Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acknowledgments: I would like to give full acknowledgment to the authors of previous versions of this chapter, Dr. William B. Geissler and the late Dr. Joseph F. Slade. Their chapter was the foundation of the current chapter; I have maintained many of their principles but have updated the chapter with my own thoughts, influenced by prime mentors of mine, Drs. Martin Posner, Scott Wolfe, and Michael Hausman. Dr. Slade was one of my mentors and teachers from residency. Most know of his contributions to hand and upper extremity surgery and his continual willingness to teach and instruct worldwide. His dedication and enthusiasm for hand surgery are legendary and are an inspiration to us all. Thank you also to Dr. Geissler, who continues to be a pioneer in our specialty. Thank you to Drs. Gregory Bain, Simon MacLean, and David Lichtman for their outstanding section on Kienböck, edited from Green’s Operative Hand Surgery seventh edition, updates. Acknowledgments to Dr. James P. Higgins, who authored a case study for this chapter available on ExpertConsult.com . I also wish to acknowledge Karthik Krishnan, MS, for editorial assistance.
The treatment of scaphoid fractures requires knowledge of the blood supply, surgical approaches, and effects that fractures and nonunions of the scaphoid have on carpal kinematics, stability, and arthritis. Vigilant care of these fractures can usually lead to a functional result for the patient. However, malunion or nonunion can potentially lead to a relentless downward spiral of wear and cartilage damage. Chronic pain and dysfunction of the wrist results, which affects both hand function and the entire upper extremity.
Within the past 2 decades, methods of scaphoid repair have been developed to minimize additional surgical trauma and optimize stabilization until healing. Minimally invasive fixation has been demonstrated to have a higher union rate than cast treatment and has relatively few complications. This approach allows the patient or athlete to return to work or sports within weeks or months, whereas a failed attempt at healing with cast immobilization can result in months of lost time, compounded by the increased complexity, cost, and complications of nonunion repair. This chapter will explore the mechanics, biologic factors, and modern treatment regimens for fractures of the scaphoid and neighboring carpal bones.
The scaphoid bone garners more interest in upper extremity surgery for its weight and size than any other bone because it is the “keystone” of the carpus. In architecture, the keystone is the central stone at the summit of an arch, locking the whole together. Likewise, the scaphoid links the carpus together. Pathologic conditions of the scaphoid can affect the entire wrist.
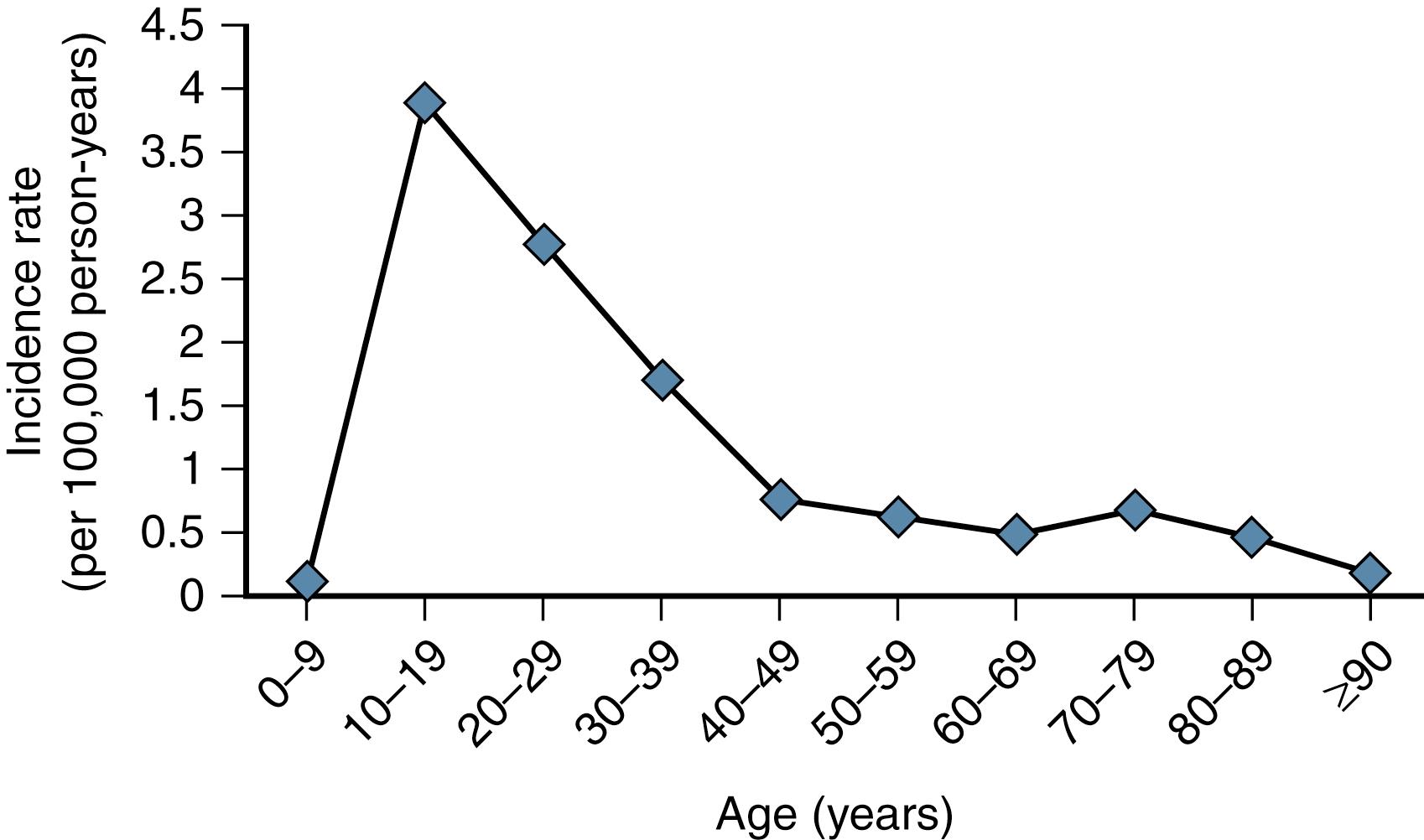
Not only is the scaphoid important, but it is also the most commonly fractured carpal bone. Scaphoid fractures account for 60% to 70% of all carpal fractures and are second in frequency of wrist fractures only to distal radius fractures. The majority of injuries are low-energy injuries, either from a sporting event (59%) or from a fall onto an outstretched wrist (35%); the remainder result from high-energy trauma such as a fall from a height or a motor vehicle injury. Howe documented that 82% of the scaphoid fractures in Norway occur in males, with an average age of 25 years (range, 11 to 79 years). The age-specific incidence in males remained significantly higher than that in females until age 60 years, at which point the incidences were similar. The annual incidence was 43 per 100,000 people. The statistics were similar in a series by Larsen and colleagues; the mechanism for scaphoid fracture was a fall in 69% of cases and a blow to the wrist in 28% of cases.
More recently, Wolf et al. studied a large U.S. military population and found a greater incidence of scaphoid fracture than the previous data had shown, 121 per 100,000 person-years. Males and the 20- to 24-year-old age group were associated with higher rates of scaphoid injury. The more active nature of the occupations of this population may explain this higher incidence. Van Tassel and colleagues showed a peak incidence in scaphoid fractures in the second decade, and few after age 50 years ( Fig. 16.1 ).
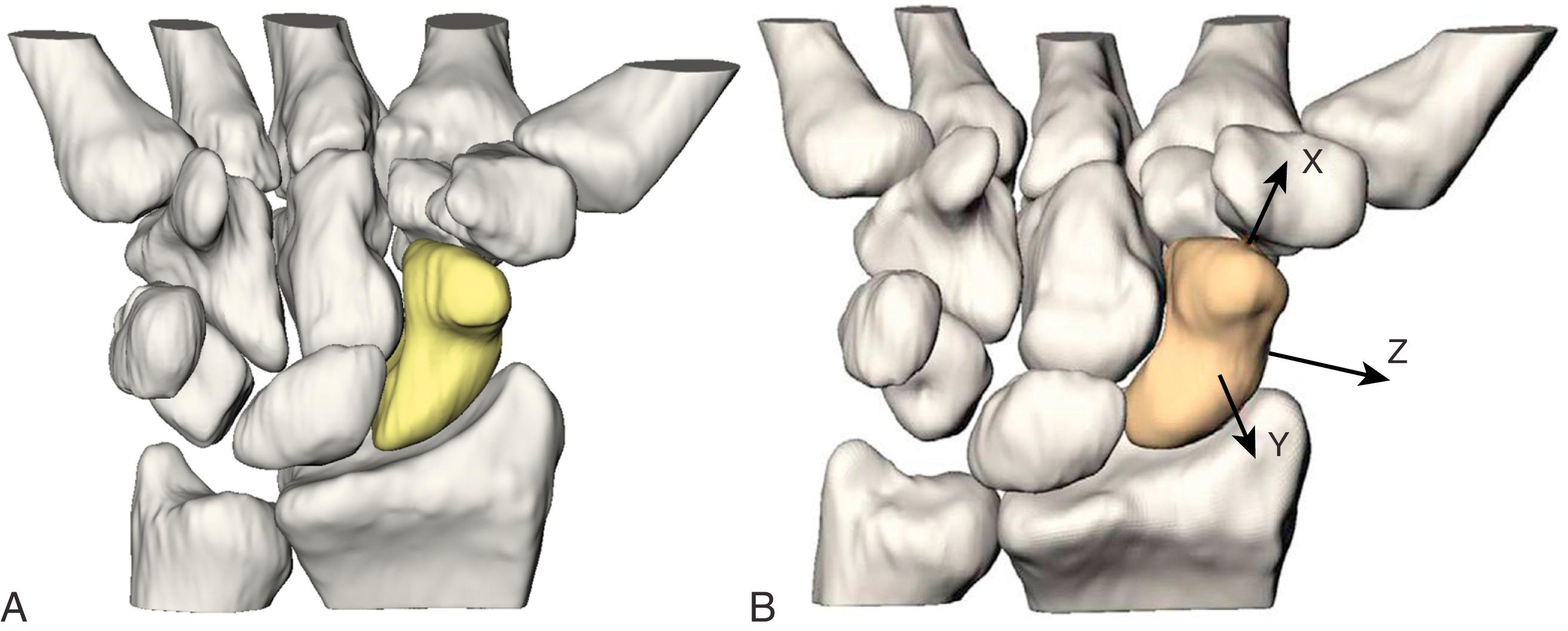
The shape of the scaphoid bone has been described with several terms: as a boat (“skaphos” in Greek), as a twisted peanut, and as bean-shaped. The complex shape of the bone can present challenges to reconstruction and fixation. Approximately 80% of the scaphoid is covered by cartilage, limiting ligamentous attachment and vascular supply ( Fig. 16.2 ). , The scaphoid is divided into three regions: proximal pole, waist, and distal pole (tubercle). The proximal pole articulates with the scaphoid fossa of the distal radius and the lunate. The scaphoid is oriented in the carpus with an intrascaphoid angle averaging 40 ± 3 degrees in the coronal plane and 32 ± 5 degrees in the sagittal plane. ,
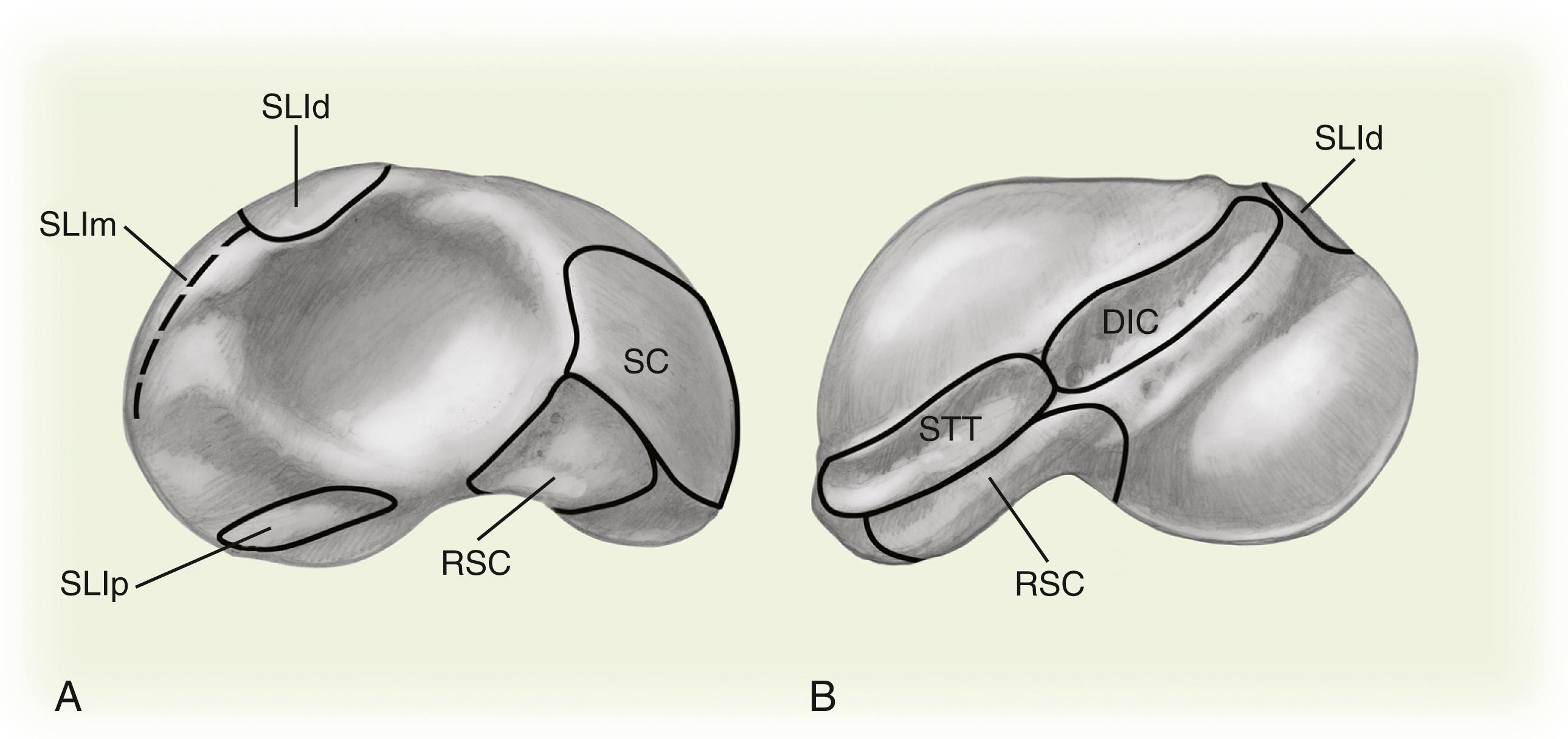
The scaphoid is the only carpal bone that bridges the proximal and distal carpal rows and acts as a tie-rod. The carpal rows are supported by stout intrinsic ligaments and reinforced by a complex system of volar and dorsal extrinsic ligaments ( Fig. 16.3 ). The scapholunate interosseous ligament (SLIL) is a stout ligament connecting the scaphoid to the lunate and is the primary stabilizer. The dorsal aspect of this ligament is composed of transverse collagen fibers, whereas the palmar ligament is composed of oblique collagen fibers inserting to the volar capsular ligaments. The dorsal portion is twice as strong as the palmar portion. Only 20 to 30 degrees of motion is possible at an intact scapholunate interval. The dorsal and palmar regions are critical in maintaining normal carpal kinematics and function of the scapholunate interval. The dorsal region resists palmar-dorsal translation and gap, whereas the volar portion resists rotation. The proximal fibrocartilaginous region is the weakest mechanically and is well suited to accept the compression and shear loads at the radiocarpal joint. The radioscaphocapitate (RSC) ligament originates from the volar radial aspect of the radius, crosses the volar concavity of the scaphoid waist, and proceeds ulnarly toward the capitate, acting as a fulcrum around which the scaphoid rotates. The scaphoid can also fracture around this fulcrum at the waist. The scaphocapitate ligament originates from the distal scaphoid at the border between the trapezoid facet and the capitate facet. It inserts into the volar waist of the capitate distal to the RSC ligament. This ligament, along with the scaphotrapezial ligament, functions as a primary restraint of the distal pole.
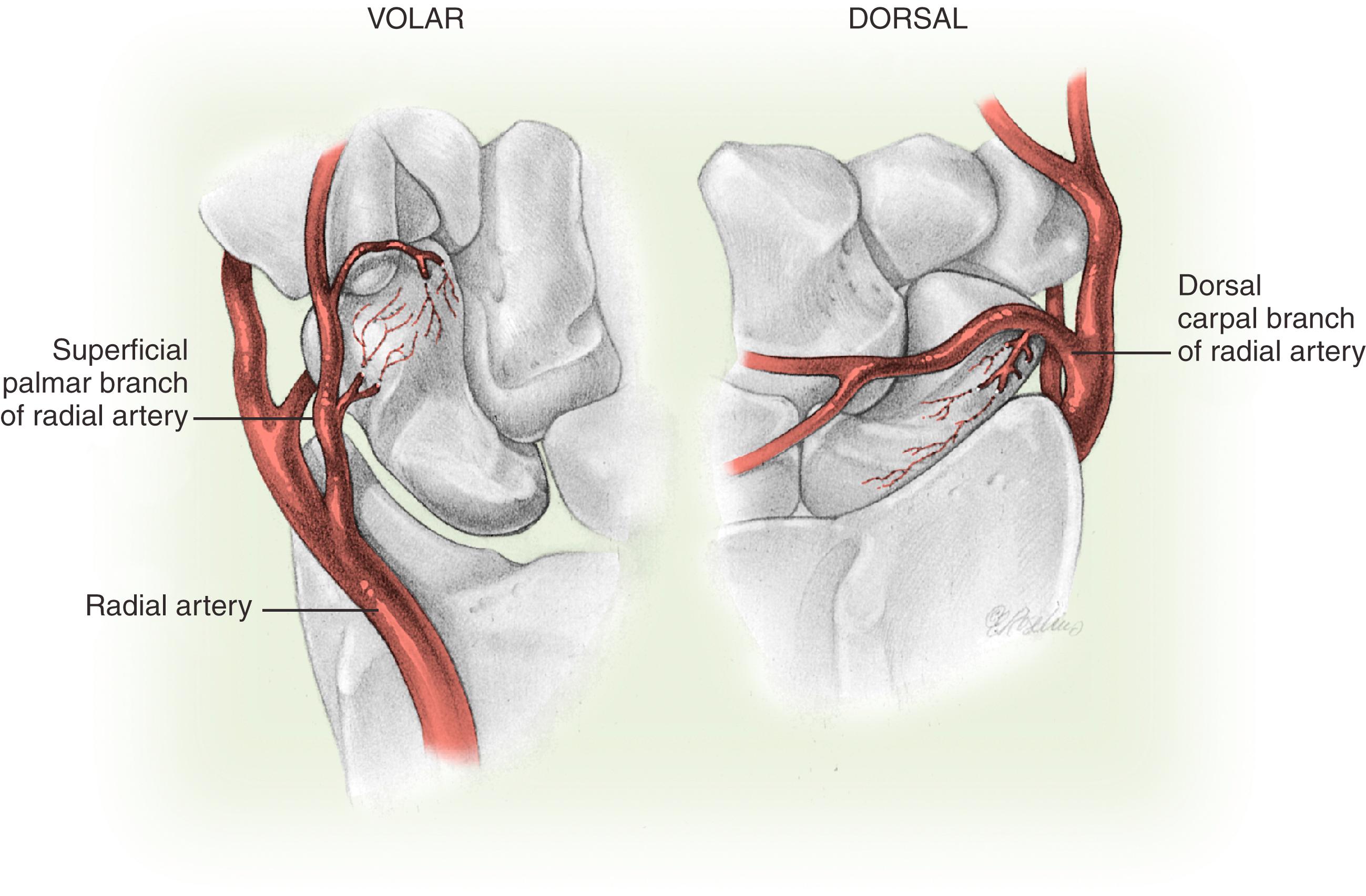
The blood supply of the scaphoid bone is not robust because it is predominantly retrograde. The major blood supply to the scaphoid is via the radial artery: 70% to 80% of the intraosseous and proximal pole vascular supply is from branches of the radial artery entering distally through the dorsal ridge of the scaphoid between the proximal and distal articular surfaces. The radial artery or the superficial palmar arch also give volar branches that enter in the region of the tubercle and provide the blood supply to 20% to 30% of the bone in the region of the distal pole ( Fig. 16.4 ). The proximal pole also gets blood supply from the radioscapholunate ligament (ligament of Testut, a neurovascular conduit) and direct scapholunate branches from the palmar and dorsal transverse carpal arches. Handley and Pooley found that the venous drainage from the proximal pole of the scaphoid was via the dorsal ridge into the venae comitantes of the radial artery.
The more proximal the fracture, the more likely the bone is to be dysvascular and the higher the risk of nonunion. Proximal pole fractures have been reported to have an incidence of avascular necrosis (AVN) of 13% to 50%. Knowledge of the vascular anatomy has implications for sensible approaches to the scaphoid. Combined palmar and dorsal approaches taking off the soft tissue at the tubercle and the dorsal ridge would not be advisable. During the dorsal approach to the scaphoid, the majority of the dorsal ridge tissue and vessels can and should be left intact.
Although the exact mechanism of fracture is not completely understood, hyperextension past 95 degrees is the usual position of injury, but other mechanisms such as axial loading have also been postulated to produce scaphoid fractures, as has hyperflexion of the wrist. With the hyperextension mechanism, a fracture of the scaphoid usually begins at the volar waist with a tensile failure; the forces propagate to the dorsal surface with compression loading, until failure occurs. In a cadaveric study, wrists placed in extreme dorsiflexion and ulnar deviation produced fractures through the scaphoid waist as the scaphoid impinged on the dorsal rim of the radius. Proximal scaphoid fractures resulted from dorsal subluxation during forced hyperextension. Carpal dislocations and scapholunate ligament tears were reproduced with wrist extension and ulnar deviation, combined with intercarpal supination.
As with any fracture, the potential for healing relies on the fracture’s location, vascularity, and stability. Nonunion occurs in 10% to 15% of all scaphoid fractures. The risk of nonunion increases with:
Delay of treatment for more than 4 weeks
Proximal pole fractures
Fracture displacement greater than 1 mm
Osteonecrosis
Tobacco use
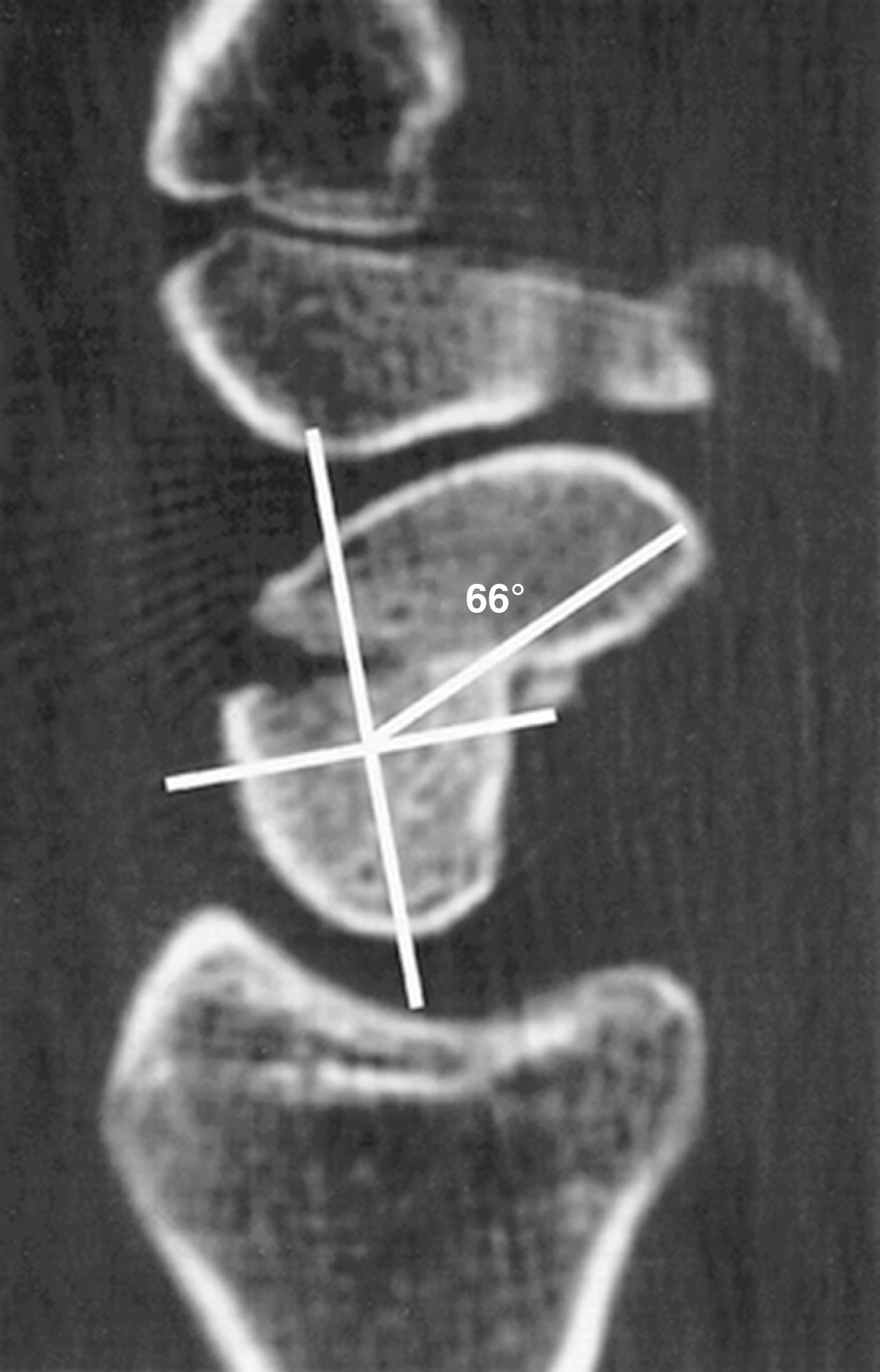
Associated carpal instability (dorsal intercalated segmental instability [DISI] with a scapholunate angle >60 degrees and a capitolunate angle >15 degrees) secondary to humpback (flexed with intrascaphoid angle >45 degrees; the normal intrascaphoid angle is 24 degrees) ( Fig. 16.5 )
For nondisplaced waist fractures treated with casting, nonunion rates are 5% to 12%. Nonunion rates for displaced scaphoid fractures treated nonoperatively are higher, reaching 50%.
