Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pelvic floor disorders are an underdiagnosed source of morbidity and significantly diminish the quality of life. Most patients are women. In any clinic that deals with pelvic floor disorders, about 80% of patients will be women between the ages of 35 and 50 years. Pelvic floor disorders are seen less frequently in men.
The demand for pelvic floor imaging is increasing. There are several factors that account for this, such as more patients seeking assistance, more physicians interested in this area, and more experience in the therapeutic options. Almost 24% of women in the United States complain of at least one pelvic disorder. The number of patients seeking assistance also increases with age, parity, and obesity. , As the population ages, the need for pelvic floor imaging is expected to increase.
The presenting symptoms of patients with pelvic floor disorders are variable. They can have anal incontinence, urinary incontinence and constipation. There may be prolapse of the rectum or vagina with a cystocele associated with any of these.
Anal incontinence ranges from constant soiling as a result of slow leakage, to the total inability to hold on to any stool in the rectosigmoid area. This latter group copes by emptying their bowel at the faintest desire to defecate. They are therefore constantly emptying their bowel to avoid accidents. Overall, about 9% of women have fecal incontinence and 3% experience pelvic floor prolapse. These signs and symptoms appear to be unrelated to the mode of delivery. However, parous women are not immune to functional pelvic floor disorders either.
The presenting symptoms of constipation are also variable. This may be described as difficulty in initiating defecation, incomplete defecation, and considerable straining with defecation. Some patients may also complain that the stool is of inadequate size, weight, or water content. Patients who have difficulty in evacuation may use digital or positional maneuvers to assist in emptying the rectum. If this is not volunteered by the patient, it is useful to ask when taking a history prior to the examination because this information could be used to enhance the study.
Seemingly unrelated symptoms, such as bloating and abdominal pain, may also be related to pelvic floor disorders. Other apparently unrelated symptoms include dyspareunia, painful defecation, perineal pain, and rectal bleeding. Solitary rectal ulcer syndrome is often suspected when there is rectal bleeding.
Preoperative identification of abnormalities causing pelvic floor disorder symptoms is important so that the appropriate surgery can be performed. This is especially true because one study has shown that there is a lifetime risk of 11% of at least one surgical procedure for pelvic organ prolapse and urinary incontinence and that the repeat surgery for recurrence is 29%.
