Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
* This chapter is modified from the original chapter written by Dr. R. Alan B. Ezekowitz and includes material written by Dr. Samuel E. Lux, IV, with his permission.
Evaluation of almost any patient who seeks medical attention, particularly in a hospital setting, includes the obligatory complete blood cell count. The vast majority of these patients do not have a primary hematologic problem. However, there are many pathophysiologic paradigms that have secondary effects on hematopoiesis and/or the coagulation system. One of the most common examples of these effects includes the anemia of chronic disease or inflammation, which is associated with a wide variety of acute and chronic illnesses, including cancer, collagen vascular disease, severe tissue injuries, renal failure, and infectious processes. A detailed discussion of the anemia of chronic disease can be found in Chapter 11 .
In this chapter, both an organ system and disease-specific approach is used. The goal of this chapter on the hematologic manifestations of systemic diseases is to focus on information that is not included in other sections of this book. For overlapping topics, the reader will be guided to other relevant chapters throughout the book.
Many of the hematologic manifestations of the various collagen vascular diseases are similar. However, the characteristics that are unique to each syndrome are emphasized in this section.
Anemia is the most common hematologic abnormality in systemic lupus erythematosus (SLE). There are multiple causes of anemia in this disease, although the anemia of chronic disease is probably the most common (see Chapter 11 ).
Acquired autoimmune hemolytic anemia (AIHA) may precede the onset of active SLE. It is typically characterized by a warm-type AIHA with a serologic pattern of immunoglobulin (Ig) G plus complement on the red blood cell surface. Although positive results on an antiglobulin test are common, true hemolysis is seen in less than 10% of patients. Chapter 13 details the pathophysiology and management of AIHA.
Mechanical hemolytic anemia may be seen in SLE-associated thrombotic thrombocytopenic purpura (TTP). Myelofibrosis has also been described in several patients with SLE; immune complexes have been suggested but not proven to be the cause of this rare complication of SLE. Aplastic anemia has been reported in association with SLE.
A reduction in the total white blood cell count is seen in most patients but is more common in adults than children. The leukopenia is usually caused by a combination of decreased numbers of granulocytes and lymphocytes. Lymphopenia may be severe in SLE and is sometimes due to IgG or IgM antilymphocyte antibodies. The magnitude of the reduction in T cells parallels disease activity and is associated with defects in cellular immunity, as measured by skin tests of delayed hypersensitivity, blast transformation in response to mitogen (phytohemagglutinin and concanavalin A), and macrophage-inhibiting factor production. Reduced numbers of regulatory T cells contribute to the loss of T- and B-cell tolerance in SLE. Unlike T-cell function, B-cell function is increased despite the decrease in the absolute number of B cells with emergence of autoreactive B cells. There is actually an increase in IgG-synthesizing peripheral blood lymphocytes, as well as elevated numbers of cells capable of binding native DNA and elevated numbers of IgM- and IgG-producing cells with antibody specificity against DNA antigen. These changes in peripheral blood lymphocytes are associated with certain morphologic alterations. A strongly basophilic cytoplasm and a high nuclear-to-cytoplasmic ratio are noted in many lymphocytes believed to be “immunoblasts.” On electron microscopic examination, inclusions are identified within lymphocytes that appear as undulating tubules characteristically associated with the endoplasmic reticulum.
Antibodies to granulocytes have been proposed as one mechanism for the granulocytopenia. Granulocyte antibodies and peripheral granulocyte destruction have been observed. Bone marrow depression of granulocyte formation may also occur. Sera from patients with SLE can inhibit mouse bone marrow colony-forming units. Qualitative abnormalities in granulocyte function have also been noted. Although several investigators have found no abnormalities in chemotaxis, others have observed abnormal migration by the skin window technique and diminished in vitro phagocytosis. These defects parallel the observed depressions in complement. The qualitative defects in granulocyte function may be the result of altered humoral factors rather than defects in the phagocytic cells themselves.
Thrombocytopenia secondary to platelet immune sensitization is the most common immune-mediated hematologic manifestation of SLE (see Chapter 34 ). Thrombocytopenia may be an initial manifestation of SLE, and consideration of SLE should be part of the evaluation of immune thrombocytopenia (ITP) in adults and adolescent females. Occasionally, ITP may manifest years before serologic evidence or clinical manifestations of SLE are detectable. Similar to primary ITP, platelet survival is shortened and there are typically increased numbers of bone marrow megakaryocytes. The platelet antibody is commonly an IgG with a molecular mass of 150,000 to 330,000 daltons, and it binds complement. ITP in patients with SLE has the same therapeutic implication as primary ITP, that is, corticosteroids or intravenous immune globulin as primary intervention, with other treatments, including rituximab and splenectomy, reserved for patients who fail these approaches.
Rarely, a qualitative defect in platelet function may also be present with an antibody that interferes with platelet aggregation. Thrombocytopenia may be seen in association with SLE and TTP.
Patients with SLE may also have a circulating anticoagulant in their plasma. The antibody-anticoagulant is directed against various phospholipids involved in phospholipid-dependent anticoagulation assays. Most investigators have reported that lupus anticoagulant is an immunoglobulin that may be of the IgG, IgM, or mixed IgG-IgM class. The pathologic significance of the lupus anticoagulant and other antiphospholipid antibodies is their association with thrombosis, mainly venous, as well as miscarriages and spontaneous abortions (see Chapter 35 ). Among adult SLE patients, 12% to 30% have anticardiolipin antibodies and 15% to 34% have lupus anticoagulant antibodies. The prevalence of thrombosis in pediatric SLE patients with lupus anticoagulant is not known. In adults, the prevalence of thrombosis is about 30%, with the predominant site being the legs in 66%, followed by the peripheral arteries in 10% and the cerebral arteries in 2%.
The partial thromboplastin time is invariably prolonged in patients with the lupus anticoagulant and is considered to be the most sensitive screening test. The inhibitor is suspected when the addition of normal plasma to the patient's plasma fails to correct the defect. The prothrombin time in patients with the lupus anticoagulant is often normal or minimally prolonged because of the influence of the inhibitor. True prothrombin deficiency may occur in patients with the inhibitor, but it is rare. Prolonged thrombin times in the absence of elevated levels of fibrin split products may occasionally be seen. The exact significance of this finding is unclear.
A minority of patients have a catastrophic form of the disease. This often fatal syndrome is defined by the clinical involvement of at least three different organs over a period of days to weeks, with pathologic evidence of multiple occlusions of large or small vessels. In general, involvement of small vessels is more common in these patients, who tend to present with an acute thrombotic microangiopathy involving the kidney, lungs, central nervous system, heart, or skin, along with disseminated intravascular coagulation (DIC). The mortality rate is 30% to 50%. Aggressive therapy with combinations of anticoagulants, corticosteroids, intravenous immune globulin, and plasmapheresis have been tried in anecdotal reports with varying success.
In children, the lupus anticoagulant is usually found coincidentally on routine preoperative coagulation screening. In the majority of cases, this lupus anticoagulant is not associated with SLE or bleeding symptoms. In these cases, the lupus anticoagulant typically resolves spontaneously within weeks to months.
