Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
| Acetylcholinesterase | AchE |
| α-fetoprotein | AFP |
| American College of Medical Genetics and Genomics | ACMG |
| American College of Obstetricians and Gynecologists | ACOG |
| Assisted reproductive technology | ART |
| Cell-free DNA | cfDNA |
| Chorionic villus sampling | CVS |
| Chromosomal microarray | CMA |
| Comparative genomic hybridization | CGH |
| Confidence interval | CI |
| Confined placental mosaicism | CPM |
| Congenital bilateral absence of the vas deferens | CBAVD |
| Copy number variant | CNV |
| Cystic fibrosis | CF |
| Deoxyribonucleic acid | DNA |
| Early amniocentesis | EA |
| First and Second Trimester Evaluation of Risk | FASTER |
| Fluorescence in situ hybridization | FISH |
| Genitourinary | GU |
| Human chorionic gonadotropin | hCG |
| Human immunodeficiency virus | HIV |
| Human leukocyte antigen | HLA |
| Individual patient data | IPD |
| Inhibin A | INHA |
| Intelligence quotient | IQ |
| Intracytoplasmic sperm injection | ICSI |
| Intrauterine growth restriction | IUGR |
| Limb reduction defect | LRD |
| Massively parallel DNA shotgun sequencing | MPSS |
| Maternal serum α-fetoprotein | MSAFP |
| Mean corpuscular volume | MCV |
| Megabase | MB |
| Meta-analysis | MA |
| Multiples of the median | MoM |
| Nasal bone | NB |
| National Institute of Child Health and Human Development | NICHD |
| Neural tube defect | NTD |
| Noninvasive prenatal screening | NIPS |
| Nuchal translucency | NT |
| Percutaneous umbilical blood sampling | PUBS |
| Polymerase chain reaction | PCR |
| Pregnancy-associated plasma protein A | PAPP-A |
| Preimplantation genetic testing | PGT |
| Quantitative polymerase chain reaction | QPCR |
| Randomized controlled trial | RCT |
| Single nucleotide polymorphism | SNP |
| Small for gestational age | SGA |
| Society of Maternal-Fetal Medicine | SMFM |
| Spinal muscular atrophy | SMA |
| Transabdominal chorionic villus sampling | TA-CVS |
| Transcervical chorionic villus sampling | TC-CVS |
| Unconjugated estriol | uE 3 |
| Uniparental disomy | UPD |
| Variants of uncertain significance | VOUS |
| Whole exome sequencing | WES |
| Whole Genome Sequencing | WGS |
| Whole-genome amplification | WGA |
The goal of genetic screening is to identify individuals or couples at risk for having a child with an inherited condition, chromosome abnormality, or birth defect . Ideally, screening should take place before conception to ensure that couples are fully informed of their reproductive options, including preimplantation genetic screening and diagnosis, or screening should be done as early as possible in pregnancy to allow couples the opportunity to consider aneuploidy screening and prenatal diagnostic testing. Genetic screening begins with a thorough personal and family history, followed by genetic counseling if indicated. Approximately 3% of liveborn infants will have a major congenital anomaly; approximately one-half of these anomalies are detected at birth and are due to a genetic cause—a chromosome abnormality, single-gene mutation, or polygenic/multifactorial inheritance. Less frequently, malformations may be due to nongenetic causes or teratogens (see Chapter 7 ). The detection of many congenital malformations is possible using ultrasonography and fetal echocardiography (see Chapter 9 ). Screening for aneuploidy, inherited disorders, and structural malformations is an integral part of routine obstetric care. When indicated and desired, amniotic fluid, placental tissue, and cord blood can be readily obtained and analyzed for chromosome abnormalities and genetic disorders. In this chapter, we review genetic history and counseling, common chromosome abnormalities, mendelian disorders, aneuploidy and carrier screening, cytogenetic and molecular genetic testing, whole exome sequencing (WES), and techniques for prenatal and preimplantation genetic diagnostic testing.
Obstetricians/gynecologists should attempt to take a thorough personal and family history to determine whether a woman, her partner, or a relative has a heritable disorder, birth defect, intellectual disability, or psychiatric disorder that may increase their risk of having an affected offspring.
The clinician should inquire into the health status of first-degree relatives (siblings, parents, offspring), second-degree relatives (nephews, nieces, aunts, uncles, grandparents), and third-degree relatives (first cousins, especially maternal). A positive family history of a genetic disorder may warrant referral to a clinical geneticist or genetic counselor who can accurately assess the risk of having an affected offspring and review genetic screening and testing options. In some cases, it may be straightforward enough for the well-informed obstetrician to manage. For example, if a birth defect such as a cleft lip and palate or neural tube defect (NTD) exists in a second- or third-degree relative, the risk for that anomaly will usually not prove substantially increased over that of the general population. In contrast, identification of a second-degree relative with an autosomal recessive disorder such as cystic fibrosis (CF) increases the risk for an affected offspring; therefore more extensive genetic counseling should be considered. Adverse reproductive outcomes such as repetitive spontaneous abortions, stillbirths, and anomalous liveborn infants should be noted. Couples who have such histories should undergo chromosome studies to exclude balanced translocations, which could impact a subsequent pregnancy (see Chapter 33 ).
Parental ages should be recorded. Advanced maternal age confers an increased risk for aneuploidy. A few studies indicate an increased frequency of aneuploidy in sperm in the sixth and seventh decades. However, risks are only marginally increased above background, and data do not indicate that the risks of having aneuploid liveborns are increased based on paternal age. A paternal-age effect is associated with a small aggregate increased risk (0.3% to 0.5% or less in men older than 40 years of age) for sporadic gene mutations for certain autosomal dominant conditions such as achondroplasia and craniosynostosis. No specific screening tests exist for anomalies associated with advanced paternal age, although some of these conditions may be detected by ultrasonography (see Chapter 9 ).
