Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A root cause of cancer is the accumulation of genetic and epigenetic defects in key cellular pathways regulating proliferation, differentiation, and death. The defects in cancer cells are of two types: gain-of-function alterations affecting oncogenes, and loss-of-function alterations affecting tumor suppressor genes. Regardless of whether the defects are genetic or epigenetic in nature, a common net consequence is dysregulation of gene expression in cancer cells. This intimate relationship between genetic and epigenetic changes has been further confirmed by findings of frequent mutations in chromatin enzymes.
More recently, genetic alterations in noncoding DNA have also been reported in cancer tissues. They contribute to tumorigenesis by affecting the regulatory elements (e.g., promoters, enhancers) that influence gene expression of key cancer-related genes, and some of them may correspond to cancer risk genetic variants in noncoding regions identified by genome-wide association studies (GWASs).
In addition to genetic mutations and genomic alterations, it is becoming more evident that clinical and pathologic studies indicate that many cancers arise from preexisting benign lesions, and numerous cooperating genetic and epigenetic defects affecting multiple independent signaling pathways are likely needed for development of most clinically recognizable cancers.
A process termed clonal selection has a key role in determining the particular constellation of genetic and epigenetic defects present in a cancer cell. Clonal selection is essentially an evolutionary process that promotes outgrowth of precancerous and cancerous cells carrying those mutations and gene expression changes that confer the most potent proliferative and survival properties on the cancer cells in a given context.
Although a sizeable and diverse array of mutations and gene expression changes have been implicated in cancer pathogenesis, the defects appear to affect a more limited number of conserved signaling pathways or networks. A relatively small collection of oncogenes and tumor suppressor genes is recurrently deranged in cancer cells of various types and includes the RAS, PIK3CA, EGFR, RAF, β-catenin, IDH1, and MYC oncogene proteins and the p53, p16 Ink4a , ARF, RB1, PTEN, APC, NF1, and ARID1A tumor suppressor proteins. The proteins that are recurrently targeted by mutations in cancer likely represent particularly critical hubs in the cell's regulatory circuitry.
Although cancer represents a very heterogeneous collection of diseases, the development of all cancers, regardless of type, appears to be critically dependent on the acquisition of certain traits that allow the cancer cells to grow in an unchecked fashion in their tissue of origin and to grow as metastatic lesions in distant sites in the body. Signature traits that are likely to be inherent in the majority of, if not all, cancers include (1) an enhanced response to proliferative and growth-promoting signals; (2) a relative resistance to growth inhibitory cues; (3) an increased mutation rate to allow for the rapid generation of new variant daughter cells; (4) the ability to attract and support a new blood supply (angiogenesis); (5) the capacity to minimize an immune response and/or evade destruction by immune effector cells; (6) the capacity for essentially limitless cell division; (7) a failure to respect tissue boundaries, allowing for invasion into adjacent tissues and organs with microenvironments that are markedly different from the one where the cancer cells arose; (8) the ability to escape cell death; (9) altered cell metabolism to support uncontrolled proliferation; and (10) the acquisition of immune cells that promote tumor progression.
Certain gene defects in cancer cells may contribute to a few or perhaps even only one of the signature traits. However, many of the gene defects and expression changes might have been selected for in large part because they exert pleiotropic effects on the cancer cell phenotype.
Despite the fact that some gene defects may arise early in the development of certain cancer types, advanced cancer cells might still be critically dependent on the “early gene defects” for continued growth, survival, and even metastasis. Studies have shown that metastatic cancers still harbor the same genetic alterations as the primary tumors. Such findings imply that agents that specifically target key signaling pathways and proteins could have use in advanced cancers, even if the signaling pathway defect arose very early in cancer development.
Genomic and epigenomic characterization of organ site cancer has identified a number of subtypes with different prognoses and therapeutic relevance.
Future studies will further clarify the role of the diverse array of genetic and epigenetic defects in cancer phenotypes, even at the level of a single cell, allowing more definitive and more specific strategies for cancer detection, diagnosis, and therapeutic targeting of cancer cells.