Untreated displaced fractures of the waist are subject to varying degrees of these forces and will usually angulate as the volar bone is reabsorbed, yielding a “humpback” flexion deformity of the scaphoid. The resultant radial column shortening and the extension of the proximal scaphoid pole releases the lunate to rotate into DISI under the influence of the attached triquetrum. Ultimate treatment of a humpback scaphoid nonunion with DISI requires both restitution of scaphoid anatomy and reversal of the secondary changes in carpal kinematics.
Untreated scaphoid nonunion will predictably progress to arthritic change, in what has been termed scaphoid nonunion advanced collapse (SNAC). Arthritic change arises at the radial styloid articulation with the distal scaphoid pole (stage I) and is followed by degeneration of the scaphocapitate joint (stage II) and ultimately the midcarpal joint (stage III). Arthritic changes have been found in 97% of the patients assessed at least 5 years after injury, with the degree of arthritic changes being proportionate to the duration of nonunion. Patients generally present with escalating mechanical pain, with limitations in range of motion. Düppe and colleagues reviewed 30-year follow-up results of scaphoid fractures treated with thumb spica short-arm casts. Ten percent of the patients developed nonunion; 60% of these demonstrated radiographic evidence of radiocarpal osteoarthrosis, while only 2% of the healed group demonstrated degenerative change.
The patient usually presents with pain on the radial side of the wrist. There may be swelling on the radial side as well. There is usually a history of trauma, such as falling on an outstretched hand, collision of the wrist against a person or heavy obstacle, or possibly a direct blow against an object. There may be limited range of motion and pain when applying extended wrist loading or positioning the wrist in extreme positions of flexion or extension.
Physical examination starts with visual inspection. Wrists with acute fractures may have swelling and bruising in the radial aspect of the wrist. There may be limited range of motion. Wrists with chronic injury may have swelling in the dorsoradial wrist. “Snuffbox tenderness” has become synonymous with scaphoid fracture, but this applies predominantly to waist fractures, which represent 70% of scaphoid fractures. The second most common type of scaphoid fracture is a proximal pole fracture, at 20%. The least common is a distal pole fracture, at 10%. Fractures tend to occur at the waist partly because the RSC ligament acts as a fulcrum over which the scaphoid waist fractures ( Fig. 16.6 ).
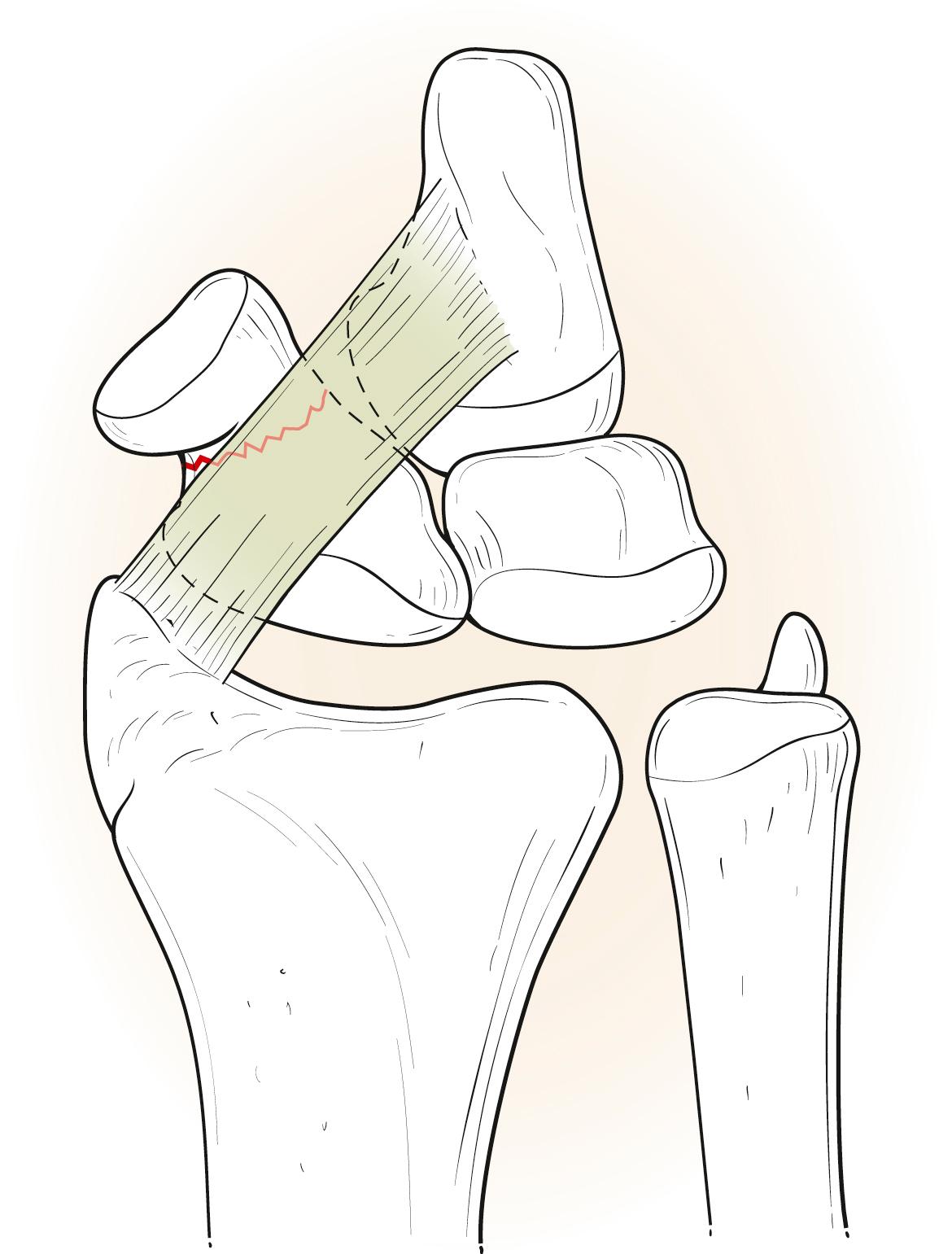
The full physical examination of the scaphoid bone should include all of its parts: the waist, distal pole, and proximal pole. To palpate the anatomic snuffbox for the waist examination, palpate just distal to the radial styloid in the “soft spot.” The distal pole should be palpated at the scaphoid tubercle on the palmar aspect of the wrist. To do this, place the index finger in the anatomic snuffbox and place the thumb on the palmar aspect just distal to the anatomic snuffbox. The prominent bone palpated is the distal pole of the scaphoid. With radial deviation of the wrist, this prominence should move palmarly toward the examiner’s thumb. This distal pole should be checked for tenderness. The proximal pole is palpated dorsally in line with the second ray just distal to the dorsal radius lip. The scapholunate ligament is in line between the second and third rays just distal to the dorsal radius lip and corresponds to the 3-4 wrist arthroscopy portal. The proximal pole is just radial to the scapholunate ligament/3-4 portal area. Pain on longitudinal compression of the thumb (scaphoid axial compression test) is also a sign of scaphoid fracture. If all three tests of anatomic snuffbox tenderness, scaphoid tubercle tenderness, and scaphoid axial compression test are positive, there is 87% to 100% sensitivity and 74% specificity for scaphoid fracture. ,
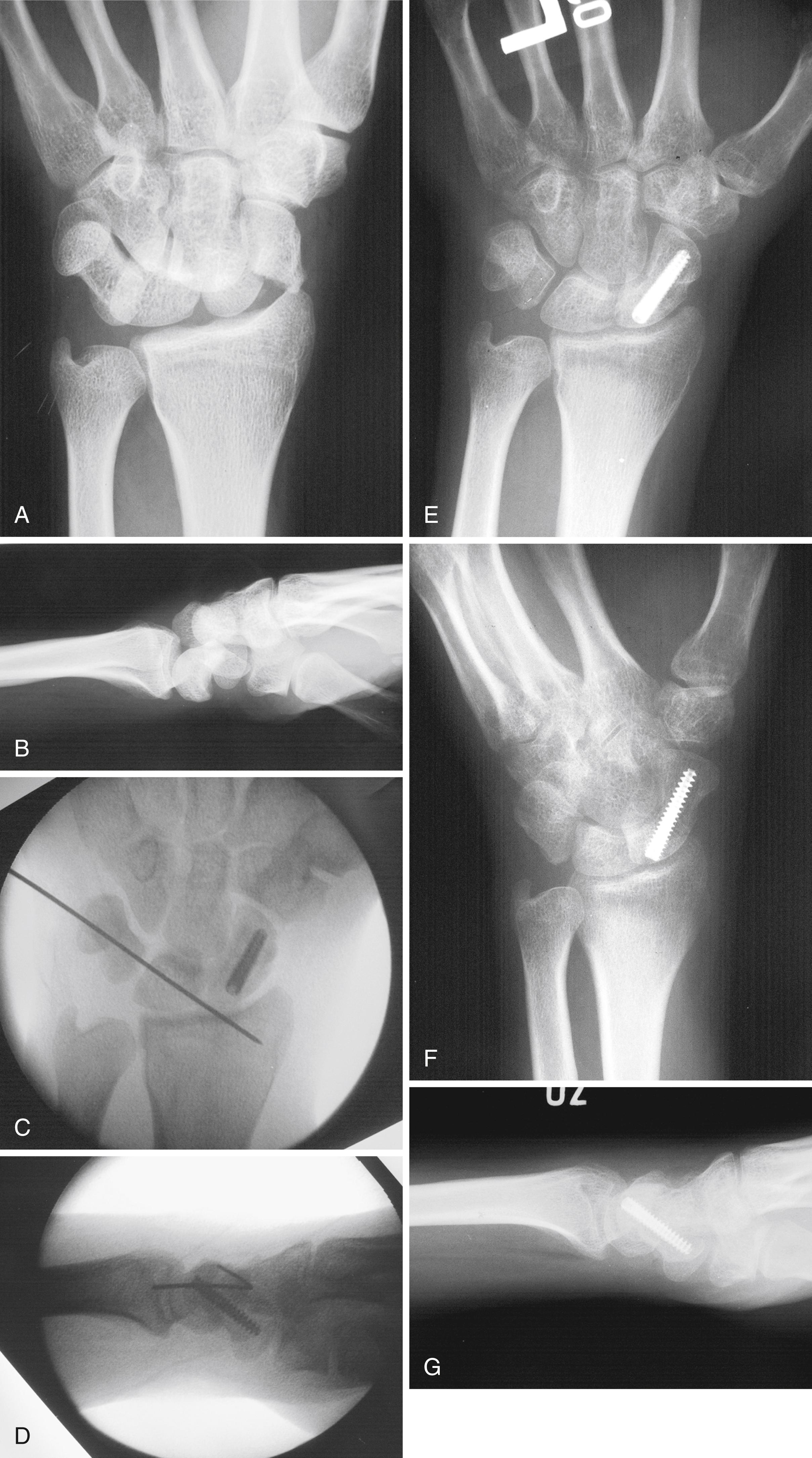
Radiographs are a mainstay in the workup of scaphoid fractures. Standard radiographic views are the posteroanterior (PA), lateral, oblique, and scaphoid views. It is important to obtain a true scaphoid pisiform capitate (SPC) lateral radiograph of the wrist, wherein the palmar cortex of the pisiform bone overlays the interval between the palmar cortex of the distal scaphoid pole and the palmar cortex of the capitate. This view allows a true assessment of carpal alignment.
A predictable scaphoid view is taken where a fist is made, with the thumb covering the dorsum of the middle phalanges of the index and middle fingers. The pronated forearm and hand are placed on the radiography table. The wrist is placed in ulnar deviation ( Fig. 16.7 ). The rationale behind this position is to take the scaphoid out of its usual position of flexion and pronation. With the thumb in the above position, the wrist is extended and supinated slightly. Ulnar deviation and wrist extension extend the proximal carpal row. This view allows a full view of the scaphoid bone with minimal overlap from neighboring bones.
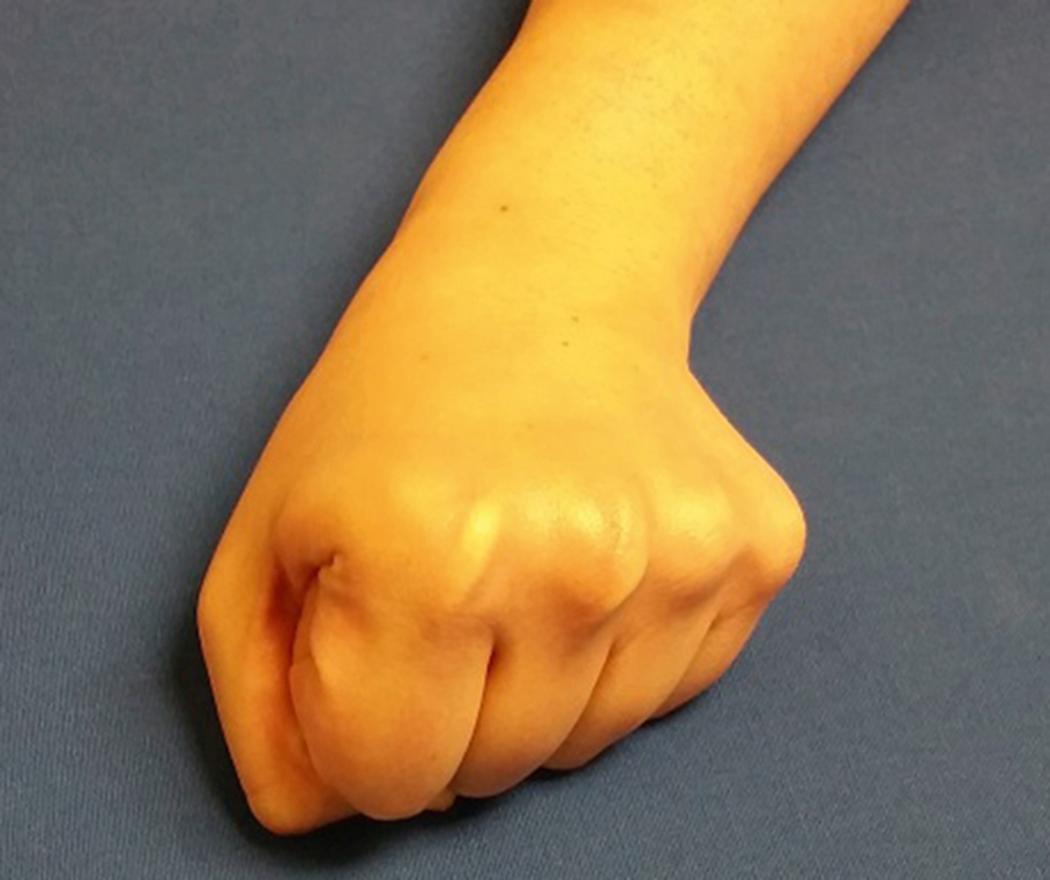
A clenched pencil view can also be useful. , This is the best view to assess associated dynamic scapholunate widening ( Fig. 16.8 ) and also shows SNAC and scapholunate advanced collapse (SLAC) wrist changes better than standard PA views. For clinically suspected occult scaphoid fractures with negative initial radiographs, the wrist may be casted and radiographs repeated in 10 to 14 days.
Computed tomography (CT) scanning helps elucidate scaphoid fracture displacement, bony morphologic findings, gapping, sclerosis, cysts, and evidence of healing. CT is particularly helpful in addressing nonunions. It is important that CT scans are taken with overlapping 0.625 mm cuts along the long axis of the scaphoid and with coronal and sagittal reconstructions. CT scans have particular utility in evaluating for healing after scaphoid surgery, especially because radiographs are often indeterminate. CT scanning has also demonstrated some utility in evaluating osteonecrosis of the proximal pole of the scaphoid. Increased radiodensity of the proximal pole is a sign of dysvascularity.
Gilley and colleagues showed that radiographs frequently underestimate fracture displacement compared with CT scan. Thirty-nine preoperative radiographs and CT scans were evaluated by two blinded readers. Of these, 26% to 33% of nondisplaced fractures on radiographs were read as displaced on CT scan. If the status of the radiocarpal or midcarpal cartilage is in question, a magnetic resonance imaging (MRI) without contrast with cartilage-sensitive sequencing may be obtained.
Scaphoid healing cannot be reliably determined by standard radiographs at 3 months ; consequently, CT provides enhanced resolution and definitive information regarding healing.
MRI may be helpful to determine whether there is occult scaphoid fracture, especially in the acute period after injury. Specificity is 90% and sensitivity is between 90% and 100%, as opposed to bone scintigraphy (i.e., bone scan), which is 92% to 95% sensitive and 60% to 95% specific. Karl and colleagues performed a study comparing cast immobilization and repeat radiographs at 2 weeks, immediate CT scan and immediate MRI for occult scaphoid fractures. They concluded that advanced imaging for suspected scaphoid fractures in the setting of negative radiographs represents a cost-effective strategy for reducing both morbidity and costs. The decision of use of CT versus MRI is a function of local costs and test performance capabilities. In a comparison of CT and MRI scans for suspected scaphoid fracture, both modalities had similar characteristics. The sensitivity, specificity, and accuracy were 67%, 96%, and 91%, respectively, for CT and 67%, 89%, and 85%, respectively, for MRI which were not significantly different.
Bone scintigraphy can be used in cases of clinically suspected scaphoid fractures. In a Cochrane study by Mallee and colleagues, bone scintigraphy was compared with MRI and CT scans in patients with negative radiographs. After 72 hours from injury, bone scintigraphy was statistically the best diagnostic test; however, it has the drawback of increased radiation exposure and may not be available at all institutions.
Scaphoid fractures have been classified by fracture location (proximal, waist, or distal), plane (transverse or oblique), and stability (stable or unstable). The goal of a fracture classification is to guide management of injuries in order to enable rapid healing with minimal complications and allow return to activities of daily living, work, and sports or hobbies. The consequences of failed healing include wrist pain, loss of wrist motion, loss of grip strength, loss of productivity, and premature articular degeneration. Of particular importance is identifying which scaphoid fractures require surgical intervention to heal. A failure to identify an unstable scaphoid fracture and treat it accordingly will predictably result in 6 months or more of additional treatment and restricted activities.
One of the earliest efforts to identify unstable fractures was to examine the scaphoid fracture plane. Russe recognized that oblique fractures were unstable and difficult to control with immobilization and that they resulted in an increased rate of nonunion. Herbert and Fisher classified scaphoid fractures according to their stability. Stable fractures, classified as type A, included incomplete fractures or fractures of the scaphoid tubercle. The authors stated that these fractures could be safely treated with immobilization with expectation of a high rate of union. All other fractures were considered potentially unstable and merited rigid fixation, a point of some controversy. Despite the assertions of these authors, however, Desai and colleagues were unable to predict fracture union with closed treatment using either the Russe or the Herbert classification system.
Cooney and colleagues attempted to further define unstable injuries. These included fractures with more than 1 mm of displacement, a lateral intrascaphoid angle of more than 35 degrees, bone loss or comminution, perilunate fracture-dislocation, DISI alignment, and proximal pole fractures. They advocated open surgical fixation for all unstable injuries.
Up to 25% of scaphoid fractures are not visible on initial radiographs. Unless the x-ray beam lies in the same plane as the fracture, the fracture line may be missed. Because delay in treating a scaphoid fracture increases the nonunion rate, the treating physician should be vigilant about confirming diagnosis. The obvious goal is to attain healing of the scaphoid fracture in an anatomic position while maintaining carpal alignment. Patients with union have better functional results. ,
Distal pole and tubercle fractures of the scaphoid are generally treated nonoperatively. The distal pole of the scaphoid is well vascularized, and distal scaphoid pole fractures have a high rate of union after 6 to 8 weeks of immobilization in a short-arm cast. The two predominant distal fracture types treated in plaster immobilization are (1) avulsion fractures from the radiopalmar lip of the scaphoid tuberosity and (2) impaction fractures of the radial half of the distal scaphoid articular surface. In a study of long-term follow-up of distal scaphoid fractures, Clementson and colleagues reported on a cohort of 41 patients treated nonoperatively in thumb spica casts. All but one healed. Median Disabilities of the Arm, Shoulder, and Hand (DASH) score was 2, median patient rated wrist evaluation (PRWE) 0, and median visual analog scale (VAS) pain score 0. CT revealed arthritis in the scaphotrapeziotrapezoid (STT) joint in 7 out of 41 wrists, none of which caused clinical symptoms.
Nondisplaced waist fractures may be treated either with casting or with surgery. There are a number of issues involved in this decision, including union rates, recovery time, cost effectiveness, age, and activity level. How to treat these fractures is a complex topic and is controversial in the literature.
For nondisplaced waist fractures, union rates are similarly high whether treated in a cast or with surgery. In a prospective randomized trial of cast versus surgery for nondisplaced waist fractures, 100% of fractures healed in both groups. In a multicenter trial, non- to minimally displaced scaphoid waist fractures showed a 98% union rate in a short-arm cast. Dias and colleagues advocate that if a scaphoid waist fracture is displaced less than 2 mm, short-arm casting is used. If a nascent nonunion is suspected by radiographs and a CT scan confirms this, then surgery is performed. This strategy produced union rates of 95%. In a multicenter randomized controlled trial of surgery versus casting for nondisplaced waist fractures, there was no difference in an early surgical group versus a cast immobilization group assessed by PRWE at 52 weeks.
Good outcome for cast treatment is not necessarily the case if all acute fractures are treated with cast immobilization. Symes and Stothard performed a systematic review of cast versus surgery for acute scaphoid fractures. Subgroup analysis was not performed as to location or displacement. The rate of nonunion was three times less for surgery, and recovery was quicker. However, there were more complications with surgery.
There is evidence that fixing nondisplaced waist fractures allows for faster healing and earlier return to work. Bond and colleagues demonstrated, in a prospective study of 25 active military personnel randomized to cast or screw treatment, that time to healing was significantly faster for the group who had surgery (7 versus 12 weeks) and the return-to-work time was also significantly faster for this group (8 versus 15 weeks).
The treatment of scaphoid waist fractures by surgery has raised the issue of cost. Would the health care system be better served by plaster immobilization of these stable fractures, or are the theoretical cost savings offset by the increased costs of surgically treating fractures that failed to heal (6% to 23% nonunion rate)? Arora and colleagues compared two groups of stable scaphoid fractures, one treated with plaster immobilization and the other with internal screw fixation. They concluded that internal screw fixation of nondisplaced scaphoid fractures had a shorter time to bony union and that the patients returned to work an average of 7 weeks earlier than patients with cast immobilization. Although it is assumed that operative treatment is more expensive, in this study, the overall cost was not found to be higher.
Davis and colleagues conducted a cost/utility analysis to weigh open reduction and internal fixation (ORIF) against cast immobilization in the treatment of acute nondisplaced midwaist scaphoid fractures. The authors used a model to calculate the outcomes and costs of ORIF and of cast immobilization, assuming the societal perspective. Medical costs were estimated using Medicare reimbursement rates, and costs of lost productivity were estimated by average wages obtained from the U.S. Bureau of Labor Statistics. ORIF offered greater quality-adjusted life-years (QALY) than casting. ORIF was less costly than casting ($7940 versus $13,851 per patient) because of a longer period of lost productivity with casting. When considering only direct costs, the incremental cost/utility ratio for ORIF ranged from $5438 per QALY for the 25- to 34-year-old age group to $11,420 for the 55- to 64-year-old age group, and $29,850 for the age group 65 years and older. They concluded that ORIF is more cost-effective than casting for acute scaphoid fractures.
Age and activity level usually help guide treatment choices as well. Younger active patients may choose surgery to return to work and activities and sports sooner. Older patients may choose cast immobilization, especially if they have comordities that increase surgical risks.
The choice of operative or nonoperative treatment must be individualized based on the discussion of pros and cons of treatment with the patient. If a cast is chosen, there are controversies about what type of cast to use. In a cadaveric study, there was motion of 1 to 4 mm in simulated scaphoid fractures treated with a short-arm thumb spica cast. Therefore the authors recommended a long-arm thumb spica cast. In a series by Dias and colleagues, 23% of patients treated with a short-arm cast with the thumb free did not unite at 12 weeks. However, in a recent multicenter, randomized control trial, non- to minimally displaced scaphoid waist and tubercle fractures were treated with short-arm casts, randomized with half immobilizing the thumb and the other half leaving the thumb free. Results showed a 98% union rate with no difference whether the thumb was included.
In two trials that compared long-arm casts versus short-arm casts and thumb spica casts versus short-arm casts, there was no difference in outcome. Alshryda and associates performed a metaanalysis of 13 level I studies (randomized controlled trial [RCT]) and concluded that for closed treatment there was no difference between short-arm and long-arm casting or between thumb spica and short-arm casting. Union rates were the same for surgical and nonoperative treatment for undisplaced fractures. Surgery was recommended for displaced fractures.
