Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Blood cells arise from the differentiating embryonic mesoderm. Human erythroid and macrophage progenitor cells have been observed in the yolk sac by days 16-19 and at day 19 in the aortic-gonad-mesonephros (AGM). After the development of the circulatory system on day 21, pluripotent hematopoietic cells localize in the AGM, placenta, and liver by days 24-28. Myeloid, lymphoid, and megakaryocytic precursors have been noted in fetal liver at this stage. Definitive erythropoiesis occurs in the fetal liver, thymus, spleen, and bone marrow. A knowledge gap exists regarding the details of in situ hematopoiesis between weeks 3 and 12, but fetal liver is recognized as the major site of hematopoiesis between weeks 6 and 16. The bone marrow assumes this role by week 24 ( Fig. 79.1 ).
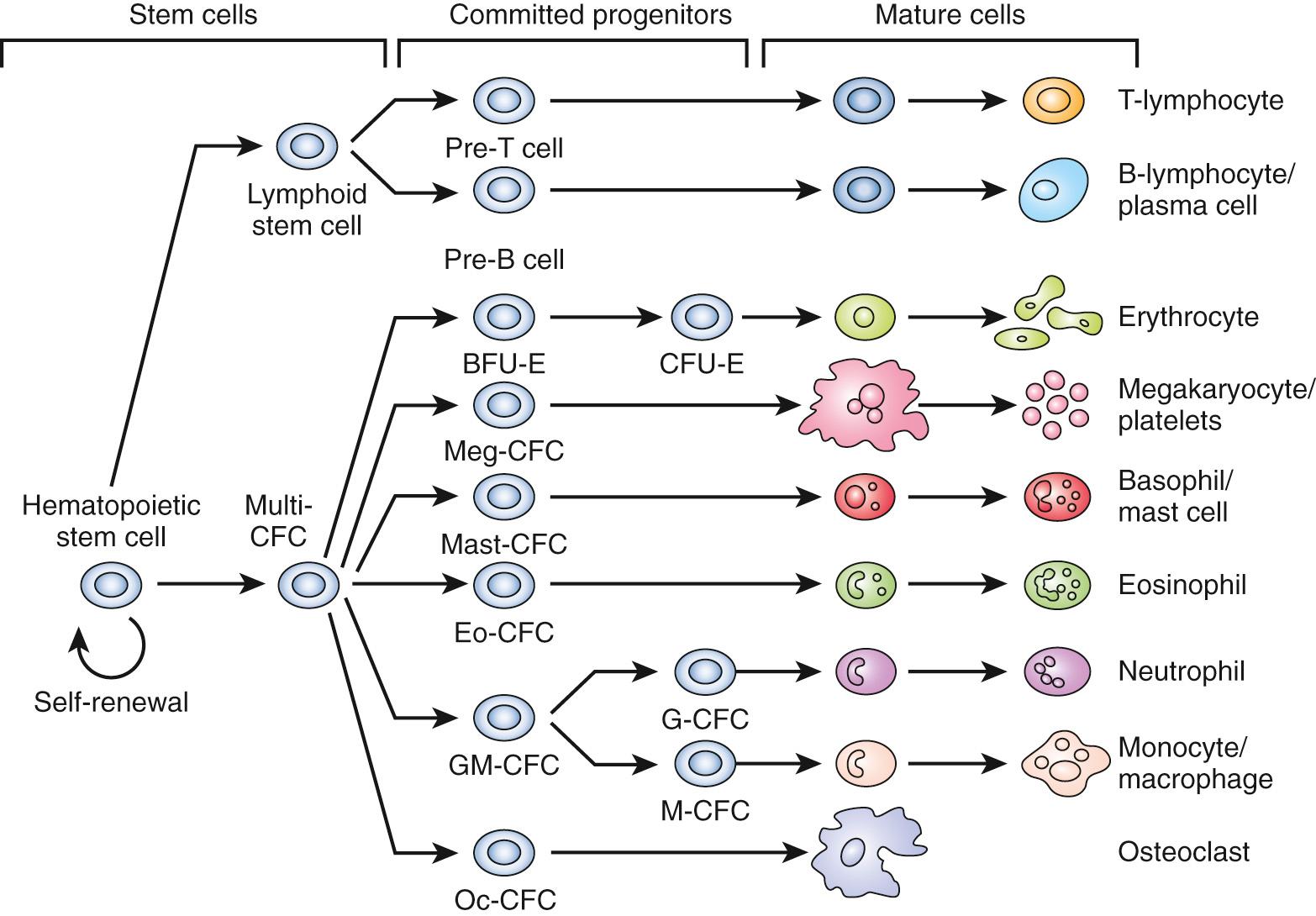
Proper blood cell formation is necessary for survival. Much has been learned about the origin and regulation of blood cells through studies of congenital and acquired defects in hematopoiesis. Studies of mouse hematogenesis identified pluripotent cells in the murine inner cell mass. These embryonic stem (ES) cells are capable of self-renewal as well as differentiation into hematopoietic cells. Human ES cells were isolated in 1998, fueling research efforts to generate pluripotent stem cells from early embryos and to perform genetic manipulation of differentiated somatic cells. Somatic cells can be reprogrammed into an ES-like state, and these induced pluripotent stem (iPS) cells are another tool researchers are using both to understand and manipulate progenitor cells.
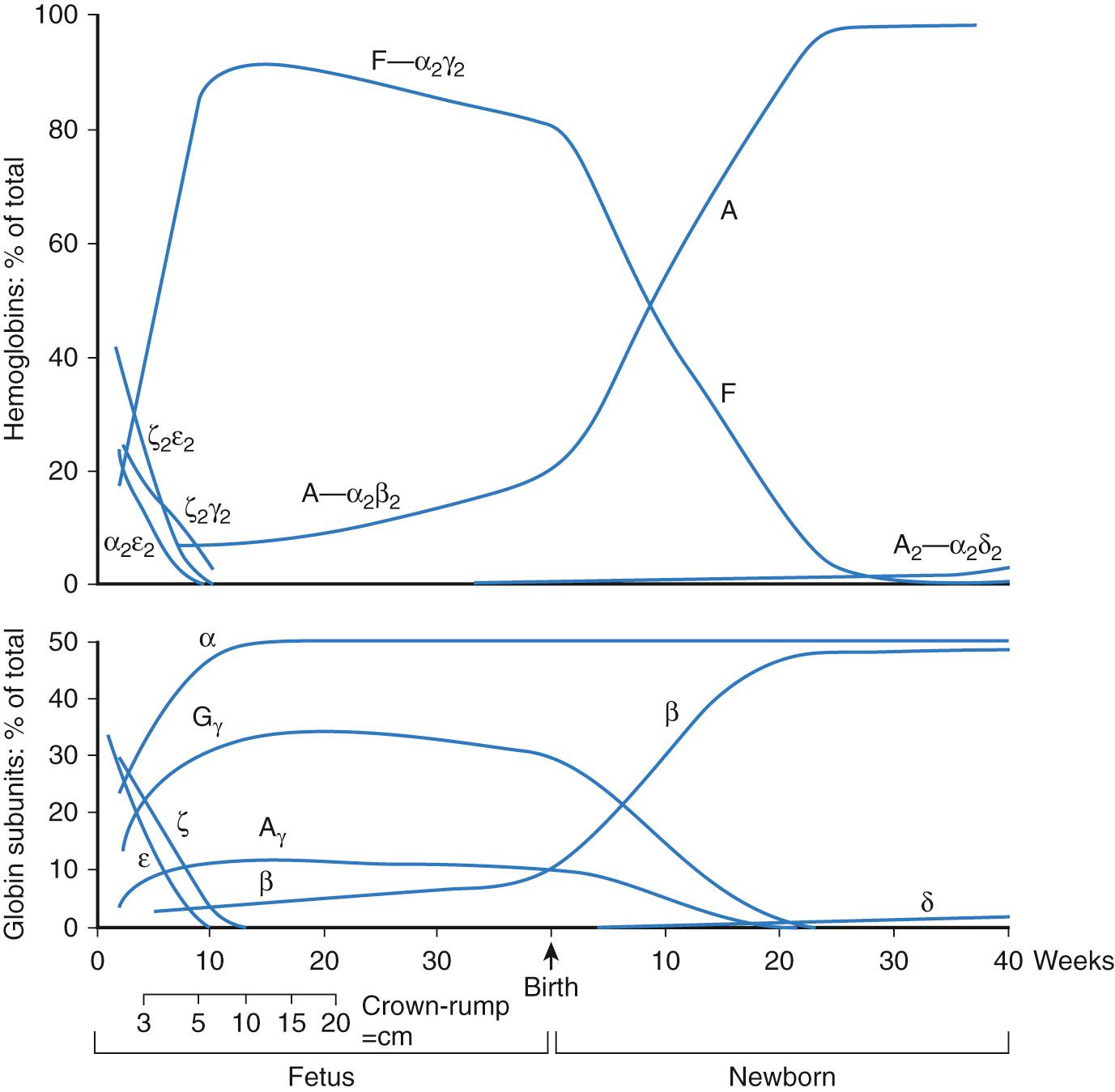
The earliest blood cells produced by colony-forming cells (CFCs) in the yolk sac are very large, primitive erythroid cells expressing embryonic globins. Subsequently, CFCs produce definitive erythrocytes expressing fetal globins and macrophages. Later still, multipotent CFCs and lymphoid progenitor cells arise. The adult-type globins are not expressed until just before birth and rapidly assume primacy afterward ( Table 79.1 ; Fig. 79.2 ). With each transition from primitive to definitive to adult erythroid cells, the mean corpuscular volume (MCV) decreases.
| Hemoglobin | Predominates | Globin Composition |
|---|---|---|
| Hb Gower 1 | Embryonic (yolk sac) | ζ 2 ε 2 |
| Hb Gower 2 | Embryonic (yolk sac) | α 2 ε 2 |
| Hb Portland | Embryonic (yolk sac) | ζ 2 γ 2 |
| Hb F | Fetal (liver) | α 2 γ 2 |
| Hb A | Adult (bone marrow) | α 2 β 2 |
| Hb A2 | Minor adult (bone marrow) | α 2 δ 2 |
| Hb Barts | Fetal-alpha thalassemia | γ 4 |
| Hb H | Adult-alpha thalassemia | β 4 |
Few hematopoietic stem cells enter the cell cycle at any given time. Most are in a resting state. Proliferation and differentiation occur within a suitable microenvironment of stroma and humoral factors. The WNT/β-catenin and Notch-δ signaling pathways drive stem cell development. Transcription factors involved in stem cell development include GATA2, RUNX1, TEL/ETV6, SCL/TAL1, and LM02. Other transcription factors such as PU.1, GFIX, C/EBPα, and GATA1 are considered to be more lineage-specific, but most also participate in lineage priming, where stem cells differentiate along a pathway depending upon cellular and environmental stimuli. Multiprotein complexes assemble and bind DNA regulatory elements to modulate transcription. Epigenetic regulatory mechanisms of transcription include DNA methylation on CpG residues and histone modification.
Factors promoting hematopoiesis include BMP4, VEGF, WNT, and FGF. Hematopoietic cytokines such as stem cell factor, fms-like tyrosine kinase receptor-3 ligand, interleukin-6, thrombopoietin, erythropoietin, and granulocyte colony-stimulating factor (G-CSF) play critical roles in the maintenance and differentiation of human hematopoietic cells. Some hematopoietic growth factors are produced in the vicinity of hematopoietic progenitors, and others are synthesized remotely ( Table 79.2 ). Few of the glycoprotein growth factors are available for clinical use, but that number is expected to increase.
| Factor | Source | Receptor | Target Cells | Effects |
|---|---|---|---|---|
| Erythropoietin (EPO) | Kidney, hepatocytes | EPO-R | E, Meg | Stimulates growth and differentiation of erythroid precursors |
| Stem cell factor (SCF) (also known as steel factor [SF], KIT ligand [KL], and mast cell growth factor [MCGF]) | Ubiquitous | KIT | E, mast cells, melanocytes, germ cells | Stimulates growth and differentiation of erythroid and myeloid precursors; enhances growth of mast cells |
| Granulocyte colony-stimulating factor (G-CSF) | Stromal cells, macrophages | G-CSF-R | N | Stimulates growth and differentiation of neutrophil precursors; activates phagocytic function of mature neutrophils |
| Granulocyte-macrophage colony-stimulating factor (GM-CSF) | Stromal cells | GM-CSF-R (α and β chains) | M, N, Eo, Endo | Stimulates growth and differentiation of neutrophils, eosinophils, and monocytes; activates endothelial cells; induces cytokine expression by monocytes |
| Macrophage colony-stimulating factor (M-CSF) | Mesenchymal cells | FMS | M | Stimulates growth and differentiation of monocytes; induces phagocytic function in monocytes and macrophages; is involved in bone remodeling |
| Interleukin 1 (IL-1) | Ubiquitous | IL-1RI, IL-1RII | T, E, B, M, S | Induces production of cytokines and prostaglandins by stromal cells, T cells, and many other cell types; induces fever |
| Interleukin 2 (IL-2) | T cells | P55, P75 | B, T, NK | Induces proliferation and activation of T, B, and NK cells; induces IL-1 expression by monocytes |
| Interleukin 3 (IL-3) | T cells | IL-3Rα, GM-CSF-Rβ | M, N, Eo, Meg | Stimulates growth and differentiation of myeloid and erythroid precursors, induces cytokines |
| Interleukin 4 (IL-4) | T cells, mast cells, basophils | IL-4R | M, Ba, B, T | Induces proliferation and activation of B and T cells |
| Interleukin 6 (IL-6) | Ubiquitous | IL-6R/GP130 | B, N | Induces activation of neutrophils; induces B-cell maturation, synergistic with IL-3 |
| Interleukin 7 (IL-7) | Stromal cells | IL-2R | B, T, meg | Stimulates T cells; induces monocytes |
| Interleukin 8 (IL-8) | Stromal cells, macrophages, T cells | IL-8R | T, N | Induces neutrophils and chemotaxis |
| Interleukin 10 (IL-10) | T cells, macrophages | IL-10R | Meg, E | Induces B and mast cells; inhibits T cells |
| Interleukin 11 (IL-11) | Stromal cells | IL-11R, GP130 | Meg | Stimulates megakaryocytes |
| Interleukin 12 (IL-12) | Neutrophils, monocytes | IL-12R | T, NK | Induces differentiation of cytotoxic T cells |
| Thrombopoietin (TPO) | Unknown | MPL | Meg | Stimulates megakaryocytes |
Some of the earliest hematopoietic growth factors discovered were referred to as colony-stimulating factors (CSFs), because in culture they stimulate progenitor cells to form colonies of recognizable maturing blood cells. The prefixes refer to the maturing cell produced. GM-CSF is granulocyte-macrophage CSF. The interleukins (ILs) were named for the fact that they are derived from, or act upon, leukocytes. Other factors are named for the cell surface receptor to which they bind, such as thrombopoietin receptor agonists that stimulate megakaryocyte differentiation and platelet production. Growth factors such as IL-3 and GM-CSF stimulate proliferation, differentiation, and survival of a broad range of precursors, including stem cells. Others such as erythropoietin and granulocyte CSF (G-CSF) are lineage restricted.
