Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Knowledge of basic anesthetic techniques and of the advantages and disadvantages of various anesthetic agents used for sedation and general anesthesia in the operating room during airway management should be incorporated into the practice of otolaryngology–head and neck (OLHN) surgeons.
Preoperative patient evaluation by the anesthesiologist can help to identify patients with a potentially difficult airway for intubation.
For a patient who is not at increased risk of aspiration and who does not have a difficult airway, induction of anesthesia and establishment of mask ventilation before paralysis is the safer and preferred technique.
The American Society of Anesthesiologists defines a difficult airway as “a clinical situation in which a conventionally trained anesthesiologist experiences difficulty with facemask ventilation of the upper airway, difficulty with tracheal intubation, or both.”
The consequences of failed airway maintenance and/or endotracheal intubation can be medically and financially devastating to the patient, the practitioner, and the health care system.
The most common adverse outcomes related to difficult airway are death, brain injury, cardiopulmonary arrest, surgical airway, airway trauma, and damage to the teeth.
Multidisciplinary airway teams can decrease emergency surgical airways and associated adverse outcomes to the patient.
The Johns Hopkins Hospital Difficult Airway Response Team (DART) Program, which has over a decade of experience (2008–18), integrates multidisciplinary operations, safety, and educational components to improve teamwork and communications, improve difficult airway management, decrease adverse events, and improve processes with innovative educational resources for airway providers.
The Johns Hopkins Hospital DART Program provides a roadmap for institutions interested in initiating a DART Program equivalent.
Dissemination of difficult airway information to the patient's next health care provider is critical, but a letter from the anesthesiologist to the patient, or even to the patient's primary care physician and surgeon, may not be sufficient to guarantee that this information is available when and where it is needed.
The electronic health record as currently implemented in the United States cannot provide 24/7 access to a patient's difficult airway information regardless of when or where it was created; however, the MedicAlert Foundation already provides this service both nationally and internationally via its National Difficult Airway/Intubation Registry.
Case presentations focus on unanticipated difficult airway, anticipated difficult airway, OLHN cases, and DART Program cases.
Airway management is the essence of clinical anesthesiology. The goal of airway management is simple: to provide the most expeditious form of management that has the lowest potential for injury and the greatest potential for control of the airway. The approach to the patient with a difficult airway varies, depending on whether airway management is elective or urgent and whether the health care setting is an operating room (OR) or some other hospital setting.
This chapter first presents the anesthetic component of basic airway management in the OR, including the use of anesthetic drugs and their advantages and disadvantages, in a manner that otolaryngology–head and neck (OLHN) surgeons can incorporate into their practices. In-depth discussions about physiology and pharmacology related to anesthesiology are beyond the scope of this chapter, but authoritative textbooks of anesthesiology are listed in the Suggested Readings for this chapter.
The American Society of Anesthesiologists (ASA) definition, incidence of occurrence, and consequences of difficult airway are discussed along with various methods to identify patients with a potentially difficult airway and advances in airway techniques and airway devices.
Complex airway management of the patient with a difficult airway/intubation (anticipated or unanticipated) demands a multispecialty approach that involves anesthesiologists, OLHN surgeons, critical care physicians, emergency department physicians, and nursing/technician support staff. The OLHN surgeon who has expertise in rigid laryngoscopy and bronchoscopy, flexible fiberoptic bronchoscopy (FOB), and surgical approaches to the airway will be uniquely qualified to take the lead surgical role, in partnership with the anesthesiologist, during management of difficult airway/intubation patients.
The Johns Hopkins Hospital Difficult Airway Response Team (DART) Program is a multidisciplinary (OLHN, anesthesiology, trauma surgery, emergency medicine) approach to in-hospital (non-OR and OR) difficult airway patients. Inception of the DART Program in 2008 reduced difficult airway–related morbidity, mortality, and sentinel events to zero. Lessons learned will be briefly presented.
The importance of disseminating patients’ critical difficult airway information to future health care providers is discussed, with a suggested solution of enrollment in the MedicAlert Foundation's National Difficult Airway/Intubation Registry as an already established way to provide 24/7 national and international access to this information.
The chapter concludes with real-life case histories that illustrate airway management principles, the use of airway techniques and devices, and the DART Program patient management approach.
The three main tasks of the anesthesiology team are to (1) keep the patient safe, (2) keep the patient comfortable, and (3) provide for optimal conditions during the preoperative, intraoperative, and immediate postoperative periods. The component qualities of a general anesthetic are loss of consciousness, amnesia, analgesia, and muscle relaxation/paralysis. General anesthesia is provided for most head and neck surgeries, hence the need for airway management. In a routine setting, the patient is brought into the OR and positioned supine. Monitors are placed as described below. Preoxygenation with 100% inspired oxygen is used to denitrogenate the patient's functional residual capacity. At that point, anesthesia is induced, and the airway is managed appropriately.
The patient's risk for aspiration of gastric contents helps determine whether management should be with standard, non–rapid-sequence induction or with rapid-sequence induction (RSI). During the preoperative history and physical examination and on the day of surgery, an important question to ask is whether the patient is at risk for aspiration of gastric contents into the airway, an event that can be potentially catastrophic. One national study found that aspiration was the single most common primary cause of death and brain damage in anesthesia events. The ASA has guidelines for preoperative fasting that are based on the time required for gastric emptying in healthy patients. Familiarity with these guidelines as an OLHN surgeon can prevent delay or cancellation of elective surgery. The summary of fasting recommendations is 2 hours for clear liquids, 4 hours for breast milk, and 6 hours for other food or beverages, including infant formula and milk. In patients with delayed gastric emptying, such as those with diabetic gastroparesis, further fasting may be necessary to reduce the risk of aspiration.
In addition to a patient's adherence to fasting guidelines, pharmacologic agents administered preoperatively may reduce the risk of aspiration. These include clear antacids (30 mL of sodium citrate), anticholinergic agents (atropine or glycopyrrolate), metoclopramide (to stimulate gastric emptying and increase lower esophageal sphincter tone), and H 2 -receptor antagonists (cimetidine or ranitidine) to decrease secretion of hydrochloric acid.
In patients without increased risk of aspiration, a controlled and stepwise approach is taken for induction and intubation. After monitoring and preoxygenation, optimally for 3 minutes, general anesthesia is induced. Once the patient is unconscious, positive-pressure mask ventilation is performed ( Fig. 5.1 ). Only after successful mask ventilation is established is a paralyzing agent given. This stepwise approach to airway management increases patient safety, because even if intubation cannot be performed successfully, the patient still can be mask ventilated and oxygenated while the effect of the paralytic agent wears off, or while alternative intubation techniques are readied. The ability to ventilate the patient is more crucial than that to intubate the patient, and bag-valve-mask ventilation is a lifesaving skill that every anesthesiologist must master. After successful mask ventilation, the paralytic agent is administered; the patient is intubated after the agent takes effect.
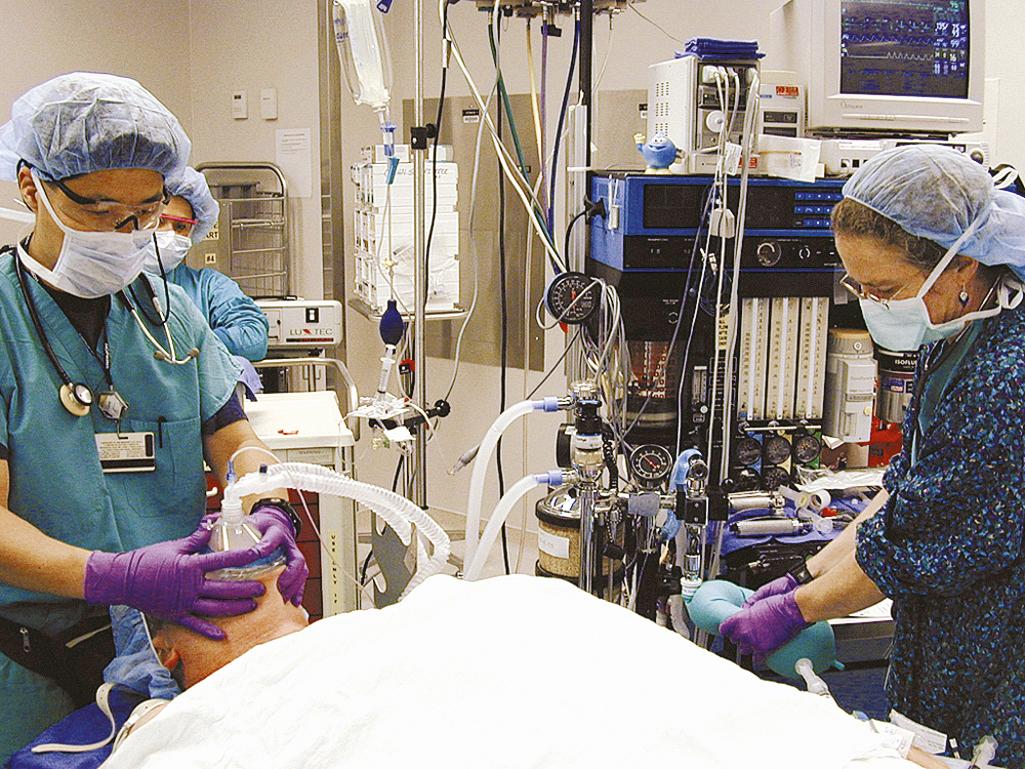
RSI and intubation are performed in patients who have an increased risk of aspiration. Such patients include those who do not meet the ASA's preoperative fasting guidelines, emergency patients who have a full stomach, and patients with a significant history of gastroesophageal reflux. During an RSI technique, mask ventilation is not performed because it can fill the stomach with air and increase the risk of aspiration even further. Instead, the paralytic agent is administered immediately after the induction agent. Cricoid pressure is maintained throughout this time, and the patient is not ventilated until the paralysis takes effect. Proper preoxygenation allows most apneic patients to maintain oxygen saturation during this brief period of time. The patient is intubated once paralysis is achieved, usually by means of direct laryngoscopy. After confirmation of proper endotracheal tube (ETT) placement by capnography or end-tidal CO 2 and auscultation of bilateral breath sounds, the ETT cuff is inflated and the cricoid pressure is released.
The risk of an RSI is that intubation may not be successful, and the ability to mask ventilate the patient has not been previously established. The most dangerous result of failed RSI could be a paralyzed patient who cannot be ventilated or intubated (see Case 8).
