Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The portion of the immune system resident within the intestine faces significant challenges. A single layer of epithelium separates the majority population of the host’s immune cells from a massive number of bacteria. It is therefore probably not surprising that the mediation and control of intestinal immunity follow rules distinct from those governing systemic immune reactivity.
The challenges faced by the intestine not only include achieving nutrient absorption but also maintaining tolerance toward dietary antigens and the enteric microbiota, while retaining the ability to react vigorously to intestinal pathogens. , Such balance of immunological response is made possible by the depth of interaction between the ancient innate immune system and the evolutionarily more recent adaptive immune system. The footprints of evolution are clearly seen within the immune system of the intestine: different classes of cells that arose in distinct evolutionary eras work together in synergy. This has led to addition of control mechanisms over time, rather than simple replacement of more archaic cell types by evolutionarily more modern successors. Dysfunction of cell types that have arisen relatively late in evolutionary history (for example T cells) can induce significant disturbance of intestinal immune homeostasis, although the effector mechanisms of the more ancient elements of the mucosal immune system function perfectly well.
An important question is why intestinal inflammation is not more common. The gut lumen contains at least as many bacterial cells as there are human cells in the entire body, while most immune cells reside in the intestine. Food contains complex dietary antigens, which if injected parenterally would evoke systemic reactions. Thus there are fundamental mechanisms that inhibit potential reactivity to dietary antigens and the gut flora. Intestinal inflammation often occurs as a consequence of breakdown of these mechanisms.
Many of the core studies have been in mice, and less is known of human mucosal immunology. The same broad principles appear to apply, as evidenced by diseases in humans with genetic disorders affecting immune function. This review will first cover components of the intestinal environment that contribute to the maintenance of mucosal immune tolerance. Later more detail will be given about individual elements and mechanisms.
Many of the cell populations that cause tissue damage and inflammation are of innate immune origin, such as macrophages, neutrophils, eosinophils, mast cells, and dendritic cells. These cells may respond rapidly but do not directly exhibit immune memory. Products of some of these cell types may cause epithelial disruption, tissue breakdown, and vascular thrombosis. Innate immune cells may respond directly to invading bacteria without prior involvement of adaptive immune cells such as T and B cells. However, these effector cell types are often recruited in response to chemotactic cytokines (chemokines) produced by T and B cells. Innate immune responses may thus be shaped by adaptive immunity.
B cells are shifted in their isotype from a default IgM polarity by the local cytokine environment within organized lymphoid tissues and cell-cell contact with T cells. , In general, immunoglobulin A (IgA) responses characteristic of the gut mucosa protect against inflammation, while immunoglobulin G (IgG) responses are more proinflammatory. Deviation toward allergic immunoglobulin E (IgE) responses may also promote inflammation by disrupting epithelial barrier and neural function.
Among T cells, CD4-expressing helper cells (T H ) produce cytokines to alter function of other cells while CD8-expressing cytotoxic cells (T C ) are capable of directly killing other cells. Among CD4 T helper cells, three major populations can drive different forms of intestinal immune reactions and inflammation—T H 1 cells (that produce the cytokines interferon-γ [IFN-γ] and interleukin [IL]-2), T H 2 cells (producing IL-4, IL-5, and IL-13), and T H 17 cells (producing IL-17). , These cell types are discussed in more detail later.