There is often a multispecialty involvement in the management of pelvic floor disorders. Radiologists, colorectal surgeons, gynecologists, and urologists may be involved. This chapter will only deal with the radiologic aspects.
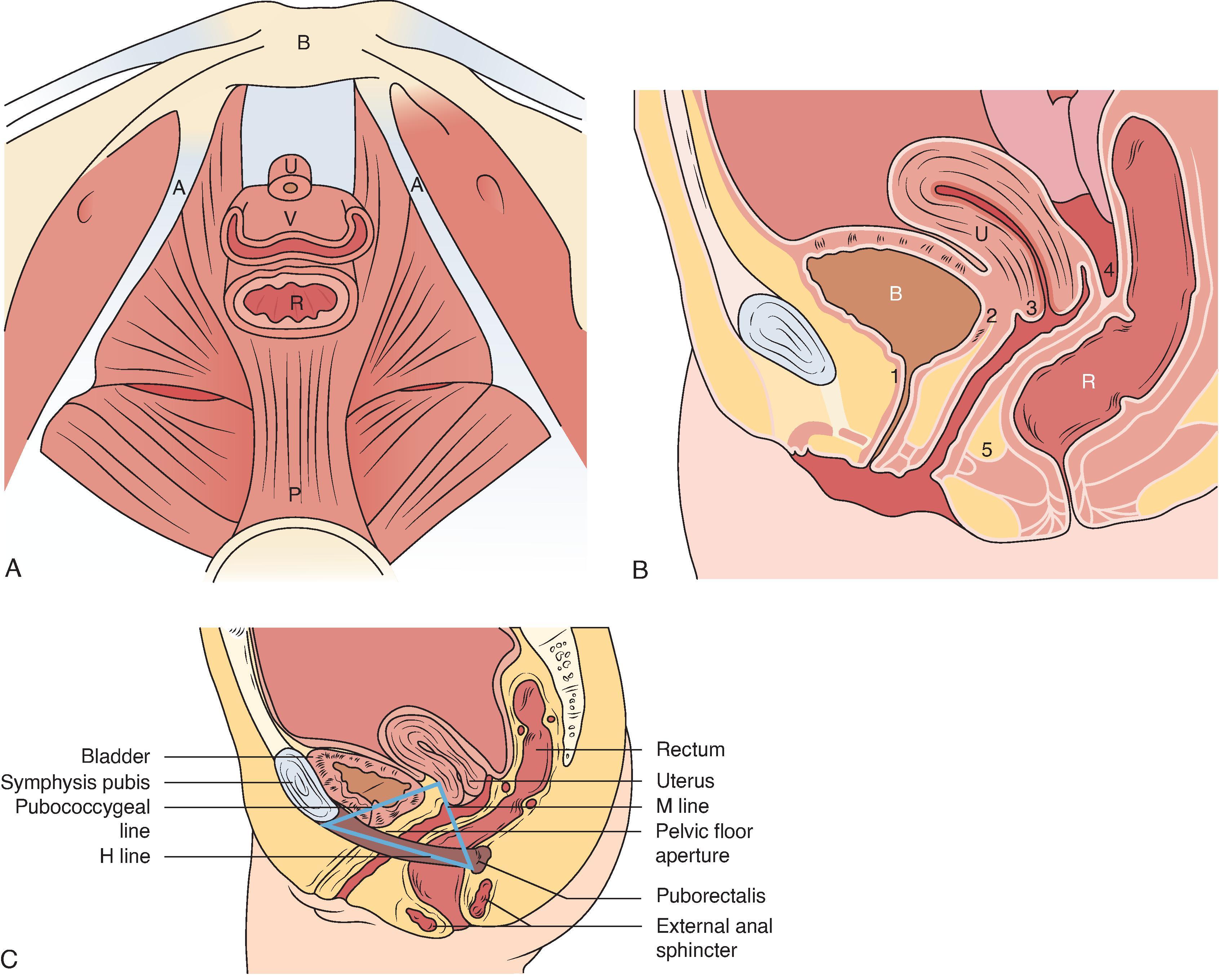
The pelvic floor comprises the levator ani, urinary and anal sphincter mechanisms, and fascial supports of the pelvic viscera. The pelvic viscera include the rectum, vagina, bladder, and urethra ( Fig. 37.1 ). An understanding of the structure and levels of pelvic floor support is important for the evaluation of pelvic floor dysfunction because this forms the basis of reconstructive surgery.
There is an interaction between the colon and rectum in normal defecation. High-amplitude propagating waves that move fecal contents into the rectum stimulate the urge to defecate. As a result, the distention that this produces relaxes the internal sphincter through the anorectal inhibitory reflex in preparation for defecation. Defecation is then initiated by abdominal straining and voluntary pelvic floor relaxation whenever desired. Once the anal canal is open, the rectum is squeezed by abdominal contraction. About one-third of the left colon and rectum is emptied in normal physiologic defecation. This is secondary to continued mass colonic contractions and likely additional proximal rectal contractions.
Pelvic floor anatomy is complex, and dynamic cystocolpoproctography (DCP) does not show the anatomic details that pelvic magnetic resonance imaging (MRI) provides. This consideration, predictions of a hypothetical increase in cancer incidence, and deaths in patients exposed to radiation and questions relating to the clinical significance of DCP findings have made pelvic floor MRI an important preoperative tool.
Several studies have compared CP and dynamic pelvic floor MRI. A recent evidence-based comparison has shown that DCP is the functional imaging study for anorectal dysfunction and pelvic floor prolapse evaluation. This is because, in most existing MR units, the patient is imaged supine rather than upright, a deficiency negated by open architecture MR magnets. This is an embarrassing examination and is made less acceptable when the patient is asked to defecate in an artificial environment and in an unnatural (supine) position where the patient is usually asymptomatic. Evacuation is pivotal for the functional evaluation of the pelvic floor. Additional important factors are economics and practicality in everyday practice. In most institutions, the additional expense incurred with MRI compared with DCP and relative lack of accessible time on an MRI unit that is subject to heavy demand by other clinical specialties are important considerations. The logistics of performing a tailored examination (drainage of an undrained bladder and emptying of rectoceles) are important practical considerations when evaluating for the full extent of pelvic organ prolapse.
