Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Four decades ago, pediatric gastritis and peptic ulcer disease (PUD) lacked a putative microbial causative agent. Pediatric flexible upper endoscopy was becoming more available. In 1983, Warren and Marshall proposed that colonization of the human stomach with an organism, now known as Helicobacter pylori , was associated with human disease, specifically PUD. The earliest reports of this bacterium in children noted its coincidence with gastritis and ulcer disease. , In February 1994, the National Institutes of Health Consensus Development Conference concluded that H. pylori represented the major cause of PUD. Later that year the International Agency for Research on Cancer Working Group of the World Health Organization classified H. pylori as a group I (definite) human carcinogen.
H. pylori is a slow-growing gram-negative, microaerophilic bacterium that colonizes the gastric mucosa. H. pylori infection is acquired during the first decade of life and unless eradicated causes lifelong infection. Large-scale analysis of H. pylori protein expression has been used to identify possible disease markers associated with the wide spectrum of putative Helicobacter -associated diseases, as well as being utilized in selecting potential vaccine candidates.
Many features of infection such as prevalence, clinical presentation and complications, diagnostic methods, and antibiotic resistance are age specific and differ between children and adults. Although H. pylori infects children worldwide, its role in certain pediatric diseases is a matter of some debate. Depending on geographic location, the majority of children infected with H. pylori are asymptomatic and only very rarely develop any complications of infection. Pediatricians and pediatric gastroenterologists must therefore avoid inappropriate investigation and potentially harmful therapies, while ensuring appropriate utilization of preventive medicine in minimizing future ill health.
To date, humans are the only established natural reservoir of H. pylori in the animal kingdom. At least five different gastric non– H. pylori Helicobacter species have been isolated from patients at endoscopy. Nonhuman primates rapidly acquire H. pylori and can be used as animal models of disease. Recent studies have shown that ancestral variants have likely been lost through the lineage extinction that would be associated with human population sizes that were too small to sustain infection. Using a new phylogenetic virtual genome fingerprinting method, a Latin American study analyzed the genome of 107 H. pylori strains from Mexico, Nicaragua, and Colombia and compared it with 59 publicly available worldwide genomes. The results of this study show an increased resolution of populations individualizing a large Latin American cluster of strains distinct from European, Amerindian, and African clusters already 500 years after the Spanish colonization. Similar studies have been performed in populations from North, Central, and South America and Portugal.
Within populations where socioeconomic disparity exists, indices of lower socioeconomic status—including low household income, lower parental educational level, higher housing density, and poor sanitary conditions—continue to influence a higher H. pylori prevalence among children in comparison with their noninfected peers. In China, Ding et al. performed a prospective, cross-sectional, population-based study examining 3491 children; consuming meals under low hygiene conditions, sharing towels, receiving prechewed food from mothers, formula feeding, and a family history of gastrointestinal disease were significantly associated with risk of infection. Coinfection among parents (especially mothers), siblings, and nonsibling household contacts are established risk factors for childhood H. pylori acquisition. Child-rearing practices may also influence the transmission of infection. International improvements in social conditions and hygiene standards may contribute to reduce the risk of H. pylori infection in children.
Direct person-to-person contact has been long thought of as a major mode of transmission. Rowland et al. showed that maternal infection was an independent risk factor for childhood H. pylori infection. Data from studies of a German birth cohort suggested an odds ratio (OR) of 13 for the effect on the index child of the mother being infected. The Pasitos Cohort Study reported that persistent H. pylori infection in older siblings increased the risks of H. pylori infection in younger siblings and suggested a unidirectional transmission of infection from mother or older sibling to younger siblings. This theory is again supported by Yokota et al., by multilocus sequence typing, identifying matching alleles in the isolate from the index child to the mother in 34% of cases and to the father in 3%. An Iranian study looked at H. pylori genotypes, where of 30 families, 10 children had H. pylori genotypes related to their mothers and only 2 to their fathers.
Studies investigating further routes of infection have been published. A study comparing serology for hepatitis A virus (HAV) and H. pylori found the adjusted odds of H. pylori seropositivity to be over two times higher, suggesting that H. pylori has the same mode of transmission as HAV. The oral-oral route has been considered in a recent review; H. pylori was detected in dental plaque, saliva, and oral mucosa. However, researchers consider that H. pylori can only be confirmed by culture as misleading polymerase chain reaction results (PCR) can occur from transient H. pylori in the mouth. Transmission via infected water is a research area gaining considerable interest. An American study of bacterial survival through a tertiary wastewater treatment facility in Ann Arbor, Michigan, showed viable H. pylori in all processed wastewater samples and in the receiving Huron River. Finally, a Norwegian study investigated 1416 patients of all age groups by stool detection and concluded that transmission might start not only in childhood, but also in adolescent years, where possible routes of transmission could be outdoor toilet use, private well water, and farm animals.