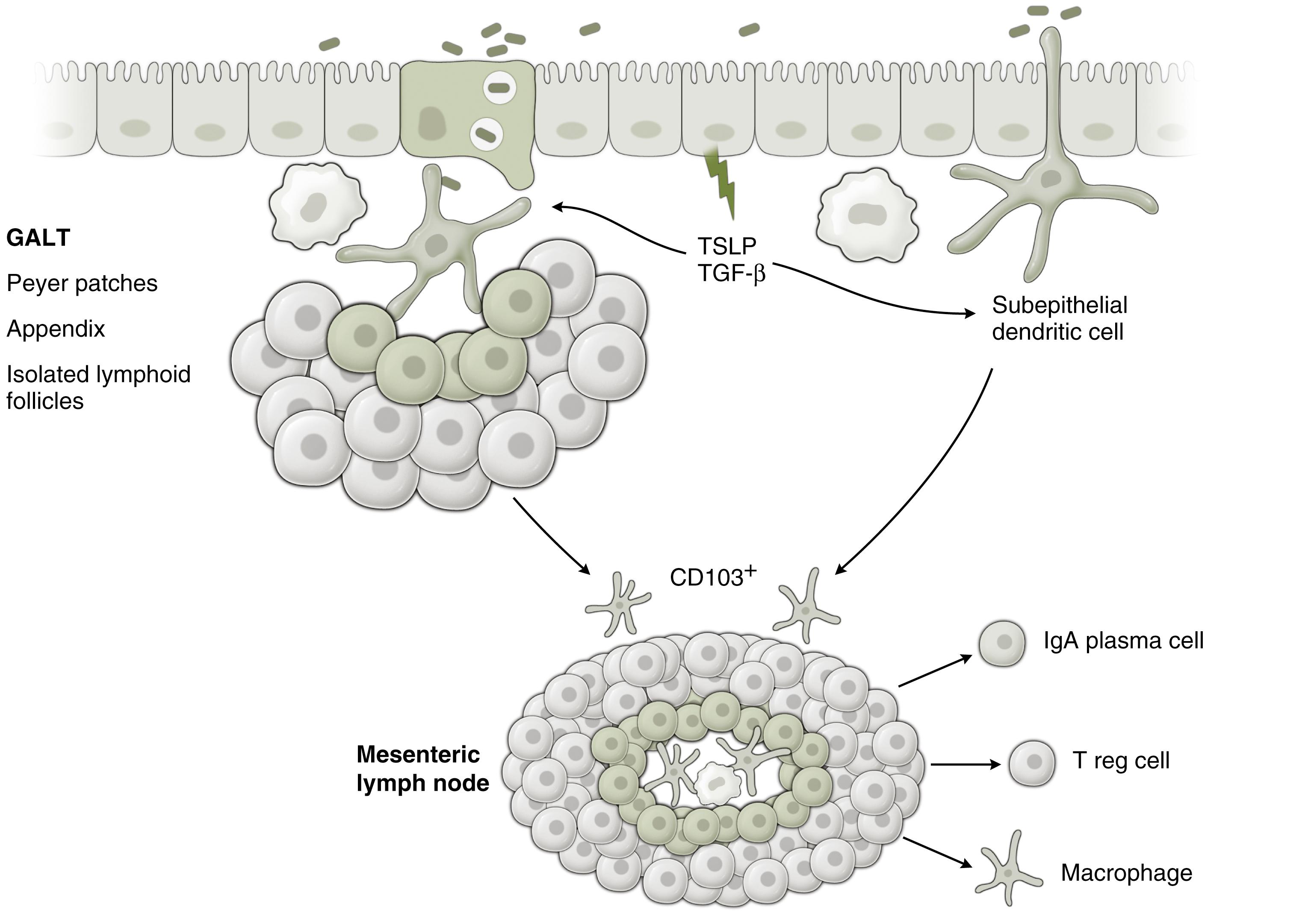
The commitment to lineage and the functional state of T cells depends critically on input from the innate immune system, notably antigen-presenting cells. These are thus the most upstream part of the gut immune hierarchy. Sensing of bacterial luminal contents by dendritic cells is critical in this process ( Fig. 5.1 ), as is the local cytokine environment that shapes dendritic cell-lymphocyte interactions. Thus T H 1 cells are generated by dendritic cells producing IL-12; T H 2 cells by those producing IL-4; T H 17 in response to transforming growth factor-β (TGF-β), IL-23, and IL-6; and regulatory T cells (t REG ) cells in response to TGF-β or IL-10. Consequently sensitization, rather than tolerance, may occur if pathogens induce local cytokine production at the time of initial priming.
