Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Headache arises from activation of pain-sensitive intracranial structures. In the 1930s, Ray and Wolff identified which intracranial components were pain sensitive and mapped the pattern of pain referral based on studies in which various intracranial structures were stimulated during intracranial surgery performed during local anesthesia. Intracranial pain-sensitive structures include the arteries of the circle of Willis and the first few centimeters of their medium-sized branches, meningeal (dural) arteries, large veins and dural venous sinuses, and portions of the dura near blood vessels. More recent data from patients during awake craniotomies also suggest that, contrary to prior belief, the pia mater and small cerebral vessels are pain-sensitive ( ). Pain-sensitive structures external to the skull cavity include the external carotid artery and its branches, scalp and neck muscles, skin and cutaneous nerves, cervical nerves and nerve roots, mucosa of sinuses, and teeth. Cranial nerves (CN) V, VII, IX, and X, in addition to cervical spine nerve/nerve roots, carry pain from these structures.
Trauma, inflammation, traction, compression, malignant infiltration, and other disturbances of pain-sensitive structures lead to headache. Superficial structures tend to refer pain locally, whereas deeper-seated lesions may refer pain imprecisely. A purulent maxillary sinus, for example, causes pain over the involved sinus, whereas within the cranial vault, nociceptive signals reach the central nervous system (CNS) largely by way of the first division of the trigeminal nerve (CN V), so an occipital lobe tumor may refer pain to the frontal head region. Infratentorial lesions tend to refer pain posteriorly because innervation of this compartment is by the second and third cervical nerve roots, which also supply the back of the head. However, posterior lesions or cervical spine pathological conditions may also produce frontal headache, because the caudal portion of the trigeminal nucleus extends down as far as the dorsal horn at the C3 level. Impulses arriving from C2 to C3 converge on neurons within the trigeminal nucleus and may refer pain to the somatic distribution of CN V.
Afferent pain impulses into the trigeminal nucleus are modulated by descending facilitatory and inhibitory input from brainstem structures, including the periaqueductal gray matter, rostral ventromedial medulla, locus ceruleus, and dorsal raphe nuclei. Opioids diminish pain perception by activating the inhibitory systems, whereas fear, anxiety, and overuse of analgesics may activate the facilitatory systems, thereby aggravating pain.
In 1988, the Headache Classification Committee of the International Headache Society introduced a detailed classification of headaches. This has been subsequently revised, most recently in 2018, into what is now the International Classification of Headache Disorders 3rd edition (ICHD-3) ( ). Its four main parts include: (1) primary headaches, (2) secondary headaches, (3) painful cranial neuropathies, other facial pain, and other headaches, and (4) appendix ( Box 102.1 ). Secondary headaches are those in which head pain is a symptom of an underlying disease affecting pain-sensitive structures, while in primary headache disorders head pain occurs in the absence of such disease. The purpose of the appendix in the ICHD-3 is to present research criteria for multiple entities that have not been sufficiently validated. If better scientific data become available, some of these may move to the main body of the classification in future revisions ( ). Careful definition of migraine subtypes and other primary headache disorders has helped rid research and clinical publications of the confusing and often poorly defined terminology of earlier work.
Migraine
Tension-type headache
Trigeminal autonomic cephalalgias
Other primary headache disorders
Headache attributed to trauma or injury to the head and/or neck
Headache attributed to cranial and/or cervical vascular disorder
Headache attributed to nonvascular intracranial disorder
Headache attributed to a substance or its withdrawal
Headache attributed to infection
Headache attributed to disorder of homeostasis
Headache or facial pain attributed to disorder of the cranium, neck, eyes, ears, nose, sinuses, teeth, mouth, or other facial or cervical structure
Headache attributed to psychiatric disorder
Painful lesions of the cranial nerves and other facial pain
Other headache disorders
Part Four: Appendix
Intracranial lesions that occupy space, or “mass lesions,” produce head pain by traction on or compression of pain-sensitive veins, venous sinuses, arteries, cranial nerves, and possibly by causing inflammation around pain-sensitive structures in the head ( ). The nature, location, and temporal profile of headache produced by an intracranial mass depend on many factors, including lesion location, rate of growth, effect on cerebrospinal fluid (CSF) pathways, and any associated cerebral edema. The intracranial mass lesion may be neoplastic, inflammatory, or cystic. Mass lesions can result in either localized or generalized head pain.
The estimated prevalence of headache in patients with brain tumors (see Chapter 74, Chapter 76 ) varies from 50% to 70%. The likelihood of developing headache is probably mediated by the tumor size, type, and location, and on patient age and personal history of a preceding headache disorder ( ; ). Infratentorial tumors and intraventricular tumors might be more likely to cause headache than supratentorial tumors. In gliomas, specifically, infratentorial and right-sided tumors are more frequently associated with headache presence at onset ( ). Progressive headaches (i.e., those with increases in intensity, frequency, and duration of pain) can be due to worsening of cerebral edema, the presence of midline shift, and hydrocephalus. Factors that increase the risk of brain tumor–related headaches include a personal history of a primary headache disorder and younger age at diagnosis ( ).
Although headache is typically not an isolated symptom at the time of brain tumor diagnosis, not uncommonly it can be the first and most severe symptom that the patient experiences ( ). In a prospective study of 211 patients with brain tumors, headache was the most frequent initial manifestation of the tumors (22% of the patients) and headache was the sole presenting symptom in 19% ( ). In a series of 527 adults with glioma, 12.5% indicated headache as a presenting symptom of their disease ( ).
There is wide variation in the characteristics of headaches attributed to intracranial tumors. Most commonly, such headaches are considered “nonclassifiable,” meaning that their phenotype does not meet the phenotypic characteristics of a primary headache disorder such as migraine or tension-type headache ( ). A headache phenotype that is “nonclassifiable” is followed in frequency by headaches that resemble tension-type headache and then migraine ( ). Specifically in gliomas, however, tension-type headache was the most common phenotype (47% of cases) in a recent series ( ). Most brain tumor headaches are felt bilaterally and are described as causing a pressure sensation. There is substantial variability in the frequency, duration, and specific location of pain. The location of headache pain does not reliably predict the location of the underlying brain tumor ( ).
Rapidly growing tumors are more likely to produce headache than indolent lesions, but slowly enlarging lesions can eventually produce pain by compromising the ventricular system or exerting direct pressure on a pain-sensitive structure. When the CSF circulation is partially obstructed, resulting in high intracranial pressure (ICP), headache is often worse when supine, aggravated by coughing, straining, and Valsalva, and is often associated with nausea and vomiting. When tumors interfere with CSF flow and cause periodic increases in ICP, the periods of elevated ICP may correlate with increasing headache severity, vomiting, decreased consciousness, or a change in respiration.
Tumors growing in the ventricular system are rare, but they can manifest dramatically. The classic presentation of a colloid cyst of the third ventricle is a sudden headache of great severity, rapidly accompanied by nausea and vomiting and sometimes by loss of consciousness. Intraventricular meningiomas, choroid plexus papillomas, and other intraventricular tumors can present in this manner if they suddenly obstruct the ventricular outflow pathways. A positional change may precipitate such an event; similarly, adoption of a different posture may rapidly relieve the headache and other symptoms. Colloid cysts of the third ventricle generally lead to slowly enlarging hydrocephalus that may result in a generalized and constant headache with superimposed episodes of catastrophic increases in headache intensity. Headaches that have a rapid onset and/or are associated with loss of consciousness should lead the examiner to seek a secondary cause.
Infiltrating tumors such as gliomas can reach considerable size without causing pain, because they may not deform or stretch the pain-sensitive vessels and nerves. Such lesions are more likely to present with focal neurological symptoms or with seizures rather than headache. Sudden worsening of the neurological state due to hemorrhage into the tumor may present with sudden headache. Infarction of a tumor can cause edema and swelling that result in a similar dramatic onset of head pain and neurological deficit.
Tumors that are intracranial but extraparenchymal (e.g., meningioma, acoustic neuroma, pinealoma, craniopharyngioma) and pituitary tumors can all produce headaches, but the clinician must carefully consider whether the headaches and these tumors are causally related or coincidental. When headaches are truly associated with these tumors, there are no specific headache patterns. Headaches can be near the lesion, referred to a more distant site in the cranium, or generalized when ICP increases. A family history of primary headaches and cavernous sinus invasion appear to be risk factors for headaches associated with pituitary nonfunctioning adenomas ( ). Meningiomas and meningeal sarcomas can invade the skull and even cause a mass externally by direct tumor spread or by overlying hyperostosis. Such tumors are often associated with localized head pain. Meningeal carcinomatosis (carcinomatous meningitis) produces a headache in most subjects, but the associated cranial nerve involvement and other neurological symptoms are generally more striking.
The headache associated with other intracranial mass lesions such as cerebral abscess and intracranial granuloma is no more specific than that due to a cerebral neoplasm.
Features that should serve as warnings that a patient’s headaches may not be of benign origin and that raise the possibility of an intracranial mass lesion are: subacute and progressive; new onset in adults; change in pattern; associated with nausea or vomiting; nocturnal or upon awakening in the morning; precipitated or worsened by changes in posture or Valsalva maneuver; associated with confusion, seizures, weakness, and/or abnormal neurological examination.
Although originally termed “migrainous syndrome with CSF pleocytosis,” several later reports used various terms, including headache with neurological deficits, CSF lymphocytosis, and pseudomigraine with temporary neurological symptoms and lymphocytic pleocytosis. This self-limited syndrome consists of one to several episodes of variable neurological deficits accompanied by moderate to severe headache and sometimes fever. Each episode lasts hours, with total duration of the syndrome being from 1 to 70 days. CSF abnormalities have included a lymphocytic pleocytosis varying from 10 to more than 700 cells/mm, elevation of CSF protein, and in some patients, elevated opening pressure. MRI and CT are normal in the vast majority of reported cases. Some patients may have evidence of gray-matter swelling or hemispheric hypoperfusion ( ; ). Results of microbiological studies are usually negative. The cause of the syndrome is unclear, although an immune response to a viral infection is speculated. No treatment alters the self-limited course of this disorder. In contrast to this syndrome, episodes of Mollaret meningitis (see Chapter 103, Chapter 76 ) are separated by months to years and are typically not accompanied by focal neurological symptoms.
Lesions that prevent free egress of CSF from the ventricular system result in obstructive hydrocephalus. If this occurs before closure of the cranial sutures, enlargement of the skull occurs, usually without producing headache. Ventricular obstruction after closure of the sutures leads to raised ICP and often to headache. The pain is often worse on awakening, occipital in distribution, and associated with neck stiffness. Vomiting, blurred vision, and transitory obscuration of vision due to papilledema may follow, as well as failing vision due to optic atrophy.
