Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() Additional content is available online at Elsevier eBooks for Practicing Clinicians
Additional content is available online at Elsevier eBooks for Practicing Clinicians
Cardiac arrhythmias encompass a large and heterogenous group of electrical abnormalities of the heart with or without underlying structural heart disease. Cardiac arrhythmias can be innocuous, can predispose to the development of potentially lethal stroke or embolus, or can present emergently with a life-threatening condition that may result in sudden cardiac death (SCD), one of the most common causes of death in the developed countries. In the United States, for example, an estimated 300,000 to 400,000 individuals die suddenly each year, with the vast majority involving the elderly; 80% are caused by ventricular fibrillation (VF) in the context of ischemic heart disease. In comparison, SCD in the young is relatively uncommon, with an incidence between 1.3 and 8.5 per 100,000 patient-years. However, tragically, thousands of otherwise healthy individuals under the age of 40 years die suddenly each year without warning signs. Most SCD in the young can be attributed to structural cardiovascular anomalies identifiable at autopsy. However, 30% to 50% of sudden death in the young remains unexplained following a complete autopsy and medicolegal investigation (see Chapter 70 ).
Potentially lethal and inheritable arrhythmia syndromes—classified under “the cardiac channelopathies” including congenital long QT syndrome (LQTS), Brugada syndrome (BrS), catecholaminergic polymorphic ventricular tachycardia (CPVT), and related disorders—involve electrical disturbances with the propensity to cause fatal arrhythmias in the setting of a structurally normal heart. These often unassuming electrical abnormalities have the capacity to cause the heart of an unsuspecting individual to develop a potentially lethal arrhythmia, leading to a sudden and early demise of an otherwise healthy individual. In fact, it is now recognized that nearly a third of autopsy-negative sudden unexplained death (SUD) in the young and approximately 10% of sudden infant death syndrome (SIDS) stem from these inherited cardiac channelopathies.
Through molecular advances in the field of cardiovascular genetics, the underlying bases responsible for many inherited cardiac arrhythmia syndromes have been identified, while other underlying genetic substrates are on the cusp of discovery. Over the past two decades, a set of themes including extreme genetic heterogeneity, reduced/incomplete penetrance, and variable expressivity have proven to be central themes among the cardiac channelopathies. However, for some disorders, important genotype-phenotype correlations have been recognized and have provided diagnostic, prognostic, and therapeutic impact.
Given the potentially devastating impact that these disorders can have on a family and their communities, we sought to illustrate the clinical description, genetic basis, and the genotype-phenotype correlations associated with these inherited arrhythmia syndromes. Specifically, this chapter will discuss cardiac channelopathies, focusing on the subset of QT-opathies [LQTS including calmodulinopathic LQTS, triadin knockout (TKO) syndrome, Timothy syndrome (TS), cardiac-only Timothy syndrome (COTS), short QT syndrome (SQTS), and drug-induced torsade de pointes (DI-TdP)] and other channelopathies [Andersen-Tawil syndrome (ATS), ankyrin-B syndrome (ABS), BrS, CPVT, early repolarization syndrome (ERS), familial atrial fibrillation (FAF), idiopathic ventricular fibrillation (IVF), multifocal ectopic Purkinje-related premature contractions (MEPPC), progressive cardiac conduction defect (PCCD), and sick sinus syndrome (SSS)].
Congenital LQTS comprises a distinct group of cardiac channelopathies characterized by delayed repolarization of the myocardium resulting in heart rate-corrected QT prolongation (QTc >480 msec as the 50th percentile among individuals with genetically confirmed LQTS; Fig. 63.1A ) and an increased risk of syncope, seizures, and SCD in the setting of a structurally normal heart. The incidence of LQTS may exceed 1 in 2500 persons. However, individuals with LQTS may not manifest QT prolongation on a resting 12-lead surface electrocardiogram (ECG). This repolarization abnormality almost always is without consequence; however, it is rarely triggered by exertion, swimming, emotion, auditory stimuli such as an alarm clock, or the postpartum period, which can cause the heart to become electrically unstable and develop a life-threatening and sometimes lethal arrhythmia, torsade de pointes (TdP) (see Chapter 70 ). Though the cardiac rhythm often returns to normal spontaneously, resulting in only transient syncope, 5% of untreated and unassuming LQTS individuals succumb to a fatal arrhythmia as their sentinel event. However, it is estimated that nearly half of individuals experiencing SCD, stemming from this very treatable arrhythmogenic disorder, may have exhibited prior warning signs (i.e., exertional syncope, family history of premature sudden death) that went unrecognized. LQTS may explain approximately 20% of autopsy-negative SUD in the young and 10% of SIDS.
LQTS is a genetically heterogeneous disorder of cardiac repolarization inherited predominantly in an autosomal dominant pattern (formerly referred to as Romano-Ward syndrome). It is rarely inherited in a recessive pattern as illustrated by Jervell and Lange-Nielsen Syndrome (JLNS), characterized by extreme QTc prolongation, high risk of SCD, and sensorineural hearing loss. Spontaneous/sporadic germline mutations can account for nearly 5% to 10% of LQTS. To date, hundreds of mutations have now been identified in LQTS-susceptibility genes responsible for a nonsyndromic “classical” LQTS phenotype. In addition, three extremely rare, multisystem disorders (ATS formerly referred to as LQT7; ABS formerly referred to as LQT4; and TS formerly referred to as LQT8) associated with marked QTc prolongation and an array of extra-cardiac manifestations have also been described and are detailed in the later sections of this chapter.
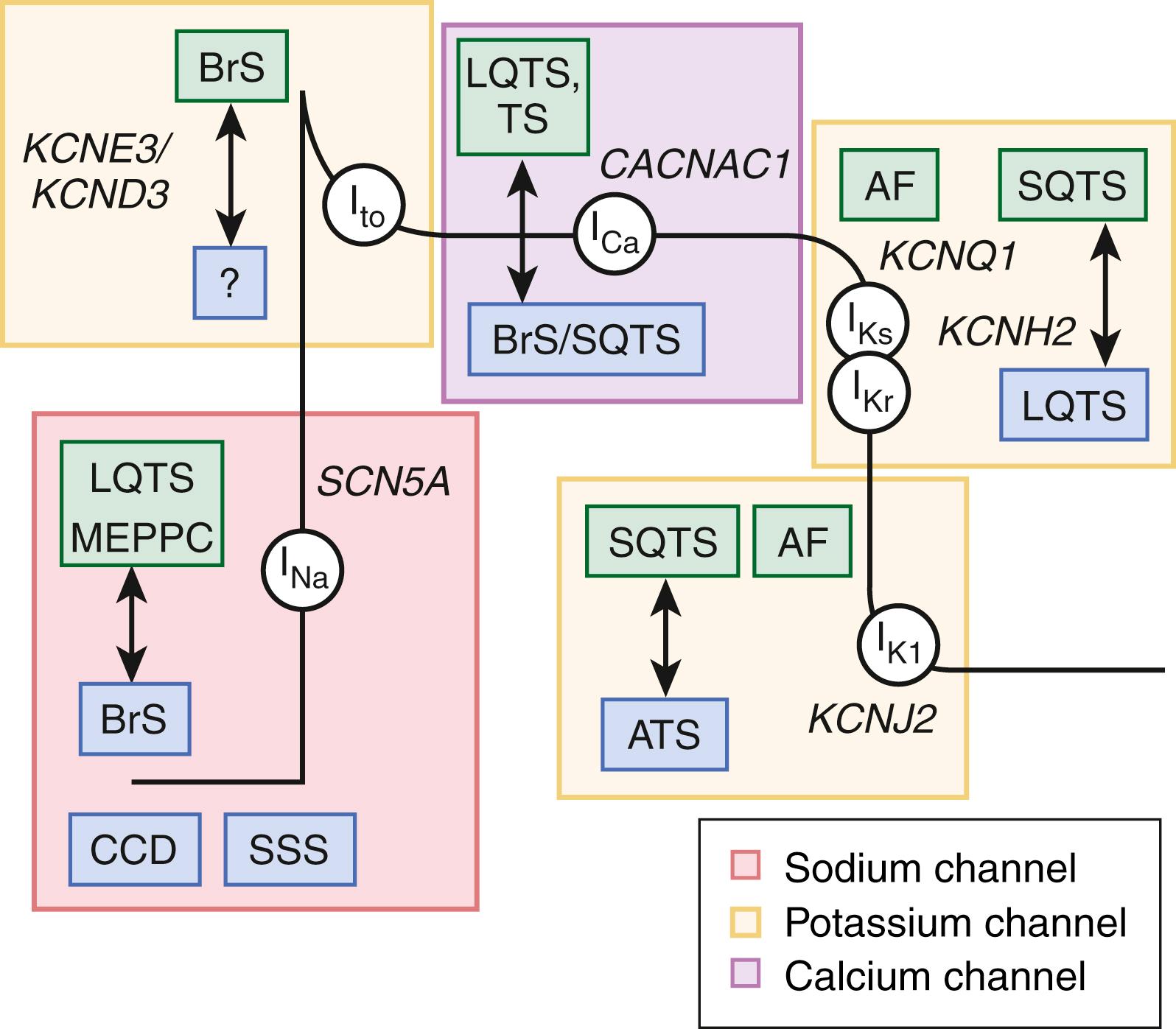
Approximately 75% of patients with a clinically robust diagnosis of LQTS host either loss-of-function or gain-of-function pathogenic/likely pathogenic variants in one of these three major/canonical LQTS genes ( eTable 63.1 )— KCNQ1- encoded I Ks (K v 7.1) potassium channel (LQT1, approximately 35%, loss-of-function); KCNH2 -encoded I Kr (K v 11.1) potassium channel (LQT2, approximately 30%, loss-of-function); and SCN5A- encoded I Na (Na v 1.5) sodium channel (LQT3, approximately 10%, gain-of-function)—which are responsible for the inscription of the cardiac action potential ( Fig. 63.2 ). Approximately 5% to 10% of patients have multiple mutations and such patients present at a younger age and with greater expressivity.