Ethnic origin should also be recorded because certain genetic diseases are increased in selected ethnic groups; this will be discussed in this chapter. Such queries also apply to gamete donors.
Although situations exist in which referral to a clinical geneticist or genetic counselor is indicated, it is impractical for obstetricians to refer all patients with genetic inquiries. Obstetricians should be able to counsel patients before performing screening tests for aneuploidy and NTDs and prior to carrier screening as well. Pivotal to counseling is communication in terms readily understood by patients. Preprinted information, videos, and select websites that cover common genetic conditions are useful and have the additional advantage of emphasizing that a given problem is not unique. Medical records should be requested and reviewed to confirm a diagnosis. It may be necessary for an appropriate specialist to examine an affected individual and order confirmatory diagnostic tests; examining first-degree relatives may also be required to detect subtle findings. This is particularly applicable for autosomal dominant disorders such as neurofibromatosis or Marfan syndrome, for which variable expressivity is expected. Accurate counseling requires a definitive diagnosis. However, the physician should not hesitate to acknowledge whether a definitive diagnosis cannot be established .
In genetic counseling, the clinician should provide accurate genetic information and outline the options for screening and testing without being prescriptive. Of course, completely nondirective counseling is probably unrealistic. Despite the difficulties of remaining truly objective, the clinician should attempt to provide information in a nondirective manner and then support the couple's decision.
A basic fund of knowledge about common chromosome disorders is essential for the obstetrician who offers genetic screening for aneuploidy or who may encounter an abnormal fetus or infant during pregnancy or at delivery. With increasing utilization of chromosome microarrays for prenatal diagnosis, it is important for obstetricians to be familiar with the clinical significance of both numeric and structural chromosome abnormalities.
The incidence of chromosome aberrations is 1 in 160 newborns. In addition, more than 50% of first-trimester spontaneous abortions and at least 5% of stillborn infants exhibit chromosome abnormalities (see Chapters 33 and 34 ). The chromosome abnormalities that generate the greatest attention are the autosomal trisomies ( Table 10.1 ). Autosomal trisomy usually arises as a result of nondisjunction that produces a gamete with 24 chromosomes rather than the expected 23 chromosomes; this results in a zygote having 47 chromosomes. This error most commonly occurs during maternal meiosis and is associated with the well-known maternal-age effect. Table 10.2 shows the year-to-year (maternal age) increase in frequency of Down syndrome and other aneuploidies. The frequency is approximately 30% higher in midpregnancy than at term, which reflects lethality throughout pregnancy. Some trisomies (e.g., trisomy 16) arise almost exclusively in maternal meiosis, usually maternal meiosis I. For a few chromosomes, the frequency of errors is relatively higher in meiosis II (e.g., trisomy 18), and in yet others, errors in paternal meiosis are not uncommon (e.g., trisomy 2). Autosomal trisomy can recur and has a recurrence risk of approximately 1% following either trisomy 18 or 21. This suggests that genetic factors perturb meiosis, a phenomenon that serves as justification for offering prenatal genetic screening or testing after one aneuploid conception.
| Autosomal Trisomy | Incidence Live Births | Clinical Features |
|---|---|---|
| Trisomy 21 | 1 in 800 | Facial features: brachycephaly; oblique palpebral fissures; epicanthal folds; broad nasal bridge; protruding tongue; small, low-set ears with an overlapping helix and a prominent antihelix; iridial Brushfield spots |
| Skeletal features: broad, short fingers (brachymesophalangia); clinodactyly (incurving fifth finger resulting from an abnormality of the middle phalanx); a single flexion crease on the fifth digit; wide space between the first two toes | ||
| Cardiac defects, duodenal atresia, neonatal hypotonia | ||
| Increased susceptibility to respiratory infections and leukemia | ||
| Mean survival extends into the fifth decade | ||
| Mean IQ is 25 to 70 | ||
| Trisomy 13 | 1 in 20,000 | Holoprosencephaly, eye anomalies (microphthalmia, anophthalmia, or coloboma), cleft lip and palate, polydactyly, cardiac defects, cutaneous scalp defects, hemangiomata on the face or neck, low-set ears with an abnormal helix, and rocker-bottom feet (convex soles and protruding heels) |
| Intrauterine and postnatal growth restriction | ||
| Severe intellectual disability | ||
| Trisomy 18 | 1 in 8000 | Facial features: microcephaly, prominent occiput, low-set and pointed fawnlike ears, micrognathia |
| Skeletal anomalies: overlapping fingers (V over IV, II over III), short sternum, shield chest, narrow pelvis, limited thigh abduction or congenital hip dislocation, rocker-bottom feet with protrusion of the calcaneum, and a short dorsiflexed hallux (hammer toe) | ||
| Cardiac defects, renal anomalies | ||
| Intrauterine growth restriction, developmental disability |
| Maternal Age | Risk for Down Syndrome | Risk for Any Chromosome Abnormalities |
|---|---|---|
| 20 | 1 in 1667 | 1 in 526 a |
| 21 | 1 in 1667 | 1 in 526 a |
| 22 | 1 in 1429 | 1 in 500 a |
| 23 | 1 in 1429 | 1 in 500 a |
| 24 | 1 in 1250 | 1 in 476 a |
| 25 | 1 in 1250 | 1 in 476 a |
| 26 | 1 in 1176 | 1 in 476 a |
| 27 | 1 in 1111 | 1 in 455 a |
| 28 | 1 in 1053 | 1 in 435 a |
| 29 | 1 in 1100 | 1 in 417 a |
| 30 | 1 in 952 | 1 in 384 a |
| 31 | 1 in 909 | 1 in 385 a |
| 32 | 1 in 769 | 1 in 322 a |
| 33 | 1 in 625 | 1 in 317 a |
| 34 | 1 in 500 | 1 in 260 |
| 35 | 1 in 385 | 1 in 204 |
| 36 | 1 in 294 | 1 in 164 |
| 37 | 1 in 227 | 1 in 130 |
| 38 | 1 in 175 | 1 in 103 |
| 39 | 1 in 137 | 1 in 82 |
| 40 | 1 in 106 | 1 in 65 |
| 41 | 1 in 82 | 1 in 51 |
| 42 | 1 in 64 | 1 in 40 |
| 43 | 1 in 50 | 1 in 32 |
| 44 | 1 in 38 | 1 in 25 |
| 45 | 1 in 30 | 1 in 20 |
| 46 | 1 in 23 | 1 in 15 |
| 47 | 1 in 18 | 1 in 12 |
| 48 | 1 in 14 | 1 in 10 |
| 49 | 1 in 11 | 1 in 7 |
a 47,XXX excluded for ages 20 to 32 years (data not available).
