Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The first recorded account of food allergy is attributed to Hippocrates, but it was not until 1921 that Prausnitz’s classic experiment initiated scientific investigation of food allergy and established the immunologic basis of allergic reactions. In his experiment, Prausnitz injected serum from his fish-allergic patient, Küstner, into his own skin; the next day he injected fish extract into the same areas and into control sites. A positive local reaction (Prausnitz-Küstner test) proved sensitivity could be transferred by a factor in serum (immunoglobulin [Ig]E antibodies) from an allergic to a non-allergic individual. In 1950, Loveless demonstrated that the patient’s history and presence of food-specific IgE antibodies were often insufficient to diagnose food allergy in the first blinded placebo-controlled food trial of patients with milk allergy. In the 3 decades that followed, standardized protocols were developed to evaluate food allergy, and the double-blind placebo-controlled oral food challenge emerged as the accepted standard for the diagnosis of food allergy.
Terminology used by investigators in the field of food allergy differs slightly in different parts of the world. The following represents current terminology in the USA. An adverse food reaction is a generic term indicating any untoward reaction that occurs following ingestion of a food or food additive and may be the result of a toxic or non-toxic reaction . Toxic reactions will occur in any exposed individual following ingestion of a sufficient dose. Non-toxic reactions depend on individual susceptibilities and may be immune mediated (food allergy or food hypersensitivity) or non-immune mediated (food intolerance). Food intolerances comprise most adverse food reactions and are categorized as enzymatic , pharmacologic , or idiopathic . Secondary lactase deficiency is an enzymatic intolerance adults (see Chapter 104 ), whereas most other enzyme deficiencies are rare inborn errors of metabolism and thus primarily affect infants and children. Pharmacologic food intolerances are present in individuals who are abnormally reactive to substances like vasoactive amines, which are normally present in some foods (e.g., tyramine in aged cheeses). Confirmed adverse food reactions for which the physiologic mechanism is not known are generally classified as idiopathic intolerances. Food allergies are usually characterized as IgE-mediated (“immediate”) or non-IgE-mediated (“delayed”); the latter are presumed to be cell-mediated.
About 8% of children and between 2% and 10% of the overall US population have food allergies. The prevalence of food allergies is greatest in the first few years of life and decreases over the first decade. The most common food allergens in young children include peanut (2.2%), cow’s milk (1.9%), shellfish (1.3%), tree nuts (1.2%), egg (0.9%), finned fish (0.6%), wheat (0.5%), soy (0.5%), and sesame (0.2%). (Reference Gupta, et al. PMID 30455345).Other than peanut and tree nuts, most childhood food allergies are outgrown by the end of the first decade. Most children who develop cow’s milk, egg, and/or peanut allergy do so in the first 2 or 3 years of life. Peanut, tree nut, sesame seed, finned fish, and shellfish allergies tend to be lifelong, but about 20% of young children with peanut allergy develop clinical tolerance. Food allergies may persist after childhood into adulthood or develop in adulthood, with the most common food allergies in adults consisting of shellfish (2%), peanut (0.6%), tree nuts (0.4%), and finned fish (0.4%). About 5% of the US population experiences limited oropharyngeal symptoms (e.g., itching and/or tingling of the lips, tongue, roof of the mouth, and throat) to raw fruits and vegetables. Most of these reactions occur in adolescents and adults who have seasonal allergic rhinitis and are due to cross-reactivity between homologous proteins in pollens (e.g., birch or ragweed pollens) and certain fruits and vegetables (e.g., raw apples, plums, cherry, kiwi, hazelnut, melons, bananas), respectively (pollen-food allergy syndrome or oral allergy syndrome). The prevalence of food allergies appears to be increasing. Studies from the US and United Kingdom indicate that the prevalence of peanut allergy has more than doubled in young children in a little over a decade. In addition, children with atopic disorders have a higher prevalence of food allergies; for example, 35% to 40% of children with moderate-to-severe atopic dermatitis have IgE-mediated food allergy.