| Gene | Locus | Protein | |
|---|---|---|---|
| Congenital Long QT Syndrome | |||
| Major LQTS Genes | |||
| KCNQ1 (LQT1) ∗ | 11p15.5 | I Ks potassium channel alpha subunit (KVLQT1, K v 7.1) | |
| KCNH2 (LQT2) ∗ | 7q35–36 | I Kr potassium channel alpha subunit (HERG, K v 11.1) | |
| SCN5A (LQT3) ∗ | 3p21–p24 | Cardiac sodium channel alpha subunit (Na v 1.5) | |
| Minor LQTS Genes (listed alphabetically) | |||
| AKAP9 # | 7q21–q22 | Yotiao | |
| CACNA1C † | 12p13.3 | Voltage-gated L-type calcium channel (Ca v 1.2) | |
| CALM1 ∗ | 14q32.11 | Calmodulin 1 | |
| CALM2 ∗ | 2p21 | Calmodulin 2 | |
| CALM3 ∗ | 19q13.2–q13.3 | Calmodulin 3 | |
| CAV3 # | 3p25 | Caveolin-3 | |
| KCNE1 # | 21q22.1 | Potassium channel beta subunit (MinK) | |
| KCNE2 # | 21q22.1 | Potassium channel beta subunit (MiRP1) | |
| KCNJ5 # | 11q24.3 | K ir 3.4 subunit of I KACH channel | |
| SCN4B # | 11q23.3 | Sodium channel beta 4 subunit | |
| SNTA1 # | 20q11.2 | Syntrophin-alpha 1 | |
| Triadin Knockout Syndrome | |||
| TRDN ∗ | 6q22.31 | Cardiac Triadin | |
| Andersen-Tawil Syndrome | |||
| KCNJ2 (ATS1) ∗ | 17q23 | I K1 potassium channel (K ir 2.1) | |
| Timothy Syndrome | |||
| CACNA1C ∗ | 12p13.3 | Voltage-gated L-type calcium channel (Ca v 1.2) | |
| Cardiac-Only Timothy Syndrome | |||
| CACNA1C | 12p13.3 | Voltage-gated L-type calcium channel (Ca v 1.2) | |
| Short QT Syndrome | |||
| KCNH2 (SQT1) | 7q35–36 | I Kr potassium channel alpha subunit (HERG, K v 11.1) | |
| KCNQ1 (SQT2) | 11p15.5 | I Ks potassium channel alpha subunit (KVLQT1, K v 7.1) | |
| KCNJ2 (SQT3) | 17q23 | I K1 potassium channel (K ir 2.1) | |
| CACNA1C (SQT4) | 12p13.3 | Voltage-gated L-type calcium channel (Ca v 1.2) | |
| CACNB2 (SQT5) | 10p12 | Voltage-gated L-type calcium channel beta 2 subunit | |
| CACN2D1 (SQT6) SLC22A5 (SQT7) SLC4A3 (SQT8) |
7q21–q22 5q31.1 2q35 |
Voltage-gated L-type calcium channel 2 delta 1 subunit Organic cation transporter 2 (↓ carnitine transport; ↑ I K1 ) Anion exchanger 3 (↑ intracellular pH; ↓ Cl − conductance) |
|
| Catecholaminergic Polymorphic Ventricular Tachycardia | |||
| RYR2 (CPVT1) | 1q42.1–q43 | Ryanodine Receptor 2 | |
| CASQ2 (CPVT2) | 1p13.3 | Calsequestrin 2 | |
| KCNJ2 (CPVT3) | 17q23 | I K1 potassium channel (Kir2.1) | |
| CALM1 | 14q32.11 | Calmodulin 1 | |
| CALM2 | 2p21 | Calmodulin 2 | |
| CALM3 | 19q13.2–q13.3 | Calmodulin 3 | |
| TRDN | 6q22.31 | Cardiac Triadin | |
| Brugada Syndrome | |||
| SCN5A (BrS1) ∗ | 3p21–p24 | Cardiac sodium channel alpha subunit (Na v 1.5) | |
| Minor BrS Genes (listed alphabetically) | |||
| ABCC9 # | 12p12.1 | ATP Binding Cassette, Subfamily C Member 9 | |
| ANK2 # CACNA1C # |
2p13.3 | Voltage-gated L-type calcium channel (Ca v 1.2) | |
| CACNA2D1 # | 7q21–q22 | Voltage-gated L-type calcium channel 2 delta 1 subunit | |
| CACNB2 # | 10p12 | Voltage-gated L-type calcium channel beta 2 subunit | |
| DLG1 | 3q29 | Synapse-associated protein 97 | |
| FGF12 # | 3q28 | Fibroblast Growth Factor 12 | |
| GPD1L # | 3p22.3 | Glycerol-3-phosphate dehydrogenase 1-like | |
| HCN4 # | 15q24.1 | Hyperpolarization-activated cyclic nucleotide-gated channel 4 | |
| HEY2 | 6q | Hes Related Family BHLH Transcription Factor With YRPW Motif 2 | |
| KCND3 # | 1p13.2 | Voltage-gated potassium channel (I to ) subunit K v 4.3 | |
| KCNE3 # | 11q13.4 | Potassium channel beta subunit 3 (MiRP2) | |
| KCNE5 # | Xq22.3 | Potassium channel beta subunit 5 | |
| KCNH2 # | 7q35–36 | I Kr potassium channel alpha subunit (HERG, K v 11.1) | |
| KCNJ8 # | 12p12.1 | Inward rectifier K(+) channel K ir 6.1 | |
| PKP2 # | 12p11 | Plakophilin-2 | |
| RANGRF # RRAD |
17p13.1 16q22.1 |
RAN guanine nucleotide release factor 1 RAD GTPase (↓ I Na ; ↓ focal adhesions) |
|
| SCN1B # | 19q13 | Sodium channel beta 1 | |
| SCN2B # | 11q23 | Sodium channel beta 2 | |
| SCN3B # | 11q24.1 | Sodium channel beta 3 | |
| SCN10A # | 3p22.2 | Sodium Voltage-Gated Channel Alpha Subunit 10 (Na v 1.8) | |
| SEMA3A | 7p12.1 | Semaphorin 3A | |
| SLMAP # | 3p14.3 | Sarcolemma associated protein | |
| TRPM4 # | 19q13.33 | Transient receptor potential cation channel, subfamily M, member 4 | |
| Early Repolarization Syndrome | |||
| CACNA1C | 2p13.3 | Voltage-gated L-type calcium channel (Ca v 1.2) | |
| CACNA2D1 | 7q21–q22 | Voltage-gated L-type calcium channel 2 delta 1 subunit | |
| CACNB2 | 10p12 | Voltage-gated L-type calcium channel beta 2 subunit | |
| KCNJ8 SCN5A |
12p12.1 3p21–p24 |
Inward rectifier K(+) channel Kir6.1 Cardiac sodium channel alpha subunit (Na v 1.5) |
|
| Idiopathic Ventricular Fibrillation | |||
| CALM1 | 14q32.11 | Calmodulin 1 | |
| DPP6 | 7q36 | Dipeptidyl-peptidase-6 | |
| IRX3 | 38p13 | Iroquois homeobox gene family transcription factor | |
| RYR2 | 1q42.1–q43 | Ryanodine Receptor 2 | |
| Isolated Progressive Cardiac Conduction Disease | |||
| GJA5 | 1q21 | Connexin 40 | |
| SCN1B SCN5A |
19q13 3p21–p24 |
Sodium channel beta 1 Cardiac sodium channel alpha subunit (Na v 1.5) |
|
| TRPM4 | 19q13.33 | transient receptor potential cation channel, subfamily M, member 4 | |
| Sick Sinus Syndrome | |||
| ANK2 | 4q25–q27 | Ankyrin B | |
| HCN4 | 15q24–q25 | Hyperpolarization-activated cyclic nucleotide-gated channel 4 | |
| MYH6 | 14q11.2 | Myosin, Heavy Chain 6, Cardiac Muscle, Alpha | |