H. pylori infection is declining worldwide and may even be less than 10% in “westernized” countries. , It is postulated that the infection will die out in due course. Notably, in some parts of the world, infection remains present in 28% to 84% of subjects, depending on the population tested.
The rate of decline seems greatest in developing countries, in contrast to data from developed countries, which are less consistent. Declining rates in Belgium contrast with stable rates in the Netherlands and Portugal, for example. , Studies from Tunisia, Turkey, the Netherlands, rural Alaska, and Israel have reported childhood H. pylori prevalence rates of 51.4%, 30.9%, 1.2%, 86%, and 32.5%, respectively. , , , A more recent study in China of 867 school-aged children revealed a prevalence rate of 24.1%. Akamatsu et al. reported a low prevalence in Japanese adolescents of 4.2%. An Icelandic study showed an infection rate of 3.4% among 205 children. However, the prevalence was 2.6% among children where both parents were born in a low-prevalence country compared to 17% amongst those who had one parent born in a high-prevalence region ( P = .026). Prevalence also differs between different ethnic groups living in the same geographic area, again reflecting differences in socioeconomic status, cultural habits, and family size. Consideration should be given to migrants and ethnic minorities within populations. There is evidence to suggest that the prevalence of H. pylori among migrants is similar to or lower than their country of origin but higher than that of their destination. Also, studies in both China and Vietnam have shown a higher prevalence in their ethnic minority groups.
Tkachenko et al. described a dramatic example in their study of seroprevalence changes in St. Petersburg, Russia. Using two cross-sectional studies in children from 1995 and 2005 (and using the same enzyme-linked immunosorbent assay [ELISA] method for anti- H. pylori immunoglobulin G [IgG]) they showed that 10 years later, the overall prevalence of H. pylori infection had decreased from 44% to 13%. Among children younger than 5 years of age, the prevalence decreased from 30% to 2%.
H. pylori is rapidly acquired early in our life cycle, generally during the early childhood years. Cross-sectional prevalence studies outnumber longitudinal studies examining H. pylori infection acquisition and loss. Many published studies have been of heterogeneous design and population mix and used diverse methods of H. pylori detection, not all of which have been validated for use in pediatric populations. Higher rates of childhood H. pylori infection earlier in the 20th century likely account for the persisting higher disease prevalence with advancing age in developed countries. This “birth cohort effect” has been unmasked by cross-sectional seroprevalence studies from around the world.
Beyond the first decade of life, the annual incidence of newly acquired infection is remarkably low. Rowland et al. described the incidence of infection in 327 Irish children between 2 and 4 years of age using the validated 13 C urea breath test (UBT). Adopting strict criteria, they showed that acquisition of H. pylori infection occurred in early childhood years and that coinfection of household contacts and prolonged use of feeding bottles, but not pacifiers, beyond the age of 2 years were risk factors for infection. Over 4 years of follow-up, 279 index children not infected at baseline contributed 970 person-years of follow-up to the study. During this time, 20 children became infected with H. pylori . The authors did not find evidence of spontaneous infection clearance in their study children. The use of serologic, stool, and urine tests to detect H. pylori in other studies of infection incidence may potentially weaken the data, but the majority conclude that the incidence of H. pylori infection is highest in children younger than 5 years of age.
Many confounding factors that influence the epidemiology of H. pylori infection are difficult to control and must be borne in mind when interpreting published data. Given that H. pylori is acquired in childhood, environmental factors that influence infection most likely exert their greatest influence during childhood. These are among the most difficult factors to control in cross-sectional or retrospective studies.
Spontaneous clearance of H. pylori infection in early childhood has been reported. Clearance of IgG seen in very young infants is more likely to represent the detection and then clearance of maternal antibodies. A low spontaneous eradication rate was noted in the recent Chinese study of children infected with H. pylori of 2.9% at 1 year. However, investigative data suggest that spontaneous elimination of H. pylori in childhood may be influenced by antibiotic use for systemic childhood infections, albeit an incomplete explanation.