Anemia in rheumatoid arthritis is caused typically by both anemia of chronic disease and iron-deficiency anemia. A high incidence of iron deficiency has historically been seen in children with juvenile idiopathic arthritis (JIA), because of gastrointestinal blood loss secondary to long-standing use of aspirin and nonsteroidal analgesics in the symptomatic treatment of this disease. The severe inflammatory nature of this disorder makes it a model for anemia of chronic disease, a sideropenic anemia with reticuloendothelial iron overload. Iron is not only loaded inappropriately into the reticuloendothelial system but also accumulates in the involved joint, possibly playing a role in joint destruction.
Microcytosis is very common in juvenile chronic forms of arthritis, with a rate of occurrence of about 40%. The degree of microcytosis relates directly to disease activity. Occasionally, macrocytic anemia develops in patients with JIA. Although abnormal vitamin B 12 metabolism has been suggested as a cause, this is not seen in children. Folate metabolism may, however, be abnormal. Some children with rheumatoid arthritis have diminished plasma and red blood cell folate levels. Increased folic acid plasma clearance and reduced protein binding have also been observed.
A few patients have had mild shortening of red blood cell survival as a result of an extracorpuscular defect. More severe hemolytic anemia is much less common than in other collagen vascular diseases. Erythroid aplasia responsive to corticosteroid therapy has been reported in a child with juvenile rheumatoid arthritis. Circulating inhibitors of erythropoiesis have also been noted.
Leukocytosis and neutrophilia are common during acute flare-ups of JIA; however, this is uncommon in the adult form of the disorder. Neutrophil chemotaxis may be mildly diminished. Phagocytosis is normal or mildly impaired. Nitroblue tetrazolium dye reduction may be increased during active phases of this disease. These minimal alterations in white blood cell function do not produce any clinical disturbances. Wound healing, for example, is normal even after surgery in patients who have not been receiving high-dose corticosteroid therapy. A peripheral blood eosinophil count greater than 5% has been noted in slightly more than 50% of children with JIA. Some will also demonstrate basophilia and plasmacytoid lymphocytes.
Secondary thrombocytosis is common in JIA, as part of the inflammatory response. A consumptive coagulopathy may be seen in association with systemic JIA. Acquired circulating inhibitor to factor VIII has been described in JIA and may cause bleeding. However, clinical problems related to coagulation abnormalities in this disorder are rare.
Felty originally described the triad of rheumatoid arthritis, splenomegaly, and neutropenia in 1924. Whether Felty syndrome occurs with JIA is unclear. Splenomegaly is seen in about 20% of all children with JIA, especially in those with the acute extraarticular exacerbations of this disease. However, splenomegaly alone does not suggest Felty syndrome.
The etiology for the neutropenia of Felty syndrome is multifactorial. The neutropenia is not always a consequence of hypersplenism per se because only 60% of adults who undergo splenectomy experience resolution of their neutropenia. In 50% of patients, a maturation arrest bone marrow pattern is demonstrated, possibly from immune humoral or cellular mechanisms. Neutropenia in association with cytotoxic lymphocytes directed against granulocyte progenitors has also been reported, as have lower than normal levels of granulocyte colony-stimulating factor (G-CSF) activity. The neutropenia of Felty syndrome may also be immunologic in origin, because some patients have IgG antibodies directed against neutrophils.
The neutropenia may be associated with serious infections. Recombinant human G-CSF and granulocyte-macrophage colony-stimulating factor (GM-CSF) both effectively raise the neutrophil count in essentially all patients with Felty syndrome. The neutrophil count decreases again once the growth factor treatment is stopped, but often it stabilizes at a level higher than it was before treatment. The drug can be used long term and may be cost-effective in selected patients with a high incidence of infections and repeated hospitalizations. Methotrexate treatment in Felty syndrome can be effective in correcting the neutropenia. Splenectomy may be indicated in those in whom G-CSF or GM-CSF is ineffective or rapid blood cell improvement is desired. A rise in the neutrophil count after splenectomy is experienced by 50% to 80% of patients.
Microangiopathic hemolytic anemia can be associated with renal disease or hypertensive crises in polyarteritis nodosa. The hemolytic process in these circumstances parallels disease activity. Anemia of chronic disease is common in polyarteritis nodosa.
Neutropenia occurs and is associated with the presence of antineutrophil cytoplasmic antibodies. Neutrophilia and eosinophilia are also common. The eosinophilia may be marked, particularly in those patients with clinically apparent pulmonary involvement.
Wegener granulomatosis, characterized by necrotizing vasculitis (particularly in the lungs and kidneys), is rare but can affect children. The disease can also occur in the neonatal period. The course of the disease is marked by fever, cough, hemoptysis, epistaxis, nasal discharge, obliteration of the nasal sinuses, and nodular pulmonary infiltrates. Renal failure may occur. A microangiopathic hemolytic anemia can develop. An associated anemia of chronic disease is common.
The white blood cell count is elevated, often coupled with an eosinophilia. The disease is characterized by marked thrombocytosis. Autoantibodies directed against cytoplasmic components of neutrophil granulocytes and monocytes have been described as a disease-specific marker for Wegener granulomatosis.
Bleeding and bruising are common manifestations of connective tissue disorders related to fragility of capillaries and perivascular connective tissue rather than disorders of clotting or platelet function. Primary hemostasis depends on the interaction of the vessel wall, platelets, and the clotting cascade. In connective tissue disorders, the connective tissues of the skin, subcutaneous tissue, and blood vessel wall have increased fragility, which portends increased bruising and bleeding. Laboratory evaluation for disorders of platelet aggregation and clotting factors is typically normal. Genetic counseling is recommended when a connective tissue disorder is suspected.
Most Ehlers-Danlos subtypes are caused by mutations in fibrillar collagens. The elastic tissue defects result in an increased bleeding tendency. Different forms of Ehlers-Danlos have been associated with platelet and coagulation defects, including disorders of platelet aggregation and factor deficiencies. It is not clear whether these hematologic defects are sporadic associations in individual patients or true associations with the subtypes of Ehlers-Danlos.
In Ehlers-Danlos syndrome, the bleeding symptoms are typically easy bruising, gingival bleeding, prolonged bleeding with surgical or dental procedures, and menorrhagia. Other common clinical findings include skin hyperextensibility, “cigarette paper–like” scars, and joint hypermobility. The vascular Ehlers-Danlos syndrome, type IV, is an autosomal dominant disorder caused by mutations in the collagen type III alpha 1 (COL3A1) gene. Type IV Ehlers-Danlos is associated with small and large vessel bleeding, including arterial bleeding, and spontaneous ruptures of the bowel, uterus, and lungs. Nonemergent surgical interventions are to be avoided in these individuals owing to the high risk for perisurgical bleeding and complications. With serious bleeding in Ehlers-Danlos syndrome patients, efficacy of desmopressin and of factor VIIa has been reported.
Marfan syndrome is not associated with fragility of capillaries or small to medium-sized arteries or veins. However, many Marfan syndrome patients report easy bruising, which has not been explained. A defective fibronectin has been proposed as the cause of the hypermobility and platelet dysfunction in some patients. Easy bruising has also been reported in individuals with osteogenesis imperfecta.