Pathogens may break immune tolerance by disrupting the epithelial barrier and/or inducing secretion of proinflammatory cytokines by resident macrophages. They may also induce expression of chemokines by the epithelium, leading to recruitment of other inflammatory cells. These newly recruited cells in turn may respond to other antigens penetrating the breached epithelial barrier, or self-antigens liberated from tissues as a consequence of tissue damage. Provided there is adequate repair of the epithelial barrier and clearance of the initiating pathogen or antigen, such inflammatory responses are normally damped down by regulatory immune responses, which are discussed in more detail later. The triggering of chronic inflammatory disorders by pathogens represents a failure of regulatory responses or of epithelial barrier repair.
The epithelium plays a very important role in mucosal immune responses. Epithelial barrier function is utterly critical in preventing immune reactions to the gut flora and antigen. Firstly, bacterial ingress is minimized by secretion of mucus by goblet cells and antibacterial peptides (such as α and β defensins) by Paneth cells. Paneth cell α-defensin production in fact shapes the composition of the bacterial flora, thus indirectly regulating mucosal T cell responses. , Two mechanisms may lead to disruption of this coordinated Paneth-cell response to the normal flora: defects in either bacterial autophagy (a process of intracellular bacterial digestion and consequent immune presentation) or intracellular bacterial response (through loss-of-function polymorphism in the NOD2 pattern recognition receptor); both lead to suboptimal immune response to bacteria and are strongly associated with the development of Crohn disease.
Secondly, tight junction integrity limits penetration of antigens past the epithelium via the paracellular route, where they might be taken up by antigen-presenting cells. In health, peptide chains longer than 11 amino acids are normally prevented from penetrating; these are too short to invoke effective T cell activation. Experimental studies of animals with leaky intestinal epithelium (caused by mutated cell adhesion genes) confirmed that epithelial leakiness alone is sufficient to drive inflammation in response to the normal flora. Human genetic disorders with impaired gut epithelial adhesion (such as tufting enteropathy and epidermolysis bullosa) are also characterized by inflammation. At a population level, increase in paracellular permeability is associated with nutritional failure, intestinal inflammation, and overall mortality in developing-world children. , Study of mucosal biopsies in persons with environmental enteric dysfunction shows clear evidence of epithelial detachment and leakiness. Pathogens may directly cause epithelial damage or may trigger local production of cytokines such as tumor necrosis factor-alpha (TNF-α) and IFN-γ, , which promote paracellular leakiness. Their action is opposed by local production of the cytokine TGF-β, which in addition to promoting tight-junction integrity also plays numerous roles in maintaining intestinal immune tolerance. , Thus either infections or local inflammatory reactions may impair epithelial barrier function and thus promote secondary inflammatory or sensitizing events.
The epithelium also functions to regulate mucosal lymphocyte populations through constitutive secretion of chemokines such as CCL25 (TECK) in the small intestine and CCL28 (MEC) in the colon. These chemokines allow retention of B and T cells that have been primed within mucosal lymphoid follicles, following their circulation via the thoracic duct and subsequent homing to the mucosa. , The epithelium also produces mediators that induce local adaptation of retained cells toward a regulatory, non-inflammatory type. However, when epithelium is stressed or activated, it produces different chemokines that attract polymorph neutrophils (IL-8), monocytes (MIP-1α), T cells (CCL20), or eosinophils (eotaxins), depending on the initiating stimulus. , One important and consistent feature of intestinal immune regulation is the different responses made by such newly recruited cells compared to the locally adapted populations. Thus the epithelium may play a critical role in determining the overall status of mucosal immune responses.
Finally, epithelial cells may play a role in antigen presentation that may promote tolerance, by presenting absorbed antigens to lymphocytes in an inherently non sensitizing manner, because these cells do not express the co stimulatory ligands required for full T cell activation. , T cells primed in this way may be rendered anergic and thus fail to respond to their antigen.