The risk of reinfection in the pediatric setting following successful eradication is low in developed countries. Generally, recolonization of the same strain within 12 months of eradication (recrudescence) rather than reinfection (colonization with a new strain, more than 12 months after eradication) is considered responsible for most documented “cases.” Rowland et al. followed 52 children prospectively for a mean of 2 years following successful eradication therapy. Reinfection was identified by UBT; age younger than 5 years was the only risk factor for reinfection identified by logistic regression. Neither socioeconomic status nor number of infected family members influenced reinfection rates.
The risk of reinfection in developing countries is less clear. A meta-analysis of studies involving predominantly adult subjects suggested that reinfection rates might be higher in developing than developed countries. Continuing poor sanitary and water standards, and household density, may account in part for the differential reinfection rates between countries at economic extremes.
In a prospective longitudinal study of 136 children in Vietnam, the risk for recurrence of H. pylori was inversely proportional to age, with the youngest children running the greatest risk. A further study from Bolivia in a high prevalence, low-income population showed a reinfection rate of 20% in children younger than 10 years, 1 year after successful therapy, compared to 8% in older children and adolescents.
These findings lend support to the observation that early childhood may be the main age of acquisition of H. pylori infection and for postponing attempts of eradication in high-prevalence areas unless motivated by medical reasons.
H. pylori is a formidable pathogen. Its natural reservoir is the human population and it is carried and transmitted asymptomatically by the majority of infected hosts. H. pylori infection and disease outcomes are mediated by a complex interplay among bacterial, host, and environmental factors.
Cytokine gene polymorphisms are important host factors that can alter H. pylori disease outcomes. Proinflammatory polymorphisms of the interleukin 1β (IL-1β) gene have been associated with the development of gastritis, predominantly involving the body of the stomach (corpus gastritis), hypochlorhydria, gastric atrophy, and gastric adenocarcinoma, but a reduced risk of duodenal ulceration. In the absence of these polymorphisms, H. pylori gastritis predominantly involves the antrum and is associated with normal to high acid secretion. Polymorphisms of the tumor necrosis factor α (TNFα) and IL-10 genes demonstrate a similar but less-pronounced association with the development of gastric cancer.
Host innate and adaptive immune responses are ordinarily under genetic influence. Harboring of genetic polymorphisms in key immunity-related genes offers an attractive explanation in part for diverse host phenotypic responses to this bacterium within given ethnic populations. Polymorphisms in IL-1B and its receptor have been linked to gastric cancer susceptibility. Individuals with the IL-1B _31∗C or _511∗T and IL-1RN ∗2/∗2 genotypes are shown to be at increased risk of developing hypochlorhydria and gastric atrophy in response to H. pylori infection. , , In children, polymorphisms in the IL-1 receptor antagonist gene, IL-1RN , but not the IL-1B gene cluster, were associated with susceptibility to duodenal ulcer (DU). This polymorphism, along with cytotoxin-associated gene A (CagA) + H. pylori strain infection, is independently associated with DU. Polymorphisms in cytokine genes including TNFα , IL-8 , and IL-10 , which result in a proinflammatory phenotype in the host, have been linked to increased severity of H. pylori disease in children and cancer susceptibility in adulthood. , Michalkiewicz et al. studied the expression of innate immunity components and cytokines in the gastric mucosa and found that INFγ expression correlates with both the density of colonization and lymphocytic infiltration in the gastric mucosa, whereas TNFα expression correlates only with bacterial density. Patients infected with the CagA positive strain had higher levels of IL-6 and IL10mRNA.
Pattern-recognition receptors (PRRs) are germ-line–encoded proteins that detect conserved microbial molecular motifs and danger signals. They include the membrane-associated Toll-like receptor (TLR) family and cytosolic nucleotide-binding oligomerization domain (Nod)-like receptor (NLR) family. Polymorphisms in TLR4, which senses bacterial lipopolysaccharide (LPS), have been linked with susceptibility to H. pylori –induced gastritis and gastric cancer. , However, these findings have not been consistently replicated, albeit in populations of different ethnicity. A study of 486 Brazilian children undergoing gastroscopy for abdominal pain failed to show an association between polymorphisms in TLR2 , TLR4 , and TLR5 and H. pylori infection or duodenal ulceration. However, children with TLR4 polymorphisms were more likely to have infection with CagA+ H. pylori strains and enhanced mucosal IL-8 and IL-10 levels as measured by ELISA. Pellino proteins are emerging as key regulators of immune signaling, including the TLR pathways. Pellino1 and Pellino2 positively regulate TLR2 driven responses to H. pylori LPS, whereas Pellino3 exerted a negative modulatory role. A variation of expression is also seen in gastric tissue; low Pellino3 levels together with high inducible Pellino1 expression may be an important determinant of the degree of inflammation triggered upon TLR2 engagement with H. pylori . Of interest, human leukocyte antigen (HLA) class I and II allele studies have not shown a predisposition to H. pylori infection, although they may affect susceptibility to gastric cancer. Obayashi et al. evaluated the immune responses of H. pylori -infected gastric mucosa obtained from 24 patients (12 children) using microarray real-time (RT)-PCR. Interestingly, OLFM4 was only upregulated in H. pylori –positive adults, while that of PIM2 was equally upregulated in both the affected adults and children.