A genetic basis for human cancer has been recognized for perhaps more than a century and has been supported by data from familial and epidemiologic studies and animal studies. However, only during the past three decades has definitive molecular evidence been accumulated to support the view that all cancer types arise from defects in the structure and/or regulation of genes. Studies from many different fields, including tumor virology, chemical carcinogenesis, molecular biology and biochemistry, somatic cell genetics, developmental biology, and genetic epidemiology, have all played critical contributing roles in clarifying the contributions of genetic and epigenetic mechanisms to cancer development. Although environmental and dietary factors have substantial roles in cancer development, it is now well established that the accumulation of multiple genetic and epigenetic alterations in a single cell plays a fundamental role in cancer initiation and progression.
The mutations that arise in cancer cells can be divided into two functionally distinct classes: oncogene and tumor suppressor gene mutations. In addition to the classic alterations of oncogenes and tumor suppressor genes, other genetic alterations occur in genes regulating transcription and translation, as well as in genes responsible for DNA repair. Although localized mutations are commonly observed, other genetic variations are also important in oncogenesis, such as copy number variation, deletion, and translocations. It is becoming more evident that these genetic changes could occur in both coding and noncoding DNA and that the latter may involve cis- regulatory regions affecting gene transcription. All of these genomic and epigenomic alterations in the initiation and progression of cancer can be broadly considered as oncogenic or “gain of function” and suppressor or “loss of function.” In addition, some of these genetic alterations may be present in an individual's germline and may predispose to particular cancers. Such mutations can also be passed on to future generations. The nature and role of certain germline mutations in cancer development are of great interest to cancer biologists because the mutations provide powerful clues about the identity of genes and pathways that play particularly critical roles in the malignant conversion of cells. Nevertheless, germline mutations in oncogenes or tumor suppressor genes likely have a major contributing role in the development of only hereditary cancer, representing a small fraction of all cancers, and the vast majority of mutations in cancer are somatic (i.e., present only in the tumor cells).
A few of the cellular genes that are recurrently affected by inherited and somatic mutations in human cancer will be discussed in more detail later. Brief mention will be made here of some general properties of the mutated genes. Oncogenes act in a positive fashion to promote tumorigenesis. Their normally functioning cellular counterparts, termed proto-oncogenes, have been found to be important regulators of many aspects of cell physiology. The proteins encoded by various proto-oncogenes can be found in virtually all subcellular compartments. The term proto-oncogene does not imply that genes of this class lie dormant in the cell with the purpose of promoting tumorigenesis. Rather, the terminology reflects the fact that mutations in cancer cells alter the normal structure and/or expression pattern of the proto-oncogene, generating oncogenic variants with altered function. In genetic terms, oncogenic alleles have gain-of-function mutations that confer enhanced or novel functions.
In contrast to the activating mutations in oncogenes, tumor suppressor genes harbor loss-of-function defects in cancer cells. Historically, the term antioncogene was sometimes used with respect to the tumor suppressor gene class. The term suggests that the primary function of the genes might be to act in direct opposition to activated oncogenes. Although many of the proteins that are encoded by tumor suppressor genes do in fact bind to and regulate the function of proto-oncogenes or function in pathways that regulate proto-oncogene activity, it is not by necessity a general principle. Hence, genes that contribute to cancer by virtue of their inactivation or loss-of-function mutations in human cancers are referred to here as tumor suppressor genes. Similarly to the proto-oncogenes, the normal functions of tumor suppressor genes are diverse, and the proteins encoded by these genes are found in essentially all compartments of the cell.