Rapidly developing obstruction due to a posterior fossa mass lesion or a ball-valve tumor, such as a third ventricular colloid cyst, can lead to a rapidly increasing headache followed by vomiting, impaired consciousness, and increasing neurological deterioration. Slowly developing hydrocephalus may result in massively dilated ventricles and may be associated with little or no headache.
Congenital obstruction of the foramina of Luschka and Magendie (the Dandy-Walker syndrome) can lead to ballooning of the fourth ventricle and deformity of the cerebellum. Minor degrees of this malformation can remain asymptomatic until later in life and then manifest with obstructive hydrocephalus and headache. Similarly, the Chiari malformation can obstruct free circulation of CSF and lead to hydrocephalus and headache and can cause symptoms via direct compression of the brainstem by herniated cerebellum ( ). This malformation can result in an occipital-suboccipital headache worsened or even initiated by a Valsalva maneuver during lifting, straining, or coughing. Thus, the Chiari malformation is one of the causes of an exertional or Valsalva maneuver–induced headache and cough headache. Other symptoms of Chiari malformation include visual phenomena (e.g., wavy lines, flashing lights, scotoma), blurred vision, photophobia, dizziness, disequilibrium, pressure in the ears, tinnitus, decreased hearing, nystagmus, dysphagia, and dysarthria. Other symptoms might be present if there is concurrent spinal cord syrinx. The clinician must be careful to differentiate between mild cerebellar tonsillar descent, a condition that is unlikely to cause symptoms, and a true Chiari malformation, a condition that can be associated with headache.
In communicating hydrocephalus, free communication exists between the ventricular system and the subarachnoid space, but CSF circulation or absorption is impaired. Obstruction in the basal cisterns or at the arachnoid granulations may follow subarachnoid hemorrhage and meningitis. Venous sinus occlusion can impair absorption of CSF. Headache may be a prominent symptom of both obstructive and communicating hydrocephalus, except in the case of normal-pressure hydrocephalus, which is generally painless (see Chapter 86 ).
Idiopathic intracranial hypertension (IIH) is a condition of increased intracranial pressure which typically manifests with papilledema and headaches and has no identifiable cause.
In recent years, the IIH treatment trial (IIHTT) has extensively expanded the available data on this condition ( ; ). Analysis of clinical profiles at baseline confirmed previous observations that IIH is almost exclusively a disease of obese young women. The mean age was 29.0 years, 97.6% were women, and the average body mass index was 39.9 kg/m 2 ( ). Headache was the most common initial symptom reported at study entry, followed by visual loss, pulsatile tinnitus, and diplopia, in that decreasing order. Frequency of most common symptoms at study entry was: headache in 84%, transient visual obscurations in 68%, back pain in 53%, and pulse synchronous tinnitus in 52% of patients. The headaches in IIH can be constant, daily, or intermittent and may or may not be classifiable according to phenotypes of primary headaches. In the IIHTT, the most common headache phenotypes were migraine in 52% and tension-type headache in 22% of patients, with the rest not fulfilling complete criteria for other primary headaches. Visual obscurations are a direct result of raised ICP leading to papilledema, while diplopia most often results from a lateral rectus palsy. Only 32% reported visual loss in the IIHTT ( ).
Most IIH patients have papilledema, which should not be confused with pseudopapilledema (e.g., drusen). An ophthalmologist should be involved in the diagnostic evaluation to confirm this and assess threat to vision if papilledema is present. One of the most common errors in diagnosing IIH is inaccurate ophthalmoscopic examination ( ). Brain neuroimaging, ideally brain magnetic resonance imaging (MRI) with contrast, is mandatory before lumbar puncture (LP) to rule out an intracranial mass as the cause for symptoms and signs. Cerebral venography (usually with magnetic resonance venography [MRV]) should also be pursued, if possible, to rule out cerebral venous sinus thrombosis (an IIH mimic) and to assess for bilateral distal transverse cerebral venous sinus stenoses, which are present in most if not all IIH patients and may aid the diagnostic evaluation. Whether these stenoses are the cause or a consequence of IIH remains a matter of debate. It has been proposed, however, that bilateral transverse sinus stenosis (or stenosis of a dominant transverse venous sinus) might lead to the following sequence of events: decreased venous outflow drainage, cerebral venous hypertension, impairment of CSF passive resorption, further incrementing CSF pressure resulting in external compression of the transverse venous sinuses, leading to worsened venous stenosis and vicious cycle perpetuation ( ). Other described brain MRI findings in IIH include: empty sella, posterior sclera flattening, optic nerve sheath distention, optic nerve vertical tortuosity, and optic nerve head enhancement ( ). None of these brain MRV and MRI findings are specific for IIH, however; they can be seen in other causes of intracranial hypertension. Normal subjects may also have bilateral cerebral venous sinus stenoses.
Following the determination that there is no intracranial mass, ventricular system obstruction, or thrombosis of a dural venous sinus, the high CSF pressure can be confirmed by LP manometry. Opening pressure via LP of at least 250 mm CSF (in adults) is required for the diagnosis of IIH ( ). Importantly, pressure should ideally be measured in lateral decubitus, which provides the most accurate readings. Opening pressures obtained while the patient is sitting are not valid. Pressures measured while prone during fluoroscopy are typically difficult to interpret because of overestimation of pressure, although some suggest a table tilt while prone may improve interpretation ( ). CSF composition should be normal in IIH. Removal of CSF to achieve a normal closing pressure relieves the headache and temporarily prevents visual obscurations. Table 102.1 summarizes IIH diagnostic criteria. Importantly, IIH mimics or “secondary pseudotumor cerebri” causes should be ruled out prior to diagnosing IIH ( Table 102.2 ).
IIH
IIH Without Papilledema (IIHWOP)
|
| Cerebral Venous Abnormalities |
|
| Medications and Exposures |
|
| Medical Conditions |
|
Prevention of permanent visual field loss is the main goal of therapy. Once the diagnosis of IIH is established, treatment in the absence of immediate threat for vision loss consists of weight loss and acetazolamide. Acetazolamide is typically started at 500 mg twice daily and is gradually increased if needed and tolerated. Most patients in the recent IIHTT tolerated >1 g/day for 6 months ( ). This trial has also provided support for the safe use of acetazolamide up to 4 g daily with weight loss for effective treatment of mild vision loss in IIH, with associated improvements in papilledema, increased ICP, and quality of life ( ). Other diuretics and topiramate are used in patients who cannot take acetazolamide, although these have not been studied in controlled fashion. The amount of weight loss required for IIH to remit is not known, but up to 15% of weight loss has been reported ( ). An ophthalmologist should follow patients together with the neurologist to properly monitor vision. Headache is usually managed according to its phenotype (e.g., migraine or tension-type), when present.
Patients who have significant visual field loss at presentation and those that have progressive visual field loss and/or progressive worsening of visual acuity despite medical management may require surgical intervention, traditionally CSF shunting or optic nerve sheath fenestration. Cerebral venous sinus stenting has been reported to improve symptoms of intracranial hypertension. The role of neurovascular stenting in IIH, however, has not yet been established. Stenting may be useful for highly selected IIH patients with venous sinus stenosis with an elevated pressure gradient in whom traditional therapies have not been effective ( ).
Of note, headaches sometimes persist even after proper management of IIH. In the IIHTT, 41% of patients reported a prior history of migraine ( ). In addition, 37% of headache sufferers at baseline were overusing symptomatic headache medications ( ). Possibly, in some cases, persistent headaches may occur on the basis of other processes (e.g., migraine with or without medication overuse headache) different from intracranial hypertension.
The headache of low CSF pressure/volume from spinal CSF leaks is characteristically orthostatic, developing or worsening when a person is upright and resolving or significantly improving with recumbency. It most commonly occurs after a LP via loss of CSF volume due to the removal of CSF for diagnostic purposes, and/or continued leakage of CSF through the hole in the arachnoid and dural layers left by the LP needle. Loss of CSF can result in brain sagging and traction on pain-sensitive structures such as bridging veins and sensory nerves. Recumbency removes the effect of gravity, and the traction headache is relieved. The headache that occurs after a spinal tap usually resolves spontaneously within a few days. Spontaneous recovery is estimated to occur in 24% of patients within the first 2 days, an additional 29% of patients within 3–4 days, and an additional 19% within 5–7 days ( ). The healing process might be hastened when the patient has relative bed rest and good hydration. When these conservative measures fail, relief can usually be obtained by the application of an epidural blood patch. An epidural blood patch consists of approximately 20 mL of the patient’s own venous blood being injected into the epidural space close to the site of the original LP. The resulting compression of the thecal sac and the presumed elevation of subarachnoid pressure presumably explains the resulting headache resolution. The increased pressure resulting from the epidural blood patch presumably causes temporary cessation of the CSF leak, thereby allowing the dura and arachnoid to heal. The success rate of a single epidural blood patch is estimated between 70% and 98% ( ).
Similar low-CSF pressure/volume headaches can occur when a spinal leak spontaneously develops, a condition still often referred to as “spontaneous intracranial hypotension” by some. Although indeed a significant number of patients with this condition have low (sometimes negative) CSF opening pressure when measured via LP, most have normal pressures ( ). Because low CSF volume may better explain the low pressure, headache, and neuroimaging findings seen in spontaneous spinal CSF leaks, “CSF hypovolemia” has been proposed as an alternative term ( ). Skull-based CSF leaks, however, rarely if ever present with the classic syndrome in the setting of spontaneous spinal CSF leaks ( ). The remaining discussion pertains to “headaches secondary to spontaneous spinal CSF leaks (SSCSFL),” the authors’ preferred term for this syndrome.
Spontaneous spinal CSF leaks are most commonly located in the thoracic or cervico-thoracic regions. Three main types have been identified in observational studies: the dural tear, the meningeal diverticulum, and the CSF-venous fistula ( ). Although a precipitating event for symptoms is often absent or uncertain, many patients with SSCSFL recall having a very minor injury, coughing, sneezing, or performing a Valsalva maneuver just prior to onset of symptoms. Most commonly headache is orthostatic, but not infrequently can be purely precipitated by Valsalva-like maneuvers (e.g., coughing, sneezing, laughing) or present as a combination of these two. A large series of SSCSFL specifically secondary to CSF-venous fistula reported an even greater percentage of patients experienced Valsalva-induced headache exacerbation or precipitation compared to orthostatic headache, features that should raise suspicion for occult CSF-venous fistula ( ). Occasionally, headaches secondary to SSCSFL are preceded by a single “thunderclap headache.” Other common symptoms of SSCSFL include auditory muffling, tinnitus, nausea and vomiting, and neck pain. Patients who have had the condition for a prolonged time can lose the orthostatic component to their headache. Such patients might have constant headaches or so-called “end-of-the-day” headaches (headache starting late in the day and getting worse as the day goes on). As additional cases have been reported in the literature, the clinical picture of CSF leaks has been found to take many forms ( ).