Proximal pole fractures are considered by many to be unstable, whether or not they are displaced, because of their small size, their tenuous blood supply, their articular location, and the relatively large moment arm across the fracture site. Rettig and Raskin reported 100% healing of 17 proximal pole fractures treated acutely with operative screw fixation through a dorsal approach. Nonunion rates for proximal pole fractures are 34% if casted and 2% if fixed operatively. Overall, there is a 7.5 times risk of nonunion for proximal pole fractures compared with fractures at the waist or distal pole. Based on known data, any proximal pole fracture, whether nondisplaced or displaced, should be fixed operatively. Because it is much more likely to gain solid fixation of the proximal pole through a dorsal approach, proximal pole fractures are best fixed with an antegrade screw technique.
Fractures of the immature scaphoid are uncommon and can be challenging to diagnose. These fractures most commonly involve the distal scaphoid and are effectively treated with cast immobilization. Fortunately, most acute pediatric scaphoid fractures heal with nonoperative treatment, and scaphoid fracture fixation in children is indicated only if there is nonunion.
The diagnosis of acute scaphoid fracture may be missed or delayed because of minimal symptoms. This is particularly true in athletic adolescents, who may return to sports prematurely after a seemingly minor injury to the wrist. The presentation of scaphoid fractures in adolescents has changed over the years and today more closely resembles the adult pattern. Malunion or nonunion may occur in patients with a missed diagnosis or delayed presentation and occasionally in patients treated promptly with immobilization. Surgical intervention should be considered for fracture nonunions in patients who are at or near skeletal maturity or in those in whom nonsurgical treatment has failed. Mintzer and Waters presented the outcome of 13 pediatric scaphoid fracture nonunions in 12 children treated over an 18-year period. The average elapsed time between fracture and surgery was 16.7 months. Four of the nonunions were treated with inlay bone graft from a palmar approach, and nine were treated with Herbert screw fixation and iliac crest bone grafting. The average time of follow-up was 6.9 years (range, 2 to 19 years). All cases went on to clinical and radiographic union. Patients had no statistically significant difference in range of motion or strength between the operative and contralateral normal wrists. The length of time for postoperative immobilization in the Herbert screw group was significantly less than that in the inlay bone graft group. Though scaphoid nonunions in children can heal with prolonged cast immobilization, the authors recommended that the treatment of scaphoid fracture nonunions in the skeletally immature patient be rigid fixation with a compression screw and iliac crest bone graft.
Masquijo and Willis presented their series of 23 pediatric (average age, 15 years) scaphoid nonunions fixed with iliac crest bone graft and screws, with a 95.6% union rate.
Weber reported on six children with nonunited scaphoid fractures treated nonoperatively. The mean age was 12.8 years (range, 9.7 to 16.3 years), and the mean follow-up time was 67 months. Five had no previous treatment, and the time to diagnosis averaged 4.6 months (range, 3 to 7 months) after injury. Treatment consisted of cast immobilization until clinical and radiologic union. Fractures united in all six children after a mean period of immobilization of 5 months (range, 3 to 7 months). All patients returned to regular activities. Although prolonged treatment with cast immobilization resulted in union of the fracture and an excellent subjective wrist score in all patients, this delay may not be well tolerated by the child or family.
Surgery is generally indicated for delayed presentation of a scaphoid fracture 4 to 6 weeks or more following injury, even in seemingly nondisplaced fractures. Patients with delayed presentation of scaphoid fractures have a higher likelihood of nonunion with closed treatment and require 4 to 6 months to heal in plaster.
Scaphoid fractures are common in competitive and recreational athletes, and such patients are reluctant to submit to the long period of immobilization and restricted activity that plaster requires. These factors may influence treatment decisions. The treating physician may permit participation in athletic activity with a playing cast. Some organizations permit casts that are protected with foam padding. Whether the forces generated by firm gripping are detrimental to healing probably depends on the fracture’s inherent stability. One study reported a faster return to play with internal fixation compared with a playing cast alone. A potential problem with plaster immobilization is compliance. Young people often will modify or remove the cast and are increasingly noncompliant with follow-up over time. The pressure to return to sports may lead the patient and coach to seek ways of shortening the period off of the field. The player’s ability to return to sports before the fracture is healed depends on the sport and its requirements. Options to be weighed will be surgery, a playing cast, and playing restrictions. The goal will be the successful union of the scaphoid fracture regardless of the patient’s athletic responsibilities. Clearly, educating the patient, family, trainer, and coach is essential. Every sport and different levels of the sport (professional, collegiate, high school) have different rules, and decisions are best customized based on individual circumstances. ,
I order CT scans on all scaphoid fractures to assess for displacement and fracture geometry. I indicate surgery for all proximal pole fractures and displaced (>1 mm) waist fractures. Distal pole fractures are almost always treated nonoperatively in a short-arm cast, unless a person’s occupation or special needs requires earlier and unrestricted mobility. For nondisplaced waist fractures, I have a discussion with the patient about the advantages and disadvantages of operative and nonoperative approaches. For nonoperative care, I treat in a short-arm cast. See Table 16.1 for the author’s preferred treatment of acute scaphoid fractures.
| Type of Fracture | Treatment |
|---|---|
| Stable Fractures, Nondisplaced (Obtain CT Scan to Ensure the Fracture Is Nondisplaced, Especially on Long Axis Scaphoid View. See Below for Definition of Displacement) | |
| Tubercle fracture | Short-arm cast for 6–8 weeks |
| Distal third fracture or incomplete fracture | Short-arm cast for 6–8 weeks |
| Waist fracture | Short-arm cast until healed, especially for pediatric patients and sedentary or low-demand patients, preference for nonoperative treatment |
| Mini-open internal fixation (APM: dorsal approach), especially for active, young, manual worker, athlete, or worker in high-demand occupation, preference for early range of motion | |
| Proximal pole fracture, nondisplaced | Mini-open internal fixation, dorsal approach |
| Unstable Fractures | |
| Displacement of more than 1 mm Lateral intrascaphoid angle of more than 35 degrees Bone loss or comminution Perilunate fracture-dislocation Dorsal intercalated segmental instability alignment (DISI with radiolunate angle >15 degrees) |
Open reduction and internal fixation, with or without bone graft |
Bone healing requires viable bone cells, an adequate blood supply, and stabilization of the fracture site. For a fixation device to be successful in providing rigid fixation of scaphoid fractures, it must be able to resist complex bending, shearing, and translational forces during normal functional loading. Because the majority of the scaphoid is covered with cartilage, fracture callus is not produced, so primary bone healing is entirely dependent on rigid stabilization of the fracture fragments until healing.
The mechanical effectiveness of internal fixation is determined by the bone quality, fracture geometry, fracture reduction, choice of implant, and implant placement. While all five of these independent variables are important, bone quality and fracture geometry are intrinsic to the patient. Fracture reduction, choice of implant, and implant placement are all under the surgeon’s control. Fracture reduction and placement of the implant in the biomechanically ideal position are the most important of the five variables.
Where and how to place the screw is controversial. Earlier literature focused on the longest central screw, where later literature focused on an awareness of the three-dimensional fracture geometry and placing the screw with all factors considered. While a central long screw is best for many fractures, it may not be best for all.
Trumble and colleagues observed that screws placed in the central third of the scaphoid were associated with significantly shorter healing times than screws placed outside of the central third axis ( P < .05). To explain this observation, McAdams and colleagues simulated scaphoid waist fractures and compared screws placed in the central axis with screws placed eccentrically. This study demonstrated that simulated fractures fixed with centrally placed screws in the proximal fragment demonstrated mechanical properties compared with screws placed in an eccentric position. Fixation with central screw placement demonstrated 43% greater stiffness, 113% greater load at 2 mm of displacement, and 39% greater load at failure.
Biomechanically, the longer the screw, the more rigid the fixation, because longer screws reduce forces at the fracture site and bending forces are spread along the threads. In a cadaveric study by Dodds and colleagues, short or long screws were placed along the central scaphoid axis after a simulated waist osteotomy, and constructs with longer screws were significantly stiffer than those repaired with short screws.
More recent literature focusing on the obliquity and location of the fracture line has called into question using a long central screw for all cases. Biomechanical modeling of preoperative CT scans demonstrated increased compression strength when cannulated screws were placed within 10 degrees of a perpendicular trajectory to the plane of the fracture. In another biomechanical study using preoperative CT scans, Kupperman et al. identified the ideal starting point and trajectory for proximal, waist, and distal pole fractures that predicted maximum “effective compression length,” a composite of screw perpendicularity and number of crossing screw threads.
When rigid fixation cannot be provided by a screw placement alone (as in extreme proximal pole fractures and nonunions), augmentation may be necessary to prevent micromotion at the fracture site. Supplemental fixation can be applied from the distal scaphoid to the capitate using a 0.045-inch or 0.062-inch Kirschner wire or a mini–headless screw. If a Kirschner wire is placed, it should be buried because it may be necessary to leave it in place for 3 months or until the bone is healed.
Implants used include Kirschner wires, headless compression screws, staples, and plates. Solid and cannulated screws are available from several manufacturers. Any implant used must reduce bending, shearing, and translational forces acting at a fracture site.
Although Kirschner wires are easy to insert, they have a narrow role for scaphoid fixation today, given the relatively insecure fixation and minimal compression afforded by these implants. Kirschner wire fixation must be supplemented with a cast until healing, and a separate procedure for Kirschner wire removal is required. In multitrauma situations or open fractures, rapid stabilization of an unstable scaphoid fracture may be expedient.
In 1954, McLaughlin described fractures of the scaphoid as “an unsolved problem.” His main interest was returning a “breadwinner” to work with a treatment that would “hold bone fragments in apposition” until healing. He reported on the fixation of scaphoid fractures using solid lag screws. The operative procedure was technically challenging, the optimal screw position was not always achieved, and the incidence of nonunion in unstable fractures was not substantially reduced over that obtained by casting alone.
Herbert and Fisher in 1984 presented the results of the first headless screw used to treat 158 patients from 1977 to 1981. The rate of union was 100% for acute fractures and 83% overall. This screw revolutionized bone fixation because it permitted compression of a fracture with two heads of differential pitches ( Fig. 16.9 ). The embedded threaded heads of headless screws are placed in the densest bone of both poles for maximum bony purchase. This paper also demonstrated that screws placed perpendicular to an acute fracture plane using compression and rigid fixation could successfully heal an acute fracture. The implant, however, is not technically easy to insert, as it required a special compression jig that was difficult to position; other centers reported lower rates of union with screw fixation of acute scaphoid fractures secondary to technical problems.
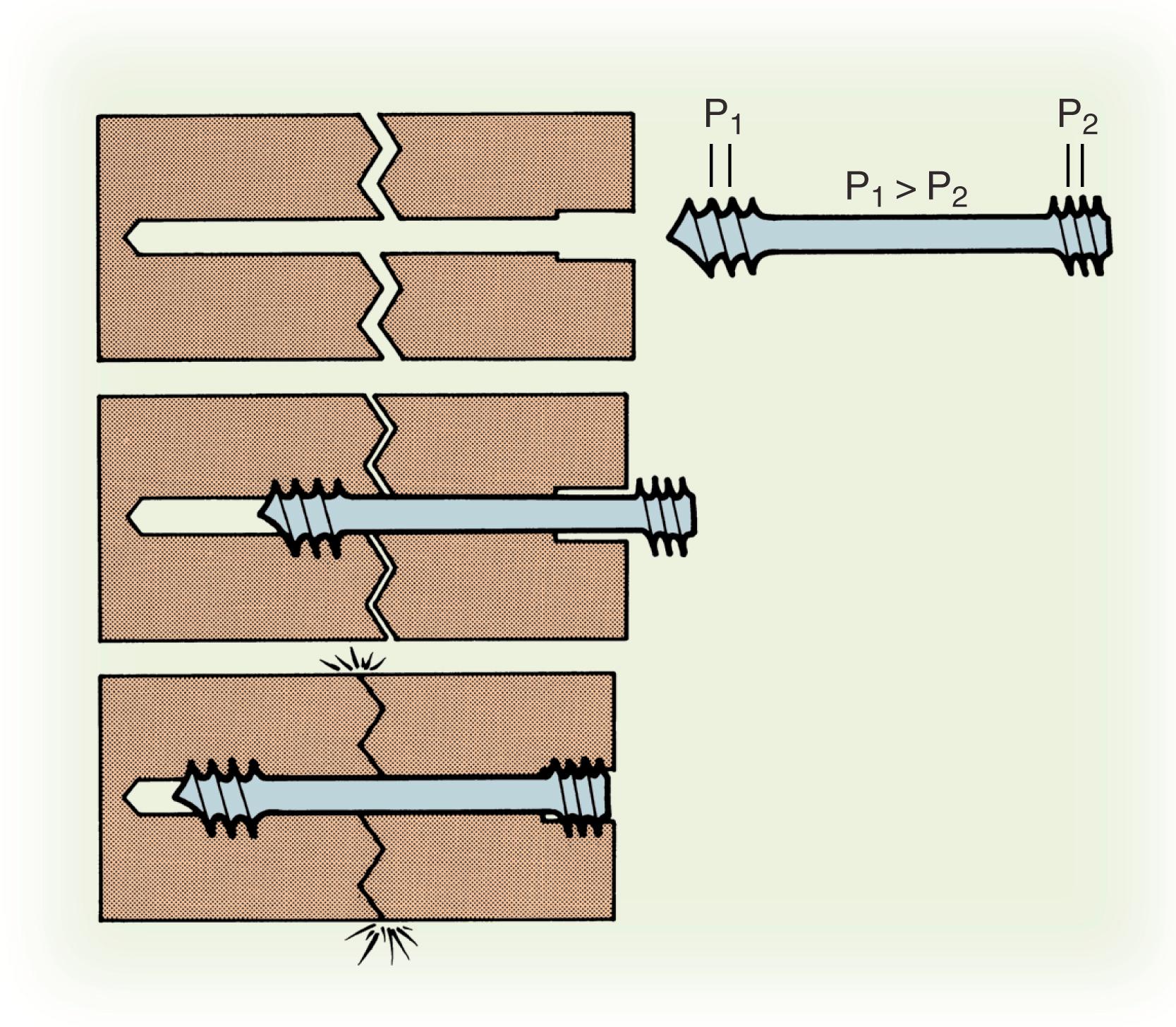
The next major development in headless screw design was the cannulated compression screw. This device greatly simplified accurate placement of the screw within the scaphoid bone by using a thin guidewire placed under fluoroscopic control. A fully threaded, variable-pitch implant, the Acutrak screw (AcuMed, Hillsboro, OR), demonstrated compression comparable to that of a standard 4-mm compression screw and greater compression compared with the Herbert screw in biomechanical testing. Several manufacturers have developed cannulated compression screws with unique advantages and disadvantages. There have been a number of biomechanical studies comparing headless compression screws. Most second-generation headless compression screws achieve adequate compression; however, the compression decreases by 50% 12 hours after placement, and the compression does not necessarily correlate with the feel of torque on the screwdriver. What is unknown is how much compression is necessary for bone healing. In a biomechanical study of different screws in cadavera, no one screw demonstrated superior mechanical properties. Currently, screw selection is still largely a matter of surgeon preference.
The use of staples has had its proponents as a means of achieving stable fixation. Early studies demonstrated simple application and satisfactory healing rates among patients with scaphoid nonunions and acute fractures but long-term degenerative changes secondary to hardware impingement. New staples with memory achieve compression after insertion as they warm to body temperature. In a series of 131 patients with acute fractures and nonunions of the scaphoid waist, all acute fractures healed and all but 2 nonunions healed. However, pain was present in 21% of cases. This method has not replaced headless screw methods.
The main advantage of the dorsal approach is that it is technically easier to obtain an ideal screw position in the central axis of the scaphoid. Many surgeons prefer dorsal mini-open screw fixation because of the ease of access, the minimal soft tissue disruption, and the ability to place a screw down the central axis. , The disadvantage of the dorsal approach is that it requires flexion of the wrist, which may theoretically displace an unstable fracture. The dorsal mini-open approach for scaphoid fracture fixation is indicated for proximal pole and waist fractures. A small incision is safer than a purely percutaneous method when approaching from the dorsal wrist. Weinberg and colleagues have shown that there is a 13% chance of tendon injury with a purely percutaneous technique.
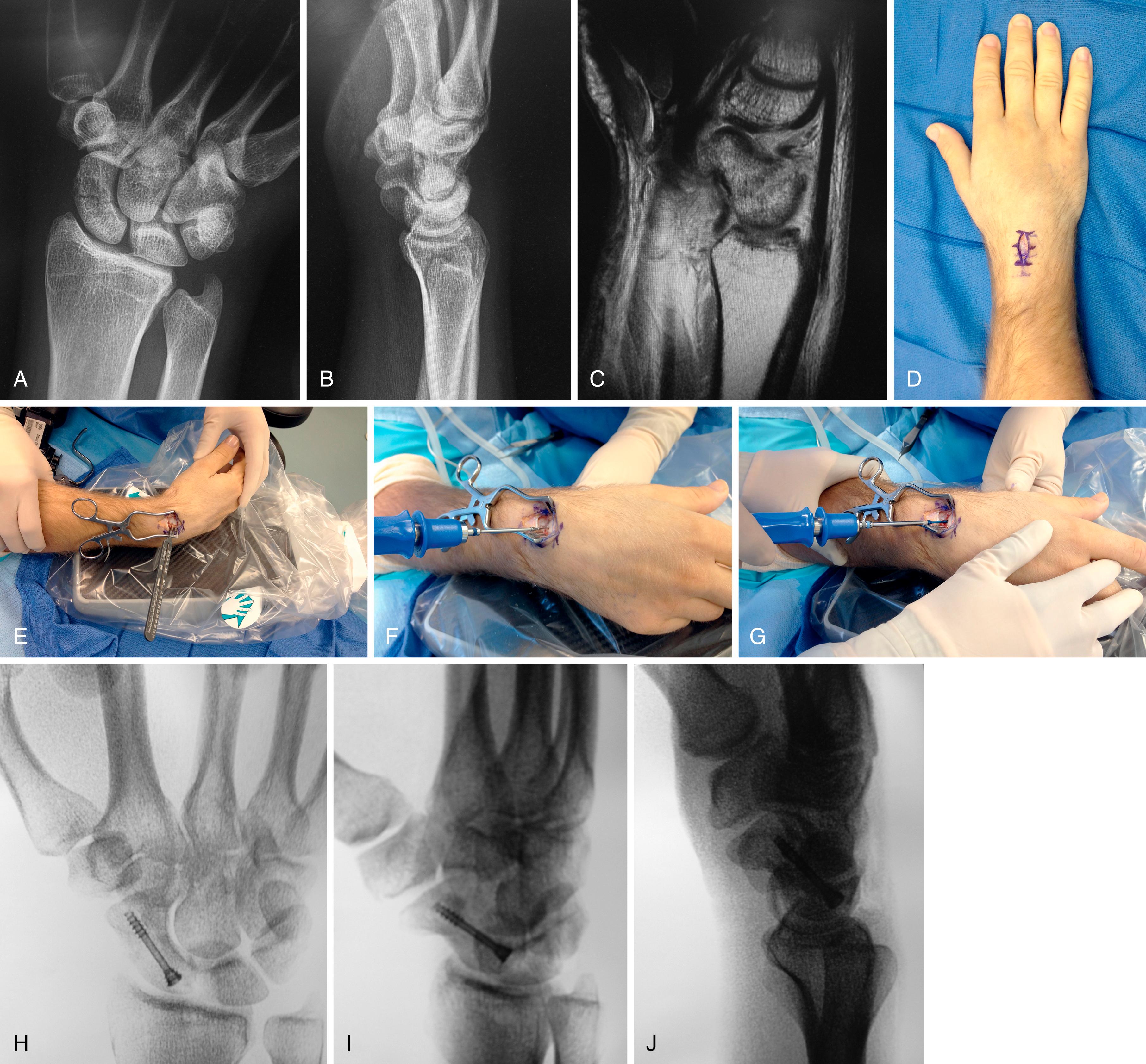
To perform the dorsal mini-open technique, a tourniquet is placed proximally on the arm and draped to allow full motion of the extremity. A 2- to 3-cm longitudinal or transverse incision is made over the proximal pole just distal to the Lister tubercle, and just radial to the area that corresponds with the 3-4 arthroscopic portal. The extensor pollicis longus (EPL) is carefully identified and the second and third dorsal compartment tendons are retracted radially. The capsule is incised off the radial rim and on the radial side of the Lister tubercle. If more exposure is necessary, the “window” approach is extended in an oblique fashion toward the lunate and just distal to the fibers of the dorsal radiocarpal ligament (see Chapter 13 and Video 13.4) ![]() .
.
Extreme care is taken to avoid disruption of the dorsal fibers of the SLIL and dorsal scaphotriquetral ligament when reflecting the capsular flap ( Chapter 13 and Video 13.4 ![]() ). Dissection in a plane tangential to the dorsal surfaces of the scaphoid and the lunate can be performed with a scalpel or Beaver blade. The distal boundaries of dissection of the scaphoid are determined by the vascular supply along the dorsal ridge, which must be preserved. Wide skin hooks are used to retract the capsule or the capsule is sutured to nearby soft tissue or skin. If the scaphoid is displaced, the hematoma should be evacuated and the scaphoid reduced with the aid of a dental pick or smooth Kirschner-wire joysticks, if necessary. The wrist is flexed and the entrance point on the scaphoid is identified 1 to 2 mm radial to the membranous portion of the scapholunate ligament and in the midportion of the scaphoid in the sagittal plane for long axis screw placement. For oblique fractures, preoperative CT scan is used to identify the optimal location of the starting point and screw trajectory to gain maximum effective compression length.
). Dissection in a plane tangential to the dorsal surfaces of the scaphoid and the lunate can be performed with a scalpel or Beaver blade. The distal boundaries of dissection of the scaphoid are determined by the vascular supply along the dorsal ridge, which must be preserved. Wide skin hooks are used to retract the capsule or the capsule is sutured to nearby soft tissue or skin. If the scaphoid is displaced, the hematoma should be evacuated and the scaphoid reduced with the aid of a dental pick or smooth Kirschner-wire joysticks, if necessary. The wrist is flexed and the entrance point on the scaphoid is identified 1 to 2 mm radial to the membranous portion of the scapholunate ligament and in the midportion of the scaphoid in the sagittal plane for long axis screw placement. For oblique fractures, preoperative CT scan is used to identify the optimal location of the starting point and screw trajectory to gain maximum effective compression length.
The surgeon should aim the guidewire for headless compression screw from the ideal starting point to the articular base of the thumb metacarpal (because the scaphoid distal pole, trapezium, and metacarpal base are collinear). Once this wire is placed, the surgeon must not extend the wrist because this will bend the wire at the radiocarpal joint and risk guidewire breakage. Fluoroscopic images are taken to assess the fracture and wire position. In order to obtain the PA images while keeping the wrist flexed, it is imperative to flex the elbow. An optimal position of the guidewire in all views (PA, lateral, and oblique) should be obtained. The optimal position is one that has a combination of being closer to perpendicular to the fracture yet has as much bone on either side of the guidewire as possible.
When the wire is ideally positioned, the wire is advanced to the subchondral bone on the distal end of the scaphoid bone and measured. The appropriate screw length is shorter than this distance by at least 4 mm. For an adult man, 20 mm is often an appropriate length. The screw should be relatively long but should definitely not be too long. If the screw is too long, it will either distract the fracture when it abuts the unyielding distal subchondral bone or protrude from the bone distally or proximally. Also, as the fracture is compressed the length of the bone will diminish. Once this length is determined, the surgeon may choose to drive the guidewire into the trapezium bone and out the thenar skin so that wire retrieval can be easily accomplished should the wire break during drilling or screw insertion. A second, antirotation wire may also be placed. The guidewire is overdrilled with a cannulated drill. Power is used for the near cortex, but then the rest is hand drilled because on power the drill can cut the guidewire, especially if there is a slight bend in the wire. If when drilling, the drill bit does not advance, then stop and get fluoroscopy to confirm that the wire is not bent and that the drill is collinear with the guide wire. Although some of the systems tout a “self-drilling” screw, drilling past the fracture can be performed as determined by fluoroscopy. After drilling, and depending on the particular screw system, countersinking the proximal hole can help avoid a catastrophic proximal pole fracture. Most systems come with a countersink. Particularly when using a tapered screw, countersinking will decrease the hoop stresses on the proximal pole to lessen the chance of fracture. After countersinking, a screw of the appropriate size is placed, making sure that it is well seated below the cartilage and into the proximal subchondral bone. A study by Hart and colleagues showed that the torque of the screw turns felt by the surgeon does not correlate with fracture compression. After the screw is placed, it is important to remove the guidewire and check all fluoroscopic views (PA, lateral, supinated, and pronated oblique views) to ensure an excellent fracture reduction and hardware position. Particularly helpful is the 60-degree pronated oblique view in demonstrated screw prominence from the proximal pole. If unsure about screw position, live fluoroscopy should be used, rotating the wrist 360 degrees. The capsule is closed with absorbable suture. The tourniquet is released, and hemostasis achieved. The skin is closed, and a well-padded short-arm splint is placed. Depending on fracture stability and patient compliance, a postoperative short-arm thermoplastic splint or short-arm cast may be placed at 2 weeks until the bone is healed; this may take up to 12 weeks following operation, especially for proximal pole fractures. Fracture healing is determined clinically by lack of tenderness and by imaging with radiographs. CT scan may be obtained at 3 months to confirm healing before releasing patients to sports and activities, provided their strength and motion is within 80% of their uninjured side.
