Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In 1867 Theodor Langhans reported the first description of a carcinoid tumor in a 50-year-old woman with a mushroom-shaped small bowel tumor that contained nesting glandular structures within a fibrous stroma. Otto Lubarsch subsequently described autopsy findings of two patients with ileal tumors in 1888, making the astute observation that they were unlikely to be carcinomas. Soon thereafter, William Ransom described the first case of carcinoid syndrome in a 50-year-old woman with severe diarrhea and attacks of wheezing, whose autopsy revealed multiple ileal and hepatic tumors. Like Lubarsch, he commented upon their peculiar biologic behavior and noted that these tumors appeared to “demonstrate very slight, local malignancy.”
Siegfried Oberndorfer coined the term karzinoide (carcinoid, meaning carcinoma-like) in 1907 during a presentation at the German Pathological Society. He emphasized salient differences between carcinomas of the ileocecal junction and those more benign-behaving and often multifocal tumors of the ileum. Although initially he viewed these as benign, he later came to realize that these tumors could have malignant phenotypes and the ability to metastasize.
In 1914 Gosset and Masson suggested that carcinoid tumors might arise in the Kulchitsky cells in the glands of Lieberkühn. The serum vasoconstrictor serotonin (5-hydroxytryptamine) was isolated and named by Rapport in 1948. Two years later, it was determined that the Kulchitsky cell produced serotonin. Lembeck soon thereafter confirmed that serotonin was produced by an ileal carcinoid and was responsible for the carcinoid syndrome.
In contrast to many other malignancies, the story of carcinoid tumors is unfinished. The first tumor-node-metastasis (TNM) staging system for organ-specific neuroendocrine tumors (NETs) was not published until 2010, and the discussion of the nomenclature surrounding these enigmatic tumors currently continues. There continue to be unresolved issues in our understanding of their pathobiology, which will be resolved in the future.
The neuroendocrine system consists of a glandular (solid) system and a diffuse system (DNES). The glandular system is composed of the pituitary, parathyroids, paraganglia, and adrenal medulla. The diffuse system comprises cells dispersed throughout the skin, thyroid, lung, thymus, pancreas, gastrointestinal (GI) tract, biliary tree, and the urogenital system. The multiplicity of neuroendocrine cells in the GI tract makes it the largest single endocrine organ.
A characteristic feature of neuroendocrine cells is the production of a diversity of biogenic amines, peptides, prostaglandins, and tachykinins. These secretory products are stored in both large dense-core vesicles and small synaptic-like vesicles. Among other functions, it appears that the protein, chromogranin A (CgA), is essential in the genesis of vesicles and regulates the biogenesis of the dense-core granules. Such granules are then secreted under control of a complex variety of stimuli. The type of secretory granule produced depends in part on the cell type. Currently there are 16 different types of endocrine cells, producing more than 100 secretory products, that have been described. Although some of these cell types are present throughout the GI tract, others have a more restricted topography ( Table 80.1 ). Some cell types are located in one organ and produce specific products that result in well-described clinical syndromes (e.g., beta cells in the pancreas and insulinomas), whereas others produce a spectrum of disease states and are in a variety of locations (e.g., enterochromaffin [EC] cells and somatostatin in the stomach, small bowel, and colon). Curiously, the common nonfunctional NET, frequently found in the pancreas, has no clear cell of origin and no obvious secretory product.