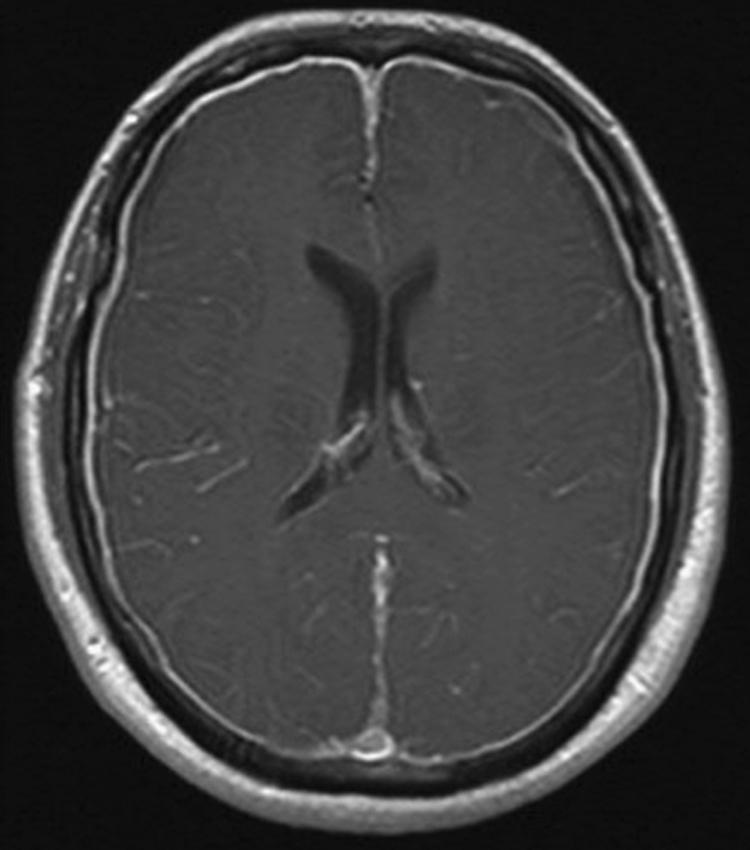
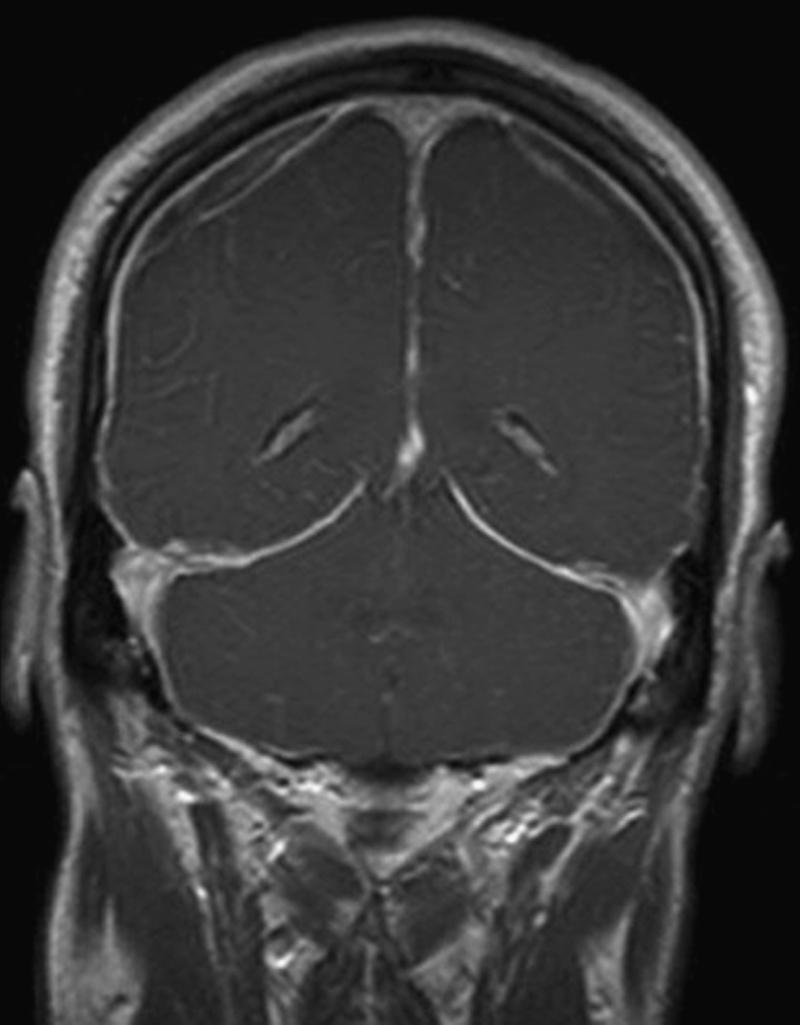
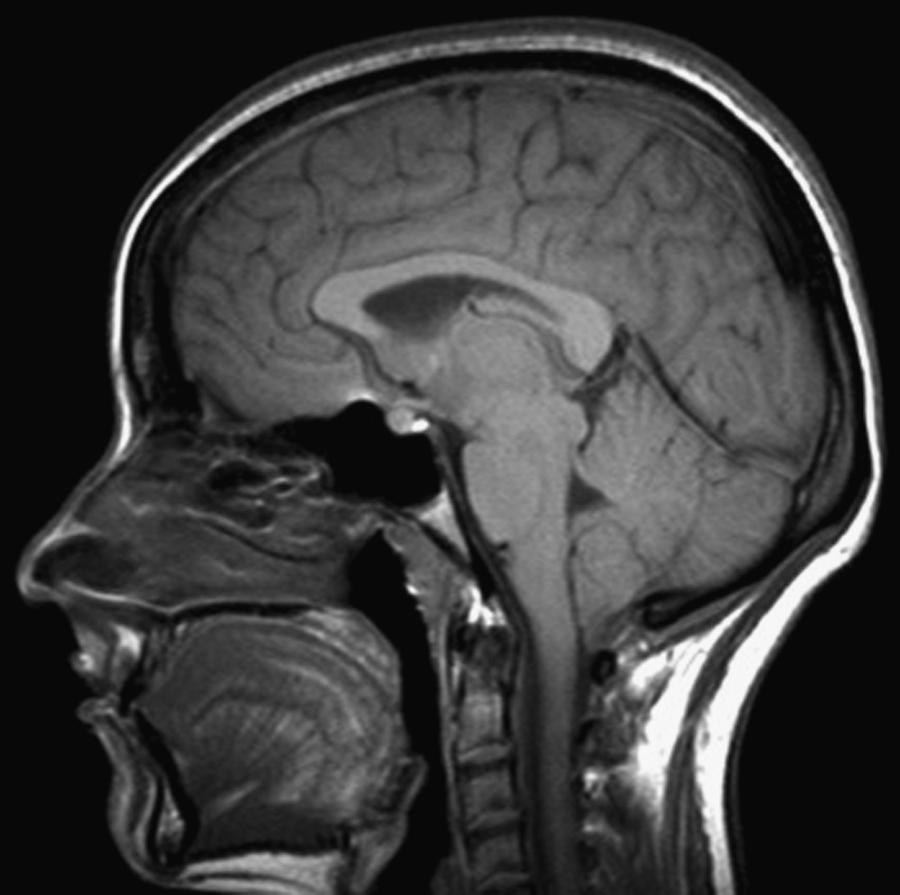
When a SSCSFL is suspected, the initial diagnostic test is brain MRI with gadolinium. MRI findings supportive of a diagnosis of SSCSFL include diffuse pachymeningeal gadolinium enhancement, brain sagging (i.e., cerebral and cerebellar tonsillar descent, inferior displacement of the optic chiasm), flattening of the anterior aspect of the pons, and venous dilation ( Figs. 102.1 and 102.2 ; ![]() eFig. 102.3 ). While an “acquired Chiari” from brain sagging can commonly be distinguished fairly easily from a congenital Chiari I malformation, sometimes this can be challenging. Subdural fluid collections (subdural hygromas and subdural hematomas) might occur in up to 50% of patients with SSCSFL ( ). The patient with classical symptoms of SSCSFL and classical brain MRI findings of the disorder might not need additional diagnostic tests prior to treatment with conservative measures or epidural blood patch. However, increasing symptom duration associates with decreased prevalence of abnormal dural enhancement; brain MRI can be normal in 20%–30% of cases ( ). When the diagnosis is uncertain a complete spine MRI and/or radioisotope cisternography (RICG) may help establish the presence of a SSCSFL although they rarely localize the exact leak site. Spine MRI findings suggestive of CSF leak include dural enhancement, dilated epidural veins, epidural venous plexus engorgement, and/or epidural fluid collections. Large longitudinal epidural fluid collections often suggest the presence of a high flow or “fast” SSCSFL. On RICG, delayed radioactive tracer ascent to cerebral convexities and/or early isotope tracer appearance in the urinary bladder are findings suggestive of CSF leak. Although measurement of opening pressure via LP can aid diagnosis if low opening pressure is found, LP should be avoided when possible due to the risk of worsening symptoms following the procedure. Importantly, a normal CSF opening pressure does not rule out a spontaneous CSF leak. If RICG is pursued, however, we typically measure opening pressure during the LP part of the procedure.
eFig. 102.3 ). While an “acquired Chiari” from brain sagging can commonly be distinguished fairly easily from a congenital Chiari I malformation, sometimes this can be challenging. Subdural fluid collections (subdural hygromas and subdural hematomas) might occur in up to 50% of patients with SSCSFL ( ). The patient with classical symptoms of SSCSFL and classical brain MRI findings of the disorder might not need additional diagnostic tests prior to treatment with conservative measures or epidural blood patch. However, increasing symptom duration associates with decreased prevalence of abnormal dural enhancement; brain MRI can be normal in 20%–30% of cases ( ). When the diagnosis is uncertain a complete spine MRI and/or radioisotope cisternography (RICG) may help establish the presence of a SSCSFL although they rarely localize the exact leak site. Spine MRI findings suggestive of CSF leak include dural enhancement, dilated epidural veins, epidural venous plexus engorgement, and/or epidural fluid collections. Large longitudinal epidural fluid collections often suggest the presence of a high flow or “fast” SSCSFL. On RICG, delayed radioactive tracer ascent to cerebral convexities and/or early isotope tracer appearance in the urinary bladder are findings suggestive of CSF leak. Although measurement of opening pressure via LP can aid diagnosis if low opening pressure is found, LP should be avoided when possible due to the risk of worsening symptoms following the procedure. Importantly, a normal CSF opening pressure does not rule out a spontaneous CSF leak. If RICG is pursued, however, we typically measure opening pressure during the LP part of the procedure.