After intubation or other airway management, invasive monitors or additional intravenous (IV) access may be placed. The ASA's Standards for Basic Anesthetic Monitoring , first released in 1986, was most recently updated in 2011. These standards include continual evaluation of the patient's oxygenation, ventilation, circulation, and temperature during all administered anesthetics. This document introduced pulse oximetry and capnometry as standards of care, thus allowing more rapid and accurate recognition of oxygen desaturation and previously unrecognized esophageal intubation. Practically speaking, basic anesthetic monitoring should include continuous oxygen analysis of the anesthetic circuit, pulse oximetry, continuous waveform capnography, tidal volume measurement, electrocardiography, and temperature measurement, as well as intermittent measurement (no less frequently than every 5 minutes) of arterial blood pressure and heart rate. In addition, the routine use of a neuromuscular blockade monitor, also called the “twitch monitor,” to assess the degree of muscle paralysis and the return of muscle strength after pharmacologic reversal of paralyzing agents has significantly contributed to improved patient safety.
This section provides a brief introduction to the array of drugs used by anesthesiologists to facilitate anesthesia and maintain control of the airway. An in-depth discussion of these agents is not within the scope of this chapter; however, certain features of these drugs that are particularly applicable to airway management are highlighted.
With the removal of thiopental from the US market, the remaining drugs used for the IV induction of general anesthesia are propofol, ketamine, and etomidate. Each of these agents works rapidly within the central nervous system to produce unconsciousness, and each has a relatively short duration of action based on redistribution of the drug away from the brain rather than on metabolism. Their near-immediate onset makes these drugs the agents of choice for the induction of general anesthesia in patients with preexisting IV access. Their varying hemodynamic profiles and unique properties allow the anesthesiologist to choose the drug most appropriate for an individual case.
Propofol is the most commonly used IV anesthetic agent. It is a highly lipid-soluble drug suspended in a milky white lipid carrier. Propofol will cause a 25% to 40% drop in blood pressure with a 15% to 20% drop in cardiac output and an equivalent decrease in systemic vascular resistance. This drop in blood pressure is often well tolerated in otherwise healthy patients and can be offset by the pressor effects of subsequent laryngoscopy and laryngotracheal intubation. However, patients with long-standing hypertension and a shift in their autoregulatory curve may have impaired cerebral perfusion after propofol administration. Induction doses of propofol cause central apnea, but propofol has inherent bronchodilating effects that favor its use in asthmatic patients. Some pain may occur during IV injection, and propofol occasionally produces myoclonic jerking. Uniquely, propofol has antiemetic properties that have enhanced its popularity.
Ketamine is a dissociative anesthetic that produces unconsciousness without ablating spontaneous respiration, swallowing, eye movement, or airway protective reflexes. The continued spontaneous respiration and presence of some airway protective reflexes may be an important advantage in some cases, such as in patients with obstructive sleep apnea; however, the airway protective reflexes cannot be relied upon completely after induction of general anesthesia. The dissociative nature of ketamine is responsible for the occasional hallucinations or emergence delirium that occurs with this agent, more commonly seen in adults than in children. Although it is a common anesthetic agent in many countries, ketamine is not used as a first-choice agent in most settings in the United States because of its potential to cause these reactions. Ketamine does remain popular in trauma and emergency surgery, because unlike propofol, it will usually increase a patient's blood pressure, heart rate, and cardiac output through sympathomimetic effects and release of stored catecholamines. These sympathomimetic effects also lead to bronchodilation. Other unique properties of ketamine include its ability to be administered intramuscularly to patients without IV access, its intrinsic analgesic properties, and its increase of salivary production; antisialagogue agents such as glycopyrrolate are often coadministered with ketamine for this reason.
Etomidate is the final IV induction agent that remains widely available. In contrast to the hemodynamic profiles of propofol (hypotensive effects) and ketamine (hypertensive effects), etomidate has neutral effects on blood pressure and cardiac output. Etomidate has no particular effect on bronchial smooth muscle and may produce apnea with an induction dose; in addition, it causes significant pain on injection and causes more myoclonic jerking than does propofol. Distinct from the myoclonus, the seizure threshold decreases; therefore, seizures may result. Etomidate will also suppress the patient's adrenal glands. The clinical significance of this adrenal insufficiency is debated in the literature, with excess mortality seen in some studies of septic and trauma patients intubated with etomidate.
Benzodiazepines are often given preoperatively for their sedative, anxiolytic, and amnestic effects. Benzodiazepines will produce anterograde, rather than retrograde, amnesia. Midazolam is the most commonly used benzodiazepine in the preoperative setting because its onset of activity is within 2 to 4 minutes. Midazolam is commonly used for sedation during awake fiberoptic intubation, and it is used as an adjunct for routine induction of general anesthesia. A benzodiazepine can also be used, in a larger dose, as the sole agent for induction of anesthesia. Despite the hemodynamic stability associated with benzodiazepine induction, the large doses required lead to prolonged sedative effects. In sedative doses, benzodiazepines produce mild respiratory depression. However, the coadministration of benzodiazepines with opioids can synergistically produce profound respiratory depression. Of note, responses to benzodiazepines can be idiosyncratic, and a standard sedative dose can produce unconsciousness and apnea in sensitive patients or even paradoxical agitation or delirium in older patients. Benzodiazepines may be antagonized at the receptor level by flumazenil, although this reversal does promote a risk of seizures.
Opioids are used intraoperatively to provide analgesia and balanced anesthesia. When given during induction of anesthesia, opioids are useful in blunting the sympathetic response to laryngoscopy and intubation. Opioids also have a sedative effect and produce a sense of well-being with decreased responsiveness to noxious stimuli. The most commonly used opioids are fentanyl, remifentanil, sufentanil, morphine, and hydromorphone. The choice among these agents depends mainly on their varying speed of onset and duration of action. Opioids produce a dose-dependent central respiratory depression with an increased PaCO 2 and a diminished respiratory drive. This respiratory depression can be offset by asking an awake patient to consciously breathe deeply. However, the combination of opioids and benzodiazepines can result in a sedated patient with central apnea who is unresponsive to instructions to breathe. Opioids can be antagonized at the µ-receptor by naloxone.
Lidocaine is sometimes used as an adjunct during anesthetic induction. Administered intravenously, lidocaine has centrally acting anesthetic effects. Although unable to induce general anesthesia by itself, lidocaine can supplement the anesthesia induced by other agents. Furthermore, lidocaine has been shown to blunt the increases in blood pressure and intracranial pressure seen with laryngoscopy and laryngotracheal intubation. Doses are kept to 1 to 1.5 mg/kg to avoid potential toxicity. Lidocaine can also be administered prophylactically into the same IV line as propofol to reduce propofol-induced venous irritation and patient discomfort.
Dexmedetomidine is a newer anesthetic agent that works as an α-2 agonist to reduce sympathetic nervous system outflow and produce a sedative effect that more nearly mimics normal sleep. Dexmedetomidine creates minimal to no respiratory depression and is therefore very useful for managing patients in whom sedation is required concurrently with a normal respiratory drive. The use of dexmedetomidine for sedation in awake fiberoptic intubations has been well described. In contrast to midazolam and opioids, dexmedetomidine must be run as an infusion, but the greater sedation that results while maintaining respiratory drive is a valuable option. This drug has also been used for sleep endoscopy to pinpoint the area of pharyngeal obstruction prior to uvulopalatopharyngoplasty. Because of its α-agonist effects, high doses of dexmedetomidine may produce initial hypertension, but the most commonly seen hemodynamic effect of the drug is bradycardia. This drug does have a slight intrinsic analgesic effect, which again sets it apart from most other IV agents.
The widely available volatile anesthetics in the United States are isoflurane, sevoflurane, and desflurane. The term volatile refers to the liquid chemical's ability to vaporize at or near room temperature. Because these agents are halogenated ethers, they are nonflammable and nonexplosive. Nitrous oxide is also an inhaled anesthetic but is nonvolatile and supplied as a gas at room temperature. The exact mechanism by which the inhaled agents exert their anesthetic effect is not well known, but they are thought to disrupt protein-protein interactions within the scaffolding of the postsynaptic density. The Meyer-Overton correlation shows that more lipid-soluble volatile anesthetics have higher potency. Differences in lipid and blood solubility, as well as some unique properties of each agent, account for the clinical choice among volatile anesthetics. The dose of inhaled anesthetics is often scaled according to minimum alveolar concentration (MAC), the inhaled percentage at which 50% of subjects do not move to a surgical stimulus.
These volatile agents are not generally used for induction of anesthesia as “mask inductions” and require several more minutes than inductions with IV anesthetics. However, in patients without preexisting IV access, such as most children who are brought to the OR electively, inhaled inductions are common practice. Another benefit of inhaled induction is the ability to maintain spontaneous ventilation while achieving deep anesthesia and unresponsiveness to stimuli. Sevoflurane is the agent of choice for inhaled induction because of its comparatively pleasant odor. Desflurane is considered noxious to the respiratory system but is less soluble and therefore more easily removed from the patient's system at the end of a surgery. Isoflurane is the most potent but slowest and longest acting of the currently available volatile anesthetics.
Nitrous oxide is not potent enough at standard atmospheric pressures to reach 1 MAC of anesthesia, even at hypoxemic gas mixtures; therefore, nitrous oxide is used only as an adjunct to general anesthesia. The benefit of nitrous oxide is that it supports hemodynamic stability, whereas the volatile anesthetics all produce a dose-dependent decrease in systemic vascular resistance and blood pressure. This drop in blood pressure is usually offset by surgical stimulation, and it may be temporized with careful administration of pressor medications as needed. The hypotension associated with volatile anesthetics occasionally becomes an issue during head and neck surgical reconstructions with flaps, during which the use of pressor agents remains controversial. When added to an inhaled anesthetic, nitrous oxide allows a reduction of the volatile anesthetic concentration and therefore minimizes hypotension.
Another benefit of nitrous oxide is its low blood and tissue solubility; this characteristic enables it to leave a patient's system rapidly at the end of surgery. However, because it can expand in a closed air space, it is contraindicated during middle ear surgery. Nitrous oxide has also been linked to increased postoperative nausea and vomiting, and it impairs methionine synthase, which may cause an increased rate of surgical wound infections. Of note, nitrous oxide is flammable, and therefore mixing it into the inhaled gas mixture to decrease inspired oxygen concentration does not reduce the risk of airway fire; in contrast, the nitrogen found in the air is nonflammable, and adding air, rather than nitrous oxide, to lower inspired oxygen concentrations does reduce the risk of airway fire.
The volatile anesthetic agents are not often used for induction of general anesthesia in adults, but they are the most commonly used agents for maintenance of general anesthesia. The preference for inhaled agents in this role relates to the anesthesiologist's ability to measure ongoing concentrations of the drug in the patient. In contrast, the IV agents can be measured only in terms of administered dosage. Thus, because rates of metabolism vary, their ongoing serum concentrations will vary throughout the case even with a steady rate of administration. For related reasons, it is difficult to time the patient's emergence from general anesthesia after infusions of IV anesthetics.