In addition to numeric abnormalities, structural chromosome abnormalities such as translocations, deletions, and duplications can occur. Individuals with a balanced translocation caused by an interchange between two or more chromosomes are usually phenotypically normal. However, such individuals are at increased risk for offspring with unbalanced gametes, which may result in recurrent pregnancy loss, fetal demise, congenital anomalies, and intellectual disability. Small, often submicroscopic deletions and duplications of chromosome material can result in recognizable syndromes, such as the 22q11 deletion syndrome, and may cause structural malformations as well as cognitive, behavioral, and neuropsychological problems.
This section reviews the common autosomal trisomies and sex chromosome abnormalities an obstetrician is likely to encounter, and we discuss the clinical significance of deletions and duplications.
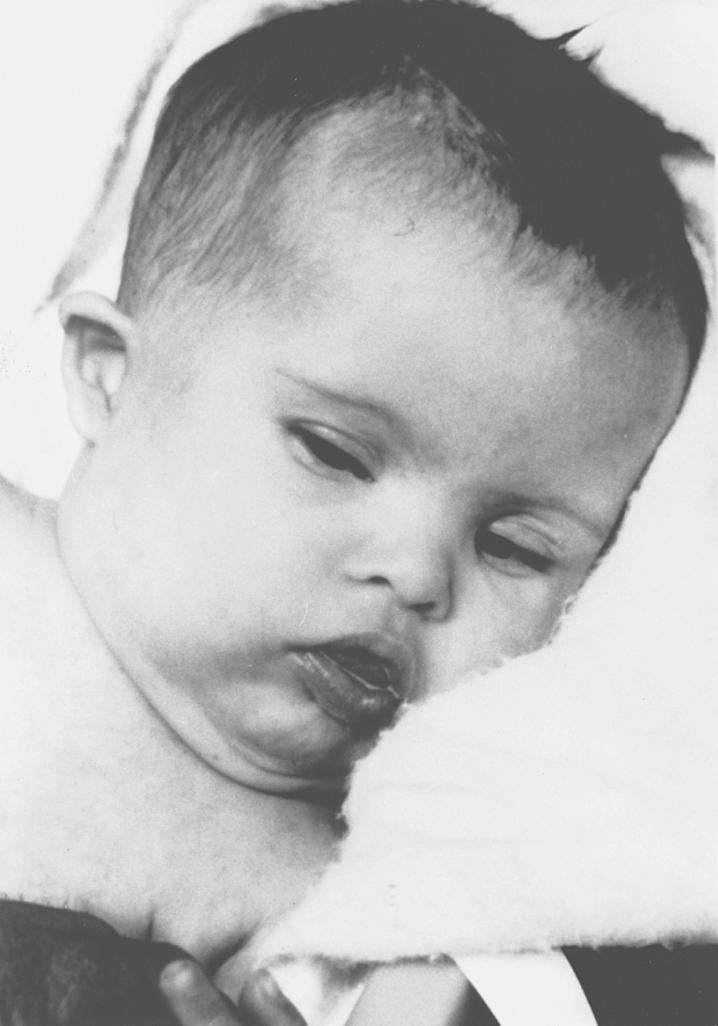
Trisomy 21, or Down syndrome, is the most frequent autosomal chromosome syndrome with characteristic craniofacial features and congenital anomalies ( Fig. 10.1 ; see also Table 10.1 ). The relationship of Down syndrome to advanced maternal age is well known (see Table 10.2 ). Approximately 95% of cases arise in maternal meiosis, usually meiosis I, and have 47 chromosomes (47,XX,+21 or 47,XY,+21). Mosaicism for chromosome 21 occurs in 2% to 4% of cases of Down syndrome and usually results in a higher IQ (70 to 80). Women with Down syndrome are usually fertile, and although relatively few trisomic mothers have reproduced, approximately 30% of their offspring are also trisomic. Men are invariably infertile.
Translocations (sporadic or familial) most commonly associated with Down syndrome involve chromosomes 14 and 21. One parent may have the same translocation, [45t(14q;21q)], referred to as a Robertsonian translocation. Empiric risks for having an offspring with Down syndrome are approximately 10% for female Robertsonian translocation carriers and 2% for male translocation carriers. A potential concern is that offspring who are diploid (46,XX or 46,XY) actually have uniparental disomy (UPD), a condition in which both of a given pair of chromosomes originate from the same parent. In a study of 65 Robertsonian translocation carriers, only one UPD case was observed (0.6%). Based on reports of UPD testing in 357 inherited and 102 de novo Robertsonian translocation cases the authors concluded that the overall risk for UPD is approximately 3%.
Other structural rearrangements that result in Down syndrome include t(21q;21q) and translocations that involve chromosome 21 and other acrocentric chromosomes (13,15 or 22). In t(21q;21q) carriers, only trisomic or monosomic zygotes are produced, and the latter presumably appear as preclinical embryonic losses. Parents who have other translocations have a low empiric risk of having offspring with Down syndrome.