The main difference in the dorsal percutaneous technique is that it is a percutaneous technique; as such the entrance point is identified by flexing the wrist and identifying the superimposed rings on fluoroscopy of the proximal and distal poles. The guidewire is placed down the center of the superimposed rings. The wire may be advanced out of the radial aspect of the thumb so the wrist can be extended for standard wrist radiographs. The wire may be further withdrawn to perform percutaneous or arthroscopic reduction before reengaging the wire in the proximal fragment. When satisfactory scaphoid reduction and wire placement is confirmed, the wrist is again flexed and the wire driven dorsally for the remainder of the procedure. The skin about the wire insertion site is opened with a No. 11 blade and blunt dissection carried out down to the capsule prior to drilling and inserting the screw. Care must be taken to avoid injury to the extensor tendons in this technique.
The goals of arthroscopy-assisted stabilization of scaphoid fractures are to reduce displaced fractures without an open incision and provide secure fixation that will permit early motion until solid union has been achieved. The early results of arthroscopy-assisted percutaneous screw fixation of displaced fractures of the scaphoid suggest that minimally invasive reduction and fracture union can be predictably obtained with good to excellent functional results in the correctly selected patient. Avoidance of open exposure limits the potential for wrist ligament injury, may help preserve the blood supply, and minimizes postoperative stiffness. However, many scaphoid fractures are now treated with mini-open techniques that create little scarring and do not adversely affect postoperative motion.
Slade et al. reviewed his results in arthroscopy-assisted fixation from a dorsal approach in 27 consecutive patients. There were 18 waist fractures and 9 fractures of the proximal pole. Seventeen patients were treated within 1 month of injury, and 10 patients were treated late. All fractures healed, as documented by CT scan.
Arthroscopy-assisted fixation of scaphoid fractures also allows for simultaneous detection of associated intracarpal soft tissue injuries. Braithwaite and Jones originally reported on four patients with a fracture of the scaphoid with complete scapholunate dissociation. As in fractures of the distal radius, associated soft tissue lesions may occur with scaphoid fractures, and arthroscopic evaluation allows detection and management. It is not known whether early arthroscopic detection and management of the associated injuries improve the final outcome. For operative details of arthroscopic-assisted percutaneous treatment of scaphoid fractures and scaphoid nonunions, the reader is referred to Chapter 17 .
The palmar approach may be used for acute waist fractures, scaphoid nonunions of the waist with humpback deformity, and the rare distal pole fracture that may require surgical fixation. An advantage of the palmar approach is that the wrist is extended, theoretically helping to reduce the fracture. A disadvantage is that it is not possible to place the screw down the true axis of the bone because the trapezium blocks the entrance of the center of the distal scaphoid ( Fig. 16.11A ).
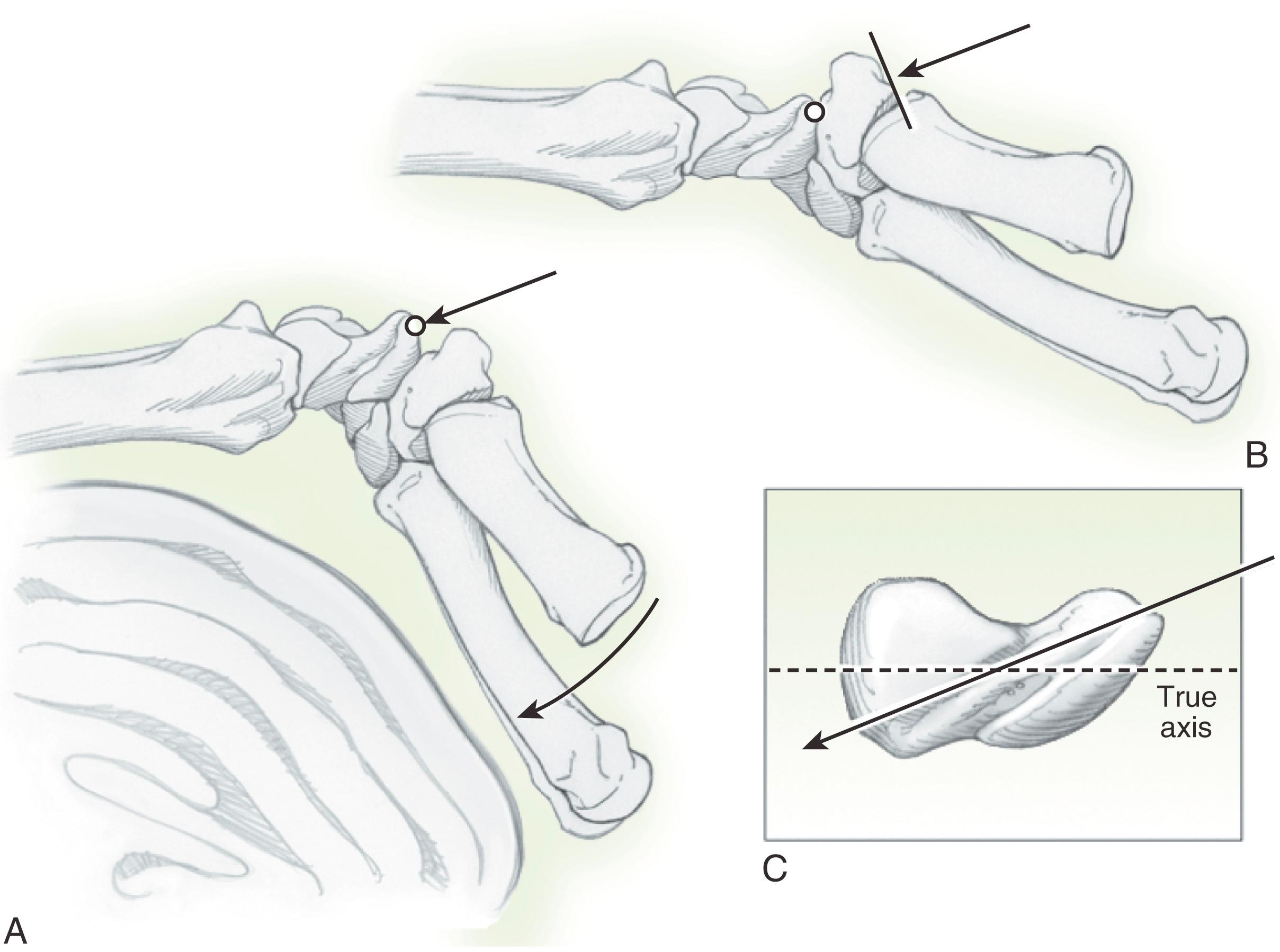
The placement of the screw is always oblique; the more proximal the fracture, the less chance of engaging sufficient screw threads in the proximal fragment. Another concern is penetration of the dorsal surface of the scaphoid bone by the screw, especially when attempting to capture more proximal fractures. The optimal radiographic views for determining dorsal screw prominence are the 60-degree pronated oblique view as reported by Kim and colleagues ( Fig. 16.12 ).
The open palmar approach is most commonly used for scaphoid nonunion and less frequently for displaced comminuted or flexed acute scaphoid fractures. In the open volar approach, a hockey-stick incision is made over the flexor carpi radialis (FCR) tendon in the distal forearm and angled across the distal wrist crease toward the base of the thumb. The FCR tendon is retracted ulnarly and the radial artery radially. The wrist is hyperextended over a bump. The wrist capsule is entered through a curvilinear incision from the volar lip of the radius to the proximal tubercle of the trapezium. The capsule and intracapsular ligaments are carefully divided and reflected sharply off the scaphoid with a scalpel. The capsule needs to be preserved, as it contains the RSC ligament and will be repaired at the close of the procedure. Some surgeons prefer to tag the ligaments at this stage with nonabsorbable sutures for later repair. The entire volar scaphoid is exposed. Reduction is performed with dental pick or by manipulation or with joysticks. Bone grafting can be performed as required for volar comminution or in subacute fractures, with the grafts harvested from the volar radius beneath the pronator quadratus by extending the incision proximally an additional 2 to 3 cm. The scaphotrapezial joint may be opened to place a guidewire more centrally in preparation for final fixation. If necessary, part of the proximal trapezium can be excised with a rongeur to clear an unobstructed path for the guidewire and screw. Rigid internal fixation can be performed with the screw of choice.
With the volar approach, it is important to know that it is impossible to put the screw down the long axis of the scaphoid. Verstreken and colleagues have popularized a transtrapezial approach that enables more central screw position. , Leventhal and associates performed a computational analysis to identify the ideal starting point (1.7 mm dorsal and 0.2 mm radial to the tip of the scaphoid tubercle) to enable the longest possible screw trajectory in the scaphoid without violating the trapezium.
Percutaneous scaphoid fixation may be performed with or without traction through a palmar approach. Fractures suitable for treatment with the volar percutaneous (distal to proximal) approach are fractures at the waist and distal pole. Humpback deformities or scaphoid collapse with a DISI deformity usually require open reduction, bone grafting, and fixation. Proximal pole fractures are best treated via a dorsal (proximal to distal) approach. An advantage of the palmar approach for percutaneous fixation is that the entrance of the scaphoid tubercle is subcutaneous; thus there are no tendons in the path of the approach. The incision may be enlarged slightly as needed to allow overdrilling of the guidewire and placement of the screw. Like the dorsal approach, a percutaneous volar approach can be extended if necessary to openly reduce the fracture or treat a nonunion.
Streli was the first to describe volar percutaneous screw fixation of the scaphoid fracture in 1970 using traction applied through the thumb and a standard Association for the Study of Internal Fixation (ASIF) screw. In 1991 Wozasek and Moser reported on an adaptation of Streli’s technique using cannulated 2.9-mm screws via a volar percutaneous approach. Later, Bond and colleagues demonstrated faster healing and return to work with modern cannulated headless compression screw fixation from the palmar approach, compared with cast immobilization.
To perform percutaneous volar scaphoid screw fixation, the patient is placed supine on an operating table and the hand is suspended vertically by the thumb using a finger trap ( Fig. 16.13 ). This position extends the scaphoid and ulnarly deviates the wrist to improve access to the distal pole of the scaphoid. A fluoroscopic imaging unit is rotated parallel to the floor and positioned so that the wrist is in its central axis. Traction from a tower permits full rotation of the scaphoid in the imaging beam. In the majority of cases, longitudinal traction is enough to reduce the scaphoid fracture. If the fracture is not reduced, Kirschner wires can be inserted and used as joysticks to manipulate the fragments into position. The quality of the reduction can then be checked radiographically.
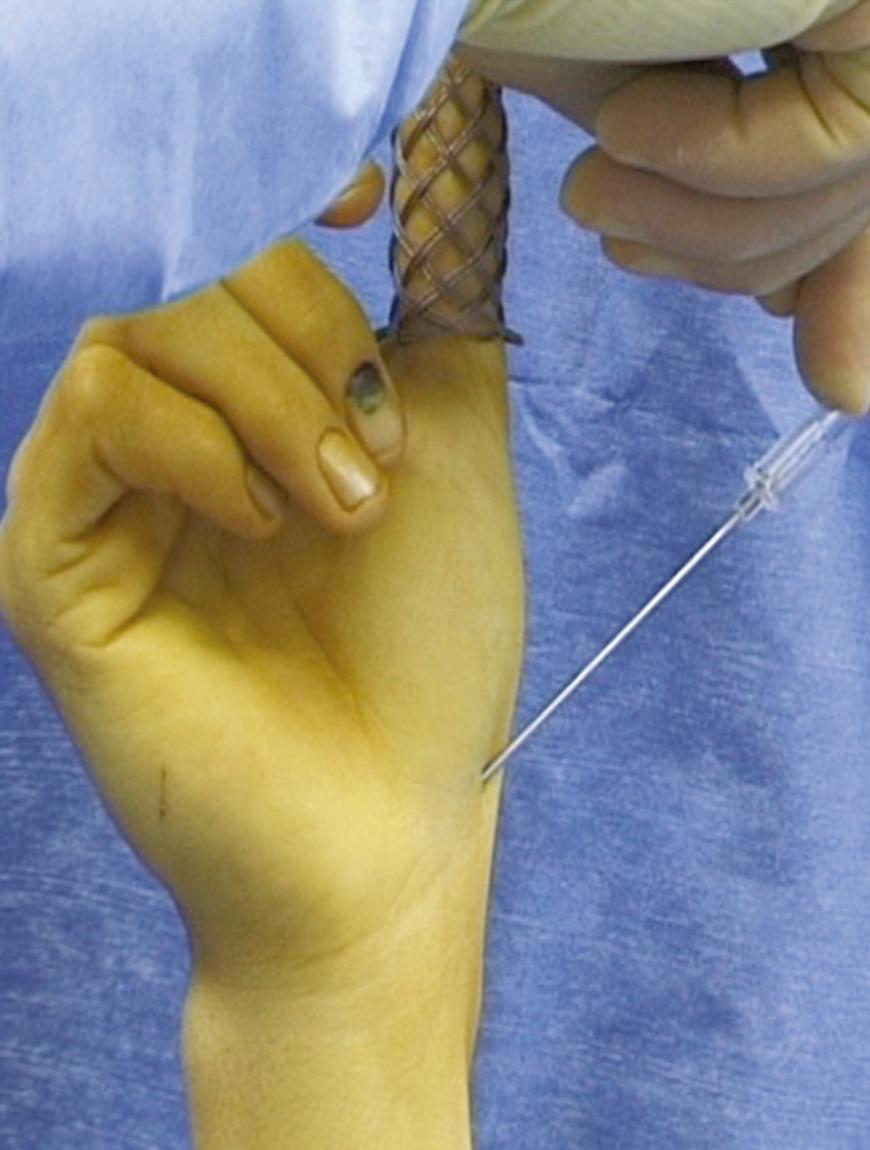
Having achieved an acceptable reduction, the most important step is to establish the entry point of the guidewire. The ulnar deviation of the wrist extends the scaphoid to make the tubercle more accessible. The entry point, the scaphoid tuberosity, is located using a 12- or 14-gauge intravenous needle introduced on the anteroradial aspect of the wrist, just radial and distal to the scaphoid tuberosity. The needle serves as a trocar to guide the wire and establish a central path along the scaphoid. The needle is inserted into the scaphotrapezial joint and tilted into a vertical position. The needle is levered on the trapezium, which brings the distal pole of the scaphoid more radially and facilitates screw insertion. The wrist is rotated in the fluoroscopic beam to confirm that the needle is aligned along the axis of the scaphoid in all planes, with the intent of directing the guidewire into the proximal pole to a point just radial to the scapholunate ligament. Leventhal and colleagues identified the ideal starting point to be approximately 2 mm dorsal and just radial to the apex of the scaphoid tubercle in order to achieve maximum guidewire length within the scaphoid.
Once the entry point and the direction of the guidewire are confirmed, the needle is impacted into the soft articular cartilage over the distal pole of the scaphoid so that the tip does not slip during insertion of the guidewire. The guidewire is passed down through the needle and drilled across the fracture; its direction is continually checked on the image intensifier and adjusted as necessary, with the goal of entering the radial aspect of the proximal pole. The position is checked in multiple fluoroscopy planes, and, if satisfactory, a longitudinal incision of 0.5 cm is made at the entry point of the wire and deepened down to the distal pole of the scaphoid using a small hemostat and blunt dissection. This is a relatively safe zone, with minimal risk to the adjacent neurovascular structures.
The length of the screw is determined using a depth gauge or by advancing a second guidewire of the same length up the distal cortex of the scaphoid and subtracting the difference between the two. The correct screw size is 4 to 5 mm shorter than the measured length, which will ensure that the screw head is fully buried below the cartilage and the subchondral bone on each end. In rare cases a second antirotation wire may be inserted parallel to the first prior to drilling and reaming. The wire is advanced into the radius or out of the dorsal skin and clamped so as to avoid loss of wire position with drilling. The 12-gauge needle is removed and the cannulated drill is passed over the wire and advanced under imaging guidance, stopping 1 to 2 mm short of the far articular surface. At this point the hand is taken out of traction so that the screw will adequately compress the scaphoid. A countersink or a trailing drill is used, depending on the particular set. A self-tapping screw is advanced over the guidewire. The final position is checked with multiple fluoroscopic views to confirm complete containment within the scaphoid. A hyperpronated PA view profiles the dorsal-radial cortical margin of the scaphoid, where a perforation of the proximal cortical bone can occur. Compression of the fracture site is confirmed radiographically on the image intensifier. The wire is removed, the skin closed with a suture or Steri-Strips, and the wound covered with a sterile compressive dressing.
A plaster splint is removed at 10 to 14 days postoperatively. The sutures are removed at this stage, and wrist radiographs (PA, lateral, 45-degree oblique, scaphoid view) are taken to confirm that screw position is satisfactory. Depending on the bone quality, fixation, and assumed patient compliance, a short-arm cast or well-molded thermoplastic short-arm splint is used for 4 additional weeks or until the fracture has healed. Hand therapy may be useful to regain hand motion and forearm rotation; no heavy carrying or weight-bearing activity is permitted. A return to sedentary work is allowed as soon as the patient feels ready or when 75% of the contralateral range of movement is achieved. When radiographic and clinical union have been achieved, the splint is discontinued and all previous activities are resumed as tolerated. CT is used to confirm healing before return to heavy lifting or competitive athletics.
Potential complications include malposition of the screw, violation of the cortical surface, and hardware protrusion within the radioscaphoid or scaphocapitate joint proximally, breakage of a guidewire, and fracture of the scaphoid during screw placement. Another potential problem is a failure to completely bury the head of the screw within the scaphoid, which can lead to STT arthrosis. This problem is avoided by selecting a screw length approximately 4 to 5 mm shorter than measured with the depth gauge. Fracture displacement can occur with guidewire malposition or in proximal pole or oblique fractures. Other risks include transient dysesthesia near scars. This is secondary to a neurapraxia of nearby sensory branches and usually resolves within 4 to 6 weeks. Nonunions or delayed unions using the volar approach have occurred with proximal pole fractures, and small proximal pole fractures should be treated using a dorsal approach.
Because of the increased risk of tendon injury (13%) with a percutaneous dorsal technique, I prefer the dorsal mini-open technique of headless cannulated screw insertion for many nondisplaced scaphoid waist and all proximal pole fractures. I precede each surgery with CT to identify the precise location and obliquity of the fracture line, and then calculate the screw entry point and trajectory to optimize the effective compression length of the screw. The guidewire is advanced from the ideal starting point to the opposite cortex, checked under fluoroscopy and measured. The appropriate screw length is shorter than this distance by at least 4 mm. I often use a screw even shorter; a 20-mm screw is often an appropriate length for an adult male. The screw should be relatively long but should definitely not be too long. If it is too long, it can distract the fracture or protrude out of the bone distally or proximally. After measuring, consider advancing the Kirschner wire into the trapezium, and when possible, through the thenar skin as a safeguard against wire breakage. I use power to drill the near cortex then hand drill past the fracture. Hand drilling minimizes the chance of shear or breakage of the guide wire. The pronated oblique view on fluoroscopy is essential, and I do a 360-degree live rotation to be certain that the hardware is entirely contained in the scaphoid.
I immobilize for 2 weeks in a postoperative short-arm splint, then cast or splint for 4 weeks. If radiographs look like there is healing, I may allow gentle wrist motion out of the splint but then obtain a CT scan usually at 10 to 12 weeks to confirm healing before return to heavy work, sports, and activities.
For the rare distal pole fracture that requires surgery and for fractures in the distal waist, I prefer a mini-open volar technique of cannulated screw fixation. The upper extremity is draped to allow for full motion of the extremity and enable adequate visualization with fluoroscopy, and the tourniquet is inflated as needed. A 1- to 2-cm longitudinal incision is made just distal to the scaphoid tubercle. I do not use a traction tower, and prefer to hyperextend and ulnarly deviate the wrist over a bump. This moves the trapezium dorsally away from the entrance point on the scaphoid bone (see Fig. 16.11B ).
If the trapezium has a particularly palmar location, a rongeur may be needed to remove bone to gain access to the entrance point. The guidewire is started as dorsally as possible on the scaphoid in the sagittal plane without impinging on the trapezium. On the coronal plane, a good landmark for the starting point is a third of the distance from the radial side of the distal pole of the scaphoid ( Fig. 16.14A ). The surgeon should attempt to drop the hand to get as close as possible to the axis of the scaphoid (see Fig. 16.14B ). Fluoroscopy is used to optimize guidewire placement.
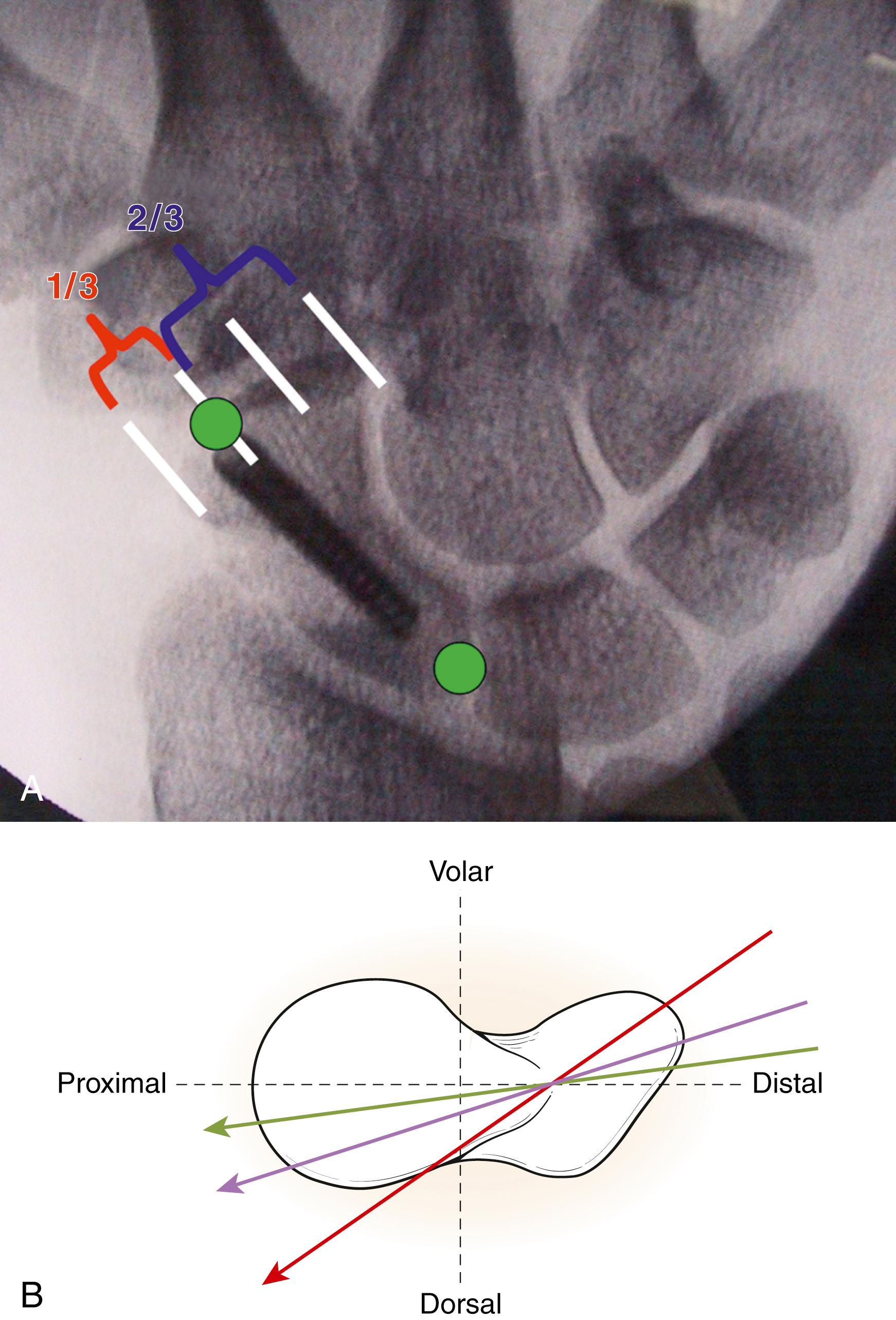
Once the guidewire is placed, multiple minifluoroscopic views are taken, including anteroposterior (AP), lateral, and oblique views. It is imperative to take 45-degree oblique views in supination and pronation to ensure that the wire is within the bone in all planes. The scaphoid and lateral views are particularly helpful to see the wire entrance and trajectory, and the 60-degree pronated oblique view is helpful to make sure the wire is entirely in the scaphoid bone. The wire should be advanced to the subchondral bone on the proximal side and measured, as above a screw at least 4 mm shorter than the measured length is selected.
Once an appropriate screw length is determined, the guidewire may be driven into the radius or out of the dorsal skin and clamped so that the wire will not come out during drilling. A second antirotation wire may be placed. The wire is overdrilled with a cannulated drill for the near cortex and hand drilled past the fracture. If during drilling there is resistance or the drill is not advancing, then I stop and get fluoroscopic images to make sure that the guide wire is not bent or the drill is not perforating out of the bone. Although some of the systems tout a “self-drilling” screw, my preference is to hand drill past the fracture as determined by fluoroscopy. After drilling, I believe it to be imperative to countersink the entrance hole. This is to decrease hoop stresses when tightening the screw, which can fracture the near fragment. After countersinking, a screw of appropriate size is placed, making sure that it is seated below the cartilage and flush with the subchondral bone. The bone with the best mechanical properties is the 2-mm subchondral shell of bone. After the screw is placed and the guidewire removed, it is important to check all fluoroscopic views (AP, lateral, 45-degree supinated, and 60-degree pronated oblique views) to ensure an excellent position of fracture reduction and hardware placement. If a tourniquet is used, it is deflated at this time. The incision is closed with sutures or Steri-Strips. A well-padded short-arm splint is placed. Depending on fracture stability and patient compliance, a thermoplastic short-arm splint or short-arm cast is placed at 2 weeks. Aftercare is as above for the dorsal approach.
