Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Genome editing is a rapidly developing field in which the genome of cells is modified with single nucleotide precision. This degree of precision is not achievable by other forms of genetic engineering, including contemporary lentiviral vectors, recombinases, or transposases. Not only is genome editing precise in the location of the changes made, but it is highly flexible and robust in the types of changes that can be made, including creating new mutations at specific genomic sites, the conversion of a single nucleotide to another nucleotide, and the insertion of cassettes of genes at specific genomic sites. Genome editing is also known as “gene editing,” but because the entire genome can be modified, not just genes, a more precise and comprehensive term is genome editing. Genome editing has become a powerful research tool and has generated tremendous excitement as a therapeutic approach to treat or cure disease. When genome editing is used therapeutically, it is a specific and precise approach that falls under the broader gene therapy umbrella. This chapter will describe the process of genome editing, explain different types of edits that can be made to the genome, use specific hematologic diseases as examples of how genome editing is being developed therapeutically, and ends with a brief discussion of technical and ethical issues of applying genome editing outside of the ex vivo manipulation of somatic cells.
The spontaneous frequency of a mutation occurring at a specific site in a nontransformed human somatic cell is on the order of 10 −8 to 10 −9 . Thus the ability to create specific mutations at a specific site in a reproducible fashion at high frequency is not generally possible. Moreover, the frequency of spontaneous targeted integration (the insertion of a DNA fragment at a specific location in the genome [also called gene targeting]) is on the order of 10 −6 (only one in a million cells will undergo this process). The identification of cells having undergone gene targeting can be increased by several orders of magnitude by using positive and negative selection strategies.
The frequencies of targeted mutation and targeted integration change dramatically if a DNA double-strand break (DSB) is created at the target site. Using contemporary methods to create DSBs, the frequency of targeted mutations, even in primary human cells, can now exceed greater than 90% of alleles and the frequency of targeted integration can routinely exceed greater than 50% of alleles. Therefore a site-specific DSB increases the frequency of targeted genome sequence changes (genome editing) by 6 to 9 orders of magnitude and is a key foundational principle of genome editing. This natural process can be manipulated experimentally or therapeutically to modify the cell’s endogenous genome, making genome editing both a powerful research tool and potential therapeutic modality.
The basis on which a DSB increases genome editing by this enormous magnitude is that a genomic DNA DSB is a dangerous event to the cell. It causes the loss of chromosomal integrity (it separates the telomere from the centromere). Dozens of DSBs occur spontaneously in all living cells per day, caused by variety of different genomic insults. Cells have developed redundant mechanisms to respond to and repair DSBs. The two most prominent are nonhomologous end-joining (NHEJ) and homologous recombination (HR).
In NHEJ, a complex of proteins holds the broken ends together while the ends are processed to allow ligases to seal the two ends back together. Conceptually, the ends are stitched back together. In general, the NHEJ process is quite accurate, with the two ends being joined precisely 70% of the time, but in the remaining instances the ends are joined after the loss or gain of nucleotides at the site of the break (insertions and deletions [indels]). The NHEJ repair pathway is active throughout the cell cycle. Inactivation of genes needed for NHEJ leads to genomic instability but not to immediate cell death.