Trisomy 13 occurs in approximately 1 in 20,000 live births. The clinical features of trisomy 13 are summarized in Table 10.1 . Most cases are caused by nondisjunction (47, +13) and are maternal in origin. Robertsonian translocations are responsible for fewer than 20% of cases and are invariably associated with two group D (13 to 15) chromosomes joining at their centromeric regions. If neither parent has a rearrangement, the risk for subsequent affected progeny is not increased. If either parent has a balanced 13q;14q translocation, the recurrence risk for an affected offspring is increased but only to 1% to 2%. The exception is a 13q;13q parental translocation in which no normal gametes are formed. For live births with trisomy 13, survival beyond 3 years is rare.
Trisomy 18 occurs in 1 per 8000 live births (see Table 10.1 ). Stillbirth is not uncommon. Fetal movement is feeble, and approximately 50% develop nonreassuring fetal status during labor. For live births, mean survival is measured in months, and pronounced developmental and growth retardation is apparent. Approximately 80% of trisomy 18 cases are caused by primary nondisjunction (47,+18). Errors usually arise in maternal meiosis, frequently meiosis II. Recurrence risk is approximately 1%.
All autosomes show trisomies, but usually trisomies other than those described previously end in abortuses. In addition to trisomies 13, 18, and 21, only a few other trisomies are detected in liveborns (8, 9, 14, 16, and 22), often as mosaics in conjunction with a normal cell line (46 chromosomes). All exhibit some degree of intellectual disability, various structural anomalies, and intrauterine growth restriction (IUGR).
Well-described genetic disorders have been associated with deletions or duplications of a number of chromosomes ( Table 10.3 ). Although some of these may be diagnosed on a routine karyotype, most will only be detected by chromosomal microarray analysis (CMA) capable of detecting deletions and duplications smaller than 5 Mb (5 million base pairs). Specific clinical features vary but may include learning difficulties, intellectual disability, neurologic and behavioral disorders, psychiatric disorders, and various congenital anomalies. De novo, large (1 Mb or greater) deletions or duplications, also referred to as copy number variants (CNVs), may contain dosage-sensitive genes and are more likely to be of clinical significance; however, even small CNVs can be significant. A growing body of literature and registries have compiled data on the outcomes of postnatal and prenatally ascertained CNVs. CMA has been recommended as a first-tier test for the postnatal evaluation of individuals with undiagnosed developmental delay, intellectual disabilities, autism spectrum disorder, and/or multiple congenital anomalies based on a review of 33 studies showing that pathogenic CNVs were found in 12.2% of the 21,698 individuals studied (10% higher than with routine karyotype). It is also important to recognize that many CNVs are of no ostensible clinical significance. In some cases the clinical significance remains unknown; these CNVs are referred to as variants of uncertain significance (VOUS).
| Chromosome Region | Syndrome | Clinical Features |
|---|---|---|
| 4p16.3 | Wolf-Hirschhorn | IUGR, failure to thrive, microcephaly, developmental delay, hypotonia, cognitive deficits, seizures, cardiac defects, GU abnormalities |
| 5p15.2 | Cri du chat | Microcephaly, SGA, hypotonia, catlike cry, cardiac defects |
| 7q11.23 | Williams | Supravalvular aortic stenosis, hypercalcemia, developmental delay, mild to moderate intellectual disability, social personality, attention-deficit disorder, female precocious puberty |
| 15q11.2q13 | Prader-Willi Angelman |
Prader-Willi: Hypotonia, delayed development, short stature, small hands and feet, childhood obesity, learning disabilities, behavioral problems, delayed puberty Angelman: Developmental delay, intellectual disability, impaired speech, gait ataxia, happy personality, seizures, microcephaly |
| 17p11.2 | Smith-Magenis | Mild to moderate intellectual disability, delayed speech and language skills, behavioral problems, short stature, reduced sensitivity to pain and temperature, ear and eye abnormalities |
| 20p12 | Alagille | Bile duct paucity, peripheral pulmonary artery stenosis, cardiac defects, vertebral and GU anomalies |
| 22q11.2 | DiGeorge (velocardiofacial) | Cardiac defects, hypocalcemia, thymic hypoplasia, immune defect, renal and skeletal anomalies, delayed speech, learning difficulties, psychological and behavioral problems |
Most deletions and duplications occur sporadically because of nonallelic homologous recombination mediated by low-copy repetitive sequences of DNA during meiosis or mitosis and are not related to parental age. Although the recurrence risk is low (<1%), a couple may desire prenatal testing in a future pregnancy due to the potential for germline mosaicism. Because CNVs can be familial, parental studies are recommended when a CNV is identified in a child or fetus. If a parent has the same CNV, then the risk to subsequent offspring is 50%. It is important to note that the phenotype of many deletion and duplication syndromes is highly variable, and even within the same family, this can range from mild to severe. In some cases a parent may appear phenotypically normal. The inability to accurately predict the outcome can lead to uncertainty and heightened anxiety; thus it is critically important that patients receive the most up-to-date information from an experienced counselor or geneticist.
The incidence of 45,X in liveborn girls is approximately 1 in 10,000. Monosomy X, or Turner syndrome, accounts for 10% of all first-trimester abortions; therefore it can be calculated that more than 99% of 45,X conceptuses are lost early in pregnancy. The error usually (80%) involves loss of a paternal sex chromosome. Mosaicism (i.e., 45,X/46,XX) is frequent.
