Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
List the different cognitive domains and their individual roles.
Describe and contrast the clinical outcome of injuries to the Broca and Wernicke areas.
Describe the ‘steps’ performed when reading in relation to areas of the brain involved.
Discuss the different clinical manifestations of frontal lobe injuries.
Discuss the different clinical manifestations of right parietal lobe injuries.
The two cerebral hemispheres are asymmetrical in several respects. Some of the asymmetries have to do with handedness, language, and complex motor activities, but other more subtle differences exist. (Limbic asymmetries are described in Chapter 33 .)
Handedness often determines the hemisphere that is dominant for motor control. Left hemisphere/right-hand dominance is the rule. Advances in ultrasound technology have made it possible for the observation of motor behaviour in the fetus, and handedness (and brain asymmetries) established before birth based on the preferred hand used for thumb sucking during fetal life.
In 96% of right-handed subjects, the left hemisphere is dominant for language, and while this asymmetry of language dominance is true for most left-handed subjects, it varies with respect to the ‘strength’ of their left-handedness. Those who are strongly left-handed have a higher incidence of right hemisphere language dominance (27%), while in those who are ambidextrous the right hemisphere is dominant in 15%. Handedness, hemispheric language dominance, and other left versus right body asymmetries may represent a polygenic trait, but are not limited to humans and functional brain asymmetries exist in the great apes and other vertebrates.
Clarifying the function of and interrelationships between brain structures has transitioned beyond the insights gathered by studying those who have suffered focal brain lesions. New techniques such as neuroimaging with functional magnetic resonance imaging (fMRI) and neurophysiologic techniques (e.g. transcranial magnetic stimulation) allow investigation of those with and without cerebral dysfunction and have substantiated some prior interpretations, but just as often have led us to question or revise them.
As exemplified by speech and language, different cognitive functions or domains consist of a network (‘connectome’) of interconnecting anatomical areas, and within each are ‘hubs’ that support integrative processing and adaptive behaviour. Critical for the broad display of normal function, hub dysfunction results in disorders that are more expansive and remote than predicted by only the ‘size’ of the cortical area involved. ( Diaschisis was a term originally suggested to signify neurophysiologic changes in an area remote from the area of injury. Now with connectomics, it signifies changes at a structural and connectivity level that may be distant from a focal brain lesion.)
Involvement of hubs and dysfunction of their network(s) are associated with or proposed to explain the clinical manifestations of disorders such as Alzheimer disease and schizophrenia. In some cases a hub may demonstrate observable morphologic differences, but in other cases such a clear relationship is absent, as the difficulty had instead disrupted its connections to another area. In addition, one anatomical area may contribute or play a role in more than one cognitive domain.
These cognitive functions or domains include the following that are the most anatomically and functionally distinct:
A language network involves cortical areas around the Sylvian fissure including Broca and Wernicke’s area.
A behavioural/emotion network includes prefrontal and cingulate cortical areas and the amygdala.
Spatial awareness is based on centres in the posterior parietal cortex, premotor cortex, supplementary cortex, and the frontal eye fields.
Face-object recognition centres are in the midtemporal and temporopolar cerebral cortex.
The executive function network includes the lateral prefrontal and orbitofrontal cerebral cortex (through its connections with other cortical areas, the prefrontal cortex contributes to and influences other cortical domains).
Working memory and learning include the hippocampus, medial temporal lobe, and prefrontal cortex.
The attention network includes the frontal, parietal, and cingulate cortex.
Our classical way of understanding language was to assign function to discrete areas of the cortex, separating language production from comprehension. At the bedside, this remains a useful conceptualisation for initial localisation and is referred to as the Wernicke-Lichtheim-(Geschwind) model (WLG) , after those individuals who pioneered clinical studies of language. While this model remains useful, language depends on multiple cortical and subcortical areas. Currently a dual stream model , a concept not limited to language, more accurately describes speech and language organisation. Speech production occurs through a dorsal stream that extends from parietal to frontal cortex of the left hemisphere and ends with acoustic speech signals ‘mapped’ onto cortical areas specific for articulation, resulting in fluent speech. The ventral stream involves the lateral temporal lobes, extending anteriorly into the inferior frontal lobe and posteriorly into the inferior parietal lobe, providing meaning to auditory information. Production and comprehension of language through these two systems are dynamically linked and both necessary for successful comprehension and communication.
