Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This work was supported by grants from NIH (NS075321, NS41509, NS103957, NS075527, NCATS U54TR001456 & Office of Rare Disease Research), American Academy of Neurology & American Brain Foundation Clinical Research Training Fellowship, the Barnes Jewish Hospital Foundation (Elliot Stein Family Fund and Parkinson Disease Research Fund), the Dystonia Medical Research Foundation, the American Parkinson Disease Association Advanced Research Center at Washington University, the Oertli Fund for Fellowship support, the Murphy Fund for Neuroimaging of Dystonia, and the Riney Foundation.
![]() This chapter includes an accompanying lecture presentation that has been prepared by the authors: .
This chapter includes an accompanying lecture presentation that has been prepared by the authors: .
Molecular imaging (positron emission tomography [PET] and single-photon emission computed tomography [SPECT]) and magnetic resonance imaging detection of blood oxygen level–dependent signals (BOLD MRI) are common functional neuroimaging tools that allow measurement of regional brain interactions in an active or resting state.
Functional neuroimaging provides new insights into pathophysiology of movement disorders.
The clinical role of functional neuroimaging remains an evolving and exciting frontier, requiring critical understanding of potential confounders of interpretation.
Potential future applications of functional neuroimaging in movement disorders include:
Improved understanding of structure-function relationships after surgical intervention
Selection of appropriate patients for neurosurgical intervention
Improved targeting of neurosurgical intervention
Ability to locate regions of neurochemical deficits to guide restorative therapeutics
Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) provide tomographic images reflecting the regional brain distribution of a variety of administered radiopharmaceuticals. PET has higher anatomic resolution and better quantification than SPECT, but SPECT is often more accessible and economical. Radioisotopes used for PET are generally relatively short lived, such as oxygen 15 ( 15 O; 2 minutes), carbon 11 ( 11 C; 20 minutes), and fluoride 18 ( 18 F; 110 minutes), and must be quickly incorporated into the desired radiopharmaceuticals, tested for quality measures, and then delivered to the subject. For many radiopharmaceuticals, this requires an on-site cyclotron and radiochemistry production facility, although commercial vendors distribute many 18 F radiopharmaceuticals at lower overhead cost. In contrast, commercial vendors distribute most SPECT radiopharmaceuticals because they incorporate much longer-lived radioisotopes—usually iodine 123 ( 123 I) or technetium 99m ( 99m Tc). This review focuses primarily on PET, but comments on SPECT are included where pertinent.
The key to molecular imaging methods is the specifics of the radiopharmaceutical. Early studies used radiotracers to measure regional glucose or oxygen metabolism. The underlying assumption is that local metabolism or flow reflects local interneuronal activity or inputs into that brain region. These measures identified changes in resting-state metabolism or blood flow that corresponded to specific clinical manifestations of various movement disorders—essentially an in vivo neuroimaging extension of the old clinical-pathologic correlation approach. Most past studies focused on specific regional deficits, but recent emphasis has moved to how specific regions or nodes may contribute to dysfunction of selected networks—for instance, identification of covariance networks (i.e., regions of the brain that have resting functional measures that correlate with one another). Patterns of covariance networks can be related to specific states or behaviors and seem to be a more sensitive means of detecting changes in brain function than limiting the search to specific regional changes.