| SCN5A | 3p21–p24 | Cardiac sodium channel alpha subunit (Na v 1.5) | |
| Ankyrin-B Syndrome | |||
| ANK2 | 4q25–q27 | Ankyrin B | |
| Familial Atrial Fibrillation | |||
| ABCC9 ANK2 |
12p12.1 4q25–q27 |
ATP Binding Cassette, Subfamily C Member 9 Ankyrin B |
|
| GATA4 | 8p23.1–p22 | GATA-binding protein 4 | |
| GATA5 | 20q13.33 | GATA-binding protein 5 | |
| GJA5 | 1q21 | Connexin 40 | |
| KCNA5 | 12p13 | I Kur potassium channel (Kv1.5) | |
| KCNE2 | 21q22.1 | Potassium channel beta subunit (MiRP1) | |
| KCNH2 | 7q35–36 | I Kr potassium channel alpha subunit (HERG, Kv11.1) | |
| KCNJ2 | 17q23 | I K1 potassium channel (Kir2.1) | |
| KCNQ1 | 11p15.5 | I Ks potassium channel alpha subunit (KVLQT1, Kv7.1) | |
| LMNA | 1q22 | Lamin A | |
| MYL4 | 17q21.32 | Myosin Light Chain 4 | |
| NKX2-5 | 5q35.1 | NK homeobox 5 | |
| NPPA | 1p36 | Atrial natriuretic peptide precursor A | |
| NUP155 | 5p13 | Nucleoporin 155KD | |
| PRKAG2 | 7q36.1 | AMP-activated protein kinase 2 | |
| RYR2 | 1q42.1–q43 | Ryanodine Receptor 2 | |
| SCN1B | 19q13 | Sodium channel beta 1 | |
| SCN2B | 11q23 | Sodium channel beta 2 | |
| SCN3B | 11q24.1 | Sodium channel beta 3 | |
| SCN4B | 11q23.3 | Sodium channel beta 4 | |
| SCN5A | 3p21–p24 | Cardiac sodium channel alpha subunit (NaV1.5) | |
∗ Denotes genes that received “definitive” or “strong” evidence designations from the Clinical Genome Resource (ClinGen).
# Denotes genes that received “disputed” or “limited” evidence designations from ClinGen.
† Denotes genes that received “moderate” evidence designations from ClinGen.
Following the discovery of the canonical LQTS-susceptibility genes in 1995 and 1996, rapid advances in deoxyribonucleic acid (DNA) sequencing technology has facilitated the discovery of new disease-susceptibility genes in scenarios (i.e., singletons and small pedigrees) not feasible with classical linkage analysis. As a result, the last two decades have seen a rapid explosion in the number of new disease-susceptibility genes, which includes the 14 minor LQTS-susceptibility genes that may underlie an additional 5% to 10% of LQTS cases ( eTable 63.1 ).
However, many of the minor LQTS-susceptibility genes (e.g., AKAP9 , ANK2 , CAV3 , KCNE1 , KCNE2 , SCN4B , and SNTA1 ; eTable 63.1 ) were discovered using hypothesis-driven, candidate-based analysis of biologically plausible genes rather than unbiased approaches (linkage analysis, next-generation sequencing-based trio/pedigree analysis, etc.). , Furthermore, many putative disease-causative variants used to establish these minor LQTS disease-gene associations (GDAs) were discovered before the true background rate of rare and presumably innocuous amino acid-altering variants was illuminated by large-scale sequencing projects such as the Genome Aggregation Database (gnomAD). Therefore, it comes as little surprise that some putative LQTS-causative nonsynonymous variants are observed at frequencies in public exomes/genomes that far exceed the anticipated contribution of the minor LQTS-susceptibility gene in which it resides (i.e., 1:250,000 or 0.0004% for a ≤1% contributor) or in some cases the estimated prevalence of LQTS as a whole (i.e., 1:2500 or 0.04%). ,
As a result, the strength of many minor LQTS GDAs is rightfully in question. To this end, the Clinical Genome Resource (ClinGen) Clinical Domain Channelopathy Working Group recently released a semi-quantitative, evidence-based assessment of the GDA strength for the 17 putative LQTS-susceptibility genes. , However, the canonical LQTS-susceptibility genes ( KCNQ1 /LQT1, KCNH2 /LQT2, and SCN5A /LQT3) and redundant genes ( CALM1-3 ) responsible for calmodulinopathic LQTS received “definitive” evidence designations, and the majority of the remaining LQTS-susceptibility genes received a “limited” or “disputed” evidence designation (see eTable 63.1 ). , As a result, the majority (9/17; 53%) of alleged LQTS-susceptibility genes have now been demoted to so-called gene of uncertain significance (GUS) status.
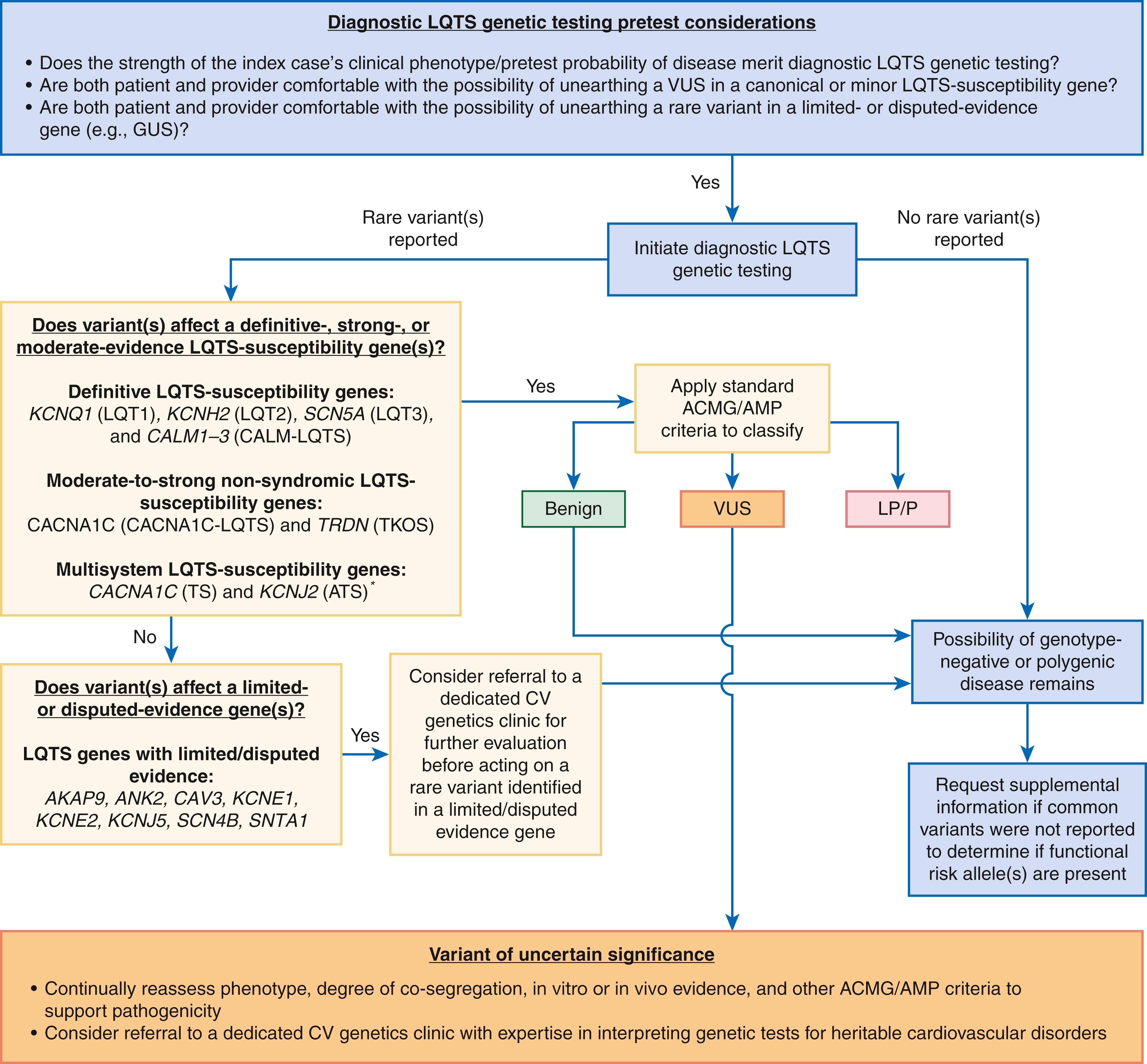
Unfortunately, owing to the limited number of sentinel variants used to establish initial GDAs, nearly all novel, nonsynonymous variants identified in a minor LQTS-GUS are destined to receive, at best, an ambiguous variant of uncertain significance (VUS) designation in accordance with the current American College of Medical Genetics and Genomics (ACMG) guidelines. , Therefore, the continued inclusion of GUS on gene panels likely elevates the signal-to-noise ratio associated with LQTS genetic testing as well as the risk of genetic testing misinterpretation, and subsequent diagnostic miscues. ,
However, in light of (1) the increasing contribution of common genetic variants (oligogenic/polygenic basis) to the genetic architecture of LQTS, particularly in the approximately 10% to 20% of individuals that remain genotype-negative, , (2) clear role of rare and common variants in some GUS, most notably the KCNE1 -encoded MiRP1 β-subunit, , in low penetrant and acquired/drug-induced forms of LQTS, and (3) potential that new evidence could elevate the ClinGen designation of a minor LQTS GUS, it is difficult to argue for the blanket removal of all limited- and disputed-evidence genes from commercial LQTS genetic testing panels at this time.