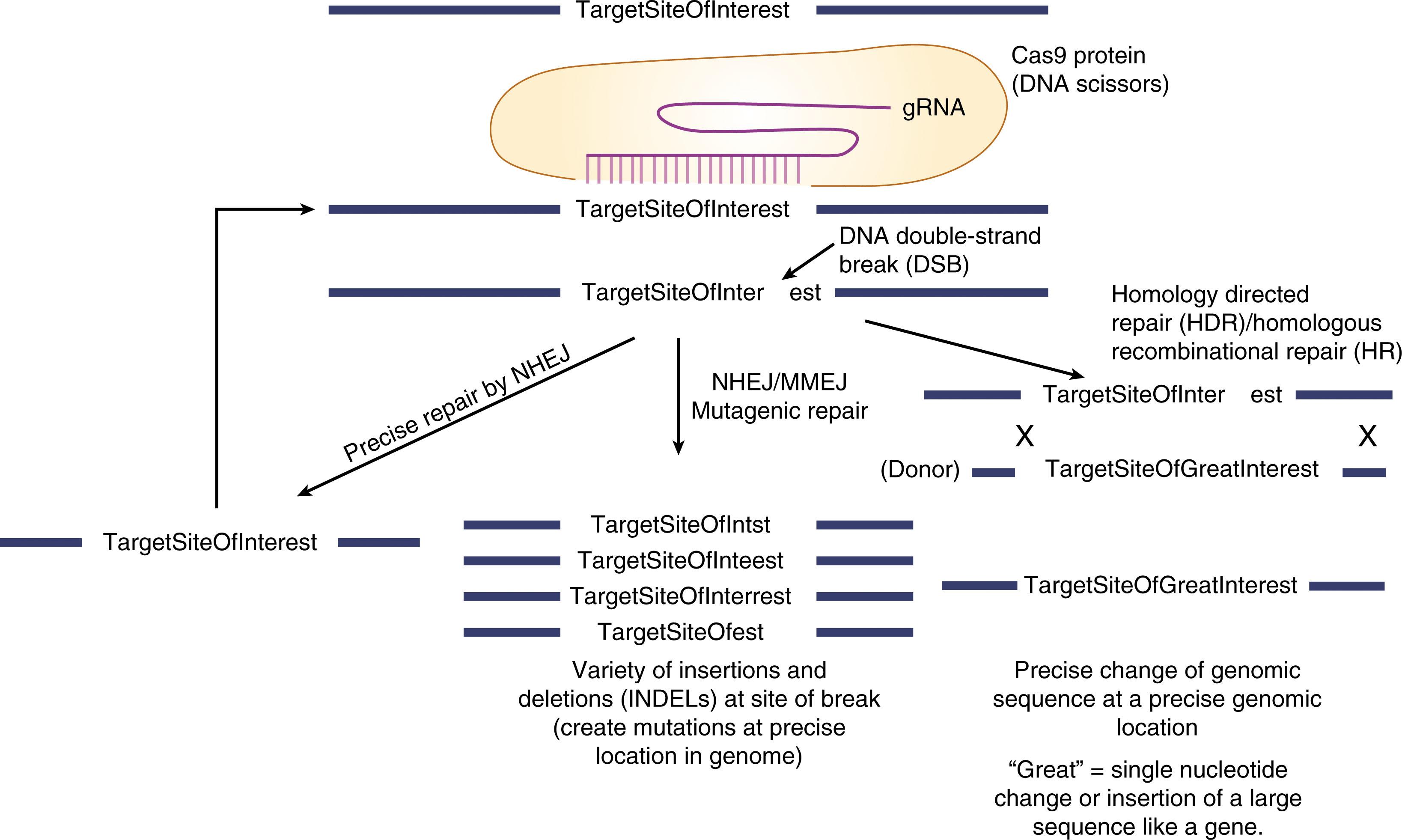
HR is a biochemically more complex process in which a much larger number of proteins are involved. In mitotic mammalian cells, the mechanism by which cells repair DSBs by HR is through the “synthesis-dependent strand annealing” (SDSA) mechanism ( Fig. 5.1 ). Conceptually, the cell uses an undamaged homologous piece of DNA as a template to synthesize new DNA and then uses the newly synthesized DNA to paste into the damaged DNA to fix the DSB. It can be thought of as a “copy and paste” mechanism. An important feature of the SDSA mechanism of repair is that the template DNA is not physically transferred into the break. Instead, it is the information in the template DNA that the recombination machinery copies and transfers into the site of the break. The normal and natural template DNA is the sister chromatid. The HR repair pathway is thought to be active only in S/G 2 of the cell cycle (when a sister chromatid is present). Mutations in important HR genes can cause cell lethality within a single round of replication.
The most well-developed genome editing system that does not require the creation of a DSB in the genome is base editing. In base editing, single nucleotide changes are made by deamination of either adenine (adenine base editors [ABEs]) and cytosine (cytosine base editors [CBEs]). The natural repair mechanisms that exist to convert deaminated adenine and cytosine back to adenine and cytosine are inhibited as part of the base editing system. In ABEs the adenine is thus converted to a guanine, whereas with CBEs the cytosine is converted to a thymidine.