Common features include primary ovarian failure, absent pubertal development due to gonadal dysgenesis (streak gonads), and short stature (<150 cm). Structural abnormalities of the X chromosome may also result in premature ovarian failure. Both the long arm and the short arm of the X chromosome contain determinants necessary for ovarian differentiation and for normal stature. Various somatic anomalies include renal and cardiac defects, skeletal abnormalities (cubitus valgus and clinodactyly), vertebral anomalies, pigmented nevi, nail hypoplasia, and a low posterior hairline. Performance IQ is lower than verbal IQ, but overall IQ is considered normal. Adult-onset diseases include hypertension, coronary artery disease, hypothyroidism, and type 2 diabetes mellitus.
Low-dose estrogen therapy is needed to induce puberty, and long-term hormone replacement is needed in adulthood. Pregnancy may be achieved with the use of donor eggs but requires careful monitoring of cardiovascular status before, during, and after pregnancy. Growth hormone treatment increases the final adult height 6 to 8 cm. Comprehensive guidelines for evaluation and clinical management of Turner syndrome are available.
Approximately 1 in 1000 males are born with Klinefelter syndrome, the result of two or more X chromosomes (47,XXY; 48,XXXY; and 49,XXXXY). Characteristic features include small testes, azoospermia, elevated follicle-stimulating hormone and luteinizing hormone levels, and decreased testosterone. The most common chromosome complement associated with this phenotype is 47,XXY.
Intellectual disability is uncommon in 47,XXY males, but behavioral problems and receptive language difficulties are common. Intellectual disability is almost invariably associated with 48,XXXY and 49,XXXXY. Skeletal, trunk, and craniofacial anomalies occur infrequently in 47,XXY but are commonly observed in 48,XXXY and 49,XXXXY. Regardless of the specific chromosome complement, patients with Klinefelter syndrome all have male phenotypes. The penis may be hypoplastic, but hypospadias is uncommon. With intracytoplasmic sperm injection (ICSI) and other assisted reproductive technology (ART), siring a pregnancy is now possible. Guidelines on evaluation and clinical management are available.
Approximately 1 in 800 liveborn girls has more than two X chromosomes. Most of these individuals have a normal reproductive system. The theoretic risk of delivering an infant who also has an abnormal chromosome complement is 50%, given half of the maternal gametes carry 24 chromosomes (24,XX). Empiric risks are much less. Somatic anomalies are uncommon but have been observed in some prenatally detected cases. The IQ of such individuals is 10 to 15 points lower than that of their siblings. The absolute risk for intellectual disability with 47,XXX does not exceed 5% to 10%, and even then, IQ is usually 60 to 80. However, 48,XXXX and 49,XXXXX individuals are invariably intellectually disabled and are more likely to have somatic malformations than individuals with a 47,XXX complement.
Presence of more than one Y chromosome is another frequent chromosome abnormality in liveborn boys (1 in 1000). Males with 47,XYY are more likely than 46,XY boys to be tall and are at increased risk for learning disabilities, speech and language delay, and behavioral and emotional difficulties. These individuals have normal male phenotype and sexual development.
Noninvasive screening for chromosome disorders such as trisomies 21 and 18 is routinely offered to women during pregnancy regardless of maternal age . Several noninvasive approaches to screening are available that use cell-free DNA (cfDNA), maternal serum analytes, and/or ultrasonography in the first and second trimesters ( Table 10.4 ). However, screening has limitations that must be taken into consideration when deciding which testing strategy best meets the patient's needs and preferences. That is, screening is not equivalent to testing, which implies a definitive answer. Pretest counseling should remind parents of the possibilities of false-negative or false-positive test results. Women with a positive screening test for aneuploidy should be referred for genetic counseling and offered an invasive diagnostic test.
| Screening Test | Trisomy 21 Detection Rate (%) | False-Positive Rate (%) |
|---|---|---|
| First-trimester NT, PAPP-A, free β-hCG | 82–87 | 5 |
| Second-trimester quad (MSAFP, hCG, uE 3 , INHA) | 81 | 5 |
| Sequential (first- and second-trimester quad) | 95 | 5 |
| Serum integrated (PAPP-A, quad screen) | 85–88 | 5 |
| cfDNA | 99 | <1 |
The newest screening test for aneuploidy is cfDNA analysis, sometimes referred to as noninvasive prenatal screening (NIPS). This method uses massive parallel sequencing analysis of cfDNA and was introduced into clinical practice in 2011 . Such testing can be performed as early as 10 weeks' gestation. Numerous studies have validated the ability of cfDNA screening for common trisomies (13, 18 and 21) and sex chromosome abnormalities with high sensitivity and false-positive rates less than 1%. However, patients should be counseled about the limitations of cfDNA analysis, including the possibility of a false-positive or false-negative test result and the necessity of confirmatory diagnostic testing to confirm screening results.
Maternal plasma contains small fragments of cfDNA (50 to 200 base pairs) derived from the breakdown of both maternal and fetal cells, primarily derived from the placenta. The concept of using cfDNA for prenatal diagnosis is not new; cfDNA has been used successfully to determine fetal sex in pregnancies at risk for X-linked disorders by identifying the Y-chromosome signal. In Europe, noninvasive testing is commonly used to determine fetal rhesus factor (Rh) status in RhD-negative women using real-time polymerase chain reaction (PCR) amplification. A similar approach can be adapted for the detection of some single-gene disorders. However, to screen for aneuploidy requires a different approach—the use of massively parallel DNA shotgun sequencing (MPSS).
Detection of aneuploidy is more difficult than for single-gene disorders because detecting fetal trisomy must reflect quantitative differences between affected and unaffected pregnancies. With MPSS technology, millions of fragments of maternal and fetal DNA are sequenced simultaneously in a single sample of maternal plasma, which is assigned to a given chromosome region and counted in comparison with a reference standard expected of a normal individual. A woman carrying a trisomy 21 fetus will have relatively more chromosome 21 counts (transcripts) than a woman carrying a normal fetus. Alternatively, some laboratories use a targeted approach that sequences specific chromosomes of interest, such as 18 and 21, and adjusts for the proportion of fetal DNA (fetal fraction) to provide a patient-specific risk assessment that takes into account maternal age. An alternative approach is to use single nucleotide polymorphism (SNP)-based sequencing, which allows for the detection of triploidy and some of the common deletion syndromes.