FLOAT NOT FOUND
In patients with the typical clinical and radiographic features of SSCSFL, treatment may be conservative with bed rest and hydration for 1–2 weeks. If this is either impractical or ineffective, treatment with an epidural blood patch is warranted. If the CSF leak site is suspected, a “targeted” patch can be attempted close to the suspected leak site, but the patch is often done “blindly” in the lumbar spine when no clear leak site is known or suspected. Although epidural blood patches are effective in a substantial number of patients, many require more than one blood patch, and some require as many as four to six blood patches ( ) with 1–2 months between individual attempts.
Patients with SSCSFL who fail to respond to conservative measures are best investigated with myelography, the most effective study at present to identify the precise spinal CSF leak site. Myelography can also be pursued even prior to attempting epidural blood patching in patients with SSCSFL when a more precise diagnosis and a more definitive treatment plan are desired. Conventional computed tomography (CT) myelography is often the first modality used. Dynamic CT myelography is an alternative modality, particularly helpful to localize high-flow leaks. Digital subtraction myelography may be particularly helpful in those with suspected occult CSF-venous fistula and in patients with SSCSFL with negative conventional CT myelogram. If myelography identifies the CSF leak site, targeted epidural blood patching can be attempted. However, CSF-venous fistulas appear to respond poorly to epidural blood patching ( ) and are often best managed with surgical repair in centers with expertise. For other resistant leaks that can be precisely localized, surgical repair may also be attempted.
Headaches are a common symptom following injuries to the head and neck. Direct causality between the injury and headache is typically difficult to prove, since there are no headache characteristics that are specific or sensitive for a diagnosis of posttraumatic headache. Thus, the interval between trauma and onset of headaches is relied upon for making a diagnosis of a posttraumatic headache. Although controversial, diagnostic criteria stipulate that headaches must begin within the first 7 days following trauma in order to be considered “posttraumatic” or within 7 days of regaining consciousness following a head injury or within 7 days of discontinuing medications that impair the ability to sense or report headache following head injury ( ). In addition, the diagnosis of headaches attributed to whiplash requires that there is neck pain and/or headache at the time of whiplash. Posttraumatic headaches are considered “acute” when they have been present for less than 3 months and “persistent” when they last longer than 3 months ( ). Posttraumatic headaches can be due to direct or indirect forces to the head and/or neck.
Headaches attributed to head injuries are typically subdivided into those associated with mild head injury and those associated with moderate to severe injury. Headache is the most common symptom following mild head injury and headaches might be more common following mild traumatic brain injury compared to moderate or severe injury ( ). Other risk factors for the development of posttraumatic headaches might include presence of pre-injury headaches, female sex, and presence of comorbid psychiatric disorders ( ).
Most often, posttraumatic headaches phenotypically resemble migraine or tension-type headaches and less often resemble cervicogenic headache or occipital neuralgia ( ). Headache may be an isolated symptom following head trauma or can be part of the post-concussion syndrome, a syndrome consisting of headache, dizziness, fatigue, cognitive dysfunction, psychomotor slowing, insomnia and personality changes ( ; ). When considering a diagnosis of posttraumatic headache, it is essential to exclude structural traumatic injuries such as cervical spine injuries, skull fractures, intracranial hemorrhages, cerebrospinal fluid leaks, and cervical artery dissections.
Treatment of post-concussion syndrome and posttraumatic headache can be difficult, and an evidence base from which to select optimal therapies is absent. Posttraumatic headaches are thus treated according to the primary headache disorder that they most resemble (e.g., a posttraumatic headache that resembles migraine is treated with medications and other therapies typically used to treat migraine). Optimizing treatment requires that coexisting symptoms such as myofascial pain and spasm, sleep disorders, and anxiety be recognized and addressed. Nonpharmacological treatments (e.g., physical therapy, biobehavioral therapy) should be considered in addition to medication therapy.
Inflammation of pain-sensitive structures such as the meninges and intracranial vessels produces the severe headache frequently associated with both meningitis and meningoencephalitis. Headache is the most common symptom in acute bacterial meningitis, occurring in nearly 90% of cases ( ). Acute bacterial meningitis characteristically produces a severe holocephalic headache with neck stiffness and other signs of meningismus, including photophobia and irritability. Pain may be retro-orbital and may worsen with eye movement. The presence of the classic triad of fever, neck stiffness, and altered mental status has a low sensitivity for the diagnosis of meningitis; however, nearly all patients present with at least two of these symptoms and/or headache ( ). Jolt accentuation of headache (i.e., worsening of headache with sudden movements) is a common feature of bacterial meningitis but its absence does not rule it out.
Chronic meningitis due to fungal or tuberculous infection may lead to headache that may be severe and unrelenting. The headache of intracranial infection is nonspecific but merits consideration, especially in immunocompromised patients. The diagnosis can be confirmed only by examination of the CSF. Further discussion of meningitis can be found in Chapters 4, Chapters 75, Chapters 76, Chapters 77 .
Sinusitis, mastoiditis, epidural or intraparenchymal abscess formation, and osteomyelitis of the skull can all cause either focal or generalized headache. The diagnosis is usually suspected in the context of other associated symptoms and signs. After craniotomy, increasing pain and swelling in the operative site may be due to osteomyelitis of the bone flap. Plain skull roentgenograms may reveal the typical mottled appearance of the infected bone, necessitating removal of the flap.
Mollaret meningitis is a rare and recurrent aseptic meningitis (see Chapter 103, Chapter 76 ). The CSF cellular response includes large epithelioid cells (Mollaret cells). The pathogenesis is unknown but may relate to the herpes simplex virus. The condition may recur every few days or every few weeks for months or years. Headache, signs of meningismus, and low-grade fever accompany each attack. Treatment is mainly symptomatic.
Headache can accompany systemic infections due to viruses (e.g., influenza), bacteria (e.g., leptospirosis) and other infectious agents (e.g., Borrelia burgdorferi ). These typically nonspecific headaches can be mild or can be a prominent symptom of the systemic infection ( ). Headaches attributable to systemic infections might be a result of the microorganisms activating pain-sensitive structures, release of inflammatory mediators, presence of fever, and/or dehydration.
Intracranial aneurysms are rarely responsible for headache unless they rupture or rapidly enlarge. Large aneurysms may produce pain by exerting pressure upon cranial nerves or other pain-sensitive structures. Such pain is most commonly associated with aneurysms of the internal carotid and posterior communicating arteries. Enlargement of an aneurysm may occur shortly before rupture, and the pain is therefore an important clinical sign.
Parenchymal arteriovenous malformations (AVMs) should be considered in a patient presenting with a cranial bruit or the classic triad of migraine, seizures, and focal neurological deficits. Headache may be a presenting symptom in about 16% of patients and is often ipsilateral to the AVM. Similar to aneurysms, the pain may increase in intensity and frequency before hemorrhage. Though photophobia and phonophobia are uncommon, large AVMs can be associated with ipsilateral or bilateral throbbing cephalgia, resembling migraine. Occipital AVMs may frequently have migraine characteristics, and it is thought that the occipital location may be linked with cortical spreading depression, causing secondary migraine headaches. The visual disturbances associated with occipital AVMs may resemble migrainous aura. MR or CT angiography can usually exclude the presence of clinically significant aneurysms and AVMs.
Both aneurysms and AVMs can produce mild subarachnoid hemorrhages that result in sentinel headaches. Such headaches may be abrupt, mild, and short-lived. More catastrophic subarachnoid hemorrhages classically present as the worst headache the patient has ever had, all the more worrisome when associated with neck stiffness or pain, transient neurological symptoms (e.g., extraocular nerve palsy), or fever. Patients having any suggestion of a sentinel bleeding episode or who describe a recent thunderclap-like headache (see thunderclap headache discussion below) require emergent examination and CT to detect the presence of subarachnoid blood. If the CT is normal, perform a LP, looking for blood or xanthochromia.
The term thunderclap headache describes a severe headache occurring with instantaneous onset (within seconds) and without warning, like a clap of thunder. While multiple processes can present like this, a subarachnoid hemorrhage is the most worrisome. Rupture of an intracranial aneurysm or AVM results in a subarachnoid hemorrhage, with or without extension into the brain parenchyma. The headache of a subarachnoid hemorrhage is characteristically explosive in onset and of overwhelming intensity. Patients may relate that they thought they were hit on the head. The headache rapidly generalizes and may quickly be accompanied by neck and back pain. Loss of consciousness may also occur, but many patients remain alert enough to complain of the excruciating headache. Vomiting often accompanies the headache, which may aggravate the pain. Extension of blood into the ventricles and basal cisterns or distortion of the midline structures can each contribute to the rapid development of hydrocephalus, which frequently worsens the headache.
Suspicion of the diagnosis is easily confirmed by an unenhanced CT scan that reveals blood in the subarachnoid cisterns or within the parenchyma and often early hydrocephalus. When a CT unequivocally shows blood in the subarachnoid spaces it is not necessary or advisable to perform a LP, because the resultant reduction of CSF pressure may cause herniation of the brain or may remotely induce further bleeding from the aneurysm. Demonstration of subarachnoid hemorrhage generally indicates the need for cerebral angiography. The timing of this procedure and the subsequent mode of treatment are detailed elsewhere (see Chapter 65 ). The headache that occurs after a subarachnoid hemorrhage may be persistent, lasting up to 7–10 days. Rarely, a chronic daily headache (CDH) may persist for months to years.
Movement aggravates a subarachnoid hemorrhage headache, and photophobia and phonophobia are often associated. Therefore, these patients require a dark, quiet room, as well as comfort measures that minimize straining with bowel movements, vomiting, and coughing.
Other conditions which can also manifest with thunderclap headache in addition to subarachnoid hemorrhage include cerebral venous sinus thrombosis, cervicocephalic arterial dissection, pituitary apoplexy, acute hypertensive crisis, spontaneous spinal CSF leaks, meningitis, embolic cerebellar infarcts, pheochromocytoma ( ; ), and reversible cerebral vasoconstriction syndromes (RCVS) ( ; ). These entities can be associated with significant neurological morbidity and may not be easily seen on the initial CT image, thus underscoring the need for MRI and magnetic resonance angiography (MRA)/MRV in this group if results of the initial workup are negative. There is also a rarely seen category of thunderclap headache, referred to as primary thunderclap headache, for which there is no underlying cause established (see the section “Other Primary Headaches” later in the chapter).