The base editing systems consist of a Cas9 (Cas9 is an enzyme that cuts DNA at specific sites to which it is guided by an attached “guide RNA” [gRNA] sequence—discussed in more detail later), which has been engineered such that it makes a break (nick) in only one strand of the DNA and is fused to a deamination enzyme and an inhibitor of a deglycosylase. The inhibitor of the deglycosylase blocks the natural repair of deaminated bases. The large Cas9-deaminase-inhibitor complex is directed to the site of base editing by complexing with its gRNA. It is the engineered sequence of the gRNA that targets the complex to the correct site in the genome.
Since the first reports of the development of the CBE and ABE systems in cancer cell lines, both have been applied therapeutically as well. These applications include making new stop codons in genes to create null versions of the gene. Because base editors do not generate a DSB, they can be applied to make multiplex edits without a high risk of causing translocations such as can occur when using multiplexing nuclease-based editing. In addition to making new stop codons, base editors have also now been applied ex vivo to genetically convert the mutation that causes sickle cell disease to a hemoglobin (Hb) variant called Hb Makaser (HbM). In contrast to HbS in which a valine is at position 6 of the HBB protein, in HbM an alanine is at position 6, a harmless change. Finally, base editing has been applied in vivo in a mouse model of a Hutchinson-Gilford progeria (a genetic premature aging disease in humans) and been demonstrated to increase the longevity and improve the symptoms.
The limit of base editing is that only single nucleotide changes can be made, although simultaneous single nucleotide changes can be made at multiple sites, and that the ABE and CBEs still do not allow every possible single nucleotide substitution to be made. Although the avoidance of a DSB has been described as a possible way to reduce genotoxicity compared with nuclease-based genome editing, the full scope of off-target effects of base editors on both genomic DNA and RNA are still being investigated.
In addition to nuclease-based genome editing and base editing systems, there are multiple other genome editing systems being developed, but they have not reached the same broad utility. These include non–nuclease-based adeno-associated virus (AAV) targeted integration systems, peptide nucleic acid–based systems, PRIME-editing systems, targeted transposase systems, and targeted recombinase systems. It is likely that other systems will continue to be discovered. The range of genomic changes that can be made using these other systems can all be made at least as efficiently nuclease-based systems, but there are several types of genomic changes that can only be made using nuclease-based genome editing. The targeted insertion of large genes in primary cells, such as hematopoietic stem cells or T cells, can only be efficiently done using engineered nucleases, for example.
For genome editing to become a practical methodology, one needed to develop nucleases that could be engineered to specifically recognize any site in the genome needed to be discovered. There are now multiple engineered nuclease platforms. Each fundamentally achieves the same effect by creating a site-specific DSB. There are three major nuclease platforms that achieve specificity by protein-DNA recognition. These platforms include engineered meganucleases, zinc finger nucleases (ZFNs), and TAL effector nucleases (TALENs). Although there is a relatively well-defined protein-DNA recognition code for TALENs, the recognition code for meganucleases and ZFNs is more obscure. These systems are workable but complex and less readily adapted to widespread use.
Fortunately, the field of genome editing was revolutionized by the discovery of a nuclease platform that achieved specificity through Watson-Crick base pairing rather than protein-DNA recognition—the CRISPR/Cas9 nuclease system. In the CRISPR/Cas9 system, the multifunctional Cas9 protein is complexed to a 99-nucleotide gRNA. The gRNA has two parts—a 3′ scaffold that allows it to complex with Cas9 and a 5′ recognition sequence that determines the target site that Cas9 will cut. By designing the 5′ recognition sequence to bind to the desired genomic target (by Watson-Crick base pairing), the gRNA can be used to bring the Cas9 nuclease to the correct location. This activates its nuclease activity and cuts the DNA. The CRISPR/Cas9 nuclease system has transformed the field of genome editing not just because of the ease of designing gRNAs to desired genomic targets but also, for remarkable reasons still not completely understood, because CRISPR/Cas9 has exhibited high on-target activity with low off-target activity in essentially every cellular/animal system it has been tested in. Refinements have been made to increase activity and specificity, but the CRISPR/Cas9 nuclease worked extremely well “right out of the box.”