As minor LQTS GUS are likely to remain on LQTS genetic testing panels for the foreseeable future, ordering health care professionals should prioritize clinically actionable (ACMG pathogenic/likely pathogenic) variants identified in ClinGen definitive ( KCNQ1 , KCNH2 , SCN5A , and CALM1-3 ), strong ( TRDN ), and moderate ( CACNA1C ) evidence genes and approach any variant (ACMG pathogenic, likely pathogenic, or VUS) identified in a ClinGen limited/disputed evidence gene with caution, as detailed in Figure 63.3 . Importantly, if any doubt exists in regards to the clinical implications of variants labeled, currently or previously, as pathogenic/likely pathogenic in a GUS or VUS in definitive, strong, or moderate-evidence LQTS-susceptibility genes, strong consideration should be given to referring the patient to a dedicated Cardiovascular Genomics Clinic with the suitable infrastructure and expertise needed to carefully interpret, continually reappraise, and if needed act on these genetic findings (see Fig. 63.3 ).
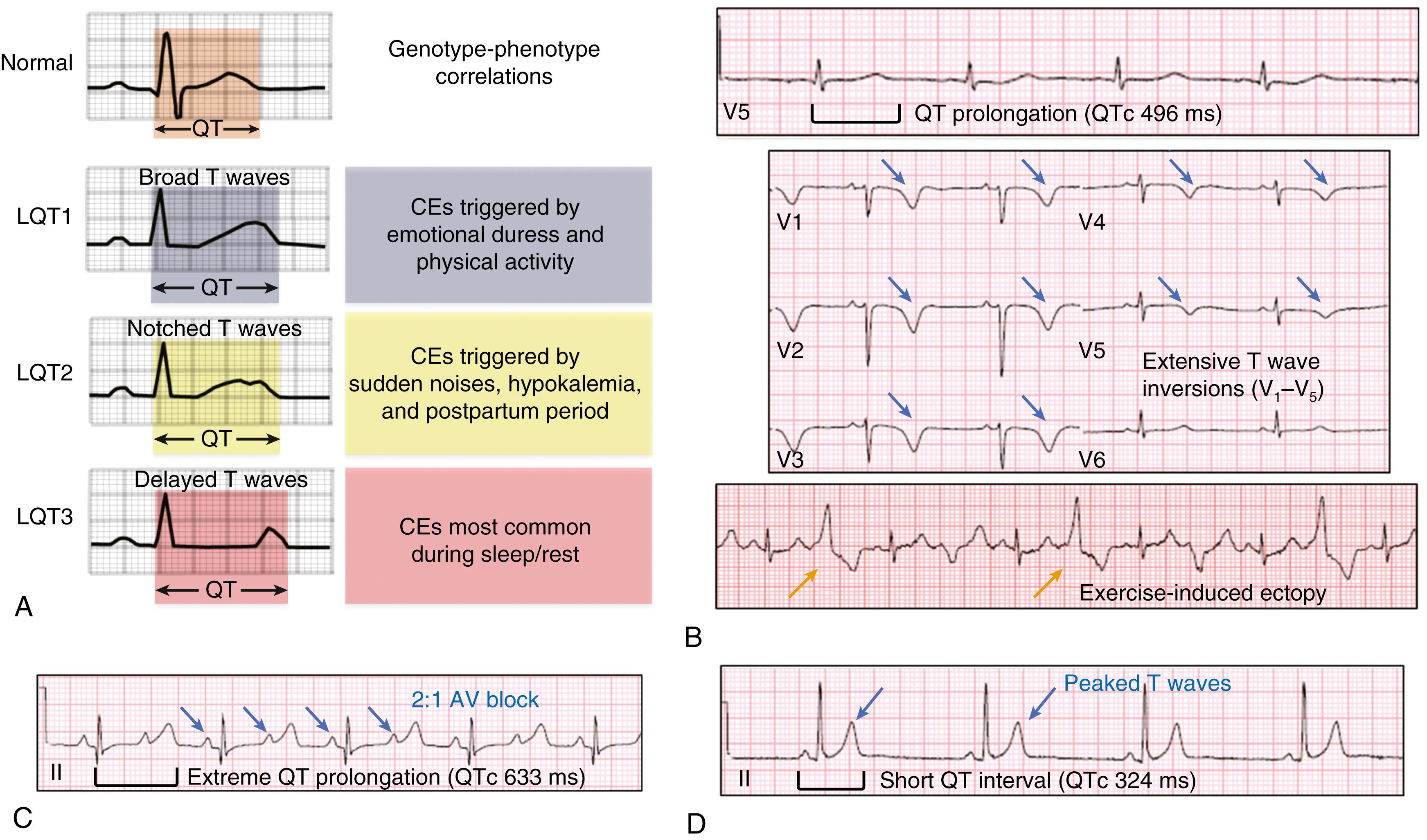
Specific genotype/phenotype associations in LQTS have emerged, suggesting relatively gene-specific triggers, ECG patterns, and response to therapy (see Fig. 63.1A ). Swimming and exertion-induced cardiac events are strongly associated with mutations in KCNQ1 (LQT1), whereas auditory triggers and events occurring during the postpartum period most often occur in patients with LQT2. While exertion- or emotional stress-induced events are most common in LQT1, events occurring during periods of sleep/rest are most common in LQT3. In a study of 721 LQT1 and 634 LQT2 genetically confirmed patients from the U.S. portion of the international LQTS registry, a multivariate analysis was used to assess the independent contribution of clinical and mutation-specific factors in the occurrence of a first triggered event associated with exercise, arousal, or sleep/rest. Among the 221 symptomatic LQT1 patients, their first cardiac event was most often associated with exercise (55%) followed by sleep/rest (21%), arousal (14%), and nonspecific (10%) triggers, whereas the 204 symptomatic LQT2 patients most often had their first event associated with either arousal triggers (44%) or nonexercise/nonarousal triggers (43%), and only 13% of the symptomatic LQT2 patients had an exercise-induced triggered first event. For LQT2 patients, the rate of arousal-triggered events was similar between male and female children, whereas there was a significantly higher rate of arousal-triggered events in women than men (26% vs. 6%, at age 40 years) following the onset of adolescence. Characteristic gene-suggestive ECG patterns have been described previously. LQT1 is associated with a broad-based T wave, LQT2 with a low amplitude notched or biphasic T wave, and LQT3 with a long isoelectric segment followed by a narrow-based T wave (see Fig. 63.1A ).