Place the guidewire in optimal position based on preoperative CT scan.
Consider using an antirotation wire.
Common error is using a screw that is too long.
Subtract at least 4 mm from the measured distance.
A common screw length for an adult male is 20 mm but may be less depending on fracture geometry.
Do not drill past the far cortex.
If feeling a lot of resistance (especially when reaming over wire), stop and look. The wire may be bent and break or the drill bit may break ( Fig. 16.15 ).
Beware of hoop stresses. Use countersinking to avoid excessive hoop stresses that can fracture the near fragment.
Consider the use of dental pick or Kirschner wires as joysticks to gain reduction.
Consider supplemental fixation of the carpus for very small proximal pole fragments ( Fig. 16.16 ).
Combined fractures of the distal radius and scaphoid are uncommon but present a challenging treatment dilemma. Scaphoid fractures may not be recognized when associated with a comminuted distal radius fracture and when untreated can result in carpal collapse, cystic degeneration, and eventual carpal degenerative arthritis. Although an isolated stable scaphoid fracture might be safely managed with plaster immobilization, the 12 to 16 weeks of immobilization required for healing are not appropriate for the treatment of the distal radius fracture. Prolonged immobilization may result in arthrofibrosis and atrophy of the forearm and hand, making the recovery of full hand function challenging.
A review of the published reports on combined scaphoid and distal radius fractures demonstrates that treatments have evolved over the past decade. Trumble and colleagues reported on six patients treated with internal fixation for ipsilateral combined fractures of the scaphoid and radius. All patients sustained a high-energy injury from a fall from a substantial height. All of the fractures united, with the radial fractures healing in an average of 6 weeks and the scaphoid fractures healing in an average of 13 weeks. Internal fixation of the scaphoid in these combined injuries allowed for earlier and more aggressive therapy to maximize wrist and forearm motion. My preferred treatment for combined distal radius fractures with scaphoid fracture is ORIF of the distal radius fracture and the mini-open approach and fixation of the scaphoid fracture with a headless compression screw. (For additional information, see Chapter 15 .)
Acute fracture-dislocations of the carpus are uncommon. Perilunate fracture-dislocations represent approximately 5% of wrist fractures and are about twice as common as pure ligamentous dislocations. Transscaphoid perilunate fracture-dislocation is the most common type of complex carpal dislocation.
Treatment of these injuries can be challenging owing to the extensive soft tissue, cartilaginous, and bony damage. Furthermore, obtaining universally excellent long-term results can be elusive. Various operative treatment options have been recommended, including dorsal, volar, percutaneous, and arthroscopic approaches.
These injuries are usually due to a high-energy impact, as may occur in motor vehicle accidents, a fall from a height, or contact sports. The mechanism of injury characteristically involves forceful wrist extension, ulnar deviation, and intercarpal supination. The injuries have been classified as greater and lesser arc injuries and can take many forms (for more information, see Chapter 13 ). My preference is to get CT scans for all perilunate injuries, as some greater arc injuries can be subtle and overlooked on plain radiographs.
Herzberg and Forissier investigated the medium-term results (mean follow-up, 8 years) of a series of 14 transscaphoid dorsal perilunate fracture-dislocations treated operatively at an average of 6 days following injury. Eleven underwent ORIF through a dorsal approach. Combined palmar and dorsal approaches were used in three cases: in two cases, ORIF, and in one case, proximal row carpectomy. All internally fixed scaphoids healed, and no carpal AVN or collapse was observed. Carpal alignment was satisfactory in most cases. Posttraumatic radiologic midcarpal arthritis or radiocarpal arthritis, or both, was almost always observed.
Nearly every combination of radiocarpal and intercarpal dislocation has been described, but few fit neatly into a particular pattern or classification scheme. Although arthroscopic techniques and fluoroscopically aided percutaneous techniques have been described, my preference for treatment is an open procedure. It is imperative to specifically ask the patient about median nerve symptoms and perform physical examination for it prior to surgery. If left untreated, median nerve compression can lead to long-term nerve deficits, hand stiffness, and, potentially, complex regional pain syndrome.
The wrist is approached dorsally primarily for treatment of the scaphoid fracture or the scapholunate tear if it is a lesser arc injury. In 3% of cases, a complete SLIL tear accompanies a scaphoid fracture-dislocation. An extended carpal tunnel approach is added if one of two situations is present: (1) The preoperative history and examination are consistent with acute carpal tunnel syndrome, or (2) a perilunate fracture-dislocation is irreducible from the dorsal approach. The lunate is visualized in the space of Poirier between the RSC ligament and long radiolunate (LRL) ligament in an extended carpal tunnel volar approach to the carpus. Using a Freer elevator or similar instrument from the volar approach and manual wrist manipulation, the lunate can be negotiated into reduction.
For associated lunotriquetral ligament injury, the lunotriquetral joint is reduced and held with two Kirschner wires. One technique that is very valuable and surgically efficient is to place one or two double-ended Kirschner wires from within the lunotriquetral joint out the ulnar side of the wrist through the triquetrum before the lunate is reduced. A similar technique can be used in the scaphoid when the SLIL is torn. It is important to protect the soft tissues (radial artery, superficial radial nerve, tendons, veins) on the radial side of the wrist. Smooth Kirschner wire drilling in oscillation mode can be helpful to decrease risk of injury to soft tissues. The lunate is reduced and the wires are driven back into the lunate from the triquetrum and scaphoid, respectively. The Kirschner wires are buried since they must remain for 12 weeks. Nonburied Kirschner wires have a higher incidence of infection if they are left in more than 6 weeks. If extended carpal tunnel release has been performed, the stronger palmar component of the lunotriquetral ligament can be sutured with nonabsorbable braided suture from the palmar side.
Occasionally the dorsal radioulnar ligament origin is avulsed off of the dorsoulnar corner of the radius. The distal radioulnar joint will be unstable and the dorsoulnar corner of the radius will be devoid of soft tissue during the exposure. It is important to repair the ligament with bone suture anchors. Repairs are generally protected with cast immobilization for 8 to 12 weeks, followed by removal of Kirschner wires and then gentle active mobilization of the wrist over the next 4 to 8 weeks ( Fig. 16.17 ).
It is imperative during reduction to restore Gilula’s lines in coronal plane and attain neutral radiolunate and capitolunate alignment and normal scapholunate angle in the sagittal plane.
Consider starting Kirschner wires from within the scapholunate joint and going out radially and ulnarly. Reduce the joint, then drive wires back in to fix the scapholunate joint and lunotriquetral joints, respectively.
Bury Kirschner wires because they should stay for approximately 10 to 12 weeks.
Beware of concomitant wrist injuries, such as dorsal radioulnar ligament injury.
The most common complications reported in the literature are delayed union, nonunion, arthritis, reduced wrist motion, and loss of strength. Prolonged cast immobilization leads to muscle atrophy, joint contracture, disuse osteopenia, lost time from work, and potential financial hardship. Closed treatment of scaphoid waist fractures may require cast immobilization for 3 months or longer, and fractures of the proximal third of the scaphoid in particular may take 6 months or longer to heal if treated closed. The incidence of nonunion in proximal pole fractures treated closed is higher than if treated with surgery. Surgical repair of scaphoid nonunion is successful in 50% to 95% of patients, depending on the vascular status and presence of arthritis or carpal collapse; successfully repaired scaphoid nonunions may require up to 6 additional months for healing and rehabilitation.
Other complications are reported. Filan and Herbert in 1996 reported on 431 patients treated with ORIF using the Herbert bone screw. They reported that 56 patients had hypertrophic scarring and 20 patients complained of postoperative pain and swelling at the donor site of a bone graft. Four superficial infections and one deep wound infection resolved satisfactorily with conservative treatment. Four patients had early signs of reflex sympathetic dystrophy after surgery. In two patients these signs resolved spontaneously, but two patients developed carpal tunnel syndrome, which required surgical decompression. Only two wrists showed instability of the scaphoid after surgery. One had sustained a tear of the scapholunate ligament at the time of injury; the other appeared to have a late rupture of this ligament. AVN developed after surgery in 20 scaphoids, all of which required further operations. In one case, a very small necrotic fragment of the proximal pole was excised and the scaphoid stabilized by dorsal capsulorrhaphy. Five wrists had a midcarpal fusion. The necrotic proximal pole was excised in 14 cases and was replaced with a stabilized silicone implant in 13 and an osteochondral autograft in one.
In another early report of headless screw fixation, Dias and colleagues investigated 88 patients with minimally displaced or nondisplaced bicortical fractures of the waist of the scaphoid in a prospective, randomized controlled study comparing plaster immobilization in a below-elbow plaster cast with early internal fixation with use of a Herbert or Herbert-Whipple screw. Complications occurred in 13 patients (30%) who had been managed operatively, and nonunion occurred in 10 of 44 patients (23%) of those treated in a cast. Most operative complications were minor and included superficial wound infection (in 1 patient), sensitive scar (in 3 patients), hypertrophic scar (in 4 patients), sensitive and hypertrophic scar (in 3 patients), hypoesthesia in the region of the palmar cutaneous branch of the median nerve (in 1 patient), and mild early complex regional pain syndrome (in 1 patient). Technical difficulty was experienced in 7 patients (16%) during surgery. In 4 of them, there was initial misplacement of either the drill or the screw. In one patient, the scaphoid tuberosity split during screw insertion.
Bushnell reported his complications with repair of scaphoid fractures using a dorsal percutaneous approach and cannulated headless screw fixation in 24 cases performed over 5 years. All cases involved nondisplaced (<1 mm) fractures of the scaphoid waist. The overall complication rate was 29%; there were five major complications (21%) and two minor complications (8%). Major complications consisted of one case of nonunion, three cases of hardware problems, and one case of postoperative fracture of the proximal pole of the scaphoid. Minor complications included intraoperative equipment breakage, with one case involving a screw and one case involving a guidewire.
Complications of internal fixation are usually due to screw placement and length. Controversy exists as to whether the screw should be placed centrally in the scaphoid or perpendicular to the fracture. , Eccentric screws decrease fixation strength and risk penetration out of the scaphoid. Rancy and colleagues reported on refracture of the proximal poles of 3 patients after successful scaphoid nonunion surgery and confirmed healing on CT scan.
The development of small, cannulated screws has permitted minimally invasive fixation of acute scaphoid fractures. There are known mechanical advantages to optimal screw length and optimal screw placement. As cited above, there are potential deleterious effects of improper screw placement, including articular protrusion, proximal pole fracture, and nonunion. Tumilty and Squire reported that the curvilinear surface of the proximal pole of the scaphoid may lead to errors in calculation of screw length and penetration into the joint. In this cadaveric study of six specimens, two screws were found to be penetrating subchondral bone. The plain x-ray films were accurate in only five of the six specimens, while 360-degree fluoroscopic views were accurate in all six. Fluoroscopy during placement of a scaphoid screw may decrease the rate of subchondral penetration.
Recently investigators have evaluated CT-generated models to assist in the development of a targeting system for implantation of screws into a reduced scaphoid fracture. Several investigative teams have demonstrated that a computer-assisted navigation of volar percutaneous scaphoid screw placement improved accuracy and required less time, less use of fluoroscopy, and diminished radiation exposure compared with traditional percutaneous techniques. , Hoffmann and colleagues showed in a cadaveric model that compared with the standard fluoroscopic technique, an electromagnetic navigation technique had a higher accuracy rate and lower rates of complications and required less operative time and radiation exposure time.
The ability to successfully treat scaphoid nonunions in part defines the abilities of a hand/upper extremity surgeon. Unfortunately, missed scaphoid fractures are relatively common because oftentimes the patient, trainer, or urgent care personnel will dismiss a wrist injury as a “sprain.” The traditional definition of scaphoid nonunion is failure of union following cast immobilization or surgical treatment of 6 months’ duration. When most patients with scaphoid nonunion present, however, they have not had any form of treatment, months to years have elapsed since the injury, and secondary deformities and carpal collapse have occurred. If scaphoid nonunion is left untreated, the natural history is carpal collapse and degenerative arthritis. In a long-term follow-up study of more than 30 years of scaphoid fractures treated with short-arm thumb spica casts, 10% had nonunion. Osteoarthritis with associated pain and weakness was present in 56% of patients with nonunion. Only 2% of the healed group had osteoarthritis. Wrist arthrosis that follows persistent scaphoid nonunion is called SNAC wrist. (For more information, see Chapter 13 .)
To minimize the incidence of arthritis, the goal of treatment is to attain a healed scaphoid with anatomic alignment. Advanced imaging, including CT and MRI, aids in the evaluation of scaphoid alignment, bone loss, scaphoid humpback deformity, carpal collapse, and osteonecrosis. Generally, scaphoid waist nonunions with severe collapse and humpback deformity must be approached volarly with interposition of bone graft and internal fixation. For proximal pole nonunions, a dorsal approach allows access to curette the nonunion site, bone grafting, and internal fixation of headless compression screw(s) from proximal to distal direction. The indications and best method of vascularized bone grafting are controversial.
When evaluating patients with scaphoid nonunion, the following issues regarding the fracture must be considered:
Where is the nonunion? At the waist or at the proximal pole?
Is the nonunion displaced?
Is there a humpback deformity?
Is there comminution, cyst formation, or cavitation?
Is DISI present?
Was there previous surgery?
Is the proximal pole dysvascular?
Is the proximal pole fragmented?
Is there arthritis (SNAC wrist)? If so, at what stage?
Is the scaphoid deformed or salvageable?
The more proximal the fracture, the more likely the proximal bone will be dysvascular. This may have implications for the choice of bone graft or other treatment.
If there is humpback deformity and carpal malalignment, ideally this should be corrected at the time of bone grafting and fixation.
If the nonunion is stable and well aligned and bone loss is minimal, limited opening in the nonunion site, curetting as necessary, and cancellous bone grafting may be appropriate, followed by internal fixation by a headless compression screw. Bone grafting can also be performed from within the drill hole, especially if gaining access to the nonunion site may cause unnecessary damage to the bone. Slade and colleagues had previously reported on successful percutaneous bone grafting and fixation for nonunions. , Imaging, even CT scanning, does not necessarily predict the stability well (Mark Cohen, MD, personal communication).
Any previous surgical treatments should be taken into account because they may make further treatment more complex. Prior surgery necessitates removal of hardware, excision of sclerotic or dysvascular bone, and abundant autogenous or vascularized bone grafting, depending on the fracture characteristics.
There is general agreement that vascularity is important, though it is widely considered that there is a spectrum of vascularity in the proximal fracture fragment, and that vascularity is inversely proportionate to the size of the fragment. The term “avascular necrosis” is widely utilized to small or sclerotic proximal poles, but true infarction of the scaphoid proximal pole is decidedly rare. Green showed that 92% of scaphoid fractures with good vascularity, as determined by punctate bleeding, healed when treated by Russe bone grafting. Union took place in 71% of patients when there was diminished vascularity in the scaphoid, but healing did not take place in any patients when the proximal pole did not demonstrate punctate bleeding. Whether these findings were related to the vascularity of the proximal pole or to the limitations of the traditional Russe technique cannot be said with certainty.
There is, in fact, no consensus on how to best determine vascularity of the proximal pole. X-rays are not adequate to assess AVN, and MRI and punctate bleeding do not correlate with healing or time to union. Histologic assessment of the excavated bone from the proximal and distal poles of the scaphoid is the gold standard for vascular assessment, but there is no preoperative or intraoperative test that correlates with histologic findings. Thus, the decision to utilize a vascular graft to augment healing capacity or expedite healing is largely empiric and at the discretion of the surgeon.
As stated above, the use of MRI to assess bony vascularity is controversial. Some authors advocate its use, whereas others present cogent arguments questioning its efficacy and utility. , , MRI findings of hypointense areas of bone on both T1-weighted and T2-weighted sequences have been suggested to be correlated with AVN. However, subsequent studies have shown poor correlation. Rancy et al. showed that there was no correlation between MRI findings, histology of bone samples taken during scaphoid nonunion surgery, intraoperative bleeding, and healing rates.
In a study of 88 patients for which imaging results were compared with findings of intraoperative bleeding, Schmitt and colleagues concluded that viability of the proximal fragment in scaphoid nonunion can be significantly better assessed with contrast-enhanced MRI than with nonenhanced MRI. Contrast-enhanced MRI has demonstrated significantly improved sensitivity (77% versus 6%; P < .001) in detecting scaphoid AVN compared with nonenhanced MRI. However, this study suggests that, even with contrast enhancement, MRI might fail to detect proximal pole AVN in nearly a quarter of cases. This might be due to ingrowth of nonspecific inflammatory tissue into the proximal pole from the nonunion site, resulting in contrast enhancement despite the necrotic bone. In another study by Dawson and colleagues, MRI with gadolinium could not predict fracture healing. Additional techniques for improving the sensitivity of MRI, such as dynamic gadolinium enhancement, are being developed and investigated.
Controversy about MRI is further complicated by differences in techniques and image quality, which depend on programming of the image acquisition parameters and reading of the images and on the MRI machines, magnet power, and specialized wrist coils used; all of these factors influence the quality of information presented.
It is likely that there is a spectrum of ischemia of the fractured proximal pole, and that the scaphoid has the ability to heal by creeping substitution when there is rigid apposition to a well-vascularized distal pole. Without a gold standard to compare with, consensus on the definition of AVN, or clear evidence that proximal pole vascularity has an effect on the healing rates of surgically treated scaphoid fractures, it is not likely that this controversy will soon be settled.
The proximal pole must not be collapsed, deformed, or fragmented, as may happen in true osteonecrosis with infarcted bone. In these cases nonunion surgery cannot be performed; either scaphoid excision or proximal pole replacement by any of several accepted methods must be considered.
If there is arthritis of the radiocarpal, midcarpal, or distal radioulnar joint, the treatment must be tailored accordingly. For instance, if early arthritis (SNAC I) is confined to the radial styloid, radial styloidectomy and scaphoid bone grafting could be considered. Radial styloidectomy should not exceed 6 mm to preserve the volar radiocarpal ligaments and prevent carpal instability. In a recent study of 21 chronic scaphoid nonunions with SNAC arthritis, all 10 proximal pole nonunions had >6 mm of exposed bone on the radial styloid, obviating the use of radial styloidectomy as safe treatment. With advanced arthritis, nonoperative management may be considered if symptoms are not severe. With increasing pain, other options may include partial denervation or salvage procedures such as scaphoid excision and four-bone fusion, proximal row carpectomy, total wrist fusion, hemiwrist arthroplasty, and total wrist arthroplasty.
Patient parameters to consider when evaluating scaphoid nonunions include:
Length of time nonunion has been present
Amount of pain or dysfunction
Activity level
Systemic comorbidities present
The longer the nonunion has been present, the more likely it is that there will be secondary issues such as arthritis, scaphoid deformity, and carpal instability. In a study by Mack and colleagues, older nonunion was associated with more severe arthritis.
The intensity, duration, and frequency of pain should be recorded in all four life domains: sleep, work, activities of daily living, and hobbies/athletic pursuits. When the only option may be a partial or complete wrist fusion, nonoperative treatment can be continued as long as the level of pain and dysfunction is tolerable. A salvage procedure or wrist arthroplasty may always be performed later.
Elderly, inactive, or infirm patients may choose to accept the pain and limited range of motion of a scaphoid nonunion. There is no mandate for operative repair because salvage operations may always be considered if escalating arthritic pain causes increasing impairment.
Scaphoid nonunion surgery can be complex and complicated, and the best efforts may be thwarted by heavy tobacco use, poor compliance, uncontrolled diabetes or inflammatory arthritis, steroid dependency, or other factors.
Several classification schemes have been proposed for scaphoid nonunion, generally based on factors such as time since injury, mobility of the fragments, cystic or flexion deformity, and degenerative change. Two such classification systems are listed in Tables 16.2 and 16.3 . Table 16.4 presents a four-part practical classification and treatment algorithm based on fracture nonunion characteristics and vascularity.
| Type | Description |
|---|---|
| Type 1 | Delayed presentation for 4–12 weeks |
| Type 2 | Fibrous union, minimal fracture line |
| Type 3 | Minimal sclerosis of less than 1 mm |
| Type 4 | Cystic formation of 1–5 mm |
| Type 5 | Humpback deformity with cystic change of more than 5 mm |
| Type 6 | Wrist arthrosis |
| Grade | Description |
|---|---|
| I | Linear nonunion without altered scaphoid form, instability, or intracarpal malalignment |
| IIA | Stable nonunion with incipient bone resorption at fracture line, without instability or malalignment |
| IIB | More or less mobile nonunion with anterior defect and proximal pole flexion on distal tubercle inducing DISI |
| III | More or less mobile displacement nonunion with instability or reducible malalignment with |
|
|
|
|
| IV | Proximal fragment necrosis with |
|
|
|
| Type of Fracture | Treatment |
|---|---|
| I. Delayed union | Mini-open rigid fixation with headless compression screw |
| IIA. Established waist nonunion, no deformity, no arthritis | Open repair and autogenous bone grafting Dorsal or volar |
| IIB. Established waist nonunion, humpback deformity, no arthritis | Volar approach Russe or Matti-Russe corticocancellous autograft Intercalated wedge autograft Hybrid Russe autograft |
| III. Proximal pole nonunion, viable | Dorsal approach Open bone grafting and fixation with headless screw Percutaneous bone grafting with headless screw Immobilize midcarpal joint with Kirschner wire(s) |
| IV. Dysvascular nonunion, waist, or proximal pole | Nonvascularized vs vascularized bone graft: dorsal or palmar approach Osteoarticular graft |
Scaphoid nonunions without substantial bone loss require only rigid fixation to heal if there is adequate perfusion. These include fractures with a delayed presentation, fibrous unions, and nonunions with minimal sclerosis (<1 mm). Stable scaphoid fractures presenting for treatment after 1 month may have already developed bone resorption at the fracture site and have a poorer union rate with casting alone. Selected stable scaphoid delayed unions without deformity or bone loss can be successfully treated with reduction and internal rigid fixation without bone grafting using techniques described for acute fractures above. Scaphoid nonunions that have minimal bone resorption of the anterior cortical bone and minimal fracture sclerosis (<2 mm confirmed by CT scan) still have the potential for healing in the early stages with screw fixation alone. Slade and coworkers successfully treated aligned nonunions without bone loss using headless screw fixation and no bone graft. All 15 patients in this case series healed at an average of 14 weeks with bridging cortical bone on CT.
Similarly, certain established fibrous nonunions stabilized with a compression screw and without a bone graft may heal. Shah and Jones examined 50 scaphoid nonunions treated with open Herbert screw fixation and noted that the scaphoid nonunions that had an intact cartilaginous envelope or a stable fibrous union healed with screw fixation alone without bone grafting. A preoperative CT scan is important to determine the amount of deformity and bone loss but gives no information as to the cartilaginous envelope.
This type of nonunion is less common but may be treated with open autogenous bone grafting (cancellous bone graft from distal radius) and headless compression screw. The screw would more commonly be placed from the volar approach because bone grafting in this scenario would be from the volar approach. See above section on volar screw placement.
The most common type of nonunion faced by the surgeon will be a midwaist fracture nonunion with fracture site resorption and varying degrees of deformity and bone loss. For this type, nonvascularized autogenous bone graft will generally suffice for healing of the bone as long as certain technical aspects are followed. A point of controversy is whether deformity must be corrected to provide an optimal outcome. In a long-term follow-up study of healed scaphoid nonunions, Jiranek and colleagues identified a higher percentage of arthritic changes in malunited scaphoid fractures. The degree of arthritis did not correlate with the activity level, satisfaction, or return to work, however. These findings were confirmed in a recent study demonstrating periscaphoid arthritis in 10 of 22 patients with asymptomatic scaphoid malunion. Although anatomic alignment is intuitively preferable for restoration of normal kinematics, some degree of malunion seems to be tolerable. , , It is therefore recommended to correct intercarpal malalignment and DISI at the time of nonunion surgery, especially in young and active patients, to avoid the otherwise probable progression of arthritis.
The procedure can be done from a volar or dorsal approach, depending on the location of nonunion. Generally, midwaist nonunion surgery is performed from the volar approach and proximal pole nonunion surgery is performed from the dorsal approach. Careful preparation of the bone fragments so as not to cause thermal necrosis with power tools is imperative. Removal of sclerotic and dysvascular bone with curettes to expose healthy bone surfaces is advantageous for both proximal and distal poles. The scaphoid is anatomically reduced and abundant autogenous bone graft packed into the proximal and distal poles. Fixation with a headless compression screw is optimal and is generally performed from distal to proximal in waist nonunions, as detailed in the Hybrid Russe Procedure section. When the lunate is malrotated into dorsiflexion (DISI), it often corrects when the scaphoid’s humpback deformity is reduced. However, if DISI is not corrected with scaphoid reduction, then reduction and temporary fixation of the lunate with a temporary 0.062-inch radiolunate Kirschner wire will facilitate accurate alignment of the scaphoid proximal pole prior to scaphoid grafting and fixation (see Fig. 16.16 ). Supplemental fixation across the scaphocapitate joint with a Kirschner wire may further stabilize the construct, especially if there is concern about the rigidity of fixation.
For nonvascularized bone graft, healing rates are identical between cancellous only and corticocancellous grafts, but cancellous only grafts demonstrated a statistically quicker time to union. There is no difference in healing or time to union between graft sources (iliac crest versus distal radius), , but the iliac crest graft has the disadvantages of another operative site, longer surgery time, more pain, and risk for cutaneous nerve injury.
