Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pelvic lesions are submitted for frozen-section intraoperative determination of the nature of an ovarian, fallopian tube, or peritoneal mass or lesion. Those frozen sections usually have very important implication for management because most are submitted for evaluation with clinical suspicion of malignancy. When malignancy is confirmed, the gynecologic surgeon usually performs a staging procedure, which generally includes omentectomy, pelvic and paraortic lymphadenectomy, and peritoneal biopsies if prior patient consent had been obtained. An intraoperative diagnosis of a metastatic process enables the gynecologic surgeon to investigate possible primary sites and modify the surgery accordingly. If, however, the pelvic lesion is determined to be benign, additional resection is not performed unless clinical suspicion of malignancy remains and further sampling is still warranted. Frozen section evaluation in pelvic organs is highly reliable.
Pathologists are advised to formulate a differential diagnosis based on the lesion’s gross appearance, age of the patient, and additional relevant clinical information prior to reviewing the frozen section slide ( Table 17-1 ). This differential diagnosis varies significantly whether the lesions are cystic or solid and the respective age of the patient. It is also very important to determine at the time of intraoperative consultation if only one site is involved and whether the process is bilateral or multifocal because the latter may indicate a metastatic process. Ovarian lesions in young patients are usually benign. In contrast to adults, malignant ovarian tumors in young patients usually are of germ cell or sex cord-stromal origin. As a general rule, malignant epithelioid tumors are more frequent in older patients, although benign processes can also be encountered in this same age group.
|
The initial approach would be to evaluate the lesion grossly. The location of the lesion—purely involving or replacing the ovary, minimally connected to the ovary, or involving the peritubal or periovarian tissue— is an essential component of the differential diagnosis. When involvement of the surface of the ovary is observed or suspected, it is beneficial to ink that surface to later easily determine it on the microscopic slides ( Figure 17-1 ). A thin-walled translucent cyst with flat inner lining can be reported as benign on gross examination without the need for frozen section. Attention should be given to not inadvertently “wipe off” the inner cyst surface by blotting it or running fingers across it to access for any projections.
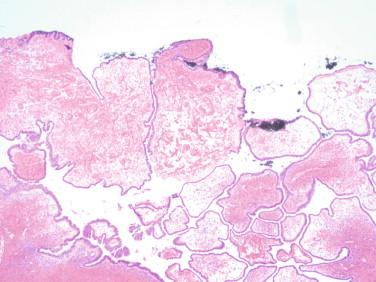
Still, one should be very cautious of gross-only examination, and the cyst should be thoroughly examined. When a frozen section is performed, the gynecologic surgeon’s interest is usually whether the lesion is benign or malignant, rather than a specific type of cyst.
The pathologist should initially observe if the cyst is unilocular or multilocular or whether a solid component is present. Serous cysts tend to be unilocular, whereas mucinous are multilocular. The differential diagnosis of a large multicystic lesion would include a borderline tumor or carcinoma. The presence of solid component should raise concern for malignancy. Evaluating the cyst contents and internal surface of the cystic wall is equally important. The presence of dark brown “chocolate cyst” content or green-brown discoloration of the cyst wall suggests endometriosis. In general, serous cysts have a clear fluid content, mucinous lesions have mucoid content, and cystic teratomas have sebaceous-type material. It is important to note the color and consistency of the cyst wall, the presence of hemorrhage, and whether there is solid component directly associated with the cyst.
Frozen section sampling is dependent on the factors mentioned above and the pathologist’s experience, as documented by intradepartmental protocols. For a lesion that is composed of a unilocular cyst with no surface involvement and no solid component, either gross-only interpretation or a single representative section would be sufficient. Sampling areas of solid or papillary components or surface involvement is crucial to exclude malignancy. It is worth noting that generally very little clinical information is provided other than the site and extent of disease. It is only in rare situations that a prior diagnosis exists or preoperative treatment was administered.
The nature of the cyst lining indicates the nature of the cyst. Occasionally, a lining may not be evident because of intracystic massive denudation or hemorrhage. Most of these cysts are generally benign, and the specific type has to be deferred to permanent section, and, even then, sometimes its nature cannot be determined. A caveat is necrosis induced by torsion that can occur in large lesions or malignant conditions.