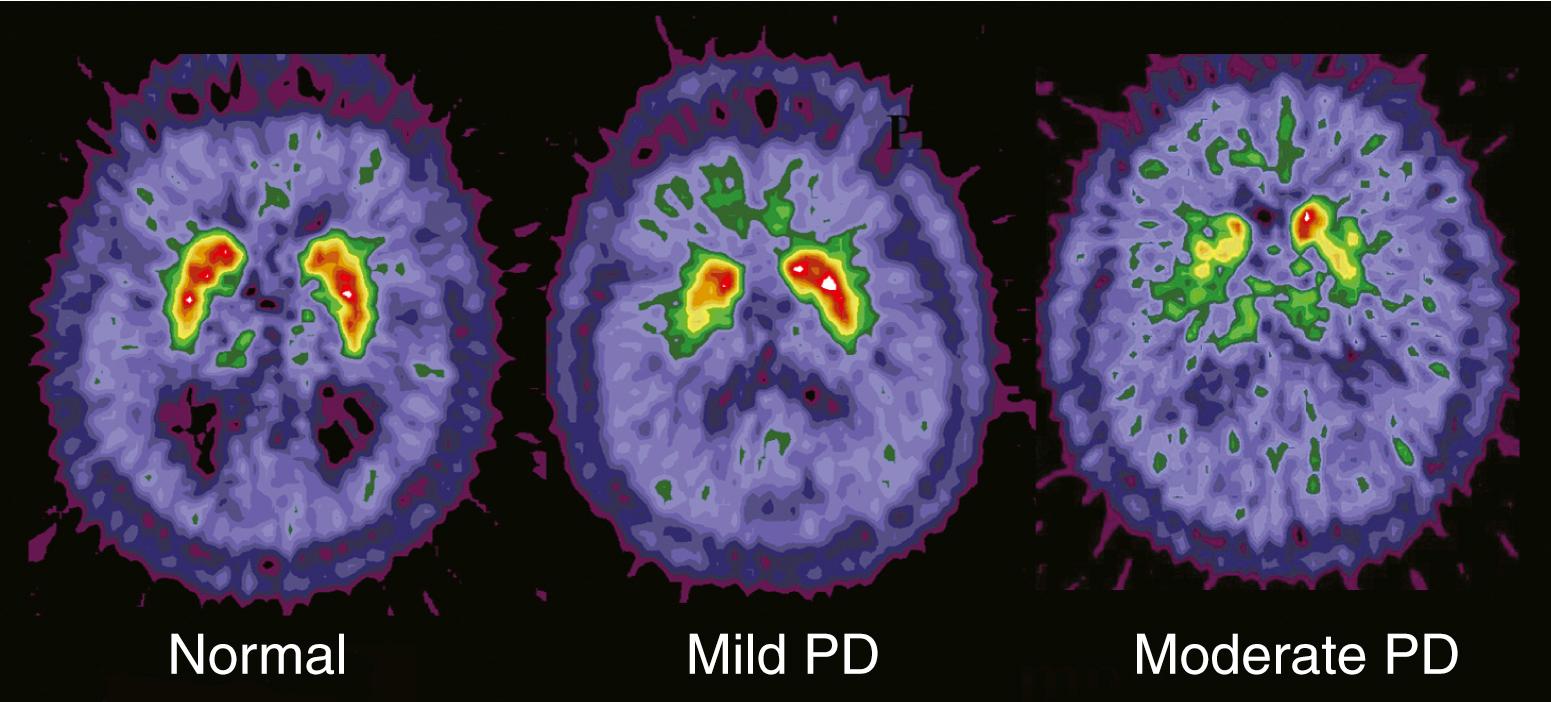
Molecular imaging permits targeting of specific neurotransmitters. For example, radiolabeled levodopa such as 18 F 6-fluorodopa (FD) allows assessment of dopaminergic pathways because this radiotracer passes through the blood-brain barrier and is trapped within the brain after conversion by aromatic acid decarboxylase into 18 F-dopamine, which is a charged molecule that does not pass through the blood-brain barrier ( Fig. 107.1 ). Because aromatic acid decarboxylase primarily resides in dopaminergic neurons, the regional distribution of radioactivity, which can be quantified with several different approaches, provides a measure of dopaminergic pathway integrity. Specifically, PET FD imaging can detect nigrostriatal neuronal loss in people with Parkinson disease (PD) ( Fig. 107.2 ). This radiotracer and a variety of other markers for dopaminergic neurons have been used to investigate the pathophysiology of multiple movement disorders, assess the efficacy of different interventions, and aid clinical diagnosis. However, many of these applications, such as neuroimaging measures of PD progression, have been misinterpreted owing to an evolving understanding of the assumptions and limitations of methods of data interpretation. Regardless, molecular imaging continues to provide important new insights into pathophysiology of various movement disorders.
