Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Niri S. Niranjan and Charles Y.Y. Loh
Perineal reconstruction is a challenging feat and there are multiple considerations for the surgeon. Discussed here are the relevant anatomy, common workhorse flaps for perineal reconstructions, our tips and tricks from experience, especially – from its conception – with the lotus petal flap, and the importance of reconstructing the perineal body.
The management of advanced pelvic malignancy is challenging and requires a multidisciplinary approach. The potential method of resection and reconstruction for total perineal defects should be discussed by a multidisciplinary team (MDT), which should consist of a colorectal surgeon, urologist, gynecologist, oncologist, radiologist, plastic surgeon, specialist nurse practitioner, physiotherapist, and psychologist. Factors that dictate the choice of method are the patient’s general condition and habits (age, sex, diabetes, atherosclerosis, obesity, smoking, and nutritional state) and the defect created (skin, pelvic viscera, pelvic muscles). Some of the patients would have already undergone surgery and radiotherapy. In these patients, the local option would have either already been used or not be possible because of radiotherapy. The patient should be fully informed of the surgical treatment, the outcome, and possible major life adjustment postoperatively (e.g., colostomy and urinary conduit). An anesthetist who is regularly involved in this type of procedure should assess the patient.
Total perineal reconstruction, including skin and pelvic viscera, may be indicated for advanced cancer and post-irradiated cancer recurrence, total perineal skin loss in extensive debilitating infection or following full-thickness burn or trauma.
The “clockface” assessment of the defect is a very useful method of considering anatomically sound options available. There are numerous perforator-based or myocutaneous flaps available beyond the clockface territory of the lotus petal flaps and extended lotus petal flaps, which serve this purpose. , These flaps are away from disease and irradiated areas of the perineum and hence will provide well-vascularized tissue for primary healing. The aim is to achieve wound closure with primary healing. Sexual function has to be sacrificed and excretory function is diverted. One should consider using a larger flap either on its own or in combination with local flaps if available. The preferred large flaps are the vertical rectus abdominis myocutaneous (VRAM) or deep inferior epigastric perforator (DIEP) flaps at 12 o’ clock; the anterolateral thigh (ALT) or transverse myocutaneous gracilis (TMG) flaps at 9 and 3 o’ clock; and the superior/inferior gluteal artery perforator (SGAP/IGAP) or posterior thigh flaps at 7 and 5 o’ clock. If and when there is a defect beyond the perineum, a larger flap like an extended VRAM, DIEP or bilateral ALTs may be considered. The bilateral TMG flap, either with a transverse or longitudinally-oriented skin paddle, is a secondary option.
The perineal region has two important components, one excretory and one sexual. Malfunction or dysfunction of either of these can affect the whole body. The perineum has a very rich blood supply, fed by both the femoral and the iliac arteries (similar to the face by external and internal carotid vessels). Branches from these vessels anastomose with each other around the urogenital and anal orifices and this enables reconstructing surgeons to use skin flaps based on the perforators from these vessels.
The soft tissue reconstruction is challenging both in terms of functional and esthetic outcomes. Local flaps based on perforators are the ideal as they replace like tissues. Regional myocutaneous flaps continue to have their place in reconstruction of the region.
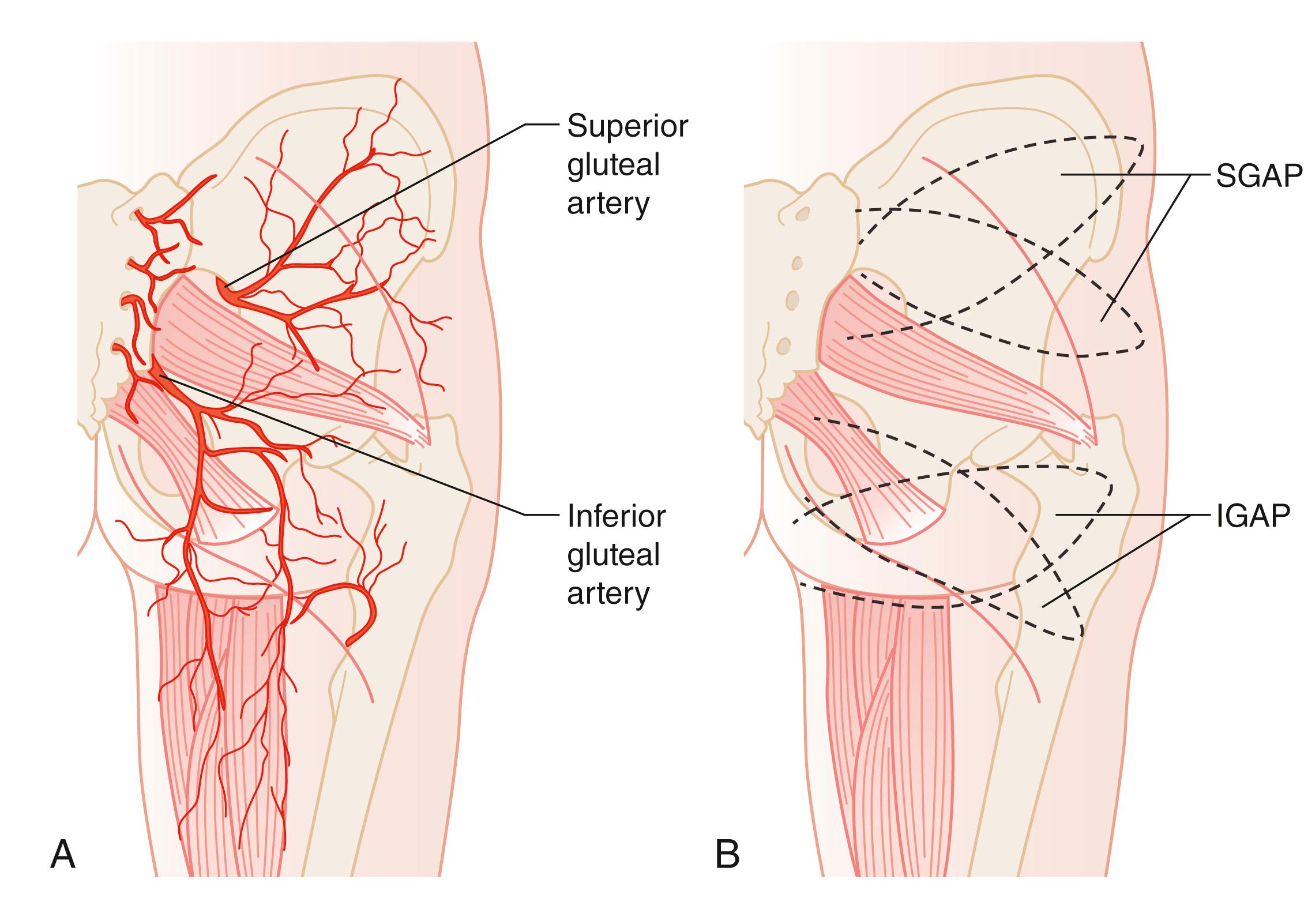
In 1889 Carl Manchot divided the cutaneous area of the perineum into anterior and posterior regions. He also described the blood supply of the perineum in great detail. Branches of the femoral and internal iliac arteries form the rich vascular network of the perineum. The anterior region is supplied by the superficial external pudendal artery and deep external pudendal artery. The obturator artery, internal pudendal artery, and branches of the inferior gluteal artery supply the posterior half of the perineum.