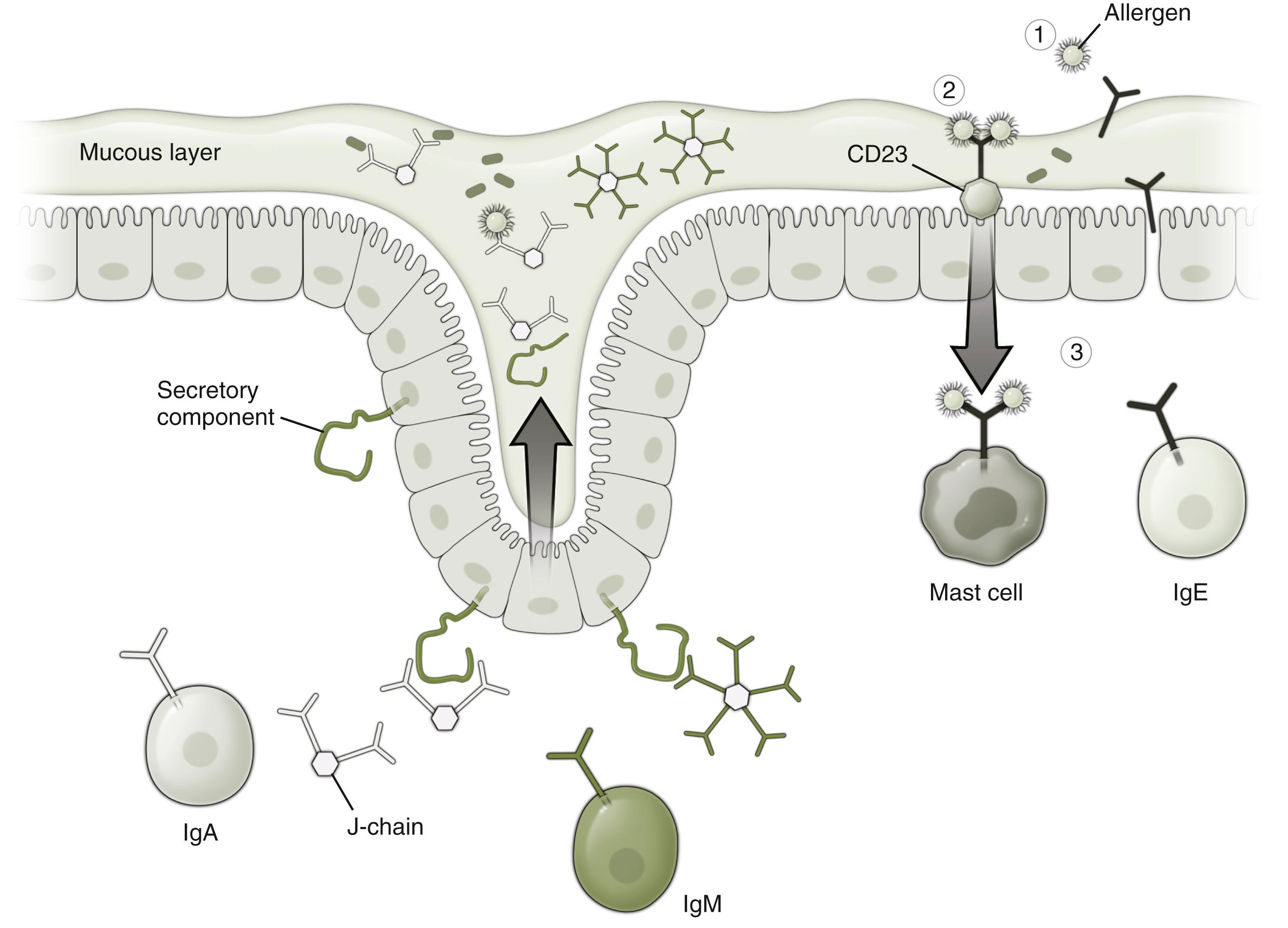
IgA is generated in response to the gut flora and other luminal antigens, following their uptake by antigen-presenting cells and transport to lymph nodes within the gut wall. These mesenteric lymph nodes are the key sites of regulation of mucosal immune tolerance (see Fig. 5.1 ) and appear to be highly important in segregating mucosal from systemic immune responses. , , IgA-producing plasma cells generated in the mesenteric lymph nodes pass into the circulation, then home back to the gut and go on to secrete specific IgA beneath the epithelium. , This secreted IgA is in turn taken up and transported through the epithelial cells into the lumen ( Fig. 5.2 ). This has two effects—secreted IgA adheres to bacteria and minimizes their invasiveness and also downregulates the transcellular absorption of antigen through the epithelial cell (enterocyte). By contrast, secreted IgE has the opposite effect of accelerating antigen uptake by the enterocyte and may also induce tight junction leakiness through triggering of subepithelial mast cells (see Fig. 5.2 ). , Thus there appears to be dynamic balance between IgA and IgE with respect of sensitization potential and maintenance of immune tolerance.