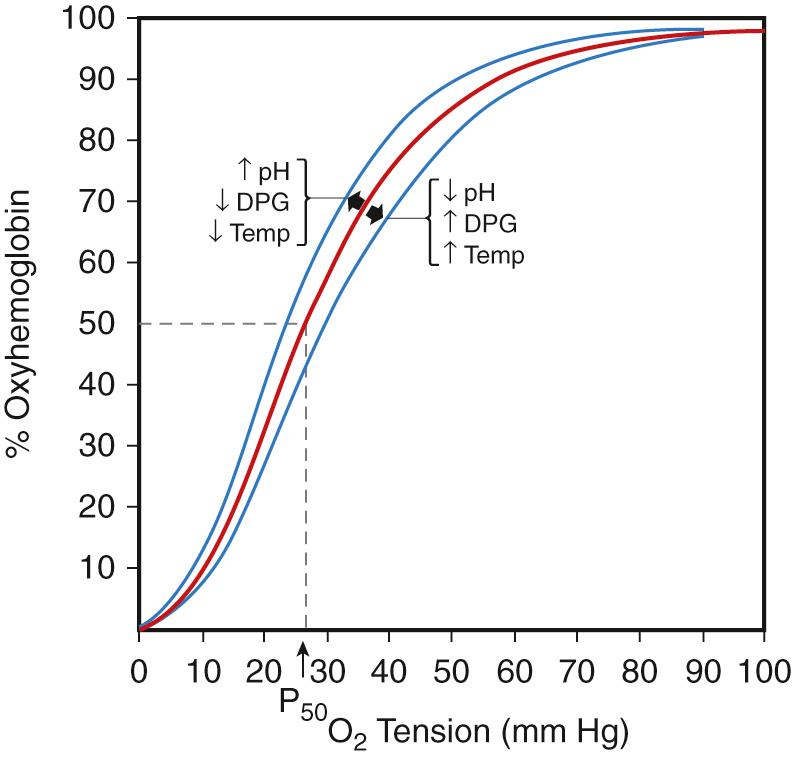
The function of red blood cells (RBCs) is to transport oxygen (O 2 ) to tissues to meet metabolic demands. Hemoglobin (Hb), the most abundant protein in erythrocytes, facilitates oxygen delivery by reversibly binding O 2 molecules. The binding of oxygen to hemoglobin tetramers is cooperative, resulting in the familiar sigmoidal oxygen dissociation curve ( Fig. 79.3 ). The affinity of Hb molecules for oxygen is influenced by a variety of factors, including temperature, pH, carbon dioxide pressure (P co 2 ), and the concentration of red blood cell organic phosphates (2,3-biphosphoglycerate or 2,3-BPG, also known as 2,3-diphosphoglycerate or 2,3-DPG). In the case of adult hemoglobin (Hb A), oxygen affinity for the molecule varies directly with pH and inversely with temperature and the concentration of 2,3-BPG. Fetal hemoglobin (Hb F) has a high oxygen affinity. Some mutations of hemoglobin affect oxygen affinity, as explained later.
The hemoglobin tetramer is composed of two heterodimers consisting of an α- and β-type globin. Different globin genes are sequentially expressed in RBC precursors, a process known as hemoglobin switching (see Fig. 79.2 and Table 79.1 ). During development, α- and β-type globin gene clusters are activated sequentially from the 5′ (embryonic) end to the 3′ (adult) end. The α-type globin genes, ζ-globin, α 1 -globin, and α 2 -globin, are located on chromosome 16 with the ζ gene 5′ to a pair of duplicated α-globin genes. The β-type genes on chromosome 11 are oriented 5′ to 3′ as ε-, G γ, A γ, δ, and β (see Fig. 79.2 ). The protein products of the A γ- and G γ-globin genes are functionally similar and differ by a single amino acid residue. Globin gene expression is controlled by cis-elements of individual globin gene promoters, proximal and distal enhancer regions, and positively acting transcription factors, such as GATA1, GATA2, NFE2, MYB, EKLF, RBTN2, and SCL. Other mechanisms that also regulate globin switching are silencers, DNA conformational changes, and DNA methylation. In fact, discovery of the ability to chemically demethylate CpG residues in the silenced γ-globin gene promoter launched translational research efforts to enhance fetal hemoglobin production in patients with β-globin defects such as β thalassemia and sickle cell anemia.
During yolk sac hematopoiesis, RBCs produce the embryonic hemoglobins (see Table 79.1 ). Hb F is the predominant hemoglobin in the fetus and neonate. Production of the major adult hemoglobin (Hb A) increases significantly between birth and 6 months of age, as Hb F production declines. Synthesis of HbA 2 , a minor adult globin, also increases gradually over the first months of life (see Fig. 79.2 ). After 6 months of age, Hb F usually constitutes less than 1% of the total hemoglobin and is unevenly distributed among red blood cells. Each of the different types of globin exhibits distinctive functional properties. Fetal erythrocytes, which contain mostly Hb F, have a higher oxygen affinity than adult red blood cells. This allows the transport of oxygen from maternal Hb A–containing erythrocytes across the placenta to fetal red blood cells. The increased O 2 affinity of fetal hemoglobin has been ascribed to the diminished interactions of Hb F with red blood cell 2,3-BPG. Embryonic erythrocytes also display a greater affinity for oxygen than adult cells.
Erythropoietin (EPO), the essential glycoprotein growth factor for erythropoiesis, binds to erythropoietin receptors on early erythroid progenitor cells and via the JAK2 signaling pathway regulates RBC production by protecting them from apoptosis. Erythropoietin is produced primarily in the fetal liver and later in the cortical peritubular cells of the kidney, so that in adults renal production of EPO is the most important. Erythropoiesis is highly responsive to blood oxygenation. Hypoxia inducible factors (HIFs), constitutively expressed EPO transcription factors, are destroyed in the presence of oxygen. Under hypoxic conditions, EPO production increases. Levels of EPO in cord blood are higher than in adult blood samples ( Table 79.3 ), but there is a dramatic decrease after birth in response to higher levels of tissue oxygenation. By 1 month of age, serum levels in healthy term infants reach their nadir. This is followed by a rise to maximal levels at 2 months of age and then a slow drift down to adult values.
| Postnatal Age (Days) | Serum EPO Level (mU/Ml) | Sample Size |
|---|---|---|
| 0-6 | 33.0 ± 31.4 | 11 |
| 7-50 | 11.7 ± 3.6 | 7 |
| 51-100 | 21.1 ± 5.5 | 13 |
| 101-150 | 15.1 ± 3.9 | 5 |
| 151-200 | 17.8 ± 6.3 | 6 |
| >200 | 23.1 ± 9.7 | 10 |
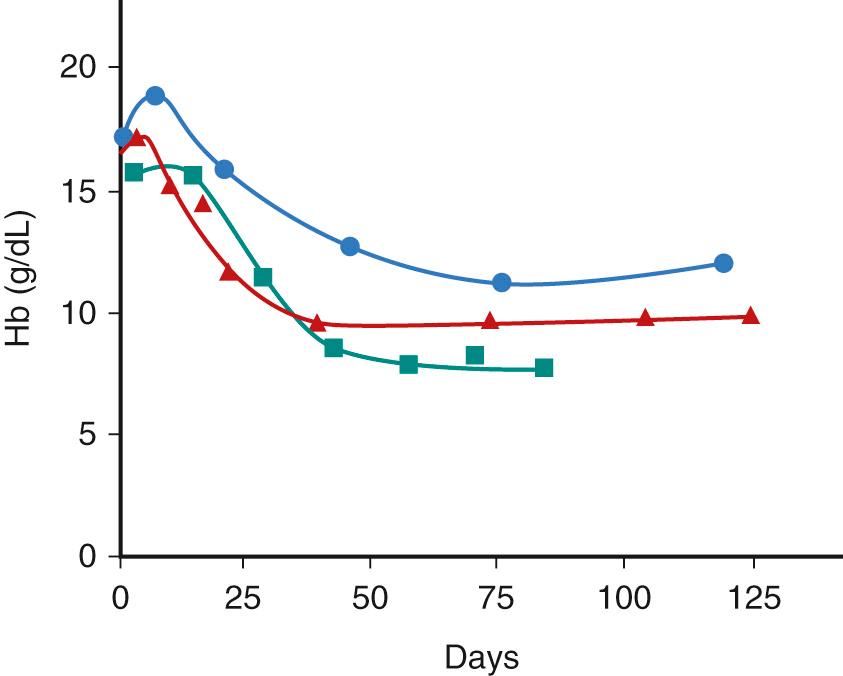
The postnatal changes in tissue oxygenation and erythropoietin production result in a physiologic anemia of infancy with a mean minimal hemoglobin concentration in healthy term infants of about 11 g/dL at 8-12 weeks of life ( Fig. 79.4 ; Table 79.4 ). Because of the shorter life span of RBCs in preterm infants with low EPO levels, the nadir is noted by 6 weeks of age and ranges from 7-10 g/dL. In very low birth weight (VLBW) and extremely low birth weight (ELBW) infants, the nadir is more than 20% below the value of the Hb at birth. In ELBW infants whose nadir falls below 7 g/dL, this so-called physiologic anemia of prematurity can be associated with pallor, tachypnea, tachycardia, poor feeding, and poor weight gain. Other causes of blood loss and suppression of erythropoiesis in the ill neonate can contribute to more severe and earlier anemia. Although preterm infants will respond to hypoxia with a rise in EPO levels, the increase is lower than that expected for term infants. The suboptimal EPO response may be because of developmental changes in transcription factors or to the site of fetal EPO production. The use of recombinant EPO in premature and sick newborn infants is discussed later.
| Age | Hb (g/dL) ± SD | RBC (×10 12 /L) ± SD | Hematocrit (%) ± SD | MCV (fl) ± SD | MCHC (g/dL) ± SD | Reticulocytes (%) ± SD |
|---|---|---|---|---|---|---|
| Days | ||||||
| 1 | 19.3 ± 2.2 | 5.14 ± 0.7 | 61 ± 7.4 | 119 ± 9.4 | 31.6 ± 1.9 | 3.2 ± 1.4 |
| 2 | 19.0 ± 1.9 | 5.15 ± 0.8 | 60 ± 6.4 | 115 ± 7.0 | 31.6 ± 1.4 | 3.2 ± 1.3 |
| 3 | 18.8 ± 2.0 | 5.11 ± 0.7 | 62 ± 9.3 | 116 ± 5.3 | 31.1 ± 2.8 | 2.8 ± 1.7 |
| 4 | 18.6 ± 2.1 | 5.00 ± 0.6 | 57 ± 8.1 | 114 ± 7.5 | 32.6 ± 1.5 | 1.8 ± 1.1 |
| 5 | 17.6 ± 1.1 | 4.97 ± 0.4 | 57 ± 7.3 | 114 ± 8.9 | 30.9 ± 2.2 | 1.2 ± 0.2 |
| 6 | 17.4 ± 2.2 | 5.00 ± 0.7 | 54 ± 7.2 | 113 ± 10.0 | 32.2 ± 1.6 | 0.6 ± 0.2 |
| 7 | 17.9 ± 2.5 | 4.86 ± 0.6 | 56 ± 9.4 | 118 ± 11.2 | 32.0 ± 1.6 | 0.5 ± 0.4 |
| Weeks | ||||||
| 1-2 | 17.3 ± 2.3 | 4.80 ± 0.8 | 54 ± 8.3 | 112 ± 19.0 | 32.1 ± 2.9 | 0.5 ± 0.3 |
| 2-3 | 15.6 ± 2.6 | 4.20 ± 0.6 | 46 ± 7.3 | 111 ± 8.2 | 33.9 ± 1.9 | 0.8 ± 0.6 |
| 3-4 | 14.2 ± 2.1 | 4.00 ± 0.6 | 43 ± 5.7 | 105 ± 7.5 | 33.5 ± 1.6 | 0.6 ± 0.3 |
| 4-5 | 12.7 ± 1.6 | 3.60 ± 0.4 | 36 ± 4.8 | 101 ± 8.1 | 34.9 ± 1.6 | 0.9 ± 0.8 |
| 5-6 | 11.9 ± 1.5 | 3.55 ± 0.4 | 36 ± 6.2 | 102 ± 10.2 | 34.1 ± 2.9 | 1.0 ± 0.7 |
| 6-7 | 12.0 ± 1.5 | 3.40 ± 0.4 | 36 ± 4.8 | 105 ± 12.0 | 33.8 ± 2.3 | 1.2 ± 0.7 |
| 7-8 | 11.1 ± 1.1 | 3.40 ± 0.4 | 33 ± 3.7 | 100 ± 13.0 | 33.7 ± 2.6 | 1.5 ± 0.7 |
| 8-9 | 10.7 ± 0.9 | 3.40 ± 0.5 | 31 ± 2.5 | 93 ± 12.0 | 34.1 ± 2.2 | 1.8 ± 1.0 |
| 9-10 | 11.2 ± 0.9 | 3.60 ± 0.3 | 32 ± 2.7 | 91 ± 9.3 | 34.3 ± 2.9 | 1.2 ± 0.6 |
| 10-11 | 11.4 ± 0.9 | 3.70 ± 0.4 | 34 ± 2.1 | 91 ± 7.7 | 33.2 ± 2.4 | 1.2 ± 0.7 |
| 11-12 | 11.3 ± 0.9 | 3.70 ± 0.3 | 33 ± 3.3 | 88 ± 7.9 | 34.8 ± 2.2 | 0.7 ± 0.3 |
The RBC count, Hb concentration, and hematocrit (Hct) increase throughout gestation, as shown in Table 79.5 . In term infants, the mean capillary hemoglobin at birth is 19.3 g/dL (see Table 79.4 ). The capillary Hct has a mean of 61 g/dL. Premature infants have lower Hb levels than do full-term infants. In addition to gestational age, Hb levels are influenced by a variety of factors that must be kept in mind when analyzing the neonate with anemia or polycythemia. One important determinant is the site of sampling: Capillary Hb values are higher than peripheral venous samples, and umbilical venous Hb results are the lowest. The interval between delivery and clamping of the umbilical cord and the height of the baby relative to the placenta can significantly affect a newborn's blood volume and total RBC mass. The placenta contains about 100 mL of blood. The mean blood volume of a full-term infant is about 85 mL/kg. Early or delayed clamping of the umbilical cord alters this mean blood volume by about 10% lower or higher, respectively. The average Hb at birth is relatively unchanged; however, 48 hours later, after redistribution of plasma volume, Hb values will reflect the lower or higher red cell mass. Racial differences also occur. One study reported significantly higher Hb, Hct, and MCV in white infants compared with black infants of similar gestational ages. Reticulocyte counts in the cord blood of infants average 4%-5%, and nucleated RBCs are evident in most cord blood samples (40,000/µL). These findings are presumed to reflect high EPO production secondary to low oxygen retention in utero. Infants who experience placental insufficiency and intrauterine growth restriction have higher than normal EPO production and an even greater degree of erythrocytosis. The mean MCV of RBCs in the newborn is increased. The RBCs of the neonate have an increased Hb content, but the mean corpuscular hemoglobin concentration (MCHC) is comparable to that of adults.