Ascorbic acid functions as a cofactor, enzyme complement, cosubstrate, and antioxidant for a wide variety of metabolic processes. Vitamin C is involved in reactions involving iron, copper, vitamin E, and folic acid. Deficient intake of ascorbic acid results in impaired collagen synthesis, mitochondrial fatty acid transport, and synthesis of neurotransmitters. Although scurvy is uncommon in developed countries, certain patient subpopulations remain at risk for this type of nutritional deficiency, such as those with neurologic impairment, psychiatric disease, alcoholism, small bowel disease, and iron-overload disorders associated with vitamin C renal wasting. Scurvy occurs when vitamin C has been eliminated from the diet for at least 3 months. Hematologic symptoms of scurvy arise mainly from defective collagen synthesis and include gingival bleeding, subperiosteal hemorrhage with painful joint bleeding, and organ hemorrhage. Scurvy is associated with a multifactorial anemia related to bleeding, dietary deficiencies, oxidative hemolysis, and altered metabolism of iron and folate. Other manifestations include poor wound healing, defective dentine formation, tooth and hair loss, and bony changes. The symptoms of scurvy, including the bleeding manifestations, rapidly respond to enteral vitamin C supplementation.
The anemia of chronic disease is common to all infections. Even common childhood infections, especially those associated with inflammation, will cause a decline in hemoglobin concentration. During active inflammation, the hemoglobin concentration declines about 13%, usually within 1 week, followed by an increase of nearly 25% during resolution of the active inflammation. The actions of hepcidin probably deprive microorganisms of iron, which is an essential nutrient for microbial replication, and may be a component of host immunity against infection. Serum levels of iron decrease after a bacterial challenge, and the lactoferrin released from stimulated granulocytes is a conduit for this iron to return to reticuloendothelial cells. Many examples of bacterial pathogenicity being dependent on iron availability (e.g., Neisseria gonorrhoeae, Neisseria meningitidis ) support the hypothesis that the anemia of infection is the body's compromise in an attempt to deprive invading microorganisms of iron.
Although some infections, particularly viral ones, such as parvovirus, cause transient bone marrow aplasia or selective erythroid aplasia, anemia from this cause is rare because of the long life span of red blood cells. In contrast, patients with chronic hemolytic anemias may experience a rapid decrease in hemoglobin concentration or an “aplastic” crisis during viral and some bacterial infections.
Severe hemolytic anemia may be observed in certain types of infections. Clostridial infections may result in a high titer of hemolysins and cause severe anemia. A similar severe anemia may result from sepsis related to other bacterial organisms, including staphylococci, streptococci, pneumococci, and Haemophilus influenzae. Immune hemolytic anemia mediated by a cold agglutinin may be observed with Listeria and Mycoplasma infections and occasionally with infections by other organisms such as Epstein-Barr virus.
Many viral illnesses may be associated with what appears to be a mild hemolytic anemia for which no pathologic mechanism has been defined. The most common morphologic finding in these circumstances is poikilocytosis. Certain viruses, such as most strains of influenza virus, contain neuraminidase activity, which is, at least theoretically, capable of affecting the sialic acid content of the red blood cell membrane. Whether this plays any significant role in the hemolysis associated with some viral diseases is not known.
Many congenital infections, including cytomegalovirus, herpes simplex, rubella, toxoplasmosis, and syphilis, produce profound hemolytic anemia in the neonatal period, even though these same agents may not significantly alter red blood cell survival at other times of life.
The white blood cell count may be normal, low, or high with infection. Viral illnesses may be associated with leukocyte counts lower than 5000 cells/mm 3 , although bacterial diseases of certain types or overwhelming sepsis of any type may also cause leukopenia. The most common viral illnesses associated with leukopenia are infectious hepatitis, infectious mononucleosis, rubella, measles, and, occasionally, influenza. Of the bacterial infections, shigellosis may produce leukopenia with a marked increase in band cell forms. Sepsis caused by meningococci, pneumococci, staphylococci, and a few other bacterial pathogens may also cause leukopenia.
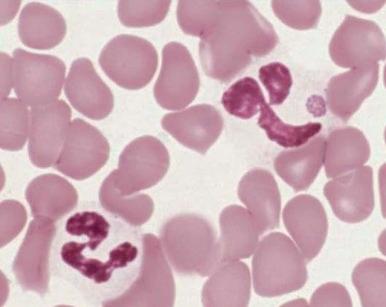
Neutrophilia, with or without an increase in band cell count, is a common result of bacterial infection. White blood cell and neutrophil counts do not differ between children of different races with bacteremia. Occasionally, viral illness also initially will be manifested as neutrophilia. A variety of morphologic changes may appear in the neutrophils of patients with infection. Döhle bodies ( Fig. 37-1 ), which are pale blue cystlike inclusion bodies usually located in the periphery of the cytoplasm of neutrophils, may appear in bacterial infections. They are occasionally associated with viral illness but are also commonly seen in patients with burns, massive trauma, and cancer, as well as in pregnancy and after the use of cyclophosphamide. In addition, Döhle bodies are seen in the May-Hegglin anomaly. Increased size of neutrophil granules (“toxic granulation”) may be found in both bacterial and viral illnesses, as well as in many of the other disorders associated with the presence of Döhle bodies. Vacuolization of the cytoplasm of neutrophils is the next most common morphologic abnormality of neutrophils in patients with significant bacteremia. In overwhelming sepsis, the organisms are frequently evident in the vacuoles of the polymorphonuclear cells. In a study of the neutrophils of patients with bacteremia, Zipursky and associates found toxic granulation, Döhle bodies, and vacuolization in 75%, 29%, and 24%, respectively, of the patients studied.
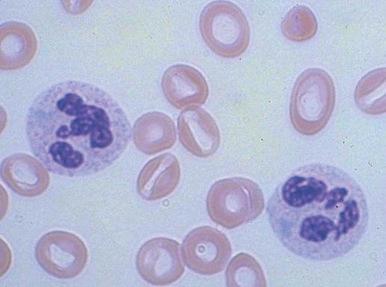
Increased neutrophil alkaline phosphatase activity and nitroblue tetrazolium dye reduction may also occur, but neither of these characteristics is specific for bacterial infection. Infections may be associated with development of the Pelger-Huët anomaly ( Fig. 37-2 ), in which granulocytes and eosinophils have one or two lobes per nucleus and assume a round, dumbbell, or peanut shape.
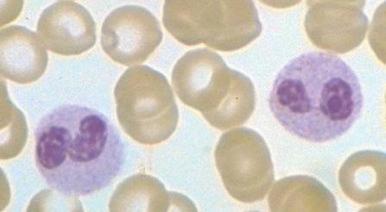
In neonates, especially those born prematurely, an increase in total white blood cell or mature neutrophil counts may not be seen in the presence of infection. In fact, a decrease in the neutrophil count often occurs. The most helpful signs of septicemia in this age group are an increase in the band cell count and the presence of toxic granulations and Döhle bodies.
