Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Heart failure (HF) is the clinical syndrome defined by signs and symptoms of elevated intracardiac pressures or depressed cardiac output, which in turn are due to either functional or structural cardiovascular (CV) abnormalities. By definition, HF presentations managed in the emergency department (ED) are acute heart failure (AHF) (ie, HF in which the signs and symptoms require unscheduled care).
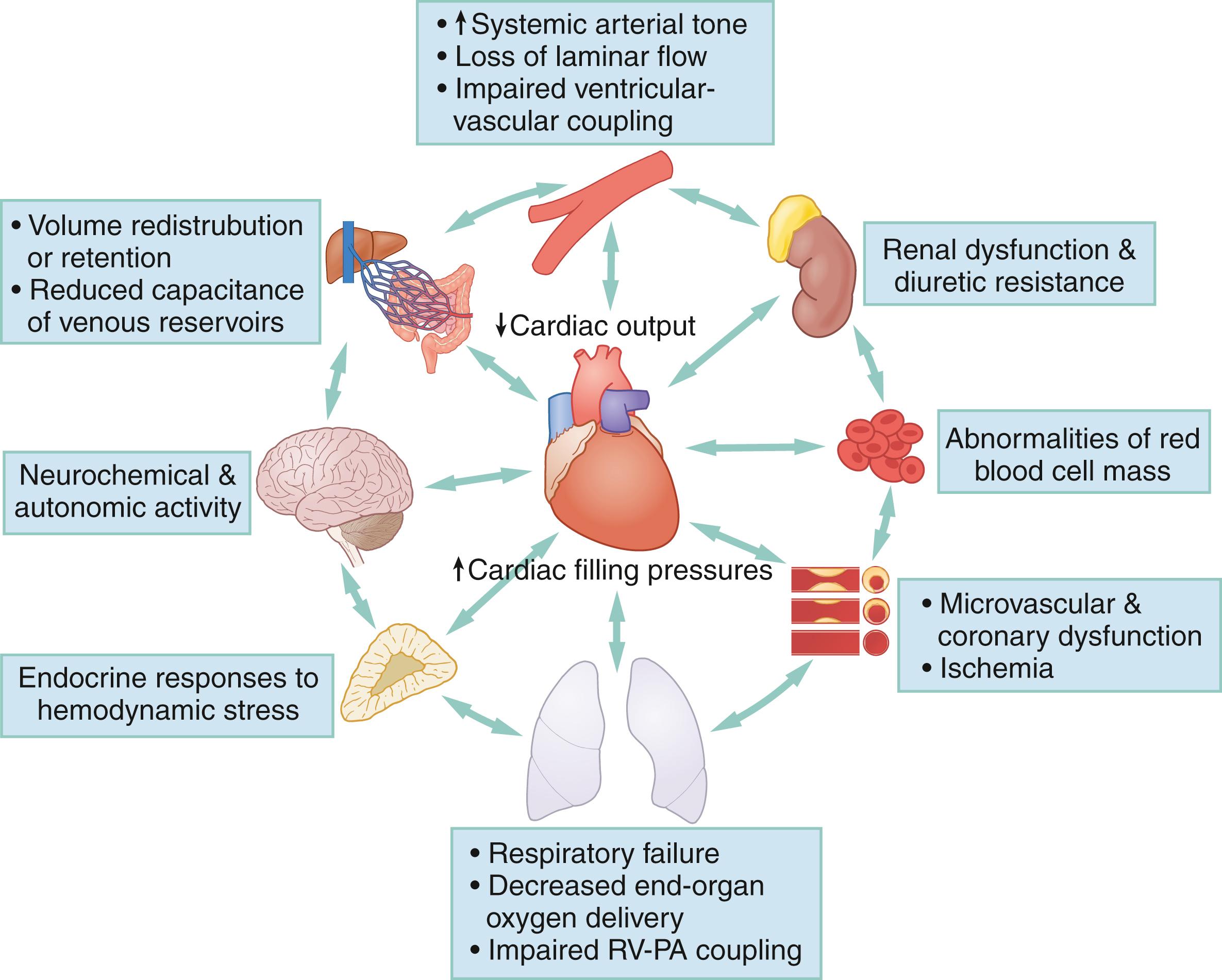
AHF is a multiorgan, multifactorial, and multiple phenotype disease state. Abnormalities in renal function, central venous volume and fluid shifts, arterial vascular tone, neuroendocrine overactivity, microvascular dysfunction, respiratory failure, or myocardial ischemia have bidirectional relationships with AHF as both potential causes and effects.
Central congestion without hypervolemia is typically due to intercompartmental fluid shifts to the central circulation from venous reservoirs with reduced capacitance (increasing preload) or abrupt increases in central arterial tone (ie, increasing afterload).
Emergency physician (EP) clinical gestalt based on history and physical exam is inaccurate for AHF diagnosis as often as a quarter of the time.
The most useful diagnostic test for identifying lung congestion due to AHF is an 8-point lung ultrasound B-line scan, which is more sensitive than chest x-ray for pulmonary edema, and has a higher positive predictive value for AHF than natriuretic peptides.
Focused cardiac ultrasound (FOCUS) for detecting reduced ejection fraction (EF) misses the 40% to 50% of AHF presentations with preserved EF. It adds little information to risk stratification because EF does not typically change with dynamic worsening of cardiac function or improvement after treatment.
A useful clinical classification of AHF distinguishes between a vascular phenotype and a cardiac phenotype, though overlap can exist. Distinguishing between systolic and diastolic dysfunction is less helpful in the ED, because EF is preserved in about half of AHF cases, and most AHF patients have both systolic and diastolic dysfunction regardless of their EF.
The “vascular phenotype” describes an AHF presentation where functional (ie, reversible) CV abnormalities such as increased vascular tone and fluid shifts predominate over structural (ie, irreversible) ones. These patients tend to be hypertensive, less likely to be hypervolemic, respond favorably to intravenous (IV) nitroglycerin, and have a better prognosis despite more abrupt symptom onset.
