Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Description: Sickle cell warrants special consideration for athletes. Inheritance of a gene from one parent results in sickle cell trait (SCT), and inheritance from both results in sickle cell disease (SCD). Other allele formations that lead to sickling include hemoglobin SC and sickle cell beta-thalassemia. Whereas SCD is a contraindication for competitive sports because of the high risk of life-threatening crisis, those with SCT are able to participate with activity modifications.
Epidemiology: SCT is present in 6%–9% of African Americans and 0.05% in the remaining US population. There have been 18 documented collegiate football–related deaths attributed to SCT (daSCT) from 1974 to 2010. After National Collegiate Athletic Association (NCAA) legislation mandating SCT screening in 2010, only one daSCT was recorded from 2010 to 2019.
Pathophysiology: Sickle cell gene causes mutation of the red blood cell (RBC) B globin molecule. When an RBC is under stress, it enters an oxidative crisis, leading to polymerization, which in turn leads to sickling. Sickled cells form vaso-occlusive clots causing ischemic insults. The vast majority of those with SCT will be asymptomatic throughout life, but there is a risk of sickling with extreme physical activity. The exact causes of sudden death because of SCT is unknown and highly debated. The end result is sickling, vascular occlusion, and perhaps exertional collapse associated with sickle cell trait (ECSAT). Factors thought to increase this risk include fatigue, poor conditioning, altitude changes, heat, and concurrent febrile illness.
Laboratory testing: Hemoglobin (Hgb) electrophoresis, high-performance liquid chromatography (HLPC), or an Hgb solubility test are the methods most often used for sickle cell identification. Sickle cell testing is included in the newborn screen for every child born in the United States. Since 2010, the NCAA has mandated sickle cell testing for all incoming athletes after a lawsuit regarding the death of a football player suffering a daSCT.
Clinical presentation: Exertional sickling (ES) is similar to heat-related illness and exertional cardiac illness and can be difficult to distinguish. In ES, muscular symptoms are typically sudden with more weakness but less pain than heat illness cramping. The legs slowly become weak, leading to collapse. Mentation is typically normal, and temperature is normal to only slightly elevated.
Evaluation and treatment: If ES is suspected, the athlete should be immediately evaluated with a complete set of vital signs. If temperature measurements are consistent with heat-related illness, they should be immediately treated as such. If that is not the case, an automated external defibrillator (AED) should be placed, intravenous (IV) fluids may be started depending on ambulance wait time, and the athlete should be closely monitored until emergency services arrive.
Prevention: For ES, it begins with determining at-risk individuals by laboratory testing. If an athlete is identified as having SCT, special attention should be paid during athletic participation. Athletes should have an individualized training program that allows for a slower build of strength and conditioning with increased frequency of rest and hydration. Education should emphasize appropriate water intake. If the athlete will be participating at higher altitude or warmer climate, a proper, gradual acclimatization program should be implemented. Coaches and staff should be aware of the athlete’s sickle cell status and be instructed to stop training immediately and have the athlete evaluated if symptoms arise.
Return to play: Athletes who collapse during exercise should be thoroughly evaluated to rule out cardiac, electrolyte, respiratory, or other metabolic etiologies of collapse. Risks and benefits to continued athletic participation should be discussed. Once the athlete has clinically recovered and laboratory work has normalized, he or she may choose to return to sport.
Description: Thalassemia is an inherited disease that is a risk factor for poor outcomes in athletes. Like sickle cell, thalassemia has a spectrum of disease presentations ranging from asymptomatic to outcomes that are not compatible with life. There are both alpha- and beta-thalassemias. Disease severity depends on how many of the four alleles are inherited and the specific alleles inherited.
Epidemiology: Roughly 5% of the world’s population has at least one thalassemia allele. These alleles initially arose in populations where malaria was endemic as a protective factor.
Pathophysiology: The disrupted ratio of alpha- and beta-globin production leads to unpaired alpha- and beta-chains that then precipitate, causing destruction of RBC precursors.
Clinical presentation: Thalassemia trait is the most common condition and is largely asymptomatic with a normal complete blood count (CBC), mild microcytosis, or mild anemia.
Laboratory testing: Includes CBC, peripheral smear, and iron panel.
Treatment: No recommended treatment for thalassemia trait. It is important to differentiate thalassemia and iron deficiency, which can have a similar clinical presentation because iron is not advised in patients with thalassemia because of the risk of iron overload. This risk contributes to the recommendation against universal iron supplementation.