| Cell Type | Primary Location | Secondary Locations | Product | Tumor Type |
|---|---|---|---|---|
| A | Pancreas | Glucagon | Glucagonoma | |
| B | Pancreas | Insulin | Insulinoma | |
| D | Stomach, jejunum, pancreas | Ileum, appendix, colon, rectum | Somatostatin | Somatostatinoma |
| EC | Stomach, jejunum, ileum, appendix, colon | Rectum, pancreas | Serotonin | Carcinoid |
| ECL | Stomach | Histamine | Gastric carcinoid | |
| G | Stomach (antrum) | Gastrin | Gastrinoma | |
| I | Duodenum, jejunum | Ileum, rectum | Cholecystokinin | CCKoma |
| L | Small intestine | Glucagon-like peptide, peptide YY, neuropeptide Y | Neuroendocrine tumor NOS | |
| N | Jejunum, ileum | Duodenum | Neurotensin | |
| PP | Pancreas | Pancreatic polypeptide | PPoma | |
| S | Duodenum, jejunum | Secretin | ||
| VIP | Pancreas | Stomach, small bowel, colon, rectum | Vasoactive intestinal polypeptide | VIPoma |
| Unknown | Pancreas | Small bowel | None known | Nonfunctional NET |
There has been significant debate and misunderstanding about the developmental origin of gut neuroendocrine cells. Gut endocrine cells have many similarities to neural cells—they produce substances with transmitter functions, have secretory granules, and have similar cellular antigens (synaptophysin and neuron-specific enolase). For this reason, it was initially postulated that they were of neuroectodermal origin and were thus initially termed neuro endocrine cells. However, it has since been convincingly demonstrated that these cells come not from the ectoderm but rather have endodermal origins. Furthermore, it has been demonstrated more recently that all four intestinal epithelial cell types (enteroendocrine cells, goblet cells, Paneth cells, and enterocytes) differentiate from common pluripotent stem cells in the intestinal crypts. These enteroendocrine cells renew themselves from a large reservoir of stem cells and continue the process of differentiation throughout life. The regulatory controls for this process of enteroendocrine cell differentiation are under active investigation and are incompletely understood. Interestingly, it appears that the regulation of differentiation of enteroendocrine cells is similar to that for cells within the nervous system. Both cell types are controlled by similar genes encoding basic helix-loop-helix (bHLH) transcription factors under the control of the Notch signaling pathway, which may have potential therapeutic implications for NETs.
The term carcinoid was coined before there was a full understanding of the tumor type. However, this term has remained in use clinically and is in part responsible for the confusion surrounding the terminology of NETs. First, the term neuroendocrine is not entirely accurate. As described previously, this term was used because it was assumed these cells derived from the neuroectoderm. Because it has since been shown that this is not the case, many have suggested the term be abandoned in favor of enteroendocrine . However, because there are many shared structural and regulatory properties between neural cells and endocrine cells, the term neuroendocrine tumor, or NET, seems appropriate, and most international groups have accepted its use.
An older term used for these enteroendocrine tumors was APUDoma (amine precursor uptake and decarboxylation), which describes a common biologic function of the cell type. These cells also have been referred to as EC or argentaffin cells because of their staining affinity for chromium and silver salts, respectively. Furthermore, those cells that required a reducing agent to stain with silver salts were termed argyrophilic cells. Those tumors that produce an active product are often referred to by the specific term for each product (glucagonoma, insulinoma, etc.) or by the term for the group that produces these products most often—islet cell tumors.
The number of terms in use for this cell type and their respective tumor types has served to generate significant confusion in the field. In fact, a group of experts participating in a summit of the National Cancer Institute identified this as one of the major hurdles to progress and commented “semantic issues continue to obfuscate the field.” For example, the term carcinoid unfortunately continues to give the inaccurate impression of the relative benignity of these lesions although many do not behave as such. The more accurate term for this group of tumors currently advocated by the World Health Organization (WHO), the European Neuroendocrine Tumor Society (ENETS), and the North American Neuroendocrine Tumor Society (NANETS) is gastroenteropancreatic neuroendocrine tumors (GEP-NETs). However, the term carcinoid continues to be in use and is best understood as a synonym for well-differentiated neuroendocrine neoplasms of the luminal GI tract.
The clinical incidence of NETs ranges from approximately 1.3 per 100,000 to 5.25 per 100,000 in a study using the 2004 data from Surveillance, Epidemiology and End Results (SEER). However, detailed autopsy studies have shown that the majority of carcinoid tumors are discovered after death from another cause. A Swedish study, from 1958 to 1969, demonstrated that only 10% of all cases were identified on surgical specimens, with 90% discovered incidentally at autopsy. In fact, 1.2% of people had a carcinoid tumor at autopsy. A second smaller but more recent autopsy study in Japan demonstrated that a similar fraction (1.6%) of patients had endocrine tumors in their pancreas on random sectioning, but when the pancreas was more carefully examined with serial sections, a full 10% of patients had endocrine tumors.