The “cardiac phenotype” of AHF involves a predominance of structural (ie, irreversible) CV abnormalities such as more severe chronic myocardial disease, myocardial ischemia, and more complex multiorgan interactions (eg, cardiorenal syndrome). These patients are often hypervolemic and require high-dose IV diuresis. They typically present with a more indolent symptomatic progression, but paradoxically are at higher risk for worse outcomes.
Noninvasive positive-pressure ventilation is the first-line approach for respiratory distress in AHF and may obviate a need for intubation in most cases.
The initial dose of furosemide in the ED for AHF is 1 to 2.5 times the patient’s total daily oral dose (or 40–80 mg if loop-diuretic naïve) and should be given IV. Although creatinine may rise after IV furosemide, it is rarely indicative of iatrogenic acute kidney injury, and patients with acute cardiorenal syndromes typically benefit from aggressive diuresis.
Inotropes are only indicated in cases of cardiogenic shock (CS), because they may increase mortality as a pharmacologic class.
Resuscitation and stabilization of CS should be followed by rapid evaluation from interjectional cardiology or cardiac surgery. While STEMI was traditionally the predominant cause of CS, it may only account for 30% of contemporary cases due to improvements in coronary intervention.
Numerous high-moderate risk features in AHF define which patients likely require admission or observation, however, low-risk factors (ie, for discharge) are less well defined. When high or moderate risk factors are absent, the patient is adherent to the appropriate guideline-directed medical therapy, and can secure close outpatient follow-up (≤1 week), a shared decision-making discussion regarding discharge from the ED may be appropriate.
Heart failure (HF) is a clinical syndrome defined by three components ( Fig. 67.1 ) :
Structural or functional cardiovascular abnormalities
Elevated intracardiac pressures or depressed cardiac output (CO) due to these abnormalities
Clinically recognizable signs or symptoms (eg, dyspnea, edema, fatigue, exertional intolerance, or others) due to the elevated intracardiac pressures or depressed cardiac output
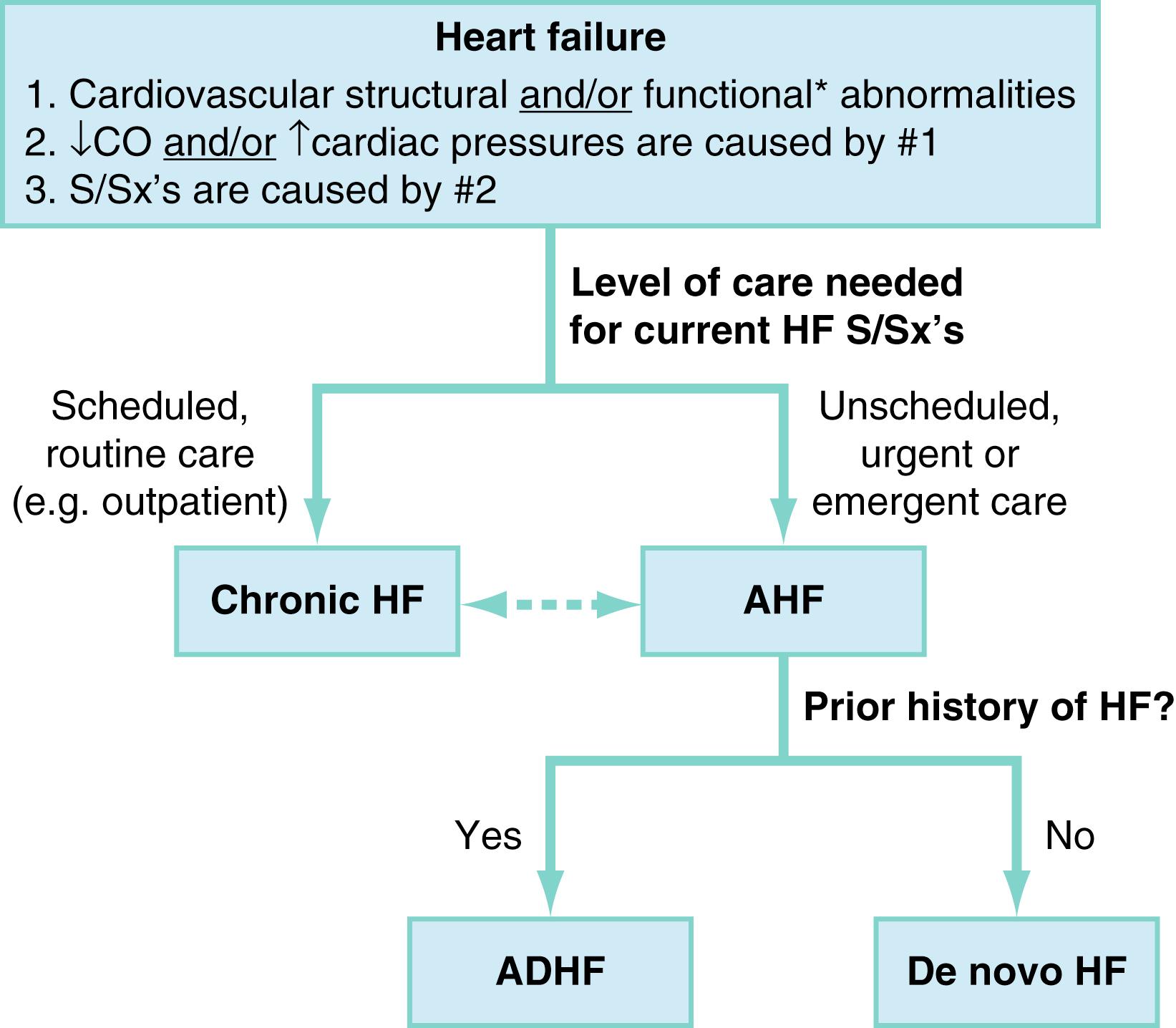
Management of clinical manifestations of HF with scheduled care is classified as chronic HF. Conversely, HF symptoms that require unscheduled care define the presentation of acute HF (AHF). Thus, when HF is managed and treated in the ED, it is, by definition nearly always AHF. Some exceptions exist (eg, a stable chronic HF patient presenting to the ED for a medication refill), however, these are relatively few, and AHF is therefore the primary focus of this chapter.