Leukocytosis may result from lymphocytosis. The most common infections producing the greatest increases in lymphocyte counts are infectious mononucleosis, cat-scratch disease, and pertussis. Many other viral illnesses, such as cytomegalovirus, rubella, mumps, and hepatitis, may also cause an increase in the lymphocyte count. Lymphopenia of T lymphocytes is a common finding after measles infection.
Eosinophilia may reflect the presence of parasitic infections. In the United States, the most common cause of marked elevations in eosinophil counts is Toxocara infestation, which is often accompanied by high titers of isohemagglutinins. Other parasites commonly causing eosinophilia include organisms belonging to Trichinella, Echinococcus, Filaria, Strongyloides, Schistosoma, Enterobius, and Ancylostoma and tapeworms other than Echinococcus. Allergic sensitization to mites may cause eosinophilia, as well as fungal infections, especially aspergillosis. Eosinophilia is not specific for infestation. Marked degrees of eosinophilia may occur in association with prematurity. An absolute eosinophilia may be expected in about 75% of low-birth-weight infants. In some, the eosinophilia is marked (3000 cells/mm 3 ) and the maximal increase seems to occur at about the time that birth weight is regained, although this is not true in all infants.
Monocytosis is occasionally reported with specific infections, especially tuberculosis, syphilis, and subacute bacterial endocarditis. Monocytosis is often noted early in the course of many infections and again on recovery, particularly if it is associated with granulocytopenia.
Basophilia is rarely seen in infection but has been reported with tuberculosis, influenza, and hookworm infestation.
DIC may be triggered by infectious processes. Of the infectious causes, gram-negative septicemia is probably most common. Meningococcus, Escherichia coli, Proteus, Pseudomonas, Aerobacter, and Klebsiella are among the most common etiologic agents recovered from the bloodstream. Gram-positive septicemia can cause a similar picture. The most common offender is Streptococcus pneumoniae, especially in asplenic individuals. Other gram-positive agents causing DIC include Staphylococcus aureus, other streptococcal species, and Clostridium. A wide range of viral infections may cause a consumptive coagulopathy that often leads to purpura fulminans. Among the most common agents are those that cause infectious hepatitis, measles, rubella, varicella, and infectious mononucleosis. Less common causes of DIC are severe mycoplasmal, rickettsial, and malarial infections.
Thrombocytopenia occurring separately from a true disseminated consumptive process is quite common in many infectious processes, especially in association with infectious mononucleosis, cytomegalovirus, rubella, measles, gram-negative bacteremia, and rickettsial diseases. Congenital viral infections and congenital syphilis and toxoplasmosis, if clinically apparent, are almost invariably associated with increased platelet turnover with or without thrombocytopenia. Pediatric immune thrombocytopenia is commonly associated with viral infections. Immune thrombocytopenia may also occur after immunization with the measles, mumps, and rubella vaccine. Corrigan found that thrombocytopenia without consumptive coagulopathy is an extremely common finding in infants and children with septicemia. In contrast, thrombocytosis is often present during the active phases of infectious processes. The platelet distribution width and mean platelet volume may be helpful in predicting whether the thrombocytopenia is due to infection. In the late neonatal period, thrombocytopenia associated with an infection dramatically increases the mean platelet volume and platelet distribution width as determined by electronic counting equipment.
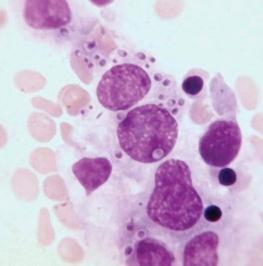
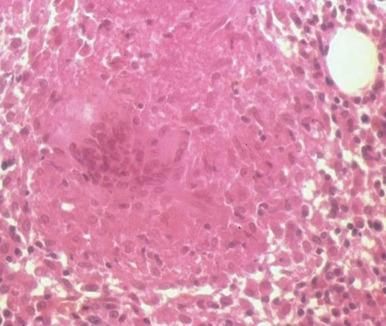
The bone marrow frequently contains clues as to the source of infectious diseases. Histoplasmosis ( Fig. 37-3 ), tuberculosis, kala-azar, Salmonella typhi, and Candida ( Fig. 37-4 ) all have been identified in marrow macrophages. Marrow granulomas may be a marker of disseminated tuberculosis or histoplasmosis ( Fig. 37-5 ). Histoplasmosis, tuberculosis, brucellosis, and Salmonella can be successfully cultured from the marrow aspirate.
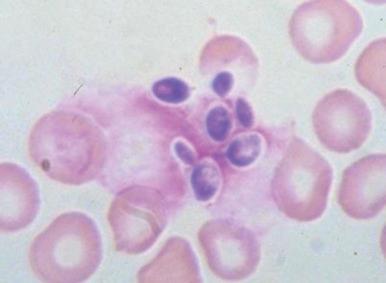
Clostridium perfringens septicemia is seen in a variety of clinical situations but must particularly be considered in patients with penetrating wounds, septic abortions, peritonitis after a perforated viscus, or cholecystitis or cholangitis; in immunosuppressed patients with gastrointestinal or hematologic malignancies; and in neonates with necrotizing enterocolitis. In patients with clostridial sepsis, severe, rapidly progressive intravascular hemolysis and microspherocytosis may occur ( Fig. 37-6 ). Hemolysis of the entire red blood cell mass has been reported. Complications include shock, acute renal failure, and death. Transfusion therapy may be ineffective. Antibiotics and hyperbaric oxygen occasionally have been used successfully to treat clostridial infections.
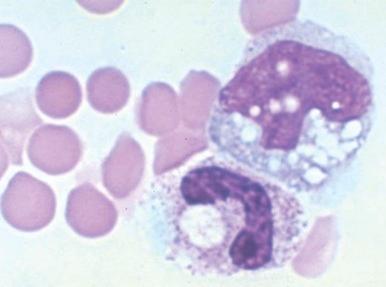
The mechanism of red blood cell damage is uncertain and may vary between patients. The bacteria produce several hemolytic toxins including α-toxin, a 43-kD protein that contains an NH 2 -terminal phospholipase domain and a COOH-terminal domain required for hemolysis, and θ-toxin, a 54-kD cholesterol-binding protein that aggregates and forms membrane pores leading to colloid osmotic hemolysis. α-Toxin appears to induce hemolysis through the activation of sphingomyelin metabolism. C. perfringens contains a neuraminidase that cleaves terminal sialic acids from red blood cell glycoproteins in some patients. The underlying galactose residues form the Thomsen-Friedenreich cryptoantigen (or T antigen). Anti-T antibodies are present in almost all adult plasma; thus T-antigen activation can lead to significant hemolysis. In infected infants and children who lack T antibodies, transfusion may lead to massive hemolysis and death. Rarely, T-antigen activation may precede the intravascular hemolysis, leading to early detection of clostridial sepsis and lifesaving therapeutic intervention.
Pertussis may cause a marked increase in the white blood cell count, with elevations to 40,000 cells/mm 3 or higher, most of which are due to an increase in the lymphocyte count. Marked leukocytosis has been associated with a more severe course. Hyperleukocytosis with white blood cell counts greater than 100,000 cells/mm 3 has been associated with the presence of immature leukocytes and obstruction in the pulmonary microcirculation. Successful leukoreduction has led to clinical improvement in a number of young infants with severe pertussis.
