Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The human body is composed of fluids and solids (proteins, fat, and minerals). Total body water (TBW) is inversely related to body fat content because fat has very low water content. Body water also contains an array of dissolved substances. Water is the largest single constituent of body composition. TBW is divided into two compartments: intracellular fluid (ICF) and extracellular fluid (ECF). ECF is further divided into the intravascular and interstitial compartments, lymphatics, and transcellular fluid. Intravascular compartment (blood volume, or BV), in turn, is subdivided into plasma volume (PV), as a part of the ECF, and red blood cell volume (RCV) as a part of the ICF, because white blood cells and platelets contribute negligibly to the total BV. Therefore, the ECF consists of plasma and interstitial fluid (ISF), so that TBW is distributed among the three major fluid spaces: ICF, plasma, and ISF ( Fig. 105.1 ).
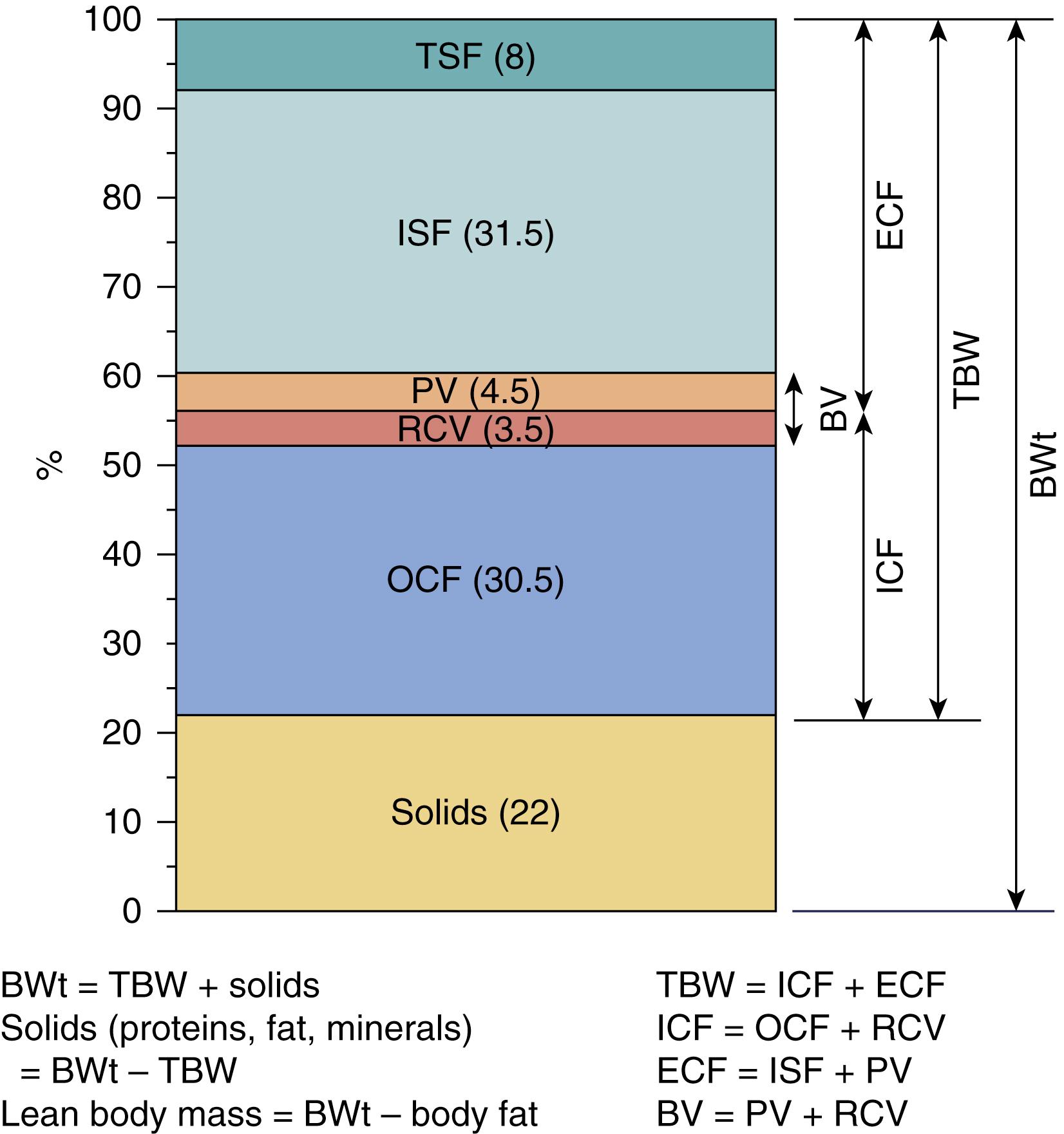
There is a constant flux of fluid among the compartments. Redistribution of fluid across the vascular endothelium under normal conditions allows overflow of excess volume from the intravascular compartment into the interstitial compartment. Consequently, volume overload of the cardiovascular system is prevented. The distribution of blood and plasma volumes is regulated by various hormones, which include the renin-angiotensin-aldosterone system, antidiuretic hormone, and atrial natriuretic factor. RCV is regulated by erythropoietin and growth factors.