Unlike the systemic immune system, which recognizes relatively small quantities of antigen and mounts a brisk inflammatory response to neutralize potential pathogens, the mucosal immune system regularly encounters enormous quantities of antigen and generally functions to suppress immune reactivity to harmless foreign antigens (e.g., food proteins, commensal organisms), only mounting a brisk protective response to dangerous pathogens when appropriate (see Chapter 2 ). The GI tract is the largest reservoir of immune cells in the body, and the gut-associated lymphoid tissue (GALT), a component of the mucosal immune system, lies juxtaposed to the external environment; it acts to differentiate organisms and foreign proteins that are potentially harmful from those that are not, and to keep the commensal microbiota compartmentalized. The mucosal immune system is separated from the intestinal lumen by a single layer of columnar epithelial cells that secrete a number of factors that contribute to barrier function, including mucins, antimicrobial peptides, and trefoil factors. The epithelial cells also transport antibodies, particularly IgA, into the intestinal lumen, where they contribute to barrier function by excluding the uptake of antigens or microbes. Just beneath this cell layer is the lamina propria of the mucosa, which is densely populated by resident immune cells, including CD4 + and CD8 + T-effector and regulatory T (Treg) cells, antibody-secreting B cells, and mononuclear phagocytes (macrophages and dendritic cells [DCs]). These scattered immune cells make up the effector sites of the mucosal immune system and function to recognize and clear pathogenic challenges from the environment. Peyer patches and isolated lymphoid follicles are situated within the intestinal mucosa, and with nearby mesenteric lymph nodes (MLN) form inductive sites where antigen-specific cellular and humoral immune responses are first generated. Specialized epithelial cells (M cells) overlie Peyer patches and contribute to the selective uptake of particulate antigens into this site. In contrast, soluble antigens are primarily taken up across the epithelial cells lining the villi and are carried into the MLNs. Lack of reactivity to our commensal flora is in part achieved by a specialized regulatory environment that may also shape the immune response to antigens derived from the diet. Antigen-presenting cells and macrophages of the intestinal mucosa are hyporesponsive to many microbial ligands and secrete high levels of immunoregulatory cytokines like interleukin (IL)-10. Both innate (natural killer cells, polymorphonuclear leukocytes, macrophages, epithelial cells, and Toll-like receptors) and adaptive immune responses (intra-epithelial and lamina propria lymphocytes, Peyer patches, secretory IgA [sIgA], and cytokines) provide an active barrier to foreign antigens. Developmental immaturity of various components of the intestinal barrier and immune system reduces the efficiency of the infant mucosal barrier; the activity of various enzymes is suboptimal in the newborn period, and the secretory immunoglobulin A (sIgA) system is not fully mature until 4 years of age. This immature state of the mucosal barrier may play a role in the increased prevalence of GI infections and food allergies seen in the first few years of life. Studies have also shown that alteration of the physiologic barrier function (e.g., gastric acidity) can lead to increased IgE sensitization in children and adults.
A highly efficient GI mucosal barrier has evolved that provides an enormous surface area for processing and absorbing ingested food and discharging waste products. This barrier uses physiologic and immunologic barriers to prevent penetration of foreign antigens ( Box 10.1 ). The physiologic barrier is composed of epithelial cells joined by tight junctions and covered with a thick mucus layer that traps particles, bacteria, and viruses; trefoil factors (TFFs; protease-resistant proteins secreted by mucus-secreting cells of the stomach [TFF1, TFF2] and intestine [TFF3]) that help strengthen and promote restoration of the barrier; and luminal and brush border enzymes, bile salts, and extremes of pH—all of which serve to destroy pathogens and render antigens non-immunogenic. Despite the evolution of this complex mucosal barrier, about 2% of ingested food antigens are absorbed and transported through the normal mature intestine and throughout the body in an immunologically intact form. In an elegant series of experiments performed more than 75 years ago, Walzer and colleagues used sera from food-allergic patients to passively sensitize volunteers and demonstrate that immunologically intact antigens cross the mucosal barrier and disseminate rapidly throughout the body. Increased gastric acidity and the presence of food in the intestine decrease antigen absorption, whereas hypochlorhydria (e.g., H 2 B- and PPI-induced) and ingestion of alcohol increase antigen absorption. These immunologically intact proteins typically do not provoke adverse reactions because most individuals have developed tolerance, but in a sensitized individual, allergic reactions will occur. Although more common in the developing GALT of young children, it is clear that cellular and IgE-mediated allergic responses to foods can develop at any age.