The transformational importance of the CRISPR/Cas9 system was codified by the awarding of the Nobel Prize in Chemistry in 2020 to Emmanuelle Charpentier and Jennifer Doudna for their discovery that the nuclease could be reduced to the two-component single-gRNA/ Cas9 protein complex. That achievement depended on vast amounts of earlier work that went into the discovery and understanding of the CRISPR system by many others who should also be recognized.
There are a wide variety of changes to the genome that can be made by simply delivering the nuclease like CRISPR/Cas9 into a cell and creating a break. When a single nuclease is delivered, high frequencies of indels can be created at a specific site. These mutations can be used to: (1) inactivate the coding region of a gene; (2) inactivate a promoter region of a gene; and (3) inactivate an enhancer or silencer element modulating a gene, among many other things ( Fig. 5.2 ).
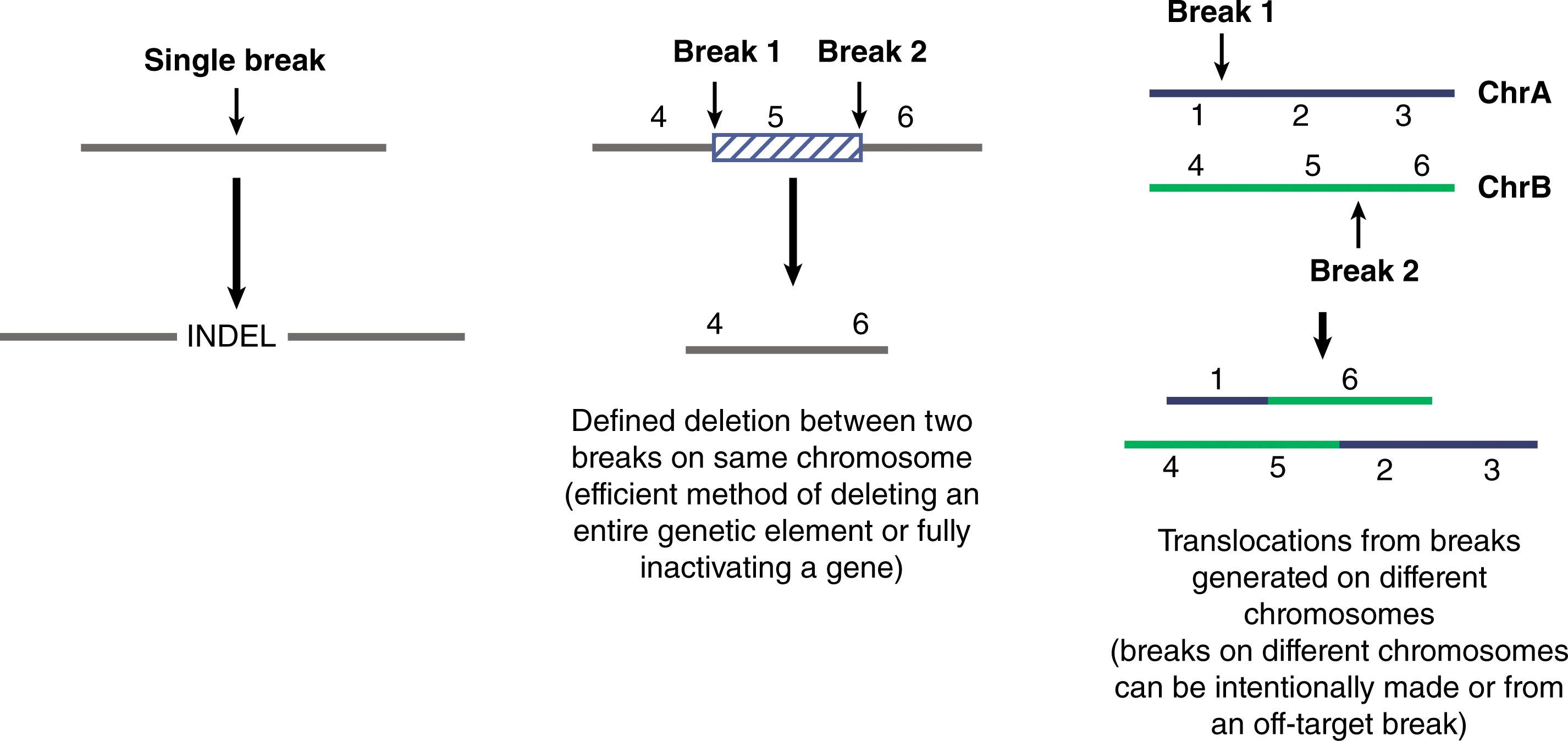
While high frequencies of indels (>90%) can be generated using a nuclease targeting a single site, some of those indels may not result in complete inactivation of the target. The repaired gene remnant may exhibit some residual activity. By delivering nucleases targeting two sites in the same genomic region, very high frequencies of defined deletions can be created (>95% when optimized). Using a two-nuclease system, defined segments of the genome can be removed, thus creating more definitive inactivation of a gene or genetic element (e.g., an enhancer or aberrant splice site). The frequency of deletions is highest when the region of the deletion is relatively short but can still be high for deletions of several thousand base pairs in some cases and can even create megabase deletions at reasonable frequency as well.