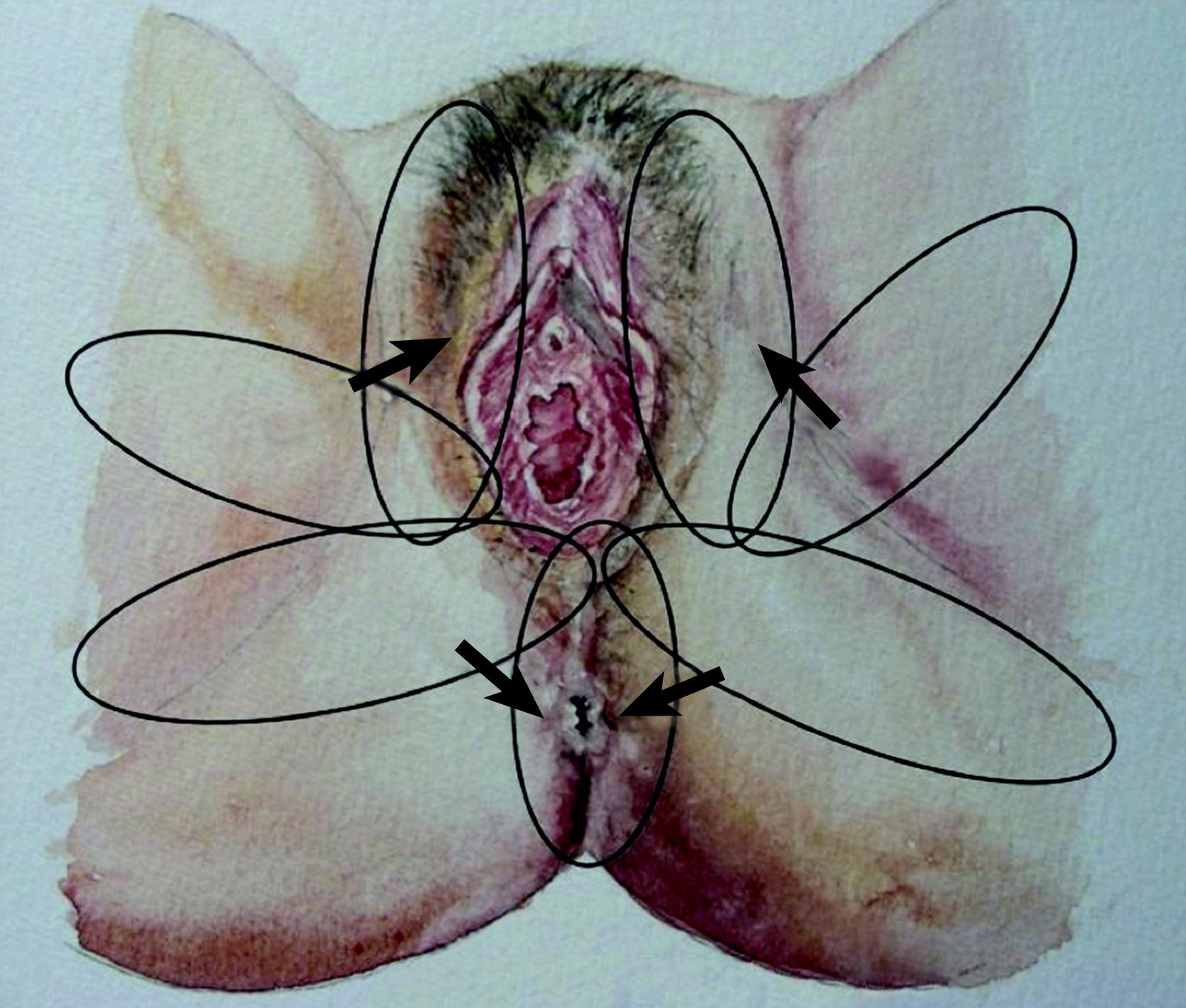
The internal pudendal artery gives out two terminal branches: the penile/clitoral branch and the perineal artery. The perineal artery supplies the labial/scrotal part of the perineum via its terminal branches, which anastomose with their contralateral counterpart. The perineal artery also gives off medial and the lateral branches. The medial branch supplies the perianal region and the lateral branch supplies an area of posterior surface of the upper thigh. All these branches anastomose with their counterpart from the opposite side and hence form a rich circle of vascular anastomoses around the orifice. This vascular network provides the basis of the perforator flaps ( Fig. 41.1 ).
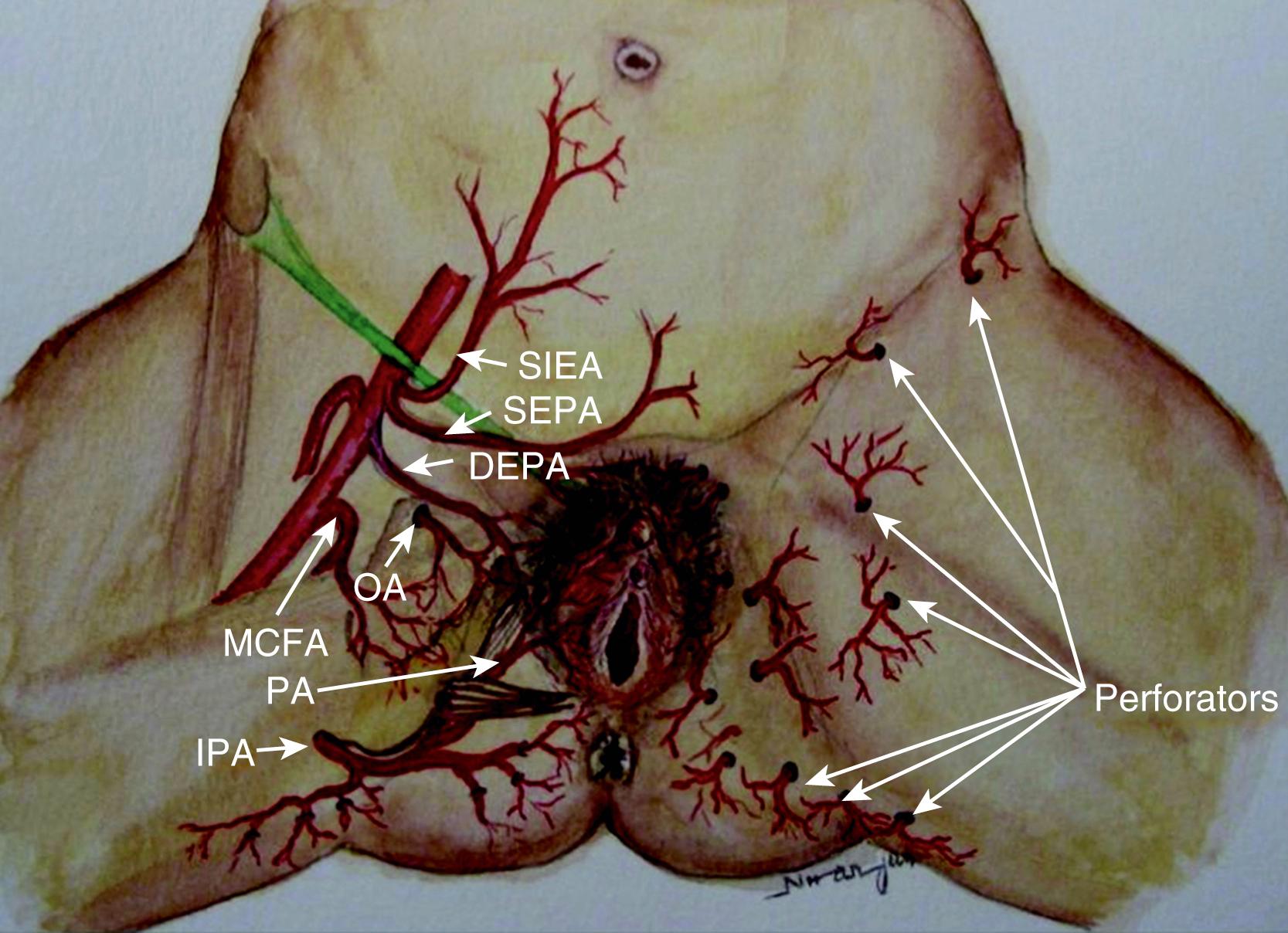
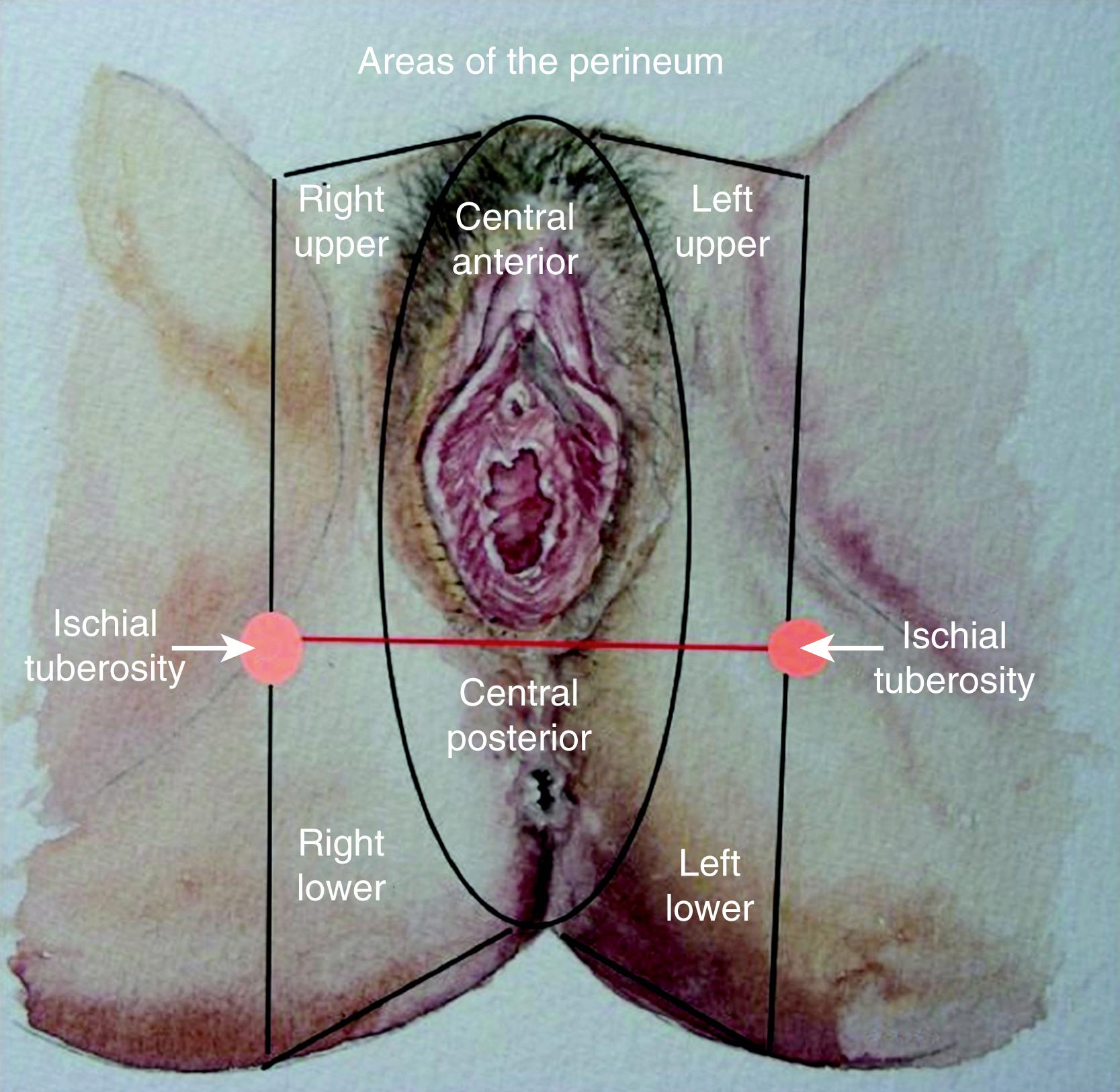
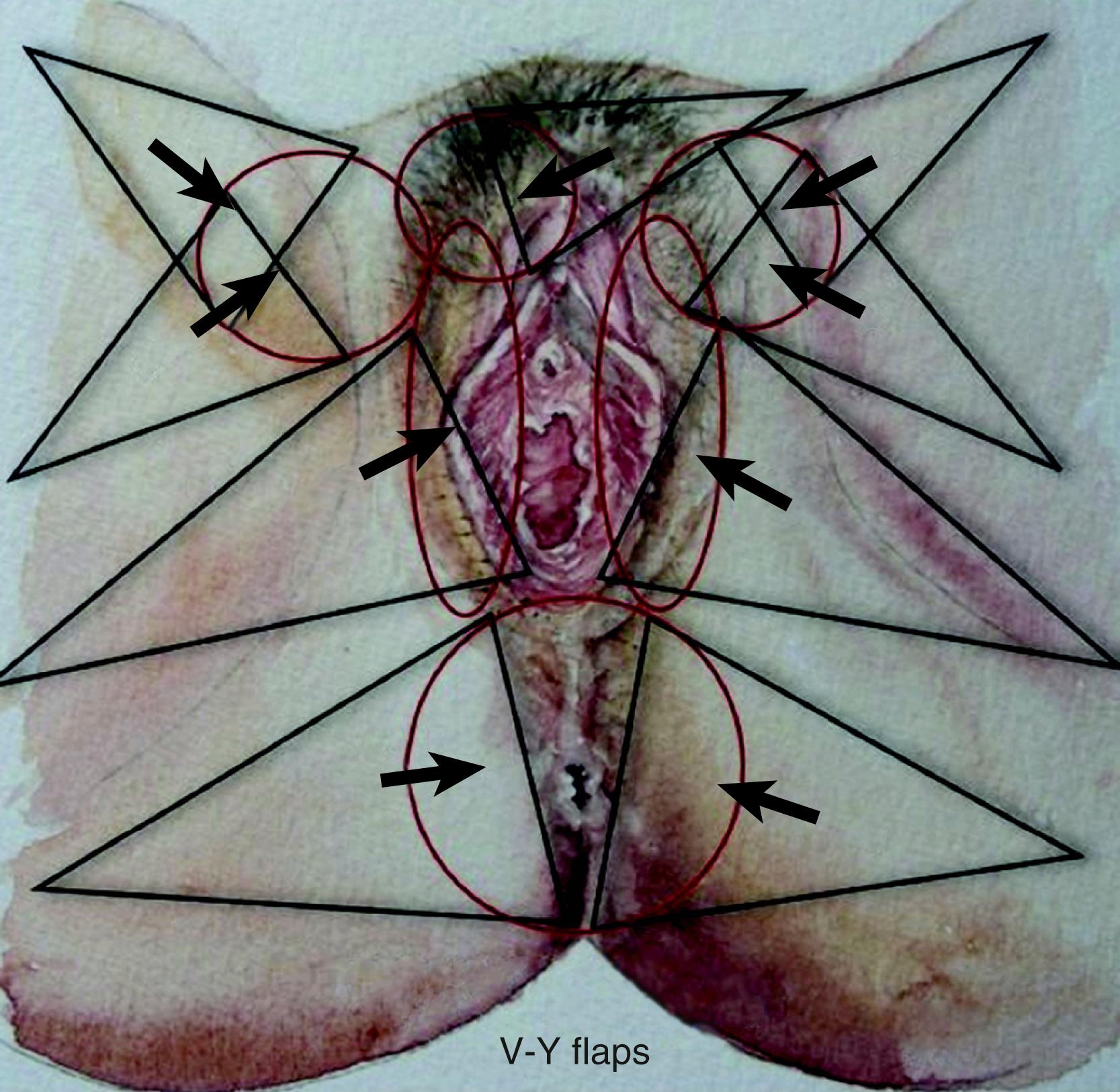
The ideal flap for any perineal defect should use reliable and robust like tissue, not be too bulky, and should have protective sensation to maintain normal function and appearance. There should also be minimal donor site morbidity. For practical purposes the perineum can be divided into right, left, and central areas. A line drawn between the ischial tuberosity divide these areas into anterior and posterior halves ( Fig. 41.2 ). The central area contains the urogenital orifice in the anterior half and the anal orifice in posterior half. In the majority of the cases the defect to be reconstructed is created in the central area, for example due to vulvar/vaginal or anal neoplasia. Other situations where flap reconstruction is used are congenital malformations and severe