In addition to the well-established role of oncogene and tumor suppressor gene mutations in cancer, it is now abundantly clear that epigenetic mechanisms play critical roles in altering the patterns and levels of expression of certain proto-oncogenes and tumor suppressor genes in cancer. For instance, in some cancers, altered transcriptional regulatory mechanisms can lead to markedly increased levels of proto-oncogene expression, akin to the level seen in cancer cells with mutational defects that alter the structure or copy number of the proto-oncogene. Conversely, gene-silencing mechanisms can exert dramatic effects on the expression of certain tumor suppressor genes in cancer cells, essentially rendering the genes functionally inactive in the absence of any mutations. It is interesting that sequence-based analyses of many different cancer types have revealed that many of the mutations in cancer cells directly affect the cancer epigenome. Specifically, the oncogenes and tumor suppressor gene mutations in cancer cells often target transcription factors, chromatin modifying proteins, and other chromatin-associated proteins, leading to potentially quite dramatic global effects on the structure and activity of chromatin, DNA methylation, histone modifications, and patterns of gene expression in cancer cells.
Finally, mutations in genes that regulate the recognition and repair of DNA damage also play critical roles in tumorigenesis. The DNA damage recognition and repair genes could be considered to constitute a distinct class of cancer genes, because at least some of the DNA repair proteins might have a more passive role in cell proliferation, differentiation, and cell survival. Their inactivation in tumor cells might lead predominantly to the acquisition of a “mutator phenotype,” with a resultant increased rate of mutations in other cellular genes. More recently, these tumors with higher mutation load, and therefore presumably generating more tumor-related neoantigens, were found to be more responsive to checkpoint immunotherapy.
Given the enormous advances during the past two decades, especially the recent use of next-generation sequencing (NGS) in profiling and characterizing gene mutations and expression defects in cancer, it will not be possible in this chapter to review in a comprehensive fashion the vast collection of genetic and epigenetic defects that have been catalogued in human cancers. Nor will it be possible to discuss in detail the possible contributions of the many different gene defects to alterations in cell signaling and cell physiology. Rather, the primary aim of this chapter will be to offer a framework for understanding the relationships between genetic and epigenetic defects in cancer cells and the impact of the accumulated defects on the cancer cell phenotype. Although some details on the identity and nature of gene defects in cancer will be offered here, the emphasis will be on concepts with biologic and clinical significance.
Oncogenic variant alleles that are present in cancer are generated from the normal counterpart proto-oncogenes by various mutational mechanisms, including point or localized mutations, gross chromosomal rearrangements, or gene amplification. Some representative oncogene mutations in human cancer are summarized in Table 14.1 . From a brief review of the data in Table 14.1 , some generalizations can be offered. First, the mutations affect proteins functioning in various compartments of the cell, including growth factor receptors, cytoplasmic signal transducers, and nuclear proteins, such as transcription factors. Second, although some oncogene mutations may be unique to cancers of a particular type, such as the specific chromosomal translocations and resultant fusion proteins that are seen in cancers of hematopoietic origin (e.g., the BCR-ABL translocation that is seen in chronic myelogenous leukemia and a subset of acute lymphoid leukemias and the PML-RARα translocation that is seen in acute promyelocytic leukemia), other mutations, such as those affecting the KRAS, β-catenin, and c-MYC genes, are found in a broad spectrum of different cancer types. Third, oncogene mutations in cancer are nearly always somatic, because only a very limited number of germline mutations in proto-oncogenes have been linked to cancer predisposition thus far. Fourth, some proto-oncogenes, such as KRAS or BCL2, are somatically altered in cancer by a single mutational mechanism, namely, point mutations in the KRAS gene and chromosomal translocations affecting the BCL2 gene. In contrast, other proto-oncogenes, such as c-MYC, may be activated by more than one mechanism in cancer, including chromosomal translocation, gene amplification, and, more recently discovered, association with superenhancer. These mutational mechanisms lead to increased levels of c-MYC transcripts and protein. In fact, specific missense mutations at threonine 58 of the c-MYC gene in some lymphomas may further enhance c-MYC protein levels by abrogating a phosphorylation-ubiquitination mechanism targeting c-MYC for proteosomal degradation. However, enhanced c-MYC protein levels can also result from alterations in c-MYC–specific microRNAs, methylation, and other noncoding regulatory elements functioning to regulate c-MYC transcription and translation.