| Variables | Group 1 23-25 Wk ( N = 40) |
Group 2 26-28 Wk ( N = 60) |
Group 3 29-31 Wk ( N = 88) |
|---|---|---|---|
| Hematocrit (%) | 43.5 ± 4.2 † | 45.0 ± 4.5 ‡ | 48.0 ± 5.0 ‡ † |
| (36.0, 43.8, 51.0) | (37.5, 45.0, 54.3) | (39.4, 47.6, 56.0) | |
| Hemoglobin (g/dL) | 14.5 ±1.6 | 15.1 ± 1.6 ‡ | 16.2 ± 1.7 ‡ † |
| (12.0, 14.7, 17.4) | (12.5, 15.0, 18.3) | (13.2, 16.1, 18.8) | |
| Mean corpuscular hemoglobin (pg) | 38.6 ± 2.2 † | 38.3 ± 2.0 | 37.3 ± 2.5 † |
| (35.0, 38.6, 43.0) | (33.4, 38.4, 43.2) | (32.0, 37.5, 40.6) | |
| Mean corpuscular volume (fl) | 115.6 ± 5.6 † | 114.0 ± 7.6 ‡ | 110.4 ± 6.6 † ‡ |
| (107.0, 114.5, 125.7) | (98.4, 114.0, 126.6) | (97.3, 111.2, 120.0) | |
| Mean corpuscular hemoglobin concentration (g/dL) | 33.4 ± 0.9 (32.3, 33.3, 34.6) |
33.6 ± 0.6 (32.3, 33.6, 34.6) |
33.7 ± 0.7 (32.5, 33.6, 34.9) |
| Red cell distribution width | 15.9 ± 1.4 (14.2, 15.6, 18.5) |
16.5 ± 1.9 (14.5, 16.0, 21.0) |
16.4 ± 1.5 (14.6, 16.0, 19.4) |
* Values are reported as mean ± standard deviation and 5th, 50th, and 95th percentiles in parentheses.
Delayed (30-90 seconds) cord clamping (DCC) has been shown to prevent hypotension, raise hematocrit, and decrease the need for transfusions in preterm infants. In addition, term infants who have had delayed cord clamping have reduced iron deficiency anemia in the first year of life but have an increased risk of early jaundice. Thus, it has been recommended that both preterm and full-term infants undergo routine delayed cord clamping.
A new population that has also shown benefit is the neonate who has needed in utero transfusion because of red cell alloimmunization. Garabedian et al. studied a series of 72 neonates who needed in utero transfusion because of alloimmunization. Thirty-six of the neonates had DCC, and 36 neonates did not have DCC. More infants without DCC Hb had anemia at birth. The rate of transfusion, maximum level of bilirubin, the rate of intensive phototherapy, and the total duration of phototherapy were similar in the two groups. Postnatal exchange transfusions (ET) were more likely performed in the group without DCC than in the group with DCC. The interval between birth and the first transfusion was higher in the group with DCC. The authors recommended DCC with duration of 30 seconds in infants at risk for neonatal anemia because of red blood cell alloimmunization, provided that the management of jaundice is optimized.
The normal life span of adult RBCs is about 120 days. The life span of RBCs in newborns at term is 60-80 days and 30-50 days in ELBW infants. In general, red blood cell survival is affected by changes related to aging (senescence) and by random hemolysis of red blood cells, or portions of red blood cells, in the spleen and the rest of the reticuloendothelial system. Some of the changes in neonatal RBCs compared with adult RBCs listed in Table 79.6 affect survival. Aging erythrocytes with declining RBC enzyme activity become progressively less tolerant of oxidative challenges during the transportation of oxygen molecules and exposure to circulating oxidants. Any additional deficiencies in the enzymatic pathways of the RBC may affect the ability of the erythrocyte to tolerate oxidative challenges and further reduce red blood cell survival. With transit through the kidneys and lungs, the RBCs experience cycles of osmotic swelling and shrinkage. Shear forces in high-pressure areas of the circulation buffet the erythrocytes. Each passage through the cords of Billroth within the spleen requires the RBCs to deform and squeeze through tiny slits in the walls of the cords or face destruction if they cannot. Congenital or acquired defects in membrane stability or decreases in the ratio of surface area to red blood cell volume will also decrease erythrocyte survival. Alterations in the deformability of neonatal erythrocytes and relative intolerance to oxidative challenges result in shorter survival for neonatal red blood cells. Random hemolysis can be increased with splenic enlargement or activation of the phagocytic system. Infants with hemolysis may have exaggerated anemia because of decreased erythropoiesis, enhanced splenic filtration, and activation of phagocytes.
| MCV (mean corpuscular volume) | ↑ |
| RBC count | ↑ |
| MCHC (mean corpuscular hemoglobin concentration) | ↑ |
| Surface area | ↑ |
| Reticulocyte count | ↑ |
| Resting cell diameter | ↑ |
| Hemoglobin F content | ↑ |
| Whole cell deformability | ↔ |
| Suction pressure for complete aspiration | ↑ |
| Sensitivity to osmotic lysis | ↑ |
| ATP utilization | ↑ |
| Glucose utilization | ↑ |
| Catalase glutathione peroxidase | ↓ |
| Susceptibility to oxidant injury | ↑ |
| Phospholipid, lipid, cholesterol content | ↑ |
| Loss of volume, surface area, and deformability with age | ↑ |
| Permeability to sodium and potassium | ↑ |
| I antigen expression on cell surface | ↑ |
| A, B, H blood group antigens on cell surface | ↓ |
| Life span | ↓ |
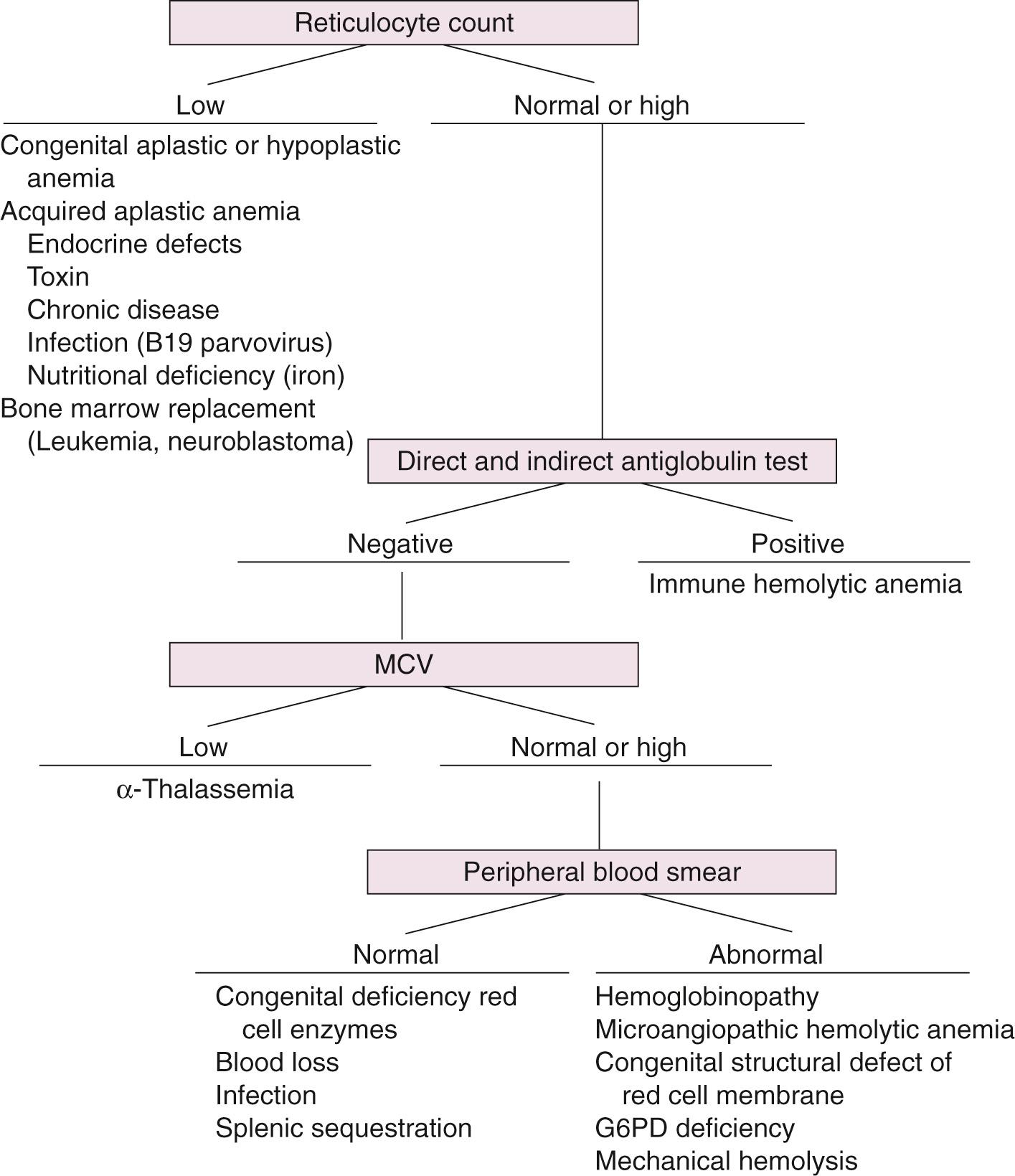
Anemia is defined by a hemoglobin or hematocrit value that is more than two standard deviations below the mean for age. In the neonate, the causes of anemia can be divided into two broad categories: anemia resulting from accelerated loss or destruction of red blood cells and anemia caused by a defect at some stage of red blood cell production ( Box 79.1 ). The defects may be congenital or acquired, and the abnormality may be intrinsic to the RBCs or extrinsic. Anemias also may be categorized on a morphologic basis. Using the normal range of the MCV for age and gestation, the anemia may be characterized as microcytic, normocytic, or macrocytic ( Box 79.2 ). Hypochromicity, abnormal RBC shapes (poikilocytes), polychromasia, and cell inclusions (e.g., basophilic stippling or Howell-Jolly bodies) also provide clues to the etiology of the anemia ( Table 79.7 ).
Hemorrhage
Fetal
Fetal–maternal
Placental
Traumatic delivery
Coagulation defects
Early umbilical cord clamping
Twin–twin transfusion
Excess phlebotomy losses
Hemolytic anemia
Immune
Alloimmune: Rh, ABO, minor blood group
Autoimmune
Nonimmune
Hemoglobinopathy
Thalassemia
Unstable hemoglobin
Red blood cell enzyme defect
Structural defect of red blood cell membrane
Mechanical destruction
Microangiopathic hemolytic anemia
Infection
Vitamin E deficiency
Congenital
Diamond-Blackfan anemia
Pearson syndrome
Fanconi anemia
Congenital dyserythropoietic anemias
Anemia of prematurity
Acquired
Parvovirus B19
Transient erythroblastopenia of childhood
Human immunodeficiency virus
Syphilis
Iron deficiency
Lead toxicity
Infection
Reticulocytosis
Folic acid deficiency
B 12 deficiency
Bone marrow failure syndromes
Diamond-Blackfan anemia
Pearson syndrome
Fanconi anemia
Down syndrome
Myelodysplastic syndrome
Liver disease
Drugs (phenytoin, mercaptopurine)
Hypothyroidism
Iron deficiency
Thalassemia
Chronic infection
Hb E trait
Sideroblastic anemia
Copper deficiency
Defects of iron metabolism
Lead poisoning
Low reticulocyte count
Acute blood loss
Infection
Parvovirus B19
Transient erythroblastopenia of childhood
Chronic disease
Drugs
Leukemia
Bone marrow infiltration
Liver disease
Renal failure
Aplastic anemia, acquired
Normal or high reticulocyte count
Blood loss
Sequestration
Red blood cell enzyme defects
Immune hemolytic anemia
Mechanical hemolytic anemia
Red cell membrane defects
Unstable hemoglobin
Hemoglobinopathy
| Morphologic Abnormality | Etiology |
|---|---|
| Acanthocytes | Alteration of lipid bilayers |
| Liver disease | |
| Abetalipoproteinemia | |
| Blister cells | G6PD deficiency |
| Basophilic stippling | Ineffective erythropoiesis: iron deficiency, lead poisoning, thalassemia, nonimmune hemolytic anemias |
| Elliptocytes | Structural defects of red cell membrane: hereditary elliptocytosis |
| Heinz bodies | Precipitated hemoglobin: normal in newborn; nonimmune hemolytic anemias |
| Howell-Jolly bodies | Splenic hypofunction or post splenectomy |
| Hypochromia | Iron deficiency, thalassemias, lead poisoning |
| Nucleated red blood cells | Normal in newborn; hemolytic anemias, semi-acute blood loss |
| Polychromasia | Normal in newborn; reticulocytosis |
| Pyropoikilocytosis | Neonates with hereditary elliptocytosis, hereditary pyropoikilocytosis, thermal injury of red cells (burn) |
| Rouleaux | Increased fibrinogen, inflammation |
| Schistocytes | Microangiopathic hemolytic anemias |
| Sickle cells | Hemoglobin SS and sickle variants |
| Spherocytes | Decreased cell membrane: volume—IgG+ hemolytic anemia, hereditary spherocytosis, artifact of area of blood smear |
| Target cells | Increased red blood cell surface: volume ratio |
| Alteration in lipid structure of red blood cell membrane | |
| Hemoglobin C, hemoglobin S, thalassemias, liver disease, abetalipoproteinemia |
The clinician begins the evaluation of anemia by taking a thorough history. Appropriate data vary with the patient's age but often include the medical and dietary history of the pregnancy, the estimated gestational age at birth, the chronologic age, the infant's diet, and details of any previous anemia, blood loss, transfusions, medications, and illnesses, as well as the family history of anemia. The physical examination should evaluate the infant's general health, growth, and development. Identification of any dysmorphic features, abnormal masses, or skin lesions can aid the diagnosis ( Table 79.8 ). The patient also should be assessed for jaundice, hepatosplenomegaly, vascular malformations, cardiovascular function, and lymphadenopathy.