These limited data suggest that in the genetically susceptible host, failure to attenuate the inflammatory response to H. pylori infection locally leads to chronic inflammation and reduced acidity. This milieu may favor subsequent colonization with non– H. pylori species and/or production of genotoxic effects. It is likely that in this era of genome-wide association studies, more elaborate research methodologies may uncover more clues to the influence of host genotype on H. pylori infection and disease.
The seemingly robust, complex acute host immune response to H. pylori infection is surprisingly ineffective at clearing this bacterium and preventing H. pylori from establishing a lifelong bacterial niche. Host and bacterial factors are clearly at play in moderating infection and disease spectrum and severity.
Antral gastritis is associated with increased stimulated acid production and predisposes to duodenal ulceration, whereas corpus-predominant or pangastritis is associated with reduced acid production and predisposes to gastric ulcer and gastric adenocarcinoma. The degree of gastric infiltration by neutrophils also correlates with the development of gastroduodenal ulcerations, and this is in part dependent on the release of damaging inflammatory mediators such as reactive oxygen species (ROS).
H. pylori is a predominantly extracellular organism that generally adheres to the gastric mucosal surface. Gastric epithelial cells, and to a lesser extent, intraepithelial myeloid cells, affect the first host responses to infection. TLRs and NLRs constitute the majority of our innate receptor arsenal. Cell-surface TLRs, including TLR2, TLR4, and TLR5, do not seem to mediate significant initial immune responses to H. pylori . Indeed, H. pylori LPS is less potent than that of Salmonella spp. or Escherichia coli , and modification of H. pylori LPS facilitates immune evasion. ,
Given that H. pylori is primarily an extracellular pathogen, it may seem intriguing that it engages NLRs at all. This interaction is in part mediated by its gene-encoded type IV secretion system (TFSS), a molecular syringe that facilitates translocation of bacterial effector proteins and products into cells. , H. pylori peptidoglycan is translocated into host cells where it engages with its receptor, NOD1. Downstream effects of NOD1 activation include instigation of NF-кB–mediated inflammatory cascades and subsequent IL-8 production.
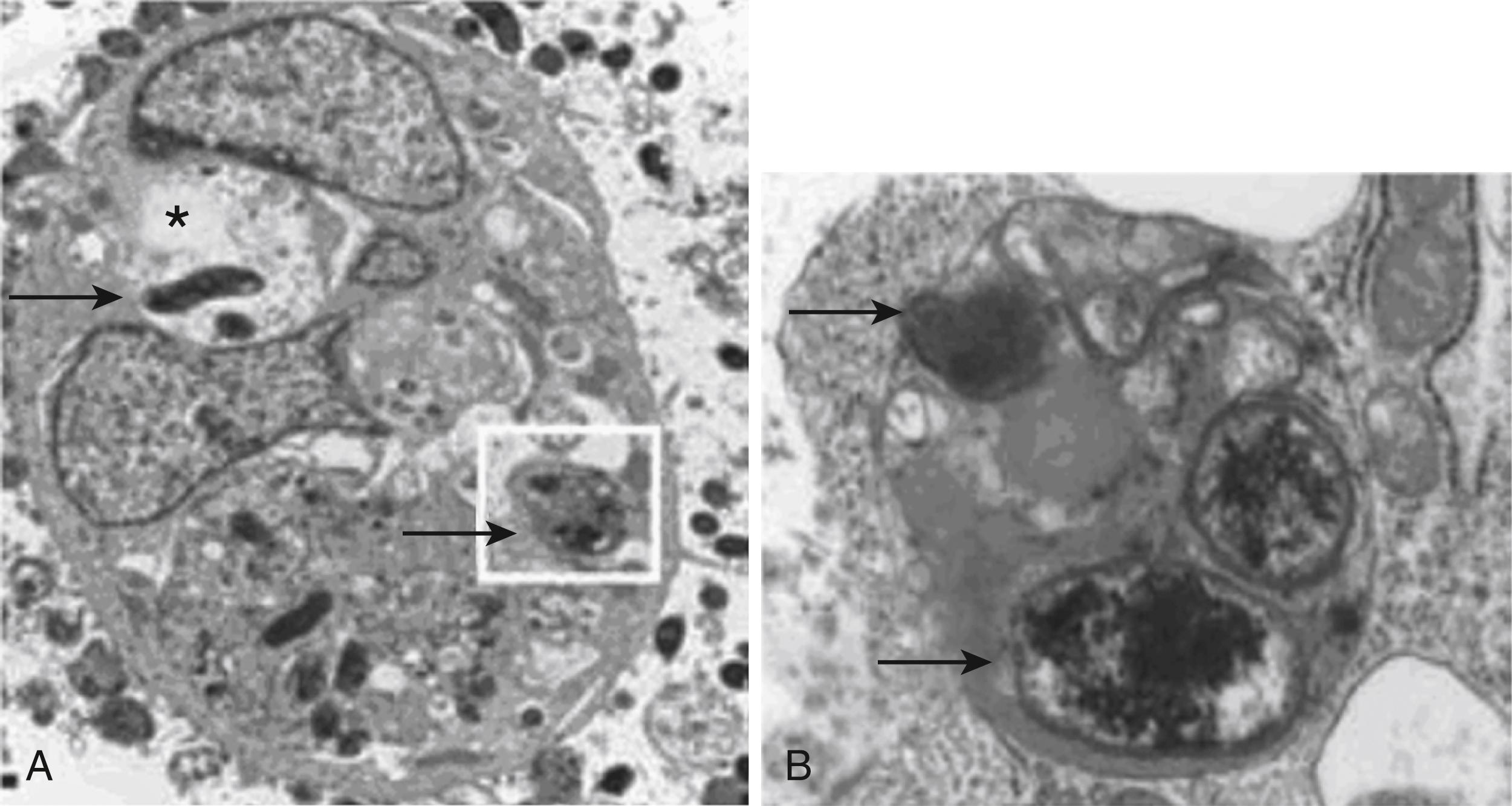
Autophagy is an evolutionarily conserved process that results in the sequestration of cytosolic components within autophagosomes that then fuse with lysosomes, resulting in the degradation of the lysosomal contents ( Fig. 27.1 ).