ICF and ECF are governed by multiple forces that include active transport, osmotic pressure, epithelial permeability, crystalloid and colloid concentrations, intravascular and interstitial hydraulic pressures, hormones, and blood and lymph circulation.
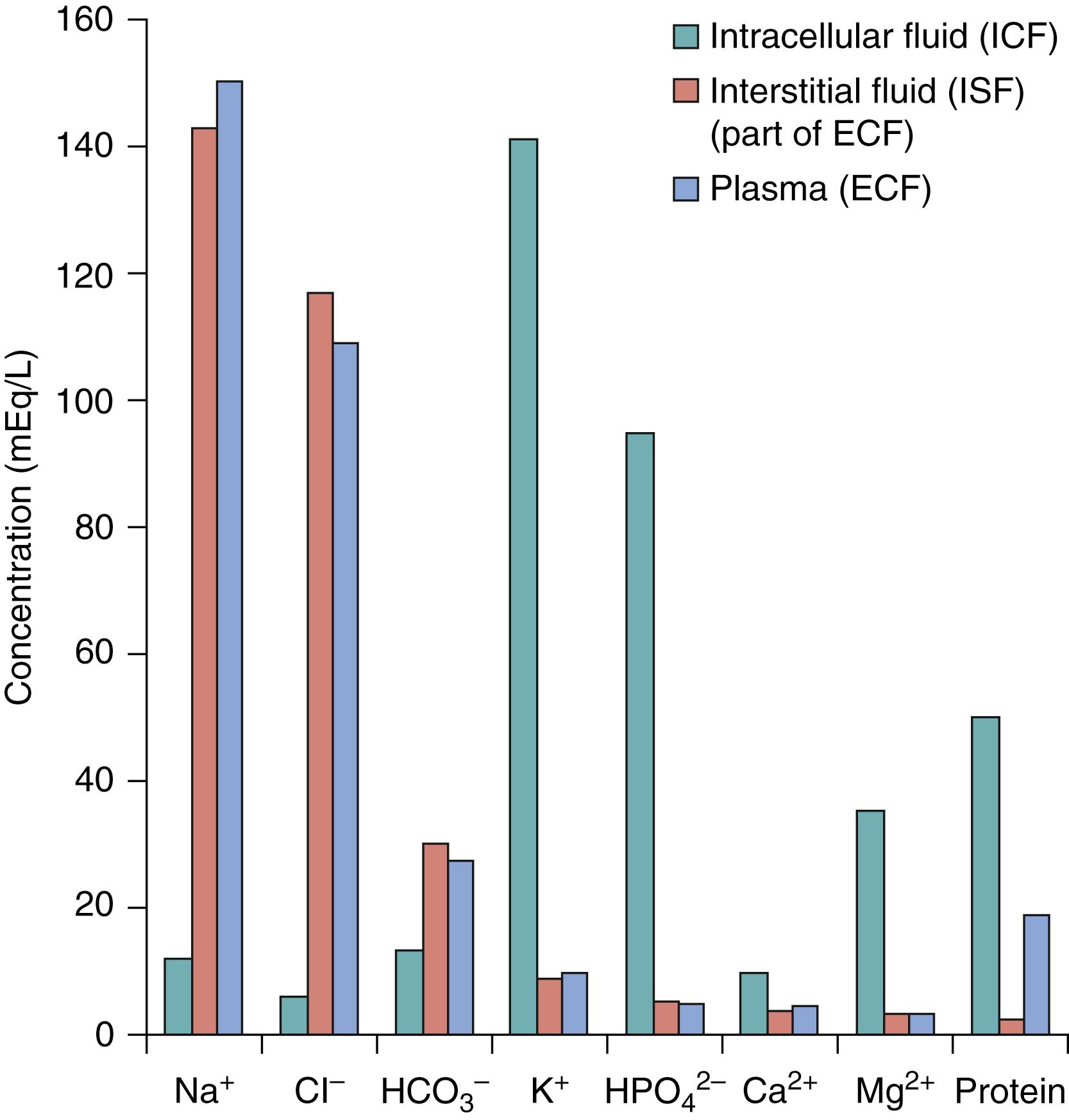
The most abundant cation is Na + , and the most abundant anion is Cl − in the ECF. The most abundant cation is K + , and the predominant anion is HPO 4 2− (phosphate) in ICF. There are more protein anions in ICF than in ECF so that ICF has a higher osmotic pressure than ISF. Normally, the higher intracellular osmotic pressure is balanced by forces that move water out of the cell, so the amount of water inside the cell does not change. When fluid imbalances between these two compartments occur, it is usually caused by a change in Na + or K + concentrations.