In terms of fixation, a systematic review of the literature showed the superiority of screw over Kirschner wires for fixation of the scaphoid. Cohen and colleagues reported on 12 patients who all healed with cancellous bone graft from the distal radius and a distal to proximal headless compression screw. The average lateral intrascaphoid angles were improved from 49 degrees to 32 degrees.
Several authors have reported on the use of plate fixation for scaphoid nonunions. Huene and Huene reported their experience with plate fixation to stabilize scaphoid fractures in 1991. The Ender blade plate is suitable for adding stability in scaphoid nonunions with AVN, cystic degeneration, and osseous size discrepancy or compromise. Plate fixation can be used with scaphoid nonunion humpback deformity. Dodds and colleagues presented a combination of volar buttress plating and a vascularized volar distal radius wedge autograft pedicled on the volar carpal artery for salvage treatment of chronic scaphoid nonunion. Ghoneim reported on healing of 13 of 14 scaphoid nonunions with an iliac crest wedge graft and a volar miniplate of 1.5 or 2 mm. Other authors have had had unsatisfactory results, however. In a series of 15 patients with scaphoid nonunion, there was 87% union but a high percentage of hardware complications and need for secondary surgery for hardware removal in one-third of patients.
In the original Matti technique, only cancellous bone was used. The Matti-Russe technique was a cancellous plug from the iliac crest and cancellous chips, and Green’s modification of the Russe technique was two back-to-back corticocancellous intramedullary iliac crest strut grafts and cancellous chips ( Fig. 16.18 ). Green reported 24 of 25 (92%) with good proximal pole vascularity united. If proximal pole vascularity was diminished, then union was 71% (10 of 14). If the proximal pole showed no bony bleeding, 0 of 5 united. In a long-term evaluation of Russe bone grafting, Barton had less success, especially in proximal pole nonunions, which demonstrated a union rate of only 54%.
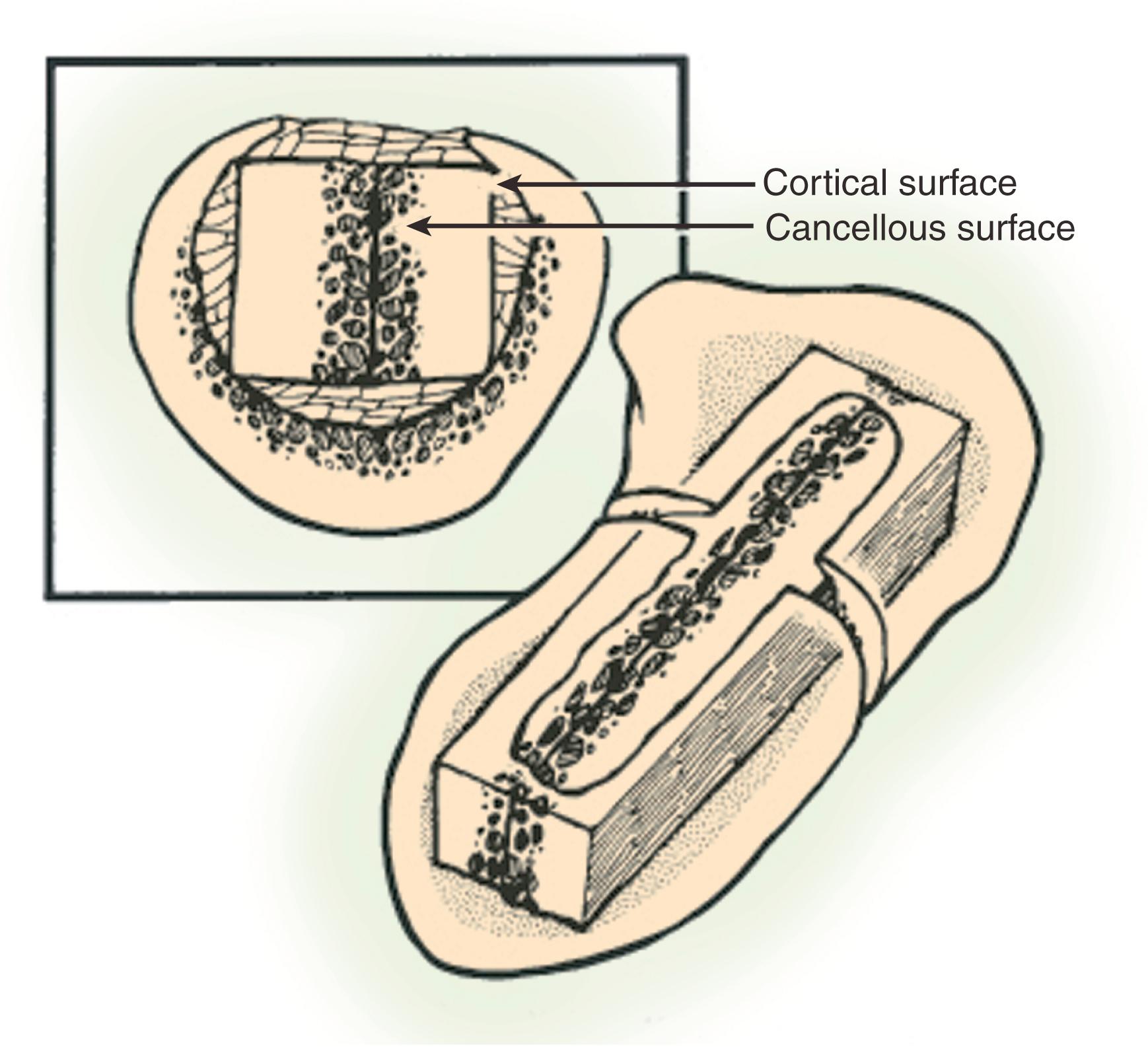
In 1984 Fernandez presented his modification of the Fisk procedure to treat scaphoid nonunions associated with carpal instability. His article described the following modifications: (1) preoperative calculation of the exact scaphoid length and form based on comparative radiographs of the opposite wrist, (2) the use of a palmar approach, (3) the insertion of a wedge-shaped corticocancellous graft from the iliac crest after resection of the pseudarthrosis, and (4) the use of internal fixation ( Fig. 16.19 ).
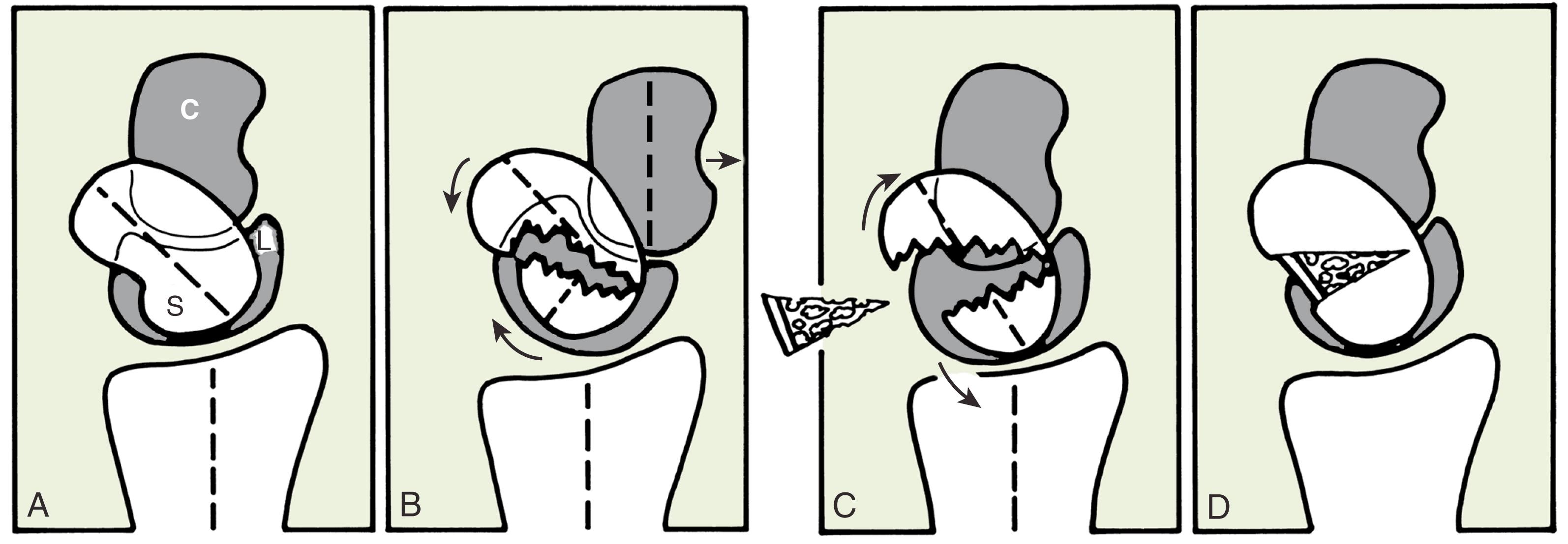
Preoperative planning is considered essential to restore the anatomic length, analyze the angular deformity, evaluate the pathologic scapholunate angle, and calculate the resection and size of the graft needed. The palmar approach reduces the danger of iatrogenic damage of the vascular supply of the scaphoid and accidental lesions of the superficial branches of the radial nerve. Iliac bone is preferred to the radial styloid graft, as proposed by Fisk, because of its better ability to resist compression forces. Internal fixation adds rotational stability so that continued postoperative plaster immobilization can be reduced to a minimum of 8 weeks.
Preoperative PA, lateral, and ulnar deviation PA radiographs of the injured wrist and the opposite normal wrist are used to determine the amount of resection and size of the graft needed. The surgeon must determine the amount of scaphoid flexion deformity, the extent of carpal collapse, and the scapholunate and lunocapitate angles ( Fig. 16.20 ). The humpback deformity can be evaluated more precisely using CT scans reformatted along the long axis of the scaphoid.
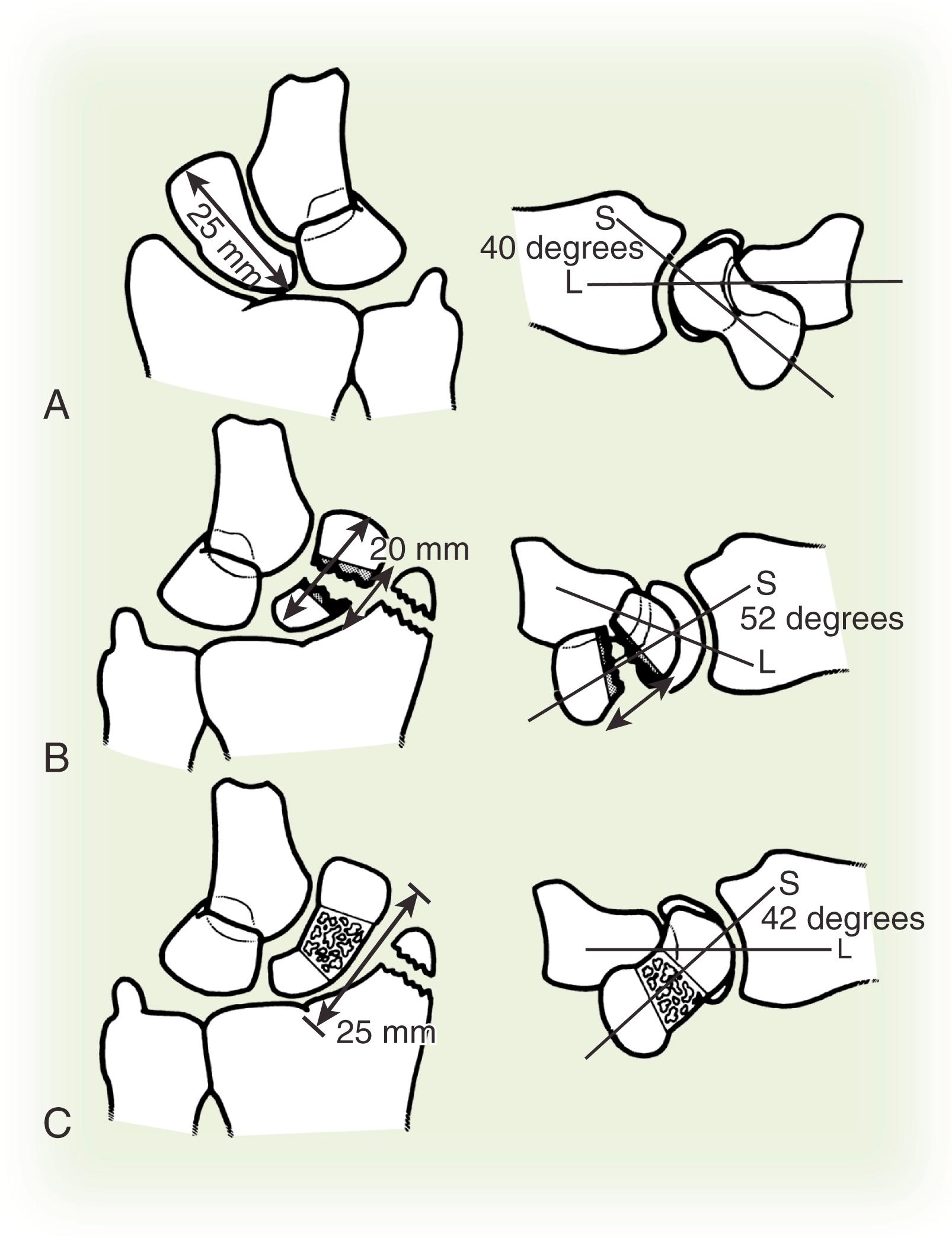
The nonunion is approached through a palmar hockey-stick incision along the FCR, extended obliquely toward the thenar eminence. The anterior capsule is incised from the distal radius to the scaphotrapezial joint to expose the volar surface of the scaphoid. The capsular flaps contain the RSC and LRL ligaments. Sclerotic or irregular borders of the nonunion site are resected with an oscillating saw to obtain flat bony surfaces. Cystic defects are curetted and filled with cancellous bone. The extended lunate is corrected by flexing the wrist until the lunate assumes a neutral position under fluoroscopy, and a 0.062-inch Kirschner wire is placed through the diaphyseal-metaphyseal junction of the radial styloid into the lunate under fluoroscopic control ( Fig. 16.21 ). The flexion deformity and shortening of the scaphoid are then corrected by distracting the osteotomy with small bone hooks or large skin hooks while hyperextending the wrist over a rolled towel. A bicortical or tricortical graft is harvested from the iliac crest, along with additional cancellous bone. The graft is kept in aspirated blood from the iliac wound until ready for use. Osteotomes of various sizes can be used to measure the depth, width, and length of the trapezoidal defect of the scaphoid, and the graft is sculpted to the correct dimensions using a saw and osteotomes. The bone graft is impacted into the defect, and scaphoid alignment and posture are checked with fluoroscopy. A guidewire is advanced from distal to proximal across the scaphoid and intercalated graft. Finally, compression screw fixation of the scaphoid is performed. Careful closure of the palmar capsule and repair of the RSC and LRL ligaments are done. A long-arm thumb spica postoperative splint is applied. Sutures are removed at 10 to 12 days, and the above-elbow immobilization is continued until the radiolunate pin is removed.
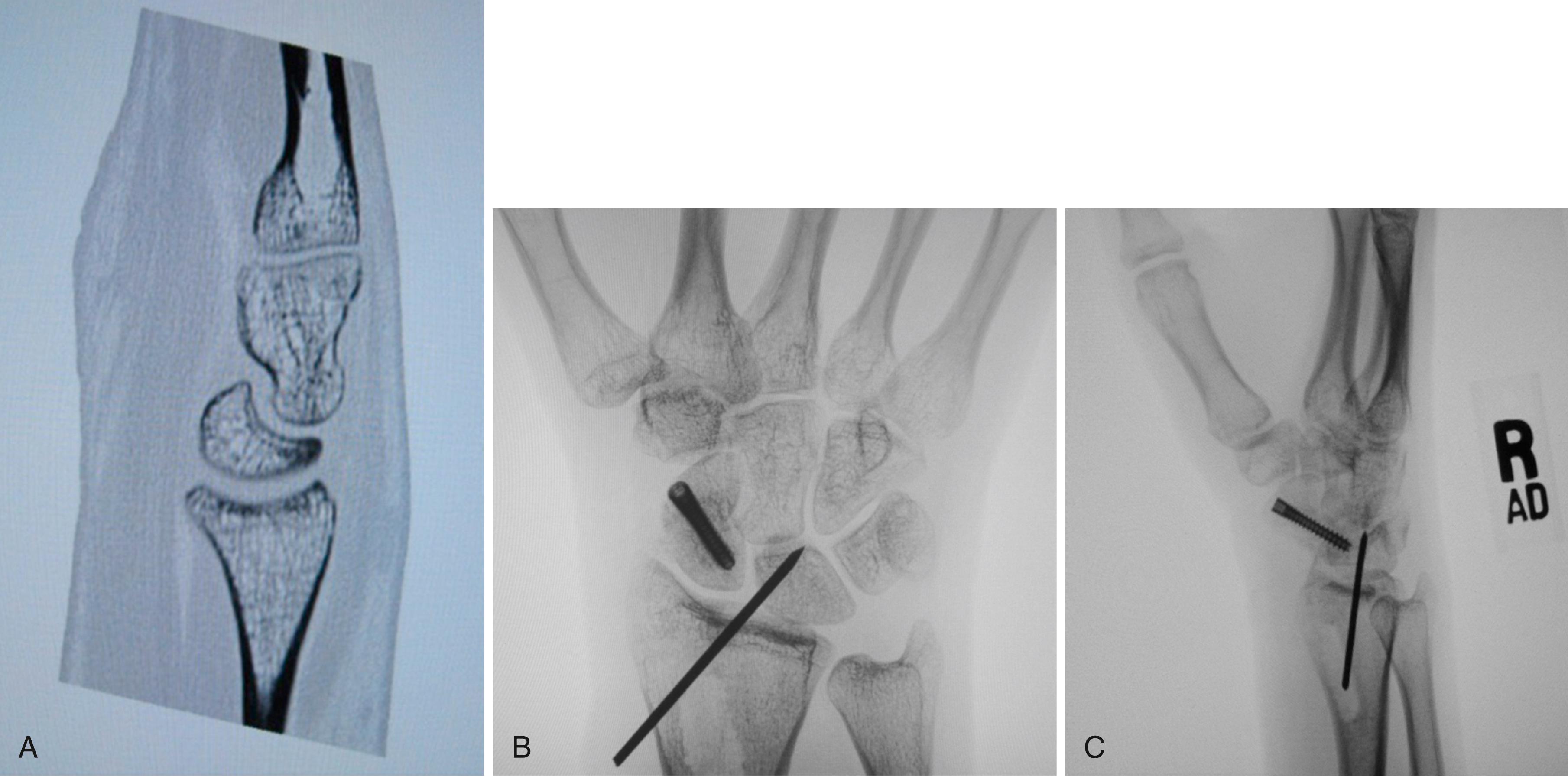
For long-standing DISI deformity that presents with a radiolunate angle greater than 15 degrees, the radiolunate transfixion wire is left in place for 4 to 6 weeks to reduce forces on the healing scaphoid. Short-arm casting is continued after wire removal until radiographic or CT evidence of trabecular healing has occurred. Fernandez’s criteria for establishing healing include the absence of pain, radiographic evidence of bridging bony trabeculae on both sides of the interposed graft, a disappearance of the osteotomy lines in conventional x-rays, and no signs of screw loosening. CT is advised to confirm union prior to return to athletic activities.
In 1990 Fernandez reported union of 19 of 20 established nonunions repaired with this technique, with an average time off work of 8.9 weeks. In a review of the pertinent literature, he determined that the most common reasons for recalcitrant nonunion were improper internal fixation techniques or the absence of bone grafting, or both.
Eggli et al. reported on a retrospective review of 37 patients with scaphoid fracture nonunions treated by interpositional bone grafting and internal fixation at an average follow-up of 5.7 years. Solid radiographic union was achieved in 35 cases. Preexisting AVN was an adverse factor for achievement of union and satisfactory outcome. Patients with preexisting degenerative changes had a significantly worse clinical outcome. The vast majority of the patients had satisfactory correction of scaphoid length and the associated DISI. Although 30 patients showed radiographic evidence of mild or moderate degenerative changes at their latest follow-up, there was no significant progression of arthrosis, and carpal collapse deformity did not progress after healing of the fracture nonunion.
The wedge graft technique as described may not routinely incorporate curettage of the proximal and distal poles to healthy cancellous bone. Removal of sclerotic, fibrotic, or cystic bone in the proximal and distal poles prior to insertion of the wedge graft and replacing with additional cancellous graft may expedite union. Volar wedge bone grafting may require 3 to 6 months to heal and may result in reduced wrist motion. In cases without punctate bleeding, volar wedge grafting with an autologous iliac crest graft, Robbins and Carter demonstrated union rates of only 30%. This makes intuitive sense, as creeping substitution would be required to entirely revascularize the interposed corticocancellous graft prior to healing and revascularization of the proximal pole. Finally, the technique is exacting, and it may be challenging to simultaneously attain excellent compression, anatomic restitution of normal scaphoid anatomy, and rigid fixation.
The hybrid Russe procedure is advantageous because it is effective for humpback scaphoid nonunions with DISI. The procedure exposes healthy cancellous bone in both scaphoid poles, uses a strut to maintain reduction of the humpback deformity, and resists collapse during screw compression. The technique incorporates abundant cancellous bone with optimal healing potential, which provides earlier healing than the standard Russe procedure. , It also avoids graft extrusion and uses local autogenous bone graft (Video 16.1 ![]() ).
).
The patient is placed in the supine position under general or regional anesthesia. The arm is cleansed with antimicrobial solution and draped using sterile technique. Exsanguination is performed with an Esmarch bandage, and an arm tourniquet is inflated to 250 mm Hg. A volar incision is made along the course of the FCR tendon and extended distally along the border of the glabrous skin of the thenar eminence. The distal incision is limited to the level of the scaphotrapezial joint in preparation for screw fixation starting in the distal pole of the scaphoid. Splitting the sheath of the FCR allows the FCR to be retracted ulnarly. Care is taken with ulnar retraction, specifically for the median nerve and the palmar cutaneous nerve of the median nerve. Use of blunt retractors and avoiding excessive force is necessary. On the radial side, the radial artery and superficial radial nerve are protected. The floor of the FCR sheath is incised longitudinally. The capsule is incised in the same line as the skin incision, being careful to incise in one clean cut so that the capsule and intracapsular RSC and LR ligaments can be repaired at closure. The scaphoid bone is exposed from pole to pole. The superficial branch of the radial artery to the superficial arch is retracted radially or divided. The nonunion is exposed with the aid of wrist hyperextension over a bump and skin hooks ( Fig. 16.22 ). All fibrous tissue at the nonunion site is removed and bone is curetted to remove sclerotic bone and fibrous tissue down to nonsclerotic bone in both the proximal and distal poles. It is important that curetting is continued in both poles until healthy bone is identified. In our experience, we are virtually always able to find this bone. , In cases of humpback deformity and DISI, the lunate may be reduced with wrist flexion under fluoroscopy, and a percutaneous radiolunate 0.062-inch Kirschner wire placed dorsally or radially for temporary fixation and to correct proximal scaphoid pole alignment. This is done if restoring the scaphoidlength and alignment does not correct the DISI deformity.