The French pathologist Pierre Broca assigned a ‘motor’ or speech production function to the inferior frontal gyrus of the left side in 1861. The principal area concerned occupies the pars opercularis and pars triangularis parts of the inferior frontal gyrus corresponding to Brodmann areas 44 and 45. Broca’s area represents a node within the anterior portion of the dorsal language stream that includes adjacent Brodmann area 47, premotor cortex (the ventral part of area 6) and the dorsal stream extends posteriorly to the supramarginal gyrus.
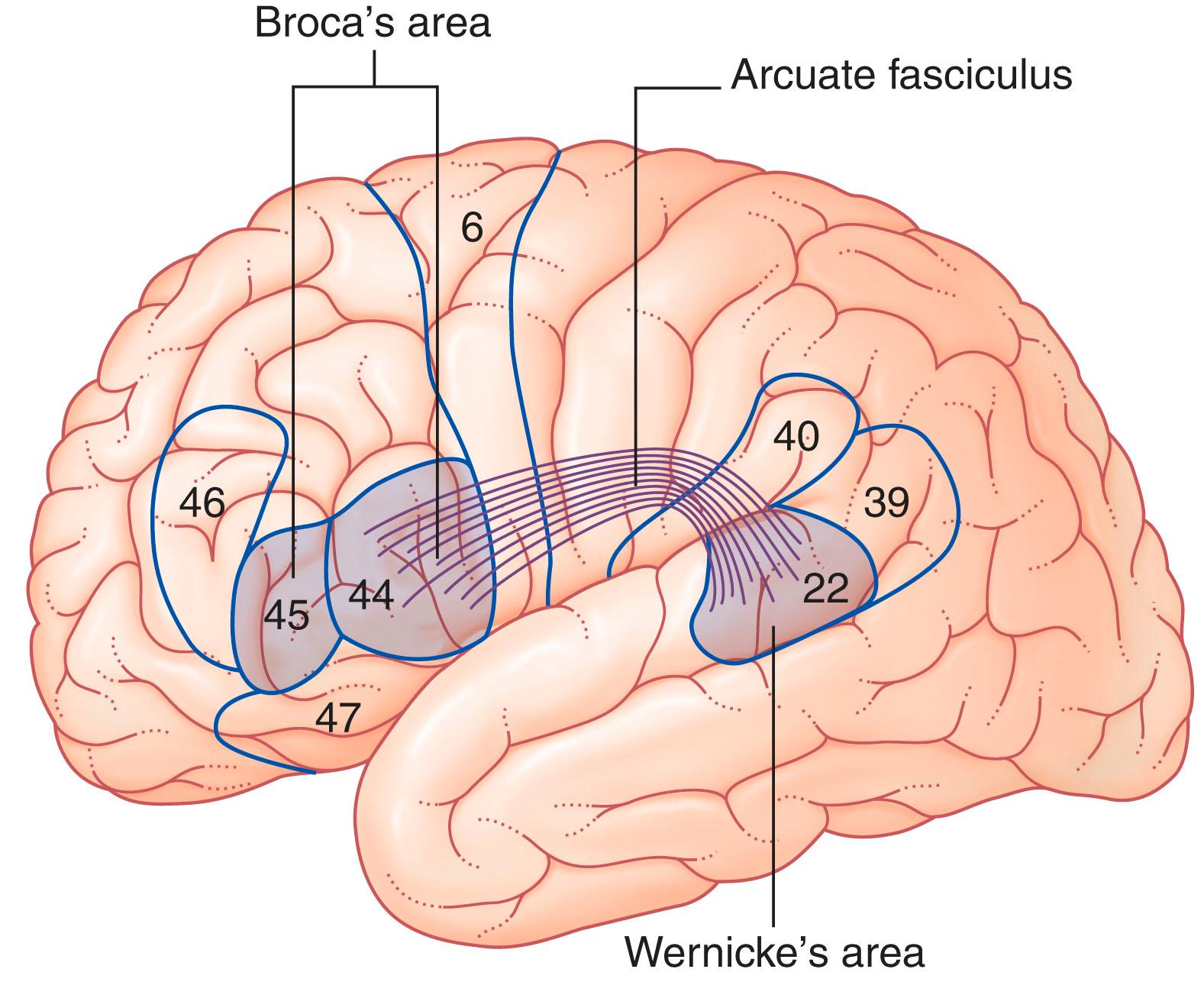
A language disorder described as a Broca or expressive aphasia is attributed to lesions involving the Broca area, where fluency (speech production) is disrupted to a greater extent than comprehension (see Clinical Panel 32.1 ), but all language disorders can be considered expressive. However, the functional role and manifestations of dysfunction attributed to Broca area lesions have extended beyond the original WLG model. Cytoarchitectonic and connectivity differences are more extensive and dynamic with functional separation into areas that serve a role in phonology (how sounds are organised and used in natural languages), syntax (arrangement of words and phrases to create well-formed sentences), and semantics (meaning of words, phrases, sentences, or even larger units).