Plasma and ISF compositions are similar but differ from the composition of ICF. The main difference between plasma and ISF is that plasma contains quite a few protein anions and ISF has very little. Plasma contains more Na + but less Cl − than ISF. The proteins stay within the plasma and do not move out of the blood into the ISF because normal capillary membranes are practically impermeable to proteins ( Fig. 105.2 ).
Fluid leaves the plasma and enters the interstitium because of the combined effects of hydrostatic and osmotic pressure gradients at the capillary membrane. The lymphatic system counterbalances this egress by pumping ISF through the lymphatic vessels back into the circulation. Thus, the distribution of fluid between plasma and ISF depends on the balance between capillary permeability and lymphatic function under steady-state conditions.
The most dramatic changes in body fluid compartments occur during intrauterine fetal growth and development and the postnatal adaptation of the neonate from the “aquatic” intrauterine to the “terrestrial” extrauterine environment.
The amount of TBW and its distribution into the body fluid compartments are markedly different in the fetus and newborn compared with the adult. The volume-regulatory mechanisms during the fetal and perinatal period are functioning at a level that is unique compared with those in later life. This chapter describes fetal, perinatal, and postnatal changes in body fluid compartments.
There are various techniques to measure body fluid volumes, such as whole-body desiccation, dynamic skinfold thickness measurement with a Harpenden caliper, bioelectrical impedance analysis, and indicator dilution techniques. The indicator dilution techniques are more valuable for clinical studies using Fick’s law of diffusion. It is essentially the restatement of the law of conservation of mass, in which a known amount of the indicator is administered to the subject, and its concentration is then measured in samples of the body fluid space of interest. The ideal indicator is devoid of toxicity and is measurable in blood or other body fluids with sufficient accuracy and reproducibility with the use of small blood samples. It rapidly reaches equilibrium in its distribution and is neither metabolized nor excreted during the desired period of study. Table 105.1 lists the various methods used to determine the volume and distribution of body water in the fetus, neonate, child, and adult.
| Body Water Compartment | Technique |
|---|---|
| Total body water (TBW) | Dilution (D 2 O, H 2 18 O, antipyrine), total body electrical conductivity |
| Extracellular water (ECW) | Dilution (bromide, sucrose, thiocyanate, thiosulfate, inulin, mannitol, 22 Na or 24 Na), bioelectrical reactance, dynamic skinfold thickness |
| Interstitial water (ISW) | ISW = ECW − PV, electrical conductivity, dynamic skinfold thickness |
| Intracellular water (ICW) | ICW = TBW − ECW, dilution ( 40 K) |
| Blood volume (BV) | BV = PV + RCV a BV = [PV/(100 − Hematocrit)] × 100 Automated blood volume analyzer (BVA, Volemetron) |
| Plasma volume (PV) | Dilution [Evans blue (T-1824), Indocyanine green, 131 I- or 125 I] |
| Red cell volume (RCV) | RCV = BV − PV, dilution (biotin, 51 Cr, 32 P, 99m Tc) |
Accurate measurement of red cell mass, plasma, blood, and ISF volumes in the fetus and newborn as well as in the adult is difficult because the methods are technically complex and time consuming, and radioactive methods are no longer considered ethical in human subjects.
When blood volume is calculated from measured plasma volume or red cell mass and hematocrit, the errors in plasma volume measurement produce major errors in blood volume determinations. Hematocrit determinations are also a potential source of error.
Large vessel (venous or arterial) hematocrits are higher than the total body hematocrit because of rheologic factors. The total-body to venous hematocrit ratio (F-ratio) is 0.87 or 0.91 in normal neonates. The ratio could be lower in severely sick neonates. Thus, both the use of an incorrectly high body to venous hematocrit ratio and the delayed withdrawal of a blood sample after injection of a plasma label result in overestimation of blood volume. Therefore, an RBC label produces a more precise blood volume estimate in the fetus than double indicator dilution techniques that separately measure RBC and plasma volumes. The most accurate estimate of plasma volume is obtained by measuring blood volume with an RBC label and then multiplying by one minus the fractional large vessel hematocrit. Thus, labeled RBCs provide better estimates of plasma, RBC, and blood volume, provided that errors due to unbound labels are minimized.
Plasma volume is measured as the dilution space of labeled high-molecular-weight substances, such as dyes (Evans Blue or indocyanine green ) or radiolabeled ( 131 I or 125 I) human serum albumin or plasma proteins, after injection into the circulation (indicator dilution technique). Plasma labels mix completely with the circulating plasma within 5 minutes.
These measurements are frequently corrected for the loss of the label from the circulation by extrapolating the concentration-time curve backward to the original time of injection. Even with this correction, plasma volume measurements are subject to large errors for two major reasons: (1) All plasma labels are rapidly lost from the circulation through the capillary membranes of organs, such as the liver, even in normal neonates, which exhibit high permeability even to high-molecular-weight substances. Extrapolating back to the time of injection does not correct for this loss because it is too rapid to be detected. (2) Most labels such as radioisotopes or dyes are not completely bound to the plasma proteins when injected into the circulation. Unbound labels are rapidly lost from the circulation and again result in an overestimation of plasma volume.