Bleeding into the subdural space is generally due to tearing of the bridging veins that cross the subarachnoid space to reach the venous sinuses. Chronic subdural hematomas may cause headache via enlargement of the lesion and may present without serious neurological signs for a considerable time. Midline shift was the most influential factor for headache in one study, which has led some to consider that the likely cause of headache may be stretching or twisting of the pain-sensitive meninges and meningeal arteries or veins ( ). Changes in personality, alterations in cognitive abilities, subacute dementia, and nonspecific symptoms such as dizziness and excessive sleepiness may be present for weeks or months. Focal seizures, focal weakness, sensory changes, and ultimately, decreasing levels of consciousness may occur. Symptoms of chronic subdural hematoma, including headache and focal neurological symptoms, may fluctuate and occur intermittently, thereby mimicking transient ischemic attacks (TIAs). Headache is the single most common symptom of subdural hematoma and often presents as a severe bitemporal pain. Headache due to subdural hematoma is more common in young people; the cerebral atrophy seen in many elderly patients may be protective in the setting of any space-occupying intracranial lesion. Subdural hematoma should be considered in an elderly person with recent onset of headaches, especially in the context of a traumatic injury of even mild severity. Once suspected, exclude the presence of a subdural hematoma with CT or MRI. Treatment of subdural hematomas is discussed in Chapter 60 . Headaches tend to resolve after resolution of the bleed.
A hemorrhage into the cerebral or cerebellar tissue is a potent source of headache of rapid onset and increasing severity. The intraparenchymal mass causes headache by deforming and shifting the pain-sensitive vascular, meningeal, and neural structures. As the hematoma enlarges, it may obstruct the normal circulation of CSF and lead to increases in ICP. Initially, the pain of a cerebral hemorrhage is often ipsilateral, but it may generalize in the presence of hydrocephalus and elevated ICP. Rupture of the hematoma into the subarachnoid space or leakage of the blood into the basal cisterns through the CSF pathways may cause the headache to intensify and may also be associated with neck stiffness and other signs of meningeal irritation. Disorders leading to cerebral and cerebellar hemorrhages are more thoroughly discussed elsewhere (see Chapter 64 ).
Cerebellar hemorrhages account for about 10% of all intraparenchymal bleeds and are neurological emergencies with potentially fatal outcomes. An enlarging hematoma in the cerebellum rapidly compresses vital brainstem structures and obstructs the outflow of CSF from the ventricular system. This leads to occipital headache followed rapidly by vomiting, impaired consciousness, and various combinations of brainstem, cerebellar, and cranial nerve dysfunction.
Cerebral infarctions and TIAs may be associated with transient head pain in up to 40% of patients. The headache may be either steady or throbbing and is rarely explosive or severe ( ). The location of the pain is a poor predictor of the vascular territory involved, though unilateral headaches tend to be ipsilateral to the infarct. Cerebral ischemia-related headache is more common in younger patients and patients who are female. It is also more common in patients with larger infarcts and infarcts in the posterior cerebral and vertebrobasilar arterial distributions ( ). A recent MRI voxel-based symptom lesion-mapping study suggests headache phenotypes may be related to specific ischemic lesion patterns ( ). In this study, pulsatile headache occurred with widespread cortical/subcortical strokes, noise sensitivity was associated with cerebellar lesions, nausea was associated with posterior circulation territory infarcts, and cranial-autonomic symptoms were related to parietal lobe, somatosensory cortex, and middle temporal cortical lesions ( ).
If a large cerebral or cerebellar infarct produces significant mass effect as a result of edema, the associated headache may worsen. Obstruction of the ventricular system frequently results in hydrocephalus and further aggravation of the pain. The pain may be pulsatile and may worsen with straining or the head-low position. Hemorrhagic transformation of an ischemic infarct may be associated with worsening of headache. As the infarct decreases in size and the phase of hyperemia subsides, headache generally eases, although in some, headache following stroke becomes chronic and often resembles tension-type headache ( ).
Paroxysmal visual and sensory disturbances commonly associated with migraine aura may mimic symptoms of cerebrovascular disease, occasionally making the differentiation between the two a challenge. The visual aura of migraine is typically a positive phenomenon, perceived with the eyes open or closed. Visual disturbances due to ischemic lesions of the visual pathway or retina are usually associated with negative phenomena such as vision loss or a negative scotoma; however, emboli to the retinal artery can result in showers of bright flashes, and calcarine ischemia can occasionally produce scintillating scotoma. While visual disturbances associated with stroke and TIA are usually abrupt and fixed, the migraine aura tends to march across the visual field over the course of a few minutes and is generally followed by headache after a latent interval. The headache associated with stroke and TIA typically has a more variable relationship to the visual disturbances.
Dissection of the cervical portion of the carotid or vertebral arteries is associated with headache, neck pain, or face pain in approximately 80% of patients. The headache may be isolated or associated with an ipsilateral Horner syndrome or stroke symptoms. An ipsilateral Horner syndrome is more common in carotid than vertebral dissections, and the sympathetic hypofunction may be due to interference with the sympathetic fibers around the internal carotid artery as they ascend from the superior cervical ganglion to the intracranial structures. In internal carotid artery dissections, the headache is typically unilateral and ipsilateral to dissection. Facial pain is common and ipsilateral cranial nerve palsies, especially of lower cranial nerves, are not infrequent. Cerebral or retinal ischemic symptoms are the initial manifestations in a minority of patients. Vertebral artery dissections present most often with headache with or without neck pain, followed by a delay of focal CNS ischemic symptoms. In uncomplicated intracranial vertebral artery dissection, the headache usually is acute in onset with a persistent and temporal feature and in many cases the pain appears to be throbbing and severe in the ipsilateral and occipitonuchal area. Additionally, the headache often is aggravated by head flexion/ rotation and relieved by head extension and being supine ( ).
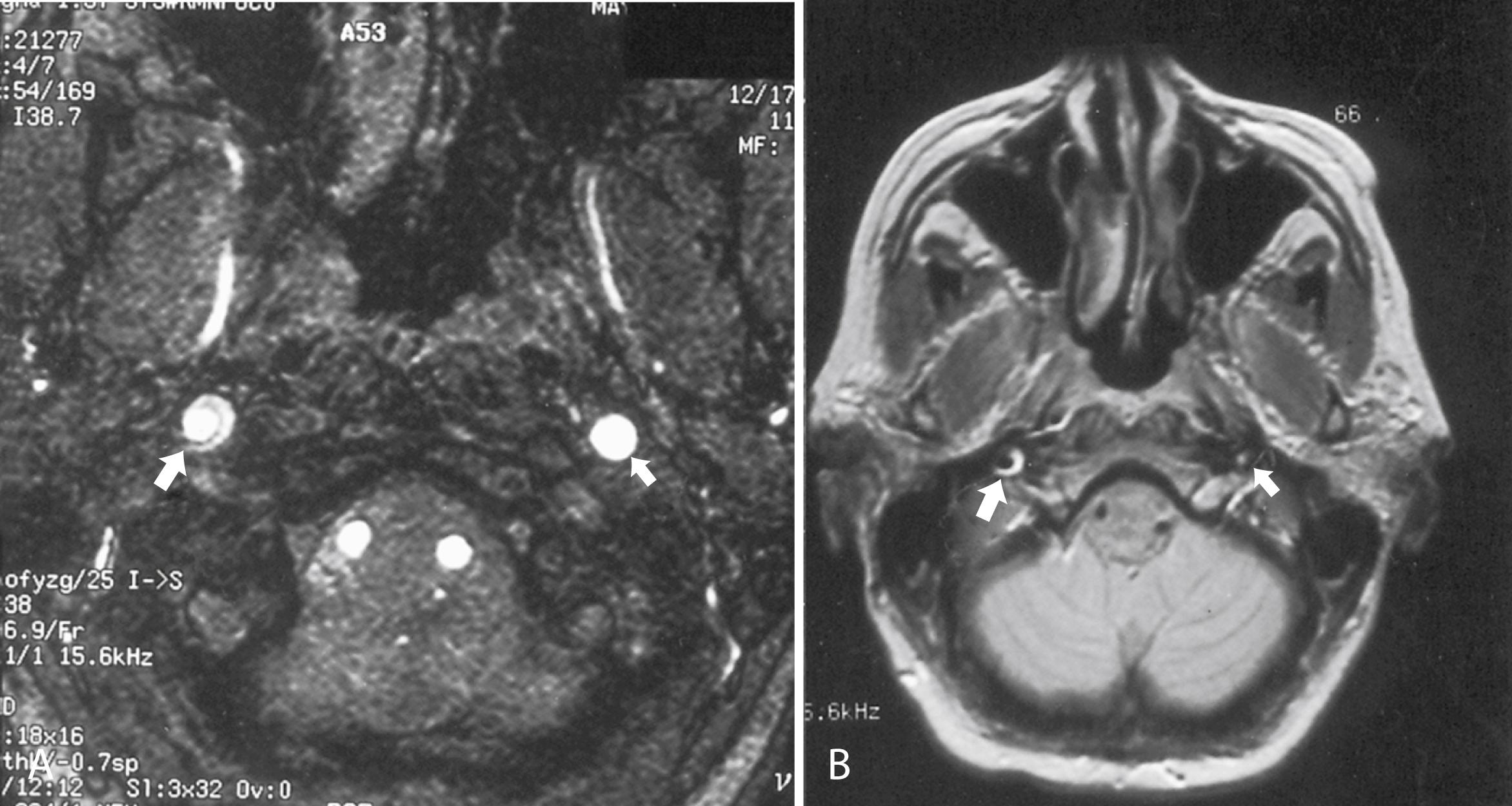
Cervicocephalic arterial dissections can result from intrinsic factors that predispose the vessel to dissection, including fibromuscular dysplasia, cystic medial necrosis, and other connective tissue disorders such as Marfan syndrome or Ehlers-Danlos syndrome. Extrinsic factors such as trivial trauma may play a pathogenic role when superimposed on structurally abnormal arteries. Severe head and neck trauma may occasionally be the proximate cause of dissection. Importantly, in patients >60 years old, pain and mechanical triggers may be absent, making the diagnosis of cervical artery dissection more challenging in these older patients ( ). MRI or MRA usually confirms the diagnosis of arterial dissection. At the level of involvement, the lumen of the artery typically appears as a dark circle of flow void of smaller caliber than the original vessel, and the intramural clot appears as a hyperintense and bright crescent or circle (in both T1- and T2-weighted images) surrounding the flow void ( ![]() eFig. 102.4 ). Catheter angiography is rarely required. The pain associated with cervicocephalic dissections is of variable duration and may require treatment with potent analgesics. Patients with evidence of distal embolization are usually treated with either antiplatelet agents or anticoagulation. FLOAT NOT FOUND
eFig. 102.4 ). Catheter angiography is rarely required. The pain associated with cervicocephalic dissections is of variable duration and may require treatment with potent analgesics. Patients with evidence of distal embolization are usually treated with either antiplatelet agents or anticoagulation. FLOAT NOT FOUND
Giant-cell arteritis is a vasculitis of elderly persons and is one of the most ominous causes of headache in this population. When unrecognized and untreated, it may lead to permanent blindness. Patients with this disorder most commonly see neurologists for new headaches of unknown cause.