These cells represent a critical component of the gut’s antiinflammatory repertoire , and are discussed in more detail later. There are several types of regulatory lymphocytes, functioning in broadly similar manner to inhibit inflammatory responses but differentiated by their pattern of surface molecule expression (e.g., CD4+CD25+ T cells) or their cytokine production (e.g., TGF-β producing T H 3 cells, IL-10 producing TR1 cells). One molecule is critical in generation of these regulatory cell types—the transcription factor forkhead box P3 (FOXP3). , , Mutations in FOXP3 cause a severe inflammatory autoimmune disorder, called IPEX syndrome, affecting the intestine and other organs, thus confirming the importance of regulatory lymphocytes in preventing gut inflammation.
There is some evidence that mucosal IgA production and regulatory T cell generation may function as a coordinated system, with regulatory T cells providing the major help for IgA responses, ensuring immunological tolerance to the enteric flora.
It is not only the ability to make regulatory responses that inhibits inflammation. Animals deficient in a wide variety of immune mediators or cell types will spontaneously develop inflammation in response to the normal gut flora. This may relate to an inability to regulate the composition of the flora, allowing overgrowth of pathogens, or because the immunodeficiency predisposes to making a pathologically skewed response to normal bacteria. A balanced immune response thus appears critical in preventing intestinal inflammation. Infants with a variety of inborn immunodeficiency disorders develop intestinal inflammation, remitting after correction of the underlying disorder by gene therapy or bone marrow transplant.
Normal mucosal tolerance cannot be established in the absence of a gut flora—animals maintained germ-free do not exhibit normal tolerance responses. , However it is important to note that different bacteria have different specific effects upon this process. Thus it is unclear how much the changes in the early gut bacterial composition of human children that have been identified since the introduction of antibiotics in the last 50 years have contributed to the increased incidence of allergic and inflammatory diseases. ,
Generation of regulatory lymphocytes within the gut is at least partly dependent on the gut flora. This depends on signals from the epithelium and directly through dendritic cells, which require input from gut bacteria via pattern recognition molecules (such as Toll-like receptors [TLRs]) in order to provide appropriate inductive signals to the T cells. , The cytokine critical in induction of regulatory T cells (T REG ) is TGF-β, which is also important in developing IgA responses and maintaining epithelial integrity. ,
It is becoming clear that specific components of the flora, rather than the overall bacterial load, may be critical in the development of normal mucosal immune responses. While much of the literature on probiotics has focused on the properties of lactobacilli and bifidobacteria species, other bacterial types appear much more important in maturing the mucosal and systemic immune systems. A carbohydrate produced by Bacteroides fragilis induced both mucosal and systemic immune shift away from T H 2 toward T H 1 responses. Segmented filamentous bacteria and clostridia appear critical in maturing mucosal T helper cell and IgA responses in mice. It thus appears that, among the myriad bacterial species found in the gut, only relatively few have shaped host mucosal immune responses during evolution.
Generation and function of regulatory T cells is also dependent on specific micronutrients—notably zinc, vitamin A, and vitamin D. Vitamin A is also important in maintenance of epithelial integrity , and generation of gut homing plasma cells within gut-associated lymphoid tissue (GALT). The consequence of micronutrient deficiency in intestinal inflammatory or allergic states may therefore be an inability to restore normal regulatory responses and thus an exaggerated inflammatory response.
The GALT is organized within three compartments—diffusely scattered through the lamina propria beneath the intestinal epithelium, within the epithelial compartment itself, and in organized lymphoid follicles such as Peyer patches ( Fig. 5.3 ).