Description: Iron plays an important role in both overall health and peak sport performance. Iron is important in oxygen transport to skeletal muscles and in in aerobic metabolism via adenosine triphosphate (ATP) production in the electron transport chain. Decreased iron stores may result in effects ranging from minimal to anemia (i.e., drop in Hgb below standard value). Patients with iron-deficiency anemia (IDA) require treatment. Iron deficiency without anemia (IDNA) seems to play a role in both subjective and objective markers relating to athletic performance. Specifics regarding evaluation and treatment of IDNA athletes is much more controversial.
Epidemiology: Prevalence of iron deficiency in athletic females and males ranges from 15% to 50% and 3% to 11%, respectively. The prevalence of athletes with true IDA is much lower, at about 2% for females and 0.1% for males. Endurance athletes are at greatest risk.
Risk factors and causes for iron deficiency in the athletic population: Once IDNA or IDA is diagnosed, the cause should be identified, if possible. Blood loss (e.g., gastrointestinal [GI], gastrourinary [GU], and menstrual losses) is the major cause of iron deficiency. Menstruating females comprise the highest percentage of those with iron deficiency. In addition to typical blood loss risk factors of the general population, there are mechanisms specific to athletes that may contribute to iron deficiency. During periods of intense and prolonged exercise, blood may be shunted from the gut to working skeletal muscles, leading to GI tract microischemia. Reports have shown that up to 85% of athletes have a positive fecal occult blood test after prolonged vigorous exercises. Hematuria caused by mechanical forces on the bladder during intense exercise and iron loss through excessive sweating have also been postulated. Lastly, hepcidin, a regulator of iron absorption, has been shown to play a part. Exercise causes an inflammatory response, which upregulates hepcidin, which then downregulates iron absorption and transfer of iron along metabolic pathways. The level of hepcidin typically peaks 3–6 hours after strenuous exercise. Additionally, relative energy deficiency in sport (RED-S) is very likely linked to iron deficiency, either by decreased amounts of iron intake through restrictive diets or by diminished iron absorption.
Clinical presentation: The severity of symptoms often correlates to the degree and acuity of iron deficiency. Some cases are asymptomatic, whereas mild to moderate cases may present with fatigue, decreased exercise tolerance, or depressed mood. Severe cases classified as IDA have symptoms that may include palpitations, shortness of breath, dizziness, or syncope and physical examination findings that may include tachycardia, increased respiratory rate, pale conjunctiva, generalized paleness, and systolic murmurs ( Fig. 31.1 ).