Block penetration of ingested antigens
Epithelial cells—single cell layer of columnar epithelium
Glycocalyx—coating of complex glycoprotein and mucins that traps particles
Intestinal microvillus membrane structure—prevents penetration
Tight junctions joining adjacent enterocytes—prevent penetration even of small peptides
Intestinal peristalsis—flushes trapped particles out in the stool
Break down ingested antigens
Salivary amylases and mastication
Gastric acid and pepsins
Pancreatic enzymes
Intestinal enzymes
Intestinal epithelial cell lysozyme activity
Block penetration of ingested antigens
Antigen-specific sIgA in intestinal lumen
Clear antigens penetrating the gastrointestinal barrier
Serum antigen-specific IgA and IgG
Reticuloendothelial system
As already noted, the dominant response in GALT is suppression, or tolerance. As first described in 1911 by Osborne and Wells, antigens ingested via the oral route induce a systemic non-responsiveness that has been termed oral tolerance . Antigens first ingested and then injected in an attempt to immunize an animal could not elicit an immune response. Similar findings have been demonstrated in humans following feeding and immunization with a neoantigen, keyhole limpet hemocyanin. Oral tolerance was shown to be an active regulatory response by the demonstration that this nonresponsive state could be induced in naïve mice through the transfer of T cells. MLNs are essential for development of oral tolerance, and surgical or immunologic ablation of MLNs prevents development of oral tolerance. Trafficking of immune cells to the intestine and from the intestine to the MLNs is regulated by expression of chemotactic cytokines (chemokines) and chemokine receptors. Expression of chemokine receptor CCR7 on DCs, which take up antigen from the intestine, is necessary for their migration from the lamina propria to MLNs, and is necessary for development of oral tolerance. Transfer of DCs derived from the intestinal lamina propria can induce tolerance in naïve animals ( Fig. 10.1 ).
CD103 + DCs isolated from the MLNs of mice and humans preferentially induce generation of gut-homing CD4 + Foxp3 + Tregs from naïve T cells. These CD103 + cells express high levels of the enzyme RALDH2, a retinal dehydrogenase that converts retinal to retinoic acid. Both intestinal homing activity and regulatory activity of the responder T cells are dependent on retinoic acid derived from CD103 + DCs. An important source of the precursor for retinoic acid comes from the diet in the form of vitamin A. In addition to the DC signals to naïve T cells, stromal cells of the MLN also express high levels of retinoic acid-generating enzymes and are important for the imprinting of factors such as intestinal homing potential .
Evidence now indicates that the commensal bowel flora (microbiota) play a major role in shaping the mucosal immune response. It has now been shown that there are approximately the same number of bacterial cells in the human body as there are human cells. The number of bacteria in a 70 kg “reference man” is estimated at 3.8×10 13 . The typical concentration of bacteria in the colon is estimated at 10 11 /mL with a rounded up order of magnitude of 10 14 . An individual’s microbiota is to some measure established in the first 24 hours after birth and depends on maternal flora, genetics, and local environment including whether birth is by cesarean section or vaginal delivery (see Chapter 3 ). The intestinal microbiota is relatively stable throughout life after reaching the adult pattern somewhere after the first year of life. In a recent study, mice with food allergy were found to have a specific intestinal microbiota capable of transferring disease susceptibility, suggesting that disease-associated microbiota may play a pathogenic role in the development of food allergy. Studies in which lactating mothers and their offspring were fed Lactobacillus suggest that probiotics may be beneficial in preventing some atopic disorders like eczema, but results from other studies are not consistent with this conclusion.
Intestinal epithelial cells (IECs) may also play a central regulatory role in determining the rate and pattern of uptake of ingested antigens. Studies in sensitized rats have indicated that intestinal antigen transport proceeds in 2 phases. In the first phase, transepithelial transport occurs via endosomes, is antigen specific and mast cell independent, and occurs 10 times faster in sensitized rats compared with non-sensitized control animals. Antigen-specific IgE antibodies bound to the mucosal surface of IECs via Fc epsilon (Fcε)RII are responsible for this accelerated allergen entry. In the second phase, paracellular transport predominates. Loosening of the tight junctions occurs as a result of factors released by mast cells that are activated in the first phase. Whereas the first antigen-specific pathway involves antibody, the second nonspecific pathway most likely involves cytokines. Consistent with this concept, IECs express receptors for a number of cytokines (IL-1, IL-2, IL-6, IL-10, IL-12, IL-15, granulocyte-monocyte colony-stimulating factor, and interferon-γ) and have been shown to be functionally altered by exposure to these cytokines.