| Gene | Activation Mechanism | Protein Properties | Tumor Types |
|---|---|---|---|
| KRAS | Point mutation | Signal transducer | Pancreatic, colorectal, lung (adeno), endometrial, other carcinomas |
| NRAS | Point mutation | Signal transducer | Myeloid leukemia, colorectal cancer |
| HRAS | Point mutation | Signal transducer | Bladder carcinoma |
| EGFR (ERBB) | Amplification, mutation | Growth factor (EGF) receptor | Gliomas, lung (non–small cell) carcinoma |
| NEU (HER2/ERBB2) | Amplification | Growth factor receptor | Breast, ovarian, gastric, other carcinomas |
| c-MYC | Chromosome translocation | Transcription factor | Burkitt lymphomas |
| Amplification | Small cell lung carcinoma (SCLC); other carcinomas; glioblastoma | ||
| MYCN | Amplification | Transcription factor | Neuroblastoma, SCLC; glioblastoma |
| MYCL1 | Amplification | Transcription factor | SCLC, ovarian carcinoma |
| BCL2 | Chromosome translocation | Antiapoptosis protein | B-cell lymphoma (follicular type) |
| CCND1 | Amplification | Cyclin D, cell cycle control | Breast and other carcinomas |
| Chromosome translocation | B-cell lymphoma, parathyroid adenoma | ||
| BCR-ABL | Chromosome translocation | Chimeric nonreceptor tyrosine kinase | CML, ALL (T cell) |
| RET | Chromosome translocation | GDNF receptor tyrosine kinase | Thyroid cancer (papillary type) |
| Point mutation | Thyroid cancer (medullary type: germline mutations) | ||
| CDK4 | Amplification | ||
| Point mutation | Cyclin-dependent kinase | Sarcoma, glioblastoma | |
| MET | Point mutation | Hepatocyte growth factor (HGF) receptor | Renal carcinoma (papillary type: germline mutations) |
| SMO | Point mutations | Transmembrane signaling molecule in Sonic Hedgehog pathway | Basal cell skin cancer |
| CTNNB1 (β-CAT) | Point mutation, in-frame deletion | Transcriptional coactivator, links E-cadherin to cytoskeleton | Melanoma; colorectal, endometrial, ovarian, hepatocellular, and other carcinomas, hepatoblastoma, Wilms tumor |
| FGF4 | Amplification | Growth factor (FGF-like) | Gastric carcinoma |
| PML-RARA | Chromosome translocation | Chimeric transcription factor | APL |
| TCF3-PBX1 | Chromosome translocation | Chimeric transcription factor | Pre-B ALL |
| MDM2 | Amplification | p53 binding protein | Sarcoma |
| GLI1 | Amplification | Transcription factor | Sarcoma, glioma |
| TTG2 | Chromosome translocation | Transcription factor | T-cell ALL |
| AKT2 | Amplification | Signal transducer (serine/threonine kinase; downstream effector of PI3K) | Pancreatic and ovarian carcinoma |
| PIK3CA | Amplification | Catalytic subunit of PI3K | Ovarian carcinoma |
| AURKA | Amplification | Centrosome-associated kinase | Breast, colon, ovarian, and prostate carcinomas gliomas |
| TMPRSS2-ERG | Chromosome translocation | Transcription factor (ETS family) | Prostate cancer |
| TMPRSS2-ETV1 | |||
| TMPRSS2-ETV4 |
In contrast to the activating mutations that generate oncogenic alleles from proto-oncogenes, inactivation of the normal function of tumor suppressor genes is critical in tumorigenesis. Akin to the proto-oncogenes, the functions of tumor suppressor genes are diverse, and proteins that are encoded by these genes reside in practically all subcellular compartments ( Table 14.2 ). Many tumor suppressor genes were identified by virtue of the fact that they are mutated in the germline of persons who are affected by a known mendelian cancer syndrome or who at the very least display a markedly elevated risk of cancer. The link between a germline-inactivating mutation in a purported tumor suppressor gene and increased cancer predisposition provides very persuasive evidence of the functional significance of the gene in the cancer process. Nevertheless, for the vast majority of tumor suppressor genes, in terms of their magnitude, somatic inactivating mutations play a far more significant role in cancer development than do germline mutations.