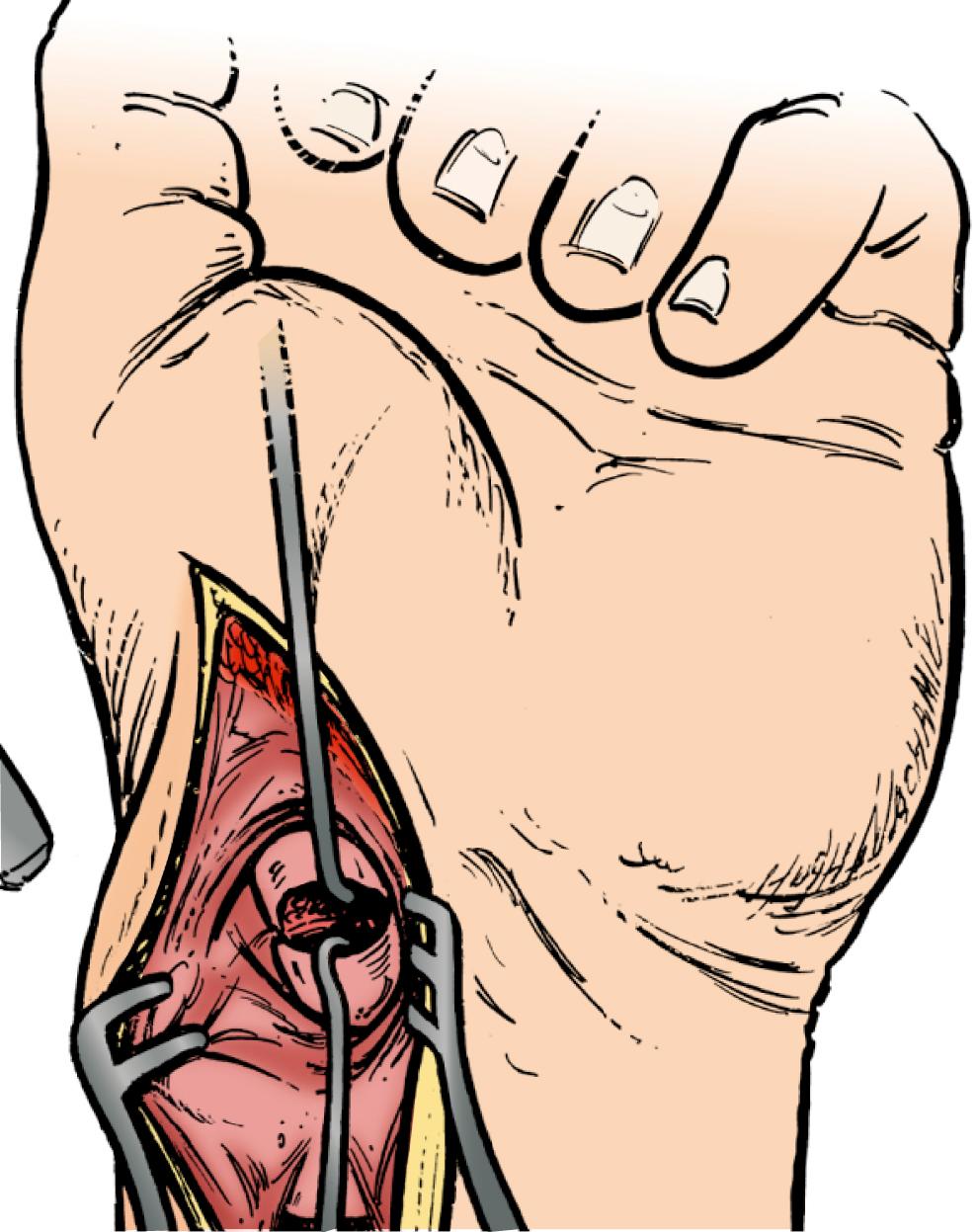
Proximal dissection is carried out by retracting the FCR to the ulnar side and incising and reflecting the pronator quadratus off the metaphysis of the radius toward the ulnar side. An oval cortical window of 20 × 8 mm is made on the volar cortex of the distal radius by first making drill holes with a 1.1 mm (0.045-inch) Kirschner wire, completing cuts with a narrow osteotome, and the cortical fragment is set aside for later use ( Fig. 16.23 ). Abundant cancellous bone graft is harvested from the radius through the oval hole. In an adolescent with an open distal radius physis, care is taken to confirm via fluoroscopy that the window does not breach the physis. A “matchstick” is fabricated from the volar cortical window of the radius and sized to act as a cortical strut (to extend and reduce the humpback deformity and restore the length of the scaphoid) ( Fig. 16.24 ). With the wrist hyperextended over a bump and using bone hooks to extend/reduce the prepared proximal and distal poles, the cortical strut is trimmed appropriately and inserted in intramedullary fashion between the proximal and distal poles. Fluoroscopy is used to assess the reduction of the scaphoid and the carpal alignment. The scaphoid may be over reduced and over extended. In this instance the cortical strut is simply removed and sculpted with a bone cutter to a small size. Once fluoroscopic imaging confirms good reduction of scaphoid and carpus, then cancellous bone graft is packed into the remainder of the nonunion site ( Fig. 16.25 ), and this is followed with fixation with a headless screw ( Figs. 16.26 and 16.27 ). Intraoperative fluoroscopy is used as needed to confirm adequate reduction and fixation. The volar capsule and radiocarpal ligaments are repaired with interrupted nonabsorbable sutures, and the subcutaneous layer and skin are closed in layers. A short-arm splint is kept in place for 2 weeks following the operation. For patients with a radiolunate wire, above-elbow immobilization is utilized to prevent wire breakage. The wire is removed in the office at 4 weeks, and a short-arm cast is applied until healing occurs. A CT scan is performed to confirm union at 10 to 12 weeks following operation, and immobilization is converted to a custom-molded removable splint.
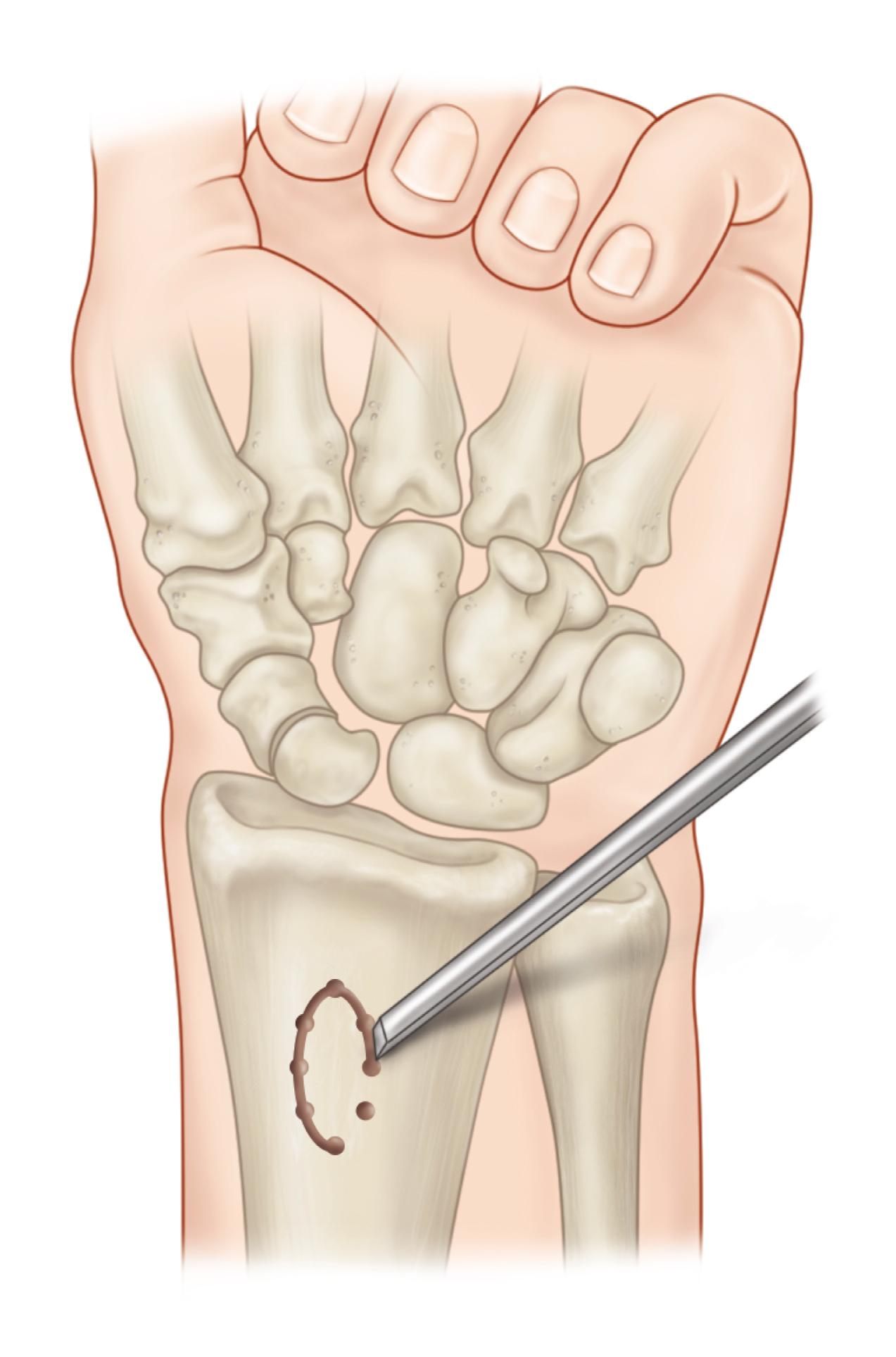
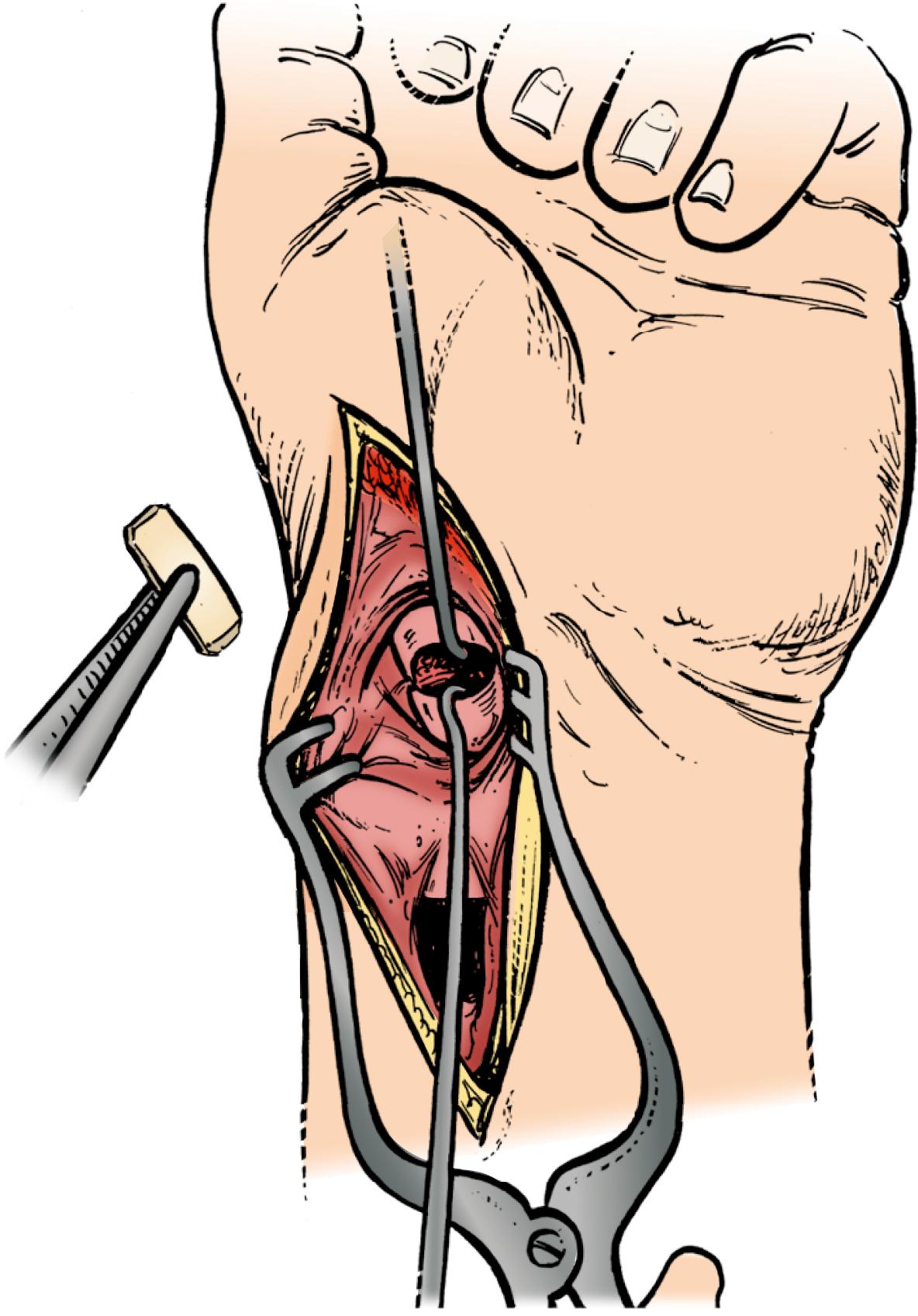
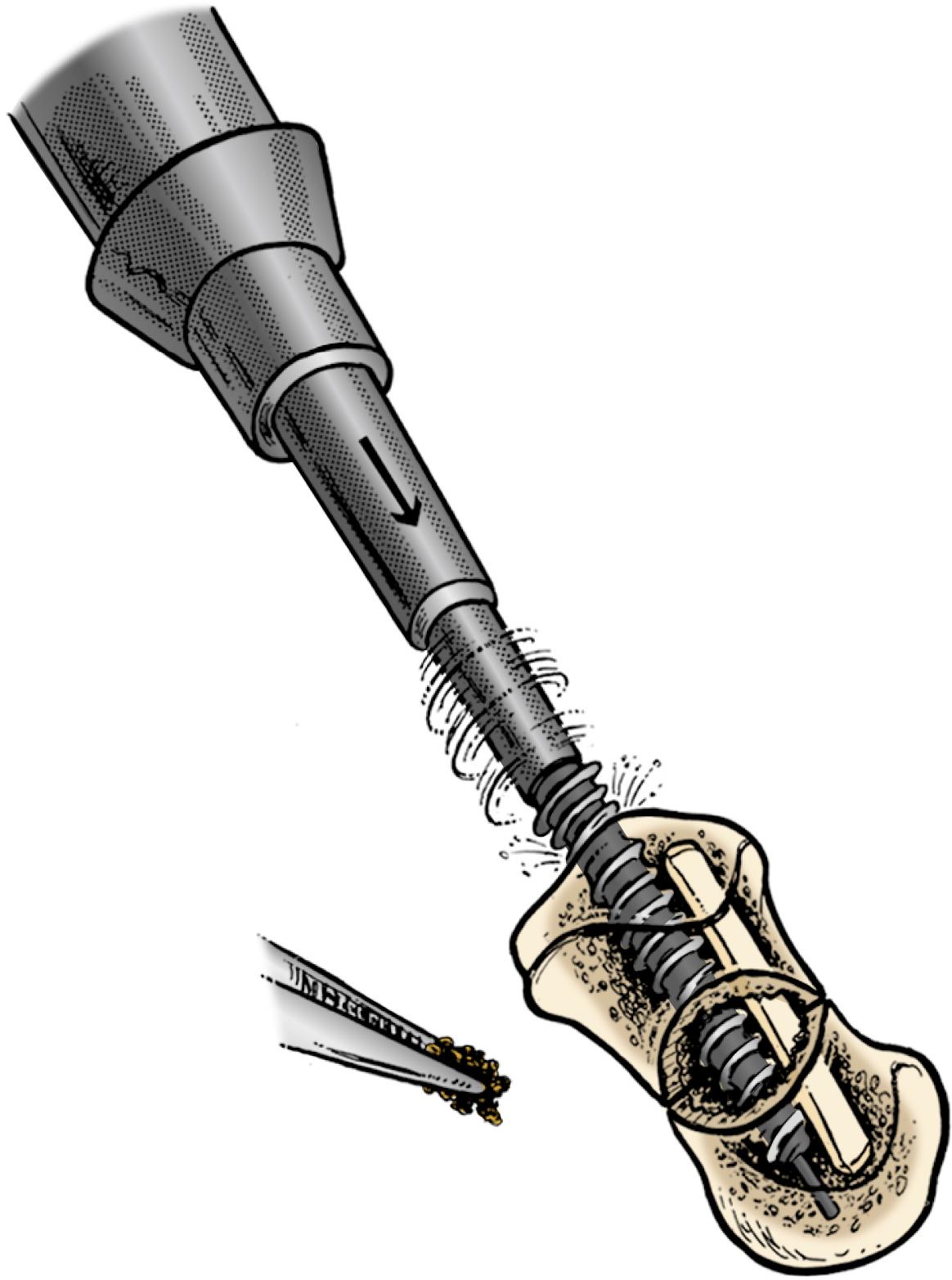
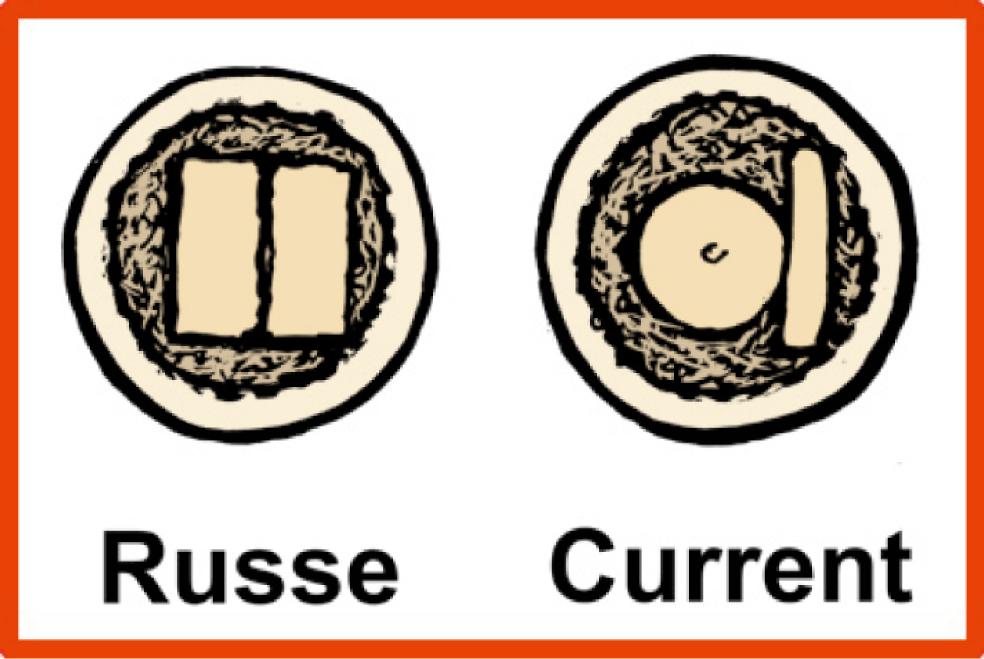
If surgery has been performed previously, this technique may still be used, but the new screw should be placed from the opposite direction ( Fig. 16.28 ). The cortical strut may be inserted down the previous screw tract.
In a series of hybrid Russe corticocancellous grafting procedures for scaphoid nonunion, we reported union in 17 of 17 patients at an average of 3.6 months. Grip strength was improved from 70% of the uninjured hand to 89% postoperatively. The mean postoperative scapholunate angle was significantly reduced compared with the preoperative angle (56 degrees postoperatively from 70 degrees preoperatively). The mean postoperative intrascaphoid angle was also reduced with surgery (35 degrees versus 65 degrees). Similarly, the mean radiolunate angle was reduced postoperatively to 0 degrees from 20 degrees preoperatively. There were no reported complications.
This technique is particularly applicable for proximal third fractures with minimal displacement and cystic cavitation without humpback deformity. The technique is similar to that described above in the acute treatment of scaphoid fractures from the dorsal approach.
The tourniquet is placed proximally on the arm and draped to allow full motion of the extremity. A small (≈2 to 3 cm) longitudinal incision is made just radial to the 3-4 arthroscopic portal. This area is also in line between the second and third metacarpal interspaces and extends proximally just radial to the Lister tubercle, crossing the dorsal lip of the radius. Mini approach (as in acute proximal pole fractures described earlier) may be used, or extended exposure may be needed depending on the clinical scenario. For an extended exposure, the EPL is carefully identified by opening the third dorsal compartment and may be transposed external to the retinaculum at the conclusion of the case. The second and fourth dorsal compartments are retracted. The capsule is incised along the dorsal rim of the radius. A mini capsulotomy may be used, or if more exposure is needed, the capsular “window” can be angled to run distal to the dorsal radiocarpal ligament, preserving this important ligament and its lunate attachment intact.
Extreme care is taken to avoid disruption of the dorsal fibers of the SLIL and the dorsal scaphotriquetral ligament when reflecting the capsular flap (see Chapter 13 and Video 13.4 ![]() ). Dissection in a plane tangential to the dorsal surfaces of the scaphoid and the lunate can be performed with a scalpel or Beaver blade. The distal boundaries of dissection of the scaphoid are determined by the vascular supply along the dorsal ridge. Care is taken not to disrupt the blood vessels entering the waist of the scaphoid. Retractors may be placed deeper to retract the capsule. The proximal pole nonunion site may not be immediately evident, and a 25-gauge needle can be inserted under fluoroscopy to confirm the location. The stability of the cartilage cap is assessed. If displaced, the scaphoid is aligned using Kirschner wire joysticks. If it is stable, then the fracture fragments and cartilage envelope should not be separated. Choices for bony preparation and grafting include access through a small dorsal window or curettage and grafting through the scaphoid screw drill hole. To make a small dorsal window, the fracture line is identified with fluoroscopy and 0.035-inch Kirschner wire holes are made to create a 7- to 8-mm diameter hole in continuity with the nonunion site. The proximal and distal poles are debrided to healthy cancellous bone, and the scaphoid is structurally left intact.
). Dissection in a plane tangential to the dorsal surfaces of the scaphoid and the lunate can be performed with a scalpel or Beaver blade. The distal boundaries of dissection of the scaphoid are determined by the vascular supply along the dorsal ridge. Care is taken not to disrupt the blood vessels entering the waist of the scaphoid. Retractors may be placed deeper to retract the capsule. The proximal pole nonunion site may not be immediately evident, and a 25-gauge needle can be inserted under fluoroscopy to confirm the location. The stability of the cartilage cap is assessed. If displaced, the scaphoid is aligned using Kirschner wire joysticks. If it is stable, then the fracture fragments and cartilage envelope should not be separated. Choices for bony preparation and grafting include access through a small dorsal window or curettage and grafting through the scaphoid screw drill hole. To make a small dorsal window, the fracture line is identified with fluoroscopy and 0.035-inch Kirschner wire holes are made to create a 7- to 8-mm diameter hole in continuity with the nonunion site. The proximal and distal poles are debrided to healthy cancellous bone, and the scaphoid is structurally left intact.
Lister tubercle is then removed with an osteotome and saved for later replacement. Cancellous bone is harvested. The harvested cancellous bone graft can be compacted in a 3-mL syringe with its plunger. The nonunion site is packed with cancellous bone graft. Guidewire and headless cannulated screw fixation is performed as is described earlier in the acute fracture section (see pages 690 and 691). Thrombin-soaked Gelfoam may be inserted into the radial defect, and the tubercle can be replaced or inverted as a lid. Use of two microheaded screws (1.2 to 1.7 mm) can also be considered, but it is important to countersink and bury these small noncannulated headed screws. Use of small bioabsorbable fixation nails has also been described. The capsule is closed with 3-0 absorbable suture, and the retinaculum is closed with 3-0 absorbable suture.
Additional maneuvers to augment stability of the construct are to place a 0.045- or 0.062-inch Kirschner wire from the distal scaphoid to the capitate and to reduce the lunate to be collinear with the radius and pin from the radius to the lunate. If used, the radiolunate pin is stabilized with an above-elbow cast and the pin removed after 4 weeks. Scaphocapitate fixation is retained until healing, which may take 10 to 12 weeks. Inoue et al. reported a successful union rate of 80% with cancellous bone grafting and Herbert screw placement through a dorsal approach.
Geissler and Slade are proponents of percutaneous bone grafting of proximal pole nonunions. Slade and Gillon reviewed their experience with percutaneous bone grafting in a series of 234 scaphoid fractures. Successful percutaneous treatment of scaphoid nonunions requires careful planning and the use of a number of imaging tools, including standard radiography, minifluoroscopy, CT or MRI, and arthroscopy. Chu and Shih reported that 14 of 15 (93%) scaphoid nonunions healed with arthroscopic-assisted use of injected bone graft substitute and a cannulated headless screw.
Selected minimally displaced or nondisplaced scaphoid fracture nonunions can be treated with percutaneous bone grafting and internal fixation. This may be particularly helpful for the small proximal pole fragment with cystic resorption at the fracture site but an intact cartilage shell. The technique for dorsal wire placement is the same as for acute fractures. The starting position for the guidewire is the proximal pole of the scaphoid. The wire is introduced into the proximal scaphoid pole using fluoroscopic imaging and is guided down the scaphoid central axis toward the thumb. The wrist is flexed to avoid bending the guidewire. The wire is advanced toward the thumb base from a dorsal to volar position until the dorsal trailing end of the wire clears the radiocarpal joint, permitting full extension of the wrist. Once the dorsal trailing end of the guidewire has been buried into the proximal scaphoid pole, the wrist can be extended for imaging to confirm scaphoid alignment and correct positioning of the guidewire. To avoid injury to extensor tendons, a mini incision over the 3-4 portal can also be made. The EPL tendon is located and retracted radially and the capsule is incised, exposing the proximal scaphoid pole. A drill guide is placed on the scaphoid proximal pole and a 0.045-inch double-cut guidewire is driven in a radial and distal direction toward the thumb base. Fluoroscopic imaging is used to confirm the correct course of the wire in the scaphoid.
Displaced nonunions may be percutaneously manipulated and reduced with the use of dorsally placed 0.062-inch joystick Kirschner wires in each fragment and a percutaneously placed hemostat, using the previously described techniques for acute fractures. Following accurate fracture reduction, placement of the guidewire, and measurement of the screw length, the wire is driven distally and out of the skin of the thenar eminence and clamped.
The scaphoid is drilled from the proximal scaphoid pole, crossing the nonunion site to the distal scaphoid 2 mm from the far cortex. The drilling of the distal scaphoid reestablishes a fresh blood supply and removes some of the devitalized bone at the nonunion site. The scaphoid wire is withdrawn distally across the nonunion site and into the distal fragment. A tiny angled curette can be passed through the proximal scaphoid portal to the nonunion site. This is done using fluoroscopic imaging. Curettage of the fracture nonunion site is performed. The outer cortex, which often has fibrous tissue, must not be violated, because this tissue acts as a net holding the percutaneously introduced bone graft. The wire is reintroduced across the fracture and out of the dorsal wound.
Bone graft can be percutaneously harvested from the distal radius or iliac crest using one of several bone-coring devices that are commercially available. Slade and Geissler prefer a 4-inch 8-gauge bone biopsy needle (Baxter Jamshidi, Deerfield, IL) to percutaneously harvest cancellous bone graft. A guidewire is percutaneously inserted into the dorsal distal radius near or at the Lister tubercle. A small incision and blunt dissection expose the bone cortex. A hand reamer is used to penetrate the cortex. The bone biopsy cannula is introduced over the Kirschner wire, the Kirschner wire is removed, and cancellous bone plugs are harvested.