Autophagy is induced in host cells in response to vacuolating cytotoxin A (VacA) to mitigate the effects of the toxin. However, persistence of VacA induces the formation of defective autophagosomes with attenuated ability to eliminate bacteria and potentially genotoxic material. In addition, a polymorphism in the autophagy gene, ATG16LI , results in both inefficient induction of autophagy in response to the toxin and increased susceptibility to infection in humans. Together these findings provide a novel mechanism for chronic host H. pylori infection and have important implications for H. pylori– mediated infection, inflammation, and carcinogenesis ( Fig. 27.2A and B ). Tanaka et al. performed a microarray analysis of 266 autophagy-related genes (ATG) showing 16 upregulated genes and 9 down-regulated genes in H. pylori –infected gastric mucosa. ATG16L1 mRNA levels were inversely correlated with H. pylori density as well as gastric atrophy in the mucosa. A further finding regarding MAP1LC3A (major regulator of autophagosome formation) shows that inactivation of this regulator disrupts the autophagy pathway and may contribute to carcinogenesis in gastric epithelial cells. There have been recent data to suggest the mammalian target of rapamycin complex 1 (mTORC1) plays a role in this autophagy process. VacA perturbations in mitochondria are linked to alteration in cellular amino acid homeostasis, which results in the inhibition of mTORC1 and subsequent autophagy.
H. pylori induces chronic gastritis in almost all hosts. Initial neutrophil recruitment gives way to mononuclear cell infiltrates with lymphocytes and macrophages and the attendant epithelial cell damage. Following H. pylori infection, enhanced levels of IL-1β, IL-2, IL-6, IL-8, and TNFα are detected in the gastric epithelium. Phagocytosis of H. pylori by macrophages results in marked IL-6 production. The precise role of TLRs in macrophage chemokine and cytokine responses to H. pylori remains unclear. As effector cells, macrophages generate nitric oxide (NO), catalyzed by the enzyme inducible NO synthase (iNOS), which is upregulated in macrophages following H. pylori infection in vitro. Through its arginase enzyme, encoded by the gene rocF , H. pylori competes with the eukaryotic macrophage for the iNOS substrate L-arginine (L-Arg) to enhance its survival. The enzyme generates urea from L-Arg, which is then utilized by urease to synthesize ammonia and neutralize the gastric luminal hydrogen chloride (HCl). Reduced macrophage NO generation therefore confers an immune evasion advantage.