debilitating infections (like hidradenitis suppurativa). Traumatic defects requiring local flaps are uncommon. For defects in the upper quadrant, donor skin flaps could come from the groin and/or mons pubis area, whilst for the lower quadrant the donor site may be from the gluteal fold and/or gluteal area. , The mid-thigh can also provide a flap for these situations. For the central area of the perineum the donor could be selected from any of the above sites. The selection depends on the site and size of the defect. There are three ways of moving local perforator flaps, namely rotation (lotus petal flaps, Fig. 41.3 ), V–Y advancement flaps ( Fig. 41.4 ), and transposition (pudendal, mons pubis flaps, Fig. 41.5 ).
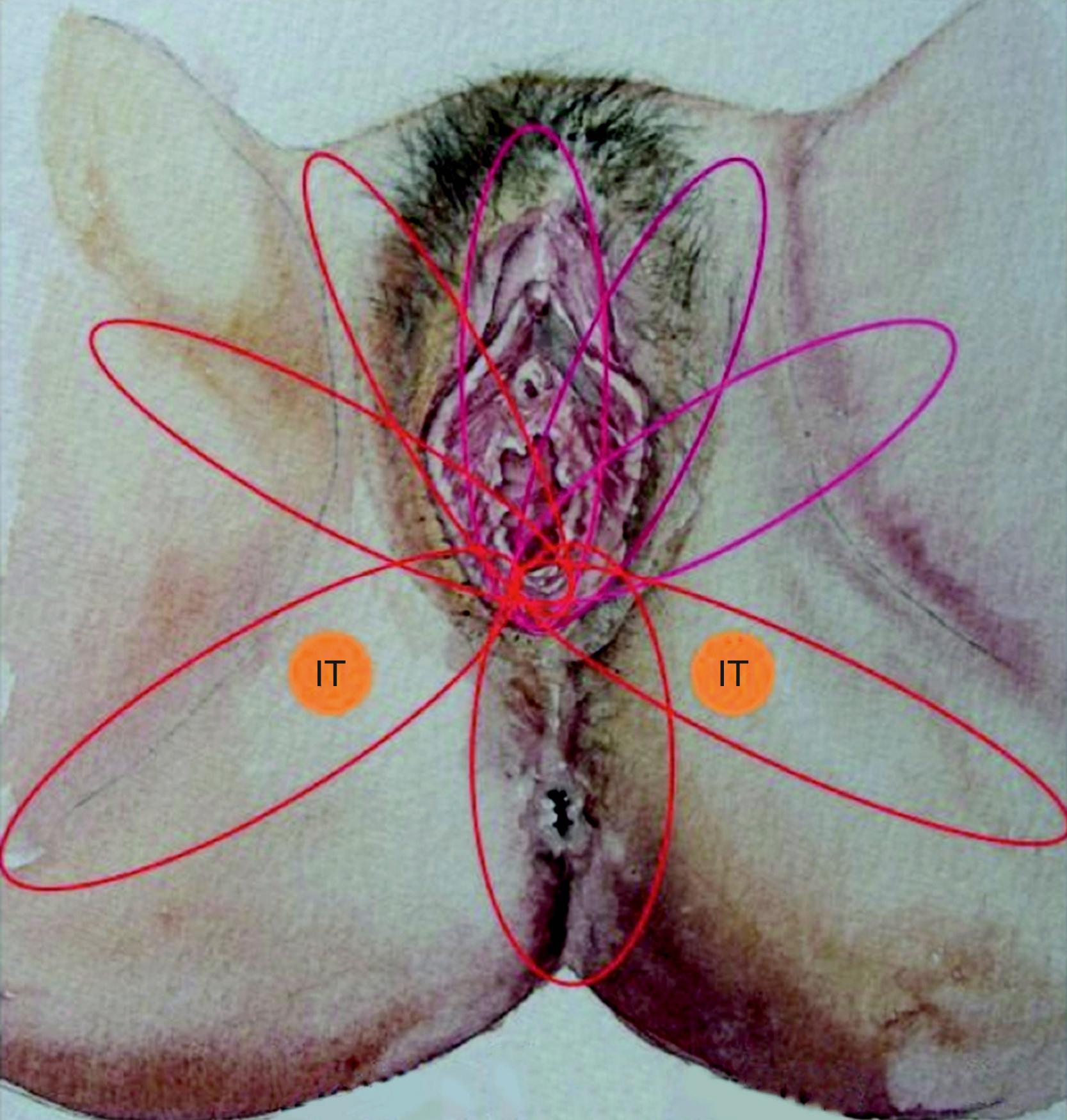
Horton et al in 1973 reported local random pattern flaps designed around the genital area. Other types of skin flaps used during the 1970s and 1980s were mostly myocutaneous flaps. Among these are, the gracillis (reported by McCrow et al in 1976), tensor fascia lata (Nahai et al in 1979 and Chafe et al in 1983) and rectus abdominus (Tobin and Day in 1988 ). These flaps were obviously bulky and accompanied by considerable donor site morbidity. The fasciocutaneous flap emerged in the early 1980s and in 1987 Wang et al advocated medial thigh fasciocutaneous flaps, whilst Wee and Joseph in 1989 described a pudendal thigh flap with an intact nerve supply for vaginal reconstruction. Woods et al modified the same flap in 1992. Where possible, the senior author attempts reconstruction using local perforator fasciocutaneous flaps, which schematically form the petals of a lotus.