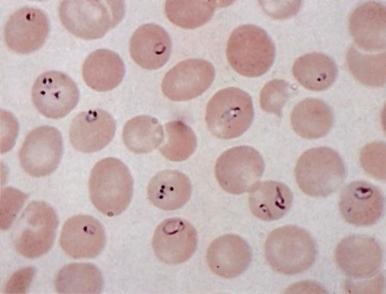
In healthy individuals, a mild hemolytic anemia is caused by Babesia microti and Babesia divergens microorganisms. Occasional cases of combined B. microti babesiosis and Lyme disease are described, attributable to the shared geographic locale of the tick vectors for these diseases. The disease can also be acquired by transfusion. Babesiosis is not life threatening except in asplenic subjects in whom the parasitized red blood cells are not contained. Then, the infection can be rapidly progressive and life threatening. In extreme cases, nearly all the red blood cells may be parasitized ( Fig. 37-7 ). Patients may have malaise, headache, fever, shaking chills, profuse sweating, jaundice, and dark urine. There may be intravascular hemolysis and, occasionally, pancytopenia and hemophagocytosis. Diagnosis is made by finding the parasites in blood smears or by serologic tests or amplification of parasitic DNA using the polymerase chain reaction (PCR) assay. Current treatment of symptomatic cases is quinine plus clindamycin, but treatment failures have been reported in asplenic patients. Exchange transfusion may reduce the parasite load and be beneficial.
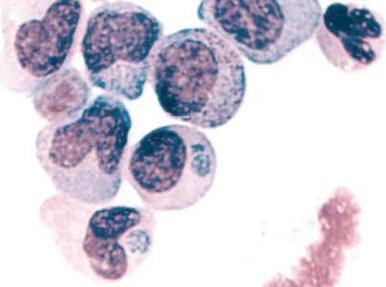
A variety of hematologic manifestations are found in infections with Ehrlichia chaffeensis, the causative bacteria of human monocytic ehrlichiosis (HME, Fig. 37-8 ), and Anaplasma phagocytophilum, the causative bacteria of human granulocytic anaplasmosis (HGA, Fig. 37-9 )). The disease is transmitted through tick bites, typically through the Lone Star tick or Ixodes scapularis, the shared tick vector of Lyme disease and babesiosis. Transmission has also been reported through blood transfusions. Symptoms are variable but are most severe in immunocompromised individuals. The obligate intracellular bacteria grow within membrane-bound vacuoles in leukocytes. General symptoms can include fever, headache, rash, and neurologic symptoms. Thrombocytopenia and leukopenia are common, often with both lymphopenia and neutropenia. Lymphopenia can be followed by an atypical lymphocytosis in the later stages of disease. Intraleukocytic morulae detected on review of the peripheral blood smear or buffy coat can be seen in up to 50% to 70% of infected individuals. Diagnosis can also be made through serologic tests or PCR assay. Doxycycline is the treatment of choice; however, rifampin has been reported to be effective in young patients.
Tuberculosis produces a variety of hematologic abnormalities. Leukemoid reactions mimicking myeloproliferative disorders are common. Bone marrow involvement in miliary tuberculosis may result in a leukoerythroblastic pattern with teardrop-shaped red blood cells, nucleated red blood cells, and myeloblasts apparent on the peripheral blood smear. Bone marrow biopsy may show evidence of granulomas. Monocytosis is common, and thrombocytopenia and pancytopenia have been reported.
Parvovirus B19, the cause of fifth disease (erythema infectiosum) in children, infects and kills early erythroid progenitors bearing the P blood group antigen. This infection, which is discussed in Chapter 6 , is not clinically significant unless the infection occurs in the setting of chronic hemolytic processes such as sickle cell disease or hereditary spherocytosis, or in patients with compromised immunity. In these situations, life-threatening red blood cell aplasia or even pancytopenia may be caused by this virus. The infection typically presents as fever, vomiting, and abdominal pain, along with pallor, fatigue, and other symptoms of anemia. Sometimes multiple family members are affected simultaneously. Parvovirus B19 infections are often associated with mild neutropenia or thrombocytopenia, and isolated cases of transient pancytopenia, hemophagocytosis, and transient myelodysplasia are reported. The virus is a particular danger to pregnant women because it can cause fetal death due to anemia and nonimmune hydrops fetalis.
Infectious mononucleosis is caused by the Epstein-Barr virus (EBV) and historically associated with the triad of (1) the classic clinical picture, (2) atypical lymphocytosis, and (3) positive results on a heterophil antibody test. Serologic testing for EBV is now often used in addition or instead of the heterophil antibody test to confirm acute infection.
EBV infection is acquired at an early age in lower socioeconomic groups. In economically privileged children, infection is often delayed until adolescence and young adulthood. About 40% of American children are seropositive for EBV by 5 years of age. In a report from the U.S. Military Academy at West Point, 63.5% of entering cadets had EBV antibody, indicative of previous infection. During the college years, the infection rate among susceptible individuals is 12% to 15% per year.
The pattern of infection in different groups of susceptible persons is probably best explained by transmission of this virus in throat secretions. EBV is present in the saliva of most patients with infectious mononucleosis, in up to 20% of healthy persons with EBV antibodies, and in 50% or more of seropositive patients receiving immunosuppressive drugs. The ease with which EBV is recovered from the oral secretions of persons with primary or reactivated EBV infection suggests that a cell type that freely permits EBV replication exists in the oropharynx. The oropharyngeal epithelial cell may be the target cell type that is productively infected in infectious mononucleosis. Transmission requires intimate contact. Intrafamilial spread, however, does occur frequently, and, in this setting, an incubation period of 4 to 6 weeks has been demonstrated. Another possible route of transmission of EBV is transfusion through white blood cells.
The classic clinical manifestations of infectious mononucleosis are generally seen only in adolescents and young adults. Younger children rarely exhibit the typical findings of this disease, and most generally have only a mild viral respiratory illness.