Mucosal dendritic cells (DCs) are an important component of our antigen-presenting cell repertoire. They express an array of innate immune receptors, and their cytokine responses influence the differentiation of T helper (Th) cells. , Transepithelial and lamina propria DCs identified in patients with chronic gastritis respond to both live bacteria and H. pylori antigens, releasing varying quantities of IL-6, IL-8, IL-10, and IL-12. In contrast to macrophages, TLR and myeloid differentiation factor (MyD88) expression in DCs is not dispensable for complete H. pylori recognition and DC activation. Endosomal TLRs that recognize microbial nucleic acids were identified as important components of DC responses to H. pylori . C-type lectin receptors (CLRs) expressed by DCs are crucial for directing immune responses to pathogens. DC-specific intercellular adhesion molecule 3 (ICAM3)–grabbing non-integrin (DC-SIGN) recognizes H. pylori fructose residues, and following pathogen binding, it triggers the expression of specific cytokines that influence T-cell differentiation. DC-SIGN binding of H. pylor i has been shown to result in enhanced IL-10 and reduced IL-12 and IL-6 expression, which could subsequently favor type 2 Th (Th2) cell polarization. A recent study by Rizzuti et al. suggests that H. pylori infection induces the activation of the signal transducer and activator of transcription 3 (STAT3) signaling pathway in dendritic cells. Activation of STAT3 blunts dendritic cell maturation and induces the production of a tolerogenic dendritic cell phenotype, once again illustrating a novel mechanism whereby H. pylori alters the host immune response to facilitate its persistence.
H. pylori also induces a vigorous mucosal humoral response that does not lead to eradication but does contribute to tissue damage. Infiltrating B lymphocytes and plasma cells give rise to H. pylori –specific IgA and IgG antibodies. CD4 + T cells help B lymphocytes to produce antibodies and also contribute to inflammation in the gastric mucosa by producing high amounts of interferon γ (IFNγ). H. pylori –specific IgG or IgA antibodies can be detected in peripheral blood from early stages of infection. The IgG and IgA responses to H. pylori in the serum and gastric mucosa have been well described and may be involved in protective immunity, but these B cell–mediated antibody responses could also be counterproductive. The development of gastric mucosa–associated lymphoid tissue (MALT) lymphoma stems from activated B cells. Chronic gastric infection with H. pylori protected splenic B cells from apoptosis, indicating a B-cell activation/survival phenotype that may have implications for MALT lymphoma. In addition to producing antigen-specific antibodies, B cells can also produce potentially harmful autoantibodies. The implications of T-cell–B-cell interactions in the pathology of the immune response remain to be fully explained.
A variety of T-cell responses have been characterized, in both the gastric mucosa and the periphery. Immature Th cells can differentiate into Th1, Th2, and Th17 functional subtypes. The data on H. pylori –induced effects on T-cell differentiation, both locally and systemically, have been difficult to reconcile. In the human gastric mucosa, H. pylori induces recruitment of CD4 + and CD8 + T cells, and murine studies have shown that the gastric inflammation is T-cell dependent, as experimentally, H. pylori does not induce gastritis in T-cell–deficient mice. , H. pylori –specific T cells with a Th1 phenotype (i.e., secreting IFNγ) have been cloned from H. pylori –infected gastric mucosa and, through the production of IFNγ, were cytotoxic to gastric epithelial cells. Conversely, it has been suggested that activation of a Th2 cell response, and production of Th2 cell cytokines like IL-4, are protective against severe pathology and curb potentially detrimental effects of Th1-related cytokines.
A significant expansion of γδ-TCR+ T lymphocytes and high concentrations of IL-10 have been observed in the peripheral blood of H. pylori –infected subjects in comparison to healthy controls. No differences were detected between infected and noninfected subjects with regard to the frequencies of CD3+, CD19+, CD4 + , and CD8 + T cells and their subsets. A mixed T-cell response, although favoring a Th2 cell profile, was reported in a study of gastric T cells from subjects with and without H. pylori infection. Using flow cytometry and RT-PCR, mixed H. pylori –specific T regulatory (T reg ) and Th cell subsets with a predominant CD4 + IL-10 + response were found in the gastric antrum. This more “tolerant” response could in part explain incomplete clearance of H. pylori and chronicity of infection. Previous investigations had reported predominant Th1 cell responses to H. pylori , which could be partly explained by methodologic differences. , , Of interest, patients in this study with ulceration had reduced T reg and increased Th1 and Th2 cell response profiles compared to those without ulceration.