| Physical Finding | Etiology |
|---|---|
| “Blueberry muffin” spots | Extramedullary hematopoiesis, replacement of bone marrow by tumor, congenital infection |
| Cardiac disease/mechanical heart valve | “Waring blender” syndrome |
| Congestive heart failure | Chronic anemia |
| Dysmorphic features | Bone marrow failure syndromes: Diamond-Blackfan anemia, Fanconi anemia, Shwachman-Diamond syndrome, Pearson syndrome |
| Down syndrome | |
| Myelodysplastic syndrome | |
| Failure to thrive | Pearson syndrome |
| Shwachman-Diamond syndrome | |
| Hepatosplenomegaly | Congenital infection, storage disorder, malignancy, hypersplenism, hemangioma, hemolytic anemia, transient abnormal myelopoiesis |
| Jaundice | Hemolytic anemia |
| Kaposiform hemangioendothelioma | Kasabach-Merritt syndrome |
| Microcephaly | Congenital infection |
| Bone marrow failure syndrome | |
| Short stature | Congenital bone marrow failure syndrome |
The initial laboratory evaluation includes a complete blood count (CBC) with RBC indices, a reticulocyte count, and evaluation of the peripheral blood smear ( Fig. 79.5 ). The results of the preliminary laboratory testing, combined with information from the history and physical examination, should dictate the need for further tests, such as hemoglobin analysis of the infant or parents, CBCs and blood smears of the parents, analysis of hepatic or renal function, direct or indirect antiglobulin (Coombs) testing, cultures or titers to identify infectious agents, a bone marrow aspirate or biopsy, osmotic fragility tests, and quantitative or qualitative testing for glucose-6-phosphate dehydrogenase (G6PD) deficiency.
The neonatal patient presents the diagnostician with a number of unique challenges. Because of the small total blood volume of the infant, who already is anemic, testing must be limited. Major pediatric medical centers can perform many of the necessary tests on very small quantities of blood, especially if the tests are appropriately batched when they are submitted. Because of the many morphologic and biochemical differences in neonatal and adult RBCs (see Table 79.6 ), some diagnoses are best made by testing the parents for evidence of disease or carrier states. At times, a definitive diagnosis can be made only with repeat testing later in infancy, when the infant would be expected to have a much higher percentage of adult-type RBCs or when the infant has recovered from an acute hemolytic crisis that may have destroyed the older, more biochemically or morphologically abnormal cells.
See Chapter 80 for discussion of liberal versus restrictive transfusion practices in preterm infants. Because the primary function of the RBC is to transport oxygen from the pulmonary bed to other tissues for release, anemia diminishes oxygen-carrying capacity and can compromise tissue oxygenation. Tissue oxygenation is a complex concept involving not only the Hb concentration but also the oxygen affinity of the Hb in the patient's red blood cells, blood viscosity, and the patient's cardiorespiratory status. The only absolute indications for rapidly correcting anemia by RBC transfusion are to restore tissue oxygenation and to expand blood volume after severe, acute loss. In most pediatric centers, the sicker patients, especially those with cardiopulmonary dysfunction, receive transfusions to maintain the Hb closer to normal for age. Neonatal exchange transfusions are also performed with the goal of replacing “doomed” infant RBCs with healthy adult RBCs, which have superior oxygen-transporting ability. This has the triple benefit of limiting hyperbilirubinemia and other byproducts of RBC breakdown, reducing the body load of maternal antibodies, and supplementing with cells that contain Hb A.
Blood loss can occur in the fetus, at birth, or in the postnatal period. The bleeding can be acute or chronic. Anemia caused by chronic blood loss generally is better tolerated because the neonate will at least partly compensate for a gradual reduction in RBC mass. Chronic blood loss can be diagnosed by identifying signs of compensation. Doppler assessment of the fetal middle cerebral artery peak systolic velocity is a noninvasive method for determination of fetal anemia, independent of the etiology. The infant may be pale and may exhibit signs and symptoms of congestive heart failure. Anemia will be present, often with reticulocytosis, hypochromia, and microcytosis.
An infant with acute blood loss may not be anemic if blood sampling is done soon enough after the acute event so that hemodilution has not yet occurred. Anemia usually develops within 3-4 hours after blood loss; repeat testing 6-12 hours after the event should reveal the true extent of the loss. In acute blood loss, the infant may exhibit signs and symptoms of hypovolemia and hypoxemia (e.g., tachycardia, tachypnea, hypotension). The RBCs should be morphologically normal. With either kind of hemorrhage, infants tend to have fewer problems with hyperbilirubinemia, because they have a reduced RBC mass. Jaundice can result if entrapped RBCs in a hematoma break down.
Internal bleeding can occur if the fetus has anatomic abnormalities or defects in the hemostatic system or with interventional obstetric procedures. A surprisingly large amount of blood can be lost within a cephalohematoma, and even greater bleeding can occur in the subaponeurotic area of the scalp (subgaleal hemorrhage), where bleeding is not limited by periosteal attachments. Traumatic or assisted deliveries and vitamin K deficiency are commonly associated with such bleeding. Full-term infants may have intracranial bleeding, which usually occurs in the subarachnoid or subdural regions. Full-term infants with intracranial hemorrhage should be evaluated for hemostasis abnormalities, because this type of bleeding is associated with qualitative and quantitative platelet defects and with abnormalities of several of the coagulation proteins. Hemorrhage into the adrenals, kidneys, liver, spleen, or retroperitoneum also can occur after difficult or breech deliveries. Splenic or hepatic rupture can occur after trauma, especially if the organs are enlarged as a result of extramedullary hematopoiesis. Occult or superficial vascular tumors can bleed and sequester large volumes of red blood cells and platelets.
Maternal factors can cause prenatal blood loss. Maternal history of vaginal bleeding, placenta previa, abruptio placentae, nonelective cesarean delivery, and cord compression are associated with anemia. Hemorrhage from the umbilical cord may be the result of intrinsic vascular abnormalities, inflammation of the cord, velamentous insertion of the cord, coagulation defects, or an unusually short cord. A normal cord can rupture during a precipitous or assisted delivery or if it becomes tangled around the infant. Accidental incision of the placenta during delivery also can result in bleeding.
Fetal cells may be found in the maternal circulation in about half of all pregnancies. At 20 weeks’ gestation, the fetoplacental volume is 30 mL, and it is rare for transplacental hemorrhage to exceed 1 mL of fetal red blood cells. By term, fetal–maternal hemorrhage (FMH) during delivery can exceed 30 mL of fetal blood, although in only 0.3% of pregnancies. Fetal–maternal hemorrhage is associated with procedures such as external cephalic version or traumatic amniocentesis. The diagnosis is made by demonstrating fetal RBCs, which contain mostly Hb F, in a maternal blood sample; therefore, the analysis must be done before the fetal cells are cleared from the maternal circulation. The test usually is performed within the first few hours after delivery. In cases of ABO blood group incompatibility, the red blood cells may be cleared more rapidly, and the test may be falsely negative.
The prevention of maternal Rh D alloimmunization requires accurate detection and quantification of fetal blood cells in the maternal circulation. If a D-negative mother has evidence of FMH, Rh immune globulin (Rh IgG) can reduce D-sensitization and the resultant hemolytic disease of the newborn. Vaginal or peripheral blood samples from women with vaginal bleeding late in pregnancy may also be evaluated for the presence of fetal blood cells. The rosette screen is a sensitive, FDA-approved screening test for FMH when the mother is D-negative. Maternal red blood cells are incubated with anti-D and then washed. Indicator D-cells are added, and in the presence of fetal D-positive cells, aggregates or rosettes will be seen by light microscopy. Confirmation of a positive screening test is performed with a quantitative method. The acid elution technique, or Kleihauer-Betke test, is the most widely available quantitative test for FMH. This method exploits the stability of Hb F–containing RBCs in acid solution relative to cells containing Hb A. False-positive test results are seen in women with any condition (including many of the hemoglobinopathies) that elevates their own Hb F level. The Kleihauer-Betke test suffers from problems with standardization and is labor intensive. Some centers now offer flow cytometry–based quantification of FMH by detecting either Hb F or Rh D or a combination. Flow cytometry can distinguish adult F cells from fetal RBCs.
In monochorionic diamniotic multiple gestations, twin–twin transfusion syndrome (TTTS) (see Chapter 21 ) can be diagnosed by ultrasound-demonstrating oligohydramnios in the donor sac and polyhydramnios in the recipient sac. Twin–twin transfusion syndrome occurs in 8%-10% of twin pregnancies with monochorionic diamniotic placentation and accounts for about half of the perinatal deaths. Twin anemia polycythemia sequence (TAPS) is defined as the presence of anemia in the donor and polycythemia in the recipient twin. Prenatal middle cerebral artery peak systolic velocity criteria have been established for diagnosis in the absence of oligohydramnios–polyhydramnios. Blood can be exchanged unequally between the fetuses through placental vascular interfetal connections. Transfusion can be problematic for the hypovolemic donor who develops oligohydramnios, but the recipient often experiences greater difficulties with hyperbilirubinemia, hypervolemia, and hyperviscosity that arise from the increased RBC mass. Cardiac, neurologic, and developmental disorders have been associated with TTTS.
Accelerated destruction of RBCs is the end point of a number of intrinsic, extrinsic, congenital, and acquired RBC abnormalities. Because the RBCs of premature infants and newborns have a shorter life span, hemolysis is defined as a process that shortens the survival of the RBCs relative to the expected life span for the infant's gestational and postnatal age. In contrast to anemia caused by blood loss, most infants with hemolysis have some evidence of indirect hyperbilirubinemia and elevated lactate dehydrogenase for age. Reticulocytosis should accompany the hemolysis, although in conditions complicated by bone marrow suppression (congenital infections, chronic illness, or nutritional deficiency) or decreased erythropoietin production, the reticulocyte count may be inappropriately low for the degree of anemia present. With maximal response, the bone marrow can compensate for RBC survival of 20-30 days without anemia. The bone marrow may show hyperplasia and a reversal of the usual myeloid-to-erythroid ratio of 3 : 1. Because optimal conditions for bone marrow response are not present in the newborn, hemolysis with anemia and hyperbilirubinemia may be evident in the neonate. In chronic hemolytic states, compensatory hypertrophy of the bone marrow may result in bony changes and other evidence of extramedullary hematopoiesis. Intrinsic hemolytic anemias are caused by inherited abnormalities of the RBC membrane, hemoglobin, or RBC intracellular enzymes. Some intrinsic hemolytic anemias also result in extrinsic damage to the RBC membrane. Extrinsic factors cause hemolytic anemia by damaging the RBC chemically, physically, or immunologically. Extrinsic hemolysis can be divided into immune and nonimmune etiologies (see Box 79.1 ); the most common causes of immune hemolytic anemia are discussed first.
Alloimmune hemolytic disease of the newborn (HDN) (see Chapter 23 ) is caused by the destruction of fetal or neonatal RBCs by maternal immunoglobulin G (IgG) antibodies. Alloimmune HDN involves the major blood group antigens of the Rhesus (Rh) and the A, B, AB, and O systems, but minor blood group incompatibilities (Kell, Duffy, MNS, and P systems) can also be associated with clinically significant disease. Particularly for Rh (D), anti-c, anti-E, and anti-K (Kell) antibodies, intrauterine or direct fetal transfusion may be indicated prenatally, and exchange transfusion may be necessary postnatally. Maternal IgG antibodies can cross the placenta, enter the fetal circulation and cause hemolysis, anemia, hyperbilirubinemia, and hydrops fetalis. In utero, the process is called erythroblastosis fetalis , whereas postnatally it is called hemolytic disease of the newborn (HDN). Maternal sensitization occurs through a prior transfusion of incompatible RBCs after fetal–maternal hemorrhage of incompatible fetal RBCs or from production of a pre-existing antibody developed against antigens from bacteria, viruses, or food. Transfer of antibodies across the placenta depends on the F c component of the IgG molecule. Because both IgM and IgA antibodies lack this component, only IgG antibodies cause hemolytic disease of the newborn. Immunoglobulin G 1 antibodies cross the placenta earlier and are responsible for more prenatal hemolysis. Immunoglobulin G 3 antibodies cross the placenta later in gestation and are responsible for more severe hemolysis postnatally.
The original description of HDN was caused by Rh D incompatibility, and it remains the most severe cause. The spectrum of clinical problems caused by Rh HDN ranges from mild, self-limited hemolytic anemia to hydrops fetalis. With the widespread use of antenatal and postpartum Rh immunoglobulin administration, Rh D sensitization is less common in pregnancies when prenatal care is received. Most cases today are caused by failure to administer Rh immunoglobulin when indicated, lack of identification of a large FMH, and chronic transplacental hemorrhage.