A number of studies have demonstrated the ability to detect fetal trisomy 21, 18, 13 and sex chromosome abnormalities using MPSS. A blinded, nested, case-control study of 4664 pregnancies at increased risk for trisomy 21 from 27 prenatal diagnostic centers worldwide validated the use of cfDNA analysis as a screening test for trisomy 21. In this study, 209 of 221 cases of trisomy 21 were detected; sensitivity was 98.6%, with a false-positive rate of 0.2%. Subsequently, Palomaki and colleagues reported that all cases of trisomy 18 in this cohort were detected, with a false-positive rate of 0.28%; however, only 91.7% of the cases of trisomy 13 were detected, with a false-positive rate of 0.97%.
Norton and colleagues conducted a multicenter, prospective cohort study—the Noninvasive Chromosomal Evaluation (NICE) study—of more than 3200 primarily high-risk women undergoing invasive diagnostic testing. For trisomy 21, the sensitivity was 100%, with a false-positive rate of 0.03%, whereas the sensitivity for trisomy 18 was 97.4% and the false-positive rate 0.07%. Furthermore, 29% of the chromosome abnormalities in this cohort were abnormalities other than trisomy 18 and 21, and hence it is important to counsel patients about the limitations of cfDNA testing.
In a cohort study of more than 2000 women who presented for routine first-trimester screening (11 to 14 weeks) with a mean maternal age of 31.8 years, Nicolaides and colleagues demonstrated that cfDNA analysis using targeted MPSS is feasible in a lower-risk population. The detection rate for trisomy 21 was 100%, with a false-positive rate of 0.1%. Norton and colleagues reported similar results in the international, blinded, prospective multicenter Noninvasive Examination of Trisomy (NEXT) study, which compared cfDNA to first-trimester screening at 10 to 14 weeks’ gestation in 15,841 women (mean age 30.7) with a singleton gestation. The positive predictive value (PPV) for trisomy 21 was 80.9% (95% confidence interval [CI], 66.7 to 90.9). Although all cases of trisomy 21 and 13 were detected, it is important to note that cfDNA testing failed to detect one in 10 cases of trisomy 18, nor would it have detected the other forms of aneuploidy found in this study population. This highlights the need to counsel patients about the limitations of screening tests to detect all forms of aneuploidy.
Another limitation of cfDNA screening is the reported assay failure rate of up to 5%. One of the reasons is a low fetal fraction; a minimum fetal fraction of 4% is required. The ability to detect the small differences between euploid and triploid fetuses depends on the relative proportion of fetal to maternal cfDNA. The average fetal fraction at 10 to 22 weeks’ gestation is 10% independent of gestational age, maternal age, race/ethnicity, or fetal karyotype. Fetal fraction decreases with maternal weight, and if cfDNA screening fails in an obese patient, it may be necessary to repeat the test on a second sample or to offer serum and/or ultrasound screening as an alternative. Further consideration should be given to offering invasive testing because recent studies have reported a higher frequency of aneuploidy when cfDNA testing fails. In the NEXT study the rate of aneuploidy among patients with no cfDNA results was 2.7%, a prevalence of 1 in 38 ; hence careful consideration should be given to offering diagnostic testing to this group of patients.
Although the false-positive and false-negative rates for cfDNA screening are lower than sequential and second-trimester screening, discordancies between cfDNA results and the fetus have been reported. A systematic review of the literature from 1997 to 2016 identified 182 cases of false-positives and 24 false-negative results. False-negative cases were reported for trisomy 21, 18, and 13 and mosaicism for these chromosomes. Approximately a third of the false-positive cases were trisomy 21 and a third trisomy 18; 26% were trisomy 13. Other aneuploidies were found in three cases and multiple aneuploidies in eight cases. This study excluded discordant sex chromosome results. A biologic or technical explanation for the discordancy between the cfDNA result and the fetus could be identified in only a third of these cases. Of these, 32% has placental mosaicism and 48% had a maternal copy number variation. Other explanations included a vanishing twin with trisomy 21, maternal chromosome abnormalities of chromosome 21 and 18, and nine cases with maternal cancer in which six had multiple aneuploidies. Maternal and fetal mosaicism for 45,X/XX can also lead to false-positive cfDNA results. These findings underscore the importance of offering confirmatory diagnostic testing prior to pregnancy termination when cfDNA indicates a high risk for a chromosome abnormality. Patients will need to be counseled that a negative cfDNA test does not ensure an unaffected pregnancy and cannot provide the diagnostic accuracy of an invasive prenatal diagnostic test, especially if fetal structural anomalies exist or with a family history of a genetic disorder.
The American College of Obstetricians and Gynecologists (ACOG) and Society of Maternal Fetal Medicine (SMFM) recommend cfDNA as one of the screening options for women with a singleton gestation at increased risk for aneuploidy, including women 35 years or older and those with fetal ultrasound markers and structural anomalies associated with aneuploidy, prior pregnancy with a trisomic offspring, positive maternal serum screening test, or a parental Robertsonian translocation carrying risk for trisomy 21. The American College of Medical Genetics and Genomics (ACMG) acknowledges that all women regardless of risk status should have the option to select cfDNA analysis.
Experience using cfDNA analysis in multiple gestations is limited, but meta-analysis suggests that cfDNA has similar sensitivity and specificity rates as in singleton pregnancies. For dizygotic twins, a higher fetal fraction (minimum of 8%) may be necessary to detect a quantitative difference. cfDNA results should be interpreted cautiously in pregnancies with a vanishing twin; results may be discordant because the vanishing twin may continue to release cfDNA into the maternal circulation. SNP-based testing may be helpful in identifying this phenomenon.