Surgery may be performed either under general or regional anesthesia. The patient is placed in the Lloyd Davies position. A urinary catheter is inserted. Prophylactic antibiotics should be given at induction and for 48 hours postoperatively. The defect is created during the resection for either cancer or infection or the debridement of traumatized tissue. Once the dimension of the defect is studied and assessed, the required flap is planned over the donor area. The flap is planned in a reverse manner by using a swab or a pattern of the defect turned towards the selected donor site in reverse. The success of perforator flap reconstruction depends on the selection of the perforator vessel at or around the base of the selected flap. Before the operation, perforator vessels are mapped using a hand-held Doppler ultrasound probe at the base of the skin flap. An exploring incision is made along one edge of this flap down to the muscle. The flap is elevated with the deep fascia and the perforating vessel is reassessed. In rotation or transposition flaps, perforators should be selected closer to the defect. However, for V–Y advancement flaps, perforators can be anywhere in and around the mid-axis of the V–Y flap.
In 1989, MacAlister introduced the term perineal body to describe the site that separates the cloacal sphincter into anal and urogenital portions. The perineal body is a complex fibromuscular mass into which many structures insert. It is bordered cephalad by the rectovaginal septum (Denonvilliers’ fascia), caudad by perineal skin, anteriorly by the posterior wall of the vagina, posteriorly by the anterior wall of the anorectum and laterally by the ischial rami. The perineal body has been compared to that of a red pine cone, with each “petal” representing insertion of the fascia or muscles of the perineum.
The perineal body is an important part of the perineum. It provides a physical barrier between the vagina and the rectum, both of which it anchors, maintaining urinary and fecal continence. When the perineal body is damaged following, for example, gynecological surgery or obstetric perineal trauma, the patient may suffer from pain, dyspareunia, aerovagina, and fecal and/or urinary urgency or incontinence. The perineal body is also an important part of the “orgasmic platform,” which can be damaged when the perineal body is damaged by an injury or disease.
Optimal primary repair followed by training of the perineal body can reduce most distressing physical and psychosexual consequences. However, if a patient requires subsequent surgery, it would be advisable to have a multidisciplinary perineal team approach (gynecologist, colorectal surgeon, urologist, and plastic surgeon). The team should also involve expertise in dealing with psychosexual problems.
The plastic surgeon’s role is usually focused on scar revision and reconstruction following tissue loss over the perineal body. This can be achieved using local perforator flaps to build a tissue bridge between the vagina and the anorectum.
The VRAM flap should be considered as first choice for patients who are undergoing abdominoperineal resection. This is an ideal flap in total perineal resection, i.e., pelvic exenteration. The perforators of the deep inferior epigastric artery are marked over the abdomen using ultrasound Doppler and/or CT angiogram. Following completion of resection, the perineal defect is re-assessed in terms of the size of skin defect and the volume of pelvic cavity. The deep inferior epigastric vessels can be inspected through the abdominal wound and the required skin paddle is re-marked working out the required length of the pedicle ( Figs. 41.6–41.8 ).