| Gene | Associated Inherited Cancer Syndrome | Cancers With Somatic Mutations | Presumed Function of Protein |
|---|---|---|---|
| RB1 | Familial retinoblastoma | Retinoblastoma, osteosarcoma, SCLC, breast, prostate, bladder, pancreas, esophageal, others | Transcriptional regulator; E2F binding |
| TP53 | Li-Fraumeni syndrome | Approximately 50% of all cancers (rare in some types, such as prostate carcinoma and neuroblastoma) | Transcription factor; regulates cell cycle and apoptosis |
| CDKN2A (p16 INK4a protein) | Familial melanoma, familial pancreatic carcinoma | Approximately 25%–30% of many different cancer types (e.g., breast, lung, pancreatic, bladder) | Cyclin-dependent kinase inhibitor (i.e., CDK4 and CDK6) |
| CDKN2A (ARF protein) | Familial melanoma (?) | Approximately 15% of many different cancer types | Regulates MDM2 protein stability and hence p53 stability; alternative reading frame of CDKN2A gene |
| APC | FAP coli, Gardner syndrome, Turcot syndrome | Colorectal carcinomas, desmoid tumors, hepatocellular carcinoma, breast (rare) | Regulates levels of β-catenin protein in the cytosol; binding to EB1 and microtubules |
| WT1 | WAGR, Denys-Drash syndrome | Wilms tumor | Transcription factor |
| NF1 | Neurofibromatosis type 1 | Melanoma, neuroblastoma | p21ras-GTPase |
| NF2 | Neurofibromatosis type 2 | Schwannoma, meningioma, ependymoma | Juxtamembrane link to cytoskeleton at adherens junction |
| VHL | von Hippel–Lindau syndrome | Renal (clear cell type), hemangioblastoma | Regulator of protein stability |
| BRCA1 | Inherited breast and ovarian cancer | Ovarian (~10%), rare in breast cancer | DNA repair; complexes with Rad 51 and BRCA2 ; transcriptional regulation |
| BRCA2 | Inherited breast (both female and male), pancreatic cancer | Rare mutations in pancreatic, others (?) | DNA repair; complexes with Rad 51 and BRCA1 |
| MEN1 | Multiple endocrine neoplasia type 1 | Parathyroid adenoma, pituitary adenoma | Nuclear protein; unknown function |
| Endocrine tumors of the pancreas | |||
| PTCH1 | Gorlin syndrome, hereditary basal cell carcinoma syndrome | Basal cell skin carcinoma, medulloblastoma | Transmembrane receptor for Sonic Hedgehog factor; negative regulator of Smoothened protein |
| PTEN | Cowden syndrome; sporadic cases of juvenile polyposis syndrome | Glioma, breast, prostate, follicular thyroid carcinoma, head and neck squamous carcinoma; many others | Phosphoinositide 3-phosphatase; protein tyrosine phosphatase |
| SMAD4 | Familial juvenile polyposis syndrome | Pancreatic (~50%), approximately 10%–15% of colorectal cancers, rare in others | Transcriptional factor in TGF-β signaling pathway |
| BMPR1A | Familial juvenile polyposis syndrome | Not known | Receptor for bone morphogenetic protein |
| MSH2, MLH1 PMS1, PMS2, MSH6 | Hereditary nonpolyposis colorectal cancer | Colorectal, gastric, endometrial, ovarian | DNA mismatch repair |
| CDH1 | Familial diffuse-type gastric cancer | Gastric (diffuse type), lobular breast carcinoma, rare in other types (e.g., ovarian) | E-cadherin cell-cell adhesion molecule |
| STK11 (LKB1) | Peutz-Jeghers syndrome | Lung adenocarcinoma; rare pancreas cancers; absent in most other cancers | Serine/threonine protein kinase |
| EXT1 | Hereditary multiple exostoses | Osteochondroma | Glycosyltransferase; heparan sulfate chain elongation |
| EXT2 | Hereditary multiple exostoses | Not known | Glycosyltransferase; heparan sulfate chain elongation |
| TSC1 | Tuberous sclerosis | Bladder carcinoma; head and neck squamous cancer; hepatocellular carcinoma | Hamartin; binds tuberin (TSC2) ; regulates cell size by inhibiting TOR function and protein synthesis |