The use of an open MRI system with patients defecating makes it functional. With an open architecture magnet, however, one must contend with images of a lower signal-to-noise ratio and soft tissue resolution. To make it a single noninvasive functional study to look at specific organ prolapses, specialized coils are needed to improve soft tissue resolution and visualize the pelvic supporting structures and fascial condensations directly. Specialized coils make dynamic pelvic MRI more intrusive. Dynamic pelvic floor MRI is an evolving technology, and its precise role in functional imaging of the pelvic floor still remains to be determined. It is likely that pelvic MRI with increased soft tissue resolution with endoluminal coils will complement the use of DCP when it is necessary to see structural details of the pelvic supportive tissues and endopelvic fascia for surgical management. A static, high-definition MRI scan gives added information about muscle and/or ligamentous tears that may alter management, information than can only be inferred with DCP.
Defecation is initiated by descent of the pelvic floor. This can be seen on proctography as the anorectal junction descending from the resting position. As descent continues, the anal canal opens in the final moments. All this happens within seconds. Rapid emptying occurs when the anal canal is completely open. With the cessation of straining, tone returns to the anal sphincter and levator ani and the canal closes. The anorectal angle becomes more acute and the pelvic floor and anorectal junction return to their normal elevated resting position (postdefecation reflex; Fig. 37.2 ).
The pelvic floor is unlike other skeletal muscles. As a result of continuous firing of motor units, even during rest, the pelvic floor has constant tone. This tone is only interrupted during defecation or urination. Therefore, a dynamic functional examination of the pelvic floor, especially to show the degree of prolapse, has to include defecation.
As with any procedure, it is extremely important to obtain a history of the patient’s symptoms. Specific questions may have to be asked, such as the use of any maneuvers that may assist the patient. This is not always volunteered because the patient may be too embarrassed to disclose them. Also, this is very intrusive, so the examination must be thoroughly explained prior to anything being done. This ensures that the patient understands the instructions given during the procedure. Getting the patient to practice some of the maneuvers, such as squeezing the buttocks while sitting on the side of a stretcher prior to instillation of any contrast, is also useful. The patient should have maximum privacy and be as comfortable as possible in the examination room. One way to achieve this is to use a remote-control fluoroscopy unit, turning the lights down low and ensuring that the patient is the only person in the examination room. In our unit, we also have blinds so that the patient cannot see the radiologist operating the remote fluoroscopy unit. Generally, if the patient is comfortable with the environment, the greater the likelihood of an accurate assessment of defecation.
Imaging of defecation was described as early as the 1950s. It was not until the 1980s that Mahieu and colleagues described a technique that interested more radiologists. , This technique has since been further modified with the addition of opacification of the small bowel, urinary bladder, and vagina. The opacification of these structures makes recognition of cystoceles, enteroceles, and sigmoidoceles easier. Preparation of the bowel with laxatives or enemas is not necessary, although some authors believe that a limited bowel preparation will be more comfortable for the patient and also provide a more standardized examination. The order of opacification is to give oral contrast first to opacify the small bowel. Usually, about 400 mL of contrast used for small bowel studies is sufficient. This is given about 45 to 60 minutes before the dynamic part of the examination. Some authors suggest waiting for up to 3 hours. How long you have to wait depends on how long it takes for the small bowel contrast being used to opacify the pelvic loops of the small bowel.
Opacification of the urinary bladder is next, with water-soluble contrast normally used for cystography. About 150 to 200 mL of contrast is enough to make the bladder recognizable. Care must be taken not to overdistend or it could mask enteroceles. Overdistention is also uncomfortable for the patient.
The vagina is then opacified. This can be done with barium paste, high-density barium, or a thick solution of 50% contraceptive gel and 50% of water-soluble contrast with the highest concentration of iodide. The vaginal contrast is introduced with a bladder irrigation syringe connected to a soft catheter. A pediatric enema catheter works well. The catheter is inserted deep into the vagina and the contrast introduced as the syringe is slowly withdrawn. Care must be taken to ensure that there is sufficient contrast opacifying the whole vagina. In the past, a contrast-soaked tampon was used. However, it was noted that the tampon acted as a strut and did not allow any enteroceles, rectoceles, or sigmoidoceles to become apparent.