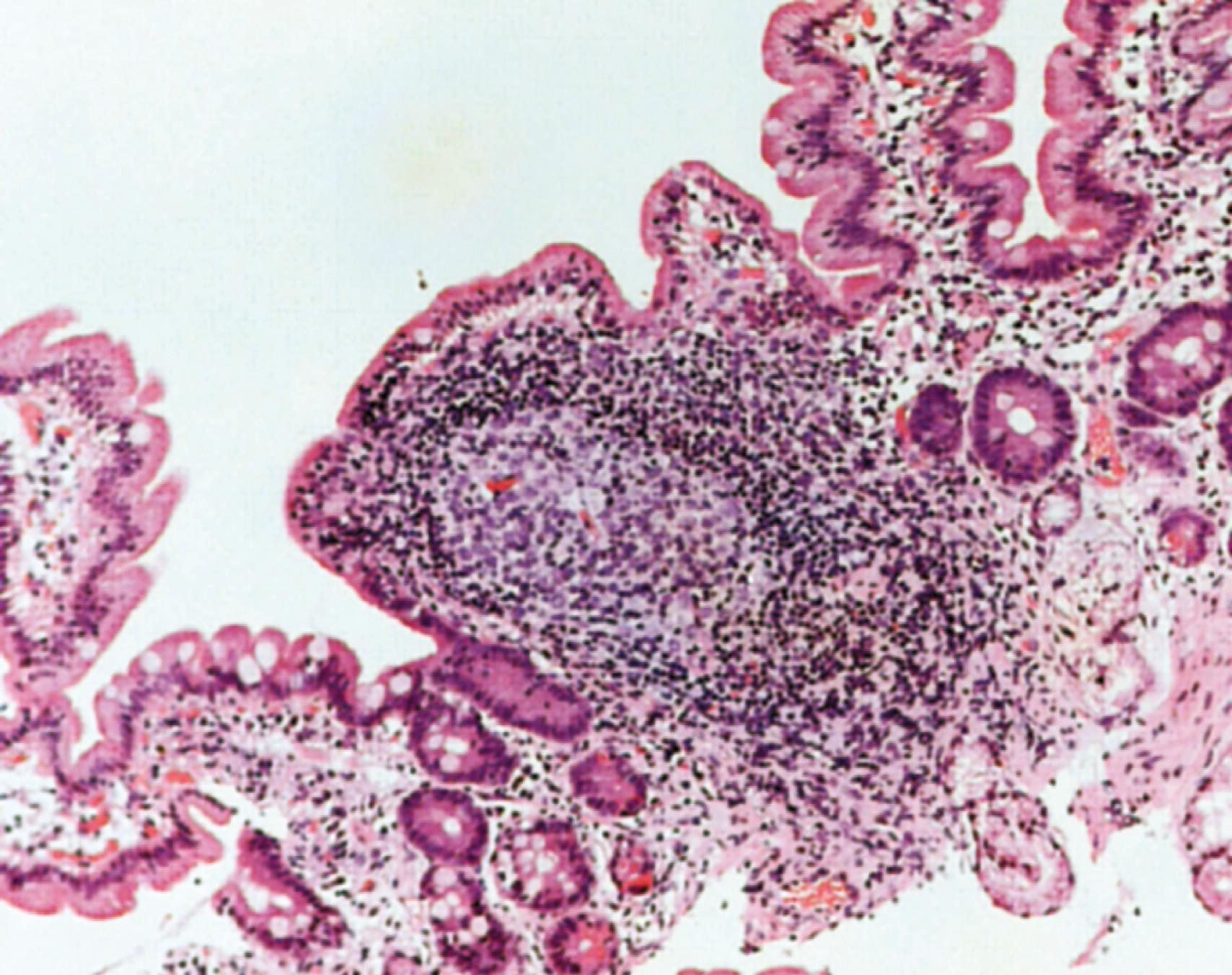
(a) The diffuse lymphoid tissue of the intestinal lamina propria is dominated by plasma cells, most of which (in health) are IgA-producing, although in early infancy IgM-producing cells are more common. T lymphocytes within this compartment are more commonly CD4+ rather than CD8+. These CD4+ cells may be subdivided functionally into T effector (T helper—T H ) and T REG cells. The T REG cells are particularly important in maintaining immune homeostasis within the intestine.