AHF may further be subdivided as decompensated chronic HF versus “de novo HF.” , The former describes AHF occurring in a patient with known chronic HF history, while in the latter the AHF episode is the patient’s first known clinical presentation of HF. De novo AHF accounted for over 50% of presentations in a large AHF registry study of Asia and Europe, but is a less common ED presentation in North America. Epidemiologic registry data from 2005 to 2014 suggest that around 28% of ED AHF presentations in the United States (US) are de novo .
It is important to note that many HF terms commonly used in the past are now considered outdated, inaccurate, or clinically misleading. “Congestive heart failure,” for instance, is no longer favored given that volume profiles in HF are heterogeneous and include many patients with normal or even hypovolemic total body water profiles. Also, the terms “systolic HF” and “diastolic HF” are no longer used to describe patient phenotypes. The following, more accurate terms are preferred: “HF with reduced ejection fraction” when the ejection fraction (EF) is less than 40%; “HF with midrange EF” when the EF is 40% to 50%, and “HF with preserved EF” when EF is greater than 50% (HFrEF, HFmrEF, and HFpEF, respectively). The terms “systolic dysfunction” (ie, decreased inotropy) and “diastolic dysfunction” (ie, impaired relaxation) still apply as physiologic concepts but are often overlapping and do not describe clinical phenotypes that warrant particular approaches to management. Combining terms to summarize a patient’s presentation, such as “acutely decompensated chronic HF with reduced EF,” is useful.
Chronic HF is highly prevalent (≈2.5% of the US population) and is strongly associated with common cardiovascular risk factors. Onset increases sharply with advancing age, such that, by age 65, the annual incidence of new chronic HF diagnosis approaches 2.1%. Other risk factors observed in population-based samples include obesity, hypertension, diabetes mellitus (DM), tobacco smoking, hyperlipidemia, low socioeconomic status, and ischemic heart disease.
Nearly 1 million admissions for AHF occur each year in the United States, and more than 80% of these originate from the ED with a nearly 50/50 split between HFpEF and HFrEF. Outcomes are poor with 30-day and 1-year mortality of 10% and 30%, respectively, after an AHF episode, and appear to be similar regardless of EF. After hospital discharge, roughly 20% of AHF patients will be readmitted within 30 days.
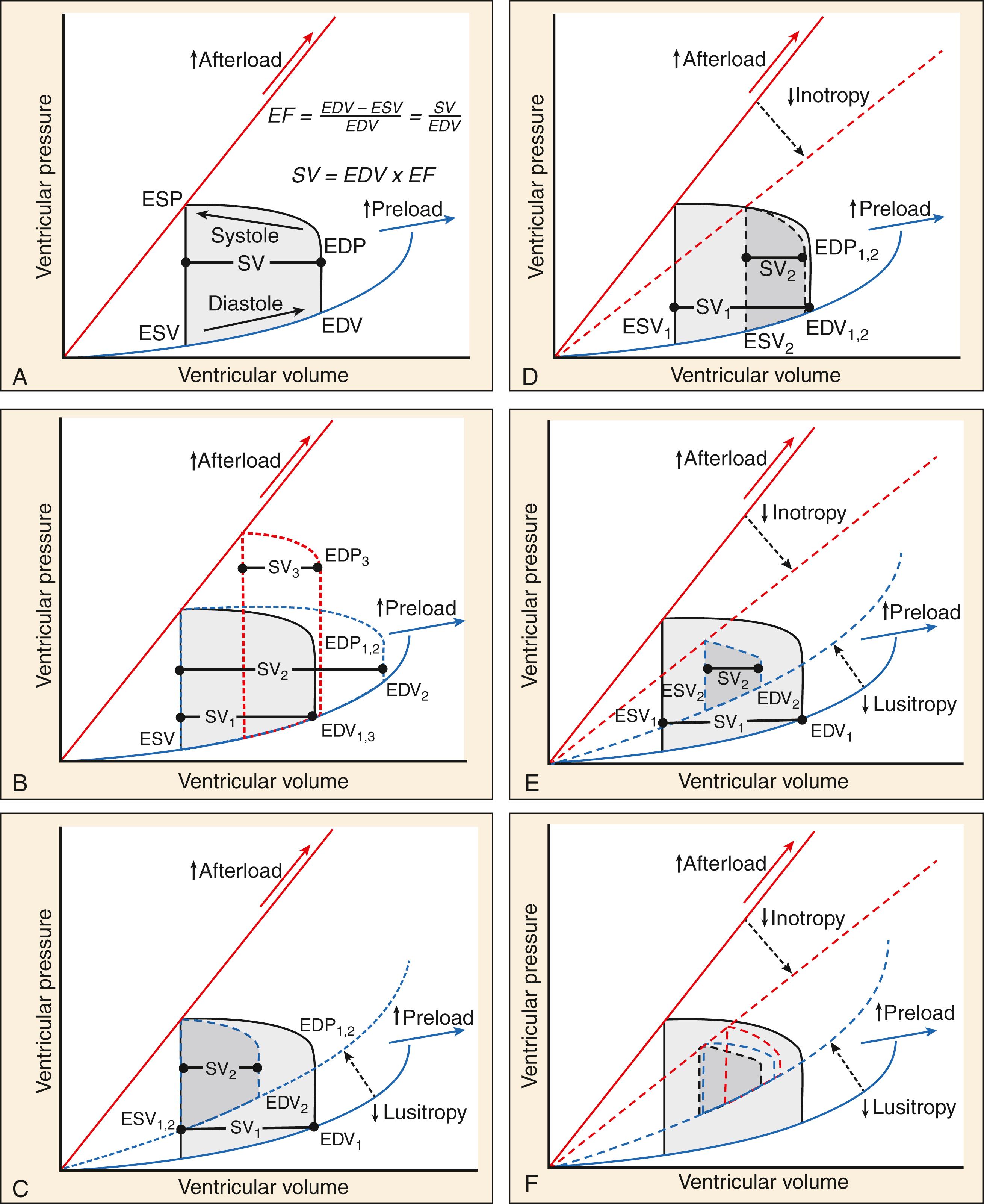
The cardiac cycle ( Fig. 67.2A ) and its relationship to CO is often described by three physiologic parameters: chronotropy, inotropy, and lusitropy. CO is the mathematical product of heart rate (HR; ie, chronotropy) and the stroke volume (SV). SV is itself the product of end diastolic volume (EDV; ie, lusitropy) and EF (ie, inotropy). Thus, mathematically
and conceptually
CO=Chronotropy × Lusitropy × Inotropy.
