Alternative | ||||
| Elbasvir (50 mg)/grazoprevir (100 mg) | 16 ‡ | F0-3, F4 | 100 | C-EDGE , |
| Genotype 1b Recommended | ||||
| Elbasvir (50 mg)/grazoprevir (100 mg) | 12 | F0-3, F4 | 99 | C-EDGE |
| Glecaprevir (300 mg)/pibrentasvir (120 mg) | 8 | F0-3 | 100 | SURVEYOR-I/II , ENDURANCE-1 |
| Glecaprevir (300 mg)/pibrentasvir (120 mg) | 12 | F4 | 100 | EXPEDITION-1 |
| Sofosbuvir (400 mg)/ledipasvir (90 mg) | 8 ∗ | F0-3 | 94 | ION-3 |
| Sofosbuvir (400 mg)/ledipasvir (90 mg) | 12 | F0-3, F4 | 97-99 | ION-1 |
| Sofosbuvir (400 mg)/velpatasvir (100 mg) | 12 | F0-3, F4 | 99 | ASTRAL-1 |
| PegIFN/RBV Treatment-Experienced | ||||
| Genotype 1a Recommended | ||||
| Elbasvir (50 mg)/grazoprevir (100 mg) | 12 ∗ | F0-3, F4 | 94 | C-EDGE |
| Glecaprevir (300 mg)/pibrentasvir (120 mg) | 8 | F0-3 | 99.7 | ENDURANCE-1 |
| Glecaprevir (300 mg)/pibrentasvir (120 mg) | 12 | F4 | 99 | EXPEDITION-1 |
| Sofosbuvir (400 mg)/ledipasvir (90 mg) | 12 | F0-3 | 95-100 | ION-2 |
| Sofosbuvir (400 mg)/velpatasvir (100 mg) | 12 | F0-3, F4 | 98 | ASTRAL-1 |
| Genotype 1b Recommended | ||||
| Elbasvir (50 mg)/grazoprevir (100 mg) | 12 | F0-3, F4 | 100 | C-EDGE |
| Glecaprevir (300 mg)/pibrentasvir (120 mg) | 8 | F0-3 | 99 | ENDURANCE-1 |
| Glecaprevir (300 mg)/pibrentasvir (120 mg) | 12 | F4 | 99 | EXPEDITION-1 |
| Sofosbuvir (400 mg)/ledipasvir (90 mg) | 12 | F0-3 | 95-100 | ION-2 |
| Sofosbuvir (400 mg)/velpatasvir (100 mg) | 12 | F0-3, F4 | 99 | ASTRAL-1 |
| NS3/4A Protease Inhibitor Plus PegIFN/RBV Treatment-Experienced | ||||
| Genotype 1 Recommended | ||||
| Sofosbuvir (400 mg)/velpatasvir (100 mg) | 12 | F0-3, F4 | 100 | ASTRAL-1 |
| Sofosbuvir (400 mg)/ledipasvir (90 mg) | 12 | F0-3, F3 | 94-96 | ION-2 |
| Glecaprevir (300 mg)/pibrentasvir (120 mg) | 12 | F0-3, F4 | 92 | MAGELLAN-1 |
| Genotype 1 Alternative | ||||
| Elbasvir (50 mg)/grazoprevir (100 mg) plus weight-based RBV | 12 ∗ | F0-3 | 96 | C-SALVAGE |
| Elbasvir (50 mg)/grazoprevir (100 mg) plus weight-based RBV | 16 ‡ | F0-3, F4 | 96 | C-SALVAGE |
| Sofosbuvir (400 mg)/ledipasvir (90 mg) plus weight-based RBV | 12 | F4 | 100 | SIRIUS Pianko et al. |
| DAA Treatment-Experienced, Excluding NS5A Inhibitors | ||||
| Genotype 1 Recommended | ||||
| Sofosbuvir (400 mg)/velpatasvir (100 mg)/voxilaprevir (100 mg) | 12 | F0-3, ¶ F4 ¶ | 97 | POLARIS-4 |
| Glecaprevir (300 mg)/pibrentasvir (120 mg) | 12 | F0-3, F4 | 99 for F0-3 89-91 for F4 |
ENDURANCE-1 EXPEDITION-1 MAGELLAN-1 |
| Sofosbuvir (400 mg)/velpatasvir (100 mg) | 12 | F0-3, § F4 § | 96 | POLARIS-4 |
| Genotype 1 Alternative | ||||