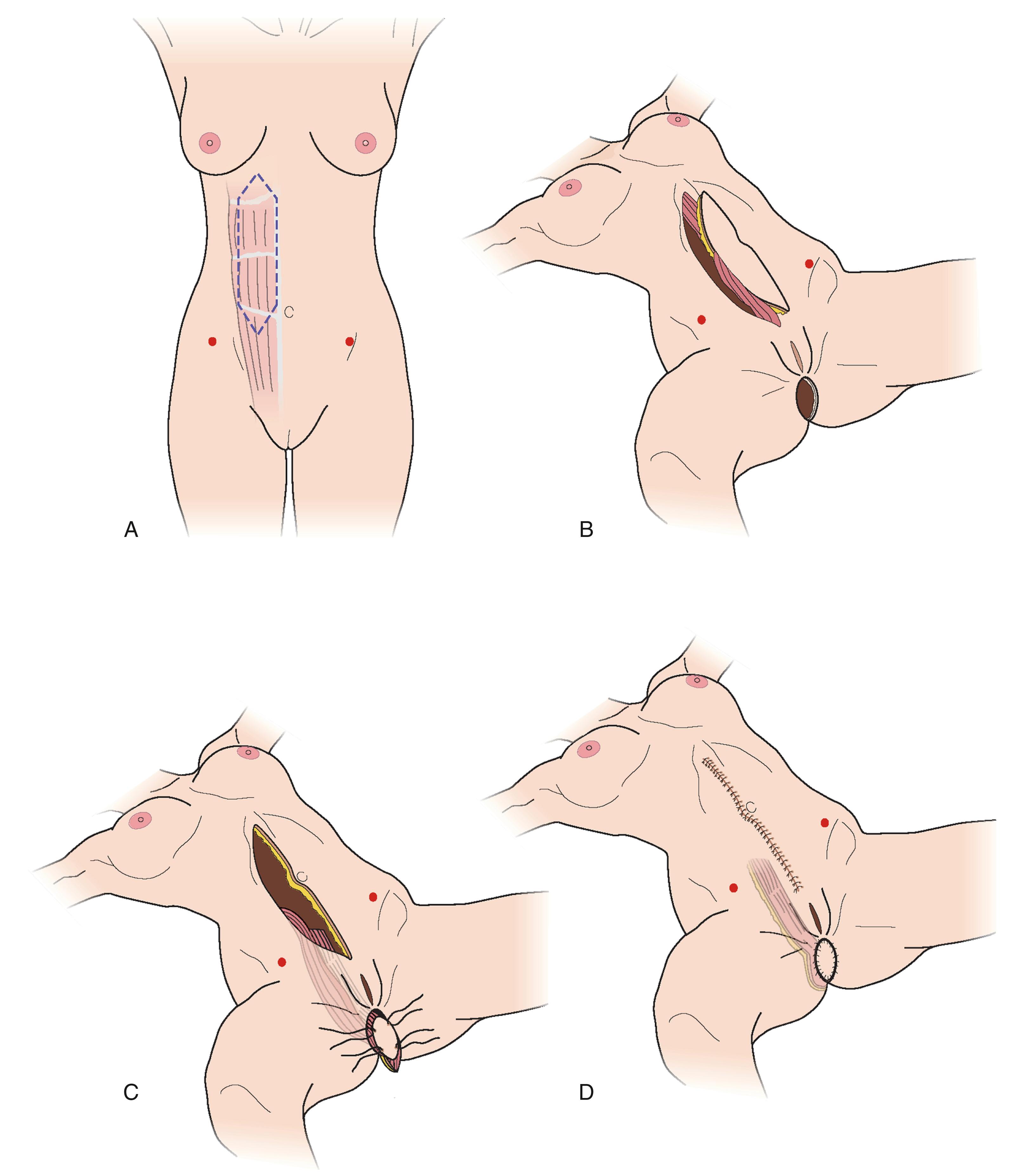
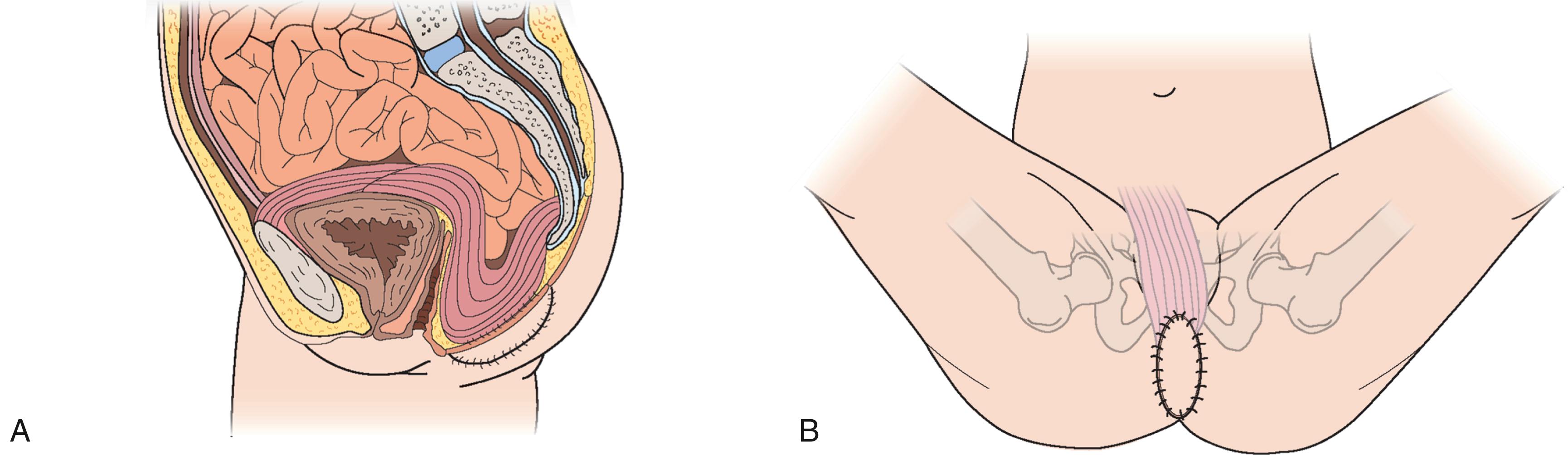
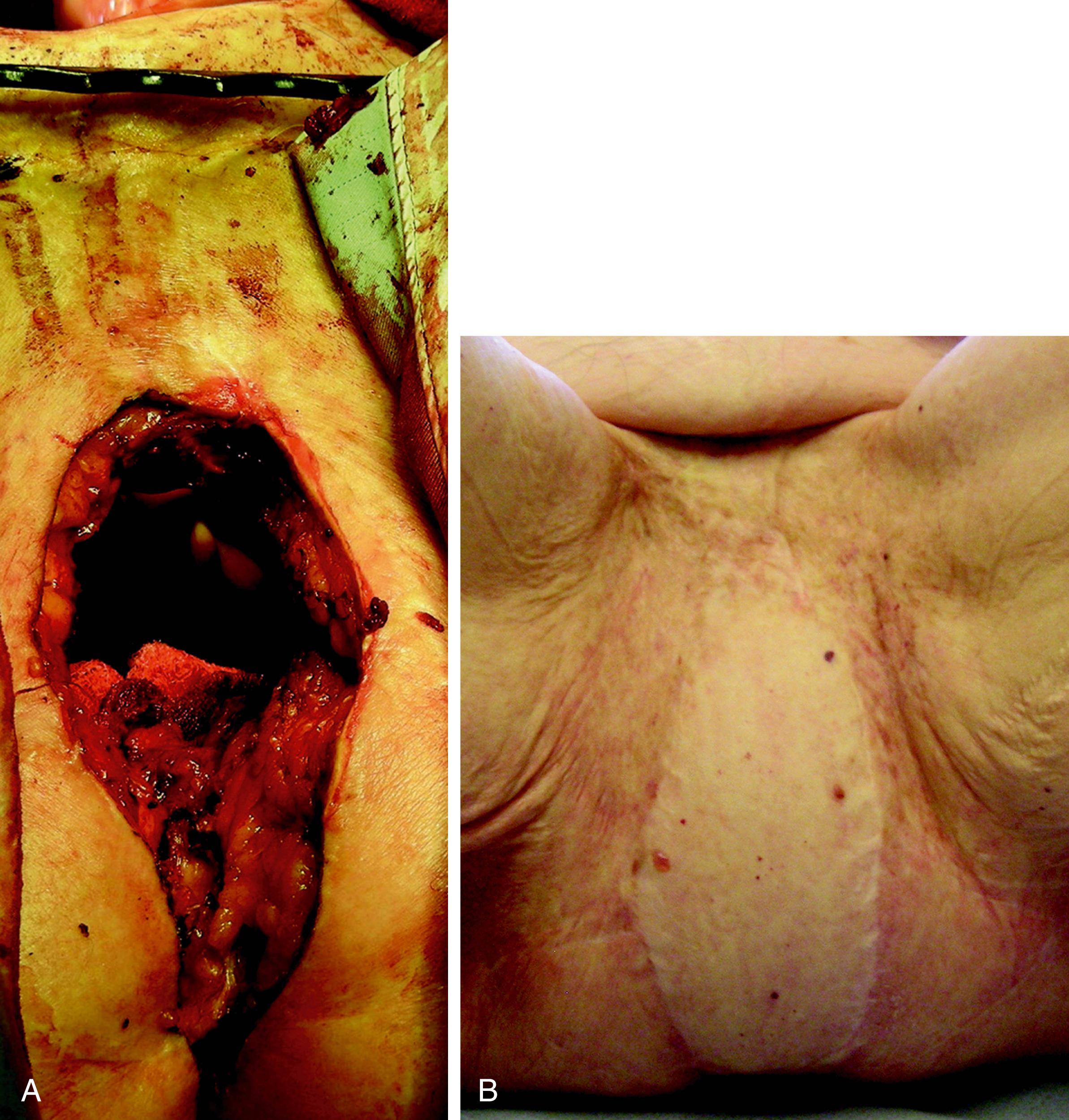
The midline incision is extended superiorly and the lateral incision of the skin paddle is incised down to the rectus sheath. The rectus sheath is incised just laterally to the lateral row of perforators and a strip of the fascia is kept intact all along the rectus muscle. The flap, consisting of muscle, a strip of fascia, and an island of skin, is elevated. The deep inferior vessel is identified and carefully dissected right down to its origin. The lower attachment of the muscle is also divided, which helps in orienting the flap in the pelvic cavity and in delivering the skin paddle through the perineal wound.
The muscle is sutured to the pelvic floor. The skin paddle is usually larger than the defect hence it is de-epithelialized, leaving the exact amount of skin required. The skin paddle is inset keeping two drains, and the abdominal wound is also closed in layers. Postoperatively, the patient is mobilized as soon as possible.
The ALT flap is now a workhorse in reconstructive surgery, first described by Song et al in 1984 as a septocutaneous flap. The major advantages of this flap are that it provides a large amount of skin for harvest and that the pedicle is close enough to reach the perineum, but far enough to be away from diseased and irradiated areas of the perineum.
The blood supply of the ALT flap is dependent upon the perforators of the descending branch of the lateral circumflex profunda femoral artery. In general, a large skin flap can be raised but a width of up to 8 cm can be closed directly and any wider skin flap requires a skin graft. The perforators are assessed for a visible pulsation and for venae comitantes. Where of septocutaneous type, the vessels traverse the septum between rectus femoris and vastus lateralis muscles. If the perforators are of a muscular type, which usually comes from the vastus lateralis, that part of the muscle may also be included to add bulk to the flap. The flap is passed under the rectus femoris muscle and through the subcutaneous tunnel to reach the perineum, making sure that there is no tension on the pedicle. The perineal wound is closed in layers with a drain and the donor area is either closed directly, or with a skin graft.
Postoperatively, the patient may be nursed in slight flexion and also abduction of the thighs to prevent any compression on the pedicle, although this is rarely necessary. The patient is mobilised after 48 hours.
The gluteus maximus muscle is a large muscle of the buttock area. It has two dominant blood supplies as a type III muscle flap. The superior and inferior gluteal arteries arise from the internal iliac artery and they emerge on either side of the pyriform muscles. The superior gluteal artery supplies the upper part of the gluteus maximus and the inferior gluteal artery supplies the lower half. The inferior gluteal artery is a direct continuation of the internal iliac artery emerging below the pyrifomis muscle, accompanying the sciatic nerve, inferior gluteal nerve, the internal pudendal vessel, and posterior femoral cutaneous nerves. The cutaneous perforators of the inferior gluteal artery, about 8 ± 4, are concentrated in the middle third of the gluteal region, parallel to the gluteal crease. The SGAP or myocutaneous flaps would be suitable for the sacral part of the defect whilst the IGAP or myocutaneous flaps would be suitable for a large part of the perineum and also in pelvic exenteration ( Figs. 41.9–41.11 ).