However, certain otolaryngologic surgeries lend themselves especially well to maintenance with IV agents. This form of anesthesia is often referred to as total IV anesthesia (TIVA). An example is a case with a shared airway, during which the ear, nose, and throat surgeon may need to remove the ETT at times to have unrestricted access to the larynx or trachea. In this case, TIVA prevents loss of administered anesthesia during the apneic portions of the surgery. TIVA has also been studied in functional endoscopic sinus surgery, during which it may result in a cleaner surgical field and less blood loss. It is possible that the propofol-mediated drop in cardiac output results in lower mucosal blood flow in the sinuses. Finally, TIVA is used in patients who are susceptible to malignant hyperthermia (MH), which is a triggered reaction that some patients experience after exposure to the volatile anesthetics or succinylcholine. MH is characterized by uncontrolled skeletal muscle metabolism that leads to hyperthermia, acidosis, rhabdomyolysis, and sometimes death. A full discussion of MH is beyond the scope of this chapter (see Case 15 for further information).
The most commonly used agent for TIVA is propofol, although mixes of ketamine and propofol may also be used to balance the hemodynamic effects. Because of its lipid solubility, propofol will accumulate in patients during prolonged or high-rate infusions, with a resulting context-sensitive half-life that makes emergence unpredictable. That being said, propofol is still considered a relatively short-acting anesthetic. Remifentanil, an ultra-short-acting opioid administered via continuous infusion, is also sometimes used as part of TIVA. Remifentanil is commonly used during suspension laryngoscopy because that procedure is quite stimulating to the patient during surgery but causes limited postoperative discomfort—a combination that makes titration of longer-acting opioids a difficult task. Remifentanil may cause hyperalgesia in the postoperative period.
Paralysis of the patient facilitates endotracheal intubation by relaxing the jaw and stopping vocal cord motion. Furthermore, paralysis is often necessary for the surgical procedure itself. The two classes of paralytic agents are depolarizing agents and nondepolarizing neuromuscular blockers .
The only depolarizing paralytic agent used in the United States is succinylcholine, which acts on acetylcholine receptors in the neuromuscular junction, activating the receptors but then occupying them, thereby prolonging the refractory period before the muscle can contract again. The activation of the muscle leads to the characteristic muscle fasciculations, and associated postoperative myalgia, after which the skeletal muscles are relaxed. Muscle fasciculation can be effectively blocked by administering a small dose (usually 1/10 intubation dose) of a nondepolarizing paralytic agent 3 to 4 minutes before the administration of succinylcholine. The major advantage of succinylcholine is its very fast onset of action. Paralysis sufficient for endotracheal intubation can be reliably produced within 45 to 60 seconds. Another advantage is its short duration of action; clinical paralysis usually dissipates within 5 to 8 minutes of an intubating dose, as the drug diffuses away from the receptor and is metabolized and deactivated by pseudocholinesterase. It has been thought that this quick reversal of paralysis would allow resumption of spontaneous respirations if positive-pressure ventilation were not successful. However, deleterious oxygen desaturation may occur before resumption of spontaneous respirations.
In addition, the small percentage of patients who are pseudocholinesterase deficient will have prolonged paralysis after administration of succinylcholine. Vigilant monitoring of neuromuscular blockade has led to increased diagnosis of patients with atypical cholinesterase activity, a genetic condition with an incidence of 1 : 2800 in the general population in the United States and a 1 : 1 male/female ratio. Confirmatory blood laboratory diagnosis is made by determining the patient's dibucaine number, which relates to the amount of normal pseudocholinesterase. Although not contraindicated in patients with pseudocholinesterase deficiency, administration should be monitored with the train-of-four (TOF) monitor to verify full return of muscle strength before extubation. Succinylcholine has also been identified as the most common muscle relaxant trigger for MH. Primary contraindications to the use of succinylcholine include known or suspected MH, increased intracranial pressure, increased intraocular pressure, and elevated serum potassium. Potassium release occurs as the muscle fasciculates, and it may be exaggerated in patients with recent burns, strokes, or other denervating conditions in which the acetylcholine receptors are upregulated. Exaggerated potassium release and subsequent hyperkalemia have been implicated in ventricular fibrillation.
The other group of paralytic agents are the nondepolarizing neuromuscular blockers. These drugs also act in the neuromuscular junction, but they prevent muscle contraction by competitively blocking acetylcholine from binding to its receptors. Many different nondepolarizing agents are available that are clinically distinct because of differences in time of onset, duration of action, and route of metabolism. None of these agents work as quickly as succinylcholine. For patients in whom succinylcholine use is contraindicated, the nondepolarizing agent of choice for RSI is rocuronium because it has an onset of action between 60 and 75 seconds. A marked disadvantage, however, is that the effect of rocuronium, when given in doses sufficient for intubation, persists for 30 to 40 minutes—a major problem if initial attempts to intubate the trachea are unsuccessful. If no other option is available, establishment of mask ventilation is then essential, despite the increased risk of aspiration. (Historically, paralysis by rocuronium could not be pharmacologically reversed for 20 to 30 minutes.)
Sugammadex (Bridion; Merck & Co, Whitehouse Station, NJ) is a newly available reversal agent that binds rocuronium and vecuronium, thus removing neuromuscular blockade. It received FDA approval in the United States in December of 2015 but has been used widely in Europe and throughout the world since 2008. Intravenous dosing is dependent upon the degree of residual motor blockade: for moderate blockade with TOF > 2, the dose is 2 mg/kg; for deep blockade with TOF 1 to 2, the dose is 4 mg/kg; and for immediate reversal of complete blockade, the dose is 16 mg/kg. The impact of this high 16 mg/kg dose on the management of patients with both anticipated and unanticipated difficult airway is potentially profound, and may ultimately shift the balance toward the use of rocuronium and away from succinylcholine in such cases. It is recommended in the package insert that women on oral contraceptive pills or other hormonal birth control be counseled to use another form of contraception for 1 week after administration of sugammadex, likely stemming from in vitro binding to steroidal compounds. Anaphylaxis has been reported in some patients administered sugammadex. At least in some cases, this reaction may be caused by the sugammadex/rocuronium complex rather than either drug alone. Because sugammadex is cleared renally, its use in patients with renal failure is not recommended. However, in a difficult airway scenario, we suggest that the risk of sugammadex may be favorable to that of anoxia or a surgical airway. Sugammadex has not been well studied in pregnancy.
The clinical effect of the muscle relaxant is followed by observation of the TOF response to nerve stimulation. Nondepolarizing muscle relaxants in sufficiently low concentrations can have their clinical effects reversed by the administration of neostigmine or another cholinesterase inhibitor; the resultant increase in available acetylcholine at the neuromuscular junction leads to a return of muscle strength. However, the cholinesterase inhibitors also lead to increased parasympathetic effects at muscarinic receptors that must in turn be treated with anticholinergic drugs such as glycopyrrolate to avoid potential side effects, including bradycardia and asystole. Sugammadex does not appear to cause these side effects and may produce superior reversal of neuromuscular blockade. However, the rate of anaphylaxis is unknown, and whether it will increase as more individuals are subject to repeated dosing is unclear. Sugammadex has been used successfully to rescue residual paralysis after the administration of neostigmine and may be particularly useful for preventing reintubation in such scenarios.
The otolaryngologist often administers topical or local medications to the surgical field to produce either anesthesia or vasoconstriction. Cocaine is a medication that does both. An ester local anesthetic, cocaine rapidly numbs mucous membranes. Its vasoconstrictive properties are unique among local anesthetics, and when applied before sinus surgery, cocaine will shrink the mucosa and reduce surgical bleeding. However, some cocaine may be systemically absorbed. In the central nervous system, cocaine prevents reuptake of neurotransmitters, including dopamine and norepinephrine. The dopamine reuptake inhibition has been implicated as part of the addictive nature of cocaine use, and its use in the OR requires monitoring. The norepinephrine reuptake inhibition from systemic cocaine absorption causes tachycardia and hypertension, as well as potential vasospasm, and can be harmful to patients with or without known coronary disease. Other local anesthetics such as lidocaine may be used in lieu of cocaine, but then a second drug is needed for vasoconstriction. Topical phenylephrine has isolated vasoconstrictive effects and can be applied separately, via soaked pledgets, or mixed with lidocaine jelly. Epinephrine may also be injected with lidocaine solutions, to both induce local vasoconstriction and limit systemic absorption of the local anesthetic.
Local anesthetics are used surgically as an adjunct to analgesia and as regional anesthesia of the airway in awake patients; they can also be used for postoperative analgesia in patients who have undergone general anesthesia. Lidocaine and bupivacaine are the most commonly used anesthetics for local infiltration or nerve blocks. The surgeon must be aware of the maximum allowable dose because local anesthetic toxicity manifests initially as central nervous system depression and seizures followed by cardiovascular dysrhythmias with the potential for ventricular fibrillation. The maximum dose of lidocaine (5 mg/kg) may be increased (to 7 mg/kg) if epinephrine is used in the solution to slow uptake from the subcutaneous tissues into the general circulation. (A 2% lidocaine solution contains 20 mg/mL, and a 70-kg patient should receive no more than 17.5 mL of this solution.) The effect of topical, local, or regional lidocaine anesthesia is additive to that of IV lidocaine administered during induction of general anesthesia; therefore, communication between the anesthesiology and surgical teams is crucial to avoid a potentially toxic overdose. Bupivacaine is also used as a local anesthetic. The maximum dose of bupivacaine (2 to 3 mg/kg) can also be given safely with the addition of epinephrine to the solution. As a word of caution, local anesthetics can produce toxic effects at much lower doses if they are administered directly into the circulation; therefore, needle location must be verified by aspiration before injection of these drugs during infiltration or regional blocks. Accidental injection into the carotid artery during extra-oral glossopharyngeal block can produce immediate seizures and loss of consciousness. The cardiovascular collapse and ventricular dysrhythmias associated with local anesthetic toxicity can be refractory to advanced cardiac life support resuscitation; cardiopulmonary bypass, if available, may be necessary to support these patients. Immediate IV administration of intralipid has also shown benefits because the lipid “sink” may limit the systemic lidocaine or bupivacaine from dissolving into the cardiac tissues.
Airway management in the last few decades has been radically advanced by an increased understanding of the pathophysiology of ischemia and the judicious perioperative use of antihypertensive agents for patients at increased risk for ischemic events. Topical anesthesia—specifically with cocaine, epinephrine in lidocaine mixtures, or phenylephrine in lidocaine mixtures—can cause a direct response of intraoperative hypertension and tachycardia. Translaryngeal intubation of the trachea stimulates laryngeal and tracheal receptors and results in a marked increase in sympathomimetic amines. This sympathetic stimulation results in tachycardia and an increase in blood pressure. In normotensive patients, this increase is approximately 20 to 25 mm Hg; however, it is much greater in hypertensive patients. In hypertensive patients on β-blocking agents, this increase in blood pressure from vasoconstriction is due to unopposed α-stimulation. Hypertension and tachycardia are also commonly seen during emergence from general anesthesia. In any patient, but particularly in those with known coronary disease or arteriovenous malformations, extreme changes in blood pressure can be harmful or fatal.