Red cell mass is determined as the dilution space of a known quantity of labeled erythrocytes (indicator dilution technique; Biotin, 51 Cr, 32 P, 99m Tc) or calculation from blood and plasma volumes. To measure red cell mass, blood is taken from the study subject, mixed with the erythrocyte label, and then retransfused into the subject. Labeled red blood cells (RBCs) do not leave the circulation for several hours, but the mixing time might be markedly prolonged compared with labeled plasma. Biotin has been validated as a red cell label in neonates because there is a consensus that the use of radioactive materials was not ethical for research studies in human fetuses and neonates. ,
ISF volume can be calculated as the difference between ECF and plasma volume. However, measurement of each of these volumes is subject to error. ECF volume has been measured as the volume of distribution of a variety of labels injected into the circulation (indicator dilution technique: bromide, sucrose, thiocyanate, thiosulfate, inulin, mannitol, 22 Na, or 24 Na).
Each of these labels has a unique distribution space that does not exactly match the biologic ECF volume. Therefore, the comparison of ECF volumes measured with different methods is difficult. Bromide and sucrose are more widely used as ECF markers in the newborn. , Bromide may not be adequate in the fetus because of transplacental losses and because it has an intracellular distribution in the fetus and neonate. Sucrose is not metabolized, does not enter cells, and has been validated in neonates and children. ,
Water is partitioned between the fetus, placenta, chorionic and amniotic membranes, and amniotic fluid (AF) during fetal life. The AF that surrounds the fetus is often considered to be an extension of the fetal extracellular space under unique volume regulatory mechanisms before birth. AF is formed from either a transudate of fetal plasma through nonkeratinized skin or from maternal plasma across the uterine decidua or placental surface. AF most likely represents trophoblastic or fetal transudation early in gestation, which is isotonic with fetal and maternal plasma but contains minimal protein. AF reflects the fetal ECF to around the 20th week of gestation because the fetal skin is permeable to free exchange of substances.
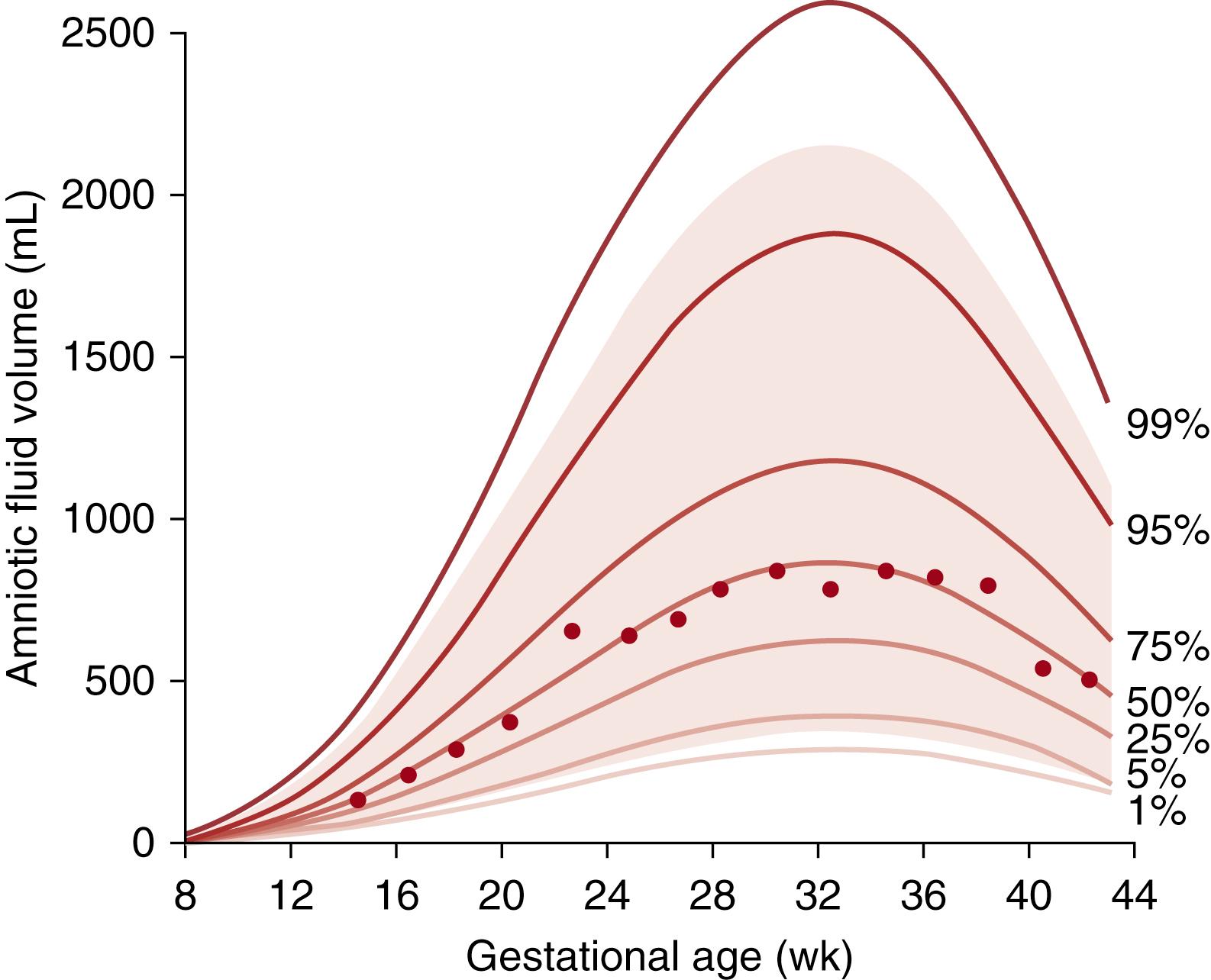
Approximately 4000 mL of water accumulates in the human uterus (2800 mL in the fetus, 800 mL in AF, and 400 mL in placenta) near term. The amniotic fluid volume (AFV) may vary from 500 mL to more than 1200 mL. Although AFV does not necessarily correlate with fetal weight, AF volume is lower in growth-restricted fetuses and higher in macrosomic fetuses.