Finally, the rectum has to be opacified. It helps to have contrast material that has the consistency of stool or at least close to it. If it is too liquid, even normal patients will find it difficult to hold in the rectum. Currently, there are no commercially made preparations available. The options are to use a thick esophageal paste such as Esobar (Bracco Diagnostics Inc., Monroe Township, NJ, USA) or the thick paste, as suggested by Mahieu and colleagues. , Three 50-mL syringes (150 mL total) of Esobar introduced into the rectum are usually enough to perform the procedure. When the syringes are filled, care should be taken to eliminate air bubbles because trapped air under pressure can cause discomfort to the patient. The syringes are easier to fill if the paste is first warmed by holding the contrast tube under hot water. If the Mahieu preparation is used, it is useful to opacify the rectal wall first with the contrast used for barium enemas. The doughy paste, if prepared as suggested, provides bulk but does not opacify the wall. Therefore, intussusceptions could be missed. The other disadvantage is that because of the consistency, it is difficult to introduce into the rectum. An orthopedic glue gun that is geared makes this thicker paste easier to introduce. To coat the rectal wall and fill the rectum with the Mahieu paste, attach a 50-cm-long and 0.5-inch-wide tube filled with high-density liquid barium to the glue gun, with a wide-bore enema tip at the end so that both contrast agents can be introduced in one maneuver. The rectum is filled until the patient reports a sensation of fullness and a desire to defecate.
Finally, if you wish to measure the anal canal or determine the location of the anus, the surface around the anus should be opacified. This can be done with petroleum jelly opacified with barium. Petroleum jelly, 50 g, with 50 g of barium works well. This is smeared around the anus and along the natal cleft.
When the patient is ready for the study after all the contrast has been introduced, the patient is asked to sit on the defecography commode. The commode design should allow filming in lateral and frontal projections. A sturdy commode also dispels any fears that the patient may have about sitting on it. There are commercially available commodes but you can build one to your own design. A commode designed by Bernier and colleagues has a ruler in the midline so that direct measurements of the midline structures can be done if necessary. If a remote-control fluoroscopy unit is used, the commode can be attached to the table top. Positional changes of the commode can then be easily made by moving the tabletop. A remote-control fluoroscopy unit is certainly preferable for this study.