A specific subset of CD4 + T cells termed Th17 cells, which are distinct from and antagonized by the classical Th1 or Th2 cells, has been described. Th17 cells produce IL-17 and play a prominent role in the development of chronic inflammation associated with inflammatory and autoimmune disorders. T-cell production of IL-17 stimulates the production of IL-1, IL-6, TNFα, and matrix metalloproteinases by fibroblasts, endothelial cells, epithelial cells, and macrophages. IL-17 upregulation occurs at both RNA and protein levels in H. pylori infection. IL-17 activates extracellular signal-related kinase 1 and 2/mitogen-activated protein (ERK1/2 MAP) in gastric epithelial cells, and IL-17 expression levels correlate with the IL-8 content and number of infiltrating neutrophils in H. pylori –infected mucosa. Furthermore, IL-23 is overexpressed in H. pylori –infected gastric mucosa, which could contribute to sustaining IL-17 production. The exact molecular mechanism by which IL-23 regulates IL-17 in H. pylori –infected mucosa remains to be ascertained, but the STAT3 likely plays a key role in IL-23–driven IL-17 production during H. pylori infection. A comparison of gastric levels of Th17- and T reg -associated cytokines between children and adults showed that in children, T reg -cell differentiation was more predominant and might help to explain the increased susceptibility of pediatric patients to infection.
H. pylori induces apoptosis both in vitro and in vivo by several mechanisms. H. pylori or its products can induce apoptosis directly. For example, VacA induces the release of cytochrome c from mitochondria. , Alternatively, the bacterium induces host immune responses, which then mediate apoptosis. For instance, Th1 cell cytokines (TNFα and IFNγ) markedly potentiate H. pylori –induced epithelial cell apoptosis. , H. pylori also upregulates expression of the Fas death receptor. , The absence of Fas signaling has been associated with less apoptosis and enhanced premalignant gastric mucosal changes. ,
Expression of gastric mucins has been proposed as a biomarker for intestinal metaplasia or prognostic factor for gastric cancer in adults. Park et al. examined the expression of gastric mucins in both infected and noninfected children from Korea. Increased mucin (MUC) 2, MUC5AC, and MUC6 were noted in infected adults with intestinal metaplasia. Of note, gastric expression with MUC5AC was decreased in children with H. pylori aged >5 years, while MUC2 and MUC6 were not affected by H. pylori status.
H. pylori infection–induced DNA damage in epithelial cells is a major contributing factor to the development of gastric cancer. Telomere shortening in genomic DNA reflects an accumulation of oxidative stress and chromosomal instability as a result of lifelong infection. Tahara et al. showed that H. pylori –infected gastric cancer tissues and the adjacent noncancerous tissues possessed shorter telomere length than H. pylori –negative gastric tissues and that the shortening of length was associated with higher expression of IL-1B and NF-kB.
MicroRNAs (miRNAs) are post-transcriptional regulators of gene expression playing a role in development, cell proliferation, and oncogenesis. Wang et al. examined 53 different miRNAs; the expression of miR-143-3p was the most significantly upregulated in H. pylori –positive gastric cancer tissues. Murray-Stewart et al. reported that miR-124 has a protective role through the inhibition of spermine oxidase (SMOX)-mediated DNA damage in H. pylori –positive gastric cancer.
The mechanisms of H. pylori –related carcinogenesis are unclear and are likely the result of both bacterial and host factors and environmental factors such as smoking, high-salt diet, and antioxidant ingestion. H. pylori disrupts the DNA mismatch repair system (DMRS). , , By leading to gastric atrophy, H. pylori may be permitting its own replacement by more genotoxic bacteria.
To promote chronic infection, H. pylori has developed an array of mechanisms to survive in the harsh acidic environment of the gastric mucosa. One of these is an “acid acclimation mechanism” that promotes adjustment of periplasmic pH in the acidic environment of the stomach by regulating activity of urease and urea influx through UreI and α-carbonic anhydrase. Previous studies indicated that the sensor histidine kinase (ArsS) two-component system regulated transcription of urease. A study using ArsS mutants extended these findings to demonstrate that ArsS also mediated trafficking of urease to the inner membrane upon acute but not prolonged acid exposure, signaling another method by which the ArsS two-component system regulates acid acclimation. H. pylori infection can cause hypergastrinemia by both reducing D-cell somatostatin production and increasing G-cell gastrin production; removal of H. pylori reverses these effects. However, the ultimate effect of infection on acid homeostasis depends on the topographic distribution of H. pylori –induced inflammation within the stomach. In antral-predominant gastritis, gastrin release leads to higher acid levels, and persistently high gastrin levels increase the parietal cell mass. This in turn results in increased acid delivery to the duodenum, inducing gastric metaplasia. H. pylori can colonize gastric metaplasia, resulting in inflammation and, possibly, ulceration.