AFV is 98% to 99% water and is regulated within a narrow range to maintain fetal fluid status throughout gestation. Fetal organ function becomes more important for the regulation of AFV as gestation advances. AFV gradually increases during the first trimester, remaining relatively stable with an average volume of 700 to 800 mL between 22 and 39 weeks of gestation. There is also a peak at 33 weeks of gestation. Thereafter, AF decreases by 8%/week with a mean volume of approximately 500 mL at 40 to 42 weeks of gestation ( Fig. 105.3 ) .
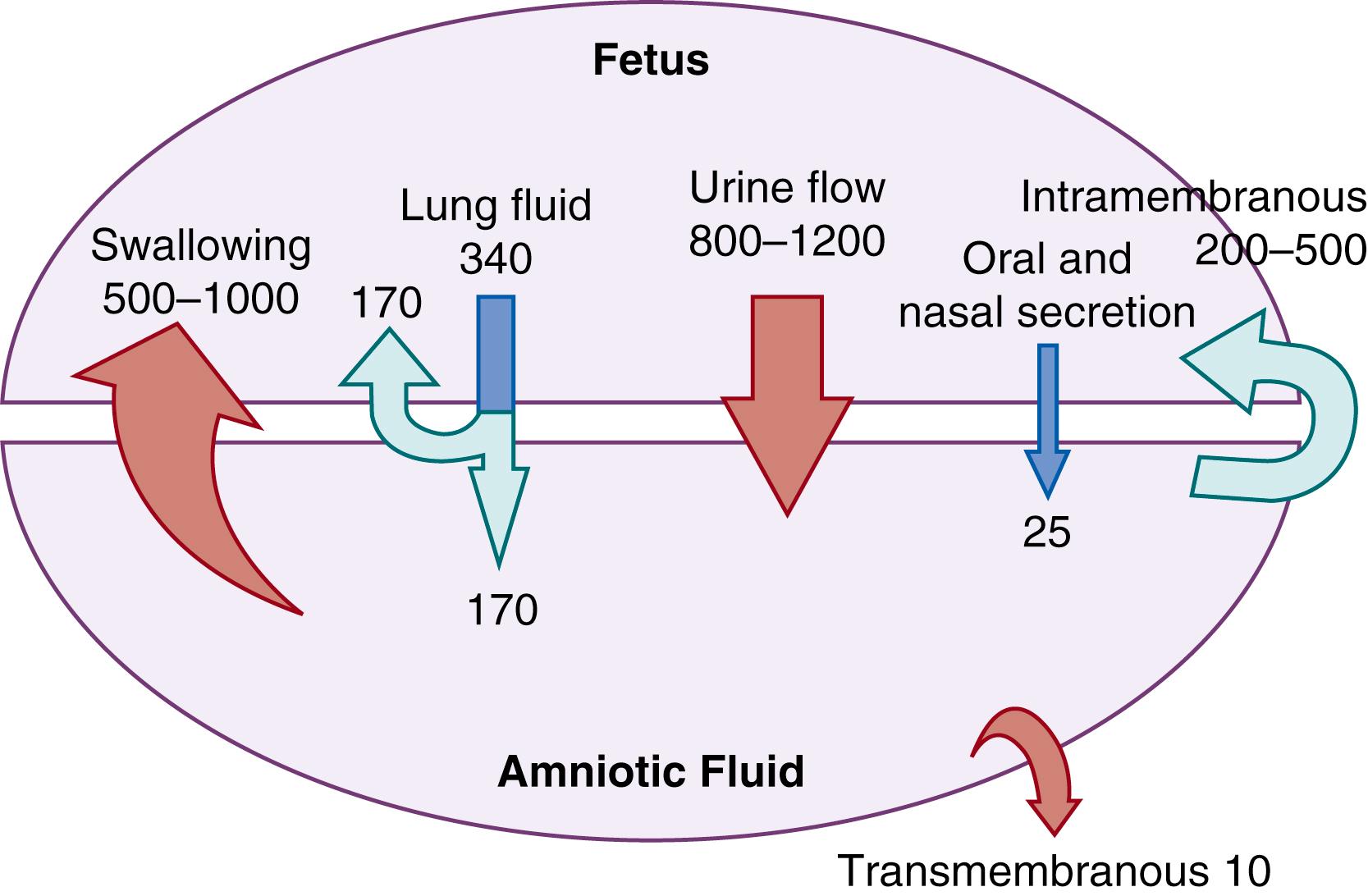
The two primary sources of AF influx during the latter half of gestation are fetal urine and lung liquid, with additional small contributions from fetal oral-nasal secretions. However, fetal urine is the main source of AF. The two primary routes of AF egress are fetal swallowing and the intramembranous absorption of water into fetal vasculature, , as schematically summarized in Fig. 105.4 .