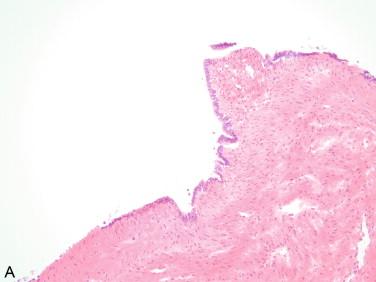
Serous cysts: A cyst containing clear fluid and microscopically composed of a single layer of cuboidal or cylindrical cells with round and small nuclei and occasional cilia represents a serous cystadenoma, which, if less than 1 cm, is sometimes called a simple cyst ( Figure 17-2 ). The latter is a nonspecific designation that should be avoided in the final diagnosis but may serve as an “adequate for management” frozen section interpretation.
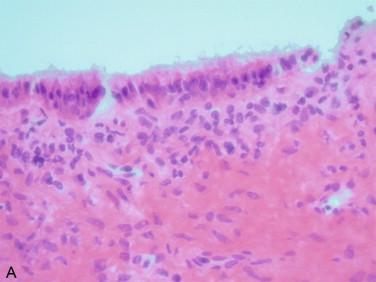
Mucinous cysts: Generically, mucinous cystadenomas are characterized by the presence of a single layer of cuboidal or cylindrical cells with intracytoplasmic mucin that displace the round and small nuclei to the base of the cells ( Figure 17-3 ). Concern for a metastatic process should be raised in the presence of bilateral lesions harboring a simple mucinous epithelium with cells showing centrally located nuclei with atypia, which occasionally may be focally severe, and including mitotic figures. Intraoperative examination of the gastrointestinal tract, pancreas, and biliary tract, and performance of an appendectomy are recommended in this situation.
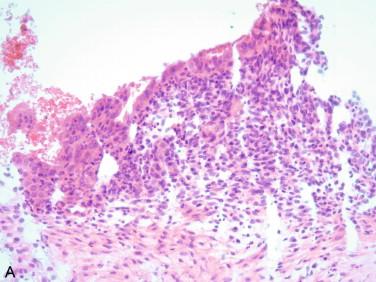
Endometriotic cysts: As noted above, endometriotic cysts generally have classic gross features. Histopathologically, endometriotic cysts are characterized by the presence of a simple endometrial-like lining with eosinophilic cells that have small, round, or oval nuclei ( Figure 17-4 ). The presence of endometrial stroma with dense small spindle cells and oval nuclei, pale to amphophilic cytoplasm, and/or recent or remote hemorrhage are necessary to confirm the diagnosis. The presence of pigmented macrophages with brown-yellow pigment is indicative of prior bleeding.
Luteal cysts: Functional luteal cysts and serous cystadenomas are the most common lesions encountered in young patients. Luteal cysts have a stratified nonepithelial lining devoid of atypia. However, frozen section artifact can cause granulosa cells to have larger and irregular nuclei that can be misinterpreted as atypical features. Cystic follicles (less than 3 cm) and follicular cysts (larger than 3 cm) are lined by granulosa cells that are characterized by the presence of cells with scant eosinophilic or pale cytoplasm and small round nuclei. Usually, luteinized theca cells with eosinophilic cytoplasm are present in the stroma surrounding the cyst. Occasionally, the granulosa cell lining is not evident, and specific typing of the cyst is not possible ( Figure 17-5 , right). Rarely, granulosa cell tumors, adult type, can be predominantly cystic and difficult to discern on frozen section. Attention should be given to the conspicuous lack of theca cells and the presence of other classic features of granulosa cell tumor (described later).
Corpus luteal cysts are lined by luteinized, large and polygonal cells with eosinophilic cytoplasm and round nuclei with nucleoli ( Figure 17-5 , left). Intracystic hemorrhage may predominate. The differential diagnosis includes other lesions with predominantly eosinophilic epithelioid cells such as luteoma of pregnancy, steroid cell tumor, and Leydig cell tumors.