However, exceptions to these relatively gene-specific T wave patterns exist, and due caution must be exercised with making a pre-genetic test prediction of the particular LQTS subtype involved, as the most common clinical mimicker of the LQT3-looking ECG is seen among patients with LQT1. This is key because the underlying genetic basis heavily influences the response to standard LQTS pharmacotherapy where beta blockers are extremely protective in LQT1 patients and moderately protective in patients with LQT2 and LQT3. Additionally, targeting the pathologic, LQT3-associated late sodium current with agents such as mexiletine, flecainide, or ranolazine represents a gene-specific therapeutic option for LQT3. Attenuation in repolarization with clinically apparent shortening in the QTc has been demonstrated with such a strategy and recently, a reduction in LQT3-triggered events using this strategy has been demonstrated. While the generalization that beta-blocker efficacy is genotype-type dependent has been well accepted, the effectiveness of beta-blocker therapy may be largely trigger-specific, rather than dependent on genotype. For both LQT1 and LQT2 patients, beta-blockade was associated with a pronounced 71% (LQT2 patients) to 78% (LQT1 patients) reduction in the risk for exercise-triggered cardiac events, but had no statistically significant effect on the apparent risk for arousal- or sleep/rest-triggered events. However, it should be noted that many symptomatic LQT1 and LQT2 patients experience a subsequent cardiac event associated with a different trigger. For example, an LQT2 patient first presenting with an arousal event or event during sleep may present subsequently with an exercise-triggered event. Therefore, beta-blocker therapy remains first-line therapy even for patients experiencing a non-exercise-associated first event.
In addition, intra-genotype risk stratification has been realized for the two most common subtypes of LQTS based on mutation type, mutation location, and cellular function. Patients with LQT1 secondary to Kv7.1 missense mutations localizing to the transmembrane-spanning domains clinically have a twofold greater risk of a LQT1-triggered cardiac event than LQT1 patients with mutations localizing to the C-terminal region. In addition, missense mutations localizing to the so-called cytoplasmic loops (C-loops) within the transmembrane-spanning domains, an area of the protein involved in adrenergic channel regulation, are associated with the highest rate of both exercise- and arousal-triggered events, but were not associated with an increase rate of sleep/rest associated events. C-loop K v 7.1 missense mutations were consistently associated with a sixfold increase in risk for exercise-triggered events compared to non-missense mutations, and a nearly threefold increase compared to N- and C-terminal missense mutations.
Patients with mutations resulting in a greater degree of K v 7.1 loss-of-function at the cellular in vitro level (dominant negative) have a twofold greater clinical risk compared to mutations that damaged the biology of the K v 7.1 channel less severely (haploinsufficiency). Adding to the traditional clinical risk factors, molecular location and cellular function are independent risk factors used in the evaluation of patients with LQTS.
Akin to molecular risk stratification in LQT1, patients with LQT2 secondary to K v 11.1 pore-region mutations have a longer QTc, a more severe clinical manifestation of the disorder, and experience significantly more arrhythmia-related cardiac events occurring at a younger age than those LQT2 patients with non-pore mutations in K v 11.1. Similarly, in a Japanese cohort of LQT2 patients, those with pore mutations had a longer QTc, though not significant among probands, non-probands with pore mutations experienced their first cardiac event at an earlier age than those with a non-pore mutation. Most recently, additional information has been gleaned suggesting that LQT2 patients with mutations involving the transmembrane pore region had the greatest risk for cardiac events, those with frame-shift/nonsense mutations in any region had an intermediate risk, and those with missense mutations in the C-terminus had the lowest risk for cardiac events. Interestingly, LQT2 patients with mutations in the pore-loop region of the K v 11.1 channel have a greater than twofold increased risk for arousal-triggered events, and LQT2 patients with non-pore loop TM region mutations have a nearly sevenfold increase in the risk for exercise-triggered cardiac events compared with patients with N-terminal/C-terminal mutations.
Incomplete penetrance and variable expressivity are clinical hallmark features of LQTS, and it has been long thought that co-inheritance of a true disease-causing mutation and either a common or rare channel genetic variant may determine the expressed severity of the disorder. For example, the co-existence of the common K897T-KCNH2 polymorphism and the A1116V-KCNH2 mutation (on opposite alleles) led to a more severe clinical course in a single Italian LQTS family. The A1116V mutation by itself produced a sub-clinical phenotype of mild QT prolongation and an asymptomatic course, while the proband hosting both variants had clinically overt disease consisting of a diagnostic QT prolongation, presyncopal episodes, and cardiac arrest. Besides cardiac ion channels, single nucleotide polymorphisms (SNPs) of non-ion channel genes like NOS1AP (the gene encoding the nitric oxide synthase 1 adapter protein), ADRA2C (alpha-2C adrenergic receptor), and ADRB1 (beta-1 adrenergic receptor) can modify disease severity in LQTS.
There is compelling evidence for a strong disease modifying effect of a 3′ untranslated region (3′UTR) KCNQ1 allele-specific haplotype in LQT1 mutation positive pedigrees; the magnitude of the effect on the QTc and symptomology go well beyond any other currently described genetic modifiers. The KCNQ1 gene encodes a single K v 7.1 ion channel alpha subunit that assemble to create a pore-forming K v 7.1 tetrameric channel. Therefore, if a patient had a heterozygous KCNQ1 mutation (i.e., one normal KCNQ1 gene allele and one mutant allele), one would expect that if both the normal and mutant gene alleles were expressed in equal amounts, then 1/16 of the K v 7.1 channels would be a normal homomeric tetramer and 1/16 of the K v 7.1 channels would be a mutant homomeric tetramer. The remaining channels would be hybrids containing both normal and mutant alpha-subunits. If expression of the normal KCNQ1 gene allele was somehow suppressed, then there would be relatively more KCNQ1 mutant alpha-subunits translated and ultimately assembled to provide more dysfunctional K v 7.1 channels, thus leading to a more severe manifestation of the disorder (see eFig. 63.1 ). The opposite would be true if the mutation containing the KCNQ1 allele was suppressed.