The x-ray beam is aimed at the region of the reproductive organs. Therefore, keeping the radiation dose as low as possible is paramount. To keep the dose low, several investigators use only videofluoroscopy. Although the dose is low, the resolution is not adequate to record subtle radiographic changes. Therefore, it is recommended that videofluoroscopy be performed during the entire examination, with spot films being taken at key events. The key spot images are lateral views of the rectum at rest, lift (squeeze), cough, strain, defecation, and postdefecation. Other ways of reducing radiation dose include digital serial acquisition with one image (or images) for 30 seconds or using digital equipment with a low-dose program. Major reductions in radiation dose can also be achieved by slow pulsed fluoroscopy. Additional beam filtration can reduce radiation without impairing image quality.
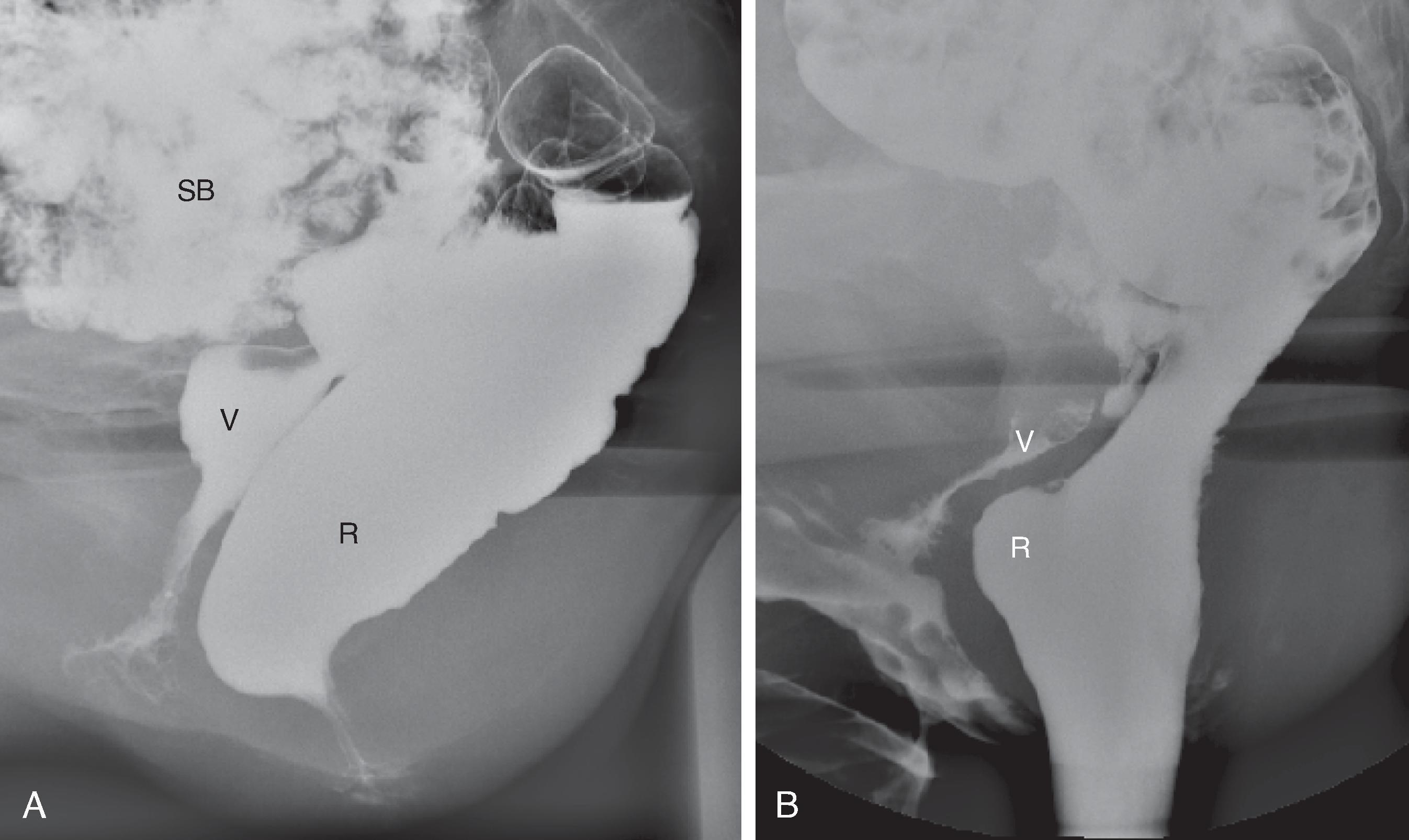
The images at rest are taken without any conscious contraction of the pelvic muscles ( Fig. 37.3A ). On lifting (squeezing), there is maximal contraction of the pelvic floor musculature. This results in elevation of the entire pelvic floor ( Fig. 37.3B ). The muscles responsible for elevation of the pelvic floor are the puborectalis and levator ani. The anorectal angle becomes acute with maximum elevation. The squeeze image demonstrates pelvic floor strength, and impaired movement may reflect puborectalis atrophy. The anal canal simultaneously lengthens. These changes can be measured and are indicators of the continence mechanism. When asked to cough, the patient should produce a powerful cough in the rest position. This action simultaneously increases the intra-abdominal pressure and contraction of the anal and pelvic floor muscles. In normal individuals, there is minimal or no descent of the pelvic floor and total continence ( Fig. 37.3C ). When asked to strain, the patient should strain downward maximally, without evacuating ( Fig. 37.3D ). This maneuver stresses the continence mechanism and provides a measure of pelvic floor descent. The anal canal shortens during straining in most women (mean change, 2 mm). Almost all patients demonstrate some perineal descent, but this is the least reliable portion of the examination because some patients paradoxically contract the pelvic floor muscles, perhaps for fear of incontinence. Straining is supposed to demonstrate the competence of the external anal sphincter.