We now consider the Broca area (and its connectivity) as not ‘language specific’, but a role it does fulfil through its connectivity with language-relevant areas. It participates in other cognitive domains such as music and a role in other actions (increasing a listener’s attention when specific utterances occur, while decreasing attention to utterances when engaged in ‘cocktail speech’, or modifying a speaker’s utterances in ways that will enhance the meaning of his or her communication to a specific listener). Similarly, its connectivity to Wernicke’s area via the arcuate fasciculus (see Fig. 32.1 ) is more extensive and includes other relevant language areas within the frontal, temporal, and parietal cortex.
Output from the Broca area does facilitate speech articulation through sequences of oral motor and respiratory activity of the adjacent premotor and motor cortex. It also serves to direct and focus attention, and to ensure appropriate behavioural interactivity (e.g. waiting your turn to speak, speaking in the appropriate tone or manner) through interaction with the dorsolateral prefrontal cortex (DLPFC), anterior cingulate gyrus, and parietal cortex. Connectivity with the temporal cortex as well as inferior parietal areas is necessary when accessing memories with respect to knowledge type and the associated phonological, syntax, and semantic forms. In view of these multiple roles, the Broca area is referred to as the Broca region , as different functional roles reflect its subparcellation.
As an ‘entrenched’ bedside clinical localisation, Broca aphasia will likely remain, but eventually replaced by a more appropriate clinical phenomenology.
The German neurologist Karl Wernicke made extensive contributions to the understanding of language processing in the late 19th century. He designated the posterior part of Brodmann area 22 in the superior temporal gyrus of the left hemisphere as a ‘sensory area’ concerned with understanding the spoken word. Lesions involving this Wernicke area in adults are associated with a Wernicke or receptive aphasia ( Clinical Panel 32.1 ), where comprehension is impaired to a greater extent than fluency of speech.
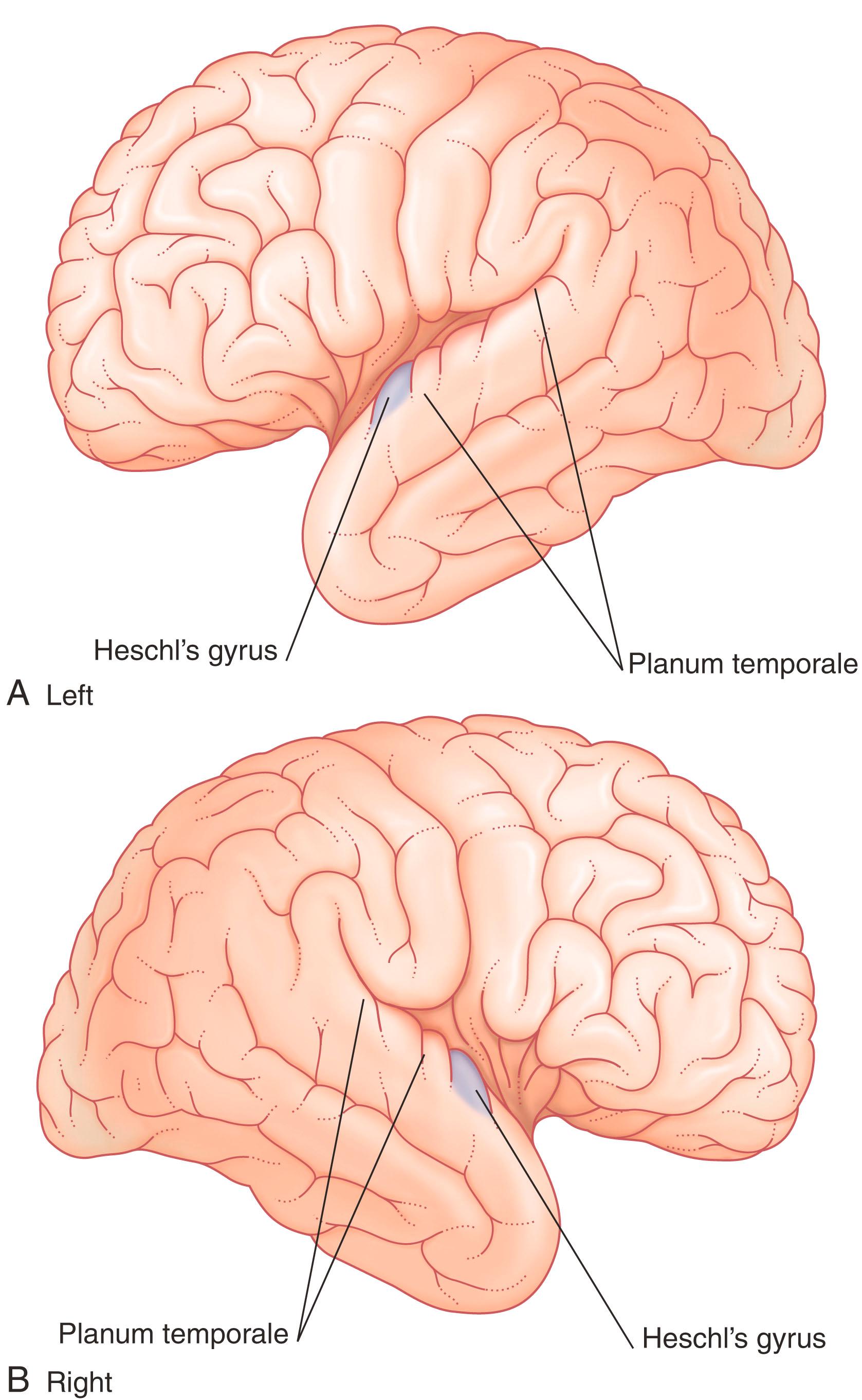