| Sofosbuvir (400 mg)/ledipasvir (90 mg) plus weight-based RBV | 12 | F0-3 ¶¶ | 98-100 | Osinusi et al. Wyles et al. |
| NS5A Inhibitor Treatment-Experienced | ||||
| Genotype 1 Recommended | ||||
| Sofosbuvir (400 mg)/velpatasvir (100 mg)/voxilaprevir (100 mg) | 12 | F0-3, F4 | 95 (1a) 100 (1b) |
POLARIS-1 |
| Genotype 1 Alternative | ||||
| Glecaprevir (300 mg)/pibrentasvir (120 mg) | 16 ∗∗ | F0-3, ∗∗∗ F4 ∗∗ | 91 | MAGELLAN-1 |
∗ If baseline HCV RNA <6 million IU/mL in HCV monoinfection only
† In patients without baseline NS5A RAS
‡ In patients with baseline NS5A RAS
§ HCV genotype 1b patients only
¶ HCV genotype 1a patients only
¶¶ Excluding simeprevir failures
∗∗ Excluding DAA regimens that included a protease inhibitor
The FDC of sofosbuvir (400 mg)/ledipasvir (90 mg) once daily for 12 weeks is approved for HCV genotype 1a infection in patients without cirrhosis based on data from the phase 3 registrations studies, ION-1 and ION-3 . ION-1 investigated HCV genotype 1 (1a and 1b) patients, including patients without and with (16%) compensated cirrhosis, treated with sofosbuvir/ledipasvir for 12 or 24 weeks with and without RBV. SVR12 rates were 97% to 99% across all treatment arms and HCV subtypes, indicating that 12 weeks of sofosbuvir/ledipasvir without RBV was sufficient. SVR12 rates were similar among HCV genotype 1a-infected patients without and with compensated cirrhosis (98% and 97%, respectively). Therefore, this regimen is recommended in both noncirrhotic and compensated cirrhotic HCV patients with genotype 1a HCV. ION-3 investigated whether the duration of therapy with sofosbuvir/ledipasvir could be shortened and compared 8 weeks of sofosbuvir/ledipasvir with and without RBV versus 12 weeks of sofosbuvir/ledipasvir in treatment-naïve HCV genotype 1-infected patients. Compensated cirrhotic patients were excluded from this study. In an intention-to-treat analysis, there was no difference in SVR12 among the treatment arms (93% to 95%), although relapse rates were numerically higher in the 8-week treatment arm irrespective of RBV use. A post hoc analysis of the 8-week RBV-free arm revealed higher relapse rates in patients with high viral loads (>6 million IU/mL) at baseline. Although this was a post hoc analysis, these data led to the recommendation that 8 weeks of sofosbuvir/ledipasvir without RBV is sufficient in noncirrhotic HCV genotype 1 treatment-naïve patients with a baseline HCV RNA level less than 6 million IU/mL. Real-world clinical experience has confirmed the similar efficacy rates of 8 and 12 weeks of sofosbuvir/ledipasvir for this group of patients.