Multiple studies have documented a significantly increasing incidence of NETs over the past several decades, with an estimated 3% to 10% increase per year over the past 35 years. The lack of consensus in nomenclature results in differences in classification and incidence reports. Although a fraction of this rise may be related to a true increase in disease frequency, much of this is probably more because of the improvements in clinical awareness of the disease, the improvement in diagnostic and imaging technology, and the changing classification systems. For example, because databases such as SEER included malignant disease only, lower-grade carcinoid tumors were not reported until 1986. Furthermore, the distribution of the primary sites of tumor has changed with time as well. For example, rectal carcinoids represented only 9% of tumors in the early SEER cohort (1973 to 1991) compared with 18% in the later cohort (1992 to 1999). Similarly, 7% of the early cohort had appendiceal carcinoids compared with 2% in the later cohort. These changes could reflect the increase in the use of sigmoidoscopy and the decrease in incidental appendectomies, respectively, rather than a true change in the sites of disease.
Although carcinoid tumors are often considered rare, they are actually the second most prevalent GI cancer, after colorectal cancer. The estimated 29-year limited-duration prevalence of NETs in 2004 was approximately 103,000, making them significantly more common than esophageal, gastric, pancreatic, and hepatobiliary cancers.
The risk factors for the development of sporadic NETs are still unknown. Not surprisingly, the risk factor shown to be most significantly associated with development of a NET is the parental history of a carcinoid tumor in an extrapulmonary site. A similar pattern was seen with history of carcinoid tumor in a sibling. In addition, there was an increase in the incidence of carcinoid tumors in the offspring of parents with cancers of the brain, breast, liver, endocrine system, and urinary tract. This finding of an excess number of cases of carcinoid tumors in offspring of parents with any history of cancer was also demonstrated in a US case-control series. In addition, this study demonstrated that a long-term history of diabetes mellitus was a risk factor for the development of gastric carcinoids, especially in women. For environmental exposures, the bulk of the evidence suggests that there is not a strong association with cigarette smoking. A large European population-based case-control study suggested an increased risk of small bowel carcinoid with occupational exposure to organic solvents and rust-preventive paint containing lead, although more evidence is needed.
Although little is known about the risk of sporadic NETs, there are four well-established genetic disorders that are strongly associated with the development of pancreatic neuroendocrine tumors (PNETs). The most recognized is multiple endocrine neoplasia type 1 (MEN1), which is associated with PNETs in approximately 65% of cases; 7% have gastric carcinoids. The PNETs in MEN1 patients are most commonly nonfunctional, but gastrinomas and insulinomas are frequently reported. The treatment strategies in these patients are complicated given the multifocality of pancreatic tumors and the need to balance a morbid operation against the potential for benefit in the context of multiple other competing medical issues.
The second genetic disorder known to be associated with PNETs is the von Hippel-Lindau (VHL) syndrome, caused by a mutation in the VHL tumor suppressor gene. Pancreatic cysts and tumors develop in the majority of patients. PNETs develop in approximately 15% of patients with VHL, are usually nonfunctional, and asymptomatic. In general, the tumors in VHL patients show indolent growth and rarely metastasize if less than 3 cm in size. For these reasons, many authors recommend routine resection only when tumors are larger than 3 cm. The third genetic disorder associated with PNETs is von Recklinghausen syndrome (neurofibromatosis 1). These patients are affected in only 10% of cases, with nearly all patients having a duodenal somatostatinoma. These generally are large periampullary tumors and generally require resection to treat local complications. The final genetic association with PNETs is tuberous sclerosis. Only a small fraction of these patients are affected, and both functional and nonfunctional tumors occur.