The most commonly used drugs for intraoperative control of hypertension and tachycardia related to airway management include the β-blockers esmolol and metoprolol and the mixed αα- and β-blocker labetalol. Most blood pressure and heart rate changes occur approximately 15 seconds after the start of direct laryngoscopy and become maximal after 30 to 45 seconds. Esmolol is especially effective for blunting these responses because of its almost immediate onset of action, ease of titration, and short half-life (9 minutes). Labetalol is comparable for attenuating hemodynamic effects but has a less immediate onset of action and a half-life of 5 hours.
Deliberate hypotension has been studied as a method to reduce bleeding during endoscopic sinus surgery. The use of sodium nitroprusside as a potent vasodilator was shown not to significantly reduce bleeding, perhaps because the vasodilation led to a drop in blood pressure but not to a drop in blood flow to the nasal mucosa. Although deliberate hypotension brought on with other agents, such as β-blockers, may better reduce surgical bleeding, deliberate induction of hypotension does pose a significant risk of cerebral hypoperfusion, particularly in patients with cerebrovascular disease. Therefore, its routine use remains risky.
Complex airway management is a multifaceted problem that involves health care providers in a variety of clinical settings. The consequences of failed airway maintenance, endotracheal intubation, or both, can be devastating to the patient, the practitioner, and the health care system. This section discusses the definition, incidence of occurrence, and consequences of a difficult airway and the identification of difficult airway/intubation patients.
Controversy regarding the definition of “difficult” exists both within and among specialties, and may depend on practitioner skill, relate to specific techniques, or be complicated by variations in patient pathophysiology. The ASA defines a difficult airway as “a clinical situation in which a conventionally trained anesthesiologist experiences difficulty with facemask ventilation of the upper airway, difficulty with tracheal intubation, or both.” Despite advances in airway management techniques and refinement of difficulty predictors, the cited 1% to 3% incidence of unanticipated difficulty has not changed and is still defined by conventional rigid laryngoscopy (using Macintosh or Miller blades). Thus, in an institution where approximately 25,000 general endotracheal anesthetic procedures are performed annually, 250 to 750 unanticipated difficult airway/intubations could potentially occur per year. Another way to estimate the incidence is by assuming that a full-time practitioner (anesthesiologist or nurse anesthetist) would encounter one unanticipated difficult airway/intubation per year; then, based on the number of practitioners reported by the Anesthesia Quality Institute, at least 85,700 unanticipated difficult airway/intubations could occur annually in the United States. A 2005 article that reviewed 35 studies (over 50,000 patients) found the rate of difficult intubation to be 5.8%. In emergent intubation that occurs outside of the OR, the incidence is 6% to 10%. An analysis of the ASA Closed Claims Database (5230 claims) from 1990 to 2007 found that 67% of difficult airway management events occurred on induction, 15% during surgery, and 12% on extubation.
However, these numbers may underestimate the true incidence because anesthesiologists may not recall the more common “near misses” as vividly as they recall the smaller number of actual difficult airway/intubations for which the outcome was suboptimal. In addition to those patients who have unanticipated difficult airway/intubations on initial presentation, certain cohorts of patients have anticipated complex airway management.
On a national and international level, the scope of this problem and its impact on patients, practitioners, and the health care system is sufficient to warrant vigorous efforts to identify and implement solutions. The ASA practice guidelines note that the most common adverse outcomes related to difficult airway are death, brain injury, cardiopulmonary arrest, surgical airway, airway trauma, and damage to the teeth. The consequences of difficult airway/intubation, with or without an adverse outcome, may be as unsettling as the event itself. The patient may perceive this as a threat to future anesthetic safety or may fail to understand the significance of the difficulty. The practitioner may also perceive a threat to professional security.
In addition, failed airway management has serious legal and financial consequences. The impact of complex airway management–related events in direct and indirect costs to the health care system is far-reaching. The anesthesia specialty is the twelfth highest with respect to the number of physicians who have paid claims for malpractice.