Attention is directed back to the scaphoid, and the biopsy cannula is inserted over the guidewire into the prepared nonunion site. The guidewire is withdrawn into the distal scaphoid pole. Guided by imaging, autogenous bone plugs are implanted through the cannula into the nonunion site using the plunger, and the graft is impacted. The guidewire is driven back across the proximal pole and out of the dorsal wound. Rigid fixation is obtained with cannulated headless screw fixation along the guidewire axis. Additional implants may be inserted to further stabilize the fracture site and prevent bone shearing until bone healing has occurred as needed, including a scaphocapitate Kirschner wire to temporarily immobilize the midcarpal joint and a radiolunate wire as needed. In some cases, extreme proximal scaphoid pole nonunions have been treated with bone grafting and compression by sandwiching a compression screw or wire from the midscaphoid to the lunate in addition to the midcarpal immobilizing wire.
Immediate postoperative care includes a bulky compressive hand dressing and a volar splint. The patient is encouraged to initiate early finger exercises to reduce swelling. The therapist fashions a removable volar splint that holds the wrist and hand in a functional position at the first postoperative visit, and the patient is started on an immediate gentle strengthening program, the purpose of which is to axially load the fracture site now secured with an intramedullary screw to stimulate healing. This early digital motion program decreases swelling and permits an early return of hand function. Patients with eccentric fractures, particularly proximal pole fractures, are restricted from wrist motion until CT scan confirms bridging bone at the fracture site at 6 weeks following operation. Although postoperative radiographs are obtained at the first postoperative visit and at 6-week intervals, a CT scan is used to confirm trabecular bridging.
When vascularized bone grafting was first introduced, the healing rate was reported to be 100%. , With time, the original enthusiasm has waned because more reports have shown much lower healing percentages. Hirche and colleagues reported a healing rate of only 75% with the 1,2 intercompartmental supraretinacular artery bone graft. Chang and associates reported a union rate of 50% with the same graft if AVN was present, and Straw and coworkers reported a union rate of only 12% if AVN was present. No distinct guidelines exist as to which cases would most benefit from vascularized bone grafting.
Previous papers reported low healing rates with the Russe technique (0% union) or volar iliac crest wedge grafting (30% union) when ischemic bone was diagnosed by the absence of punctate bleeding. Many of these patients were treated with Kirschner wire fixation before the advent of headless screw fixation. Modern methods of rigid internal fixation have called into question the relative importance of ischemia versus fixation strength as the key parameter influencing healing of scaphoid nonunions. Whether removal of necrotic or dysvascular bone and replacement with healthy autogenous bone and rigid fixation is preferable to retention of ischemic bone and implantation of a vascular graft to augment creeping substitution has not been answered by the current scientific evidence. In fact, few papers report the histologic findings of surgically treated nonunions, making comparative studies impossible. There is substantial controversy as to how to best assess vascularity of the proximal pole, whether by preoperative MRI with or without contrast or by the presence or absence of bleeding bone intraoperatively. Rancy and colleagues have shown no correlation between any preoperative assessment or the eventual outcome of healing. Two systematic reviews demonstrated no evidence to support a difference in healing rates between vascularized and nonvascularized grafts for scaphoid nonunions. , Given all of these unanswered questions, the role of vascular grafting for scaphoid nonunion cannot be stated with certainty; the various options available and current best evidence will be reviewed.
In 1979, Hori and colleagues performed canine studies to demonstrate the efficacy of an implanted arteriovenous pedicle to treat osteonecrosis. Sunagawa and colleagues, in a subsequent canine study, compared nonvascularized (conventional) grafts with arteriovenous pedicle graft implantation and quantitatively assessed bone blood flow, fracture healing, and bone remodeling. They found that 73% of the vascularized grafts and none of the conventional grafts healed. At 6 weeks, bone blood flow in the proximal pole was significantly higher on the side of the vascularized graft. Quantitative histomorphometry of the avascular proximal segment demonstrated significantly higher levels of fluorochrome-labeled osteoid- and osteoblast-covered trabecular surfaces on the vascularized graft side. These data supported the clinical application of pedicled vascularized bundle implantation in the treatment of carpal osteonecrosis including proximal pole scaphoid nonunions ( Fig. 16.29 ).
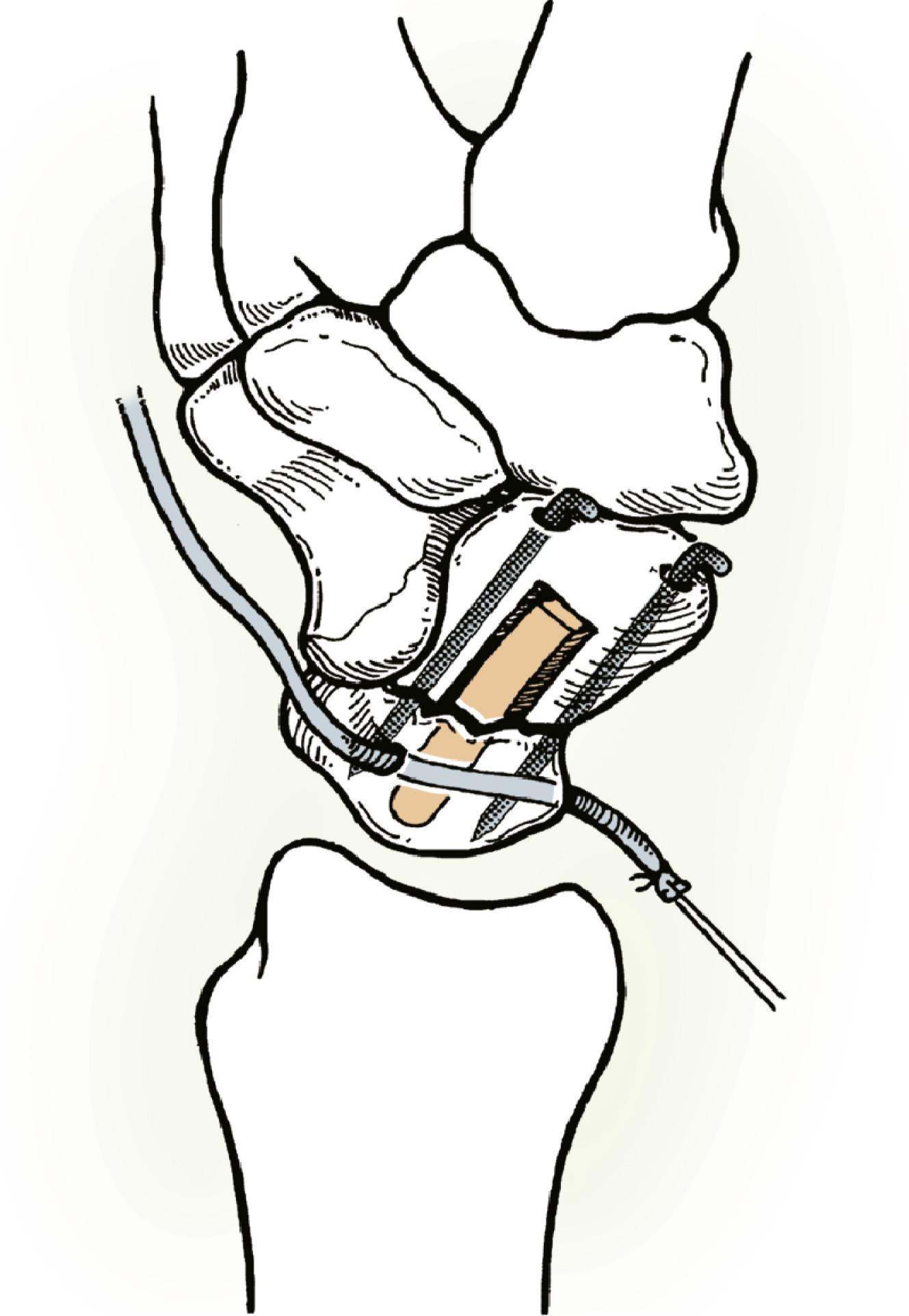
The technically correct term is vascularized bone flap, either rotational flap or free flap, although in the literature it has been named vascularized bone graft. To avoid confusion and use of a term used previously, either term will be used. The context of the discussion will make it clear.
Since 1986 there has been great interest in vascularized bone grafting to treat bony nonunion including the scaphoid. Shi and Xu reported on an experimental study and clinical uses of the fasciosteal flap for bone healing. In 1991, Zaidemberg and colleagues demonstrated a consistent vascularized bone graft source from the distal dorsoradial radius for the treatment of scaphoid nonunions using latex injection techniques. In a preliminary series of 11 patients, these authors reported 100% success.
Although when to use vascularized bone grafting is controversial, many authors discuss using it when there is lack of punctate bleeding witnessed intraoperatively. Another indication may be persistent nonunion following previously attempted surgical treatment. Some of the more common vascularized bone graft choices are the following:
1,2 intercompartmental supraretinacular artery (ICSRA) pedicle (Zaidemberg)
Volar carpal artery pedicled graft (Mathoulin)
Dorsal capsular pedicle (Sotereanos)
Free medial femoral condyle graft (Doi, Bishop and Shin, Higgins)
The vessels supplying the nutrient arteries to the dorsal radius are best described by their relationship to the extensor compartments of the wrist and the extensor retinaculum. The 1,2 ICSRA is superficial to the extensor retinaculum and lies between the first and second compartments. The 2,3 intercompartmental supraretinacular artery (2,3 ICSRA) lies superficial between the second and third compartments. Both arteries are at areas where the extensor retinaculum is firmly attached to bone, allowing nutrient arteries to penetrate the cortex. In addition to the superficial vessels, two vessels are deep to the extensor tendons on the floor of the fourth and fifth dorsal compartments. These are the fourth and fifth extensor compartment arteries (ECAs). The 1,2 ICSRA branches from the radial artery 5 cm proximal to the radiocarpal joint and rises dorsally to lie on the extensor retinaculum between the first and second compartments.
The 1,2 ICSRA graft for scaphoid nonunion described by Zaidemberg and colleagues ( Fig. 16.30 ) is most useful for scaphoid nonunion, but it has a relatively short pedicle. A dorsoradial incision is centered over the radiocarpal joint between the first and second compartments. This allows good exposure of the dorsoradial scaphoid. Branches of the superficial radial nerve need to be identified and protected. The 1,2 ICSRA is found coursing up dorsally from the radial artery to lie superficially on the surface of the extensor retinaculum between the first and second compartments ( Fig. 16.31 ). The first and second compartments are incised at their attachments to bone near the 1,2 ICSRA. The 1,2 ICSRA is carefully mobilized as a pedicle ( Fig. 16.32 ). Care is taken not to elevate the pedicle off the bone more than 10 to 15 mm proximal to the joint line because this is the area where the nutrient vessels begin to penetrate the cortex. The pedicle is freed almost to the radial artery at the level of the first compartment.
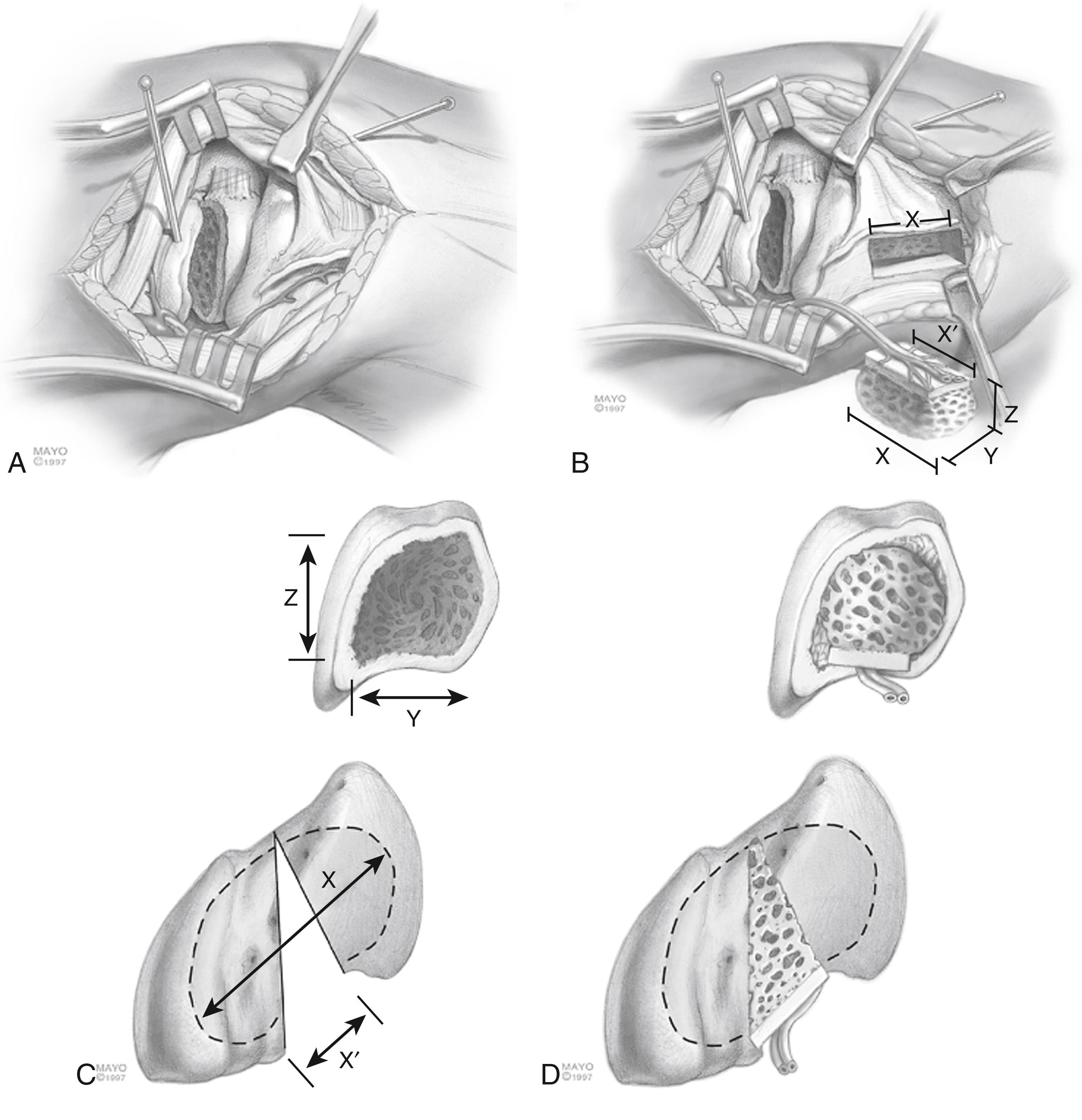
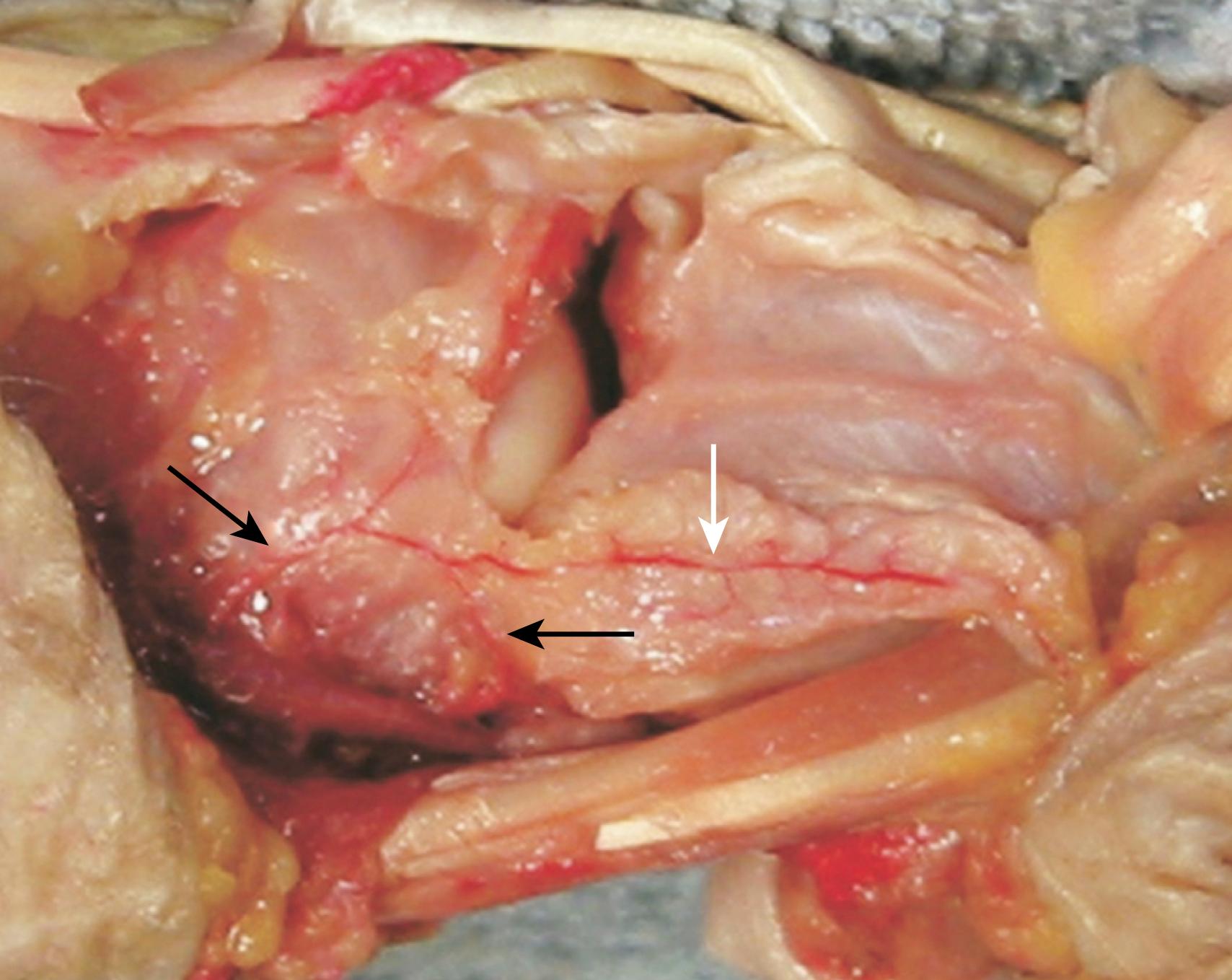
After elevation of the pedicle, the scaphoid nonunion is approached through a radial longitudinal incision at the capsule. The nonunion site is identified and exposed. Ideally, a rectangular slot is created, bridging the fracture site in the scaphoid that will receive the bone graft. If the fracture is in the proximal pole, a slot can be created in the distal fragment, and the proximal pole can be curetted or burred out of avascular bone. In such a case, the graft would slide into the concavity of the proximal pole and fit into the slot in the distal fragment. Radial styloidectomy can improve visualization but is often not needed. If the scaphoid is foreshortened with a humpback deformity, a vascularized graft may be placed as a volar wedge graft (see Fig. 16.30 ). This requires a wider exposure, which may be increased with a radial styloidectomy as necessary.
Once the nonunion site is prepared, the pedicled graft is carefully lifted from the radial metaphysis using small osteotomes. The 1,2 ICSRA is ligated proximal to the bone graft, and the graft is checked for flow by deflating the tourniquet. The graft is passed under the radial wrist extensors and impacted gently into place. If a significant concavity exists in either the proximal or distal fragment, additional cancellous bone is harvested from the distal radius to fill out the deep area of concavity in the scaphoid. Once the graft is in place, a screw is placed down the scaphoid and supplemental internal fixation may be used. Alternatively, if the scaphoid fragments are loose and grossly unstable with respect to each other, internal fixation can be placed first to stabilize the scaphoid as a single unit, and the vascularized graft can be impacted into place. The screw should be placed in the volar third of the scaphoid to reduce the chance of dislodging the graft.
Outcomes of the 1,2 ICSRA vascularized bone graft have had an evolving history. Initial reports were encouraging, with some authors reporting union rates of 100%. , Later investigations were less favorable, with healing rates of only 71%, and, if AVN was present, only 50%. Moreover, failures occurred in 64% if humpback deformity and DISI were present. The conclusion was that 1,2 ICSRA grafting is not suitable for scaphoid nonunion with AVN and carpal collapse. Straw and colleagues reported on Zaidemberg’s technique, which they used to treat 22 established scaphoid fracture nonunions, 16 of which were found to have avascular proximal poles at surgery. The union rate was a dismal 27%. If AVN was present, the union rate was a mere 12.5%. A confounding element of this study was poor fixation of a single Kirschner wire. Boyer and colleagues showed poor healing with the 1,2 ICSRA when there was AVN, with 6 of 10 (60%) achieving union. For a humpback deformity and AVN, Henry showed 100% union in 15 patients; this is in contrast to a level I study by the Mayo group in which only 4 of 10 1,2 ICSRA grafts healed, compared with 12 of 12 medial femoral condyle grafts. In more recent studies, union rates of 75% to 95% have been reported , and 94.7%. The high variability of success may be attributed in part to inconsistent definitions and diagnoses of AVN.
Kuhlmann and colleagues were the first to describe the vascularized bone graft pedicled on the volar carpal artery, which lies between the palmar periosteum of the radius and the distal part of the superficial aponeurosis of the pronator quadratus. It is harvested from the distal radius along with a 5-mm-wide strip of fascia and periosteum, using a volar approach. A trapezoidal graft can be harvested and placed palmarly in the case of humpback deformity.
Dailiana et al. reported its use in nine patients with scaphoid nonunion; union was achieved in 100% of cases. However, there was no preoperative carpal collapse and only 1 patient had AVN. Gras and Mathoulin reported on the use of this vascularized bone graft in 111 patients, 47 of whom had DISI. None had AVN. The union rate was 96% for primary nonunions and 90% for secondary nonunions ( Fig. 16.33 ). Sommerkamp and colleagues reported 100% healing in 15 patients with humpback deformity and avascular proximal pole using the palmar radiocarpal artery vascularized bone graft.
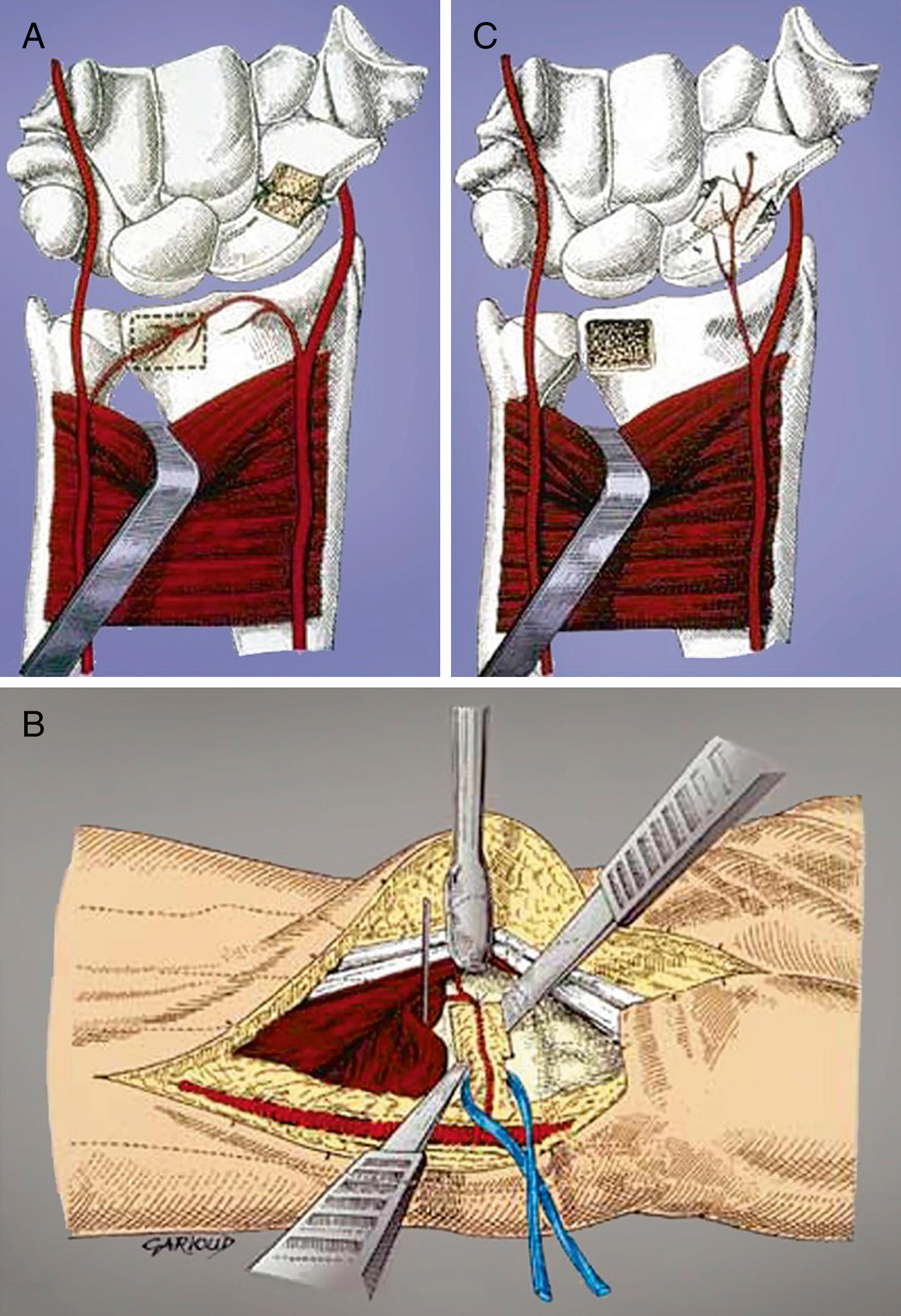
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here