The Rh antigens are inherited as a linked group of two genes, RHD and RHCE , located on chromosome 1. Persons are typed as Rh negative or positive based on the expression of the major D antigen on the RBC, and their RBCs will also express C or c and E or e antigens. Rarely, Rh deletions of D, C/c, and E/e loci can occur. Anti-c and anti-E are emerging as the most frequent causes of HDN as the incidence of Rh(D) disease decreases. The incidence of Rh disease depends on the prevalence of Rh-negative antigens in the population studied ( Table 79.9 ). Even in white populations, only a small percentage of pregnancies are affected because not all women who are Rh negative develop antibodies, Rh immunization of the mother does not usually occur during the first pregnancy, and some of the second infants will be Rh negative.
| Population Group | Percent Affected |
|---|---|
| European whites | 11-21 |
| US whites | 14.4 |
| Indians (India) | 8 |
| US African Americans | 5.5 |
| Native Americans | 0 |
| Chinese | 0 |
| Japanese | 0 |
Because the interaction of the anti-D IgG with a D-positive RBC does not usually involve complement, hemolysis is extravascular. Hyperbilirubinemia and jaundice can occur in the first day of life when the placenta is no longer available to clear bilirubin from hemolyzed RBCs. The peripheral blood smear may show anemia, reticulocytosis, and macrocytosis. Microspherocytes are usually not seen in Rh disease. A direct antiglobulin (Coombs) test should demonstrate anti-D IgG. Partial or total exchange transfusions may be necessary to reduce the load of antibody and remove the antibody-coated cells. Management of hyperbilirubinemia is discussed in Chapter 91 .
Although the incidence of blood group O mothers delivering babies of blood group A or B is about 15%, ABO hemolytic disease is estimated to occur in only 3% of pregnancies and requires treatment with exchange transfusion in less than 0.1% of pregnancies. In contrast to Rh hemolytic disease, ABO hemolytic disease tends to be less severe, and the severity does not depend on birth order. Most anti-A and anti-B antibodies are IgM molecules, which do not cross the placenta. However, maternal anti-A or anti-B IgG antibodies, which may have been raised against A or B substances occurring in food or on bacteria or in response to fetal–maternal hemorrhage, can cross the placenta and react with the sparsely distributed A or B antigens on the neonatal RBCs. Hemolysis is primarily extravascular. Infants may develop anemia, reticulocytosis, and hyperbilirubinemia within the first 24 hours of life. The hallmark of ABO hemolytic disease (in contrast to Rh disease) is the presence of microspherocytes on the peripheral blood smear. The direct antiglobulin test (DAT), or Coombs test, should be at least weakly positive for anti-A or anti-B; however, because of the sparse distribution of antigenic sites on a newborn's RBCs, ABO hemolytic disease may be present even without a positive result on the DAT. The maternal serum should have high titers of IgG directed against A or B (positive indirect antiglobulin test or IAT, which is the indirect Coombs test). In the absence of clinical hemolytic disease, laboratory evidence of erythrocyte sensitization should not be considered HDN.
The incidence of HDN for minor blood group antigens is related to the antigenicity of the particular blood group antigen and the expression of that antigen on fetal RBCs. Maternal antibodies against minor blood group antigens develop after exposure from a transfusion or prior pregnancy or from contact with bacteria or viruses that express the antigen. Hemolytic disease of the newborn has been associated with the minor group antigens Kell, Duffy, MNS system, and P system. Most clinically significant HDN not attributed to ABO or Rh incompatibility is caused by anti-Kell, anti-E, and anti-c. Kell HDN may require intrauterine intervention. The severity of anti-Kell HDN is owing both to hemolysis and suppression of erythropoiesis in progenitor cells. Screening for minor group antibodies is recommended for all women in the 34th week of pregnancy: Routine screening with cells containing only high-frequency antigens would not detect antibodies to low-frequency antigens. The diagnosis and treatment of hemolytic disease are identical to those for Rh hemolytic disease. Identification of low-frequency antibodies associated with HDN is especially important for mothers who plan additional pregnancies. The role of IVIG in treatment of neonatal hemolytic disease, especially ABO incompatibility, is discussed in Chapter 91 .
Hydrops fetalis is the most severe consequence of hemolysis, but anemia generally is less problematic than hyperbilirubinemia in the acute phase of illness. Hyperbilirubinemia may be evident within the first 24 hours of life. Anemia can occur in the first few weeks in both those who received exchange transfusions and those who required only phototherapy for management of hyperbilirubinemia. The anemia can be caused by ongoing hemolysis, hypersplenism, inadequate replacement by transfused red blood cells, and shortened survival of transfused red blood cells. Because the half-life of IgG molecules is about 28 days, hemolysis should resolve within the first 3 or 4 months. Resolution often occurs sooner if the antibodies are cleared by adhering to RBCs or by exchange transfusion. Thrombocytopenia and bleeding can complicate hemolytic disease. Usually, thrombocytopenia without other laboratory evidence of disseminated intravascular coagulation (DIC) is noted, but DIC can be triggered by massive hemolysis or shock and acidosis.
The other immune hemolytic diseases of early childhood are relatively rare. Autoimmune hemolytic anemia occurs, as do anemias associated with infections, drugs, and immunodeficiency syndromes. IgG antibodies, with or without complement, are often directed against one of the Rh erythrocyte antigens. These antibodies are most active at 37°C and often are called warm autoantibodies . The IgG-coated cells, with or without the assistance of complement, are cleared by the spleen and, to a lesser extent, by the liver. Congenital infections (syphilis, cytomegalovirus, rubella, toxoplasmosis, herpes, HIV, hepatitis) and acquired infections can cause hemolytic anemia and bone marrow suppression with reticulocytopenia (see Chapter 48, Chapter 49, Chapter 50 ). Immunoglobulin M autoantibodies can cause disease and are usually referred to as cold agglutinins , because they are most active at between 0°C and 30°C. These antibodies, with complement, coat RBCs and are usually cleared in the liver. Intravascular hemolysis is less common. The best-known causes of cold hemagglutinin disease are Mycoplasma pneumoniae , adenovirus, and Epstein-Barr virus. Immunoglobulin A–mediated hemolysis is quite rare but is remarkable for its severity ( Table 79.10 ).
| Mechanism | Infectious Agent |
|---|---|
| Hemolysin production | Clostridium perfringens |
| Direct invasion of RBCs | Malaria |
| Alteration of RBC surface: | |
| Adherence to RBCs | Bartonella organisms |
| Neuramidase alters antigenic phenotype | Influenza virus |
| Cold agglutinin | Epstein-Barr virus |
| Absorption of capsular polysaccharide | Haemophilus influenza |
| Microangiopathy | Any agent causing disseminated intravascular coagulation or hemolytic-uremic syndrome |
| Enhanced oxidative stress | Campylobacter jejuni enteritis in neonates with a diminished cytochrome-b 5 reductase system |
The natural history of autoimmune hemolytic disease in infancy is that of a rapid onset of anemia with hyperbilirubinemia, splenomegaly or hepatomegaly, and hemoglobinuria (with intravascular hemolysis). Initially, reticulocytopenia may be noted, especially if antibodies are directed against RBC progenitors in the bone marrow, but a brisk reticulocytosis usually follows. Resolution within 3-6 months is common. Anemia with reticulocytosis and spherocytosis may be seen on the peripheral blood smear, but the diagnosis depends on demonstration of antibody-coated cells, with or without complement, in the DAT and antibodies in the serum by the IAT. Therapy includes treatment of underlying infection, removal of the offending drug, and supportive measures to limit hyperbilirubinemia. Response to corticosteroids is common, as is complete recovery. Steroids are less effective in IgM-mediated disease. A subset of patients younger than 2 years, and with a slower onset of disease at presentation, develops chronic hemolytic anemia. Difficulties with identifying compatible blood during a hemolytic crisis, as well as the usually self-limited nature of the disease, restrict blood transfusions to cases in which severe anemia impairs tissue oxygenation.
During its lifetime, a normal RBC traverses the circulation thousands of times, enduring tremendous mechanical and metabolic stress with each passage. The smallest capillaries have an internal diameter that is smaller than the diameter of the RBC; thus the blood cells must deform to squeeze through the capillaries and then return to their normal shape as they enter distal venules. During their rapid transit, the RBCs must endure tremendous fluctuations in pH, P o 2 , and osmotic pressure. The lipid bilayer that forms the cell membrane is the site of numerous biologic functions mediated by integral proteins. It also is the attachment site for the proteins of the cytoskeleton, which confer the shape and stability necessary for proper membrane function. Some abnormalities in the membrane-cytoskeleton unit result in morphologic defects of RBCs ( Fig. 79.6 ). Because abnormally shaped RBCs are removed from the circulation by the reticuloendothelial system, many of these defects cause some degree of hemolytic anemia. Analysis of these congenital defects has led to an understanding of the erythrocyte membrane and cytoskeleton. The more common erythrocyte cytoskeletal defects originally were described by morphologic and clinical criteria. With better understanding of the cytoskeleton at a molecular level, it became clear that various mutations in genes coding for a few structural proteins are responsible for a family of inherited hemolytic anemias with overlapping morphologic, clinical, and genetic features. Mutations in the genes encoding α-spectrin, β-spectrin, ankyrin, protein 4.1, glycophorin C, protein 4.2, and band 3 have been reported in patients with hereditary defects of the red blood cell membrane/cytoskeleton. Inherited hemolytic anemias caused by RBC membranopathy, enzymopathy, and hemoglobinopathy are generally diagnosed by using conventional methods, including RBC morphology, membrane protein analysis, Hb electrophoresis, and measurement of RBC enzyme levels. In the genetic era, Sanger sequencing has been useful for detecting genetic mutations that cause inherited hemolytic anemias. Sanger sequencing is widely applied to identify disease-causing mutations in cases where traditional testing has failed or when a patient has been extensively transfused, leading to confounding biochemical and other testing findings caused by mixed RBC populations. Recent advances in genetic technology using next generation sequencing have enabled better identification of various genetic mutations that can cause these conditions.
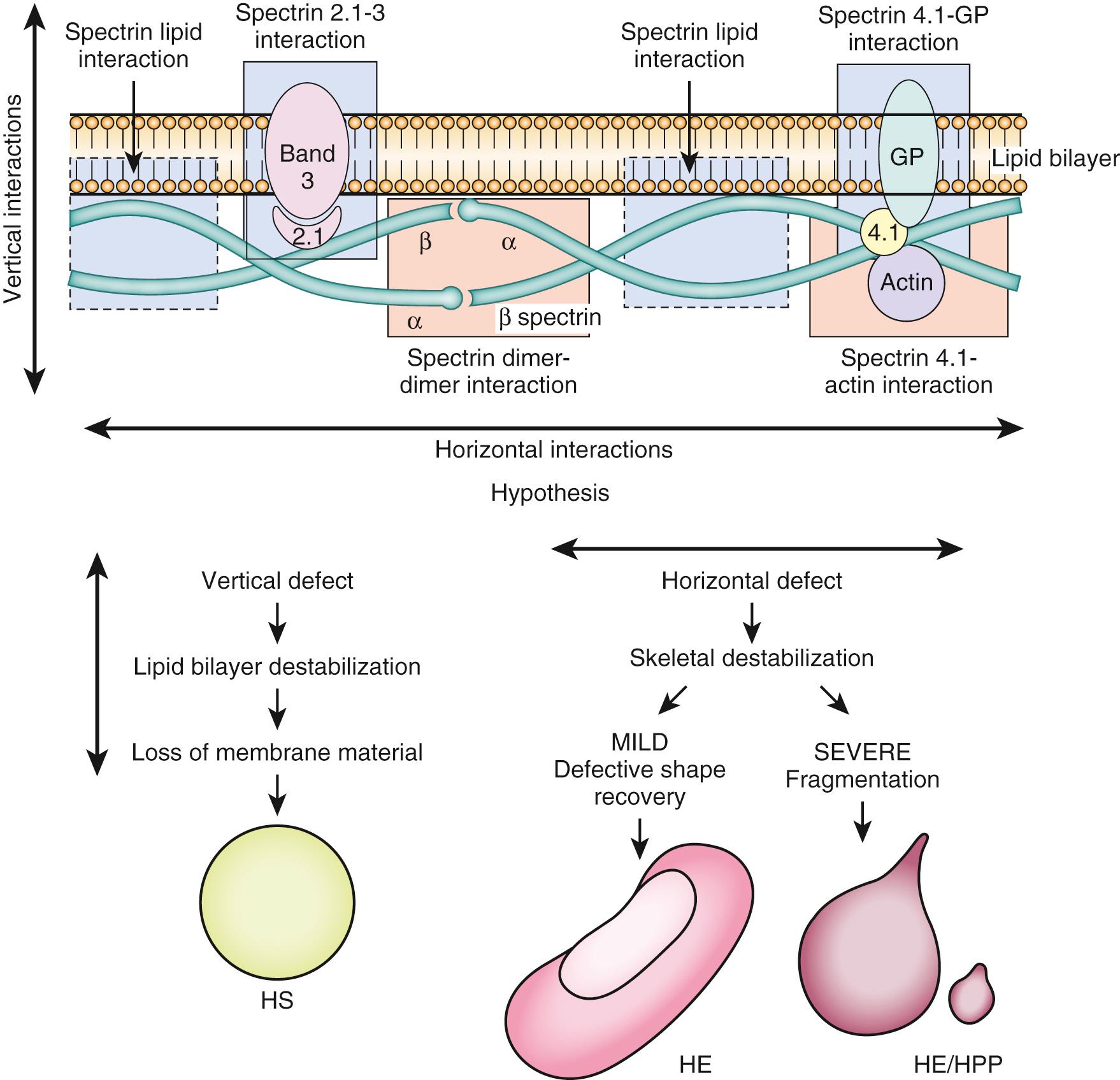
Membrane defects associated with hemolytic anemia may present in the neonate with hepatosplenomegaly, hyperbilirubinemia, and hemolytic anemia. Aplastic crisis, attributed to drugs or an infectious agent such as parvovirus B19, or splenic sequestration, may cause life-threatening anemia in patients with hemolytic anemia. The more severe cases of hemolytic anemia can require splenectomy, although surgery generally is delayed beyond the first few years of life.