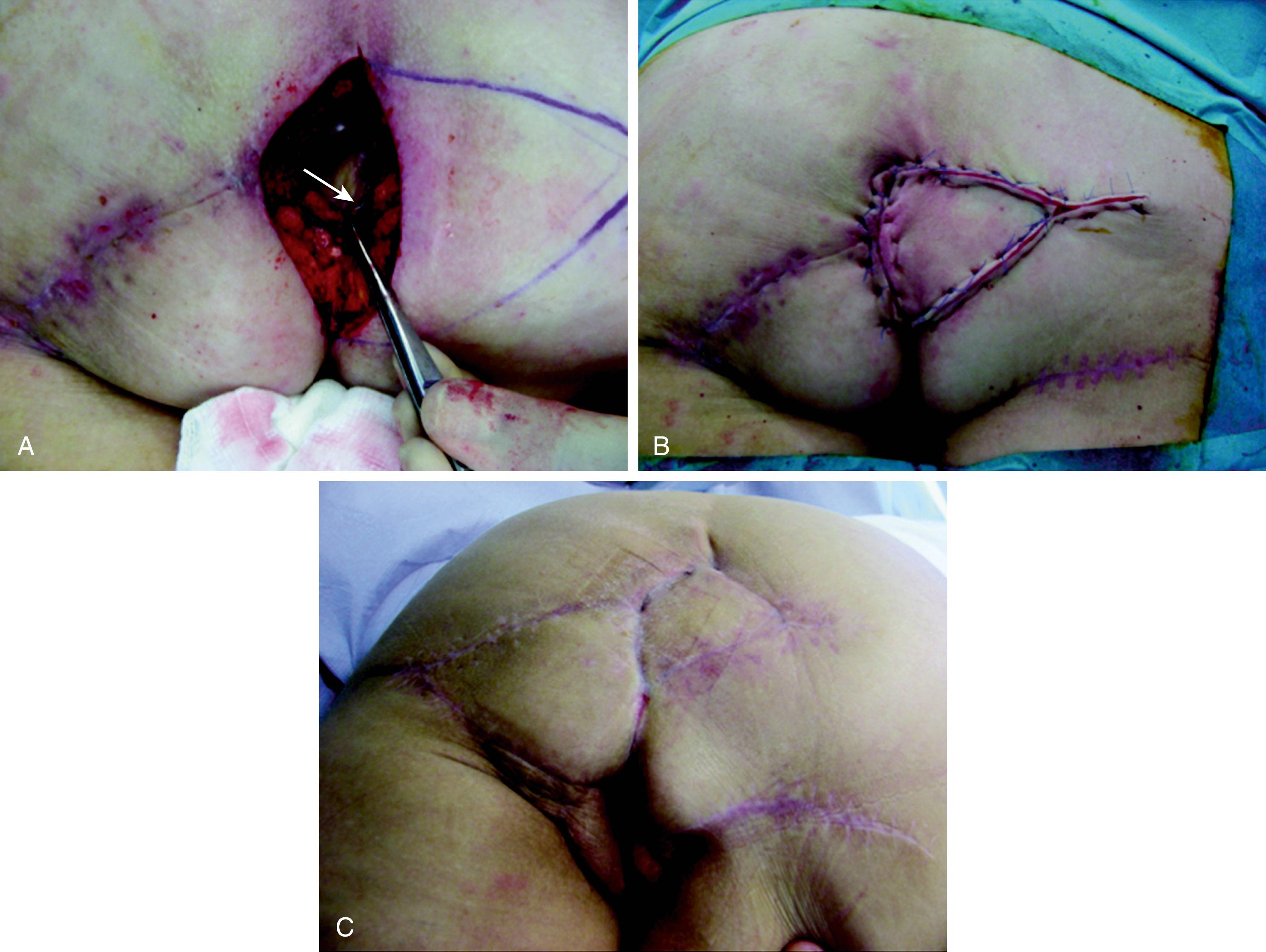
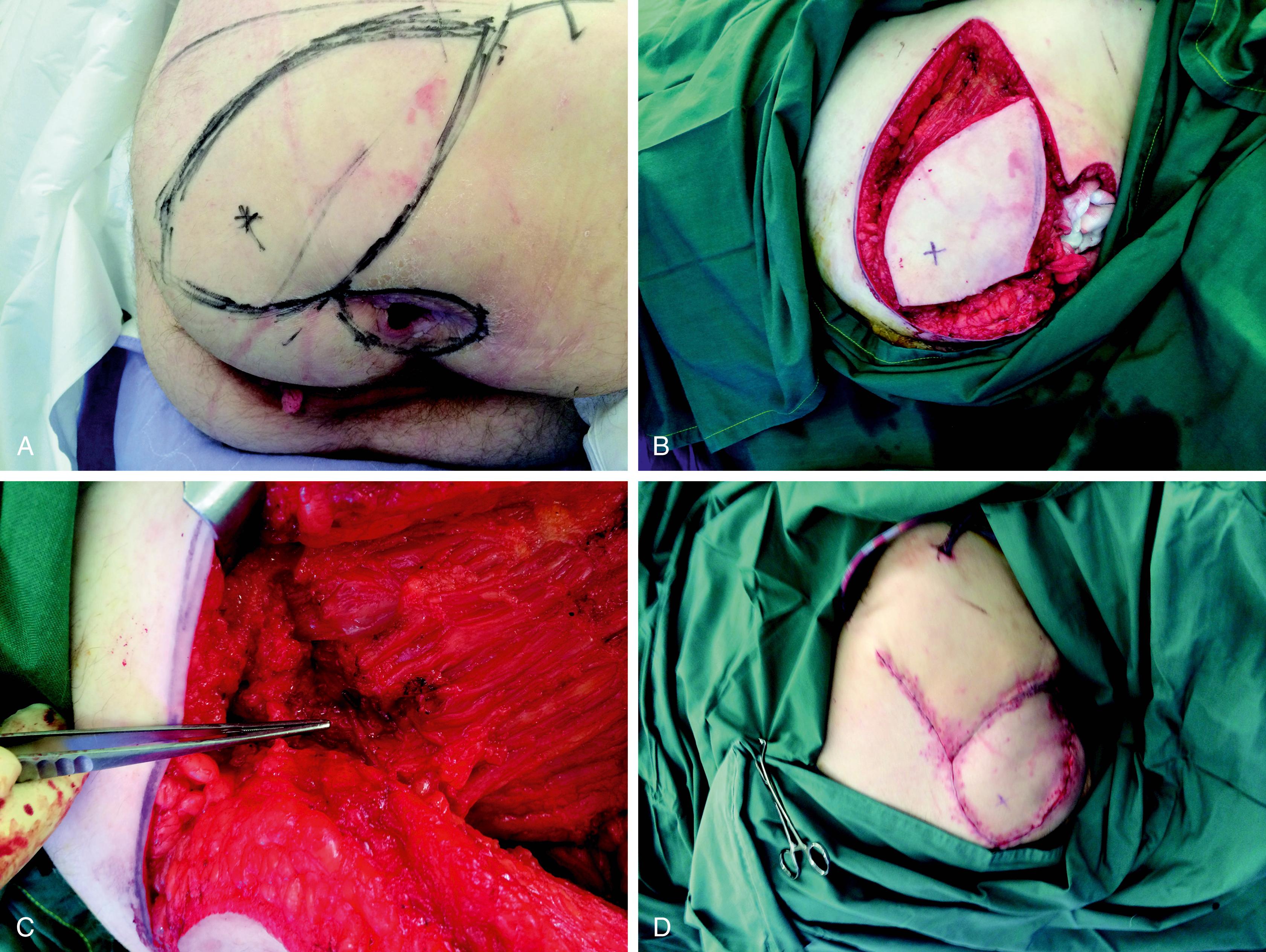
These flaps are considered when VRAM or ALT flaps are not possible for reconstruction. When chosen for abdominoperineal reconstruction, the surgery requires two different positions of the patient. Initially, the resecting surgeon will carry out the abdominal part of the operation, including colostomy and urinary conduit, and the abdominal wound is closed. The patient is then positioned in the prone, jack-knife position. Using ultrasound Doppler, the lateral groups of perforators of the IGA are selected in the ellipse of the skin so that the pedicle is long enough to reach without tension. The skin flap is elevated from the lateral aspect and a small portion of the gluteus maximus muscle may be included, which not only adds to the bulk but also includes a number of perforators for the flap. Once the flap is islanded, the pedicle is dissected, ligating all other branches of the IGA. When the flap is transposed over the perineum, the deep layer is sutured with the pelvic floor and the skin is sutured in two layers of the perineum, with two drains.
The blood supply of this flap arises from the descending branch of the IGA. This flap contains the posterior cutaneous nerve of the thigh, which makes it a sensate flap. The course of the artery and the perforators can be mapped out by using ultrasound Doppler or by CT angiography. This helps in planning the required flap. A line is drawn from the ischial tuberosity to the lateral epicondyle of the femur. The skin paddle is centered at the midpoint of this line, with the width of the skin paddle being either the amount that can be closed directly or more if skin grafting is required. It is also possible to take two flaps or to use this in combination with other flaps. The flap is elevated by incising its lateral border and extending it towards the gluteal crease to expose the pedicle and following it up to its origin. Extra length can be obtained by dissecting the IGA. The flap may then be tunneled under the skin to the perineal defect and inset.
The postoperative regime is as described before. The donor scar of the IGAP flap does cause some discomfort for the patient during sitting for about 4–6 weeks postoperatively.