In addition, the mucosal lamina propria contains numerous dendritic cells and macrophages, most of which are locally adapted to their antigen-rich environment. During inflammatory responses, increased expression of chemotactic cytokines (chemokines) and other proinflammatory mediators leads to recruitment of additional T and B cells, monocytes/macrophages, and other cell types such as polymorphonuclear neutrophils, eosinophils, and mast cells. , The pattern of cellular recruitment will depend on the polarity of T cell responses induced following antigen presentation to the T cells by dendritic cells or macrophages. , , These cells from the innate immune system are finely attuned to local microbiological influences, and therefore components of the gut flora may have a profound effect on overall immune responses within the intestine.
(b) The intraepithelial compartment contains populations of lymphocytes that are uncommon elsewhere in the immune system. , Among T cells, around three quarters of the intraepithelial lymphocytes (IELs) are CD8+ (i.e., cytotoxic T cells). Minority T cell populations (type b IELs), whose true function in man is uncertain, include cells expressing neither CD4 nor CD8 (CD4-CD8- T cells), cells expressing CD8 with two α chains rather than the usual αβ combination (CD8αα cells), and cells with the T cell receptor composed of γ and δ chains (γδ cells) rather than the α and β chains usually found in circulating T cells (αβ cells). There is also a significant population of natural killer (NK) and NK-T cells in this compartment. They may be involved in distinct mechanisms of antigen presentation based on enterocyte expression of non classical major histocompatibility complex (MHC) molecules. ,
Both T cells and NK cells jointly provide a surveillance role for the intestinal epithelium and may be induced to cause cell death of enterocytes in circumstances of infection or local production of the cytokine IL-15.
(c) Organized lymphoid follicles occur throughout the intestine. They are most numerous in the terminal ileum, where they cluster to form macroscopically visible aggregates named Peyer’s patches, after Johann Conrad Peyer who reported them in 1677, thinking them to be glands producing digestive juices. The follicles do not have afferent lymphatics and are notable for unusually permeable overlying epithelium, due to the presence of M cells (so called because of their ultrastructural appearance of microfolds). This permeability ensures penetration of luminal antigens to a subepithelial pocket containing large numbers of antigen-presenting dendritic cells. These lymphoid follicles are therefore able to sample and respond to a wide variety of luminal antigens, both bacteriological and dietary. Efferent lymphatics from the Peyer patches drain to the mesenteric lymph nodes, the major site of mucosal immune induction.
The appendix is a specialized intestinal region with dense aggregation of lymphoid follicles. It appears to be important in mucosal immune priming, as appendicectomy protects against later development of ulcerative colitis, and neonatal appendicectomy prevented later development of colitis in mutant mice. , The appendix is now thought to function as a immune-mediated reservoir for the indigenous host flora, allowing repopulation of the colon after infection. , The bacteria adhere to biofilms, enriched in mucus and defensins from the innate immune system and IgA from the adaptive immune system. The similarity of such biofilms in mammals and non mammalian vertebrates suggests an ancient origin for immune support of indigenous bacterial species. The mucus biofilm is capable of excluding bacteria from the colonic epithelial surface in health and may indeed deliver tolerogenic signals to the mucosal immune system, although this barrier becomes defective in intestinal inflammation. Blind outpockets of the distal gut, similar to the appendix, have arisen by convergent evolution across many unrelated species, suggesting a more important function than had previously been ascribed to the appendix.
Bacterial translocation into organized mucosal lymphoid follicles has been studied in resected appendix tissues from human infants. This gives an insight into the initial reactions to early colonizing bacteria within mucosal lymphoid tissue. Bacterial translocation within the appendiceal mucosa was identified in all specimens from infants aged over 2 weeks, with whole bacteria identified beneath follicular epithelium, within follicles, and in efferent lymphatics. Few lymphoid follicles were present at birth, increasing rapidly upon colonization, with germinal centers identifiable by 4 weeks. IgM plasma cells increased rapidly from 2 weeks, declining from 6 weeks as IgA plasma cells began to dominate, reaching their peak at around 10 weeks.