The clinical manifestations of giant-cell arteritis result from inflammation of medium and large arteries. Table 102.3 summarizes clinical symptoms in 166 patients examined at the Mayo Clinic between 1981 and 1983 ( ). Headache was the most common symptom, experienced by 72% of patients at some time and the initial symptom in 33%. The headache is most often throbbing, and many patients report scalp tenderness. Headache is associated with striking focal tenderness of the affected superficial temporal or, less often, occipital artery. One-third of patients with headache may have no objective signs of superficial temporal artery inflammation.
| Symptom | Patients with Symptom (%) | Patients in whom it was Initial Symptom (%) |
|---|---|---|
| Headache | 72 | 33 |
| Polymyalgia rheumatica | 58 | 25 |
| Malaise, fatigue | 56 | 20 |
| Jaw claudication | 40 | 4 |
| Fever | 35 | 11 |
| Cough | 17 | 8 |
| Neuropathy | 14 | 0 |
| Sore throat, dysphagia | 11 | 2 |
| Amaurosis fugax | 10 | 2 |
| Permanent vision loss | 8 | 3 |
| Claudication of limbs | 8 | 0 |
| Transient ischemic attack/stroke | 7 | 0 |
| Neuro-otological disorder | 7 | 0 |
| Scintillating scotoma | 5 | 0 |
| Tongue claudication | 4 | 0 |
| Depression | 3 | 0.6 |
| Diplopia | 2 | 0 |
| Tongue numbness | 2 | 0 |
| Myelopathy | 0.6 | 0 |
∗ Some patients had coincident onset of more than one symptom.
More than half of patients with giant-cell arteritis experience polymyalgia rheumatica , which is the initial symptom in one-fourth. Fatigue, malaise, and a general loss of energy occur in 56% of patients and are the initial symptoms in 20%. Jaw claudication is common and the initial symptom in 4% of patients. Tongue claudication is rare.
Amaurosis fugax is one of the most ominous symptoms in giant-cell arteritis; 50% of affected patients subsequently become partially or totally blind if untreated. In the Mayo Clinic series, 10% of patients experienced amaurosis fugax, and 35% of those cases were bilateral. Horizontal or vertical diplopia also occurs in giant-cell arteritis.
Some 14% of patients have a neuropathy, which is a peripheral polyneuropathy in 48%, multiple mononeuropathies in 39%, and an isolated mononeuropathy in 13%. Limb claudication occurs in 8% of patients and usually involves the upper limbs. TIAs and strokes occur in 7% of patients, and the ratio of carotid to vertebral events is 2:1. Vertigo and unilateral hearing loss can occur. An acute myelopathy, acute confusional state, and subacute stepwise cognitive deterioration are rare manifestations.
About 49% of patients with histologically verified giant-cell arteritis have physical signs of superficial temporal artery inflammation, including erythema, pain on palpation, arterial nodularity and/or thickening, or reduced pulsation on the affected side. Rarely, ischemic necrosis of the scalp and tongue occurs. Almost a third of patients have large-artery bruits or diminished pulses, which usually affect the carotid artery. The upper-limb arteries are more commonly affected than those in the lower limbs.
Ocular findings in giant-cell arteritis may be striking. In patients with amaurosis fugax, sludging of blood in the retinal arterioles may be observed. With infarction of the optic nerve, vision loss precedes the funduscopic signs of an anterior ischemic optic neuropathy by up to 36 hours. During the acute stage, there may be optic disc edema, optic disc pallor, and resulting visual field defects which tend to be altitudinal. Optic disc edema is commonly followed by the gradual development of optic atrophy. Restrictions in eye movements may indicate involvement of specific extraocular muscles. Oculosympathetic paresis (Horner syndrome) occasionally occurs.
Up to one-third of patients have clinically significant large-artery disease. The most common causes of vasculitis-related death are cerebral and myocardial infarction. In fatal occurrences, vertebral, ophthalmic, and posterior ciliary arteries are involved as often and as severely as the superficial temporal arteries. Rupture of the aorta is rare. In patients with peripheral neuropathic syndromes, ischemic infarction of peripheral nerves due to vasculitis is demonstrable. Intracranial vascular involvement is rare.
The laboratory abnormality most often associated with giant-cell arteritis is elevation of the erythrocyte sedimentation rate (ESR) (mean, 85 ± 32 mm in 1 hour with the Westergren method), which has a sensitivity of about 84%. C-reactive protein levels may be more sensitive than the ESR, though one study showed that both can be normal in 4% of biopsy-proven patients ( ). Patients are usually anemic (mean hemoglobin value 11.7 ± 1.6 g/dL) and show a mild thrombocytosis (mean platelet count 427 ± 116 × 10 3 /μL). As all of these laboratory tests are nonspecific, the confirmatory diagnosis rests on a temporal artery biopsy. However, the sensitivity for this procedure is low, with a high false-negative rate of 15%–40% ( ).
Imaging, particularly with temporal artery MRI or ultrasound, may supplement the diagnostic investigation and may be helpful in decision making about proceeding to biopsy. The superficial cranial arteries along with the mural and luminal properties can be investigated with a contrast-enhanced, high-resolution, temporal artery MRI ( ). Doppler ultrasound has been advocated by some, but its practical value is difficult to assess because of the heterogeneous study findings and high operator dependence for image acquisition ( ). Currently, temporal artery imaging is not considered to support the diagnosis with as much certainty as temporal artery biopsy ( ). An angiogram of the aortic arch vessels may show long segments of smoothly tapered stenosis and occlusions of subclavian, brachial, and axillary arteries. Fluorodeoxyglucose positron emission tomography (FDG-PET)/CT may supplement the diagnostic investigation by identifying vessel inflammation. The spatial resolution of FDG-PET is best for vessels greater than 4 mm in diameter, so it is most useful when involvement of larger vessels, such as the aorta or the subclavian, vertebral, and carotid arteries, is suspected.
The histopathological features of arterial lesions include intimal proliferation with consequent luminal stenosis, disruption of the internal elastic membrane by a mononuclear cell infiltrate, invasion and necrosis of the media progressing to panarteritic involvement by mononuclear cells, giant-cell formation with granulomata within the mononuclear cell infiltrate, and (variably) intravascular thrombosis ( ![]() eFig. 102.5 ). Involvement of an affected artery is patchy, with long segments of the normal unaffected artery flanked by vasculitic foci known as skip lesions , which may begin to normalize within days after treatment. For these reasons, biopsy specimens of the superficial temporal artery should be generous (4- to 6-cm-long specimens), multiple histological sections should be taken, and bilateral biopsy considered. Employing these strategies may increase the diagnostic yield of temporal artery biopsy up to 86%. FLOAT NOT FOUND
eFig. 102.5 ). Involvement of an affected artery is patchy, with long segments of the normal unaffected artery flanked by vasculitic foci known as skip lesions , which may begin to normalize within days after treatment. For these reasons, biopsy specimens of the superficial temporal artery should be generous (4- to 6-cm-long specimens), multiple histological sections should be taken, and bilateral biopsy considered. Employing these strategies may increase the diagnostic yield of temporal artery biopsy up to 86%. FLOAT NOT FOUND
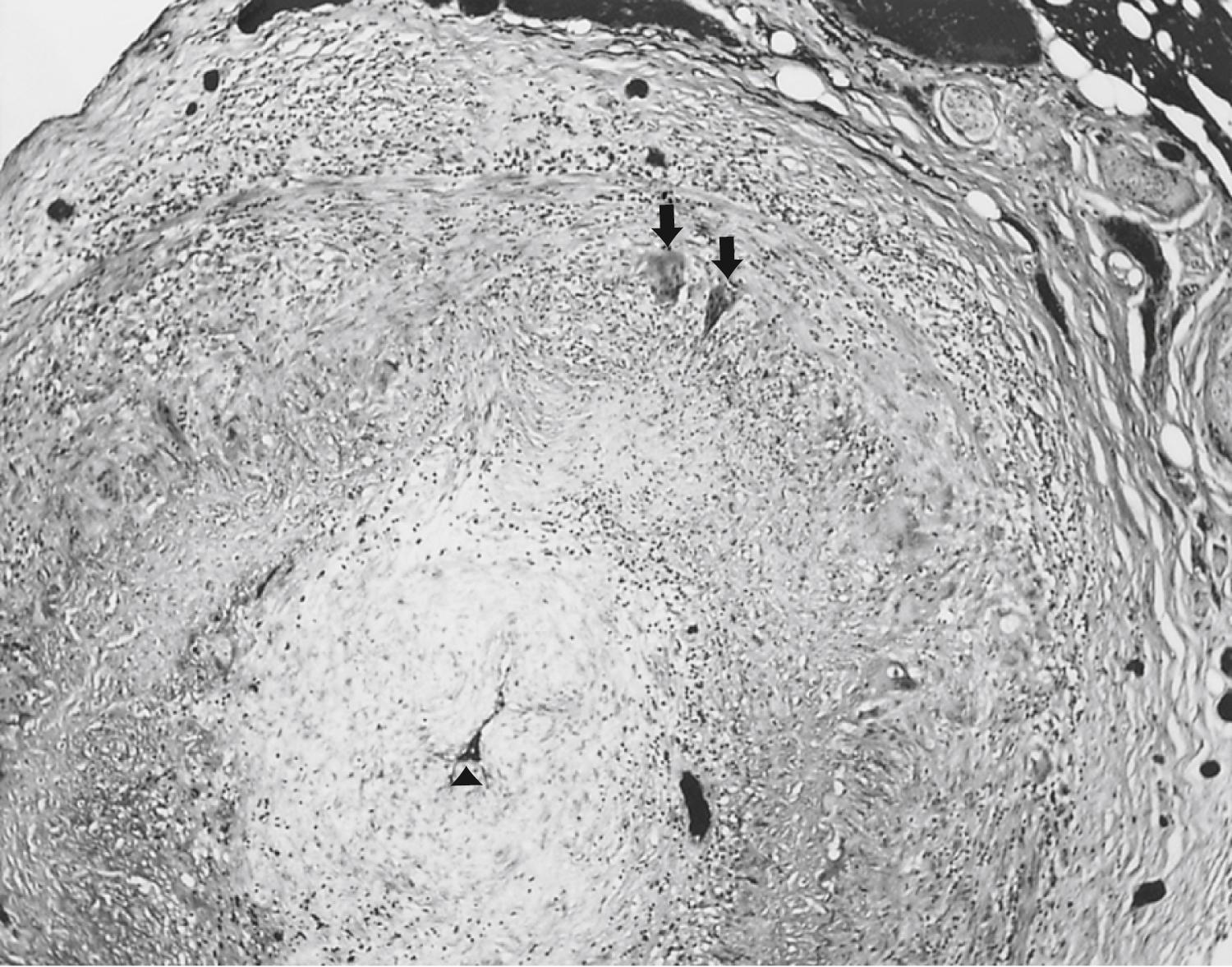
Giant-cell arteritis is an idiopathic autoimmune disease. Although vasculitic processes are often systemic, giant-cell arteritis is usually more focal than polyarteritis nodosa and characterized by a mononuclear cell infiltrate with giant-cell formation, suggesting differences in immunopathogenesis. No distinctive antigen has been identified to explain the particular tropism of giant-cell arteritis, although the possibility that the immune reaction is directed against the internal elastic lamina (which is absent from cerebral vessels shortly after they pierce the dura) may explain the paucity of intracranial involvement. Lymphocytes sensitized to the purported antigen infiltrate the internal elastic lamina and release a host of lymphokines which attract a mononuclear cell infiltrate. Activated macrophages release lysosomal proteases and may transform into epithelioid and multinucleate giant cells. T cells themselves, by antibody-dependent cell-mediated cytotoxicity or natural killer cell actions, may also be involved. In addition, the demonstration of antibody and complement deposits at the internal elastic lamina suggests that humoral mechanisms are involved.