Orticohea in 1972 and McCraw et al in 1976 described the gracilis myocutaneous flap. The gracilis myocutaneous flap is a type II muscle flap, the blood supply of which is from the ascending branch of the medial circumflex femoral artery. The skin flap paddle orientation is usually in the line of the gracilis muscle, but in recent years, following the paper from Yousif et al in 1992, a transverse orientation of the cutaneous paddle has been shown to be more reliable ( Fig. 41.12 ). This is now called a TMG or TUG flap. The muscular artery for the gracilis enters the muscle at about 8–10 cm from the pubic tubercle. The gracilis muscle can be used as a myocutaneous flap, or a muscle flap in combination with other skin flaps.
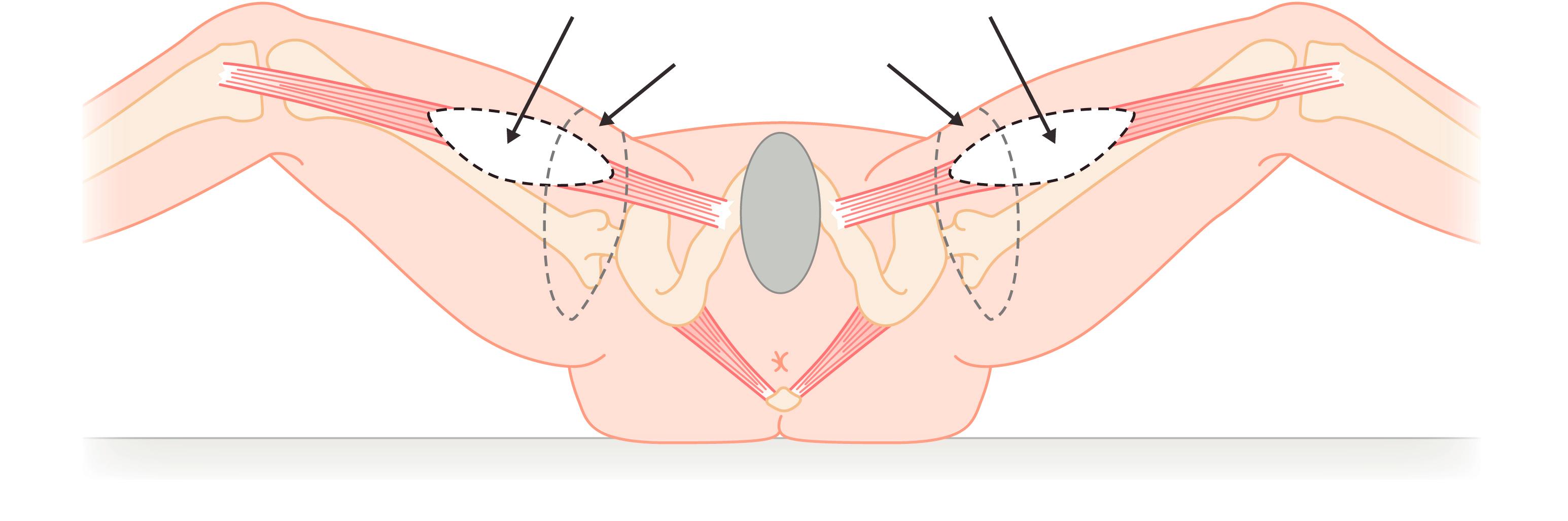
The gracilis myocutaneous flap is indicated for abdominoperineal resection with surgery carried out in the Lloyd Davies position. A skin paddle is drawn about 1–2 cm below and parallel to the groin crease and the inferior line is drawn depending on the amount of skin available for direct closure. This gives an ellipse measuring 18–20 cm in the anterior posterior direction.
The flap is elevated from anterior to posterior in the subfascial plane until the intramuscular septum between adductor longus and gracilis is encountered. The great saphenous vein is protected and preserved (i.e., not included in the flap). The adductor longus muscle can be identified easily and the fascia over the adductor longus muscle is included with the flap. The gracilis muscle is below adductor longus and the septum between the two can be easily separated by finger dissection, exposing the distal part of gracilis. The pedicle of gracilis, ascending branch of the medial circumflex profunda femoris artery, and the vein can easily be seen under the fascia.
Postoperatively, the patient is kept in a supine position with the legs slightly abducted, with a pillow under the knees to keep them flexed. After 24–48 hours, the drains are removed and the patient is mobilized.
The gracilis muscle may also be considered for functional transposition for perineal reconstruction by preserving the nerve to the muscle.
Perineal reconstruction is complex and involves a multidisciplinary approach. The ablative surgery often leaves the patients with significant and sometimes difficult lifestyle changes. Adherence to basic reconstructive surgery principles with provisions of a watertight, and reliable reconstruction offer patients the best chance of meaningful recovery and quality of life. The lotus petal flap and the V–Y perforator fasciocutaneous flaps have become workhorses in perineal reconstruction. The perineum is basically divided into an anterior and posterior half by a line joining the ischial tuberosities. When one-half of the perineum requires reconstruction, any one of the petals of a lotus petal flap would be sufficient. When both halves or the whole of the perineum require reconstruction, an extended petal of the lotus petal is selected according to the required size and volume. Generally, one needs three structures to reconstruct when the whole of the perineum is resected: pelvic floor, dead space, and skin cover. The first can be reconstructed either by acellular dermal matrix or de-epithelialized skin flaps. The second needs bulky but vascularized muscle or a de-epithelialized myocutaneous or fasciocutaneous flap, and the last, a skin flap.
Adeyemi A. Ogunleye and Gordon K. Lee
Congenital anomalies of the genitalia result from a variety of genetic and prenatal causes. Abnormal genitalia are seen in patients with 46,XX chromosomes, 46,XY chromosomes, and mixed sex chromosomes. The expressions of these chromosomal abnormalities are the results of complex biological interplay. The manifestations range broadly and can affect multiple areas of human anatomy and physiology. In males, testosterone promotes development of the epididymis, vas deferens, and seminal vesicle; and Müllerian-inhibiting substance (MIS) prevents the maturation of the uterus, cervix, and upper vagina. In females, the absence of MIS allows maturation of the uterus, cervix, and upper vagina. At approximately the 9th week of gestation, normal males have produced enough testosterone to allow synthesis of DHT (dihydrotestosterone). DHT then induces fusion of the genital folds and promotes growth of the genital tubercle.
A defect in any of the genes or environmental milieu that affect this developmental process can result in abnormal genitalia. Certain developmental problems, such as bladder exstrophy, can simply disrupt normally developing anatomy. Other developmental problems, specifically those disrupting gender-specific development, result in anatomy that lies on a spectrum between normal female and male external genitalia. The Prader scale, originally described by Andrea Prader in 1954, is often used to describe this anatomy. It is a 5-level scale where 1 represents normal female external genitalia with clitoromegaly and 5 represents normal male external genitalia.
The type of reconstruction required for each patient varies depending on the cause of ambiguous genitalia and the resulting appearance. The most common causes of anomalous genitalia in those patients undergoing feminization procedures are congenital adrenal hyperplasia and mixed gonadal dysgenesis. Gender assignment in those with anomalous genitalia is crucial although the timing of surgery is a controversial issue. Some have suggested that the birth of ambiguous genitalia is a neonatal emergency and requires immediate intervention. Others have advocated delaying surgery until the patient can give informed consent. Most physicians, however, suggest performing surgery prior to the age of 3. Performing surgery during this earlier time increases compliance with dilatations and decreases parental concern about their child’s gender. The goals in vaginal reconstruction of ambiguous genitalia include preserving fertility, adequate sexual function, and eliminating the risk of malignancy in the intraabdominal gonads.
Acquired vaginal defects are commonly due to oncological resections, although other causes include trauma and infection. Rectal adenocarcinoma frequently is cited as the most common indication for vaginal resections, accounting for approximately half of all reconstructions. Other causes include anal squamous cell cancer, cervical carcinoma, bladder or urethral transitional cell, bladder or urethral squamous cell cancer, vaginal or vulvar squamous cell cancer, endometrial adenocarcinoma, and trauma. , According to Crosby et al, the most common surgical procedures resulting in vaginal defects are abdominoperineal resections and pelvic exenterations.
In 2002, Cordeiro and colleagues published a classification system to define full-thickness vaginal defects. Type I defects include those involving the anterior, lateral, or posterior walls of the vagina. Type IA defects involve the anterior or lateral vaginal wall, whereas type IB defects involve the posterior wall. Type II refers to circumferential vaginal defects. Type IIA defects encompass up to two-thirds of the upper portion of the vagina while type IIB defects are the result of circumferential total vaginectomies. The most common vaginal defect is a Cordeiro type IB or full-thickness posterior wall defect. ,
Vaginal reconstruction has positive effects on the psyche and has been shown to improve wound healing. Placing healthy, well-vascularized tissue in a previously radiated area prevents enteroperineal fistulas and limits dead space, thereby reducing pelvic fluid collections and risk of postoperative abscess and infection.
In cases of vaginal agenesis where a dimple of vaginal mucosa is present, often nonsurgical techniques are preferred to surgical creation of a neovagina. The Frank procedure involves the use of serial vaginal dilators. Using the dilator, pressure is placed on the vaginal dimple for 30 minutes to 2 hours per day. Over time the width and length of the dilator are increased to allow for a neovagina that is appropriately sized for sexual activity. Some discomfort is expected during dilatations, but after dilatations are complete, only intermittent dilatation is required if sexual activity is frequent enough to maintain patency. Edmonds et al document a 94.9% success rate with dilatation treatment in patients with Mayer–Rokitansky–Küster–Hauser syndrome. Ingram described a modification of the Frank procedure in which the serial dilators are attached to a bicycle seat upon which the patient sits. The modified positioning allows for improved patient comfort during serial dilatations.