Recall that the second criterion of the HF definition requires that CO be reduced or intracardiac cardiac pressures be increased; however, these two defining hemodynamic insults tend to coexist and are distinctly related.
Lusitropy and inotropy ( Fig. 67.2 C–E) may be chronically depressed due to underlying chronic structural or functional cardiac changes, as in the setting of ischemic heart disease or hypertensive heart disease, or acutely impaired by a new hemodynamic stressor such as hypertensive emergency, infection, volume redistribution or overload. As shown in Figure 67.3 , AHF is also an inherently multiorgan process, with bidirectional feedback between cardiac dysfunction, vascular tone, and multiple organ involvement.
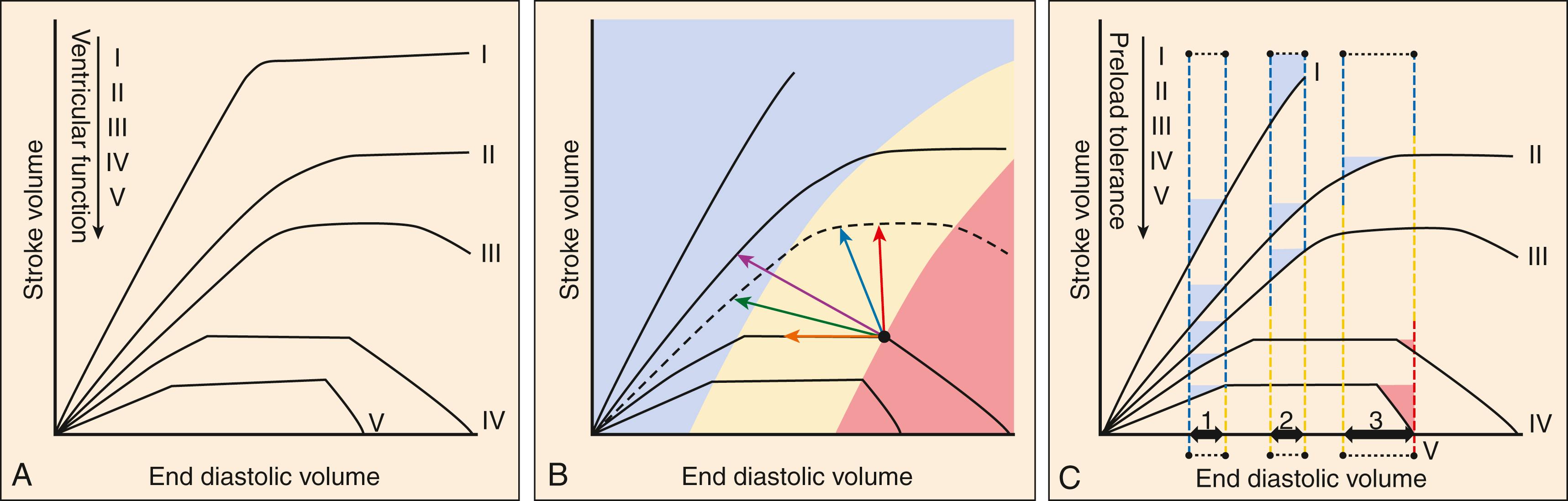
SV is classically modeled by the Frank-Starling mechanism, which describes a relationship between stroke volume and EDV ( Fig. 67.4A ). In the idealized scenario, increasing EDV leads to increasing SV because stretching myocardial sarcomeres further apart in diastole allows them a further distance to contract during systole, increasing inotropy. This model describes the preload-SV relationship well under conditions where EDV is far below the heart’s maximum capacity. Eventually, an inflection point is reached where sarcomeric stretch is maximized and increases in inotropy level-off despite further increases in EDV, noted as the flat portion of a Starling curve. If EDV increases even further, sarcomeres are stretched into abnormal configurations that inhibit inotropy and produce systolic dysfunction. Even brief and transient exposure to excessive left ventricular (LV) EDV can trigger a cascade of stretch-related insults to myocardial function, including subendocardial ischemia with cardiomyocyte injury and necrosis with associated cardiac troponin [cTn] release. Further or sustained stretch results in myocardial stunning and sarcomere proteomic remodeling, culminating in clinical deterioration that manifests as AHF. , , ,
Repeated exposures to increased LVEDV (and resultant LVEDP) cause fibrosis and myocardial hypertrophy that ultimately lead to a stiff, noncompliant ventricle. The net effect is an impairment of lusitropy (ie, diastolic dysfunction) that serves to limit the degree of myocardial stretch during future elevations protecting the myocardium from further stretch-induced myocyte death. However, this comes with a tradeoff, because the ensuing diastolic dysfunction prevents accommodation of increased LVEDV, contributing to onset of symptomatic HF. In the early stages, a certain degree of diastolic dysfunction may be subclinical, or only induced upon stress with exercise, acute hypertension, or hypoxia. , As diastolic dysfunction progresses, demand from activities of daily living may be superimposed on diminishing cardiac reserve associated with other physiologic changes, including decreased myocardial oxygen reserve, abnormalities in nitric oxide signaling, decreased aortic and pulmonary artery compliance, decreased ventricular volume, right ventricular dysfunction, and worsening interventricular and ventricular-circulatory coupling, resulting in depressed tolerance of increased preload and afterload. ,
Depressed SV resulting from acute diastolic or systolic dysfunction can be theoretically compensated for by increased chronotropy. However, the time between systolic cycling sets a “hard stop” on the upper limit of cardiac filling time and, at a certain point, further augmentation of CO through increased chronotropy becomes limited by diminished lusitropy ( Fig. 67.5 ). Thus while increasing heart rate has early adaptive benefits, persistent sinus tachycardia in the setting of AHF is generally an ominous sign that CO has been stressed to its limit. This also explains why increased or decreased chronotropy (as in tachy- or brady-arrhythmias) is a relatively common precipitant of AHF.