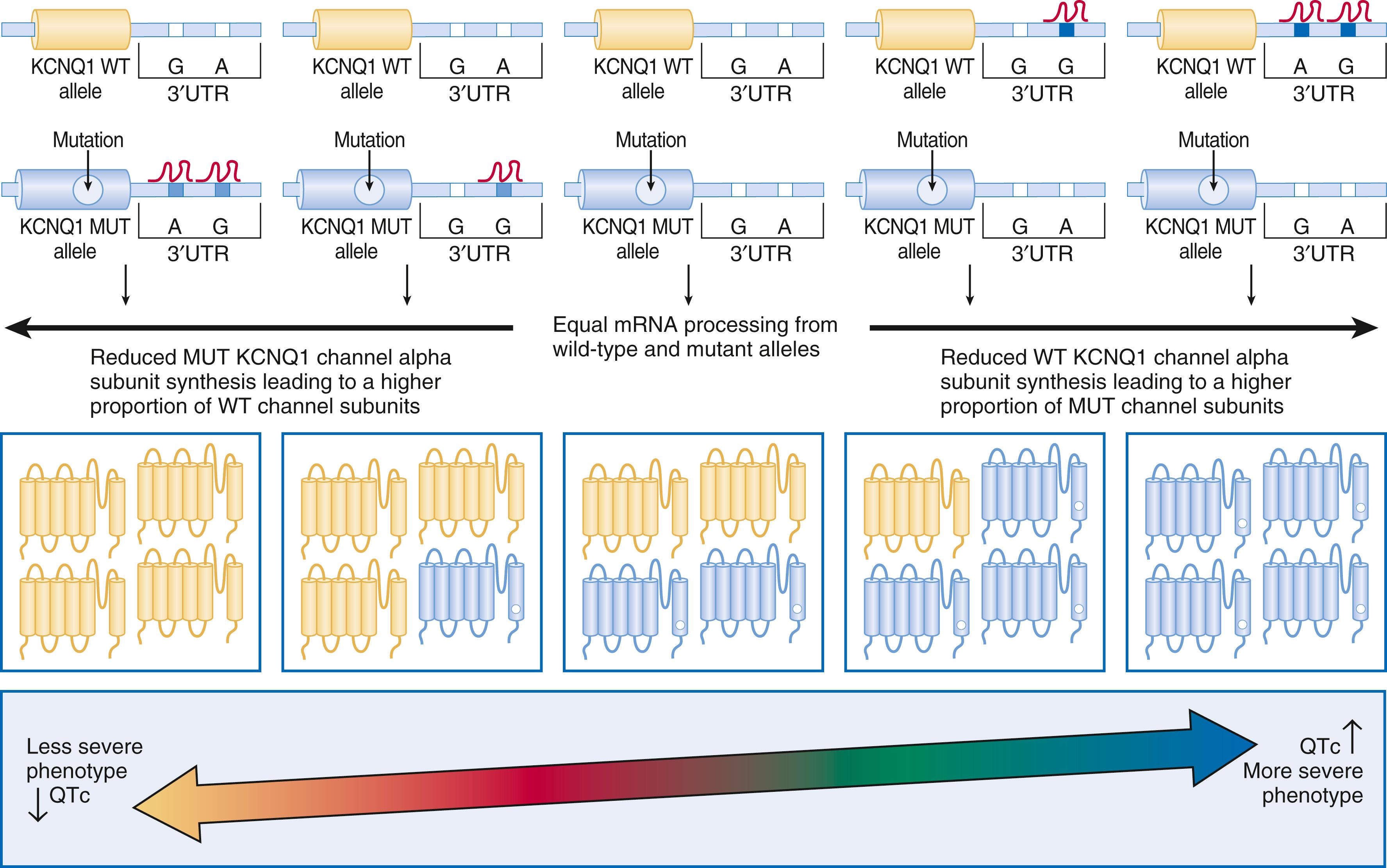
Most genes have a 3′UTR that generates an mRNA transcript containing regions of cis-regulatory binding sites for small noncoding microRNAs (miRNAs) that bind to the transcript and ultimately inhibit that gene’s expression. Naturally occurring genetic variation within these 3′UTRs (miR-SNPs) can either abolish existing or creating new miRNA binding sites. SNPs in the KCNQ1 3′UTR create a “suppressive” haplotype by generating new miRNA binding sites that suppress the expression of the KCNQ1 allele in which they reside. Inheritance of the “suppressive” haplotype residing on the normal “healthy” allele produced a more severe LQT1 phenotype, whereas the inheritance of the “suppressive” haplotype residing on the same allele as the KCNQ1 mutation gave a less severe LQT1 phenotype (shorter QTc and fewer symptoms). This intriguing discovery both explains a significant component of reduced penetrance and variable expressivity that is a common feature of arrhythmia syndromes, and also represents a paradigm shift in our thinking about disease-modifying genetic-drivers of mendelian disorders (as one of the most important genetic determinants of disease severity in LQT1 appears to be the 3′UTR KCNQ1 haplotype on the allele inherited from the unaffected “non-LQTS” parent).
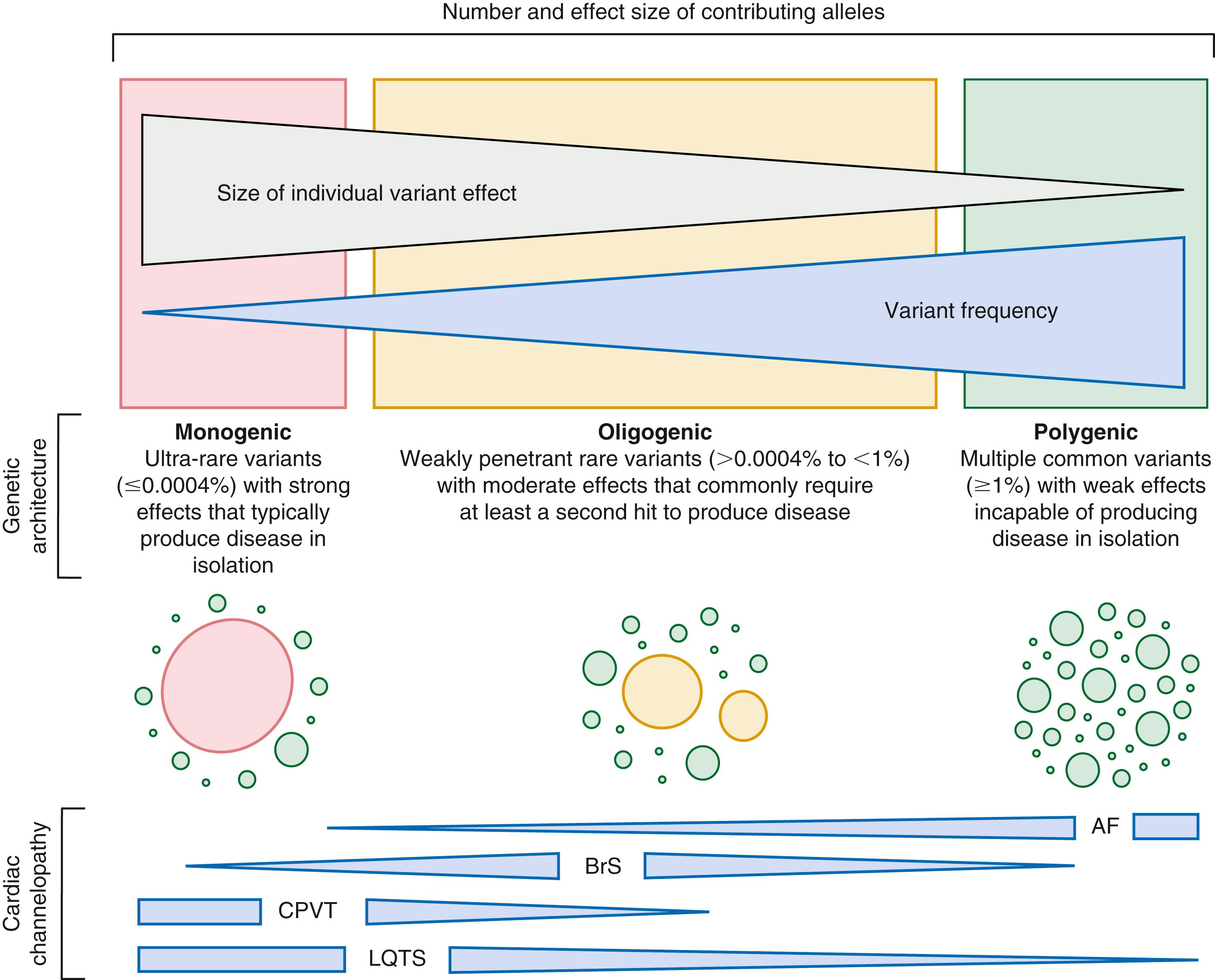
In line with the concept that common variants within noncoding regions of the human genome may impact the penetrance and expressivity (a.k.a. clinical variability) of rare LQTS pathogenic/likely pathogenic variants, recent studies have demonstrated that an aggregate polygenic risk score (PRS) comprised of common variants that influence QTc duration at the level of population can explain 2% to 15% of the clinical variability observed among LQTS patients. , Although the clinical utility of these PRSs in the management of genotype-positive LQTS patients remains unclear, they do provide intriguing and potentially clinically relevant insights into the genetic architecture of the 10% to 20% of LQTS patients that remain genotype-negative for monogenic LQTS.
Of note, the recent rare disease genome-wide association study (GWAS) by Lahrouchi et al. provided compelling evidence, by way of a 68 SNP/common variant–weighted PRS, that genotype-negative LQTS likely represents a polygenic subtype that arises secondary to the accumulation of multiple QTc-prolonging common genetic variants. Interestingly, in comparison to patients with canonical LQTS (i.e., LQT1-LQT3), these genotype-negative patients had longer aggregate QTc intervals and a similar rate of event-free survival. These observations are in line with those from other genetic heart diseases considered, at least initially, to be predominantly mendelian/monogenic such as familial hypercholesterolemia and BrS.
LQTS, like many cardiovascular disorders, appears to have a more complex genetic architecture than anticipated initially that likely includes monogenic, oligogenic, and polygenic subtypes ( Fig. 63.4 ). As our understanding of the genetic architecture underlying LQTS continues to grow, it appears increasingly likely that commercial gene panel-based LQTS genetic tests in use today require an overhaul to better accommodate assessment of the 10% to 20% of patients that have oligogenic/polygenic subtypes, and the genetic background that influences the penetrance and expressivity of canonical LQTS-causative variants.
In the early 2010s, three independent, unbiased exome sequencing studies implicated heterozygous sporadic/de novo pathogenic variants in the biologically redundant CALM1 , CALM2 , and CALM3 genes that collectively encode calmodulin (an ubiquitously expressed and essential calcium-handling protein) in infants/young children with extreme QT prolongation (i.e., >600 msec) and adrenergic-triggered life-threatening ventricular arrhythmias. Subsequently, pathogenic variants in CALM1-3 have also been identified in patients with CPVT , IVF , and CPVT/LQTS overlap phenotypes. However, recent data from the multicenter International Calmodulinopathy Registry indicate that LQTS (49%) and CPVT (28%) phenotypes predominate.