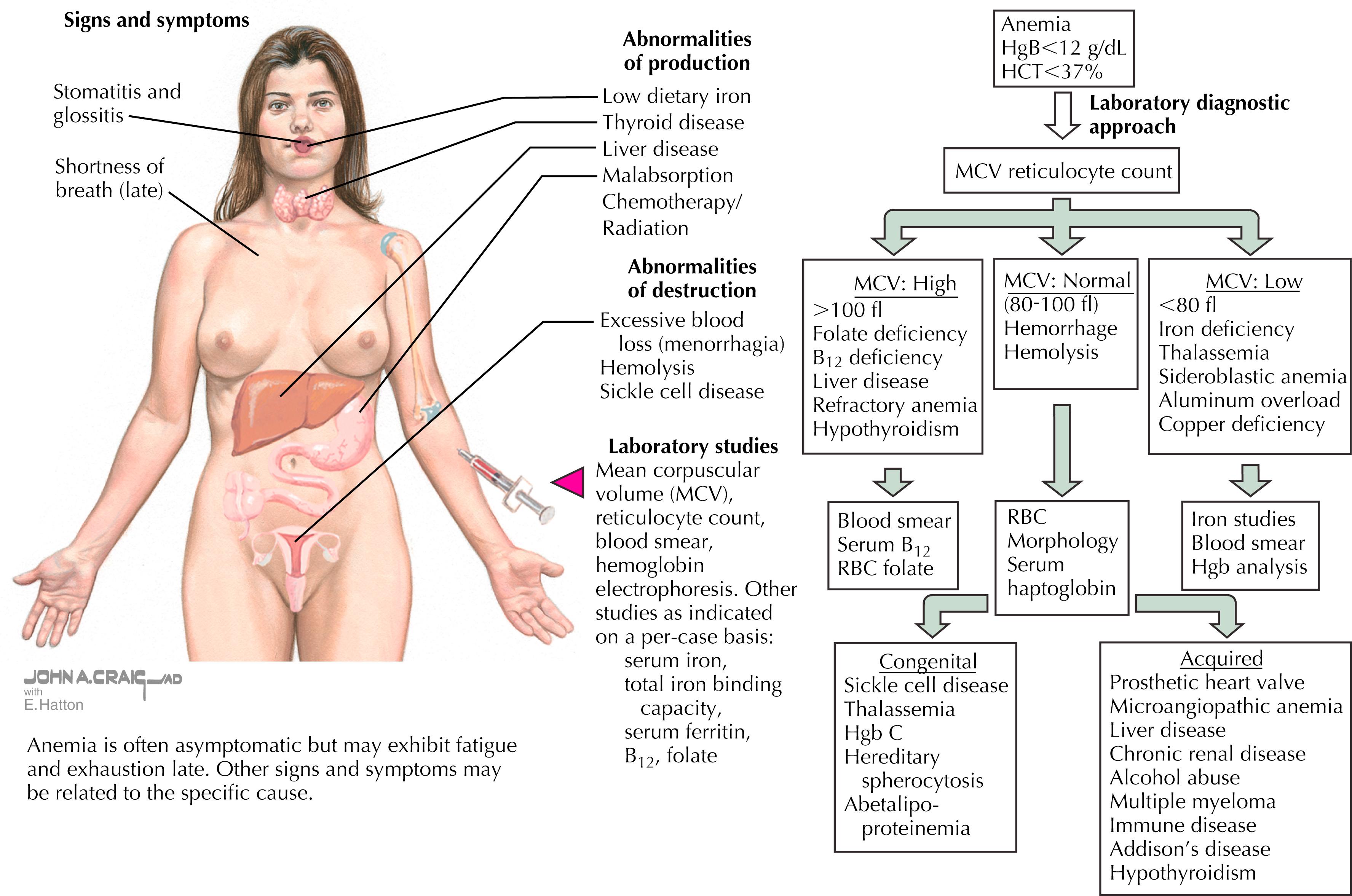
Laboratory studies: Initial laboratory work includes CBC, serum ferritin, plasma iron, total iron binding capacity (TIBC), and transferrin; fecal occult blood test or urinalysis if history suggests. The laboratory tests should be drawn in the morning, not within 48–72 hours of vigorous exercise, and not in the time of sickness or infection. This is important, as increased levels of inflammation may affect the results and make it difficult to assess. There are no guidelines regarding screening of asymptomatic individuals, but many institutions do screen those at higher risk.
Diagnosis: Iron deficiency is divided into three stages:
Stage I: Low ferritin
Stage II: Low iron and/or low transferrin and increased TIBC + low ferritin
Stage III: Low hemoglobin (female Hgb <12, males Hgb <14) + stage II findings
The World Health Organization defines iron depletion at a serum ferritin <15 for adults. The ferritin cutoff for iron deficiency in the athletic population remains controversial. Studies show a decrease in athletic performance when serum ferritin <20 and perhaps a subjective decrease in fatigue and mood when ferritin <30. Stage III iron deficiency is related to decreased oxygen transport on hemoglobin, which certainly affects athletic performance. It is thought that with stage I and II, there may be impaired function of oxidative enzymes and respiratory proteins that results in decreased aerobic power and diminished exercise performance.
Treatment: Indicated in IDA and considered in IDNA. The literature suggests treating iron deficiency to keep serum ferritin >20. If a diagnosis is made, repeat testing should be done in 6- to 8-week intervals until adequate iron status is achieved. Typically 100% of iron is obtained through digestion. The two sources of iron are free iron and heme iron (found in animal products). Heme iron is much more readily absorbed by the gut; thus, vegetarians should take care in monitoring their iron consumption. As iron becomes deficient in the body, the body upregulates absorptive pathways to increase iron bioavailability. Recommended dietary iron intake is around 14 mg/day.
Supplementation: For those who require it, 40–80 mg of elemental iron is recommended once daily. Consider every-other-day dosing, addition of vitamin C, and the avoidance of coffee and calcium at the time of dosing to aid in absorption. In more extreme or refractory cases, IV iron can be considered. The major side effect is GI symptoms, which occur in up to 70% of people. It may be reasonable to discontinue supplementation once iron stores have normalized; however, if the athlete’s cause of iron deficiency is chronic, continued supplementation may be considered. Universal iron supplementation, although often performed, is not recommended.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here