With pangastritis or corpus-predominant gastritis, H. pylori infection suppresses acid production, both directly and indirectly. Inflammatory mediators inhibit parietal cell acid secretion and enterochromaffin-like cell histamine production. , Reduced acid secretion further increases gastrin levels, thereby promoting gastric epithelial cell proliferation. Epithelial cell characteristics become altered, leading to progressive gastric gland loss, and thus gastric atrophy. Gastric atrophy increases the risk of gastric ulceration and noncardia gastric adenocarcinoma.
H. pylori infection can affect the expression of the appetite- and satiety-controlling hormones leptin and ghrelin. In children, an inverse relationship between serum ghrelin concentration and the severity of H. pylori –induced gastritis has been demonstrated. H. pylori infection leads to a reduction of the density of gastric ghrelin–positive cells. The decrease in ghrelin is associated with neutrophil activity, chronic inflammation, glandular atrophy, and low serum pepsinogen level. H. pylori infection decreases gastric D cells that produce somatostatin, and the subsequent loss of gastric G-cell inhibition is a mechanism for hypergastrinemia in H. pylori –infected individuals. H. pylori eradication has been shown to lead to an increase in body weight. The underlying mechanism has been suggested to be of gastric hormonal origin, with both ghrelin and leptin contributing to this effect. Although the prevalence of H. pylori infection was previously shown to be significantly higher in lean rather than obese patients, there is neither a sound scientific basis nor robust data to support the hypothesis that H. pylori is a protective factor against obesity. ,
The H. pylori genome (1.65 million base pairs [bp]) codes for more than 1500 proteins, including enzymes that modify the antigenic structure of surface molecules, control the entry of foreign DNA into the bacterium, and influence bacterial motility. , These factors are essential for H. pylori to colonize humans effectively. More than 100 bacterial genes are required for gastric colonization, and their expression can be upregulated within the stomach. , H. pylori lacks a genetic mismatch repair system to control the confidentiality of replication, which results in a high mutation rate. Genetic diversity of H. pylori has likely resulted from endogenous mutations and recombination. Many H. pylori isolates have a hypermutator phenotype, which favors the emergence of variants after selective pressure; the rapid development of high-level resistance to commonly used antibiotics such as clarithromycin is one such example. H. pylori are also highly competent for uptake of DNA; thus the H. pylori genome continuously changes during chronic colonization by acquiring fragments of DNA from other H. pylori strains. In essence, each host is colonized not by a single clone, but by a variety of usually closely related organisms.
After ingestion, H. pylori colonizes the gastric mucous layer and can adhere to and invade gastric epithelial cells. Gastric acidity, motility, nutrient availability, and host immune responses are but some of the barriers to colonization. H. pylori has adapted remarkably to many of these barriers. Motility of H. pylori depends on the presence of up to six functional unipolar flagella. Studies indicate that proper assembly of flagella requires peptidoglycan-degrading enzymes that promote the correct localization and function of the flagella motor. The ferric uptake regulator, Fur, which regulates iron homeostasis, acid acclimation, and oxidative response, has been shown to be important for H. pylori colonization. Recently, Lee et al. reported that Fur positively regulates the flagellar motor switch in the H. pylori strain J99. H. pylori regulates cell motility by responding to chemotactic cues, which then alter flagellar activity. Chemotactic (Che-) mutants have altered colonization patterns . H. pylori senses environmental chemical cues via four chemoreceptors: Tlp A, B, C, and D. Using isogenic chemoreceptor mutants, Rolig et al. demonstrated that T1pD is necessary for H. pylori to survive and grow in the infected and inflamed antrum but not elsewhere in the murine stomach. Huang et al. reported a new and independent role for two chemoreceptors as acid sensors. By videomicroscopy techniques, they showed that H. pylori mutants lacking TIpB are able to detect and swim away from an acid gradient. H. pylori can hydrolyze urea to generate ammonia and modify the local pH. Its urease activity is governed by a unique pH-gated urea channel. H. pylori urease binds substrate with a much higher affinity than that of other bacterial species. Nickel is essential for gastric colonization by H. pylori. Fischer et al. identified a novel H. pylori nickel transport system NiuBDE, which is required for nickel-dependent urease activation and acid survival for gastric colonization.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here