The fetus produces hypotonic urine of 300 mL/kg/day after mid-gestation. This reduces AF osmolality and provides a large potential force for the outward flow of water across the intramembranous pathway (AF to fetal circulation). AF osmolality declines from 290 mOsm/kg in the first trimester to approximately 255 mOsm/kg near term, which favors water transfer from the amniotic cavity into the fetal blood across the fetal placental surface. , Maternal and fetal osmolality are very similar, which limits the quantity of water transfer to and from the fetus under normal conditions. The chemical composition of AF also changes during gestation. AF urea, creatinine, and uric acid increase during the second half of pregnancy because of increased renal excretion.
The volume of fetal lung fluid depends on the developmental stage of the lungs. Lung fluid volume is very low early in gestation and increases rapidly with the accelerated lung growth late in gestation. This large volume suggests that lung fluid could be a potential water source for the fetus. The fetus secretes isotonic lung fluid of 60 to 100 mL/kg/day at term, which serves to expand the airways and promote normal lung growth. This fluid is formed by active secretion of chloride ions into the alveolar spaces, resulting in a progressive accumulation of fluid in the lungs as gestation progresses. Pulmonary transepithelial chloride secretion appears to be the major driving force responsible for the production of liquid in the fetal lung lumen. Since the secretion of fetal lung fluid far exceeds that needed for lung volume expansion, the excess lung fluid exits via the trachea. The fluid appears to exit the trachea intermittently rather than making a gradual egress. At least half of secreted lung fluid is immediately swallowed, and approximately half flows into the amniotic cavity. Removal of AF occurs by fetal swallowing and the intramembranous absorption of AF into the fetal blood through the fetal surface of the placenta.
Fetal swallowing is the major mechanism by which fluid is removed from the amniotic cavity, comprising 500 to 1000 mL/day. Factors known to increase fetal swallowing include decreased amniotic osmolality, increased fetal plasma osmolality, and increased AFV.
The total turnover of the AF (1170 mL/day, urine production plus lung fluid egress and minus volume swallowed of 750 mL/day) leaves a surplus influx of fluid into the amniotic cavity of at least 400 mL/day. This difference is compensated for by another pathway, termed the intramembranous pathway . The daily AF turnover of 1000 mL in the near-term fetus is actually higher than the absolute AFV of 700 to 800 mL. Therefore, AFV must be highly regulated to avoid oligohydramnios or polyhydramnios.
Regulatory mechanisms for AFV act at three levels:
Transfer of water between mother and fetus across the placenta. Transplacental transfer is dependent on the existence of hydrostatic and/or colloid osmotic pressure differences between fetal and maternal vessels. Placental water transfer between mother and fetus can serve as a reservoir to stabilize fetal ECF volume and blood pressure. The intravascular infusion of large volumes of crystalloid solutions in fetal sheep results in the transfer of large amounts of water and solute across the placenta to the mother, either against or in the absence of chemical concentration gradients. , These observations suggest that a normal fetus can protect itself against both volume and salt overloads by rapid transfer of excess to the mother. Reduced fetal blood pressure releases fetal angiotensin. This increases the resistance in the fetal placental precapillary vessels and reduces fetal placental blood flow, thereby promoting water transfer from mother to fetus to restore fetal blood pressure. However, the placenta or any other single fetal structure does not control the amount of water that enters the fetus. The combined physiologic properties of the fetal heart, kidneys, somatic tissues, and placenta contribute to the control of the fetal volume status.