A large part of the difficulty in arriving at a standard classification scheme for GEP-NETs has been their widely variable biologic behavior coupled with their locations throughout the GI tract. To further complicate the situation, until recently the WHO classification for NETs used a system that incorporated both grading and staging information in a single system. Although it provided some prognostic information, this schema did not allow for more advanced tumor stages with metastatic disease to benefit from the significant amount of clinical information that derives from grade alone. The 2000 version of the WHO classification used the terms well-differentiated NET with benign behavior , well-differentiated NET with uncertain behavior , well-differentiated NE carcinoma with low-grade malignancy , and poorly differentiated NE carcinoma . In contrast, the 2004 version described well-differentiated endocrine tumor , well-differentiated neuroendocrine carcinoma , and poorly differentiated carcinoma . These terms are still encountered in pathology reports because the protocols for NET specimen examination published from the College of American Pathologists in 2010 still advocate the use of these earlier staging systems.
The most important distinction for classifying NETs is to separate those that are well differentiated from those that are poorly differentiated. This degree of differentiation is reflected by tumor grade. In general, well-differentiated tumors include low and intermediate grades, whereas poorly differentiated tumors are high grade. The grading scheme, now accepted by the WHO, ENETS, and NANETS, is determined by markers of the tumor's proliferative index: the mitotic rate and the Ki67 labeling index ( Table 80.2 ). The mitotic rate is reported as the number of mitoses per 10 high-power fields (HPFs). The Ki67 antigen is an important marker of cellular proliferation and mitotic activity and is detected in all phases of the cell cycle except G0. A monoclonal antibody (MIB-1) that binds to the Ki67 nuclear antigen is used to estimate the percentage binding to 2000 cells in the area of highest nuclear activity.
| Grade | Differentiation | Descriptor | Mitotic Count (Per 10 High-Power Field) | Ki67 Index (%) | |
|---|---|---|---|---|---|
| Low grade | Well differentiated | Neuroendocrine tumor OR neoplasm | <2 | AND | ≤2 |
| Intermediate grade | Well differentiated | Neuroendocrine tumor OR neoplasm | 2–20 | OR | 3–20 |
| High grade | Poorly differentiated | Neuroendocrine carcinoma small or large cell | >20 | OR | >20 |
No formal TNM staging system existed for NETs until the American Joint Committee on Cancer (AJCC) published one for each anatomic site in 2010, in parallel to the TNM system published under the Union for International Cancer Control (UICC). Prior to this publication, ENETS had made recommendations for a staging system, already in use in Europe. These two systems have considerable overlap but there are important differences as well, particularly for pancreatic and appendiceal primaries. For the AJCC system in general, stage I disease includes small tumors (≤1 cm for small bowel and stomach; ≤2 cm for colon and appendix) without invasion beyond the submucosa. Stage II disease includes local disease only but with larger tumors and deeper depth of invasion. Stage III disease includes advanced local disease with T4 tumors (those that have penetrated the serosa in gastric and small bowel tumors; those that are growing into nearby structures in appendiceal and colon tumors) and locoregional tumors with nodal disease. Stage IV disease requires evidence of distant metastasis. The College of American Pathologists protocols recommend continued use of the older classification schemes and terminology on clinical pathology reports.
The carcinoid syndrome is a well-described constellation of symptoms that results from an excess of the neurohumoral factors released by some NETs. Symptomatically, it is characterized by episodic flushing, which primarily involves the face and torso, lasting only minutes. It is often described as having a dark red to purple hue and can be accompanied by a burning sensation of the skin. These episodes can include bronchospastic symptoms of wheezing and dyspnea. Another characteristic and more debilitating symptom is the presence of a secretory diarrhea and associated abdominal pain and cramping.
From a mechanistic standpoint, these symptoms result in part from serotonin excess that occurs from the conversion of dietary tryptophan to serotonin and subsequently 5-hydroxyindoleacetic acid (5-HIAA). Excess serotonin results in hypoalbuminemia and nicotinic acid deficiency from an effective tryptophan deficiency, diarrhea as a result of stimulation of intestinal motility and secretion, and fibrotic processes in the bowel mesentery and the cardiac valves. In addition, there is an excess of histamine production that can lead to flushing and pruritus. Excessive bradykinin production can lead to vasodilation and flushing, and other polypeptides, such as neurokinin A, may contribute to both flushing and diarrhea. The carcinoid syndrome requires the presence of liver metastases as the portal venous circulation permits the liver to clear the bioactive products before they can arrive in the systemic circulation, although it has been described in patients with retroperitoneal invasion.