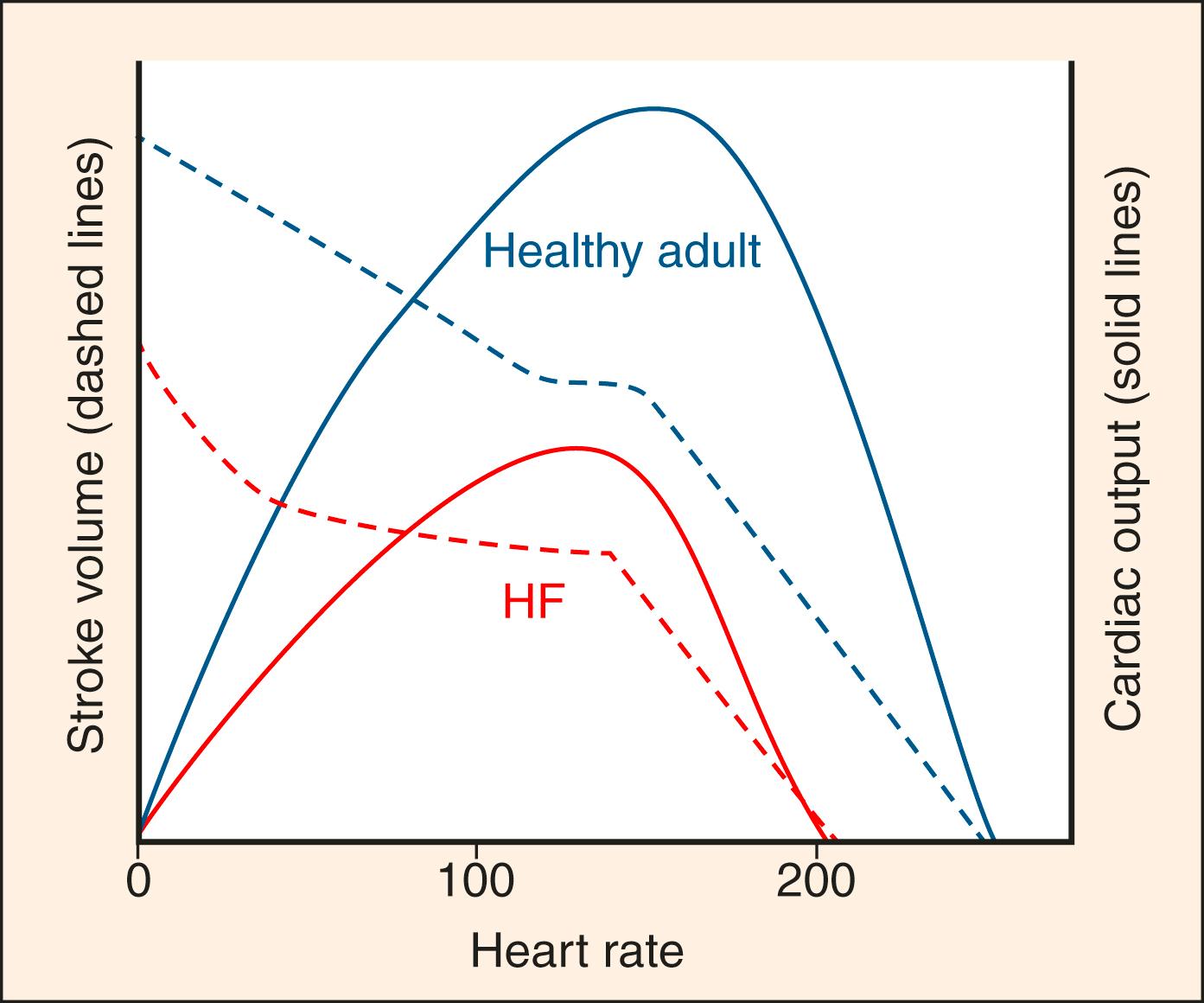
Patients with chronic HF (eg, curves IV and V in Fig. 67.4 ) tend to stay near or beyond the inflection point of the Starling curve (red and yellow zones Fig. 67.4 ) because of their chronically impaired diastolic and systolic dysfunction. Even subtle increases in loading parameters (rightward shifts on the curves) can lead to fulminant AHF. Additionally, with relatively normal baseline cardiac function (curve III Fig. 67.4 , curves I–II Fig. 67.6A ), extremes that impede forward flow (eg, hypertensive emergency) can precipitate AHF through a large rightward shift on the curve. Multiple neurohormonal effects result from and contribute to loading conditions and filling pressures. , Natriuretic peptides (NPs) are especially important in this regard and are upregulated in HF where they result in natriuresis and diuresis, vasodilation, and antifibrotic effects that can reverse remodel the heart. Produced directly by the heart, atrial-NP (ANP), B-type NP (BNP), and the N-terminal fragment of BNP’s prohormone (NT-proBNP) are clinically useful for diagnosis and prognosis of HF.