Some laboratories offer screening for select microdeletion syndromes, although this has not been endorsed by most professional societies due to limited experience and insufficient information on test performance. Individually these are rare disorders (1 in 3000 to 1 in 50,000), so the PPV is expected to be low. Petersen et al. reported a combined 13% PPV for select microdeletion syndromes and a 21% PPV for the 22q11.2 deletion, with higher false-positive rates than for common aneuploidies. A SNP-based cfDNA test for the 22q11.2 deletion demonstrated a sensitivity of 97.8% and specificity of 99.75% on a sample of 10 affected fetuses and 390 controls. That the mean gestational age was higher (21.7 weeks) and the fetal fraction higher (16.6%) may have affected the test performance. Expanded cfDNA screening is likely in the future but will require pretest and posttest counseling to ensure patients understand the limitations of screening.
First-trimester screening is an excellent screening option for women at low risk for aneuploidy and can be performed between 11 and 14 weeks using a combination of biochemical markers, pregnancy-associated plasma protein A (PAPP-A) and free β–human chorionic gonadotropin (β-hCG), and ultrasound measurement of the nuchal translucency (NT), a sonolucent space present in all fetuses behind the fetal neck. The detection rate for trisomy 21 is greater than 80%, with a false-positive rate of 5% compared with a 70% detection rate based on NT measurement alone . In trisomy 21, PAPP-A levels are typically reduced, whereas hCG levels and the NT measurement are increased. First-trimester screening is comparable or superior to second-trimester screening alone, and, most importantly, it provides parents with the option of earlier diagnostic testing in the event the screen indicates that the fetus is at high risk for aneuploidy. However, mandatory training and quality assurance for the NT measurement is critical. The incorporation of the other sonographic markers such as the presence of a nasal bone (NB), reverse ductus venosus flow, and tricuspid regurgitation has been proposed to increase the detection rates further. In general, these markers are not used except in specialized centers.
Although NT alone has a lower detection rate for trisomy 21 (64% to 70%) and is not recommended as a primary screening test, it is important to note that an NT greater than 4 mm always was associated with an abnormal noninvasive screen in the National Institute of Child Health and Human Development (NICHD) First and Second Trimester Evaluation of Risk (FASTER) trial. Therefore women with an NT exceeding this threshold should be offered diagnostic testing and forego serum analyte testing. Only 8% of pregnancies with NT greater than 3 mm had a screen-negative test. When an increased NT measurement is associated with a normal karyotype, fetal loss rates are increased and other fetal anomalies—in particular congenital cardiac defects—and genetic syndromes are observed. A targeted ultrasound examination during the second trimester and fetal echocardiography are recommended when the NT measurement is 3.5 mm or greater and the fetal karyotype is normal.
The most widely used second-trimester aneuploidy screening test is the so-called quad screen, which uses four biochemical analytes—α-fetoprotein (AFP), hCG, unconjugated estriol (uE 3 ), and dimeric inhibin A (INHA). Performed between 15 and 22 weeks’ gestation, the detection rate for trisomy 21 is approximately 75% in women who are younger than 35 years of age and more than 80% in women 35 years or older, with a false-positive rate of 5%. For trisomy 18, using only the first three markers provides a detection rate of approximately 70%. Serum screening does not detect other age-related forms of aneuploidy such as Klinefelter syndrome (47,XXY).
Serum hCG and INHA levels are increased in women carrying fetuses with Down syndrome. Levels of AFP and uE 3 in maternal serum are lower in pregnancies affected with Down syndrome compared with unaffected pregnancies. Typically, levels of AFP, uE 3 , and hCG are reduced in trisomy 18. A simple approach to detect trisomy 18 is to offer invasive prenatal diagnostic testing whenever serum screening for each of these three markers falls below certain thresholds (maternal serum -fetoprotein [MSAFP], 0.6 multiples of the median [MoM]; hCG, 0.55 MoM; uE 3 , 0.5 MoM). Using these thresholds would detect 60% to 80% of trisomy 18 fetuses with a 0.4% amniocentesis rate. Calculating individual risk estimation on the basis of three markers and maternal age, Palomaki and colleagues reported that 60% of trisomy 18 pregnancies can be detected with a low false-positive rate of 0.2%. The value of individual risk estimates is that one in nine pregnancies identified as being at increased risk for trisomy 18 by serum screening would actually be affected.
Confounding factors influence serum screening, and adjustments for gestational age, maternal weight, ethnicity, diabetes, and number of fetuses is necessary. Weight adjustment is needed because without adjustment, dilutional effects would result in heavier women having a spuriously low value, whereas thinner women would have a spuriously elevated value. In women with type 1 diabetes mellitus, a population at increased risk for NTDs, the median levels of MSAFP, uE 3 and hCG are lower than in nondiabetic women. In black women, who have a lower risk for a fetal NTD, the median MSAFP is higher than in other ethnic groups. Maternal smoking increases MSAFP by 3% but decreases maternal serum uE 3 and hCG levels by 3% and 23%, respectively. Maternal serum hCG is higher and MSAFP is lower in pregnancies conceived in vitro compared with pregnancies conceived spontaneously. Algorithms typically take these confounders into account prior to the report being conveyed.
Several approaches have been proposed using the combination of both first- and second-trimester screening to increase the detection rate over that achieved by screening in either trimester alone, with detection rates of 88% to 96% with false-positive rates of 5% reported. A caveat is that independent screening (i.e., using both first- and second-trimester screening tests to assess the risk separately and independently) is not recommended because of unacceptably high false-positive rates.