The onset of typical illness is usually a subtle prodrome consisting of fatigue, malaise, sweating, feverishness, and anorexia. Headache, nausea, and vomiting are seen frequently. The most common symptom during this period is a sore throat, which begins slowly and increases in intensity over a 1-week period. The usual findings of infectious mononucleosis then follow ( Table 37-1 ). The course of fever often follows a specific pattern, with no temperature elevation in the morning but daily afternoon or evening peaks of 38.3° C to 39.4° C (101° F to 103° F). Occasionally, higher temperatures are observed. The fever usually lasts about 2 weeks.
| Symptom or Sign | % |
|---|---|
| Adenopathy | 100 |
| Malaise and fatigue | 90-100 |
| Fever | 80-95 |
| Sweats | 80-95 |
| Sore throat, dysphagia | 80-95 |
| Pharyngitis | 65-85 |
| Anorexia | 50-80 |
| Nausea | 50-70 |
| Splenomegaly | 50-60 |
| Headache | 40-70 |
| Chills | 40-60 |
| Bradycardia | 35-50 |
| Cough | 30-50 |
| Periorbital edema | 25-40 |
| Palatal enanthema | 25-35 |
| Liver or splenic tenderness | 15-30 |
| Myalgia | 12-30 |
| Hepatomegaly | 15-25 |
| Rhinitis | 10-25 |
| Ocular muscle pain | 10-20 |
| Chest pain | 5-20 |
| Jaundice | 5-10 |
| Arthralgia | 5-10 |
| Diarrhea or soft stools | 5-10 |
| Photophobia | 5-10 |
| Rash | 3-6 |
| Conjunctivitis | 5 |
| Abdominal pain | 5 |
| Gingivitis | 3 |
| Pneumonitis | 3 |
| Epistaxis | 3 |
Lymphadenopathy is seen in all patients. Symmetric, moderately enlarged, discrete, slightly tender nodes, especially in the posterior cervical region, are most characteristic. Adenopathy is common in the axillary, epitrochlear, and inguinal areas. The nodes are not matted and do not show signs of heat, redness, or fluctuation. Rarely, enlargement of the mediastinal glands constitutes the only evidence of adenopathy and may be confused with a lymphomatous process.
Splenomegaly occurs in more than 50% of patients with infectious mononucleosis. The spleen is usually just barely palpable but on rare occasion may be quite large. It is smooth, soft to firm, and sometimes slightly tender. In some patients, splenomegaly persists for months, but the most common situation is resolution by 2 to 3 weeks after onset of the illness.
Hepatomegaly occurs in about 20% of patients, and clinical jaundice is seen in 10%. The jaundice is invariably mild. Despite the low rate of occurrence of apparent hepatic dysfunction, virtually all patients with infectious mononucleosis demonstrate abnormal liver enzyme levels. Except in rare patients with acute liver failure, the hepatitis associated with this illness is self-limited. There is no evidence that chronic liver disease or cirrhosis results from infectious mononucleosis.
Rashes occur occasionally, and their manifestation follows no particular pattern. The rash may be a diffuse, faint, erythematous or maculopapular eruption, or it may be urticarial, scarlatiniform, petechial, or herpetiform. In general, the rashes of infectious mononucleosis have no unique features and are of little or no help for diagnosis. Circulating immune complexes and complement sequence activation occur only when rashes are present. If ampicillin is administered to patients with infectious mononucleosis, a rash will develop in 69% to 100%. Other penicillins may cause this response but less often. This rash is not an allergic reaction, and these antibiotics may be administered subsequently without ill effect.
Only a few patients with infectious mononucleosis have no pharyngitis. Almost all demonstrate hyperplasia of the pharyngeal lymphoid follicles. The presence of an exudate is common, and membrane formation occurs frequently. The inflammation may be severe enough to cause respiratory obstruction. Peritonsillar abscesses may complicate the course of the illness. A palatal enanthema is seen in about a third of patients and consists of crops of sharply circumscribed petechiae, symmetrically distributed at the junction of the soft palate. Unfortunately, such petechiae are not specific for infectious mononucleosis and have been described in rubella and other viral disorders. In fact, the entire pharyngeal manifestation of infectious mononucleosis is clinically indistinguishable from that of streptococcal disease.
Periorbital edema is not rare and occasionally leads to the erroneous suspicion that renal disease or hypoproteinemia is present. The edema is self-limited and lasts only a few days.
Much attention is paid to the complications associated with infectious mononucleosis, although their overall occurrence is low ( Table 37-2 ). Hematologic complications of infectious mononucleosis include disturbances resulting in anemia, granulocytopenia, thrombocytopenia, and occasional coagulation defects. The rate of occurrence of these hematologic abnormalities is shown in Table 37-3 .
| Type of Complication | Diagnosis or Description of Abnormalities |
|---|---|
| Neurologic | Bell palsy, cerebellar syndrome, encephalitis, encephalomyelitis, encephalomyelopathy, Guillain-Barré syndrome, meningitis, meningoencephalitis, myelitis, optic neuritis, peripheral neuritis, psychosis, radiculoneuritis, ataxia, positive Babinski sign, coma, convulsions, diplopia, extraocular palsy, facial diplegia, hemiplegia, hyperesthesia, meningismus, mental confusion, nystagmus, papilledema, psychotic reaction, ptosis, respiratory paralysis, positive Romberg sign, seizure, status epilepticus, scotomas |
| Cardiac | Electrocardiographic changes, myocarditis, pericarditis |
| Ocular | Conjunctivitis, diplopia, cyclic edema, hemianopia, lacrimal pericyclitis, nystagmus, optic neuritis, ptosis, retinal edema, retinal hemorrhage, retroorbital pain, scotomas, uveitis |
| Respiratory | Laryngeal obstruction, peritonsillar abscess, pharyngeal edema, pleural effusion, pleuritis, pneumonitis |
| Hematologic | Acquired hemolytic anemia, agranulocytosis, eosinophilia, fibrinolysis, pancytopenia, splenic rupture, thrombocytopenia |
| Digestive | Esophageal varices, gingivitis, hepatic dysfunction, hepatic necrosis, jaundice, melena |
| Renal | Hematuria, hemoglobinuria, nephritis, nephrotic syndrome, porphyrinuria, proteinuria |
| Other | Bullous myringitis, endocervicitis, orchitis, otitis media, pancreatitis, porphyria, rashes |
| Findings | % Positive |
|---|---|
| Lymphocytosis, relative and absolute | 100 |
| Atypical lymphocytes, definite * | 100 |
| Epstein-Barr virus antibody in serum | 100 |
| Heterophil antibody | 80-100 |
| Liver enzyme abnormalities | 80-100 |
| Leukocytosis | 60-80 |
| Neutropenia | 60-80 |
| Hyperbilirubinemia | 30-50 |
| Bone marrow granulomas | 50 |
| Slight thrombocytopenia | 25-50 |
| Increased cold agglutinins | 10-50 |
| Occult hemolysis | 20-40 |
| Hyperuricemia | 15-20 |
| Leukopenia | 10-20 |
| Severe thrombocytopenia with bleeding | Rare |
| Positive direct Coombs test results | Rare |
| Significant anemia (usually caused by hemolysis) | Rare |
* Twenty percent or more of white blood cells in peripheral blood.
AIHA can occur with EBV infection and developed in approximately 3% of West Point cadets with infectious mononucleosis. When hemolysis does occur, it usually begins 1 to 2 weeks into the course of the illness. The majority of occurrences terminate in less than 1 month, and chronic hemolysis is rare. Although usually mild, hemolysis occasionally occurs rapidly and can then result in severe anemia. Jenkins reported the first instance of hemolytic anemia in infectious mononucleosis that was mediated by the temporary induction of a high-thermal amplitude cold agglutinin of anti-i specificity. Since then, several series have verified the high incidence of anti-i antibody as the cause of immune-mediated hemolysis in infectious mononucleosis. It should be noted that although hemolysis is not common in infectious mononucleosis, the presence of anti-i is seen in as many as 50% of all patients. Not all instances of immune hemolysis in infectious mononucleosis are caused by anti-i antibodies. Anti-N antibodies have also been reported, and in some patients the nature of the antibody has not been identified.