Although the carcinoid syndrome is a dramatic and well-known presentation of NETs, it is uncommon and occurs in only 10% to 20% of patients. The most common presentations of GI NETs are periodic abdominal pain and cramping, intermittent small bowel obstruction, and GI bleeding. Up to one-third of patients are asymptomatic and have their tumor detected incidentally. The most difficult group of patients and those with the longest delay in diagnosis are those who present with more protean symptoms, such as psychiatric disorders, depression, food allergy, lactose intolerance, and menopausal symptoms. It is the very nature of carcinoid disease that makes it so difficult to diagnose, namely, that it is located in the segment of the GI tract that is not easily accessed endoscopically, that it is submucosal in nature and grows extrinsically, and that it causes symptoms that may not be easily referable to the abdomen, such as flushing. The most important aspect of making the diagnosis in these cases is the suspicion of its presence, without which the patient will have delays in diagnosis and treatment.
In the evaluation of the patient with a suspected carcinoid tumor, it is essential to consider the differential diagnosis so as not to miss other more common alternative diagnoses. This should include considering infectious causes (such as appendicitis or terminal ileitis), vascular causes (mesenteric ischemia or vasculitis), mechanical causes (adhesions, volvulus), neoplastic causes (adenocarcinoma, lymphoma), and inflammatory causes (Crohn disease, celiac disease). Other causes of flushing should also be considered and evaluated when this is a predominant symptom. These include such diverse etiologies as renal cell carcinoma, mastocytosis, panic attack, menopause, autonomic neuropathy, medications, and pheochromocytoma.
In patients in whom other diagnoses have been ruled out or those in whom the likelihood of a GEP-NET seems particularly high, we proceed first with measurement of a 24-hour urine sample for 5-HIAA. This has a sensitivity of 75% and specificity of more than 90%, but it requires adequate preparation to achieve these statistics. Specifically, foods with high tryptophan content, such as avocados, bananas, and walnuts, are proscribed. Some medications can disrupt the test and lead to either falsely high (e.g., acetaminophen, guaifenesin, nicotine) or falsely low (e.g., heparin, aspirin, isoniazid) values. Measurement of urine 5-HIAA is useful mainly for midgut tumors and is much less helpful for tumors from the foregut and hindgut.
The measurement of serum CgA is a useful test in patients with suspected NETs. This can be elevated in both functional and nonfunctional tumors, and its sensitivity varies according to the NET site and tumor burden. This test is particularly sensitive to the assay technique, and it is advised that serial measurements be performed. In addition, there are many nonneoplastic causes of elevated CgA that should be considered, including impaired renal and hepatic function, hypergastrinemia secondary to proton pump inhibitors or atrophic gastritis, and inflammatory bowel disease.
Blood serotonin measurements can also be useful in making the diagnosis of a GEP-NET, particularly when the urinary 5-HIAA yields equivocal results. Other pancreatic peptides should be measured as indicated by the clinical picture, including gastrin, vasoactive intestinal peptide, somatostatin, insulin, and glucagon. Other tests that can be helpful in the diagnosis, in rare cases, include pancreatic polypeptide, neuron-specific enolase, pancreastatin, and neurokinin A.
If clinically suspected, a gastric, duodenal, or rectal carcinoid is best evaluated endoscopically, both with standard techniques and endoscopic ultrasound. However, standard axial imaging with computed tomography (CT) and magnetic resonance imaging (MRI) remains the mainstay of the evaluation of intraabdominal complaints. Although routine abdominopelvic CT imaging may detect large NETs, it is much less sensitive for the detection of small bowel luminal lesions. However, this can be improved by performing CT enterography, magnetic resonance (MR) enterography, or enteroclysis. The value of CT and MRI is that they can simultaneously detect hepatic metastases, examine regional lymph node basins, and specify the mesenteric vasculature that is often involved ( Fig. 80.1 ). Their ability to detect PNETs is also excellent, with hypervascular masses often being seen best on arterial phase imaging. For these reasons, contrast-enhanced CT scan is the imaging modality of choice for the majority of intraabdominal NETs.