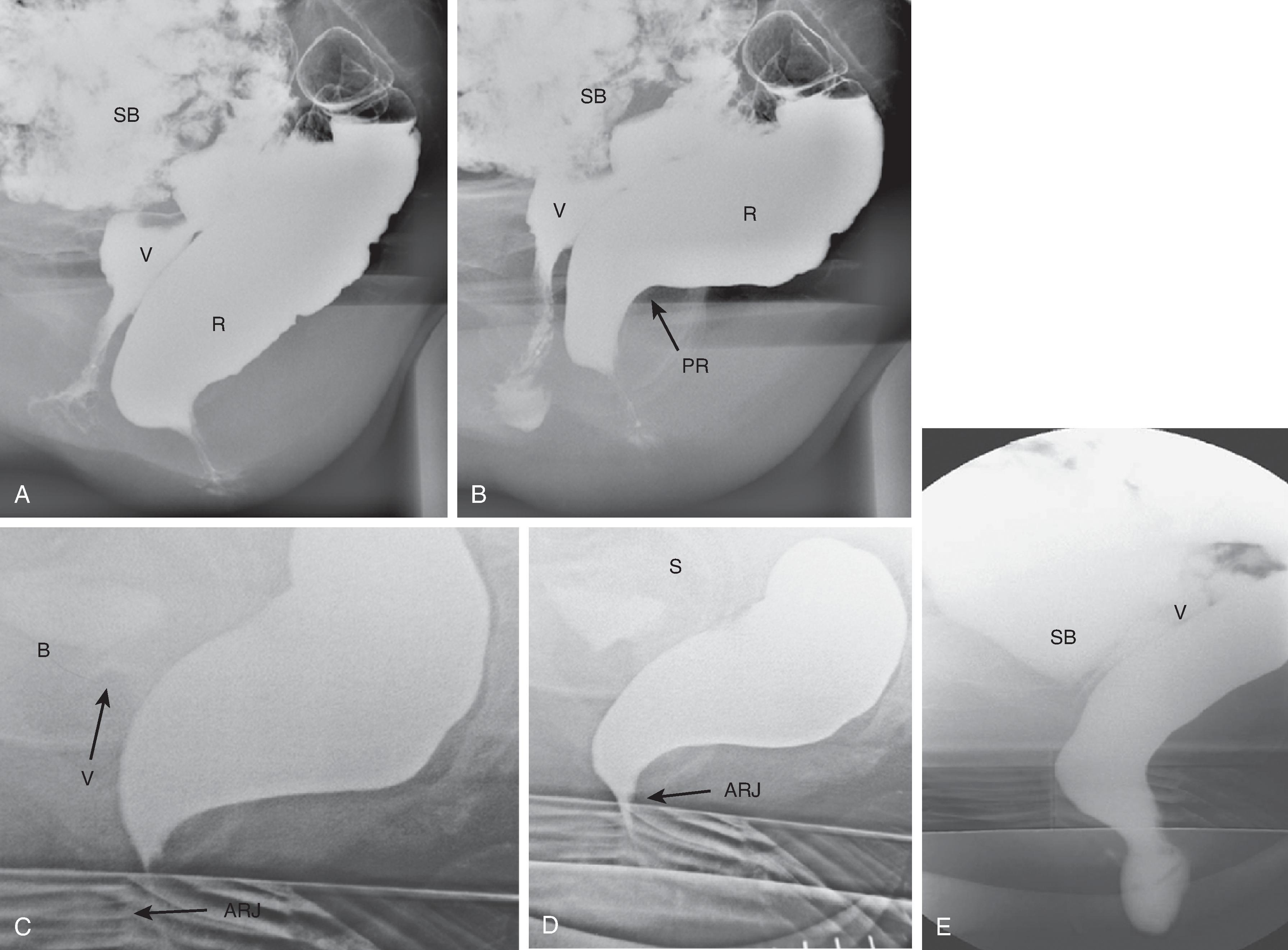
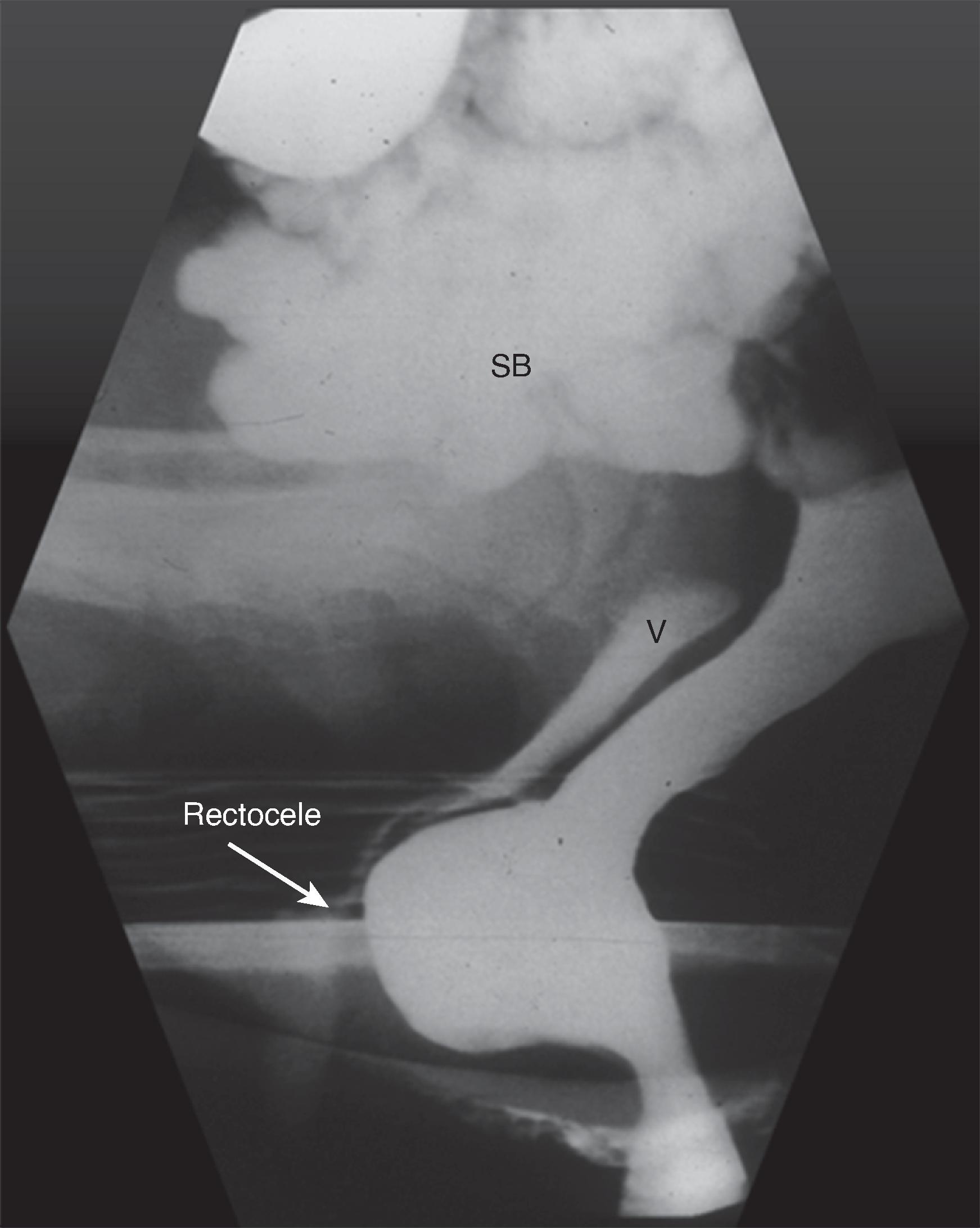
Finally, spot films are obtained during defecation and postdefecation straining ( Fig. 37.3E ). These films give a measurement of pelvic floor descent and an estimate of the completeness of emptying. Morphologic abnormalities such as intussusception and rectoceles are often visualized at this time, and the changes contributing to difficulty in defecation can be analyzed. Rectoceles are outpouchings of the rectum beyond the expected contour of the rectal wall ( Fig. 37.4 ). They are often normal and more common in women. If a rectocele does not empty completely during defecation, it may produce a feeling of incomplete evacuation of the rectum.
Measurements taken from spot films include the anorectal angle, anal canal length, level of the anorectal junction, and descent and elevation of the anorectal junction.
The anorectal angle ( Fig. 37.5 ) is measured between the axis of the anal canal and the tangent to the posterior wall of the rectum. It can be difficult to measure because the posterior wall of the rectum is often not clearly delineated, and the angle becomes highly subjective. Caution should therefore be exercised when assessing its significance. At rest, this angle is usually no greater than 120 degrees in control subjects. However, the angle has a normal range of 70 to 134 degrees, with a mean of about 95 degrees. When the patient squeezes or lifts the buttocks, the anorectal angle decreases to a mean of 19 degrees in women (range, 6 to 26 degrees) and 28 degrees in men (range, 12 to 45 degrees). This angle increases during straining and defecation. The mean anorectal angle during straining in normal young women is 103 degrees, with a range of 75 to 108 degrees. In men, the mean angle is slightly lower, 98 degrees, with a range of 67 to 123 degrees.