Aplastic anemia has been reported to follow the onset of infectious mononucleosis. Aplastic anemia caused by EBV infection has also been reported after bone marrow transplantation. Localization of EBV in the bone marrow of some patients with aplastic anemia supports a causative role of the virus in these aplastic anemia patients.
Granulocytopenia is common during the acute phase of infectious mononucleosis. It is rarely severe but, on occasion, is associated with a secondary bacterial infection. Bone marrow myeloid hyperplasia with myeloid arrest is the most typical finding. Spontaneous resolution is the rule.
Immune thrombocytopenia occurs rarely in infectious mononucleosis. Most occurrences of thrombocytopenia are mild. The signs and symptoms of severe occurrences are similar to those of primary ITP. Severe hemorrhagic complications are rare. Treatment of the thrombocytopenia of infectious mononucleosis in children is guided by bleeding symptoms. Treatment approaches are similar to primary ITP with first-line therapies with corticosteroids and/or intravenous immune globulin.
The incidence of the neurologic complications reported varies from 0.37% to 7.3%, depending on the series. A rare occurrence in adolescence is the “Alice in Wonderland” phenomenon in which objects are visualized in a very distorted fashion, with exaggerations in size, being either too large or too small. A transverse myelopathy characterized by a sudden onset of profound weakness of the lower extremities and urinary retention may complicate the clinical course of infectious mononucleosis.
Cardiac complications are rare and occur in 1% to 6% of reported series. They usually consist of only nonspecific T-wave changes or minor conduction abnormalities. Myocarditis and pericarditis are rare. Liver function abnormalities are rarely severe, although enzyme changes are common. Primary EBV infection has been associated with a Reye syndrome–like illness. Severe liver dysfunction causing death is a common complication of EBV infection in the X-linked lymphoproliferative syndrome. Hepatic dysfunction is uniformly present with this disorder at the time of death and is the cause of death in about a third of such patients. An unusual manifestation of infectious mononucleosis is intense jaundice. It generally results from a combination of hemolysis and mild hepatitis. Spontaneous rupture of the spleen may occur.
Respiratory difficulties usually consist of upper airway obstruction. Transient interstitial infiltrations, some with effusions, have been recorded. Infectious mononucleosis should be considered in the differential diagnosis of any child with pleural effusions. Renal complications of infectious mononucleosis generally consist of hematuria associated with a mild nephritis. Not all occurrences have been clearly separated from poststreptococcal glomerulonephritis. Severe rhabdomyolysis can be associated on rare occasion with EBV infection. Eye findings in infectious mononucleosis are unusual but may be significant when they do occur. Severe retinochoroiditis is such an ocular complication. In some patients with infectious mononucleosis, lethargy, particularly daytime lethargy, is seen for prolonged periods, often longer than a year.
X-linked lymphoproliferative syndrome is associated with fatal or severe infectious mononucleosis, acquired hypogammaglobulinemia, and malignant lymphoma. Details about X-linked lymphoproliferative syndrome can be found in Chapter 24 .
A variety of malignancies have been linked to EBV, including clonal T-cell proliferations. In patients who undergo transplantation, a spectrum of lymphoproliferative diseases may occur as a result of activation of EBV. These diseases may vary from an infectious mononucleosis–like polyclonal B-cell proliferation to a monoclonal B-cell lymphoma. EBV infection can also result in a hemophagocytic syndrome. The total spectrum of the rare complications and unusual syndromes associated with EBV infection have been reviewed by Timár and colleagues.
Atypical lymphocytosis is the hallmark of infectious mononucleosis ( Fig. 37-10 ). Several attempts have been made to classify these abnormal cells on morphologic grounds, the best known method being that of Downey and McKinlay. However, it is clear that atypical lymphocytes cannot be easily classified into separate categories and that a spectrum of cell types exists. In general, the atypical lymphocytes of infectious mononucleosis are large, but they vary in size considerably. Their outlines are irregular, and many cells show a characteristic tendency to flow around adjacent erythrocytes. Nuclei are large and usually eccentrically located and pleomorphic, with abundant coarse chromatin and occasional nucleoli. The cytoplasm is generally abundant and typically basophilic. Cytoplasmic vacuoles may be seen. These types of cells are not morphologically specific for infectious mononucleosis and can be seen in other viral infections and after administration of a number of medications.
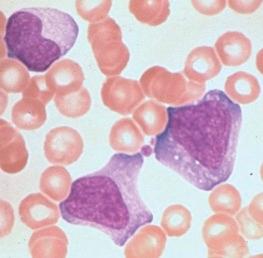
The vast majority of atypical lymphocytes from patients with infectious mononucleosis are thymus derived. These atypical cells possess human T-lymphocyte–specific antigens, as well as sheep erythrocyte receptors. T lymphocytes appear to lack receptors for EBV, and it would appear that only B lymphocytes are infected by this virus. A possible unifying interpretation of the role of T lymphocytes is that these cells represent an immune reaction that protects against this potentially oncogenic virus. Increased numbers of B cells are found during the first week of illness and decline to normal levels in 3 weeks. T lymphocytes reach their peak later, usually 10 to 14 days after the onset of symptoms, and remain elevated for 5 weeks. There may be an early reversal of the ratio of T to B lymphocytes, with a subsequent increase in the percentage of T cells during the second through fifth weeks of illness. It is possible that both T and B cells may be “transformed” into atypical lymphocytes—B cells by infection with EBV and T cells by an immunologic response to viral antigen itself—or the B cells may respond to altered antigens on their surface. EBV-infected B lymphocytes account for only a minority of the atypical lymphocytes found in peripheral blood. In the very early stages of symptomatic illness, however, nearly 20% of all B cells in the circulation may be infected with the virus. The majority of atypical lymphocytes are T lymphocytes. Natural killer (NK) cell activity has been shown to be present during the acute phase of infectious mononucleosis. Interferons, which are inducers of NK cell activity, may have an inhibitory effect on the outgrowth of EBV-infected B lymphocytes in vitro. Significant anergy and diminished lymphocyte responsiveness in vitro to mitogens and antigens exist during the first week of illness. These lymphocyte changes are reflected in a great increase in uric acid turnover, with 55% of infected patients having serum uric acid levels of 8 mg/dL or higher.
Patients with infectious mononucleosis have lymph node pathologic findings that are easily confused with lymphoma. The nodal architecture is distorted by large, dark lymphoid cells, and the capsule may be infiltrated. Reed-Sternberg cells have been reported on several occasions. Pathologic changes are not confined to lymphoid tissue, however. Perivascular cuffing of the brain vasculature, inflammation of the liver, and inflammatory infiltration of the kidney and bone marrow have been repeatedly observed.