Teratoma and other germ cell tumors: A teratoma can be confidently diagnosed grossly if the cyst has white-to-yellow sebaceous content and associated hair structures. A frozen section analysis is generally not required in classic cases. However, if the cyst wall has a solid area, we recommend sampling of this area to rule out the possibility of monodermal components, immature elements, or other germ cell components. In many cases, these solid areas are composed of cartilage, calcification, bone, or even teeth. In some instances, when the solid areas are submitted for frozen section, only fibrous and edematous stroma are observed, precluding a definitive histologic diagnosis of teratoma. It is advisable that a section with the classic cyst wall should always be submitted in addition to the more solid areas to ascertain the diagnosis of teratoma. Mature cystic teratomas contain elements derived from more than one germ cell layer, while skin and skin appendages predominate ( Figure 17-6 ).
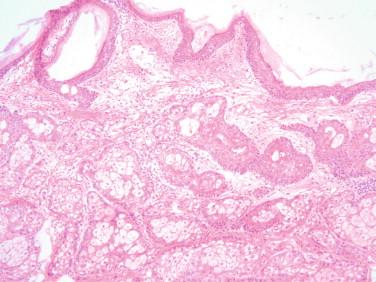
Rete cysts: Rete cystadenomas are present in the hilum and are lined by cuboidal cells devoid of cilia in a serrated arrangement. Rete ovarii can be hyperplastic or cystic and might mimic a lesion histologically.
Other cysts: Occasionally the cysts are devoid of lining, and, if no other worrisome features are seen, the designation “benign cyst, type undetermined” may be appropriate. Also luteal cysts can be devoid of lining but contain abundant bloody content and “benign hemorrhagic cyst” can be the diagnosis pending permanent sections ( Figure 17-5 right).
Serous cysts: A diagnosis of papillary adenofibroma or cystadenofibroma can be rendered for a serous cyst with papillae that are very fibrotic, large, and communicating with an underlying fibrotic mass or nodule devoid of epithelial cytologic atypia. The presence of epithelial atypia or small detached papillary clusters usually devoid of stroma is an important feature that even raises concern for a serous borderline tumor.
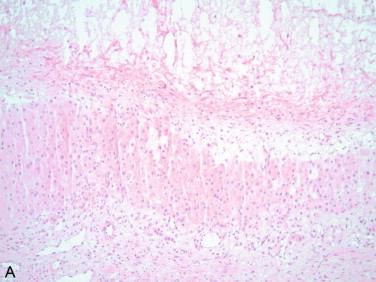
Serous borderline tumors (atypical proliferative tumors) should be suspected if the inner serous cyst lining shows mild atypia, evidenced by slightly larger and irregular nuclei, nuclei stratification and overlapping, and most importantly the presence of small papillary structures detached from the cyst wall ( Figure 17-7 ). By convention, 10% of these features should be present for a borderline tumor diagnosis; however, this assessment cannot be made on a single frozen section slide. Still, a diagnosis of serous borderline tumor can be rendered if the papillary areas are noted grossly, and microscopically large papillary structures with edematous stroma accompanied by other cellular features noted above are present. After intraoperative evaluation in young patients, a limited staging may be performed. Complete staging is generally reserved by gynecologic oncologic surgeons for postmenopausal patients. If a definitive diagnosis of serous borderline tumor is not possible, communicating the uncertainty to the gynecologic surgeon and establishing a management plan is essential. Possible terminology to report the frozen section might be “serous cyst with focal proliferation, serous borderline tumor cannot be excluded.”
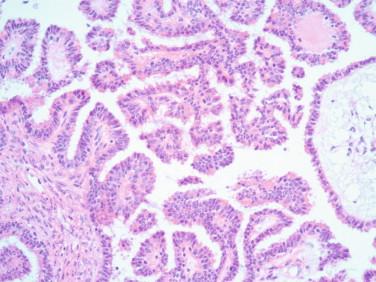
The presence of severe atypia and easily identified mitotic figures could indicate a serous carcinoma in the absence of definitive invasion. Most of these tumors have solid areas that are easily recognized; however, rarely we have encountered serous tumors that at low magnification resemble a borderline tumor, but at higher magnification severe atypia and mitotic figures are easily identified that are not compatible with a borderline tumor ( Figures 17-8, 17-9 ). Again, in this setting, staging would be performed if patient consent had been given. Extensive sampling of the cyst wall for permanent sections is recommended to ascertain the absence of a more advanced lesion.