Elevated intracardiac pressures are a defining characteristic in AHF (see Fig. 67.1 ), and even brief episodes can impair the normal cardiac cycle and lead to irreversible systolic or diastolic dysfunction. , Thus, episodes of AHF negatively impact the long-term prognosis of patients with chronic HF. This is of particular relevance to ED management, with a physiologic justification for a “time-to-treatment” concept in AHF, similar to many other time-sensitive conditions seen in emergency medicine. Given the potential benefit of limiting myocardial damage through earlier normalization of intracardiac pressures, organizations such as the Heart Failure Society of America (HFSA), the Society for Academic Emergency Medicine (SAEM), the American Heart Association (AHA), and the American College of Cardiology (ACC) have all recommend that AHF treatment be started as quickly as possible after ED arrival. Broadly speaking, the major classes of ED AHF therapies work by targeting the physiology underlying elevated intracardiac pressures either by moving the Starling curve leftward with diuretics or venodilators, moving the curve upward to a higher level of efficiency for a given EDV with inotropes or arterial vasodilators, or both. A simple but useful hemodynamic classification distinguishes between cardiac (ie, primary pump failure predominates) and vascular (ie, acutely increased preload or afterload predominates) phenotypes of AHF. The ED management differs depending on which phenotype predominates.
Congestion in AHF is very specifically the congestion of the central vasculature (ie, the vena cava, great arteries, and proximal organs such as the heart, lungs, and kidneys). Central congestion can occur from true volume overload associated with excessive fluid intake or retention, typical of the cardiac phenotype. Generally, this is an indolent process ( Fig. 67.7A ). When rapid decompensation occurs in AHF, fluid typically shifts from venous reservoirs to the central circulation in a process largely independent of total body water, more typical of the vascular phenotype. This, in part, explains why classic signs and symptoms of fluid overload may be absent in patients with AHF.
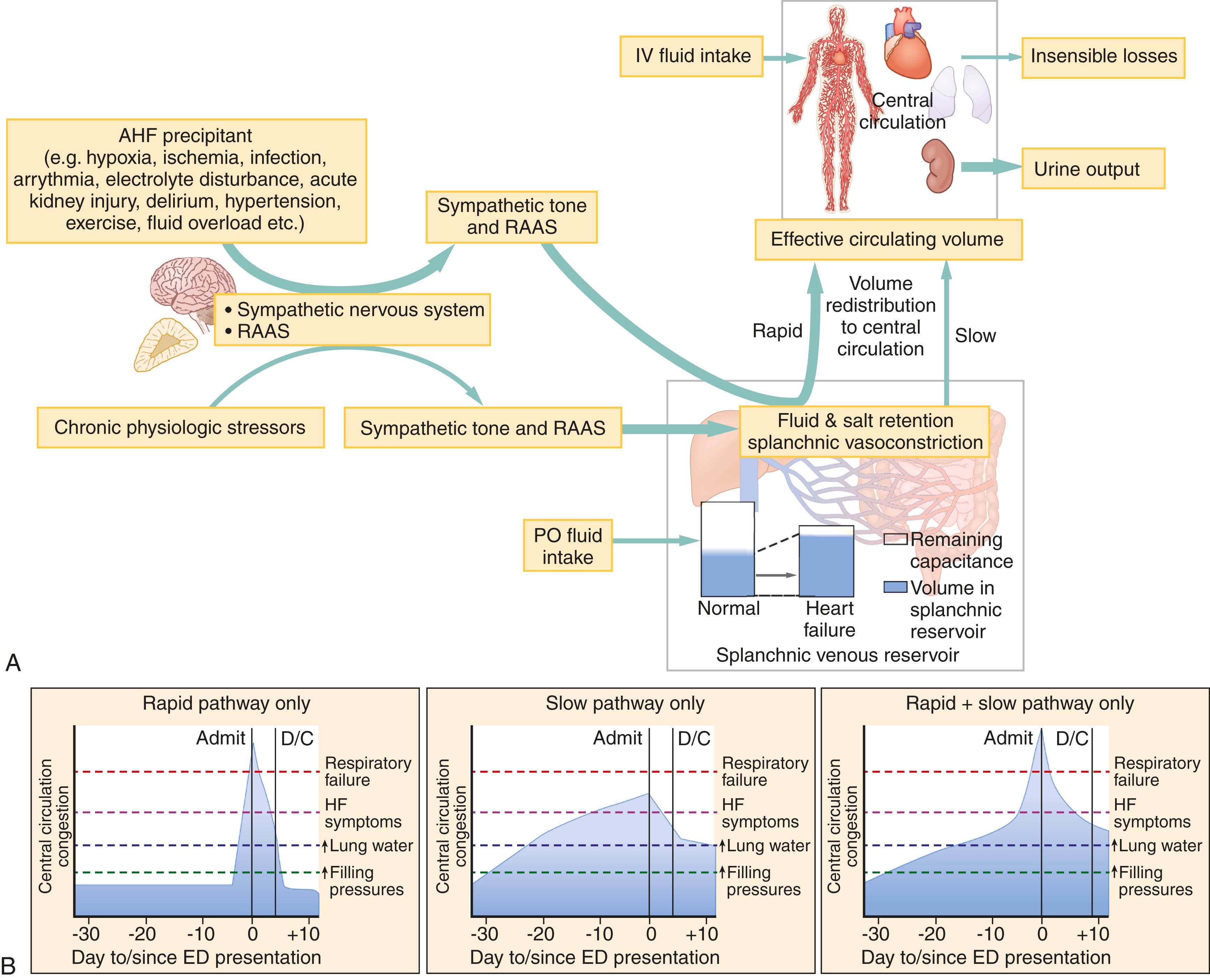
One of the largest and most critical venous reservoirs in the body is the splanchnic circulation, containing 20% to 50% of total blood volume at any given time. The splanchnic vessels dilate or contract in response to central circulation baroreceptors and sympathetic tone, and the renin-angiotensin-aldosterone system (RAAS). Under normal physiologic conditions, regulation of fluid from the splanchnic to central circulation via the hepatic veins acts as a buffer to maintain central volume. In stress situations, volume can be rapidly mobilized from the splanchnic circulation to the central circulation by vasoconstriction. When HF is present, neurohormonal mediators are chronically activated, leading to basal splanchnic vasoconstriction and a reduction in the reservoir’s buffering capacity. As a result, rapid fluid shifts can develop in response to any number of AHF precipitants (eg, infection, acute hypertension, ischemia, etc.), leading to central congestion even when total body water is normal ( Fig. 67.7B , first curve). Experimentally, denervation of the splanchnic vasculature in AHF patients dramatically lowers intracardiac filling pressures while increasing cardiac output.