Oral tolerance of humoral and cellular immunity has been demonstrated in rodents and humans. Feeding of keyhole limpet hemocyanin to human volunteers resulted in T-cell tolerance but priming of B cells at both mucosal and systemic sites. Failure of human infants to develop oral tolerance, or the breakdown of oral tolerance in older individuals, results in development of food allergy. Young infants are more prone to develop food-allergic reactions because of the immaturity of their immunologic system and, to some extent, their GI tract (see Box 10.1 ). Exclusive breast-feeding promotes development of oral tolerance and may prevent some food allergies and atopic dermatitis. The protective effect of breast milk appears to be due to several factors, including decreased content of foreign proteins, the presence of sIgA (which provides passive protection against foreign protein and pathogens), and the presence of soluble factors (e.g., prolactin), which may induce earlier maturation of the intestinal barrier and the infant’s immune response. The antibacterial activity of breast milk is well established, but the ability of breast milk sIgA to prevent food antigen penetration is less clear. Low concentrations of food-specific IgG, IgM, and IgA antibodies are commonly found in the serum of normal persons. Food protein-specific IgG antibodies tend to rise in the first months following introduction of a food, and then generally decline even though the food protein continues to be ingested. Persons with various inflammatory bowel disorders (e.g., celiac disease [CD], food allergy) frequently have high levels of food-specific IgG and IgM antibodies, although there is no evidence these antibodies are pathogenic. Antigen-specific T cell proliferation in vitro alone does not represent a marker of immunopathogenicity but simply reflects response to antigen exposure.
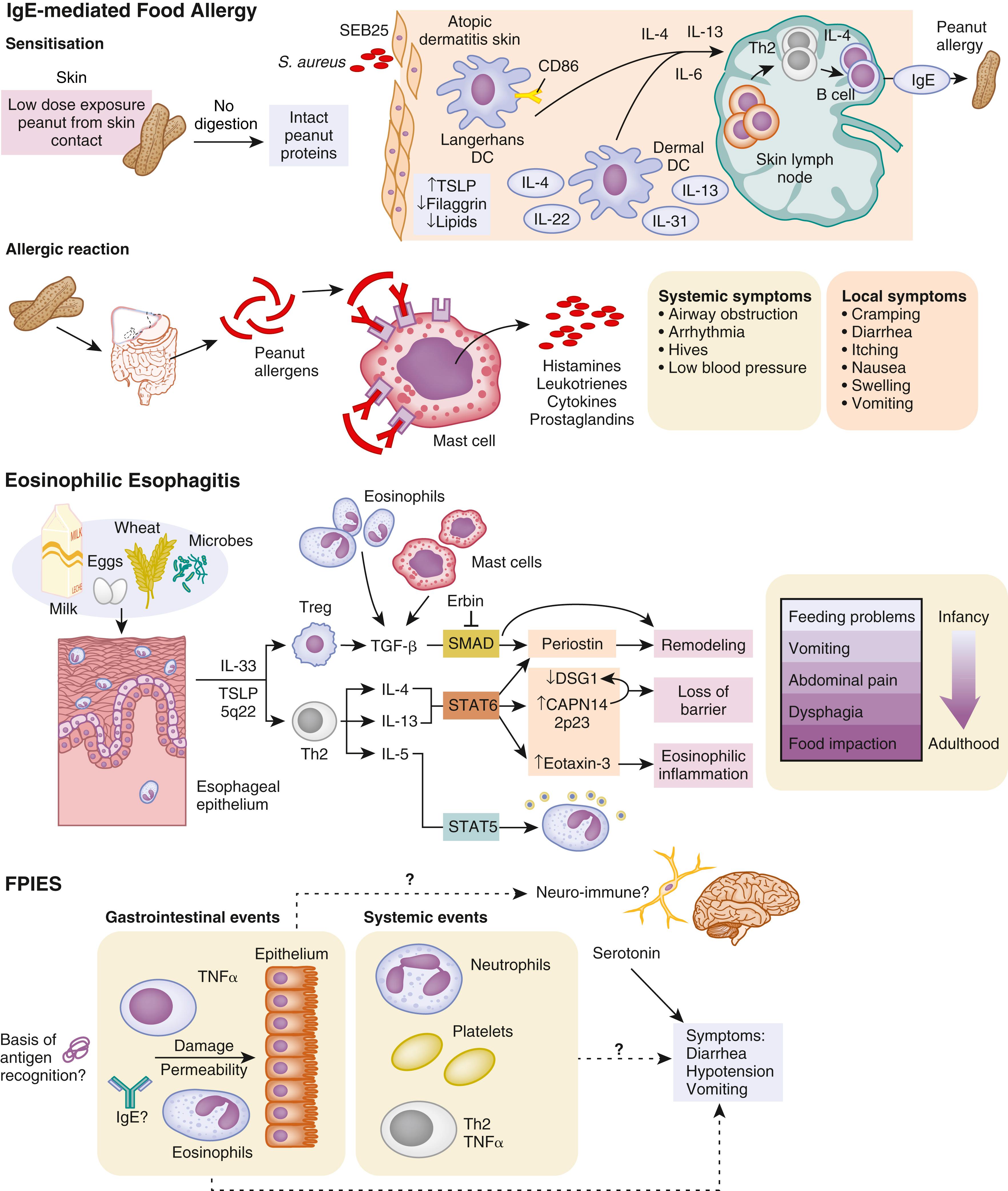
In genetically predisposed individuals, antigen presentation leads to excessive Th2 responsiveness (i.e., lymphocytes that secrete IL-4, IL-5, IL-10, and IL-13), resulting in increased IgE production and expression of FcεI receptors on a variety of cells. These IgE antibodies bind high-affinity FcεI receptors on mast cells, basophils, and DCs, as well as low-affinity FcεII (CD23) receptors on macrophages, monocytes, lymphocytes, eosinophils, and platelets. When food allergens penetrate mucosal barriers and reach IgE antibodies bound to mast cells or basophils, the cells are activated, and mediators (e.g., histamine, prostaglandins, leukotrienes) are released that induce vasodilation, smooth muscle contraction, and mucus secretion, which