The reconstructive surgeon must consider multiple factors when planning the surgical creation of a neovagina. The location and size of the defect is the most important factor. However, it is also valuable to consider the patient’s position on the operating room table. The oncological surgeon may have completed the procedure with the patient in the prone or supine position. This may influence the options that are available without repositioning the patient. The type of incision, particularly whether a laparotomy incision was performed, and whether the initial incision remains open or has been closed, can alter the operative plan. The desired neovagina length and width should be based on the patient’s postoperative goals for sexual activity. The need for a connection between the neovagina and the uterus, when present, must be considered in congenital reconstruction.
Small defects of the vagina are amenable to primary closure if they do not significantly alter the length or width of the vagina. For larger defects, which are superficial or partial thickness, a skin graft can be considered. A vaginal conformer is often used to help promote take of the skin graft, which provides compression and immobilization of the graft to the underlying wound bed. In the pediatric gynecology literature, the preferred method for surgical creation of a neovagina in patients with vaginal agenesis is the Abbé–McIndoe procedure. First documented in 1938, this involves creating a space between with rectum and bladder and inserting a skin graft-lined mold to create the neovagina. This procedure requires the use of a mold or vaginal conformer for 6–12 months postoperatively and dilatations to maintain a vagina that can be used for sexual activity. Fussey et al. have also described the use of continuous negative pressure therapy to aid in skin graft take during vaginoplasty.
The pedicled rectus abdominis myocutaneous flap is the workhorse of vaginal reconstruction. Cordeiro described the use of the rectus flap for both partial posterior vaginal defects and circumferential upper two-thirds defects. The myocutaneous flap can be designed and harvested with a vertical or transverse skin paddle. The senior author (G.K.L.) prefers the vertical rectus abdominis myocutaneous (VRAM) flap as it is aligned with the midline incision that is most often used for a laparotomy incision, and, therefore, provides the greatest length of flap and greatest reach for the vaginal and perineal defect. The flap is based upon the deep inferior epigastric artery and can be passed directly into the pelvis/vaginal defect. Authors have recently described the harvest of the flap as a perforator flap for vaginal reconstructions noting decreased donor site morbidity. However, incorporation of the rectus muscle provides additional bulk and blood supply, which can be helpful in eliminating pelvic dead space and facilitating healing.
The pedicled gracilis myocutaneous flap , based on the medial femoral circumflex vessels, is another versatile option for vaginal and perineal reconstruction. The flap can be harvested with a transverse or longitudinal skin paddle. Cordeiro favored the longitudinal skin paddle for total circumferential vaginal defects while the transverse skin paddle can be used for labial reconstruction ( Fig. 41.13 ). It is important to note past reports of necrosis of the gracilis skin paddle when designed distally on the muscle down the thigh. Surgeons should note that the gracilis muscle tapers distally and becomes more tendinous, and that the blood supply of an overlying skin paddle at this level becomes less reliable. Hence, a skin paddle that incorporates the proximal major skin perforator, which enters at approximately 8–10 cm below the pubic ramus, is important for maintaining maximal perfusion.
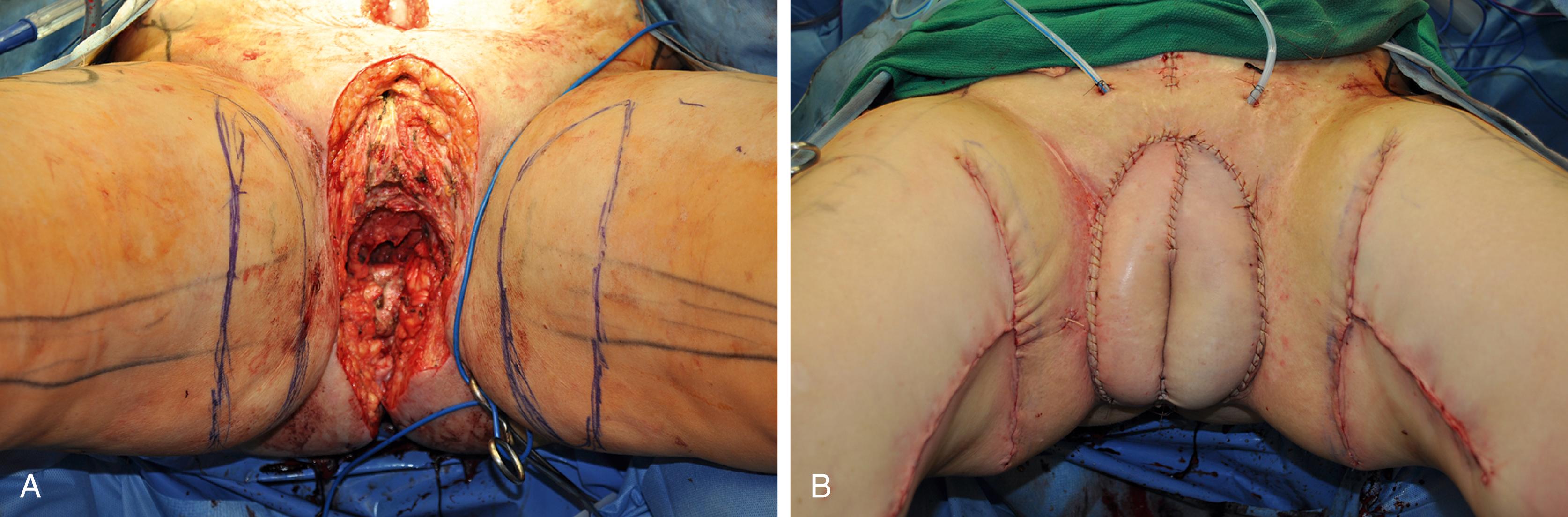
The pudendal thigh flap was first published by Wee and Joseph in 1989, and is often referred to as the “Singapore flap.” The flap is an innervated fasciocutanous flap based off perforators from the internal pudendal artery, which travel from posterior to anterior. The skin flaps are designed over the proximal medial thigh and harvested deep to the fascia of the adductor muscles. Proponents of this flap cite the benefit of an inconspicuous donor site and more reliable blood supply than the gracilis myocutaneous flap. This technique has been used in the reconstruction of both congenital and acquired defects. This flap is of limited bulk, and should be considered for defects that do not require a large amount of dead space obliteration.
Intestinal conduits , including the colon and jejunum, have also been utilized for vaginal reconstruction. This type of reconstruction is more widely documented for congenital defects. Erman Akar et al described their experience with vaginal reconstruction for congenital defects (33 of 34 patients) using a free vascularized jejunal flap. At a mean follow-up of 50 months, the authors noted satisfactory vaginal depth and diameter. However, six patients noted jejunal hypersecretion post-reconstruction and 10 patients required two lengths of jejunum opened along its antimesenteric border in order to increase luminal diameter. In contrast, the benefit of the colon in vaginal reconstruction is its larger diameter in comparison to the jejunum. Lima et al reviewed their 34-year experience treating 46 cases of vaginal agenesis with sigmoid colon interposition. While they noted no cases of excessive mucus production, eight patients had stenosis, six of whom required operative intervention. Of note, 34% of the group was sexually active at a mean follow-up of 12 years. Anecdotally, some patients may report a foul odor with the use of intestinal conduits as a result of mucus secretions.
Omental flaps , which have been used to treat rectovaginal and vesicovaginal fistulas as well as to close off the pelvic inlet after pelvic exenteration, can be modified for vaginal reconstruction. Authors have documented the use of a cylindricized omental flap based off the left gastroepiploic artery with a skin graft for neovaginal creation. The minimal donor site morbidity and ease of flap harvest are among the benefits of this flap. However, like other skin graft-based reconstructions, stenting is necessary for up to 6 months postoperatively to maintain adequate vaginal depth and to prevent strictures and stenoses.
The anterolateral thigh (ALT) flap has emerged as another option for vaginal and perineal reconstruction. Based on the descending branch of the lateral femoral circumflex artery, the ALT flap is a versatile flap for reconstruction. The flap can be harvested as a fasciocutaneous, an adipofascial, or a musculocutaneous flap. This versatility is beneficial for reconstruction in the perineum where dead space obliteration may be required or thinner pliable tissue may be ideal. The flap is tunneled beneath the rectus femoris and sartorius to improve pedicle length.
The most common complication after vaginal reconstruction is delayed wound healing or dehiscence. Other more devastating complications include: flap necrosis, fistula formation, and pelvic abscesses. Strictures may develop that require dilatations or revision surgery. Donor site infections may also be seen, as vaginal reconstruction is not a clean procedure. It is advised to use a separate clean set of instruments for flap harvest outside of the abdominal cavity if practical. After vaginal reconstruction with a rectus abdominis myocutaneous flap or an intestinal conduit, neovagina prolapse can occur which becomes bothersome to the patient. This may require secondary correction with sacral suspension.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here