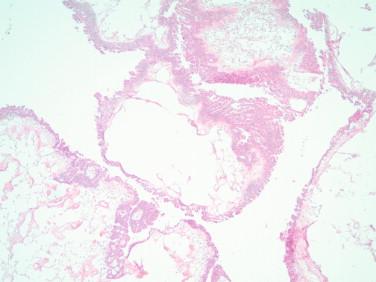
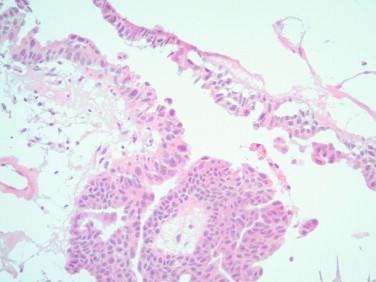
Mucinous cysts: Classic mucinous borderline tumors show a complex multicystic proliferation lined by stratified epithelium and cells with larger, elongated, and mildly atypical nuclei and pale cytoplasm with less mucin than their benign counterpart ( Figures 17-10, 17-11 ). Occasional mitoses are seen. The presence of goblet cells indicates the more common intestinal-type borderline tumor. An endocervical-like borderline tumor has a lining that resembles the endocervical epithelium with mild atypia and no goblet cells. The presence of moderate or severe atypia in a complex multicystic proliferation indicates an intraepithelial carcinoma component. The designation mucinous carcinoma is reserved for tumors with clusters or single eosinophilic cells invading the stroma with a desmoplastic and/or inflammatory reaction (destructive or invasive pattern), or a complex proliferation of back-to-back cystic or glandular structures devoid of stroma encompassing an area larger than 5 mm (expansile or confluent pattern) ( Figure 17-12 ).
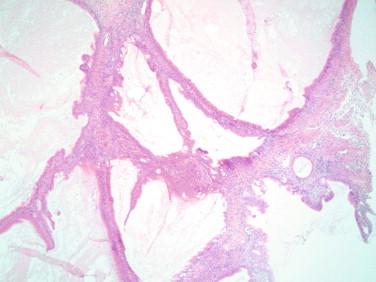
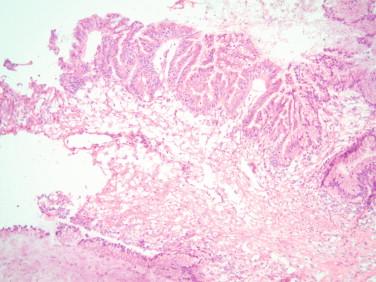
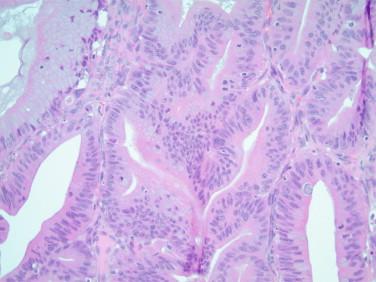
As previously mentioned, the presence of mucinous epithelium with significant atypia and mitotic figures should also raise the possibility of a metastatic mucinous carcinoma, especially if there is bilateral involvement. A metastatic process should also be suspected if cellular pools of mucin are seen in the stroma (pseudomyxoma associated with appendiceal mucinous carcinoma), or the cystic or glandular proliferation has a garland pattern and includes central dirty necrosis. The latter is usually typical of metastatic colorectal carcinoma. An algorithm described by the Hopkins group predicts that primary lesions are unilateral and larger than 10 to 12 cm, whereas bilateral lesions, especially if smaller than 10 cm, are most likely of metastatic origin.