The two-nuclease approach for targeting sites on different chromosomes can generate high frequencies of translocations. For research this can be a useful method to interrogate the role of specific translocations in development and oncogenesis. For therapeutic purposes, such translocations need to be evaluated as a potential safety issue.
HR-based genome editing requires that both a nuclease and a donor DNA molecule be provided to the cell. In HR-based editing, the cell’s natural HR machinery uses the engineered undamaged donor as the template for the repair of the nuclease-induced break. To differentiate HR that repairs a DSB using the sister chromatid as a natural form of repair from genome editing HR, the term “homology-directed repair” (HDR) has been coined to describe the application for genome editing ( Fig. 5.3 ). In nuclease-based gene targeting, the donor vector needs to have flanking homology arms of greater than 400 bp (although shorter can work). These relatively short homology arms are in contrast to the several thousand base pair homology arms needed for mouse ES cell gene targeting.
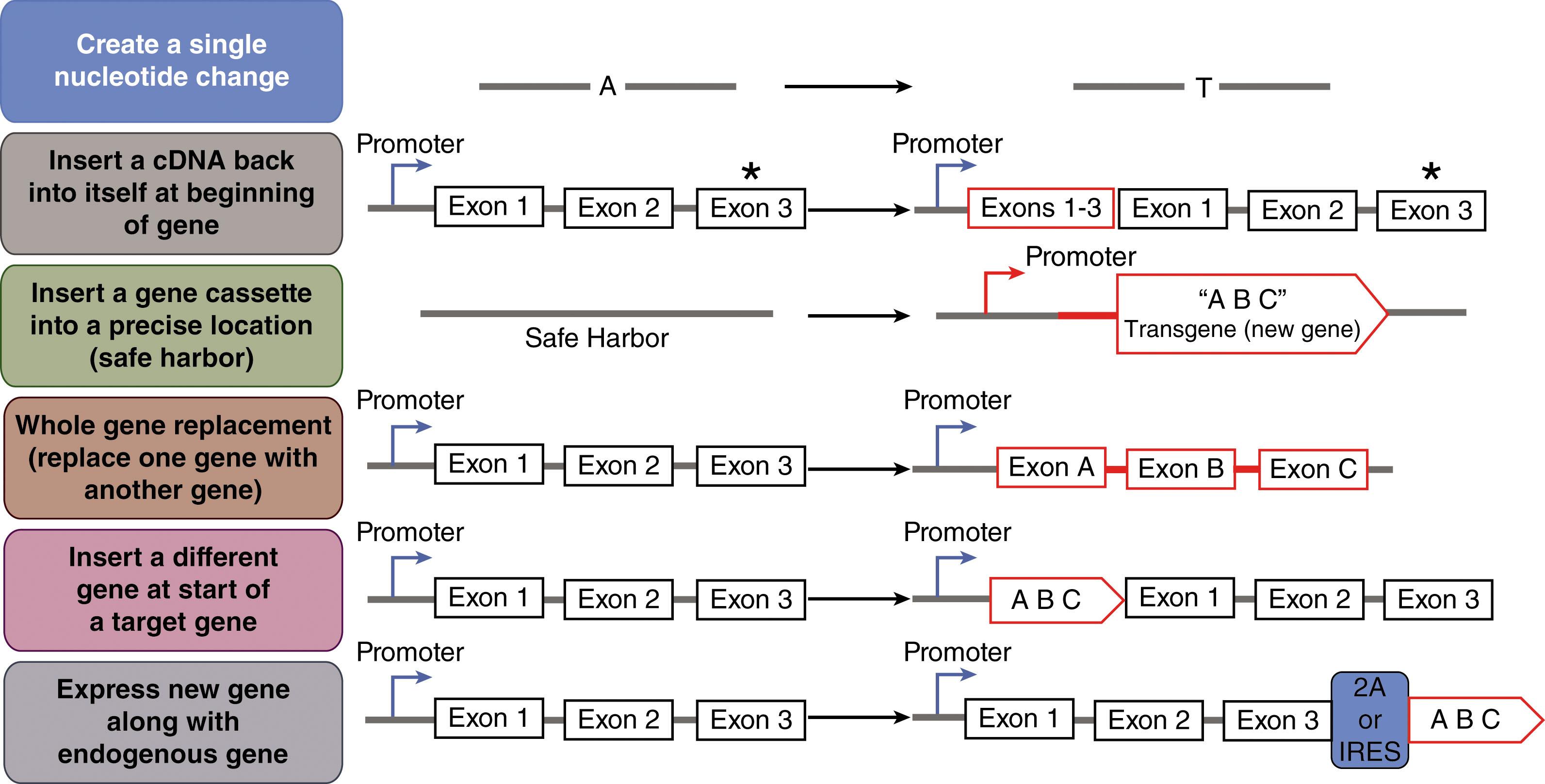
There are a wide variety of changes that can be made using HDR-based genome editing that can be used for both research and therapeutic purposes. These include:
Making single nucleotide changes to the genome, such as to revert a disease-causing genetic single-nucleotide variant;
Inserting a short stretch of nucleotides at specific location, such as to add an epitope tag to a protein;
Inserting a full complementary DNA (cDNA) or partial superexon cDNA back into a gene to functionally correct downstream mutations;
Inserting one gene into another gene or replacing one gene with another gene. In this form of synthetic biology, the genome is being reprogramed to express the inserted gene under the control of the target gene’s regulatory apparatus.
Inserting one gene into another gene without disrupting the target gene. Thus the knockin gene is now expressed along with the target gene in the cell.
Inserting promoter-gene cassette(s) into a safe harbor locus. A safe harbor is defined as a genomic location that if DNA is inserted into it, there would be no deleterious consequence. A safe harbor can be universal, in which it would safe to insert in all cell types or can be cell type specific, where insertion is safe in that cell type but not in another. There are many possible safe harbor options, but each needs to be carefully evaluated.