An analysis of the ASA Closed Claims Database (5230 claims) from 1990 to 2007 found that death was the most common outcome in anesthesia claims and that difficult intubation represented 27% of adverse respiratory events related to those claims. In addition, persistent intubation attempts cause swelling and bleeding. Each attempt increases the likelihood of failure to intubate and increases the rate of complications, including permanent brain damage or death, up to 70%.
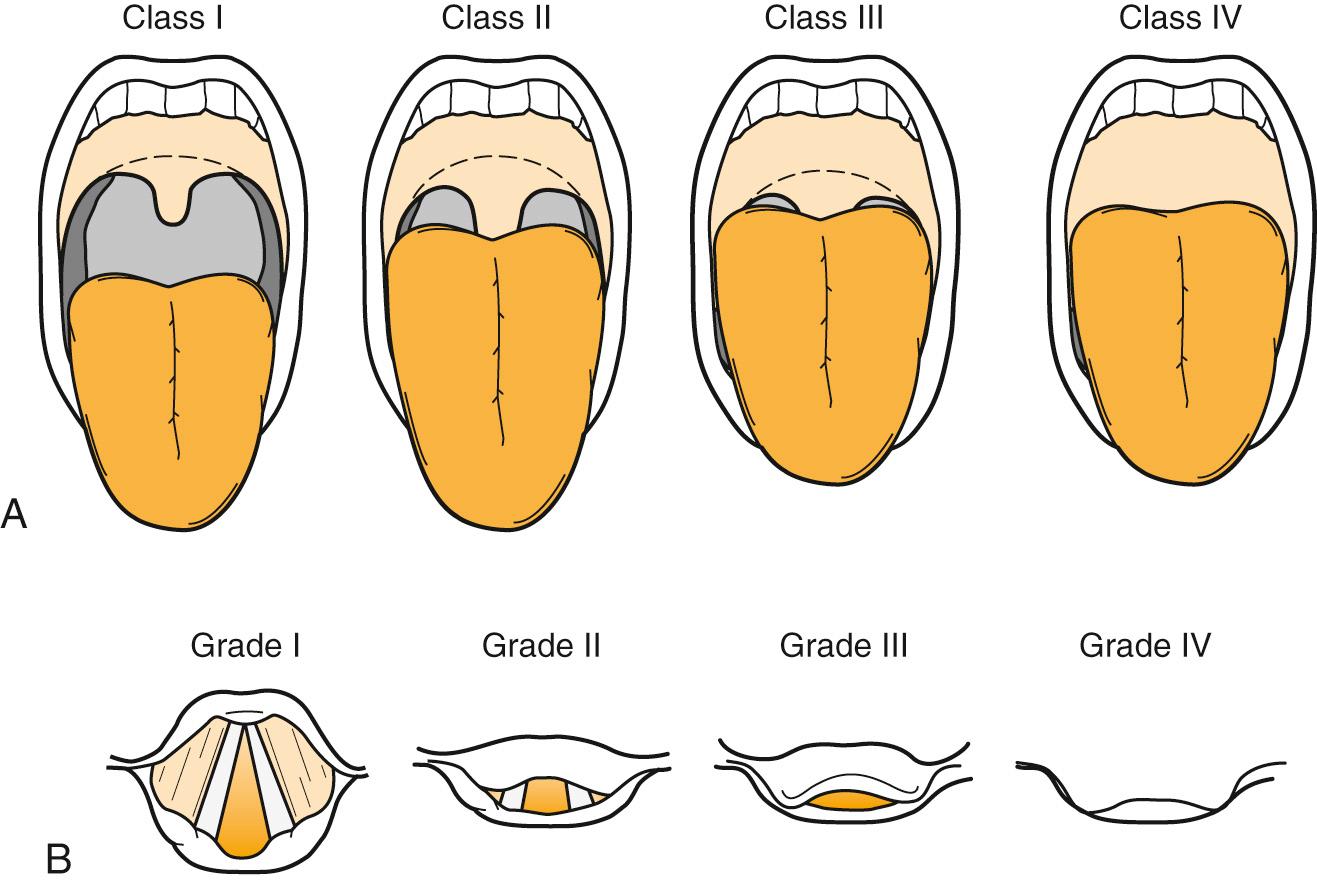
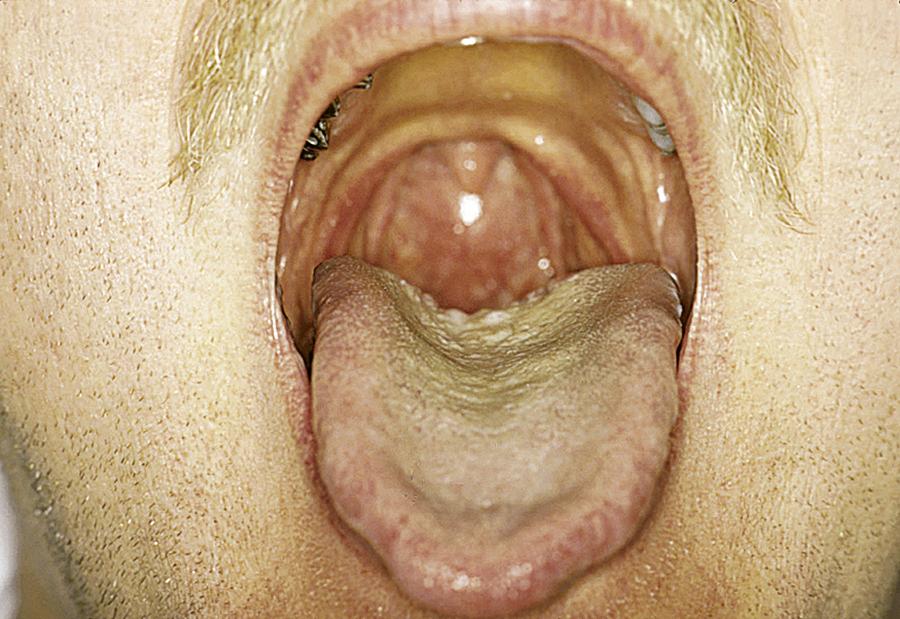
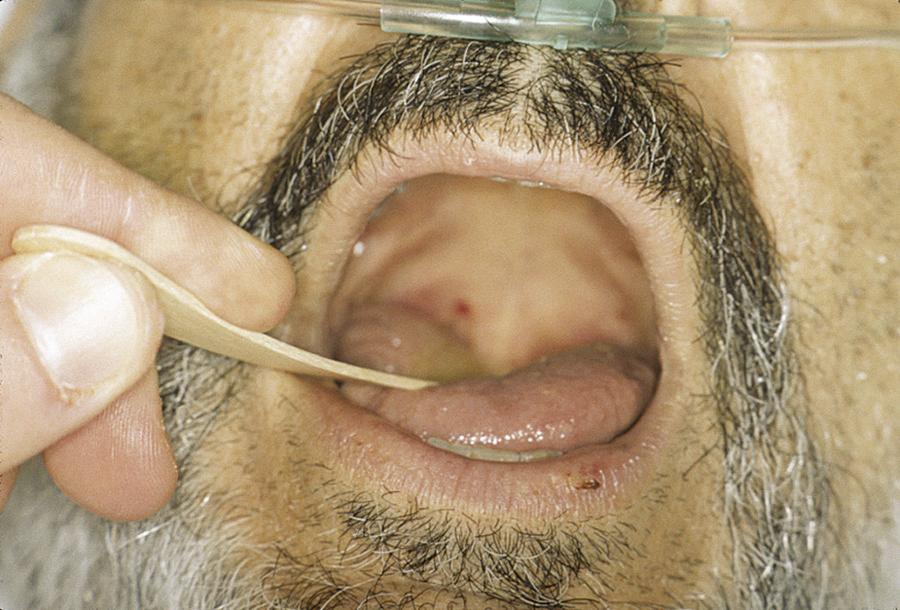
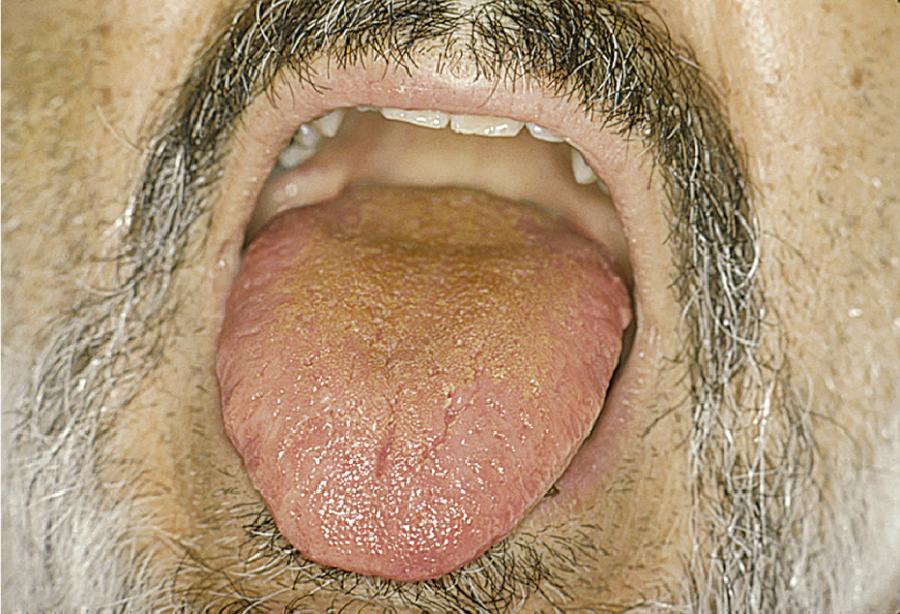
Practitioners may anticipate difficult intubation for some patients based on a history of previous difficult intubation or physical examination findings suggestive of a difficult intubation. In 1985, Mallampati developed a system of three classes to predict a difficult airway based on the position and visibility of certain anatomic structures—the uvula, fauces, and soft palate—in the oral cavity and oropharynx. The original system was revised in 1987 by Samsoon and Young to have four classes (modified Mallampati). Cormack and Lehane developed a system of four grades to predict a difficult airway based on the position and visibility of certain anatomic structures—specifically, the glottic aperture, posterior arytenoids, and epiglottis—in the laryngopharynx. Some other physical predictors of anticipated difficulty with conventional direct laryngoscopy (Macintosh/Miller blades) include prominent overbite, receding chin, a large tongue, a narrow mouth opening (interincisor distance or gap), short neck, limited neck flexibility, or obesity. Other ways to predict a difficult airway use a measurement of the thyromental distance or the sternomental distance. Various prediction models such as correlation of the Mallampati oral view, classes I through IV, to the Cormack and Lehane laryngoscopic view, grades I through IV, have been proposed, but none offer 100% sensitivity for prediction of a difficult airway ( Figs. 5.2–5.5 ). One study found that the modified Mallampati score was inadequate as a stand-alone test but could be part of a multivariable approach for predicting a difficult airway. Another study found that the most useful predictor was a combination of the Mallampati classification and the thyromental distance.
According to the 2013 ASA practice guidelines, “the anesthesiologist should have a preplanned strategy for intubation of the difficult airway” based on the ASA Recommendations for Strategy for Intubation algorithm, the type of surgery being performed, the patient's condition, and the skills and the preferences of the anesthesiologist. This section discusses various airway techniques and devices that were available in the past, and current ones that can be used during implementation of an airway algorithm.
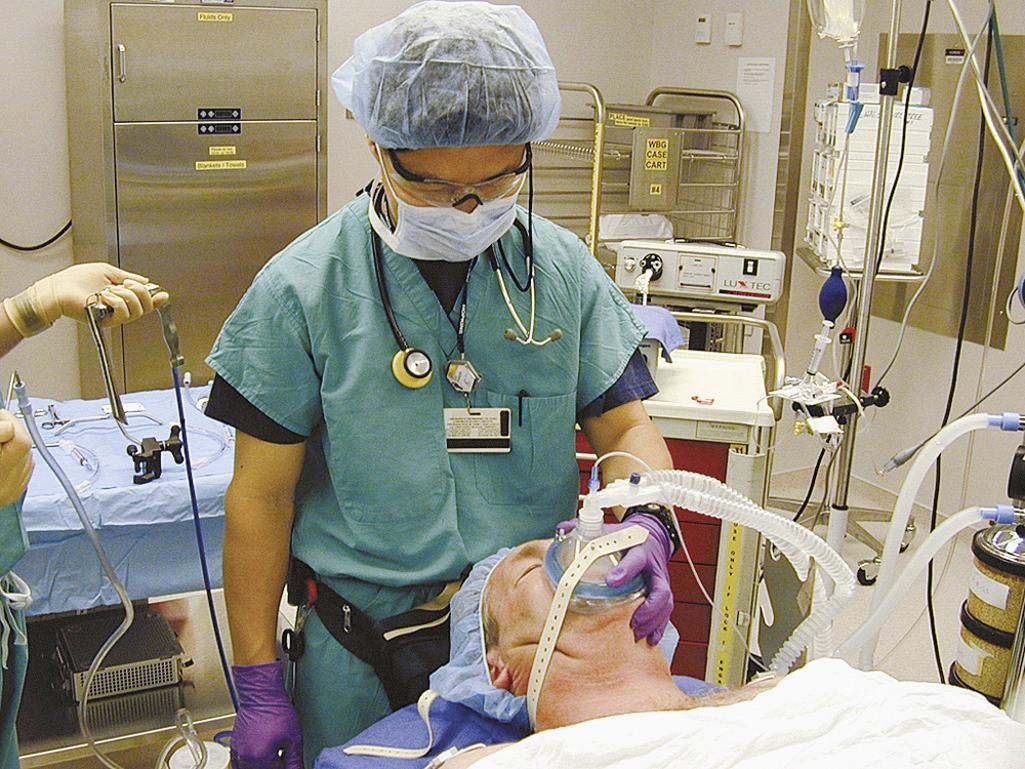
In the early 1990s, most anesthesiologists in the United States had three airway management techniques available to them: (1) awake blind nasal intubation, (2) awake nasal fiberoptic intubation, and (3) awake or asleep conventional laryngoscopy. Select difficult patients would undergo spontaneous ventilation “breathe-down” inductions without paralysis to facilitate optimal conditions for the OLHN surgeon to attempt to secure the patient's airway with either rigid laryngoscopy or bronchoscopy. Complications of this technique included laryngospasm, aspiration, lost airway because of inability to maintain spontaneous ventilation, or difficult positive-pressure mask ventilation and inability for the OLHN surgeon to intubate. During that same period of time, OLHN surgeons used the airway management techniques of awake and asleep surgical airway, awake FOB, and asleep rigid laryngoscopy and bronchoscopy ( Fig. 5.6 ). Facemask ventilation was a standard approach with general anesthetics that do not require intubation ( Fig. 5.7 ).
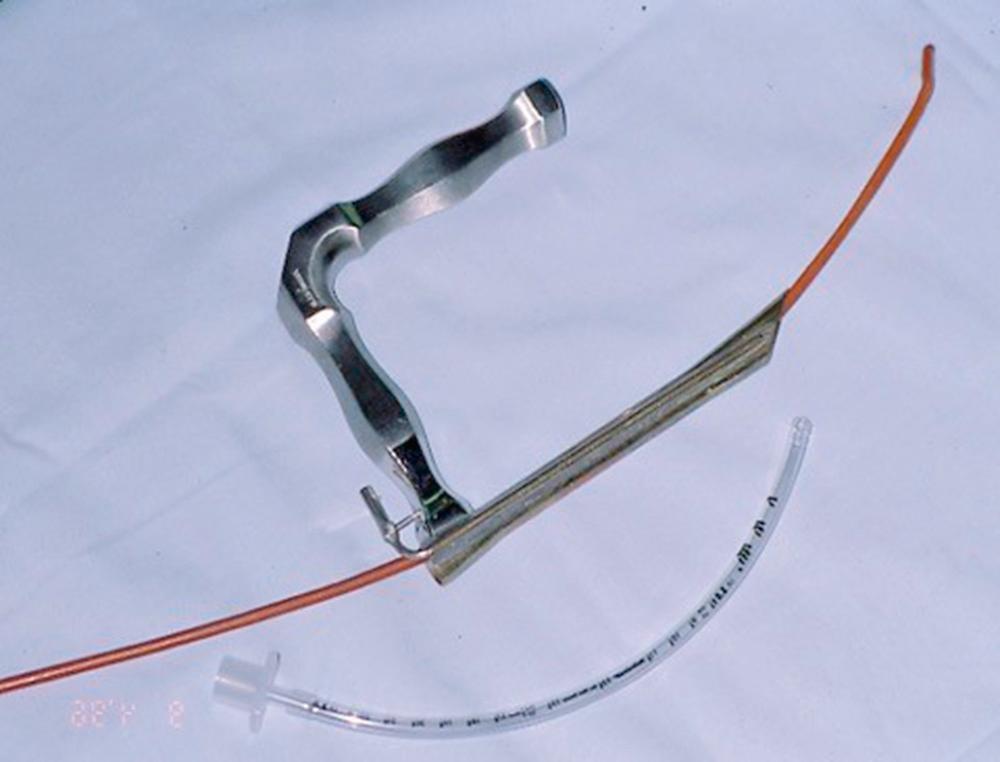
A significant invention in the history of airway management was that of the laryngeal mask airway (LMA). It was introduced in the United States in the early 1990s as an alternative to elective facemask ventilation; however, the value of the LMA as a rescue device for the most devastating situation—cannot intubate/cannot ventilate—was quickly realized. In the 1993 ASA Practice Guidelines for Management of the Difficult Airway , the LMA was the airway device that routed the difficult airway algorithm into the significant branch points. In the 2003 ASA practice guidelines, the LMA was promoted as a first-choice device for cannot-ventilate rescue options. For two decades, the “family” of LMAs—Classic, ProSeal, Flexible, and Fastrach—had a major impact on elective difficult airway management. Specifically, many practitioners decided that if they could mask the patient with an LMA, definitive placement of an ETT could be achieved with either FOB or ETT exchanger assistance. As always, a backup plan was a necessity because those techniques could be difficult and unsuccessful, as described in Case 8.