Hereditary spherocytosis (HS) is characterized by the appearance of spherocytes on the peripheral blood smear, accompanied by a hemolytic anemia of varying severity and splenomegaly. Hereditary spherocytosis occurs predominantly in those of Northern European ancestry. Inheritance usually is autosomal dominant, but autosomal recessive and autosomal dominant inheritance with reduced penetrance also have been reported. Spontaneous mutations account for about one-fourth of HS. The defects alter the vertical stability of the cytoskeletal attachment to the lipid bilayer so that pieces of untethered membrane are removed in the spleen. Defects or deficiency in ankyrin are the most common, although defects in spectrin, band 3, and protein 4.2 also occur. Nondeformable spherocytes with a decrease in the surface area are more susceptible to lysis with exposure to the metabolic and deformation stresses of the splenic sinuses or with incubation in hyposmolar solutions. The osmotic fragility test exposes the suspected cells and normal control cells to progressively more hypotonic solutions and compares their ability to resist lysis.
The findings on the peripheral blood smears in hereditary elliptocytosis and hereditary pyropoikilocytosis are distinct ( Fig. 79.7 ). Hereditary elliptocytosis (HE) is an autosomal dominant condition characterized by elliptical RBCs called ovalocytes, which is more common in people of African and Mediterranean origin. In hereditary pyropoikilocytosis (HPP), the RBC morphology is bizarre, marked by fragments, elliptocytes, and spiculated cells. The disorders are caused by defects in cytoskeletal proteins, most commonly α- or β-spectrin, which weaken the horizontal interactions necessary for cytoskeletal stability and reversible deformability (see Fig. 79.6 ). The deformed RBCs are prematurely cleared by the reticuloendothelial system. Hemolysis in HE generally is milder than in hereditary spherocytosis. The majority of those with HE are symptomatic. Hereditary pyropoikilocytosis is caused by homozygous or compound heterozygous defects. Some neonates with HE may exhibit hemolysis with HPP morphology on blood smear, but later in life their red blood cells will appear as typical elliptocytes. Hereditary pyropoikilocytosis is associated with more severe hemolysis and hyperbilirubinemia, often requiring exchange transfusion or phototherapy in the neonatal period. Examination of blood smears from each parent may be helpful in making this diagnosis.
Southeast Asian ovalocytosis (SAO), or stomatic elliptocytosis, is a mild hemolytic variant of HE, which is common in Southeast Asia, likely because it confers malaria resistance. Defects in band 3 are the most common.
Spherocytic elliptocytosis , characterized by spherocytes and elliptocytes and a mild hemolysis, occurs in those of European ancestry.
Membrane lipid abnormalities can be inherited but most often are acquired. Their presence, detected by morphologic abnormalities on the peripheral blood smear, is most important as the signal of an underlying disease. Target cells form when the surface-to-volume ratio of the red blood cell increases, either because of poorly hemoglobinated cells (iron deficiency, thalassemias, Hb C) or because lipids are added to the RBC membrane (liver disease with intrahepatic cholestasis, lecithin-cholesterol acyltransferase deficiency). Acanthocytes form when the membrane lipid composition between the inner and outer leaflets of the bilayer is altered, as in liver disease and abetalipoproteinemia.
The mature RBC, lacking a nucleus, polyribosomes, and mitochondria with which to carry out protein synthesis, must circulate through extremes of pH, P o 2, and osmotic gradients while maintaining deformability and integrity. Red blood cells metabolize glucose through the Embden-Meyerhof pathway and hexose monophosphate shunt to provide energy for maintaining ionic pumps, to reduce methemoglobin, and to synthesize small molecules such as adenine, guanine, pyrimidine nucleotides, glutathione, and lipids. The most commonly defective enzyme, glucose-6-phosphate dehydrogenase (G6PD), has been extensively evaluated at the clinical, biochemical, and molecular level. Many other enzymatic defects have been reported, but the rarity of the disorders has hindered investigation. Enzymatic defects of the RBC known to be associated with hemolytic anemia are listed in Box 79.3 . A few of the enzyme deficiencies cause abnormalities in other tissues. The end result of these defects is hemolytic anemia of varying severity, sometimes called hereditary nonspherocytic anemia . The RBCs may be morphologically normal and usually produce normal results on osmotic fragility testing, but they have a shorter life span. The milder cases cause little difficulty in the neonatal period. Some defects are associated with chronic and/or severe hemolysis, necessitating intermittent or chronic blood transfusions.
Glucose-6-phosphate dehydrogenase *
* Most common defect worldwide.
Pyruvate kinase †
† Second most common defect.
Hexokinase
Glucose phosphate isomerase
Phosphofructokinase
Aldolase
Triose phosphate isomerase
Glyceraldehyde-3-phosphate dehydrogenase
Phosphoglycerate kinase
2,3-Diphosphoglycerate mutase
Enolase
Glutathione synthetase
γ-Glutamyl cysteine synthetase
Glutathione reductase
Glutathione S-transferase
Glutathione peroxidase
Elevated adenosine deaminase levels
Pyrimidine 5′-nucleotidase
Adenylate kinase
Glucose-6-phosphate dehydrogenase deficiency is the only common defect of RBC enzymes and can be associated with severe hemolysis, anemia, and hyperbilirubinemia in the neonate, although many are asymptomatic. This enzymatic defect is common in infants with bilirubin encephalopathy. Occasionally, the hyperbilirubinemia is severe, because of an interaction of G6PD deficiency with the (TA) 7 promoter polymorphism of the UDP-glucuronosyltransferase 1A1 (UGT1A1) bilirubin-conjugating enzyme. The prevalence among some ethnic groups suggests a selective advantage, because deficient cells are resistant to malaria. As an enzyme in the pentose phosphate pathway, G6PD supplies NADPH, and by producing hydrogen ions, promotes reduction of glutathione. Deficiency limits the cell's ability to recover from oxidative stress. The gene is inherited as an X-linked recessive. Males are affected most commonly. Females may also be affected, either because of homozygosity or compound heterozygosity, or in cases in which X-inactivation is unbalanced. In the United States, African-American males are the most commonly affected. The A-minus variant, common in Africans, results in a mild reduction in both catalytic activity and stability. The B variant, common among Mediterranean, some Asian, and Ashkenazi Jewish populations, involves a severe reduction of enzyme activity, resulting in chronic, moderate-to-severe hemolytic anemia that could prove fatal in the face of severe oxidant challenge. In affected patients, the oldest RBCs are most deficient, resulting in a normal or minimally shortened erythrocyte life span, but exposure to oxidant drugs and toxins or a febrile episode can precipitate severe hemolysis. Hemolysis occurs within 24-48 hours of exposure and is accompanied by abdominal pain, vomiting, diarrhea, low-grade fever, jaundice, splenomegaly, hemoglobinuria, and anemia.
Neonates may present with hemolytic anemia and hyperbilirubinemia within 24 hours of life and are susceptible to developing methemoglobinemia. Management involves avoidance of precipitating agents, hydration, phototherapy and, if needed, transfusion (partial volume exchange or simple). Common drugs to be avoided or used with caution in this disorder include antimalarials, sulfonamides and sulfones, nitrofurans, anthelminthics, ciprofloxacin, methylene blue, acetaminophen, aspirin, and vitamin K analogues. An excellent resource for complete food and drug lists, as well as family education, is at https://www.g6pd.org (accessed May 4, 2018). Enzyme levels are highest in younger cells. Evaluation immediately after a hemolytic episode could be inconclusive because the older, abnormal cells have been destroyed. Repeat quantitative testing at a later time, as well as evaluation of maternal enzyme levels, can be useful. Some states and countries include GPD deficiency screening as part of their newborn screening program, using biochemical qualitation, biochemical quantitative assays, and DNA-based PCR screening for common mutations.
Vitamin E is a fat-soluble antioxidant that reduces the peroxidation of polyunsaturated fatty acids by reactive oxygen species during oxidative enzyme activity. Preterm infants and infants with low birth weight have low serum and tissue levels of this vitamin. Patients who have chronic fat malabsorption are the most likely to develop symptomatic vitamin E deficiency. A deficiency state in preterm infants and those with very low birth weight has been described, characterized by hemolytic anemia, reticulocytosis, thrombocytosis, chronic lung disease, intracranial hemorrhage, and retinopathy of prematurity. However, present evidence does not support routine supplementation with intravenous or high-dose vitamin E in neonates.
The thalassemias are a group of hereditary anemias that arise from quantitative defects in the synthesis of globin chains. In their milder forms, thalassemias are among the most common heritable disorders. The α-thalassemias are most common in the Chinese subcontinent, Malaysia, Indochina, and Africa. β-Thalassemia is common in Mediterranean and African populations but also has a higher incidence in China, Pakistan, India, and the Middle East. Hemoglobin E is common in Southeast Asia. The four α-thalassemia syndromes (silent carrier, α-thalassemia trait, Hb H disease, and hydrops fetalis) are caused by diminished production of α-globin protein because of deletion in one, two, three, or four of the α-globin genes, respectively. Although only two genes for β-globin production are inherited, there are four clinical classifications for β-thalassemia: silent carrier, β-thalassemia trait, thalassemia intermedia, and thalassemia major. The blood smear shows hypochromic microcytes, target cells, and nucleated RBCs. Bizarre RBC shapes and fragments are seen in more severe disease. The clinical outcome in this disease is a result of complex interactions involving the type of genetic defect, the degree of β-globin production, and the ratio of α-globin chains produced relative to the number of β-globin chains ( Table 79.11 ). Abnormal hemoglobin tetramers can accumulate in the bone marrow and reticuloendothelial system, resulting in ineffective erythropoiesis. Mutant hemoglobins can precipitate to form inclusion bodies that adhere to cell membranes, promote oxidative damage to the membrane, diminish cell deformability, and shorten RBC survival. Coinheritance of α- and β-globin defects may result in a less severe thalassemia, because the ratio of α- to β-globin chains is rebalanced.
| Thalassemia Defect | Product | Result | Outcome |
|---|---|---|---|
| Absent or reduced globin production | α:β Globins ratio altered | Precipitation of excess globins | Abnormal hemoglobin pattern |
| RBC membrane damage | Hemolysis Thrombosis |
||
| Ineffective erythropoiesis | Basophilic stippling Hypochromia Microcytosis Reticulocytosis |
||
| Anemia | Bone marrow expansion Extramedullary hematopoiesis Iron hyperabsorption |
Prenatal diagnosis strategies are available for both α- and β-thalassemia. If evaluation of the parents’ RBCs provides evidence of thalassemia, molecular detection strategies can be used. Fetal tissue from chorionic villus sampling (10-12 weeks’ gestation) or amniocentesis (15-20 weeks) can be analyzed by a number of DNA-based methodologies (see Chapter 10 ). After 18-20 weeks’ gestation, fetal reticulocytes may be tested for globin chain biosynthetic ratios, although sampling carries higher risks.
Because the production of α-globin chains predominates from midgestation onward, defects in synthesis can be detected at birth. Before the debut of molecular analysis, the various α-thalassemia syndromes were assigned according to clinical criteria ( Table 79.12 ). Hydrops fetalis, the most severe and rarest form of α-thalassemia, occurs when an infant inherits deletions of both α-globin genes from each of the parents (see Chapter 23 ). Such an infant produces small quantities of Hb Portland to maintain in utero viability. The excess γ and β chains form tetramers with themselves, resulting in functionally useless Hb Barts (γ 4 ), present in the newborn, and Hb H (β 4 ) present in the older infant. The fetus with four α-globin gene defects experiences congestive heart failure in utero secondary to severe anemia and, in the absence of fetal transfusion, becomes grossly hydropic (hydrops fetalis). There is evidence of extensive extramedullary hematopoiesis and placental hypertrophy. Most are stillborn at 30-40 weeks’ gestation or die shortly after delivery. Infants with hepatosplenomegaly, jaundice, and a moderate hypochromic, microcytic hemolytic anemia should be evaluated for deletion of three α-globin genes, Hb H disease. At birth, large amounts of Hb Barts may be seen as well as some Hb H. Incubation of the RBCs with brilliant cresyl blue reveals many small inclusions. Treatment is supportive and includes supplementation with folic acid, avoidance of oxidant drugs, transfusion of RBCs, and attention to iron hyperabsorption and transfusional iron overload. Hypersplenism, characterized by leukopenia, thrombocytopenia, and worsening anemia, may necessitate splenectomy, although this usually is deferred beyond the first few years of life and is associated with infectious risk as well as increased thrombosis.
| α-Thalassemia Genotype | Number of α Genes Deleted | Phenotype |
|---|---|---|
| αα/αα | 0 | Normal |
| αα/α− | 1 | Silent carrier |
| α−/α− αα/− − |
2 | Alpha thalassemia trait, trans |
| Alpha thalassemia trait, cis | ||
| α−/− − − −/α cs α |
3 | Hemoglobin H |
| Hemoglobin H-Constant Spring | ||
| − −/− − | 4 | Hydrops fetalis |
| β-Thalassemia Genotype | Phenotype | Clinical Implications |
| β/β | Normal | Normal |
| β/β + β/β 0 |
β-Thalassemia minor (trait) | Microcytosis |
| Hypochromia | ||
| Increased Hb F, Hb A2 | ||
| Basophilic stippling | ||
| Occasional target cells | ||
| β +/ β + β + /β 0 β E /β + β E /β 0 |
β-Thalassemia intermedia | Moderate microcytosis |
| Moderate-severe anemia | ||
| Moderate hypochromia | ||
| Basophilic stippling | ||
| Target cells | ||
| Increased Hb F, Hb A2 | ||
| Variable transfusion dependence | ||
| Hepatosplenomegaly variable | ||
| Iron hyperabsorption | ||
| β 0 /β 0 | β-Thalassemia major | Severe microcytosis, hypochromia, target cells |
| Increased Hb F, Hb A2 | ||
| Transfusion dependence | ||
| Hepatosplenomegaly | ||
| Iron hyperabsorption | ||
| Transfusional iron overload | ||
| Hemoglobin E Genotype | Phenotype | Clinical Features |
| β/ε | Hb E Trait | Microcytosis |
| ε/ε | Hb E Disease | Microcytosis |
| Mild anemia | ||
| ε/β + | Hb E/β + -Thalassemia | Moderate microcytosis |
| Moderate anemia | ||
| ε/β 0 | Hb E/β 0 -Thalassemia | Moderate-severe microcytosis |
| β-Thalassemia intermedia |
α-Thalassemia trait is characterized by a mild anemia with microcytosis, hypochromia, and erythrocytosis. Hemoglobin Barts is mildly elevated at birth. After the neonatal period, there is no simple laboratory evaluation that is diagnostic for α-thalassemia trait. Alpha globin gene analysis can be ordered to confirm clinical suspicion. The diagnosis usually is made in an iron-replete patient with a normal hemoglobin electrophoresis and appropriate family history for α-thalassemia. As the name implies, silent carriers of α-thalassemia could have slightly microcytic RBCs but usually are asymptomatic. Several states now use molecular testing for HBA1 and HBA2 , the genes coding for α 1 - and α 2 -globin, respectively.