Regulation of flow between the amniotic cavity and the fetus. Increasing or decreasing intramembranous absorption appears to be the main mechanism regulating flow between the amniotic cavity and fetus. Intramembranous water movement into the fetal circulation is driven by the large osmotic difference between fetal plasma and AF, because the fetal vessels on the placental surface are bathed in AF. The primary mechanism that drives intramembranous volume flow is not passive osmosis. Instead, it has been suggested that AF with all its dissolved solutes is transported across the amniotic membrane via bulk flow transfer by a yet unspecified vesicular transport mechanism. Aquaporins (AQPs) in the placental and fetal membranes may also play a role in AF absorption. AQP 1, 3, 8, and 9 are the major AQPs in the placental and fetal membranes. Fetal membrane AQP1, and placental AQP1 and AQP9 expression, were negatively correlated with AFV. Placental AQP3 expression was positively correlated with AFV in pregnant mice.
Changes in maternal hydration: maternal oral hydration with water or intravenous hypotonic fluid significantly increases AFV in oligohydramnios because of maternal osmotic changes, rather than maternal volume expansion.
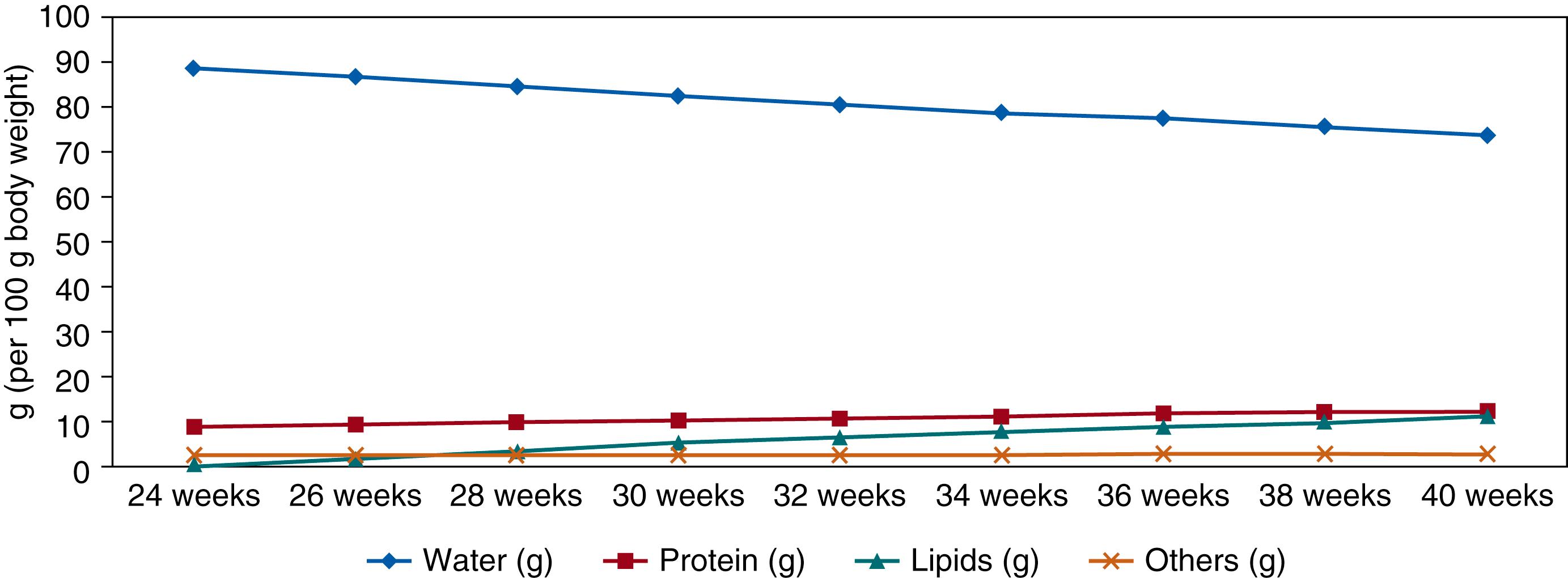
Friis-Hansen in 1961 reported the developmental changes in the relative fluid volumes in humans from early in the fetal period through adulthood. As shown in Fig. 105.5 approximately 95% of the fetus is water in the early fetal period. The water proportion of total body weight gradually decreases throughout the fetal period to reach 86% at 27 weeks of gestation, of which the majority is in the ECF (60%), and 78% water with 44% in ECF, 34% in ICF at term. This reduction in TBW is a result of the accumulation of body solids during growth. Body solids increase in the first two thirds of gestation because of the accretion of protein and minerals, but little fat deposition occurs. During the last trimester of gestation the proportion of body solids increases from 14% to 24% of body weight because of the deposition of body fat from 2% of body weight at 27 weeks of gestation to 10% to 15% of body weight at term. The calculated body composition of the reference fetus for various gestational ages is indicated in Fig. 105.6 .