However, the 2013 ASA practice guidelines changed the algorithm by replacing an LMA with a supraglottic airway (SGA) device for cannot intubate/cannot ventilate situations. The guidelines also recommend video-assisted laryngoscopy, such as with a GlideScope (Verathon, Bothell, WA), as the initial approach to intubation. Although video laryngoscopy frequently enables excellent visualization of the larynx, difficulty with placement of the ETT and oropharyngeal injury from the rigid stylette are well described. Positioning the tip of the indirect video laryngoscope parallel to the lumen of the trachea and carefully maintaining normal laryngeal position makes it easier to place the ETT without resorting to flexible bronchoscopy.
Table 5.1 presents a brief review of many of these devices and identifies their primary use by OLHN and anesthesiology. Some airway devices, like the SGA, are readily available, require minimal practitioner education or training, and are inexpensive. Other devices and techniques—such as FOB, surgical airway, specialized rigid laryngoscopes, videolaryngoscopes, and fluoroscopic-assisted intubation—are available primarily in tertiary care centers, may require extensive practitioner skill, and may be relatively expensive.
| Technique | Visualization | Site | Awake/Asleep | Contraindications | Use by OLHN | Use by Anesthesiology | Comments |
|---|---|---|---|---|---|---|---|
| Facemask | None | Oral | Both | Full stomach | − | + | To optimize nasal/oral airways, headstrap |
| Video-assisted laryngoscopy | Indirect | Oral | Both | Limited jaw opening | + | + | Variations include C-Mac, GlideScope, McGrath |
| Supraglottic airway devices | None | Oral | Both | Full stomach | − | + | Variations include LMA, ILMA, flexible LMA, ProSeal, Air-Q, Fastrach |
| Blind nasal | None | Nasal | Both | Nasal pathology, coagulation status | − | + | |
| Digital | None | Oral | Both | Limited jaw opening | − | + | |
| Lighted stylet | None | Oral, nasal | Both | Nasal or laryngeal pathology, large neck/mass | − | + | Requires transillumination at sternal notch |
| Conventional laryngoscopy | Direct | Oral | Both | Limited jaw opening | − | + | Macintosh/Miller blades |
| Endotracheal tube guides (Eschmann, Frova, Arndt, Aintree) | Direct or none | Oral, nasal | Both | + | + | Adjunct to conventional and rigid laryngoscopy intubation with LMA and extubation techniques | |
| Rigid laryngoscopy (Hollinger, Dedo) | Direct | Oral | Both | Severely limited jaw opening | + | − | Laryngoscope commonly used for difficult airway intubation |
| Rigid bronchoscopy | Direct | Oral | Both | Severely limited jaw opening | + | − | |
| Fiberoptic bronchoscopy | Direct | Both | Both | Blood or oral secretions | − | + | |
| Rigid fiberoptic laryngoscope (Bullard, Upsher, Wu) | Indirect | Oral | Both | Blood or oral secretions | − | + | Combination of fiberoptic and conventional laryngoscopy |
| Retrograde intubation | Indirect | Neck | Both | + | + | ||
| Percutaneous cricothyroidotomy | Indirect | Neck | Both | Neck pathology, technique | + | + | OLHN assisted with FOB/direct visualization |
| Cricothyroidotomy | Direct | Neck | Both | Neck pathology, technique | + | − | |
| Tracheotomy | Direct | Neck | Both | Neck pathology, technique | + | − | |
| Transtracheal jet ventilation | Oral, neck | Both | Via angiocath attachment to a rigid laryngoscope, ventilating ETT changers | + | + | ||
| Combitube | Indirect | Oral | Both | Limited jaw opening | − | ? |
No approach to the management of a difficult airway is complete unless its scope embraces both OR and non-OR locations within the hospital. This section describes the efforts of John Hopkins Hospital (JHH) to create and sustain a multidisciplinary DART Program and includes lessons learned from over a decade of experience (2008–18).
The ASA first developed and released practice guidelines for the management of difficult airway patients in 1993. After that release, several individual departmental airway projects were initiated at JHH. In 2008, realizing that effective teamwork, communication, and coordination among health care providers could help prevent adverse patient care events, and based on the Joint Commission's call for a collaborative multidisciplinary approach to patient care through formal teamwork training, The JHH Department of Anesthesiology and Critical Care Medicine (ACCM), Department of Otolaryngology–Head and Neck Surgery, Trauma Surgery, and Emergency Medicine set out to develop the JHH DART Program. Its goal was to improve operational coordination and patient care, to identify system defects using in situ patient simulations, and to ensure sustainability in practice and knowledge through innovative educational programs.
In the 3-year period before implementation of the DART Program, several adult and pediatric actual and near-miss events related to urgent/emergent difficult airway management occurred outside of the OR. A root-cause analysis showed inconsistencies in several critical points of the process, including lack of a coordinated method to simultaneously page specialty providers who had difficult airway expertise, lack of access to specialty difficult airway equipment at the patient's bedside, and lack of training/experience with specialized airway techniques. In addition, the assigned roles of providers from multiple specialties were unclear during the event. These actual and near-miss events outside of the OR involved four departments: ACCM, OLHN, Emergency Medicine, and Trauma Surgery. Each department had attending physicians who were nationally or internationally recognized experts in difficult airway management, but their expertise was not utilized effectively as part of a multidisciplinary team. The overall finding of the root-cause analysis was that the major factor in airway event morbidity and mortality was the lack of a systemic approach to communicating and responding to difficult airway events.
The JHH DART Program initiative was created to recruit leaders from the departments involved in the actual and near-miss events to develop a comprehensive program that included operational, safety, and educational components. To facilitate cooperation among faculty members from each department and to support their individual academic careers, joint faculty appointments were awarded to physicians who became active members of the DART Program. Two to three members from each department received such appointments. These individuals had the primary responsibility for teaching other faculty, residents, and nursing and support staff within an organized educational program that included a month-long elective for senior anesthesiology residents. These members also provided consultation, both electively and urgently, for patients who required difficult airway management. They were also encouraged to become members of the Society of Airway Management (SAM) and to participate in its annual meeting.
To illustrate the staged DART Program implementation process, we present highlights from DART Program years 1 to 3, the 5-year DART Program review, and a decade of DART Program—lessons learned:
DART Program year 1 focused on reorganization of the emergency paging system, standardization of DART carts and their locations, and initiation of 24/7 attending physician coverage. An in-house phone number with a centralized page operator was agreed upon to activate the Code Team. If Code Team members decided it was necessary to activate the DART, the same “universal” phone number was used, and it prompted an equipment specialist to bring the DART cart to the patient's bedside. Eight DART carts were equipped based on input from DART team specialists and were modeled on carts used in ORs. They were strategically located hospital-wide, mapped to specific clinical locations based on human factors, time-to-delivery response (10 minutes or less metric), and elevator locations. Elevator over-ride keys were attached to each cart. Each cart contained equipment for performing rigid bronchoscopy, rigid bronchoscopy with a Dedo or Hollinger laryngoscope, flexible FOB, LMA placement, LMA/Aintree catheter intubation, surgical airway, or tracheotomy ( Fig. 5.8 ). The DART members also carried a standardized code bag that contained basic airway equipment and included ETTs, LMAs, oral airways, laryngoscopes, stylets, medications—and again, an elevator over-ride key. An ACCM Quality Assurance and Encounter Report was created to track all difficult airways in the OR and all codes and DART calls in the rest of the hospital. A DART Oversight Team, composed of risk management, a human factors engineer, a safety officer, and a senior DART member from each department, performed a daily review of all DART events. Difficult airway patients were identified with a wristband and were triaged to enrollment in the MedicAlert Foundation's National Difficult Airway Registry, as they had been in the past. An additional focus was on the education of providers. The DART Oversight Team conducted in situ simulations, modeled after actual DART events, that proactively addressed complex airway management in various hospital settings. A biannual DART Program Difficult Airway Course was developed for senior members of each department. The course included lecture topics that covered airway assessment, airway techniques, trauma airway management, pediatric airway management, team training, and decision-making (crisis management, critical language, and situational awareness). Simulations of a difficult airway, trauma airway, and pediatric airway were conducted. Skills training included bag-mask ventilation, direct laryngoscopy, tracheal tube introducers, SGAs, fiberoptic intubation, Hollinger scope intubation, videolaryngoscope intubation, and cricothyroidotomy with a pig trachea model. Over 100 residents participated in this multidisciplinary airway course, and participation was then extended to fellows. During DART Program year 1, 488 codes were called, and of those, 49 were escalated to DART calls (44 adult, 5 pediatric); 13 of these were transported to the OR for airway management. However, during this same year, no difficult airway sentinel events occurred.