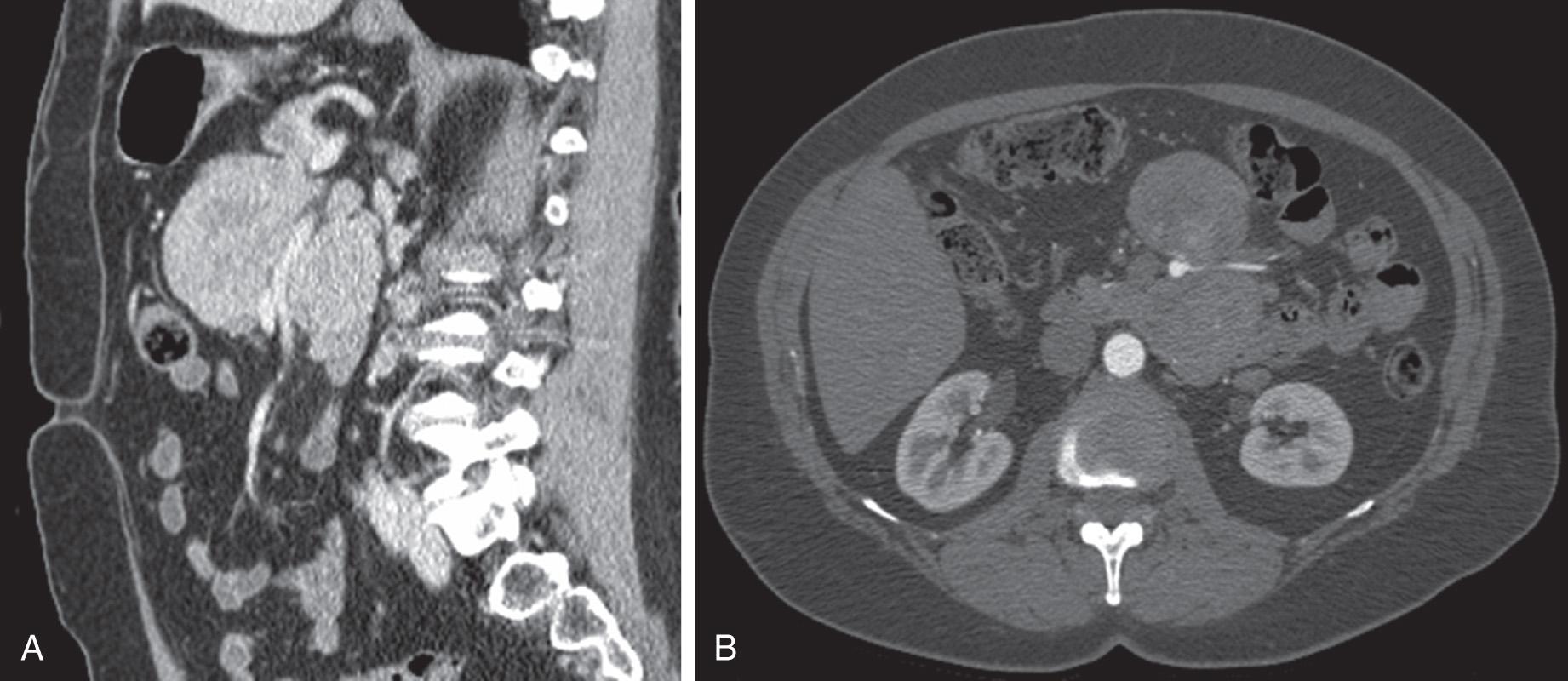
Somatostatin receptor scintigraphy (Octreoscan) involves the use of 111 In–diethylenetriaminepentaacetic acid (DTPA)-labeled octreotide to locate small tumors that overexpress somatostatin receptors (particularly subtypes 2 and 5). This is most useful for PNETs, with the exception of insulinomas. This has more recently been coupled with CT imaging to provide improved localization of areas of uptake. It is thought to be the most sensitive imaging modality for detecting metastatic disease. Other imaging modalities to consider include the use of intraoperative or endoscopic ultrasound for difficult-to-locate pancreatic lesions.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here