From a pathophysiological perspective, it is interesting to note that all LQTS-causative CALM1-3 mutations described to date localize within or proximal to calcium-coordinating residues of the C-terminal lobe (C-lobe) of calmodulin and impart a marked reduction in calcium-binding affinity. , Although calmodulin is known to regulate a number of cardiac ion channels, the predominant effect of LQTS pathogenic/likely pathogenic CALM1-3 variants appears to be loss of calcium-dependent inactivation (CDI) of the L-type calcium channel (LTCC) resulting in unrestrained calcium influx (i.e., LTCC/I CaL gain-of-function). In contrast, CPVT pathogenic/likely pathogenic variants in CALM1-3 localized at the N-terminal (N-lobe) and C-lobe increase RyR2-binding affinity/single channel open probability and spontaneous calcium release from the sarcoplasmic reticulum rather than calcium-binding affinity.
Unfortunately, the calmodulinopathies (such as CALM-LQTS and CALM-CPVT) are frequently refractory to conventional LQTS- and CPVT-directed therapies (beta-blockers). At present, it is not known whether the varying levels of neurodevelopmental delay observed in a minority of patients with a calmodulinopathy (17%) are secondary to anoxic brain injury in the setting of recurrent cardiac arrhythmias or are related to developmental effects of perturbed calcium-handling in the central nervous system. Regardless, a combination of pharmacologic, sympathectomy, and device-related therapies is typically required and even then is often inadequate. As such, the calmodulinopathies represent an important target for gene-therapy and other targeted precision medicine approaches.
In 2015, Altmann et al. described a rare autosomal recessive form of LQTS characterized by transient/consistent QT prolongation with extensive precordial (V 1 –V 4 ) T wave inversions (see Fig. 63.1B ), severe and often refractory exercise-induced ventricular arrhythmias during childhood. In addition, they described mild-to-moderate proximal skeletal myopathy secondarily due to either homozygous (p.D18fs∗13-TRDN and p.K147fs0∗-TRDN) or compound heterozygous (p.N9fs∗5-TRDN and p.K147fs∗0-TRDN) frame-shift/null pathogenic/likely pathogenic variants in TRDN -encoded triadin (a key structural component of the cardiac release unit [CRU]). , Interestingly, some of the same TRDN null variants (p.D18fs∗13-TRDN and p.N9fs∗5-TRDN) were implicated previously in recessively inherited CPVT, suggesting that Triadin knockout syndrome (TKOS) may represent a unique clinical entity with clinical characteristics of both LQTS and CPVT.
To this end, a recent study from the International TKOS Registry attempted to clarify the phenotype observed in individuals with homozygous/compound heterozygous TRDN null variants. Whereas the mean QTc observed in TKOS patients was 472 ± 34 msec, exercise-induced ectopy was observed in 89%. Furthermore, 90% of TKOS patients experienced SCA/SCD, and 74% suffered breakthrough cardiac events despite a myriad of medical, surgical (sympathectomy), and device therapy highlighting the malignant nature of the distinct TKOS clinical phenotype.
Like calmodulinopathies, TKOS is primarily a disorder of calcium-handling. Although the TKOS patient-specific human induced pluripotent stem cell-derived cardiomyocytes (iPSC-CM) have yet to be characterized, insights gleaned from ventricular arrhythmia-prone TRDN null mice suggest that complete ablation of triadin, as would be expected in TKOS patients, disrupts the approximation of the T-tubule and junctional sarcoplasmic reticulum within the cardiac dyad and reduces the expression of key proteins such as RyR2, calsequestrin2, and junctin reducing the co-localization of the LTCC/RyR2 and RyR2/Calsequestrin2 in the CRU. The resulting remodeling of the CRU leads to reduced sarcoplasmic reticulum calcium release and impaired LTCC CDI that ultimately leads to calcium overload in the sarcoplasmic reticulum. These molecular events likely contribute to an underlying proarrhythmic electrophysiological substrate capable of triggering delayed afterdepolarization- and/or early after-depolarization-mediated ventricular arrhythmias. This explains the distinct and particularly malignant clinical phenotype, with elements of LQTS and CPVT, observed in TKOS patients. ,
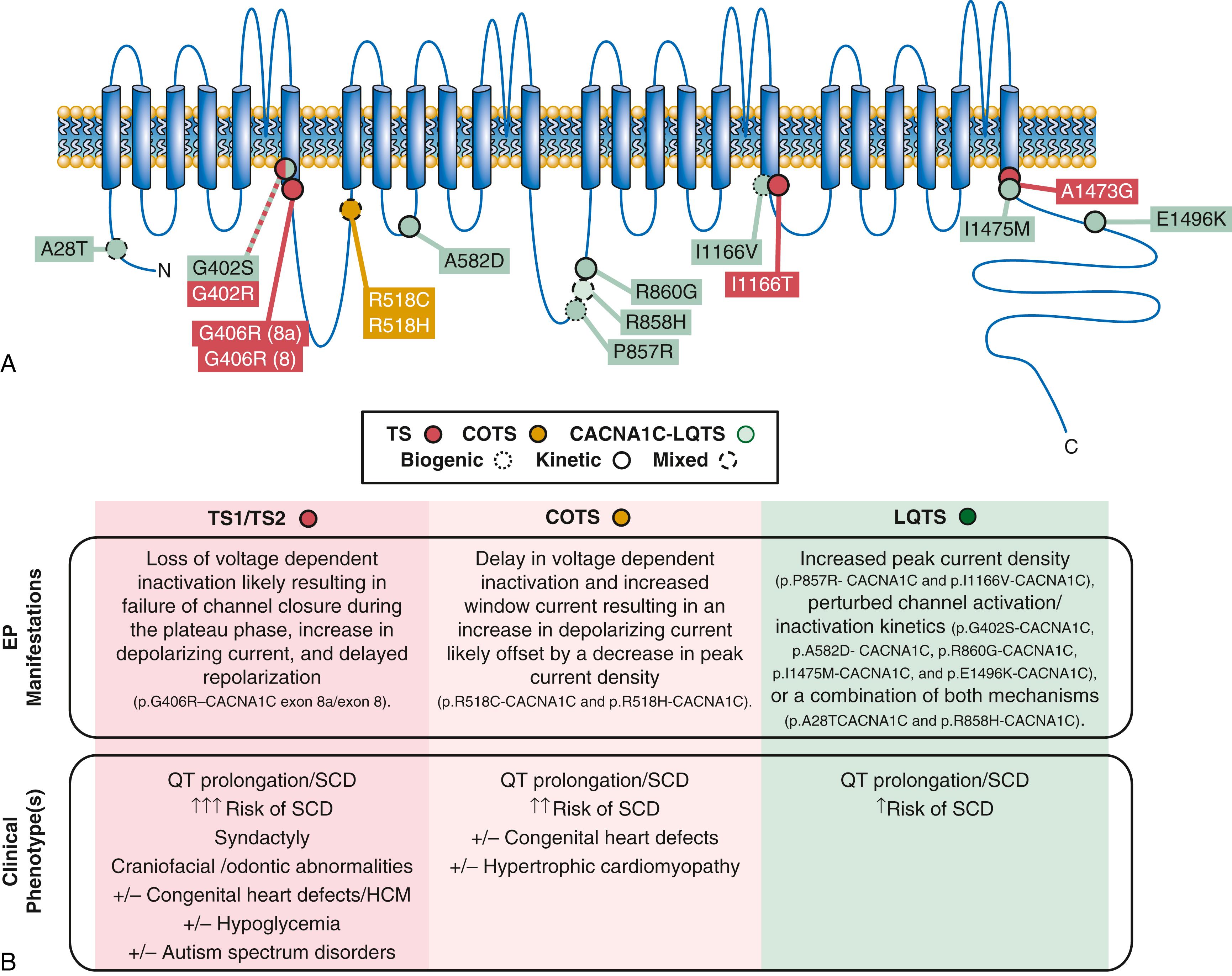
Timothy syndrome (TS) is an extremely rare (<30 patients described worldwide) multisystem, highly lethal arrhythmia disorder, associated with both cardiac and extracardiac abnormalities. The typical cardiac manifestation of TS includes fetal bradycardia, extreme prolongation of the QT interval (QTc >500 msec) often with macroscopic T wave alternans and 2:1 atrioventricular block at birth (see Fig. 63.1C ). These abnormalities often coincide with congenital heart defects or cardiomyopathies. Extracardiac abnormalities often consist of simple syndactyly (webbing of the toes and fingers), dysmorphic facial features, abnormal dentition, immune deficiency, severe hypoglycemia, and developmental delay (including autism). Currently, most TS patients die before reaching puberty. While the majority of TS has been described as sporadic/de novo occurrences, few cases with somatic mosaicism associated with a less severe phenotype have recently been described. For example, the CACNA1C mutation may be present in the patient’s skeletal muscle, but could only be present in traces or even completely absent in other cell types of the human body (i.e., absent in heart, blood lymphocytes), where the patient may present with simple syndactyly.