The incidence of biopsy-confirmed giant-cell arteritis ranges between 9.5 and 29.1 per 100,000 per year, significantly increases after 50 years of age, and peaks in the eighth decade ( ). It is the most common vasculitic process in both Europe and North America, appears to be most common among individuals of Scandinavian descent, and is significantly less common among Asians. The reported female-to-male ratio in giant-cell arteritis is as high as 4:1.
Once giant-cell arteritis is suspected, histological confirmation should be obtained, and treatment started immediately. Treatment consists of oral corticosteroids given initially in high doses and gradually tapered over months. Treatment should not be withheld pending the result of temporal artery biopsy. Prednisone may be initiated at 40–60 mg/day and continued for 1 month, after which time, start a cautious taper of less than 10% of the daily dose per week. If, at the time of presentation, ischemic complications are imminent or evolving, parenteral high-dose corticosteroids should be given until these complications stabilize. Intravenous (IV) pulse corticosteroids, typically in the form of methylprednisolone 1000 mg/day for 3 days, has been advocated for patients with transient, partial, or complete vision loss at presentation ( ). Some studies have shown antiplatelet therapy with low-dose aspirin to be associated with a lower risk for developing visual loss and cerebrovascular infarcts ( ). A Cochrane review of the literature published in 2014, however, found that there is no evidence from randomized controlled trials to determine the safety and efficacy of low-dose aspirin as an adjunctive treatment in giant-cell arteritis ( ). The adjunctive use of anticoagulants for patients with ischemia may be tried, but their efficacy in this setting is unproven.
Disease activity must be monitored both clinically and by monitoring the ESR. A flare of symptoms accompanied by an increase in the ESR mandates increasing the corticosteroid dose at least to the last effective dose and often boosting it temporarily to a higher level. Relapses generally reflect too rapid a taper, and resumption of a more slowly tapering regimen is indicated after the relapse has stabilized. Some patients may require continuation of low-dose (7.5–10 mg/day) prednisone for several years, although complete withdrawal remains the eventual goal. There is some evidence that treatment with methotrexate 10 mg/wk may be an effective adjunctive treatment that allows for more rapid tapering of the prednisone dose. Recently, the US Food and Drug Administration (FDA) approved the use of tocilizumab as a steroid-sparing agent for giant-cell arteritis treatment.
The multitude of well-known adverse effects associated with exogenous corticosteroids (e.g., vertebral body compression fractures, myopathy, a confusional state, among others) may influence management by prompting a more rapid taper, thereby exposing the patient to the risks that accompany a relapse of the vasculitis.
The clinical onset of giant-cell arteritis may be acute, subacute, or chronic. Although the median duration of symptoms before diagnosis is 1 month, patients may rarely present with a history of up to several years of polymyalgia rheumatica.
Within days of corticosteroid treatment, symptoms and laboratory abnormalities may begin to normalize. With tapering doses, relapses may occur and may present as a reactivation of prior symptoms or with new symptoms altogether. Neurological complications, including neuropathies and cerebrovascular events, are not always preventable by corticosteroid administration and have a median onset of 1 month after initiation of treatment. Similarly, large-artery involvement can occur up to 7 months after initiation of treatment.
Although the occurrence of amaurosis fugax often brings a patient with undiagnosed giant-cell arteritis to medical attention, permanent loss of vision rarely occurs with adequate treatment. In patients with acute and incomplete loss of vision, some visual function may return with immediate institution of corticosteroid therapy, but this is rare.
Sleep apnea may result in both an independent headache type and may also be an aggravating factor among individuals with migraine. Individuals with nocturnal or morning-predominant headaches should be asked about sleep apnea risk factors, such as snoring and observed apneic episodes. A body mass index greater than 35 kg/m 2 , and neck circumference greater than 40 cm, further increase the likelihood of obstructive sleep apnea. The mechanism may involve hypercarbia and/or hypoxemia.
Despite common belief, mild to moderate hypertension does not directly cause headache, as demonstrated by a lack of correlation between headache diaries and 24-hour ambulatory blood pressure analysis. Conversely, hypertensive emergency is commonly associated with headache, where a diagnosis of posterior reversible encephalopathy syndrome should also be considered. In a patient with a short-duration headache associated with diaphoresis and palpitations, the possibility of pheochromocytoma should be pursued. Similarly, headache may be a sign of pre-eclampsia during pregnancy.
Cardiac cephalalgia occurs as a direct result of myocardial ischemia and may present in the complete absence of chest pain. The headache is characteristically brought on by exertion, improved with rest, and unlike most primary headache disorders, improved by nitroglycerin. Failure to identify this diagnostic entity may be associated with dire consequences. A cardiac evaluation should be considered in patients over the age of 50 who present with new headaches (especially if exertional) and vascular risk factors.
In the absence of injection of the conjunctiva or other obvious signs of eye disease, headache and eye pain rarely have an ophthalmic cause. The maxim is that a white eye is rarely the cause of a monosymptomatic painful eye. Acute angle-closure glaucoma is a rare but often dramatic event. The patient may present with extreme eye and frontal head pain with associated vomiting. The sclera is injected, the cornea is cloudy, the globe is stony hard, and unlike cluster headache, the pupil is fixed in mid-position.
Refractive errors, imbalance of external eye muscles, amblyopia, and “eyestrain” are not causes of headache in most instances. In children and teenagers, however, refractive errors, especially hyperopia, can produce dull frontal and orbital headaches from straining to achieve accommodation at school. Myopic children are unaffected. Trochleitis may produce a periorbital headache and be either idiopathic or secondary to an autoimmune disorder. The headache is characteristically aggravated by vertical ductions of the eye, as the tendon of the superior oblique muscle runs through this structure. Cluster headache, migraine, and other primary headaches, as well as carotid artery dissection, can cause orbital and retro-orbital pain. Each is discussed elsewhere in this chapter.
Acute purulent rhinosinusitis causes local and referred pain. The distribution of the pain depends on the sinuses involved. Maxillary sinusitis causes pain and tenderness over the cheek. Frontal sinus disease produces frontal pain; sphenoid and ethmoidal sinusitis causes pain behind and between the eyes, and the pain may refer to the vertex. Acute rhinosinusitis is commonly associated with fever, purulent nasal discharge, and other constitutional symptoms. The pain is worse when the patient bends forward and is often relieved as soon as the infected material drains from the sinus. Chronic rhinosinusitis is considered to be a risk factor for CDH, where the headache most often resembles chronic tension-type headache in features ( ). Intracranial infections may occur as a complication of untreated sinusitis. Acute infection involving the sphenoid sinus can be especially dangerous because of its close proximity to the cavernous sinus.
Commonly, migraine headaches are erroneously diagnosed as sinus headaches, because they are associated with cranial autonomic symptoms, have prominent facial involvement, and/or are triggered (e.g., by a change in altitude/weather, an exposure to pollens, or a seasonal predilection). Most patients with a diagnosis of “sinus headaches” have migraine headaches ( ).
Malignant tumors of the sinuses and nasopharynx can produce deep-seated facial and head pain before involving cranial nerves or otherwise becoming obvious. Trigeminal sensory loss is an important neurological sign which is associated with neurological involvement, often by perineural spread. MRI scanning is the optimal technique for detecting these cryptic lesions.
In 1934, Costen first drew attention to the temporomandibular joint (TMJ) as a cause of facial and head pain. Until recently, Costen syndrome was a rare diagnosis. During the past 2 decades, however, interest in disorders of the TMJ, the muscles of mastication, and the bite as they relate to headaches has been increasing. Painful temporomandibular dysfunction is most common between the ages of 35 and 45, after which spontaneous resolution is often seen. Mechanical disorders of the joint, alterations in the way the upper and lower teeth relate, and congenital and acquired deformities of the jaw and mandible can all produce head and facial pain and are very occasionally responsible for the episodic and chronic pain syndromes seen by neurologists.
The neurologist evaluating head or facial pain should be familiar with the criteria for identification and localization of TMJ disorders. Temporomandibular joint pain should relate directly to jaw movements and mastication and commonly associates with tenderness in the masticatory muscles or over the TMJ on palpation. Anesthetic blocking of tender structures should confirm presence and location of the pain source. A sudden change in occlusal relationship of the teeth, restriction of mandibular movement, and interference with mandibular movement (clicking, incoordination, and crepitus) are all symptoms and signs suggestive of TMJ dysfunction.
Bruxism, teeth clenching, and chronic gum chewing are important in the production of pain in the masseter and temporalis muscles. Arthritis and degenerative changes in the TMJ, loss of teeth, ill-fitting dentures or lack of dentures, and other dental conditions can all lead to the TMJ or myofascial pain dysfunction syndrome, which manifests as facial and masticatory muscle pain. Head pain and facial pain, even when associated with the above-discussed criteria, require full evaluation, which should include a detailed history and examination, appropriate radiographs, and laboratory studies to exclude other more serious causes. If TMJ dysfunction is thought to be the source of pain, further evaluation and treatment are in the province of the appropriate dental specialist. Even when TMJ dysfunction is believed to be responsible for facial or head pain, conservative management with analgesics, anti-inflammatory agents, application of local heat, and nonsurgical techniques to adjust the bite generally provide relief. Before using surgical modalities on the TMJ or mandibles, the diagnosis must be secure and other causes of head and facial pain excluded by appropriate investigations.