A version of HDR is to use a single-stranded oligonucleotide (ssODN) as a template rather than a gene targeting donor vector. The mechanism by which ssODNs are used by the cell to repair a DSB is not through the standard recombination pathway but instead through a pathway called “single-stranded template repair” (SSTR). SSTR does not require canonical HR genes such as Rad51 but instead requires genes in the Fanconi anemia family. The nonnuclease targeted integration of single-stranded AAV vectors also requires genes from the Fanconi anemia family. Presumably SSTR-based editing, like HR based editing, is harnessing a natural pathway that cells use to repair DSBs, but the natural function of SSTR, unlike HR, has not been well established.
Although all engineered nucleases are designed to have single site specificity, as proteins with biochemical properties, there is the possibility that because off-target binding (determined by standard on- and off-rates), they might create DSBs at unintended genomic sites. Because DSBs have the potential to create genotoxicity, the measurement and minimization of potential off-target breaks have been an important part of the genome editing field.
There are multiple different methods to identify potential off-target sites, including bioinformatic methods, in vitro biochemical methods, and cellular-based methods. Each of these methods has its own strengths and weaknesses, and currently the approach to evaluating the specificity of nucleases used for translational purposes is to use a combination of approaches. In contrast to knockdown approaches using short-hairpin RNAs (shRNAs), it is remarkable that there have been no reports of off-target effects confounding an experimental result, and thus, for research purposes, using bioinformatics to identify guides with low probability of off-target cutting based on sequence homology has been adequate. The gold standard for measuring potential off-target effects is to quantify the frequency of indels using amplicon deep sequencing from the cell population of interest. Currently the sensitivity of this approach is reliable only down to the 0.1% level.