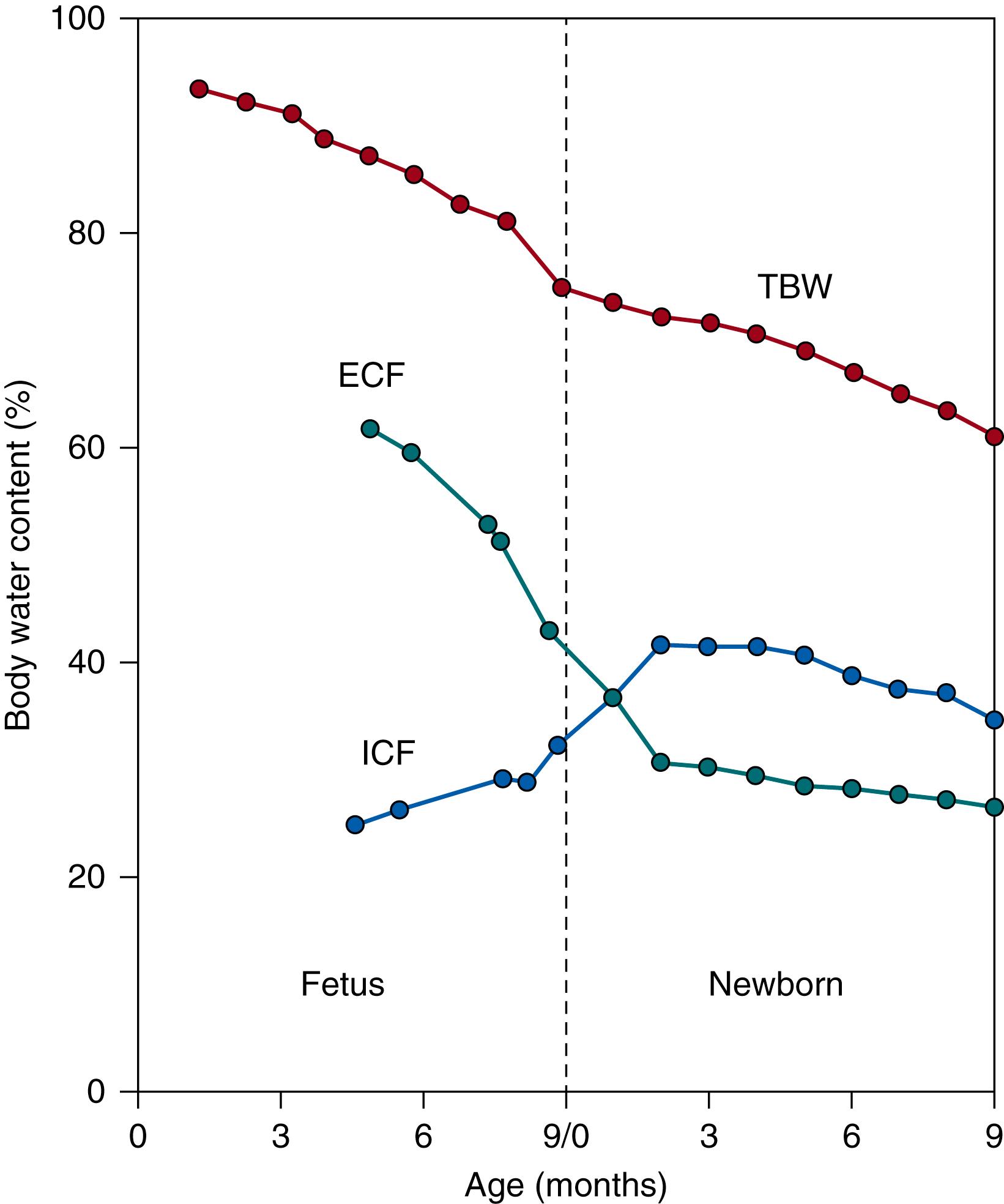
Major changes occur in the body fluid distribution into ECF and ICF, which coincide with the reductions in TBW during fetal life. ECF volume is extremely large early in gestation, accounting for 62% of body weight, and is more than twice as large as the ICF volume. The large ECF space during fetal life is because growth occurs by cell division rather than by cell growth and results in tissues with small cells surrounded by a large ECF layer. Enlargement of cell size during normal intrauterine growth in the second half of gestation increases the intracellular space from 25% to 32% of body weight from mid-gestation to term, whereas over the same interval ECF volume decreases from 62% to 43% of body weight. Decreases in hyaluronan as gestation advances also contribute to reductions in ECF water content because it is major constituent of the extracellular matrix. Hyaluronan has a high water binding capacity and is the most abundant component of the fetal extracellular matrix during the early phase of rapid cell multiplication. The reduction in ECF mainly results from loss of ISF because plasma volume per unit of body weight does not change at different gestational ages. ICF increases approximately in proportion to body weight in the first few weeks of postnatal life. ICF continues to increase as a percent of body weight until it exceeds that of ECF, at three months of life. Hormonal, renal, and cardiovascular mechanisms are the main factors influencing ECF volume regulation in the fetus and neonate.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here