Pulpitis and root abscess generally produce dental pain that a patient can localize. The cracked tooth syndrome results from an incomplete tooth fracture, most commonly involving a lower molar. The initial pain is usually sharp and well localized, but thereafter the pain is often diffuse and hard to locate. After a fracture, the tooth is sensitive to cold. Pain may be felt in the head and face ipsilateral to the damaged tooth. With time, infection develops in the pulp, leading to extreme and well-localized pain. Confirmation of the diagnosis and treatment of the cracked tooth require the expertise of a dentist.
Cervicogenic headache is often a controversial diagnosis with potential medicolegal implications. Many common cervical spine pathologies, such as degenerative spondylosis, occur just as often in individuals with or without headache. Therefore, the diagnosis rests on establishing the cervical spine as a pain generator either through clinical signs, or a diagnostic nerve block. Cervicogenic headache should be strongly suspected as a diagnosis when there is occipital headache, especially when unilateral, and associated with constant neck pain.
Migraine in particular frequently presents with pain in the occipital and nuchal regions, which are innervated by the greater occipital nerve. Furthermore, muscle hypersensitivity and tenderness, restriction of neck movements, and hyperalgesia may accompany the pain. Similarly, pain of cervical origin or cervicogenic headache is prominent in the occipital region but may also spread to trigeminal territories. The referral of pain observed in cervicogenic headache and migraine reflects the convergence of trigeminal and cervical afferents onto the same neurons in the trigeminal-cervical complex. Despite this anatomical overlap, the provocation or exacerbation of the headache by neck movement, a persistent rather than intermittent headache, and lack of photophobia, phonophobia, and nausea, are features that may be helpful in distinguishing cervicogenic headache from migraine. Diagnostic blocks performed accurately and under controlled conditions are the only currently available means by which a cervical source of pain can be established. A positive response to occipital nerve block should be interpreted with caution, however, given the fact that many primary headaches, including migraine and cluster headache, may respond to this procedure. The use of intra-articular steroids and long-acting anesthetics may provide relief that can last several months, and complete relief of headache can occasionally be achieved by radiofrequency neurotomy in patients whose headache stems from the C2 to C3 zygapophysial joint ( ). Physical therapy may also be helpful in the treatment of cervicogenic headache.
Overuse of acute medications by patients with frequent headache may lead to a daily headache syndrome, now known as medication overuse headache (MOH) . Previously referred to as rebound or medication-induced headache, this syndrome is induced and maintained by the very medications used to relieve the pain. Diagnostic criteria according to ICHD-3 are designed to improve sensitivity, requiring only the presence of chronic daily headache (CDH) in the setting of exposure to an overused analgesic ( ). The risk for development of medication overuse headache varies with individual substances. Opioids, butalbital-containing compounds, and some combination analgesics appear to have the highest risk; triptans carry moderate risk, and nonsteroidal antiinflammatory drugs (NSAIDs) the lowest risk. In fact, migraine prevention guidelines include recommendations for daily NSAID exposure in the preventive treatment of migraine ( ). Further, there is longitudinal epidemiological evidence that NSAID use among individuals with <10 headache days per month is associated with a dose-dependent reduction in the development of chronic migraine ( ).
The population prevalence of CDH associated with acute medication overuse has been estimated to be 1.4% ( ). The proportion of patients in the population with CDH who overuse acute medications ranges from 18% to 33%, indicating that medication overuse is not necessary for the development of CDH, nor is the overuse of acute medication synonymous with MOH. In other words, tapering and discontinuing the overused medication does not always return the patient to an episodic pattern of headache.
The most frequently overused acute medications include analgesics, opioids, butalbital-containing products, ergotamine, and triptans, alone or in combination. The delay between the frequent intake of these medications and the development of CDH appears to be shortest for triptans (1.7 years), longer for ergots (2.7 years), and longest for analgesics (4.8 years). The duration of withdrawal symptoms after discontinuation and the recidivism rate are also shortest/lowest for triptans and longest/highest for analgesics.
The pathogenesis of MOH is unclear. A leading hypothesis suggests facilitation of central trigeminal sensitization caused by a medication-induced impairment of descending inhibition of nociceptive trafficking. In rats, chronic morphine exposure increases the pain, facilitating “on” cell activation in the rostral ventromedial medulla (RVM) that may alter the balance between the descending inhibition from the nucleus reticularis dorsalis (NRD) and the facilitation from the RVM in favor of a pro-nociceptive increased descending facilitation from the RVM ( ; ). Similar neural adaptations may contribute to opiate-induced MOH in humans by increasing the responsiveness of the nociceptive system, as well as increasing the transmission of pain signals at the medullary dorsal horn ( ). Animal studies have found that sustained or repeated administration of triptans can also induce pro-nociceptive neural adaptations, enhance responses to established triggers of migraine headache, and lower cortical spreading depression threshold, the latter of which can increase the activation of the trigeminal nucleus caudalis ( ; ). Some individuals may possess a genetically determined liability to medication overuse. A FDG-PET study in patients with chronic analgesic overuse in migraine sufferers demonstrated persistent hypometabolism of the orbitofrontal cortex (especially in patients overusing combination analgesics) even after withdrawal of the overused medication. Persistent orbitofrontal hypofunction is known to occur in substance abuse ( ).
Treatment of MOH is challenging and requires aggressive nonpharmacological and appropriate acute and preventive headache treatment. Rigorous controlled data are lacking, with current evidence supporting preventive therapy alone, withdrawal of overuse analgesics alone, and the combination of the two. It is generally considered that lifestyle modifications such as limiting or eliminating caffeine consumption, exercise, and establishing regular mealtimes and sleep schedules can be beneficial for some patients. Depression, anxiety, and sleep disturbances occur in more than half of patients and must be addressed. Training in relaxation techniques and biofeedback may be helpful, especially if stress or anxiety is a frequent provocative trigger. Patients should always be provided with support and close follow-up, particularly during the first 8 weeks after treatment is initiated.
Pharmacological treatment involves tapering or discontinuing the overused medication. Abrupt drug withdrawal is the treatment of choice except with barbiturates, benzodiazepines, and opioids. Typical withdrawal symptoms last 2–10 days (mean 3.5 days) but may persist for 2–4 weeks. In most patients, the withdrawal can be managed on an outpatient basis. Patients with coexistent medical or psychiatric illnesses and overuse of agents containing opioids, benzodiazepines, and barbiturates may need hospitalization or withdrawal in a controlled environment. Prednisone 60 mg daily for 5 days as a transitional and short-term treatment during the withdrawal phase to reduce withdrawal symptoms can be considered and may decrease the need for acute treatment during this time ( ; ). Preventive medication aimed at the underlying primary headache disorder should be started from the outset while initiating the taper of the overused substance.
Studies have indicated a high rate of relapse following withdrawal of acute headache medications in patients with presumed MOH. One prospective study reported a relapse rate of 41% in the first year and 45% after 4 years ( ). In another report with 4 years of follow-up, only one-third of patients initially treated for CDH and analgesic overuse were successful in refraining from chronic overuse of medication.
To avoid MOH relapse, in general, it is best to avoid the use of opioids and/or butalbital for the regular management of primary headache disorders. To prevent relapse, limit NSAIDs, aspirin, or acetaminophen use to ≤14 days/month and limit combination analgesics, triptans, ergot derivatives, or opioids to ≤9 days/month. Although data are limited, the effectiveness of headache preventive medications may be decreased by overuse of acute medications ( ).
The term migraine derives from the ancient Greek word hemikranios , which means “half head,” underscoring the unilateral distribution of head pain that is present in about 60%–75% of people with migraine ( ; ). Although not all people with migraine experience all potential phases of a migraine attack, the migraine attack can consist of up to four phases: the premonitory phase, aura, headache phase, and postdrome. In addition to head pain, the “headache phase” consists of a combination of photophobia, phonophobia, osmophobia, cutaneous allodynia, nausea, and vomiting. Although osmophobia is not part of the formal diagnostic criteria for migraine, when present, is considered to be highly specific for the disorder ( ). Box 102.2 shows the classification of different encountered forms of migraine, including episodic migraine with aura, episodic migraine without aura, and chronic migraine ( ).
Migraine without aura
Migraine with aura:
Migraine with typical aura
Migraine with brainstem aura
Hemiplegic migraine
Retinal migraine
Chronic migraine
Complications of migraine:
Status migrainosus
Persistent aura without infarction
Migrainous infarction
Migraine aura-triggered seizure
Probable migraine:
Probable migraine without aura
Probable migraine with aura
Episodic syndromes that may be associated with migraine:
Recurrent gastrointestinal disturbance
Benign paroxysmal vertigo
Benign paroxysmal torticollis
A survey of a sample of 20,000 households estimated 27.9 million migraine patients in the United States. More than 90% of patients report an impaired ability to function during migraine attacks, and 53% report severe disability requiring bed rest. Approximately 31% of patients with migraine missed at least 1 day from work or school in the preceding 3 months due to migraine ( ). Indirect costs of migraine related to decreased productivity and lost days of work have been calculated to be $13 billion per year; estimates are that the equivalent of 112 million bedridden days per year are due to migraine ( ). The World Health Organization has declared migraine to be among the most disabling medical conditions experienced worldwide.
Migraine has a 1-year prevalence of 12% in the general population, including 18% of women and 6% of men ( ; ). Migraine afflicts prepubescent boys and girls with a similar frequency. At puberty, the incidence of migraine increases sharply in both boys and girls, but preferentially so in girls. Peak migraine prevalence for both sexes occurs in the fourth decade of life, during which time approximately 24% of women and 7% of men have migraine ( ). Migraine tends to manifest with fluctuating frequencies of attacks throughout one’s life, with a typical trend towards milder and less frequent migraines late in life. The lifetime prevalence of migraine is about 33% in women and 13% in men ( ).
Due to headache and other migraine symptoms, migraine causes substantial pain and disability. During a migraine attack, the vast majority of migraineurs have at least mild disability and about half have severe disability, often requiring rest in a dark and quiet room ( ). Overall, migraineurs have lower physical, emotional, and social quality of life.
There is a genetic predisposition for developing migraine. Compared to the general population, first-degree relatives of people who have migraine without aura are about twice as likely to develop migraine without aura, while first-degree relatives of people who have migraine with aura are about four times more likely to have migraine with aura ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here