The upper surface of the Wernicke area includes the planum temporale (temporal plane) ( Fig. 32.2 ) that comprises the superior temporal gyrus (posterior portion of Brodmann area 22) just posterior to the primary auditory cortex (Heschl gyrus) and the supramarginal gyrus, area 40. The planum temporale facilitates spatiotemporal discrimination and identification of auditory stimuli that are crucial for speech ( phonemes ; the smallest unit of speech in a language that is capable of conveying a distinction in meaning), as well as being involved in or modulated by auditory attention when selecting stimuli from the left versus right ear. (The volume of cerebral cortex in the planum temporale is larger on the left side in 65% of right-handed subjects but does not match the more than 90% left-hemisphere dominance for speech.)
The Wernicke area is linked to the Broca area through association fibres of the arcuate fasciculus that curve around the posterior end of the lateral fissure within the underlying white matter (see Fig. 32.1 ). Additional pathways of connectivity for language areas are delineated by magnetic resonance imaging (MRI) in humans (and suggested by tract-tracing studies in monkeys) between the frontal, temporal, parietal, and occipital cortex. These occur through the uncinate, superior, and inferior longitudinal fasciculi and extreme capsule (see Fig. 2.20 ).
These multiple pathways further support the concept of a ventral stream (analogous to visual processing) for language. The ventral stream relates auditory information to meaning as well as to proper syntax phrase construction.
Like the clinical use of the term Broca aphasia, Wernicke aphasia will likely remain in the clinical realm for some time. However, lesions restricted to the Wernicke area are associated with difficulty in speech production, word retrieval, and speech that is associated with paraphasic (substitution of the correct word by a similar speech sound, incorrect word, or nonsensical word) errors, but word comprehension remains intact. The syndrome of Wernicke aphasia (paraphasic speech production and comprehension impairment) results from injury to the Wernicke area and additional areas within the lateral temporal lobe and parietal cortex.
The angular gyrus (area 39) belongs descriptively to the inferior parietal lobule. The left angular gyrus receives a projection from the inferior part of area 19 (the lingual gyrus, shown in Fig. 2.5 ), and itself projects to the planum temporale. It is commonly included as a part of the Wernicke area.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here