In 2004, Splawski et al. identified the molecular basis for this highly lethal arrhythmia and named it Timothy syndrome (TS) after Katherine Timothy, Drs. Keating’s and Splawski’s study coordinator who meticulously phenotyped these cases. Remarkably, in all 13 unrelated patients where DNA was available, Splawski identified the same recurrent sporadic de novo missense mutation, p.G406R-CACNA1C, in the alternatively spliced exon 8A of the CACNA1C -encoded cardiac LTCC (Ca v 1.2), which is important for excitation-contraction coupling in the heart and mediates an inward depolarizing current in cardiomyocytes (see eTable 63.1 , Fig. 63.2 ) similar to the cardiac sodium channel Na v 1.5. Through alternative splicing, the human L-type Ca channel consists of two mutually exclusive isoforms: one containing exon 8A and the other with exon 8. A year later, they described two cases of atypical TS with similar features of TS yet without syndactyly. As with other TS cases, these two atypical cases were identified as having sporadic de novo CACNA1C mutations in exon 8. One case hosted a mutation analogous to the classic TS mutation, p.G406R-CACNA1C , whereas the other case hosted a p.G402R-CACNA1C missense mutation. All three mutations confer gain-of-function to the LTCC/Ca v 1.2 channels through impaired channel inactivation and reside very near the end of the S6 transmembrane segment of domain 1 in the beginning of the intracellular loop between domain I and II of the Ca v 1.2 alpha subunit.
In 2011, Gillis et al. identified a novel CACNA1C mutation, p.A1473G-CACNA1C , in a single patient with a prolonged QT interval, dysmorphic facial features, syndactyly, and joint contractures consistent with TS. In 2015, Boczek et al. identified a novel CACNA1C mutation, p.I1166T-CACNA1C , in a patient exhibiting a TS phenotype with QT prolongation, patent ductus arteriosus, seizures, facial dysmorphism, joint hypermobility, hypotonia, hand anomalies, intellectual impairment, and tooth decay. Patch-clamp analysis of p.I1166T-CACNA1C demonstrated a novel electrophysiological phenotype distinct from the loss of inactivation seen with the previously established TS mutations. Instead, p.I1166T-CACNA1C electrophysiological studies illustrated a loss of current density and a gain-of-function shift in activation, leading to an increase in window current. Interestingly, both p.I1166T-CACNA1C’s and p.A1473G-CACNA1C’s topological position (a few amino acids away from the S6 transmembrane segment of the domain III and IV, respectively) in the channel architecture is very similar to the position of the three original TS mutations (S6 segment of domain I).
In 2015, Boczek et al. used exome sequencing to identify a novel CACNA1C mutation p.R518C-CACNA1C that was most likely responsible for the observed phenotype in a large pedigree with concomitant LQTS, hypertrophic cardiomyopathy (HCM), congenital heart defects, and sudden cardiac death. None of the patients had extracardiac phenotypes, such as those observed with TS. A subsequent CACNA1C exon 12 specific analysis in 5 additional unrelated index cases with a similar phenotype of LQTS and a personal/family history of HCM identified 2 additional pedigrees with mutations at the same amino acid position; either p.R518C-CACNA1C or p.R518H-CACNA1C. Patch-clamp studies on both revealed a complex Ca v 1.2 electrophysiological phenotype consisting of loss of current density and inactivation in combination with increased window and late current. All three pedigrees hosting p.R518C-CACNA1C/p.R518H-CACNA1C presented with this unique and atypical phenotypic sequela consistent with cardiac-only Timothy syndrome (COTS).
The spectrum of QT-opathies associated with LTCC/Ca v 1.2 gain-of-function currently encompasses nonsyndromic LQTS (i.e., CACNA1C-LQTS/LQT8), COTS, and TS. The electrophysiological mechanisms and clinical phenotypes that differentiate this spectrum of LTCC/Ca v 1.2-mediated disorders are detailed in Figure 63.5 and are the focus of several recent comprehensive reviews. ,
Short QT syndrome (SQTS), first described in 2000 by Gussak et al., is associated with a short QT-interval (usually ≤320 msec) on a 12-lead ECG ( Fig. 63.1D ), paroxysmal atrial fibrillation (AF), syncope, and an increased risk for SCD. Giustetto et al. analyzed the clinical presentation of 53 patients with SQTS from 29 families (the largest cohort studied to date). They found that 62% of the patients were symptomatic, with cardiac arrest being the most common symptom (31% of patients) and frequently the first manifestation of the disorder. A fourth of the patients had a history of syncope, and nearly 30% had a family history of SCD. Symptoms including syncope or cardiac arrest most often occurred during periods of rest or sleep. Nearly one-third presented with AF. SCD was observed during infancy, suggesting the potential role for SQTS as a rare pathogenic basis for some cases of SIDS.
SQTS is most often inherited in an autosomal dominant manner; however, some de novo sporadic cases have been described. To date, mutations in six genes (see eTable 63.1 ) have been implicated in the pathogenesis of SQTS, including gain-of-function mutations in the potassium channel encoding genes KCNH2 (SQT1), KCNQ1 (SQT2), and KCNJ2 (SQT3) and loss-of-function mutations in CACNA1C (SQT4), CACNB2b (SQT5), and CACNA2D1 (SQT6) encoding for LTCC alpha, beta, and delta subunits, respectively (see eTable 63.1 , Fig. 63.2 ). However, despite the identification of these SQTS-susceptibility genes, the proportion of SQTS expected to be SQT1-6 genotype positive and that awaiting genetic elucidation are unknown. It is estimated that over 75% of SQTS remains elusive genetically.
While there are insufficient data to clearly define genotype-phenotype correlations in SQTS (probably fewer than 60 cases have been described in the literature to date), gene-specific ECG patterns are beginning to emerge. The typical ECG pattern consists of a QT-interval of ≤320 msec (QTc ≤340 msec) and tall, peaked T waves in the precordial leads with either a short ST segment present or no ST-segment at all. The T waves tend to be symmetrical in SQT1 but asymmetrical in SQT2-4. In SQT2, inverted T waves can be observed. In SQT5, a BrS-like ST elevation in the right precordial lead could be observed as well.
Owing to the prematurely small sample size, a recent report revealed that SQTS patients with KCNH2 mutations have a shorter QT and a greater response to hydroquinidine therapy than patients with a non-KCNH2 mediated SQTS. Based on a clinical variable analysis of 65 mutation-positive SQTS patients among 132 SQTS cases previously reported in the literature, Harrell et al. indicated that patients with KCNH2 -mediated SQTS (SQT1) exhibit a later age of onset of manifestation, whereas patients with KCNQ1 -mediated SQTS (SQT2) have a higher prevalence of bradyarrhythmias and AF.
Drug-induced long QT syndrome (DI-LQTS) and torsades de pointes is a multifactorial clinical entity that tends to surface in the setting of multiple modifiable (electrolyte abnormalities such as hypokalemia, co-administration of multiple QT-prolonging drugs, drug accumulation due to renal/hepatic impairment or inhibition of cytochrome P450 metabolism) and non-modifiable (female sex, underlying genetic disposition, structural heart disease, and diabetes) risk factors (see Chapter 64, Chapter 9 ). The estimated incidence of DI-LQTS/DI-TdP is drug-dependent, while class III anti-arrhythmic agents range between 1% and 8% depending on the drug and dose. DI-TdP and subsequent sudden death are rare events; however, the list of potential “QT-liability” or “torsadegenic” drugs is extensive and includes both anti-arrhythmic drugs (quinidine, sotalol and dofetilide) and many noncardiac medications (antipsychotics, methadone, antimicrobials, antihistamines, and the gastrointestinal stimulant cisapride [see https://www.crediblemeds.org for a comprehensive list]).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here