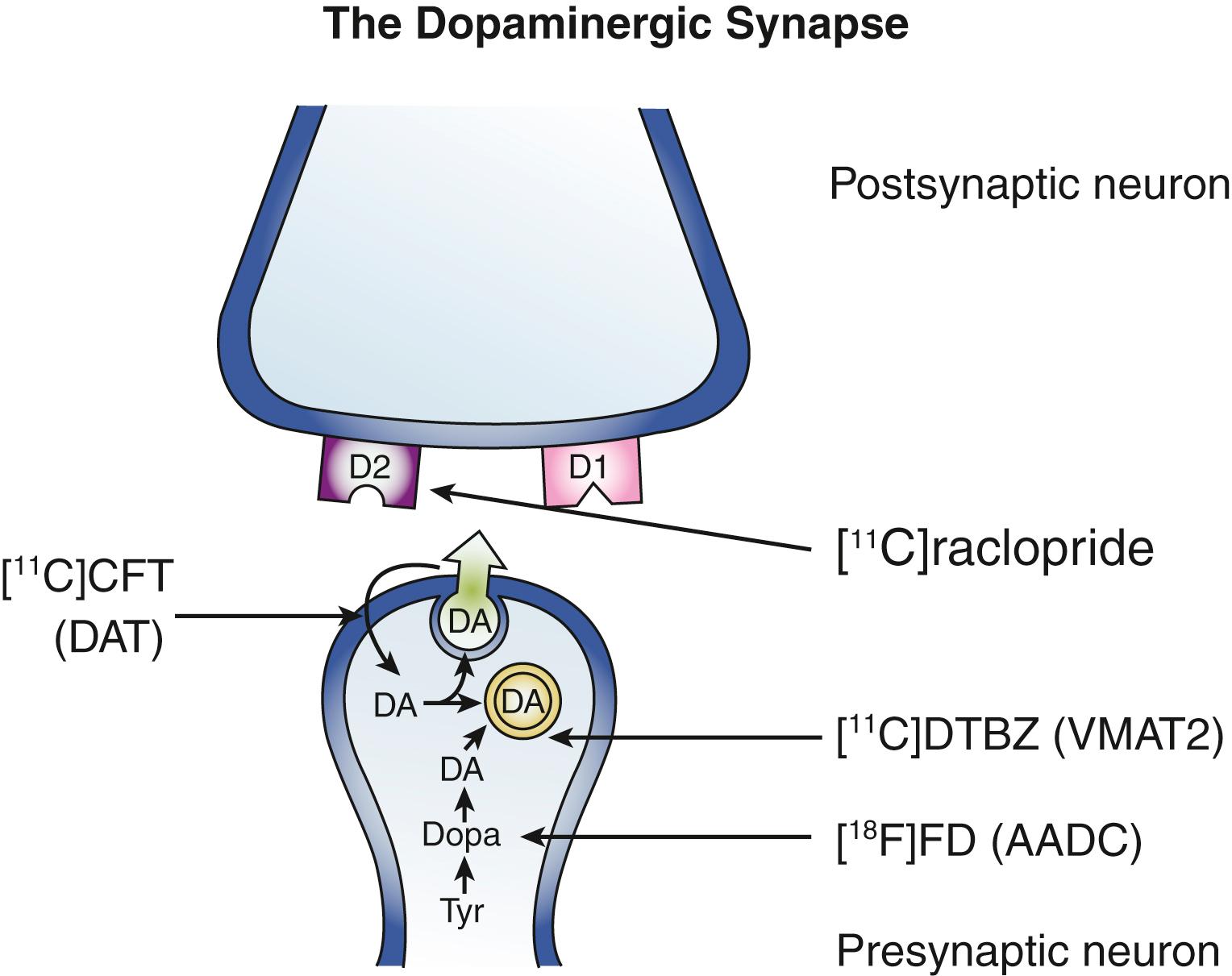
PET or SPECT also can target selected neuroreceptors such as various dopaminergic receptors including D 1 -like or D 2 -like receptors, and serotonergic, cholinergic, GABAergic, or noradrenergic receptors. These imaging techniques do not clearly distinguish whether neuroreceptor targets reside on presynaptic or postsynaptic terminals. Alternatively, radiopharmaceuticals can selectively label transporters of various transmitters such as the membranous dopamine transporter (DAT, labeled for PET or SPECT), vesicular monoamine transporter type 2 (VMAT2), or more recently the vesicular acetylcholine transporter (VAChT) (see Fig. 107.1 ). Radiopharmaceuticals also can label second-messenger signaling mechanisms such as PDE10A, which is key for postsynaptic signaling cascade systems related to D 1 -like or D 2 -like receptors. , Finally, tracers may detect abnormal protein deposition (e.g., N -methyl-[ 11 C]2-(4′-methylaminophenyl-6-hydroxybenzothiazole, also known as Pittsburgh compound B [PiB], or [ 18 F]-AV-1451) related to neurodegenerative processes. These approaches have potential for helping with clinical diagnosis or assessing target engagement of therapeutic interventions but must be applied with great caution. Some radiopharmaceuticals may be displaced by varying levels of endogenous neurotransmitters or ongoing drug treatment, which can confound interpretation of identified changes in receptor measurements that could indicate either a pathophysiologic difference in the relevant receptor or an effect of the available number of specific binding sites due to competition from either the endogenous or exogenous competitor. However, this type of competition can also be an advantage for a different type of experimental paradigm, as discussed in the next paragraph.