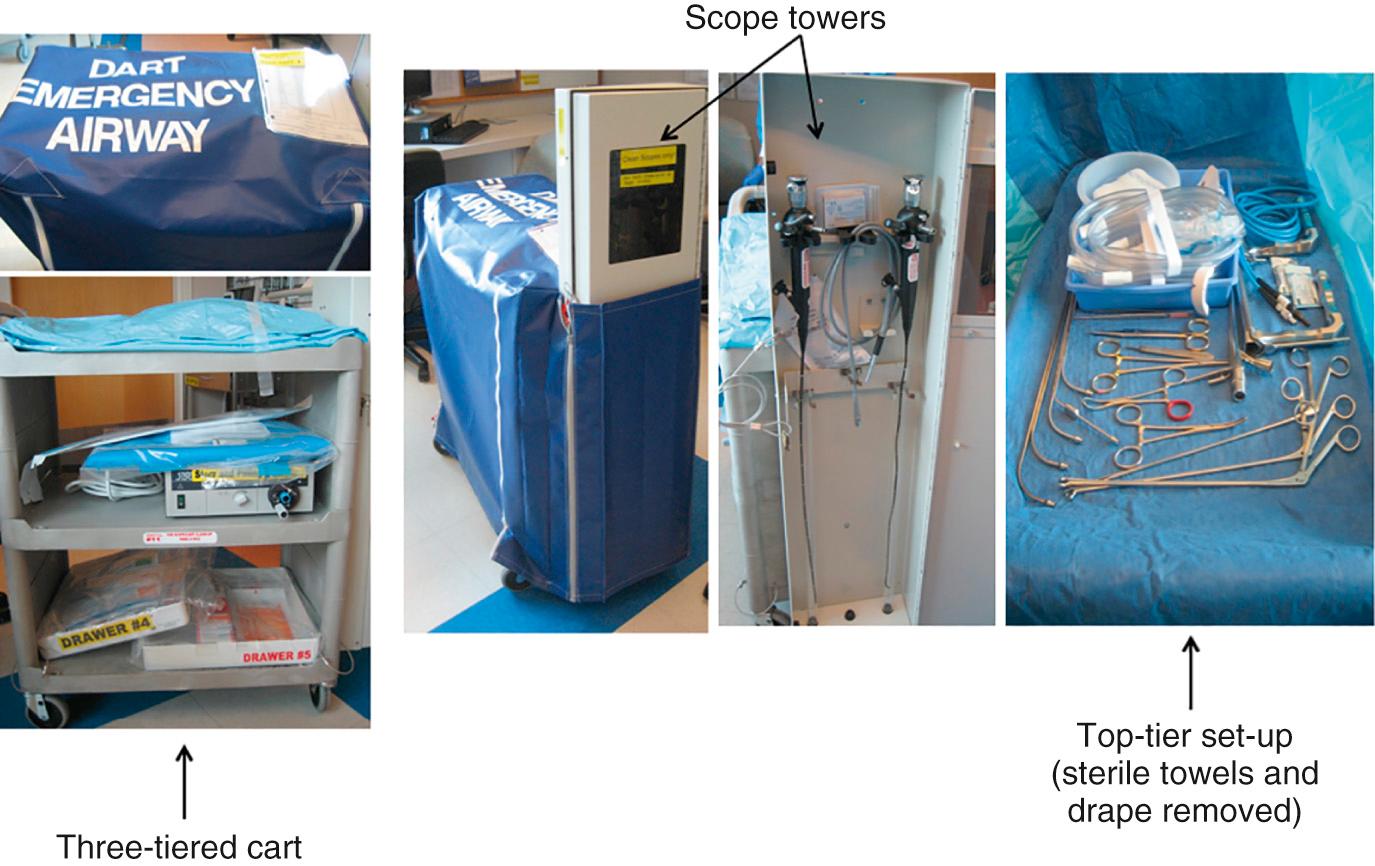
DART Program year 2 focused on practice-based learning and systems-based process improvement, quarterly multidisciplinary case conferences, and expansion of the DART cart “fleet.” The number of DART carts was increased to 11 based on a review of the previous year's DART cart usage and time trials for delivery within the timed metric. Also that year, in situ simulations were conducted in three clinical areas that had been identified as frequent DART-call locations. During DART Program year 2, 898 codes were called, and of those, 81 became DART calls (75 adult, 6 pediatric); 20 of these were transported to the OR for airway management. Although the number of DART calls increased by 65.3% compared with that in the previous year, again, no difficult airway sentinel events occurred.
During DART Program year 3, the emphasis was on the DART Program Difficult Airway Course, which expanded to a quarterly offering to include senior house staff from each of the four departments, respiratory therapists, nurses, and support staff. During DART year 3, 975 codes were called, 82 became DART calls, and 10 of those were transported to the OR for airway management. Again, no difficult airway sentinel events occurred during DART year 3.
Overall, during the first 3 years of the DART Program, a total of 2361 codes were called, and of those, 216 became DART calls (196 adult, 16 pediatric). During 19% of those patient events, the DART team chose to transport the patient to the OR for definitive airway management. In the OR, providers had the benefit of skilled nursing support, improved illumination and physical space, and additional equipment. During those 3 years, there were no difficult airway sentinel events.
In 2015, a comprehensive DART Program 5-year review was published: “Difficult airway response team: a novel quality improvement program for managing hospital-wide airway emergencies.” An overview of the DART Program and implementation process was included with operations cost analysis, DART cart inventory list, airway event data analysis, in situ simulation results, and an outline of policies and procedures recommended to other institutions seeking to develop a customized DART Program.
During the first 5 years of the DART Program, there were no airway-related deaths, malpractice claims, or sentinel events for adult patients. Between 2008 and 2013, 360 (7.5%) of 4738 code activations were escalated to DART activations; of those, 29 (8%) patients received surgical airways and 62 (17.2%) were transported to the OR for definitive airway management.
DART activation risk factors included angioedema and anaphylaxis, active airway bleeding, head and neck tumor, limited cervical spine mobility, laryngectomy/tracheostomy, history of difficult airway, and body mass index greater than 30 ( Box 5.1 ). The most frequent airway management techniques were direct laryngoscopy (conventional MAC/Miller laryngoscopy), FOB intubation (awake or as an asleep fiberoptic/LMA/Aintree intubation technique). DART OLHN members, utilizing the rigid laryngoscope (Hollinger/anterior commissure laryngoscope), reduced the need for a surgical airway in many patient encounters. Emergent surgical airway cricothyroidectomy techniques were standardized between OLHN and trauma surgery. No patient adverse events were reported in these events.
Angioedema
Active airway bleeding
Head and neck tumor
History of difficult airway
Limited cervical spine mobility
Tracheotomy and laryngectomy
Body mass index >30
The DART Program Difficult Airway Course was given 18 times and trained over 400 senior residents and providers. In addition, 20 combined anesthesiology/OLHN problem-based DART case conferences were presented, and yearly hospital-wide institutional DART Program grand rounds were presented.
In a commissioned editorial titled “Use your SMARTs (some kind of multidisciplinary airway response team) for emergent airway management outside the OR,” Joffe equated widespread implementation of a multidisciplinary airway response team to prevent loss of a life with the earlier widespread implementation of advanced cardiovascular life support (ACLS) to save a life. Death from a lost airway is a preventable death (Dr. Andy Ovassapian, founder of Society for Airway Management).
The DART Program has sustained clinical operation results of no claims or sentinel events for adult patients and further developed innovations in the safety and educational programs. Highlights of experiences and lessons learned include:
“Who's in charge?” and clarifying roles of multidisciplinary attendings at DART events.
Once DART members arrive at a patient event, debriefings are immediate and concise. Airway management plans A, B, C, and D are identified, and attendings optimize patient care by working together. Key components of “Who's in charge?” are: (a) critical language/situational awareness in crisis management and (b) primary role for each specialty attending. Leadership is defined but can be passed between experienced members of the team as their procedural requirements change. It is imperative that the acting team leader not perform a specific procedure, but rather focus on receiving and distributing information to guide the team. Open communication of information important for decision-making is encouraged from all team members. Once specific tasks are agreed upon, closed-loop communication is critical for the leader and team members (sometimes referred to as followers) ( Box 5.2 ).
| Crisis Management Concepts | Airway-Specific Crisis Management Language |
|---|---|
| Leadership | Catch phrase |
| Command team | “We can't ventilate and we can't intubate.” |
| Value-independent input | Situational awareness |
| Followership | “This patient is desaturating and becoming bradycardic.” |
| Contribute to situational awareness | Shared cognition: “ED will perform RSI with OLHN prepping neck for surgical airway.” |
| Verbalize observations | Assertive communication |
| Workload management | Diverse and independent input valued |
| Proper allocation of tasks | “I can't intubate—please start a surgical airway.” |
| Avoid work overload | Closed loop communication |
| Prioritize | “Etomidate 20 mg and succinylcholine 150 mg have been given.” |
| Avoid nonessential tasks |
ED, Emergency department; OLHN, otolaryngology—head and neck; RSI, rapid sequence induction.
Roles of DART attendings are clarified and summarized:
Anesthesiology attending: physiology and pharmacology, bag-mask ventilation, and noninvasive airway techniques (e.g., supraglottic devices, MAC/Miller laryngoscopy, videolaryngoscopy, and fiberoptic intubation). Emergency cricothyroidotomy is reserved for an event in which the DART surgical members have not arrived, an airway needs to be established immediately, and this is the technique of choice.
OLHN attending: noninvasive techniques (e.g., FOB and intubation, rigid laryngoscopy and rigid bronchoscopy) and surgical cricothyroidotomy.
Trauma surgery attending: noninvasive techniques (bronchoscopy) and surgical cricothyroidotomy.
Emergency medicine attending: physiology, pharmacology, bag-mask ventilation, noninvasive airway techniques, and surgical cricothyroidotomy.
DART practitioners agree on a standardized approach for airway management.
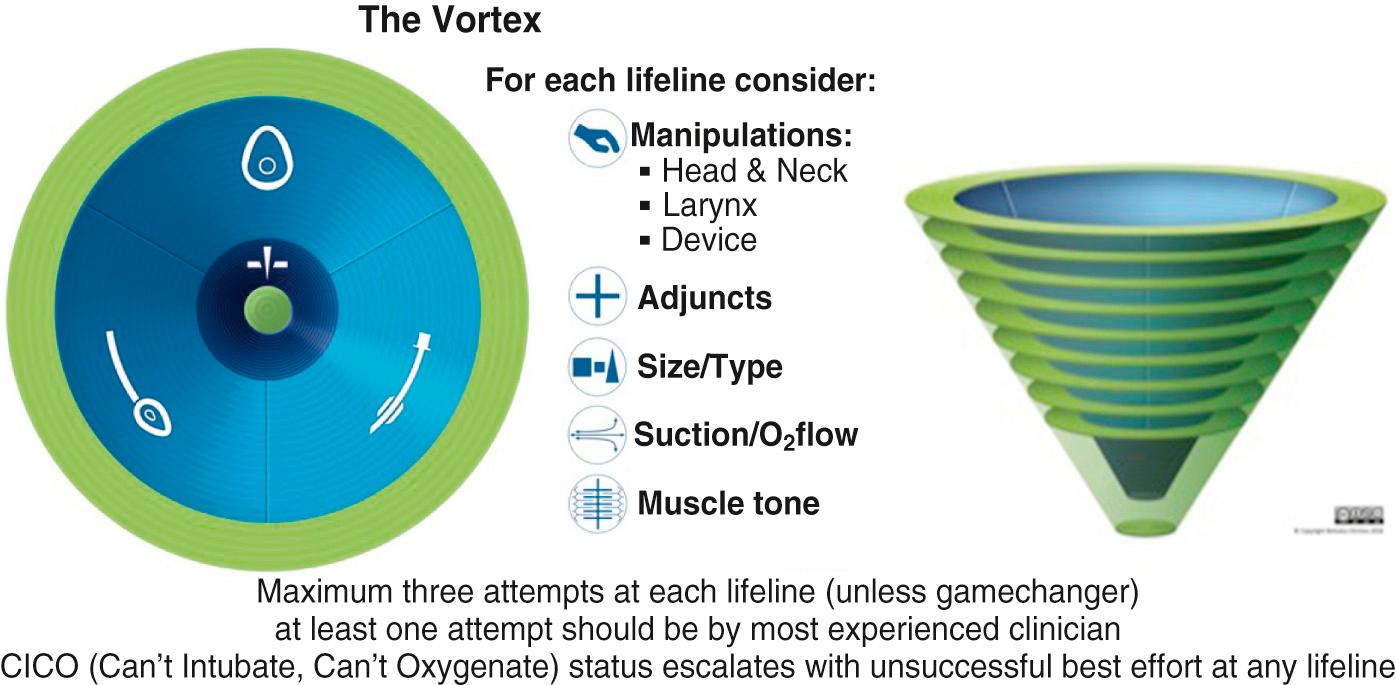
Though each DART specialty adheres to its own difficult airway management algorithms, the DART Program adapted a combination of the ASA guidelines ( Fig. 5.9 ) and the Vortex approach to difficult airway management ( Fig. 5.10 ). Introduced by Chrimes in 2016, the Vortex approach provides a graphic visualization of airway management that begins with noninvasive techniques—advising no more than 3 attempts at each—and progresses to a standardized surgical technique if noninvasive ones are unsuccessful. This visual cognitive aid supplements the airway plans A, B, C, and D at DART events, is gaining widespread usage in clinical operations, and is a focus of education in the DART Airway Courses.