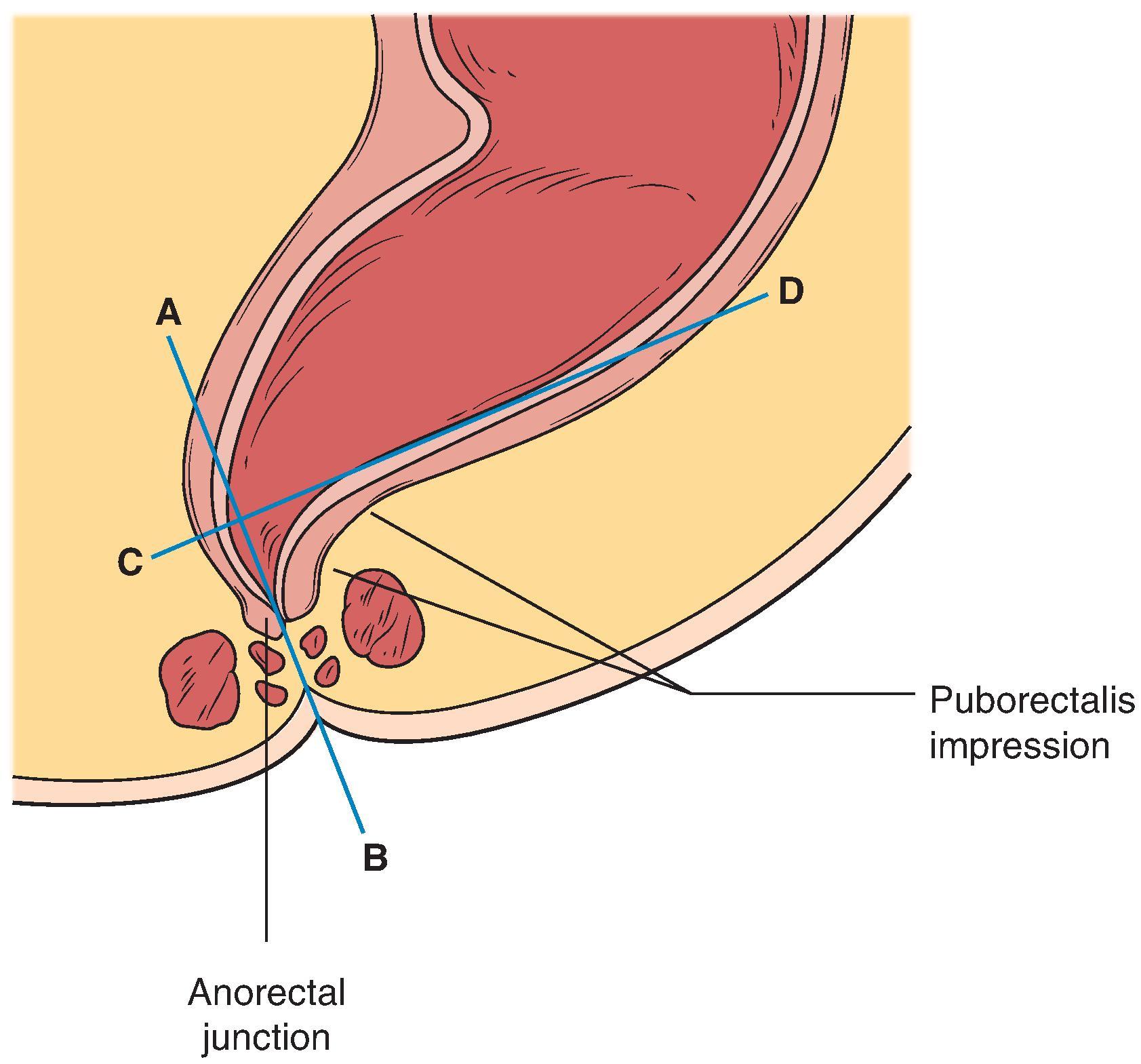
The anal canal length is easily measured. It is the distance traversed by the parallel borders of the anal canal before they form the diverging walls of the distal rectum. At rest, the mean length of the anal canal is 16 mm in women (range, 6 to 26 mm) and 22 mm in men (range, 10 to 38 mm). During lifting (squeezing), the anal canal lengthens to a mean of 19 mm in women (range, 9 to 26 mm) and 28 mm in men (range, 12 to 45 mm). During straining, the canal shortens slightly and has a mean length of 14 mm in young women (range, 6 to 20 mm) and 17 mm in young men (range, 9 to 27 mm).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here