Because of the importance of specificity, there have been several engineered variants of the Cas9 derived from Streptococcus pyogenes (the most used source for Cas9 for ex vivo editing) with increased specificity. The increased specificity has been obtained by targeting different biochemical properties of the Cas9 protein. However, some of these variants may have been overengineered and have lost on-target activity in the process of creating increased specificity. Care must then be used to identify which Cas9 is best designed for the specific project. Using these engineered specificity variants, there are now multiple examples of no measurable off-target indels being created using a given gRNA.
The potential for off-target breaks induced by a nuclease should also be put into context of the cell’s robust ability to repair the wide variety and large number of DNA insults it is challenged with each day. These include tens of DSBs at random sites and thousands if not tens of thousands of other lesions, most of which occur randomly in the genome, including in tumor suppressors and oncogenes. In contrast to lentiviral vectors, there has been no description of a well-designed engineered nuclease causing lesions in a tumor suppressor or oncogene as an off-target event.
Genome editing, especially with the discovery and development of the CRISPR/Cas9 system, has become a powerful research tool in hematology. It is a genetic tool that now gives researchers the opportunity to genetically modify blood cells, both by NHEJ and HR, at frequencies that were once only available to yeast researchers. It has become the new standard to create genetically engineered models of human disease because zygote editing in animals has become so efficient. Animal models of human disease can now be more rapidly made in mice (including in different genetic backgrounds) and, even more importantly, beyond mice to other species. These nonmice animal models are likely to be more informative to the human condition than what was possible using in-bred laboratory mouse models.
The CRISPR/Cas9 system has also given researchers a tool to do whole genome genetic screens using libraries of gRNAs that target different parts of the genome. These screens were first focused on the coding regions of protein-coding genes but are now being applied to other parts of the genome as well. These screens allow mutations to be created simultaneously in a wide variety of genes and then the population of cells to be screened for changes in phenotype. The specific genes identified by CRISPR/Cas9 knockout screens are different than those identified by shRNA knockdown screens but usually identify the same pathways—thus these two types of genetic screens are complementary to each other.
Although most of the broad screens have been done in transformed cell lines, it is also now possible to perform such screens in primary cells, especially human T cells, by limiting the breadth of the screen. Although there is some compromise in breadth, by avoiding the artifacts of screens in transformed cells that have been adapted to grow in a laboratory environment, often for decades, it is possible that understanding of the functioning of primary human blood cells will proceed more rapidly.
Hematopoietic stem cell transplantation (HSCT), whether allogeneic or autologous, has been a validated curative approach to a wide variety of malignant and nonmalignant diseases since the late 1960s and early 1970s. In the past decade, the utility of hematopoietic cell therapy (HCT) to deliver genetically engineered T cells to fight cancer (notably chimeric antigen receptor [CAR] T cells) has also been established. Delivering ex vivo genome-edited hematopoietic stem and progenitor cells (HSPCs) or HCT (e.g., CAR-T cells) via HCT is building on this well-established therapeutic foundation and has the potential to make HSCT and HCT both safer and more effective.
However, two key principles need to be considered for ex vivo genome editing for HSCT- and HCT-based therapies. The first is that there is still a need to create niche space for the cell product prior to infusion. Currently for HSCT, this requires the use of myeloablative, genotoxic chemotherapy and, for HCT, pharmacologic depletion of T cells. The requirement for niche clearance creates toxicity and is presently needed for genome-edited cell therapies. It is possible in the future that genome editing could lead to approaches to make niche clearance safer and more effective. The second is the need to preserve potency and safety of the cell product after ex vivo manufacturing. Currently, genome editing requires cells to be maintained outside the body in an unnatural environment, and care must be taken to ensure that in the process of genome editing, the target cell population does not lose its biologic potency. For HSCs, for example, that would be the ability maintain the hematopoietic system for the lifetime of the patient by undergoing self-renewal and the ability to differentiate into all of the hematopoietic lineages.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here