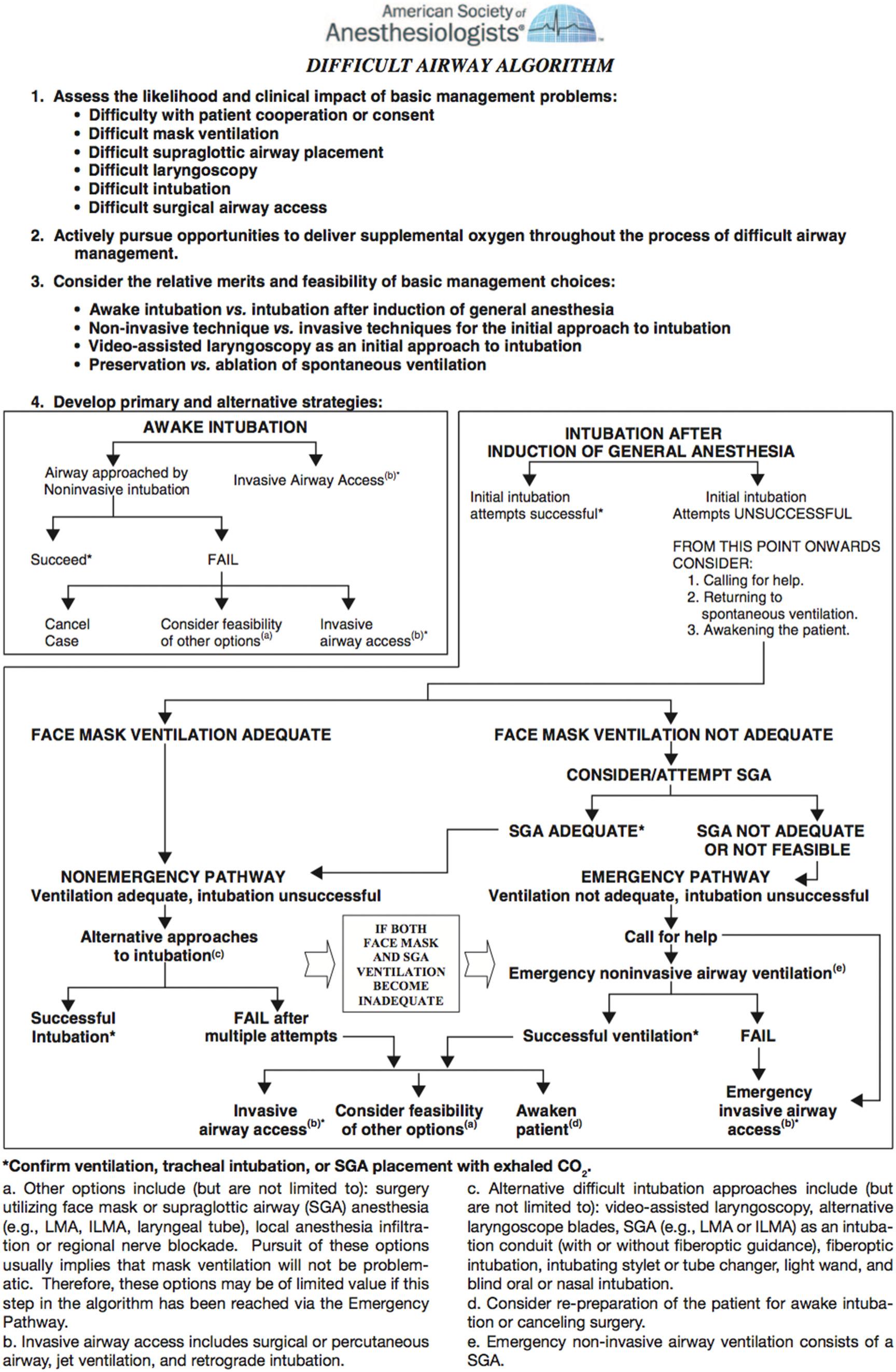
The gold standard for awake intubation and select asleep airway management techniques remains FOB and intubation; therefore, maintaining these skills is essential.
Awake fiberoptic intubation can be the technique of choice for patients with complex pathophysiology: angioedema, head and neck pathology (e.g., limited mouth opening, base-of-tongue tumors), and in some cases, extreme morbid obesity. Acquiring and maintaining skills for awake fiberoptic intubation is essential for all members of a DART Program.
A surgical airway is not a failed airway.
With the initiation of the DART Program, some clinicians held the view that a surgical airway would reflect a failure on the part of the DART providers. Rather, for the past decade of DART, 5 to 10 surgical airways have been performed yearly—by attendings, early and proactively—with no associated morbidity or mortality. DART attendings standardized the surgical airway technique, and all housestaff are taught the same approach ( Fig. 5.11 ).
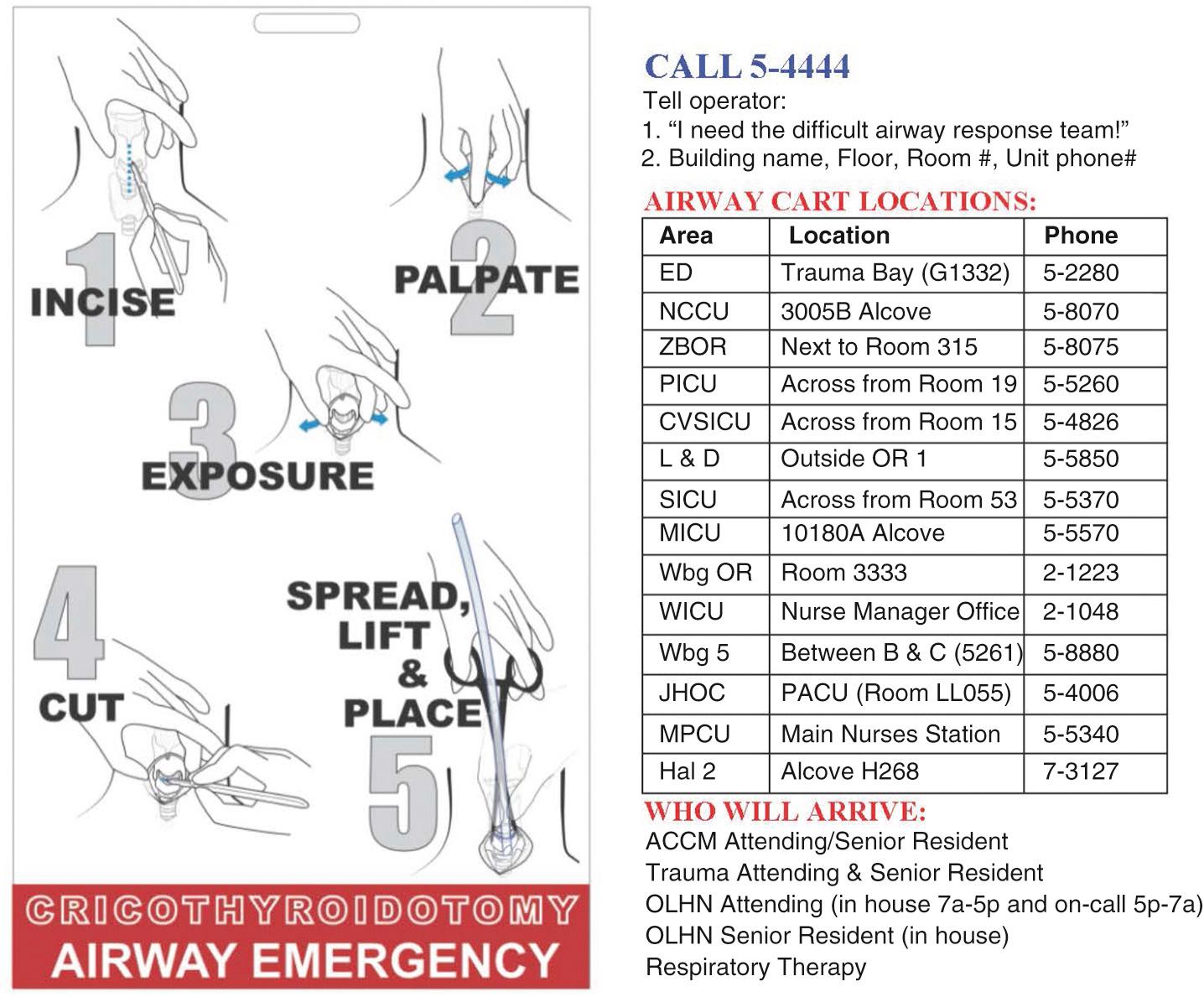
Keep pace with airway management innovations.
Critically important to process improvement for the DART Program was introducing new airway management techniques and educational programs that reflected clinical operations. Airway techniques were first introduced into the ORs for elective use and to allow practitioners to gain expertise before adding to DART carts (e.g., technique of LMA/FOB/Aintree and videolaryngoscopy). Educational programs that have been incorporated into the DART Airway Course include ORSIM bronchoscopy simulator (Airway Simulation Limited, Auckland, New Zealand) and the Difficult Airway Algorithm and Rescue Cricothyrotomy (DAARC Game) Web-based program.
Collect and use data to continually improve.
A DART Oversight Team reviews each DART event. Feedback for operations, safety, and education is provided to health care team members. Improvements have resulted in updated DART carts, improved institutional paging systems, creation of a pediatric DART Program, and improvements to the DART Airway Program.
Pediatric DART Program is different from Adult DART Program.
Although an adult DART Program can support pediatric patients in institutions that accommodate both adults and children, a pediatric DART Program has unique challenges and solutions to providing quality care to patients. Solutions include comprehensive consultation services upon admission to the institution with concise documentation and proactive airway plans A, B, C, and D, recognition that the OR is the ideal location for difficult airway management, and having an OR ready 24/7 for airway management.
The aftermath of adverse airway events: first, second, and third victims.
All institutions should have support services for health care providers involved in adverse airway events. The concept of victim includes: first victim—patient/friends/family; second victim—health care provider(s) involved in care; third victim—institution at large. Many institutions have resilience programs.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here