β-Thalassemia is not usually diagnosed at birth unless blood loss or RBC destruction has created an unusually high demand for replacement of fetal RBCs with adult. The disease generally manifests after 6 months of age, when a microcytic anemia persists beyond the time course for physiologic anemia. The more severe manifestations of the disease traditionally were called Cooley anemia , but these have been separated into thalassemia major and thalassemia intermedia (see Table 79.12 ). Affected patients exhibit splenomegaly, poor growth, microcytic hemolytic anemia with ineffective erythropoiesis, target cells on the peripheral blood smear, and extramedullary expansion of erythropoiesis. An abnormal hemoglobin analysis reveals elevations of Hb F and Hb A 2 and a decrease in Hb A.
Patients are categorized as having thalassemia intermedia if they are able to maintain a hemoglobin level greater than 7 g/dL without routine transfusion. Thalassemia major patients require regular transfusions and iron chelation therapy to maintain a hemoglobin value adequate for growth and development. Iron overload is a significant cause of morbidity and mortality starting in the second decade of life. Evidence of hypersplenism may necessitate splenectomy. Bony deformities from expansion of extramedullary hematopoiesis occur in incompletely transfused patients.
β-Thalassemia trait is characterized by abnormalities on the blood smear: microcytosis, hypochromia, erythrocytosis, and the appearance of elliptocytes and target cells. Hemoglobin analysis reveals a mild elevation of Hb A 2 and Hb F, although this is not observed if the genetic mutation also interferes with production of δ- or γ-globin or if Hb A 2 and Hb F production are depressed because of coexistent iron deficiency anemia. The silent carrier state of β-thalassemia is associated with normal findings on the peripheral blood smear and hemoglobin electrophoresis and usually is identified through family studies of patients with a more severe β-thalassemia syndrome.
Hemoglobin E causes one of the most common hemoglobinopathies in the world. This thalassemia syndrome, characterized as a qualitative and quantitative hemoglobinopathy, is particularly abundant among people of Southeast Asian ancestry, especially individuals from Laos, Cambodia, and Thailand. Hemoglobin E is generated by a single nucleotide substitution, Glu26Lys, in the coding region of the β-globin gene. The β-globin monomers associate normally with a pair of α-globin proteins to form a functional hemoglobin tetramer with essentially normal stability and oxygen-dissociation characteristics. Persons with Hb E are anemic because the mutation creates a cryptic splice site in the β-globin message. During RNA processing, a portion of the message is spliced into an abnormal, unstable transcript. Consequently, the total level of β-globin messenger RNA is reduced, resulting in a thalassemia phenotype. Hemoglobin E trait results in mild microcytosis. Those who are homozygous for Hb E have chronic, mild to moderate, microcytic anemia but are otherwise well; however, co-inheritance of an Hb E allele from one parent and a β-thalassemia allele from the other produces a moderate to severe anemia that can be transfusion dependent. Hemoglobin levels in these patients range from 2-7 g/dL, and children with this condition can develop hepatosplenomegaly and growth failure. Hemoglobin E-β 0 thalassemia disease now is the most common form of transfusion-dependent thalassemia in the United States and other parts of the world. Hemoglobin E co-inherited with Hb S results in a sickle phenotype.
Inefficient erythropoiesis is the main mechanism underlying the complications in thalassemia major, such as anemia, splenomegaly, bone malformations, and pulmonary hypertension. New knowledge has become available on pathways that contribute to the pathogenesis and clinical manifestations of this disease. Clinical trials with JAK2 inhibitors (ruxolitinib) and TGF-β family member inhibitors, sotatercept and luspatercept, are currently studying the benefits of these agents in decreasing ineffective erythropoiesis and splenomegaly.
Another modality of treatment for hemoglobinopathies is gene therapy, which is showing its safety and potential efficacy in both preclinical and early clinical studies. Gene therapy is now a reality, with seven patients cured of their β0/βE thalassemia or with significant amelioration from β0/β0 thalassemia and one patient with SCD, while others are showing modest transgene expression.
In the fetus, switching from one type of globin chain expression to another occurs several times (see Fig. 79.2 ), and during the first few months of the postnatal period, the final switch to the adult-type hemoglobin occurs. Some hereditary defects in fetal globin gene products can produce a hemolytic anemia that resolves as β-globin gene expression predominates. Similarly, a neonate who is asymptomatic at birth can develop a hemolytic anemia over the subsequent months as production of β-globin–containing hemoglobin assumes major importance. Because α chains are produced from the fetal period onward, defects in α-globin are present at birth. If the combination of the mutant α- and the γ-globin is more unstable than that of α- and β-globin or α- and δ-globin, the infant experiences a transient hemolytic anemia that resolves as Hb A predominates.
More than 500 structurally different hemoglobin variants have been reported. The clinically important variants are listed in Box 79.4 . Most involve a single amino acid substitution in one of the globin polypeptide chains owing to a single nucleotide change in DNA. Other variants are the result of gene deletions or duplications caused by unequal crossover.
Most mutations causing unstable hemoglobin affect β-globin and involve amino acid substitutions in hydrophobic residues around the heme pocket. These amino acid replacements alter the hydrophobic interior of the molecule, predisposing the hemoglobin to instability and precipitation as small round inclusions known as Heinz bodies. Because the amino acid substitutions often involve replacing one neutral amino acid with another, the hemoglobin electrophoretic mobility may not change. Patients usually develop anemia and jaundice in late infancy as β-globin synthesis increases. Mutations in fetal or embryonic globins can cause symptoms at birth but not later in life. The diagnosis is confirmed by supravital staining for Heinz bodies, which are usually present, and hemoglobin analysis after isopropanol precipitation.
Sickle cell disease is a common medical problem in the United States, where 1 in 400 newborns is affected. By definition, sickle cell anemia refers to the doubly heterozygous inheritance of abnormal genes that code for the substitution of valine in place of glutamic acid at position six in the β chain of hemoglobin (Hb SS). There are also a number of sickle hemoglobinopathies in which one gene coding for Hb S is inherited along with a second abnormal β-globin gene coding for other hemoglobins, such as Hb C, D, O Arab , or β-thalassemia. The clinical course of affected patients may be indistinguishable from, or milder than, those of Hb SS disease. As with the thalassemias, prenatal diagnostic techniques are available. Newborn screening programs have been instituted in many parts of the world, including most of the United States. Samples of cord or capillary blood are subjected to testing, usually hemoglobin electrophoresis, high pressure liquid chromatography, or isoelectric focusing. Infants with abnormal results on the newborn screen are referred for confirmatory testing and disease counseling ( Fig. 79.8 ; Box 79.5 ).
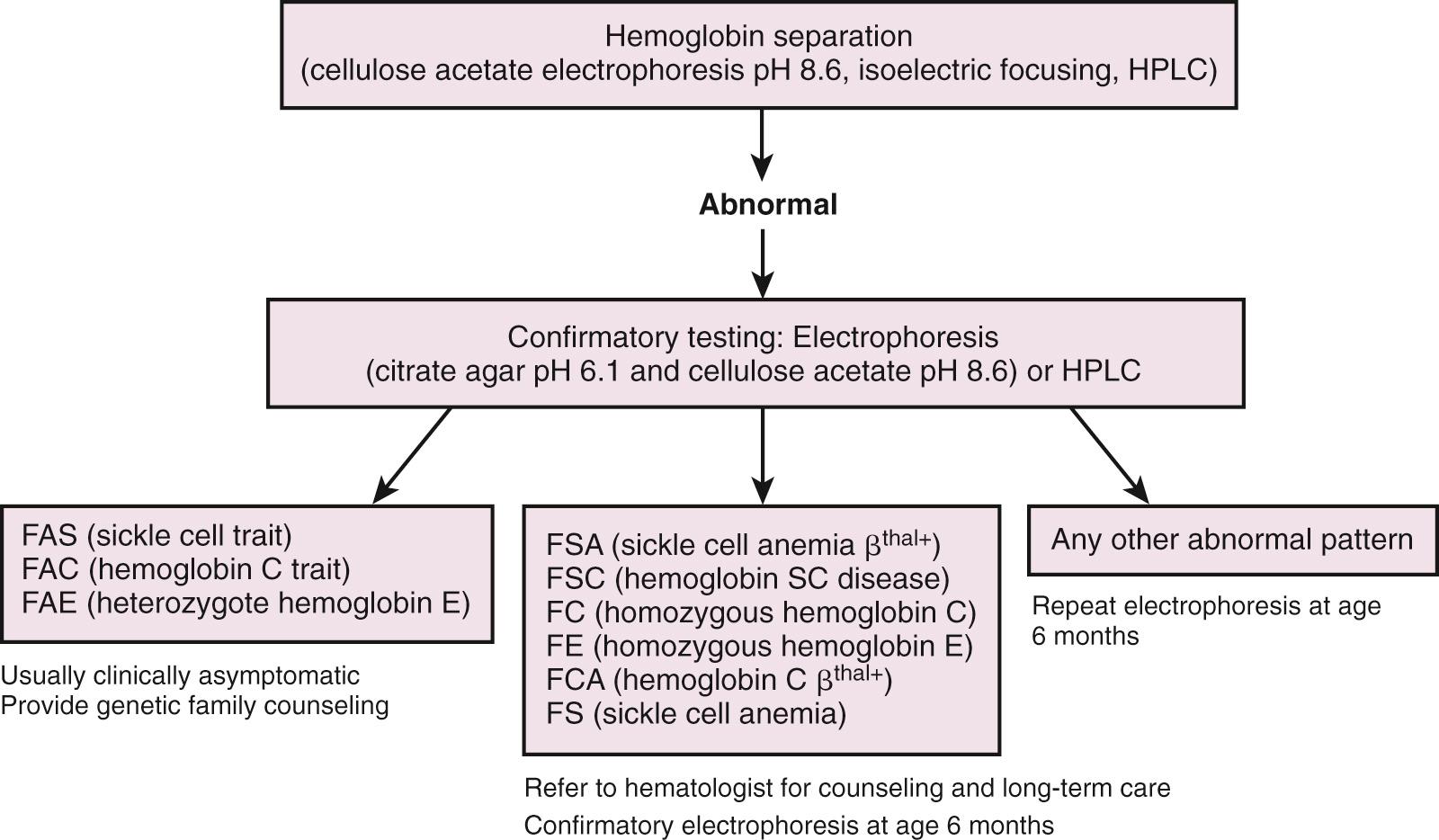
| Hemoglobin Pattern | Interpretation |
|---|---|
| FA | Normal |
| FAS | Sickle cell trait |
| FSC | Sickle C disease (Hb SC) |
| FS | Sickle cell disease or sickle β 0 -thalassemia |
| FSA | Sickle β + -thalassemia |
| FVA | Hb variant with β + -thalassemia |
| FAV | Heterozygous or trait variant 2%-8% Barts—α-thalassemia trait 25%-50% Hb Barts—hemoglobin H disease |
| FV | Hb variant with β 0 -thalassemia |
| FVV | Double heterozygous variant |
| FSV | Sickle Hb and variant Hb SO arab and Hb SD are sickle hemoglobinopathies FS and Hb Barts—sickle cell disease with α-thalassemia |
| F | Very premature infant Absence of β globin production |
The clinical hallmark of Hb SS disease is hemolytic anemia with reticulocytosis and the appearance of irreversibly sickled cells on the peripheral blood smear. The onset of signs and symptoms correlates with the decrease in Hb F levels and increasing expression of the mutant β-globin gene products during the first year of life. Neonates generally are asymptomatic, although older infants are at risk for several disease-related complications. Because of splenic dysfunction, infections with certain bacteria, such as pneumococcus, are a major cause of mortality and morbidity in infants and young children. A suddenly pale, listless infant may be experiencing life-threatening anemia caused by hepatic or splenic sequestration. Aplastic crisis, triggered by infection or drugs, also may occur. Vaso-occlusive crisis of the infant's hands and feet, the hand–foot syndrome (dactylitis), manifests with pain, warmth, and swelling of the hands and/or feet.
Identification of affected newborns through newborn screening programs with enhancements in parent education and supportive care such as penicillin prophylaxis, immunization against S. pneumoniae and H. influenzae , and early identification of hepatic or splenic sequestration have decreased the death rate for infants and children. The natural history of sickle cell anemia includes painful vaso-occlusive events, acute and chronic lung disease, stroke, overwhelming sepsis from splenic dysfunction, avascular necrosis of joints, retinopathy, and transfusion-related iron overload. The National Heart, Lung and Blood Institute (NHLBI) issued management guidelines for sickle cell disease in 2002, which are available at https://www.nhlbi.nih.gov/files/docs/guidelines/sc_mngt.pdf . Treatment of the sickle hemoglobinopathies has been largely supported using penicillin prophylaxis; vaccinations; blood transfusions with extended RBC phenotyping; cross-matching to decrease the incidence of transfusion-associated alloimmunization; and hydration, narcotics, and nonsteroidal anti-inflammatory drugs for painful crises. Hydroxyurea decreased the number and severity of vaso-occlusive pain events in infants and young children with Hb SS and Hb SB 0 thalassemia in the randomized, placebo-controlled BABY HUG study. Stem cell transplantation, the only established cure for sickle cell anemia, has been limited by the availability of suitable donors and the uncertainty in predicting which children will express a more severe phenotype. Ongoing studies using reduced intensity conditioning regimens and alternative donor options, as well as risk stratification of sickle cell patients, may allow larger number of patients to undergo stem cell transplantation in the future.