| TSC2 | Tuberous sclerosis | Head and neck squamous cancer | Tuberin (see previous row regarding TSC1 ) |
Another important point to consider is that much of the attention for tumor suppressor genes has been focused on demonstrating that cancer cells carry biallelic inactivating mutations. Clearly, a diverse array of mechanisms can inactivate gene function, including nonsense, frameshift, and nonconservative missense mutations, as well as splicing mutations and gross deletions of the gene or even the chromosome region that contains the gene. In a number of cases, studies of the chromosomal mechanisms associated with tumor suppressor gene inactivation in cancer tissues, such as loss of the parental heterozygosity (i.e., loss of heterozygosity) that is present in normal tissues, have even been used to infer the existence of tumor suppressor genes, in particular, chromosomal regions, before the actual identification of the tumor suppressor gene of interest. The emphasis on defining biallelic inactivating mutations in tumor suppressor genes has been stimulated in large part by the Knudson hypothesis, which predicted that recessive genetic determinants played a critical role in retinoblastoma and many other cancers and that inactivation of both alleles of a tumor suppressor gene was needed to abrogate tumor suppressor gene activity. Nevertheless, as will be discussed in a bit more detail in the following sections, a variety of observations indicate that epigenetic (nonmutational) mechanisms might play a prominent role in inactivating tumor suppressor gene function in sporadic tumors. Furthermore, for certain tumor suppressor genes, inactivation of only one of the two alleles of a tumor suppressor gene might significantly impair cell growth regulation or programmed cell death. For example, a number of mutant p53 proteins that carry missense mutations likely potently interfere via dominant negative mechanisms with the wild-type p53 protein in the cell because p53 functions as a homotetrameric protein and all subunits must be wild-type for fully intact p53 function in transcriptional regulation. For other tumor suppressor proteins, such as the cyclin-dependent kinase inhibitory protein p27, reduction of protein levels to 50% of the levels present in normal cells might result in significant detrimental effects on the ability of the cell to appropriately regulate growth.
On the basis of a simple consideration that a cancer involves a large number of genetic and epigenetic alterations that arise in normal cells during the many years of life, it would seem quite unlikely that any adult cancer may arise as the result of single gene defect. Even in persons who are strongly predisposed to cancer as a result of a germline mutation in a specific oncogene or tumor suppressor gene, the vast majority of cells in a person never develop into cancer or even display definitive morphologic changes akin to those that are seen in benign tumors. Therefore, any model for cancer must incorporate these data, which suggest that cancers likely arise as the result of the accumulation of multiple genetic and epigenetic defects in an affected cell. Another issue to consider is that clinical and histopathologic data indicate that the development of nearly all cancers, regardless of the organ site, is often, if not invariably, preceded by precancerous phases or stages in which the neoplastic cells manifest increasingly disordered patterns of differentiation and morphology.