The heterophil antibody is so named because the antigen to which the antibody reacts is found in more than one species. The antibody, like anti-i, is an IgM macroglobulin. It agglutinates sheep red blood cells and can be removed completely from serum by preincubation with beef red blood cells but not with guinea pig kidney. Heterophil antibody titers usually increase after the third day of illness, peak at 2 weeks, and may remain positive for several months before ultimately becoming negative (unlike the antibody specific for EBV). This traditional Paul-Bunnell serologic agglutination method has been largely replaced for screening purposes by the spot test, in which finely ground guinea pig kidney or beef red blood cell stroma is added to serum on a slide, followed by a drop of horse cells. Results of the test are considered positive if agglutination occurs in the presence of guinea pig kidney (which absorbs out Forssman antibody but not heterophil antibody) but are negative with beef red blood cell stroma. The spot test requires only 2 minutes and is 96% to 99% accurate. Results of both the Paul-Bunnell test and the spot test are usually negative in preschool-aged children, in whom heterophil antibody production is limited. This age group does produce diagnostic levels of EBV-specific antibody.
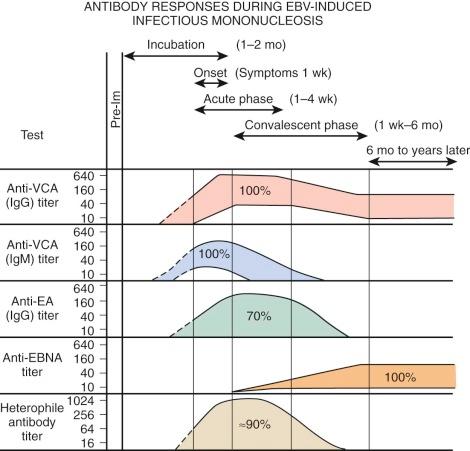
EBV is a herpeslike DNA virus. It is a relatively complex virus, and a variety of virus-associated antigens have been described. Antibodies to viral capsid antigen (VCA) and early antigen (EA) are detected early after the onset of EBV-associated infectious mononucleosis. Levels of antibodies to VCA reach their peak at about 3 weeks after the onset of clinical illness. Their levels decline somewhat thereafter, but they remain for life. Antibodies to EA usually last 2 to 4 months. EA has two components that are differentiated by their immunofluorescent staining: “D” for diffuse and “R” for restricted staining. A technique for determining EBV-specific IgM has been described. EBV-specific IgM almost always occurs in the acute phase of infectious mononucleosis. It rarely persists more than 2 to 3 months. During this period, virus shedding from the oropharynx is easily demonstrable.
Figure 37-11 shows the characteristic antibody patterns observed in young adults with EBV-induced infectious mononucleosis. Before infection, no antibodies are present. During the acute phase of illness, high titers of IgM and IgG antibodies to VCA are seen. IgM antibodies are transient and disappear after 1 to 2 months. Antibodies appearing against EA disappear after a few weeks to months. Antibodies against Epstein-Barr nuclear antigen are the last to appear and are seen 1 to 2 months after the illness. In young infants, VCA IgM is found in only 60% of patients and EA antibody is identified in only 50%. The only persistent antibody response should be VCA IgG and antibodies to Epstein-Barr nuclear antigen.
There is no evidence that bed rest or rest in general shortens the clinical course of infectious mononucleosis. Patients will determine their own level of activity. The most significant risk during acute illness is splenic rupture, but its incidence is extremely low.
Corticosteroid therapy is often used for infectious mononucleosis. Although these agents may produce improvement of symptoms, enhancement of general well-being, and reduction of fever, their use for these purposes should be restricted. Less controversial is the use of corticosteroid therapy for patients with airway obstruction secondary to tonsillar hypertrophy, severe hemolytic anemia, or thrombocytopenia and bleeding.
In the early 1980s, a newly described constellation of symptoms that resulted from immune compromise and led to death from opportunistic infections and unusual malignancies was recognized. These early descriptions of what is now known as acquired immunodeficiency syndrome (AIDS) heralded the beginning of what has become one of the major medical, public health, and social issues of our time. Identification of the human immunodeficiency virus (HIV), a retrovirus, as the etiologic agent that transmits the disease has led to major advances in understanding the pathogenesis of AIDS. At least two types of HIV, HIV-1 and HIV-2, exist; both are considered lentiviruses based on similarities in genetic composition, mechanisms of replication, and interaction with their hosts. A characteristic of lentivirus infections is that they cause slowly progressive disease with an incubation period of months to years before clinical symptoms appear. The worldwide spread of AIDS has led to a plethora of information about the biology of the virus and its epidemiology and incidence projections in different countries, as well as a concerted search for effective means of prevention and treatment.
The first occurrences of AIDS in the pediatric population in the United States took place in children born to mothers infected with HIV or in children, especially neonates, who had received blood products contaminated with HIV. The AIDS pandemic in Africa and, in particular, southern Africa has reached catastrophic proportions. The incidence of the disease in eastern Europe, India, and Thailand and throughout most of the Third World poses many political, social, and medical challenges, a discussion of which that is beyond the scope of this chapter.
Transmitted primarily through contact with infected lymphocytes and monocytes, HIV may be found in blood, semen, and vaginal secretions. Isolation of the virus from 13- and 15-week abortuses, placenta, and cord blood provides strong support for intrauterine infection. Maternal-fetal transfusion at delivery is another possible route of infection. Postpartum transmission via breastmilk, although possible, is rare. Accordingly, in countries in which safe feeding substitutes are not readily available, breastfeeding should not be curtailed; however, in developed countries where alternatives exist, breastfeeding by HIV-infected women is discouraged.
The rate of transmission from an untreated mother to child is estimated to be about 25% based on several studies from the United States and Europe. Transmission from mother to child can be significantly reduced by the use of antiretroviral therapy during pregnancy, labor, and the neonatal period.
Major advances in screening and testing of the blood supply in the United States after 1985 led to a reduction in the risk of receiving a contaminated single transfusion in 1988 to 1 in 250,000. The first screening test is a questionnaire to exclude people who engage in high-risk activities. The next step is an enzyme-linked immunosorbent assay (ELISA) for detection of antibodies to HIV antigens. In rare individuals who are infected but seronegative, HIV can escape detection, but sensitive screening tests detect the viral genome by PCR assay. Tragically, individuals with bleeding disorders such as hemophilia A and B who received clotting factors from a large donor pool for a single infusion had an increased likelihood of acquiring AIDS before modern blood screening practice and factor preparation.
Infection with HIV results in a spectrum of disease from an asymptomatic state to severe immunodeficiency involving multiple organ systems. The heterogeneity of symptoms is explained by the underlying pathophysiologic course in that (1) the incubation period may be weeks, months, or years; (2) the direct cytopathic effect on target cells, predominantly CD4+ lymphocytes, monocytes, and accessory cells, results in dysregulation of the immune system; and (3) the viral elimination phase that is accompanied by a host response to infection varies with the immune competence of the host. Because many children are infected congenitally, they show signs early in life and often have constitutional symptoms such as unexplained diarrhea, fever, night sweats, generalized lymphadenopathy, and hepatosplenomegaly. Failure to thrive and developmental delay are often prominent early manifestations of HIV infection in children. Susceptibility to recurrent infections by common bacterial and viral pathogens, as well as to opportunistic infections, increases the index of suspicion that the underlying disorder is AIDS. These and other manifestations of HIV infection are summarized in Box 37-1 .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here