lead to symptoms of immediate hypersensitivity. These activated mast cells also may release a variety of cytokines (e.g., IL-4, IL-5, IL-6, TNF-α, platelet-activating factor), which may induce the IgE-mediated late-phase inflammation. Various symptoms have been associated with IgE-mediated allergic reactions: generalized (shock); cutaneous (urticaria, angioedema, pruritic morbilliform rash); oral (lip, tongue, and palatal pruritus and edema); GI (vomiting, diarrhea); and upper and lower respiratory (nasal congestion, laryngeal edema, and wheezing associated with ocular pruritus and tearing). A rise in the plasma histamine level has been associated with development of these symptoms after blinded food challenges. In IgE-mediated GI reactions, endoscopic observation has revealed local vasodilation, edema, mucus secretion, and petechial hemorrhage. Cell-mediated hypersensitivity reactions are believed responsible for eosinophilic esophagitis (EoE) and eosinophilic gastroenteritis (EG) (see Chapter 30 ). Activated T cells secrete IL-5 and other cytokines, attracting eosinophils and inducing the inflammatory response that causes delayed onset of symptoms. Expansion studies of T cells from biopsy specimens of milk-induced EoE patients have revealed large numbers of CD4 + Th2 cells.
In summary, the GI tract processes ingested food into a form that can be absorbed and used for energy and cell growth. During this process, non-immunologic and immunologic mechanisms help destroy or block foreign antigens (e.g., bacteria, viruses, parasites, food proteins) from entering the body proper. Despite this elegant barrier, antigenically intact food proteins enter the circulation, but in the normal host are largely ignored by the immune system, which has become “tolerized” to these non-pathogenic substances.
A number of GI food hypersensitivity disorders have been described ( Box 10.2 ). Clinically, these disorders are generally divided into two main categories: IgE-mediated and non–IgE (cell)-mediated hypersensitivities. A number of other disorders may result in symptoms similar to food-allergic reactions, and these must be excluded during evaluation ( Box 10.3 ).
GI allergy
Infantile colic (minor subset)
Pollen-food allergy (oral allergy syndrome)
Eosinophilic esophagitis
Eosinophilic gastritis
Eosinophilic gastroenteritis
Allergic eosinophilic proctocolitis
Dietary protein-induced enteropathy
Celiac disease
Dermatitis herpetiformis
Food protein-induced enterocolitis syndrome
Cow’s milk-induced occult GI blood loss and iron deficiency anemia of infancy
GERD
Infantile colic (subset)
IBD
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here