It is particularly important to recognize that the gut is an organ of huge evolutionary longevity—indeed well-developed gastrointestinal tracts can be identified in fossils of organisms from the Cambrian period 600 million years ago. Thus immunological tolerance of gut luminal contents must have been established prior to the development of any adaptive immune responses. Many products of innate immunity, in addition to defensins, including C-type lectins, surfactants, and cathelicidins, contribute to shape the host’s immune response to the flora and indeed the composition of the flora itself. ,
Several cells of innate immune lineage play roles in presenting antigens to lymphocytes of the adaptive immune system. Their own pattern of activation helps to shape subsequent adaptive immune responses. Professional antigen-presenting cells, such as dendritic cells, macrophages, and B cells, can efficiently take up antigen (by phagocytosis or specific receptor-mediated uptake) and then present fragments of that antigen complexed with class II MHC molecules to naïve T cells. , The consequent antigen-specific T cell response will be shaped by both expression of co stimulatory molecules and secretion of cytokines by the antigen-presenting cell.
Dendritic cells are the most efficient activators of T cells because of their constitutive expression of co stimulatory molecules such as B7-1 (CD80) or B7-2 (CD86). There is important functional heterogeneity within populations of professional antigen-presenting cells—thus both dendritic cells and macrophages may exist as locally adapted resident populations or represent recently recruited cells, derived from bone marrow, which exhibit a more pro inflammatory phenotype. Such local adaptation is one of the key mechanisms underpinning the maintenance of immune tolerance within the intestine.
The intestinal epithelium can contribute to antigen presentation, processing ingested antigen and presenting using both classical and non classical MHC molecules. However, the enterocyte does not express co stimulatory molecules such as B7-1 or B7-2, so this form of antigen presentation does not activate lymphocytes but may render them anergic—incapable of proliferation and activation.
Other cell types in the intestinal mucosa may act as non professional antigen-presenting cells, including fibroblasts and vascular endothelial cells. Their interactions with lymphocytes may become functionally important in inflammatory states but are unlikely to play a role in the normal maintenance of immune tolerance. This review will thus focus first on the primary interactions between innate and adaptive immune cells in establishing and maintaining tolerance to dietary antigens and the enteric flora.
Dendritic cells play a central role in the maintenance of immunological tolerance within the intestine through their primary role of taking up antigens and presenting them to lymphocytes. They provide an important means of sampling luminal contents—both microbial and dietary in origin. Three distinct mechanisms have been identified by which such sampling may be affected.
Firstly, dendritic cells cluster in the subepithelial region of organized lymphoid follicles, such as Peyer’s patches. Specialized epithelial cells in the surface epithelium, so-called microfold or M-cells, are much more permeable to luminal antigens than are normal epithelial cells. Such focal epithelial leakiness allows ingress of luminal antigens of all kinds. However, there may be some specificity in uptake, as M cells express the lectin glycoprotein-2, which allows selective adherence and uptake of fimbriated bacteria. Bacterial or dietary components crossing the M cells are then taken up in turn by dendritic cells. These may present processed antigen to T cells within the local area of the lymphoid follicle (see Fig. 5.1 ). In addition, it has been demonstrated that dendritic cells in Peyer patches may phagocytose live bacteria that have penetrated through M cells and may then migrate to the regional draining mesenteric lymph nodes (see Fig. 5.1 ). It is here that fundamental adaptive immune responses may occur, including generation of antigen-specific IgA and regulatory T cells (see Fig. 5.1 ). ,
Secondly, it is now known that subepithelial dendritic cells, situated away from organized lymphoid follicles, may insinuate processes between adjacent enterocytes to sample luminal contents (see Fig. 5.1 ). , This appears to be a coordinated mechanism, involving induced focal breakdown of the mechanisms that normally maintain tight junction integrity.
Thirdly, antigen may be transported through the enterocyte following uptake either by IgG, which is shuttled back and forth across the epithelium by the neonatal Fc receptor for IgG, or by IgE, which is taken up by induced luminal expression of the low affinity IgE receptor CD23 (see Fig. 5.2 ). , This antibody-mediated uptake will thus be antigen-specific, rather than the less selective uptake across Peyer patches or by intraluminal dendritic cell sampling.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here