Sequential screening begins with first-trimester screening. A woman is informed of the adjusted risk for aneuploidy based on the first-trimester results. If her risk is high (greater than 1 in 50), she is offered genetic counseling and diagnostic testing. If the risk is low or moderate, a second-trimester screening test is performed with results of both the first- and second-trimester screening tests used to generate a final adjusted risk for trisomies 21 and 18. This is called the stepwise approach . With contingency screening, not all women will proceed to second-trimester screening because this occurs only with an intermediate risk; if the risk is low after the first-trimester screening, no further testing is indicated. Detection rates of the contingency approach are approximately 90%, with low positive screening rates (2% to 3%). Malone and colleagues compared several different first- plus second-trimester contingent sequential approaches and concluded that the optimal method was contingency screening, in which patients were divided into three groups: (1) women whose calculated (NT, PAPP-A, hCG) first-trimester risk was greater than 1 in 30 would undergo chorionic villus sampling (CVS); (2) women whose risk was less than 1 in 1500 would undergo no further testing; and (3) all other women would undergo second-trimester serum testing. Using this approach, only 21.8% of the cohort would need second-trimester testing to detect 93% of trisomy 21 cases with a 4.3% false-positive rate; 65% would be detected in the first trimester, with only 1.5% of patients having CVS procedures.
Integrated screening has the highest theoretic (modeled) detection rate (93% to 96%) among this group of serum-analyte based tests, but with this approach first-trimester screening, results are withheld until the second-trimester screen is completed. The individual receives only a single adjusted risk for trisomy 21 and trisomy 18 based on the results of both the first- and second-trimester screen. The obvious disadvantage with this approach is that the individual does not have an option of a diagnostic procedure in the first trimester even if screening would have indicated a high risk for trisomy 21 or 18. In addition, theoretic detection is not always reached because some patients fail to return for their second-trimester screening.
Serum integrated screening is an acceptable alternative when an NT measurement cannot be obtained and is not available. With this approach, first-trimester PAPP-A and second-trimester serum analytes are used to determine the risk for trisomy 21. The patient receives one adjusted risk after the second-trimester screen is completed. In the FASTER trial, the sensitivity of this approach was 88%.
In dizygotic pregnancies, each twin has an individual risk for trisomy 21; analysis of the European National Down Syndrome Cytogenetic Registry found that dizygotic pregnancies were one-third more likely to have at least one fetus with trisomy 21 than age-adjusted singleton pregnancies. The pregnancy-specific and fetus-specific risks should be the same for monozygotic twins as for singleton pregnancies, although the study found the actual risk to be approximately a third that of a singleton pregnancy. Down syndrome screening using multiple serum markers is less sensitive in twin pregnancies than in singleton pregnancies. Using singleton cutoffs, one study showed that 73% of monozygotic twin pregnancies but only 43% of dizygotic twin pregnancies with Down syndrome were detected, given a 5% false-positive rate. Decreased sensitivity in detecting trisomy 21 in dizygotic twins reflects the blunting effect of the concomitant presence of one normal and one aneuploid fetus. Thus patients with twins should be informed that the detection rate by serum screening is less than that in singleton pregnancies. First-trimester screening identifies approximately 70% of Down syndrome pregnancies; NT measurement alone has been shown to be as effective a screening test for higher-order multiple gestation as it is for twin gestations. Addition of NB assessment to the NT measurement increased the detection rate to 87% at a screen-positive rate of 5% and to 89% when serum analytes were included in a retrospective study of 2094 twin pregnancies. Although the experience is limited, cfDNA analysis may prove to be a good alternative for screening multiple gestation. Meta-analysis suggests similar sensitivity and specificity to those of singleton gestations.
Second-trimester ultrasonography may detect anomalies associated with aneuploidy, such as cardiac defects or duodenal atresia (see Chapter 9 ). In 1985, Benacerraf and colleagues showed a significant association between the thickness of the fetal nuchal skin fold and the presence of trisomy 21. Other markers that have been used in the genetic sonogram include the NB length, short femur or humerus, echogenic intracardiac focus, echogenic bowel, and pyelectasis. As markers were studied in larger numbers of women, it became possible to assign likelihood ratios to each marker and to do a formal risk adjustment from the a priori risk to an ultrasound-adjusted risk. However, most of the markers perform poorly as individual predictors of Down syndrome, having a very low sensitivity and a high rate of false-positive results. Furthermore, the test performance depends on the skill of the examiner and the subjective assessment required for several markers, particularly echogenic intracardiac focus and echogenic bowel. Most women who undergo a genetic sonogram have no markers of aneuploidy, but women should be informed that the absence of markers does not rule out the possibility of Down syndrome or other chromosome abnormalities.
The incidental finding of “soft markers” such as echogenic intracardiac focus or pyelectasis in otherwise low-risk pregnancies can cause a great deal of anxiety in the expectant parents. Opinions vary as to what to do when these markers are noted in a low-risk patient. A reasonable approach is to refer such a patient for consultation with an expert to evaluate the presence of other markers in the context of other screening tests (i.e., multiple marker screening or cfDNA analysis).
Most chromosome disorders are detectable in utero with the availability of CMA. Thus any pregnant woman could undergo an invasive procedure, if so desired, to assess robustly the chromosome status of the fetus. However, for most couples, the risks of an invasive procedure outweigh the diagnostic benefits. Many will therefore elect noninvasive screening with the understanding that the sensitivity is less than 100% and that the screen is intended only to identify pregnancies at increased risk for the three common trisomies and sex chromosome abnormalities. On the other hand, in the setting of a “positive” screening test or sonographic malformation, an invasive diagnostic procedure is strongly recommended. In this section, we review the indications for prenatal diagnostic testing for chromosome abnormalities and also discuss the types of tests currently available.
ACOG recommends that all women, regardless of age, should be offered screening or diagnostic testing for aneuploidy including the option of a CMA. Prenatal cytogenetic testing can be as basic as a routine G-banded karyotype, or in some cases a more targeted approach such as fluorescence in situ hybridization (FISH) may be recommended.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here