Heterozygotes for Hb S (sickle cell trait) were thought to be clinically unaffected, but there are reports of hematuria, decreased urinary concentrating ability, and occasional reports of sudden death related to high altitude and extreme exercise. Associations have been made between sickle cell trait and venous thrombosis, renal medullary carcinoma, and a more severe course of diabetes mellitus.
Patients suspected of having hemoglobin disorders are screened with a CBC and hemoglobin analysis. Depending on the results and whether there are genetic counseling issues to consider, molecular testing for globin abnormalities may be indicated. Patients with ambiguous hemoglobin results or unusual phenotypes are candidates for DNA testing. Couples seeking genetic counseling should be offered DNA testing when both prospective parents are suspected of having cis α-globin deletions or both have β-thalassemia traits. In addition, individuals with β-thalassemia trait or hemoglobin E trait may have coincident α-thalassemia trait that is masked by the microcytosis associated with these β-globin disorders. In such cases α-globin DNA testing might be advised to evaluate risk to offspring of hemoglobin Bart's hydrops fetalis. Prenatal genetic testing should be offered to couples at risk for having an offspring with clinically severe hemoglobin disorders.
Although the prevalence of iron deficiency anemia is decreasing in the United States, it remains a major cause of anemia in infants and children, and it has been associated with adverse neurocognitive outcomes. Factors associated with the declining rates of iron deficiency anemia include the increasing number of infants receiving breast milk, the use of standard iron-supplemented infant formulas and iron-supplemented baby cereals during the first year of life, and the delay of the introduction of cow milk into the infant diet until after 1 year of age.
Iron is an essential nutrient. Most iron is bound to the heme proteins, hemoglobin, and myoglobin. The storage proteins ferritin and hemosiderin contain most of the rest of the iron. A small percentage is bound in enzyme systems such as cytochromes and catalase. In healthy adults, most of the body's iron is recycled from the breakdown of RBCs. Little iron needs to be absorbed from the intestines, and little is lost through fecal or urinary excretion. Because neonates must rapidly expand muscle mass and blood volume, they require a much higher percentage of dietary iron intake. The amount of iron stored in the neonate's body is proportional to birth weight. Eighty percent of a term infant's iron is acquired during the third trimester. For a full-term infant, this should be sufficient for the first 4-6 months of life. Preterm infants and those born small for gestational age have a much faster rate of postnatal growth and are at risk for iron deficiency within the first 3 months of life ( Box 79.6 ). Infants born anemic and those who have accelerated iron losses from repeated laboratory testing, trauma, surgery, or anatomic abnormalities may also become deficient. Although both breast milk and cow milk contain iron, the bioavailability of the iron in breast milk is much superior. The American Academy of Pediatrics (AAP) Committee on Nutrition updated its recommendations for diagnosing and preventing iron deficiency and iron deficiency anemia in 2010 ( Table 79.13 ). Full-term infants who are breastfed should not require iron supplementation until 4-6 months of age. At 4 months of age, human milk–fed infants should receive iron supplementation until they are eating iron-containing foods. Two daily servings of an iron-fortified cereal will meet this requirement. Infant cereal fortified with iron should be one of the earliest solid foods introduced. After 6 months of age, one daily serving of a vitamin C–rich food should be introduced. Because the protein in cow milk can cause occult gastrointestinal bleeding in the neonate and because of the poor bioavailability of iron in cow milk, it is recommended that babies avoid cow milk until after 1 year of age. In general, preterm infants who are fed human milk require iron supplementation after 1 month of age, either by a supplement added to the breast milk or iron given directly to the infant. An exception may be made for infants who have received multiple blood transfusions and are thus at risk for iron overload rather than deficiency. A thought-provoking, randomized, placebo-controlled trial of daily oral iron supplementation in very low birth weight infants (<1500 g) of less than 32 weeks’ gestational age, who were managed on a liberal transfusion strategy, did not demonstrate an increase in the hematocrit, reticulocyte count, or transfusion requirements at week 36 postmenstrual age.
Prematurity
Small for gestational age
Anemia at birth
Fetal–maternal hemorrhage
Twin–twin transfusion
Perinatal hemorrhage
Iatrogenic or other ongoing blood loss
Insufficient dietary intake or malabsorption
Early introduction of cow milk
Erythropoietin use
Maternal conditions: severe iron deficiency, diabetes
| Birth Status | Intake | Minimum Daily Recommendation | How Supplied |
|---|---|---|---|
| Term, healthy | Human milk | 1 mg/kg per day after 4 mo of age until addition of complementary iron-rich foods | Iron supplement until addition of complementary iron-rich foods |
| Term, healthy | >Half human milk | 1 mg/kg per day after 4 mo of age until addition of complementary iron-rich foods | Iron supplement until addition of complementary iron-rich foods |
| Term, healthy | Standard infant formula | 1 mg/kg per day after 4 mo of age | Supplied by standard infant formula and/or addition of complementary iron-rich foods |
| Preterm | Human milk | 2 mg/kg per day beginning at 1 mo of age and until 12 mo of age | Supplement added to human milk or given separately until addition of complementary iron-rich foods |
| Preterm | Standard infant formula | 2 mg/kg per day beginning at 1 mo of age and until 12 mo of age | Supplied by full feeds of standard infant formula and/or complementary iron-rich foods |
With improved survival in the smallest patients, additional knowledge gaps and opportunities for investigation are identified in diagnosing, treating, and preventing iron deficiency and iron deficiency anemia ( Box 79.7 ). Although severe iron deficiency in children is fairly simple to diagnose, milder deficiency may not yet have caused anemia or may be difficult to distinguish from other causes of microcytic anemia, especially in patients with chronic or acute illness. The AAP recommends that testing for anemia by hemoglobin estimation should occur at approximately 12 months of age. In addition, there should be an assessment of risk factors for iron deficiency/iron deficiency anemia at that time. Earlier and/or more frequent assessments should occur in situations of high concern. A careful dietary history should be taken, and consideration should be given to special circumstances such as ongoing blood loss, malabsorption, early or excessive cow milk intake, and birth history. In early iron deficiency, the bone marrow stores are depleted and the RBC distribution width increases. Subsequently, the iron transporter levels fall, resulting in lower serum levels of iron, ferritin, and transferrin in the term infant. Finally, erythrocyte production is affected. A hypochromic, microcytic anemia becomes apparent. Screening test results such as serum iron, total iron-binding capacity, ferritin, and transferrin saturation may be inconclusive because of acute or chronic illness. It is unclear what testing is optimal in the premature infant. The AAP favors the reticulocyte Hb concentration (CHr) and the serum transferrin receptor 1 (TfR1), because these tests are not affected by inflammation or infection, but these tests are not widely available. A therapeutic trial of iron for presumptive iron deficiency anemia is a cost-effective strategy. Ferrous sulfate, in a dose of 2-6 mg/kg per day of elemental iron, may be given for 1 month. If the hemoglobin rises by at least 1 g/dL (in the absence of ongoing blood loss), the diagnosis of iron deficiency is made, and the iron supplementation is usually continued for 2-3 more months or until 1-2 months after the hemoglobin is in the normal range. Screening for lead exposure should be done as part of routine well-baby care and particularly considered if a hypochromia and microcytosis are detected.
Optimal lab testing for premature infants
Optimal formulation of iron supplementation
Optimal iron preparation
Optimal dose and interval of supplementation
Duration of iron supplementation
Incidence and proper management of adverse effects of iron supplementation
Our knowledge of iron biology has been enriched substantially by the recent discovery of mammalian iron transport proteins (DMT1, ferroportin, mitoferrin 1, and ZIP14), heme transporters (HRG1and FLVCR1), iron chaperones (PCBPs), ferrireductases and ferroxidases (DCYTB, STEAP3, PrPc, and hephaestin), and the iron-regulatory hormone hepcidin. The physiologic function of these proteins has been studied to a great extent and the implications of their up-and-down regulation.
We have learned in depth the mechanisms through which iron is transported in the gut. Iron is transported out of the enterocyte and into portal blood via ferroportin (SLC40A1) located on the basolateral membrane. Ferroportin transports only Fe2+, whereas transferrin in portal blood will bind only Fe3+. Efficient transfer of iron to portal blood transferrin is thought to involve an oxidation step catalyzed by a ferroxidase. The best characterized intestinal ferroxidase is hephaestin, a membrane-anchored homologue of the plasma ferroxidase, ceruloplasmin.
Greater than 95% of iron in plasma is bound to its circulating transport protein transferrin, which delivers most of its iron to erythrocyte precursors (i.e., erythroid progenitor cells of the bone marrow that differentiate into mature RBCs). Each day, ~25 mg of iron is taken up into these cells to support the daily production of 200 billion new RBCs. Erythrocyte precursors take up iron nearly exclusively from transferrin via transferrin receptor 1 (TFR1). The quantitative importance of this uptake is illustrated by the fact that an estimated 80% of total body cellular TFR1 is located in the erythroid marrow of an adult human. When transferrin binds to TFR1 at the cell surface, the complex is internalized into endosomes, which become acidified, causing iron to dissociate from transferrin. The endosomal ferrireductase STEAP3 (six-transmembrane epithelial antigen of the prostate 3) reduces the liberated Fe3+ to Fe2+, which is subsequently transported into the cytosol via DMT1. Despite being the most avid consumers of iron in the body, erythrocyte precursors abundantly express ferroportin at the plasma membrane and are therefore able to export nonheme iron. Systemic iron depletion has been shown to increase ferroportin expression in erythrocyte precursors, leading to the hypothesis that upregulation of ferroportin in iron deficiency serves to provide iron to nonerythropoietic tissues.
The hepatically produced peptide, hepcidin, is an important regulator of iron homeostasis. It inhibits the transport of iron across the gut mucosa as well as the transport of iron out of macrophages. Hepcidin is an acute-phase reactant: in inflammation, increased hepcidin results in decreased iron absorption. Overexpression of hepcidin in genetically modified mice results in iron deficiency anemia. This has led to interest in human defects of hepcidin overexpression that may result in the inability to absorb iron (i.e., refractoriness to oral iron). Conversely, the intestinal hyperabsorption of iron in thalassemias is associated with low hepcidin levels.
The physiologic anemia of infancy can be exaggerated in the sick or premature infant by frequent blood sampling: the smaller the infant, the proportionally greater the volume of blood that is withdrawn for laboratory testing. Most RBC transfusions in neonates occur within the first 3-4 weeks of life, with the majority being in the first 2 weeks. Physiologically lower levels of EPO in neonates provided the rationale for the pharmacologic use of erythropoietin to reduce the volume and risks of blood transfusions. Recombinant erythropoietin products are commercially available and have been used for decades in the treatment of adults with renal disease and in cancer-associated anemia. The Food and Drug Administration (FDA) mandated black box warnings for EPO because of increased risks of death, myocardial infarction, stroke, venous thrombosis, and cancer progression in adult patients. These complications have not been seen in neonates. Two approaches to EPO therapy have been systematically reviewed in neonates and reported in a 2017 Cochrane database systematic review. The early approach, defined by the use of EPO before day 8 of life, has been shown to reduce (but not eliminate) RBC transfusions and donor exposures but did not impact morbidity and mortality measures. The later use of EPO (on day 8 of life or after) resulted in a reduction in the number of blood transfusions but not the total volume of blood, per infant, so that meaningful clinical outcomes were not affected by the use of EPO. Because most infants received RBC transfusions very early and before being enrolled in the clinical trials evaluating this question, the authors concluded that the critical effort should be aimed at preventing donor exposures in the very first few days of life. Stable and larger preterm infants have a better response to EPO therapy when compared with ELBW infants. Erythropoiesis requires iron. Even though EPO administration has been shown to reduce hepcidin levels, thereby increasing intestinal iron absorption, ferritin levels have been reported to drop with EPO use. Supplementation of between 1 mg/kg per day and 10 mg/kg per day elemental iron has been used to lessen the risk of iron deficiency.
Transient erythroblastopenia of childhood (TEC) is an acquired, transient, normocytic, hypoplastic anemia with a peak incidence at ages 2-3 years. It is exceedingly rare in neonates. The etiology of TEC is unknown but may be the result of viral injury to erythroid precursors. Familial cases have been reported. The degree of anemia can range from moderate to severe. Transient erythroblastopenia of childhood is a diagnosis of retrospect. Treatment consists of supportive measures, including RBC transfusion if needed, until bone marrow recovery. Most patients recover in 1-2 months. The condition may be confused with Diamond-Blackfan anemia and parvovirus-induced anemias.
Parvovirus B19, which proliferates in human erythroid precursors, causes several diseases. The majority of adults in the United States have antibodies to the virus. The virus produces fifth disease. The rash and joint symptoms associated with this generally minor illness are caused by vascular deposition of antibody complexes generated in response to the infection. In patients with underlying hemolytic anemias, parvovirus infection can cause a transient, severe aplastic crisis. It can also cause chronic anemia from a persistent infection in erythroblasts in immunocompromised hosts. Chronic parvovirus anemia responds to administration of intravenous immunoglobulin. The virus binds to blood group P-antigen on the surface of erythroid precursors, is internalized, and then replicates, disrupting normal erythroid differentiation. Endothelial cell P-antigen is postulated to participate in placental transmission of the virus. This antigen is also found on fetal cardiomyocytes, a finding consistent with the fact that the fetus infected with parvovirus can develop myocarditis. Infection rate during pregnancy is estimated to be in the 3% range. Parvovirus infection during pregnancy can result in anemia, hydrops fetalis, fetal loss, or congenital infection. The rate of transplacental transmission of the virus has been estimated at 33%. Initial reports of high rates of stillbirth and fetal loss have been modified; it is recognized that most of the mortality occurs during the first 20 weeks of gestation. Severe thrombocytopenia occurs in more than one-third of cases and can complicate intrauterine transfusion. Parvovirus-induced hydrops can be detected by ultrasound, and treatment of suspected hydropic infants includes intrauterine RBC transfusion. Parvovirus infection of the fetus may persist after birth as a cause of congenital RBC aplasia.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here