In addition to resting-state studies, one can image the effects of activation on regional blood flow or metabolism. Activation can result from physiologic manipulation (e.g., movement of a hand or sensory input, , pharmacologic challenge), detection of the effects of an administered unlabeled drug, or another stimulus such as deep brain stimulation (DBS). , The same strategy can be applied to neuroreceptor imaging studies. For example, one could either give a drug to increase synaptic dopamine release or have subjects perform a task that causes dopamine release and image the effects on striatal uptake of a radioligand such as 11 C-raclopride, a dopamine D 2 -like radioligand that is particularly sensitive to endogenous dopamine levels.
MRI detection of blood oxygen level–dependent (BOLD) signals in the resting state or with activation is free from ionizing radiation, requires no complicated radiopharmaceuticals, and is widely available. The BOLD signal reflects a weighted ratio of oxyhemoglobin (oxygenated and isomagnetic) and deoxyhemoglobin (deoxygenated and paramagnetic) in a volume of imaged tissue. Initially, this was applied to task activation studies that were either block design or event related. A block design includes a series of BOLD acquisition frames during periods of rest alternating with periods of activation. The BOLD signals during activation are compared with those at rest to determine the change in BOLD signal. Event-related design is similar except that periods of activation and rest are much shorter; in fact, one could use a flashing light for 100 milliseconds alternating with a resting state with the light off for 200 milliseconds and then pull the appropriately temporally related BOLD signal epochs to analyze the response to the stimulus. Each of these approaches requires calculating or estimating the response delay to identify the correct epoch of imaging data. This response delay reflects the time from the onset of activation or stimulus to the time of brain response. Of course, one also can use this same approach to detect the response to a drug—so-called pharmacologic activation. The challenge in these types of studies is that the pharmacologic activation is a block design, as one cannot turn the drug on and off repeatedly unless it has an extremely short pharmacodynamic effect—not likely to be fast enough for these types of studies. This one-block-on and one-block-off design can complicate data analysis if the baseline values of the BOLD signal are not stable. Nevertheless, such studies have been completed. Alternatively, one can use arterial spin labeling (ASL) MRI rather than BOLD imaging to detect responses to drugs or other activations. ASL has similarities to blood flow imaging with PET but does not require radiopharmaceuticals. All of these MRI-based task activation approaches have the advantage of permitting many measures per participant, thereby improving signal-to-noise ratio or allowing evaluation of multiple behavioral conditions within individual subjects.