In contrast, patients with the cardiac pathophysiology typically have gradual fluid accumulation superimposed on long-standing and intractable hemodynamic derangements. AHF patients with slow onset of symptoms tend to have less pronounced pulmonary edema on arrival and delayed dyspnea relief with worse in-hospital and postdischarge outcomes. Thus, clinicians should not rely entirely on a patient’s apparent clinical severity at presentation to determine how much decongestion they may need ( Fig. 67.7B ).
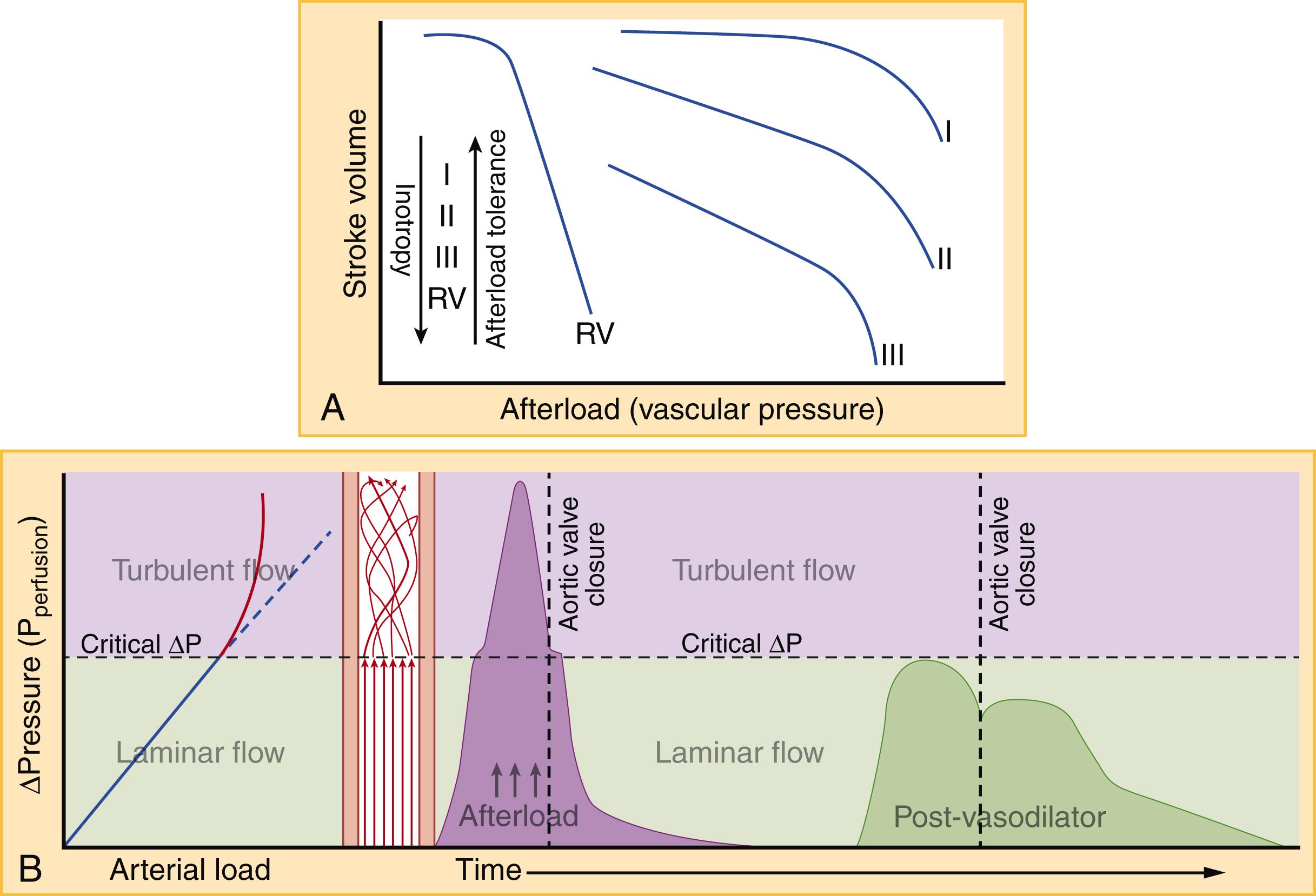
Afterload is the pressure against which the ventricle must contract to eject blood. In practical terms, this means the aortic and pulmonary artery (PA) pressures for the LV and right ventricle (RV), respectively. The coupling of ventricular and vascular function is a dynamic relationship. As afterload rises, SV will gradually decline until extremes in ventricular pressure are reached and a precipitous worsening of ventricular function ensues ( Fig. 67.6A ). The RV is significantly more pressure-sensitive and volume-tolerant than the LV, though chronic elevated pressure can cause hypertrophic remodeling similar to the LV. The ratio of oxygen consumption to stroke work increases as afterload rises (a phenomenon referred to as ventricular-vascular decoupling), burdening a heart with already diminished oxygen capacity at baseline. Whether intrinsic or exogenous, rises in afterload are often abrupt. Clinical deterioration rapidly follows, resulting in the onset of “flash” pulmonary edema. Though sometimes described as a distinct entity, flash pulmonary edema is the most severe form of afterload-mediated or hypertensive AHF.
In addition to perturbations in preload and afterload, the heart itself can contribute to AHF through disorganized contraction and relaxation. Each ventricle can be divided into individual myocardial segments (eg, apex to base, anterior/posterior, septal/free wall), which under idealized circumstances all contract and relax simultaneously. In reality, some segments within a single ventricle may contract or relax slightly before others, and even slight asynchrony reduces the mechanical efficiency of contraction. The reasons for segmental asynchrony include relatively fixed myocardial lesions, such as a scar from past MI, and more acute, dynamic causes such as localized demand ischemia, stretch-related myocyte toxicity, coronary microvascular dysfunction, or acute MI. ED treatment of AHF, including noninvasive positive-pressure ventilation (NPPV), diuresis, and vasodilation, can significantly improve segmental contraction synchronization. Failure to improve mechanical synchrony on point-of-care echocardiography (POC echo) after ED AHF treatment is associated with adverse AHF outcomes.