Given this background, it would appear that cancers arise from accumulated defects affecting multiple genes and pathways, and precancerous (benign) precursor lesions harbor fewer of the key gene defects than established cancers. One question, therefore, is how many rate-limiting defects or “hits” are required for cancer development. Although a definitive answer to this question cannot be given at this point, some estimates can be offered. Most common cancers show dramatically increased incidence with increasing age. On the basis of analysis of the age-specific incidence of a number of common cancers and some straightforward assumptions about the rate of mutations and the size of the target cell population, it was argued as early as the mid-1950s that most common epithelial cancers arise as the result of four to seven rate-limiting events. It was inferred that these rate-limiting events might represent mutational events. Moreover, benign lesions harboring fewer gene defects are often shown to have an age-incidence distribution that was shifted roughly one to two decades earlier in life than cancers arising in the corresponding organ or tissue site. Nevertheless, confounding the use of age-incidence data to model the number of rate-limiting mutations were questions about the weight of importance of certain key biologic pathways underlying the multiple hits models. On the other hand, studies of the genetic alterations of pediatric cancers that occur much earlier and are perhaps less complex in terms of their genetic signature may offer insights into key cancer target genes (see Chapter 92 on pediatric solid tumors).
As previously noted, most if not all cancers arise from preexisting precancerous populations of cells, and multiple genetic and epigenetic defects are likely needed for conversion of a normal cell to a cancerous cell. Molecular studies of various cancer types and their corresponding associated precancerous lesions have yielded some fundamental insights into the processes likely to be critical in the emergence of the cancer. First, although normal tissues and tissues from noncancerous disease states display polyclonal (balanced) cell populations, the neoplastic component that is present in benign lesions and cancers displays a clonally related cell population, consistent with the notion that neoplastic transformation of one or a number of different cells within a tissue give rise to all daughter cells or subclones that are present in the tumor. This is further established by NGS deep sequencing in studying intratumor heterogeneity. Second, in tumors in which it has been possible to analyze both cancer cells and associated precancerous cell populations, a subset of the somatic gene defects present in the cancer are clonally represented in the precancerous cell population. Other somatic gene defects appear to be acquired during progression from the precancer subclones to the dominant subclones in the cancer.
These molecular findings in benign and malignant tumors are essentially consistent with a model initially proposed by Foulds and subsequently advanced by Nowell ( Fig. 14.1 ). In brief, the clonal evolution model predicts that cancers arise as the result of successive expansions of clonally related cell populations. The successive expansions are driven by the gradual or punctuated acquisition of mutations and gene expression changes that endow a particular cell and its progeny with a selective advantage over cells that do not harbor the gene defects. In essence, clonal selection is an evolutionary process that allows the outgrowth of precancerous and cancerous cells that carry mutations and gene expression changes that confer the most potent proliferative and survival properties on the cancer cells. However, it is important to note that the specific constellation of genetic and epigenetic changes that are present in precancerous and cancerous cells is context-dependent and certainly varies considerably from one cancer type to another and even most certainly varies to a significant degree among patients whose cancers display quite similar clinical and histopathologic features. The clonal evolution model has biologic and clinical ramifications, just a few of which will be mentioned here. First, the clonal gene defects that are present in a cancer can be traced in precancerous lesions from the same organ site with a goal of attempting to clarify the preferred order in which gene defects arise in the natural history of a particular cancer type. The particular order in which defects accumulate during the initiation and subsequent progression of one cancer type often differs from that in another cancer type. As a result, a genetic or epigenetic change that is critical in tumor initiation in one cancer type might contribute to tumor progression in another tumor type and vice versa. Second, defects that arise at “early” stages of tumorigenesis might play a vital role not only in tumor initiation but also in the aggressive behavior of advanced stage cancers. Third, the model predicts that the acquisition of further genetic heterogeneity will be a common and important factor in primary cancer lesions and metastases. It is becoming more evident that genetic heterogeneity plays a significant role in resistance to chemotherapy and targeted therapy and the emergence of aggressive cell populations in patients with advanced cancer.