The FDC of elbasvir (50 mg)/grazoprevir (100 mg) once daily for 12 weeks is also approved for HCV genotype 1a infection based on data from the phase 3 C-EDGE study. Treatment-naïve patients with HCV genotypes 1, 4, and 6 were randomized to receive elbasvir/grazoprevir for 12 weeks versus placebo for 12 weeks followed by 12 weeks of elbasvir/grazoprevir. The SVR12 rate in patients with HCV genotype 1a infection was 92%. SVR12 rates were similar across all groups enrolled in the study (94% in noncirrhotics and 97% in compensated cirrhotics). SVR12 rates were significantly lower in HCV genotype 1a patients with baseline NS5A RAS, particularly in HCV genotype1a patients with cirrhosis. Therefore, treatment extension to 16 weeks is recommended in noncirrhotic HCV-1a patients with baseline NS5A RAS to elbasvir. This combination is not recommended in cirrhotic HCV-1a patients with NS5A RAS to elbasvir.
The FDC of sofosbuvir (400 mg)/velpatasvir (100 mg) given once daily for 12 weeks is a pangenotypic regimen approved for HCV genotype 1a infection based on the ASTRAL-1 study. This study evaluated sofosbuvir/velpatasvir for 12 weeks in patients with HCV genotypes 1 through 6, without and with compensated cirrhosis (19% cirrhotic patients). The overall SVR12 rate in HCV genotype 1a infection was 98% and was similar in patients who did and did not receive RBV. Overall SVR12 rates were comparable in patients with compensated cirrhosis (99%). Interestingly, with this regimen, the presence of baseline NS5A RAS (>15%) did not impact SVR12 rates.
The NS3/4A protease inhibitor glecaprevir (100 mg) plus the NS5A inhibitor pibrentasvir (40 mg) is a pangenotypic regimen available as an FDC tablet administered as 3 tablets once daily for 8 weeks in noncirrhotic HCV genotype 1a infection based on the SURVEYOR-I/II and ENDURANCE-1 trials. Both 8 and 12 weeks of this FDC regimen were evaluated. SVR12 rates were 99% for the 8-week arm and 99.7% for the 12-week arm, demonstrating noninferiority in noncirrhotic HCV genotype 1a-infected patients. Therefore, 8 weeks of glecaprevir/pibrentasvir is recommended for noncirrhotic patients with HCV genotype 1a infection.
The same FDC regimen of glecaprevir/pibrentasvir for 12 weeks has also been evaluated in compensated cirrhotic patients in the EXPEDITION-1 study. SVR12 rates were 98% in HCV genotype 1a-infected patients with compensated cirrhosis; only 1 patient experienced relapse.
The same 4 recommended IFN-free DAA regimens for HCV genotype 1a patients are also recommended to treat HCV genotype 1b infection, all of which have similar efficacy (see Table 80.5 ).
The FDC of sofosbuvir (400 mg)/ledipasvir (90 mg) once daily for 12 weeks is also approved for HCV genotype 1b infection in patients without and with compensated cirrhosis based on data from the phase 3 registrations studies, ION-1 and ION-3. As mentioned earlier, SVR12 rates in the ION-1 study were 97% to 99% across all treatment arms and were similar irrespective of HCV subtype and fibrosis stage. Similar to HCV genotype 1a infection, a post hoc analysis of the ION-3 study demonstrated that therapy could be shortened to 8 weeks only in noncirrhotic patients with an HCV RNA level less than 6 million IU/mL. Real-world clinical experience has subsequently demonstrated similar efficacy rates for 8 and 12 weeks of sofosbuvir/ledipasvir.
The FDC of elbasvir (50 mg)/grazoprevir (100 mg) once daily for 12 weeks is also approved for HCV genotype 1b infection based on data from the phase 3 C-EDGE study. SVR12 rates were 99% in HCV genotype 1b patients (higher than in HCV genotype 1a infection), and was similar among patients without and with compensated cirrhosis. Baseline NS5A RAS did not affect SVR12 rates in HCV genotype 1b-infected patients.
The FDC of sofosbuvir (400 mg)/velpatasvir (100 mg) given once daily for 12 weeks is also approved for HCV genotype 1b infection based on the ASTRAL-1 study. The SVR12 rate in HCV genotype 1b-infected patients was 99% and was similar in patients who did and did not receive RBV and among noncirrhotic and cirrhotic patients.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here