Right ventricular dysfunction (RVD) has been increasingly recognized as an important pathologic and prognostic marker in both chronic HF and AHF. RVD identifiable on ultrasound may be present in 28% to 46% of AHF cases and significantly declines in the first 24 hours after initial ED treatment. Missed antihypertensive medication within 7 days, ED PPV, COPD history, LVEF, lung ultrasound congestion severity, and right ventricular systolic pressure (RVSP) are significant predictors of RVD. RVD evident on POC echo is rarely recognized despite a high prevalence and potentially greater prognostic implications than LVEF. RVD in AHF is associated with higher all-cause mortality in AHF even after adjusting for LVEF and other measures of LV function. More importantly, the association between RVD and AHF mortality strengthens when pulmonary hypertension is absent. The relationship between acute RV and LV dysfunction is bidirectional and inextricably interdependent ( Fig. 67.8 ). Although the most common cause of right heart failure is left heart failure, RVD can precede, precipitate, or be concurrent with LV dysfunction in AHF. This is evident given that the path through the right heart, pulmonary circulation, left heart, and systemic circulation is a closed circuit and RV CO must equal LV CO. As a consequence, any hemodynamic derangement within the circuit will, on a beat-to-beat basis, adversely impact biventricular functionality. Additionally, the LV and RV share a common wall (the interventricular septum), meaning acute changes on one side do not occur in isolation.
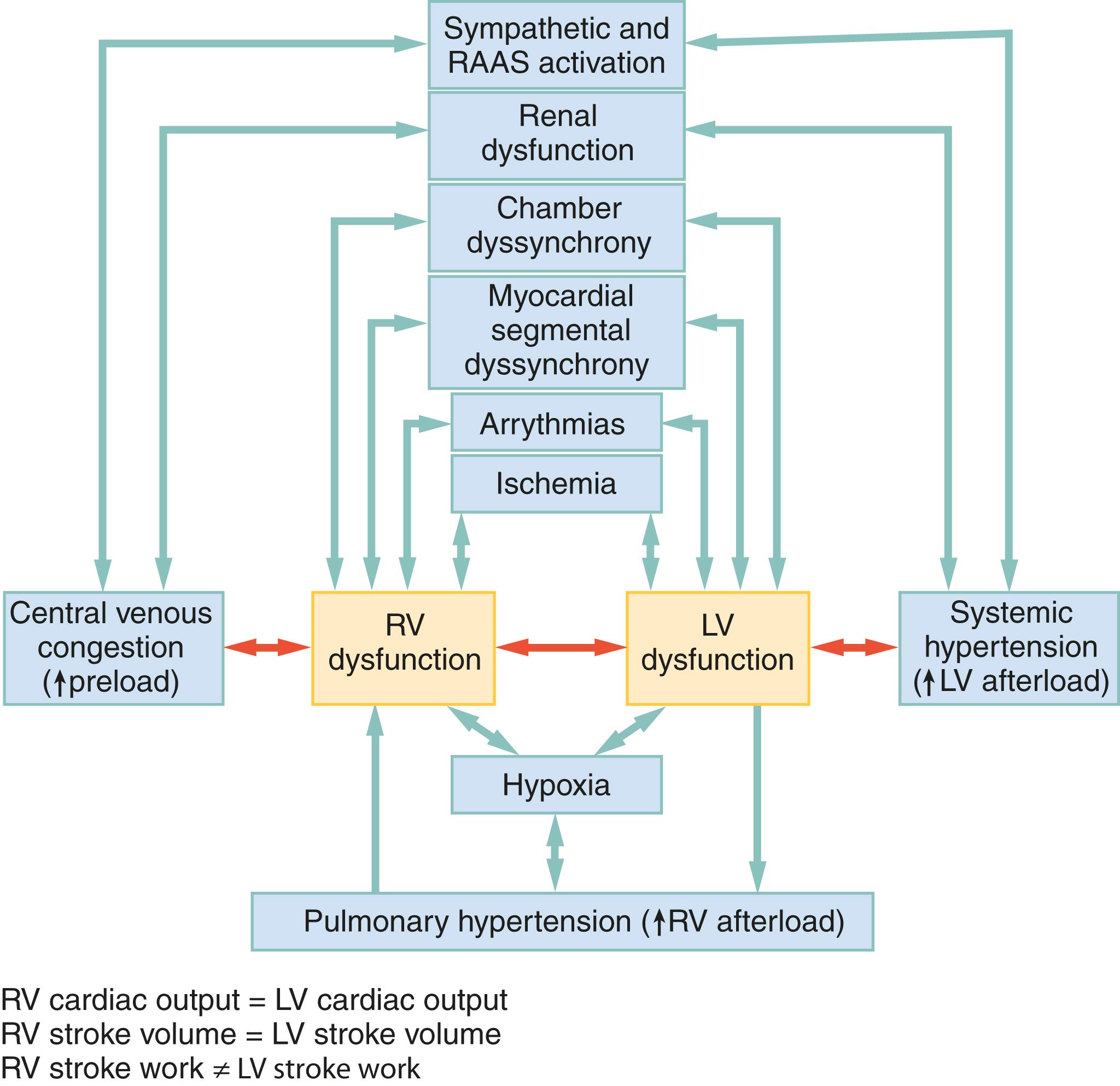
RVD in AHF can result from factors intrinsic and extrinsic to the RV itself (see Fig. 67.8 ). Some AHF therapies affect the RV as much as or more than the LV, as suggested by the observation that improvement in RV stroke-work (but not LV stroke-work) during an episode of AHF is associated with decreased rates of death, transplantation, ventricular assist device implantation, or HF-related rehospitalization at 6 months. Moreover, RVD independently predicts hyponatremia, worsening renal function after hospitalization, longer hospital length of stay, and higher rates of myocardial fibrosis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here