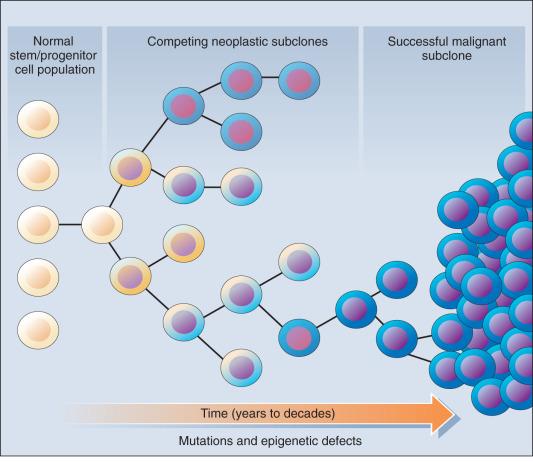
Recent comprehensive sequence-based analyses of cancer cell populations from individual patients in which the tumor cells under study were distinct from one another in location and/or time has begun to reveal that quite dramatic intratumoral genetic heterogeneity may be a “rule” in cancer, rather than an exception. Certain, perhaps key, initiating genetic lesions (e.g., truncal mutations) might be shared among all neoplastic clones, but geographically distinct regions of large primary tumors may have very distinct mutation profiles (e.g., branched mutations) from those in other portions of the primary tumor, and the metastatic cell populations may have considerable mutational divergence from the nonmetastatic cells. Accordingly, it seems that quite extensively branched evolutionary growth may be an important feature in both primary tumors and metastatic lesions, with multiple competing clonal populations evolving through divergent and convergent mutational mechanisms. This more recent view of the potentially quite extensive intratumoral genetic heterogeneity in any given cancer and the contributions of intratumoral heterogeneity to tumor progression contrasts with some earlier views. Before the more recent studies, it was suspected that the cell populations in many primary cancers might be more homogeneous, wherein somatic mutations were accumulated in a more stepwise fashion as a result of multiple sequential clonal sweeps of each variant cell population in the primary cancer, with metastases perhaps most often arising from the clonally dominant cell population in the primary tumor.
It is important to note that clonal somatic mutations are often presumed to have a causal role in promoting further tumor outgrowth or progression because somatic mutations can become clonal (i.e., present in all neoplastic cells) by only a limited number of mechanisms. For instance, the genetic alteration itself could have been selected for because it provided the neoplastic cell with a growth advantage, allowing it to become the predominant cell type in the tumor (clonal expansion). Genes with critical roles in promoting clonal outgrowth in a given cancer have been termed drivers. Genes that are mutant in a significant fraction of cancers and for which other lines of evidence link them to the cancer process can more readily be classified as drivers . Alternatively, a somatic mutation, when detected, might have arisen essentially coincident with another, perhaps undetected, alteration that was the crucial change underlying clonal outgrowth. Somatic mutations of this latter type have been termed passengers. However, studies have shown that some of these passengers, which serve as metabolic and housekeeping genes, can be targeted therapeutically, leading to tumor suppression. Therefore, on the basis of extensive data from large-scale sequencing analyses that reveal large numbers of distinct genes that are each mutated in only a minority of cancers of a given type, sorting out drivers and passengers might not be entirely straightforward based solely on sequencing data and will likely require a significant body of functional studies and data. Indeed, increasingly even noncoding alterations have been shown to play important roles in causing and maintaining the cancer phenotypes by introducing new cancer-causing promoters and enhancers that lead to altered gene expression (see the later discussion of noncoding mutations). Given this discussion about the potential uncertainties associated with linking specific somatic mutations to cancer development, it is even more challenging to assign causal significance to any given gene expression change in precancerous and cancerous cells. In particular, the ambiguities arise when considering whether a gene expression or epigenetic change merely reflects the relative difference between cancer and its matched normal tissue or is truly causally involved in the cancer process. Nonetheless, if a specific gene or epigenetic alteration can be shown to promote tumorigenesis or neoplastic transformation in a variety of in vitro and in vivo tumor models or if the same gene or chromosomal region is recurrently altered by particular epigenetic changes in tumors, then it might be reasonable to infer that the particular defect might indeed have a causal (driver) role in tumorigenesis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here