Endometriosis and associated malignancy: On rare occasions, the endometriotic lining can have cells with larger and irregular nuclei consistent with atypical endometriosis. While these changes can be degenerative or reparative in nature, it is recommended to further evaluate these areas of atypia and exclude any associated solid or polypoid component because these findings may be indicative of malignancy. The presence of a polypoid lesion or a solid nodule or mass in a classic endometriotic cyst is very concerning for hyperplasia or malignancy, including an endometrioid or clear cell carcinoma ( Figure 17-13 ). Endometrioid carcinoma resembles its endometrial counterpart. Clear cell carcinoma is characterized by the presence of severely atypical cells with large and irregular nuclei and pale or clear cytoplasm.
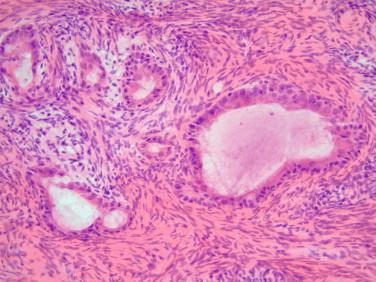
Functional cysts versus sex cord tumors predominantly cystic: Sex cord tumors, especially granulosa cell tumors, adult type, can rarely be predominantly cystic, mimicking a functional cyst. When encountering large cysts with a granulosa type lining, it is necessary to search for theca cells, sometimes luteinized, to confirm the benign etiology. If absent, additional sections, especially of solid areas, would be necessary to rule out a malignancy. Classic Call-Exner bodies or any of the many patterns of granulosa cell tumor would be helpful for a diagnosis.
The granulosa cell tumor, juvenile type, can also be predominantly cystic and usually harbors a myxoid or edematous background. The tumor cells are present in solid and/or macrofollicular patterns, whereas classic Call-Exner bodies are very rare. This diagnosis is not usually made on frozen section but can be suspected in the right clinical context and if a broad differential diagnosis is formulated prior to reviewing the frozen section slide.
Frozen sections are performed on solid ovarian neoplasms to determine if the lesion is malignant, especially in postmenopausal patients. A diagnosis of malignancy is sufficient to initiate proper staging with a few exemptions (occasional metastatic processes and widespread disease, including lymphoma). As with benign lesions, the initial approach is to evaluate the lesion grossly considering the following scenarios.
Gross examination of the cut surface of a solid neoplasm is very helpful in formulating a differential diagnosis prior to taking sections for intraoperative examination. Epithelial malignancies are usually tan to brown and fleshy and show areas of necrosis and hemorrhage. Sex cord-stromal tumors (granulosa or pure Leydig tumors) are predominantly yellow, as are thecomas. Fibromas are usually white and fibrous. Sampling should avoid excessively necrotic or hemorrhagic areas. Usually only one section is necessary to provide a diagnosis or narrow the differential diagnosis. For heterogeneous lesions, two to four sections might be essential. Sampling cystic components, if present, can in some cases determine origin and type of tumor at hand.
It is important to determine grossly if the lesion involves the ovary or adnexal soft tissue. Unusual solid tumors that mostly arise in adnexal soft tissue or broad ligament area and rarely involve the ovary include, among others, leiomyomas, adenomatoid tumors and female adnexal tumor of probable wolffian origin (FATWO). Classic surface epithelial tumors, especially serous neoplasms, can also arise in adnexal soft tissue areas adjacent to the ovary. This exercise might also determine if the mass involves fallopian tube fimbria or the transition zone between the ovary and fallopian tube, sites that are currently believed to harbor the source of a significant number of ovarian and peritoneal serous carcinomatosis.
Involvement of the ovarian surface by malignant epithelial neoplasms upstages the tumor to at least stage Ic, similar to the presence of malignant ascites. More importantly, it may indicate the presence of additional involvement of the peritoneal surface. In the presence of surface involvement, the gynecologic surgeon would need to perform a very thorough examination and sampling of the pelvic and abdominal cavity for possible additional lesions or implants. Surface involvement is best appreciated prior to inking the surface of the specimen. Once identified, applying ink to this area assists with confirming this finding on frozen and permanent sections.
Solid tumors are analyzed using a combination of clinical and gross features including patient age (fertile versus postmenopausal), laterality of the lesion, gross features, and determining the type of lesion under the microscope. In general these tumors can be categorized as follows: functional lesions, surface epithelial tumors, stromal and sex cord tumors, germ cell tumors, and other miscellaneous lesion including metastasis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here