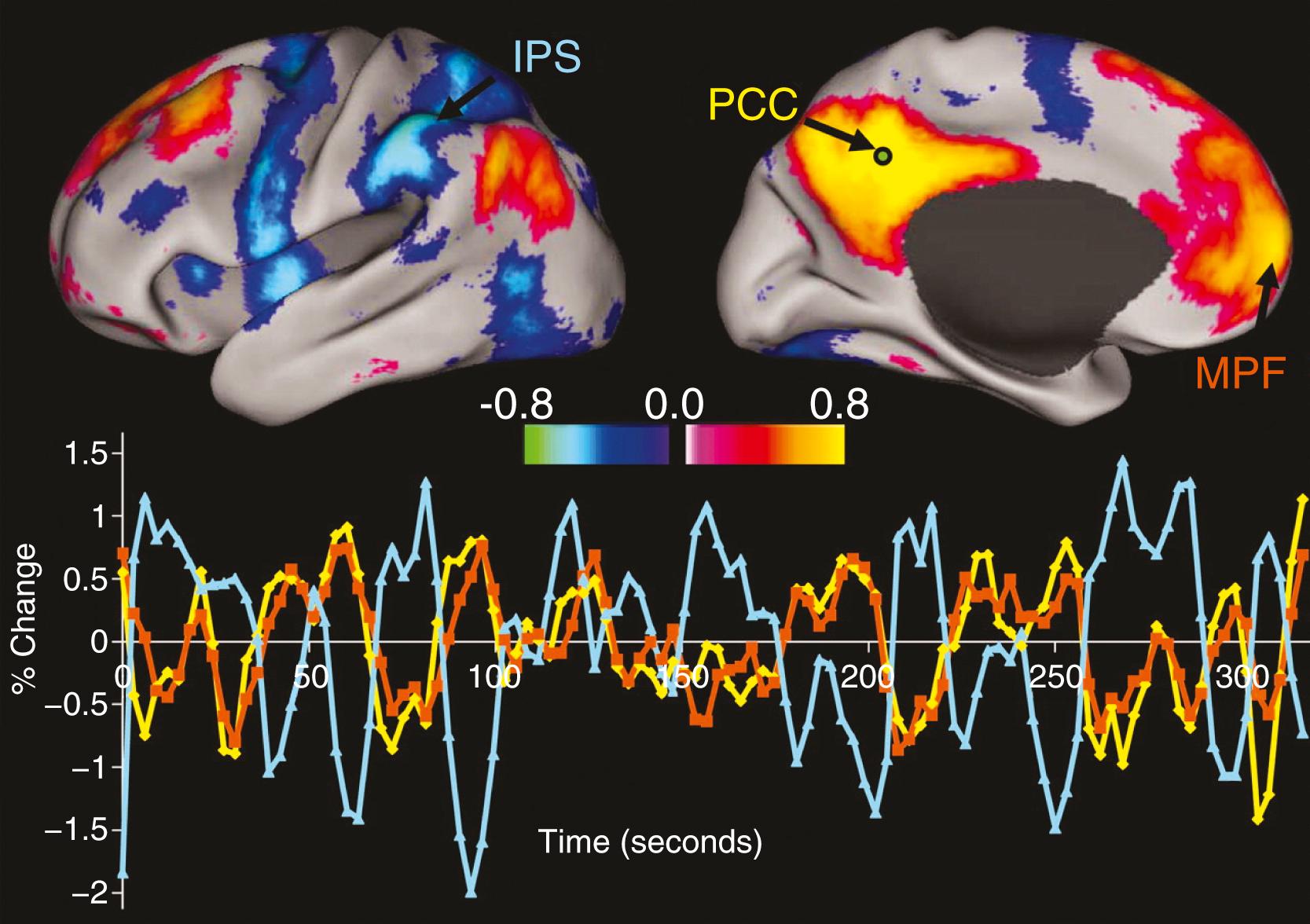
Resting-state functional connectivity MRI (rs-fcMRI) methods avoid task-based confounders. These rs-fcMRI studies also depend on detection of BOLD signal, but in this case focus is on the nature of BOLD signal with a subject “at rest” during scan acquisition. At rest, the BOLD signal in each voxel of the brain seemingly appears to have low-level noise, but closer inspection reveals that selected regions of the brain may have resting-state BOLD signals that correlate temporally with one another, as originally discovered by Biswal and colleagues. These can be either positive or negative correlations. These correlation-based networks define resting-state networks (RSNs) that have functional relevance. For example, the sensorimotor RSN includes the same regions that have correlated resting-state BOLD signals as those regions that respond to a motor task. A variety of RSNs have been identified; perhaps the most studied one is the default mode network (DMN) that includes a group of regions that become less active during almost any task. Interesting to note, the functional status of the DMN may underlie many pathologic conditions including Alzheimer disease, but also changes in people with PD. RSNs may be identified with different approaches, but the two major methods include seed-based methods and independent component analysis (ICA). Seed-based analyses start with selected brain regions (which can be one or hundreds) and then for each region identify all other brain regions that have correlated activity at rest with that seed region ( Fig. 107.3 ). Alternatively, ICA permits a data-driven approach not dependent on initial identification of selected regional seeds. Each of these approaches has advantages and disadvantages, with camps on either side arguing the advantages of one over the other. For instance, these methods suffer from reduced sensitivity due to multiple comparisons corrections, whereas a whole-connectome approach avoids such data reduction and permits direct comparisons of whole-brain RSNs.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here