Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Fibrous tumors of infancy and childhood can be divided into two large groups: (1) lesions that correspond to similar lesions in adults in terms of clinical setting, microscopic picture, and behavior (e.g., nodular fasciitis, palmar/plantar fibromatosis, abdominal/extraabdominal fibromatosis) and (2) fibrous lesions that are peculiar to infancy and childhood and generally have no clinical or morphologic counterpart in adult life. The lesions in the second group are less common, and because of their unusual microscopic features, they pose a special problem in diagnosis. In fact, the microscopic picture often does not accurately reflect the biologic behavior, and features such as cellularity and rapid growth may be mistaken for evidence of malignancy, sometimes leading to unnecessary and excessive therapy. Therefore, accurate interpretation and diagnosis of fibrous lesions peculiar to infancy and childhood are essential for predicting clinical behavior and for selecting the proper forms of therapy ( Table 8.1 ).
| Histologic Diagnosis | Age (Years) | Location | Solitary | Multiple | Regression |
|---|---|---|---|---|---|
| Fibrous hamartoma of infancy | B-2 | Axilla, upper arm | + | − | − |
| Inclusion body fibromatosis | B-2 | Fingers, toes | + | /- | + |
| Hyaline fibromatosis | 2-A | Dermis, subcutis | − | + | − |
| Gingival fibromatosis | B-A | Gingiva, hard palate | + | + | + (after tooth extraction) |
| Fibromatosis colli | B-2 | Sternocleidomastoid muscle | + | Bilateral | + |
| Lipofibromatosis | B-4 | Musculature | + | − | − |
| Congenital/infantile fibrosarcoma | B-2 | Musculature | + | − | − |
| Calcifying aponeurotic fibroma | 2-A | Hands, feet | + | − | + |
Fibrous hamartoma of infancy is a distinctive, benign neoplasm that most frequently occurs during the first 2 years of life. This lesion was first reported by Reye in 1956 as a subdermal fibromatous tumor of infancy . In 1965, Enzinger reviewed a series of 30 cases from the files of the Armed Forces Institute of Pathology (AFIP) and suggested the term fibrous hamartoma of infancy to emphasize its organoid microscopic appearance and its frequent occurrence at birth and during the immediate postnatal period.
The lesion usually develops during the first 2 years of life (median age: 10 months) as a small, rapidly growing mass in the subcutis or reticular dermis. A literature review of 197 cases found that 91% arose within the first year of life. Rare lesions have been reported in older infants and children. In the recent study of 145 cases by Al-Ibraheemi et al., the patients ranged in age from birth to 14 years (mean age: 15 months); six cases were congenital. As with other fibrous tumors in children, it is more common in boys, who are affected two to three times more often than girls. The mass is often freely movable; occasionally it is fixed to the underlying fascia but only rarely involves the superficial portion of the musculature. These lesions grow rapidly from the outset up to about age 5 years. The growth of the lesion then slows but does not cease or regress spontaneously.
Most fibrous hamartomas occur above the waist; the most common location is the anterior or posterior axillary fold, followed in frequency by the upper arm, thigh, inguinal and pubic region, shoulder, back, and forearm, although the anatomic spectrum continues to widen as larger clinicopathologic studies are published. In the Al-Ibraheemi study, 69 cases arose in typical sites (e.g., axilla, back, upper arm), but 76 arose in unusual locations. Few cases have been described in the feet or hands, a feature that helps distinguish this lesion from inclusion body fibromatosis, lipofibromatosis, and calcifying aponeurotic fibroma. Virtually all cases are solitary, with only rare reports of multiple lesions in the same patient. There is no evidence of increased familial incidence or associated malformations or other neoplasms, although there are reports of this lesion arising in the setting of a congenital nevus. Antecedent trauma is occasionally reported at presentation but is likely unrelated to pathogenesis.
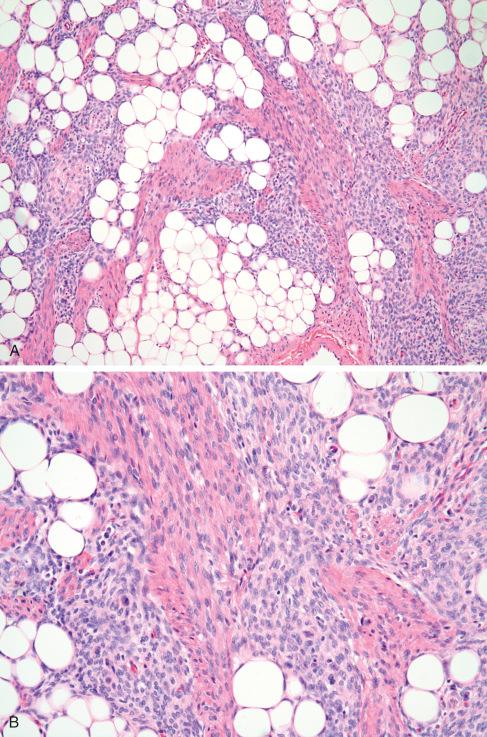
The excised lesion tends to be poorly circumscribed and consists of an intimate mixture of firm, gray-white tissue and fat. In some cases the fatty component is inconspicuous, whereas in others it occupies a large portion of the tumor, thereby resembling a fibrolipoma. Most measure 3 to 5 cm in greatest diameter, but tumors as large as 15 cm have been reported. Fibrous hamartoma of infancy is characterized by three distinct components forming a vague, irregular, organoid pattern ( Figs. 8.1 and 8.2 ):
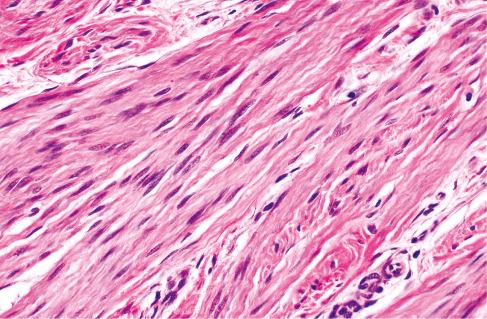
Well-defined intersecting trabeculae of fibrous tissue of varying size and shape and composed of well-oriented spindle-shaped cells (predominantly myofibroblasts) separated by varying amounts of collagen ( Figs. 8.3 and 8.4 )
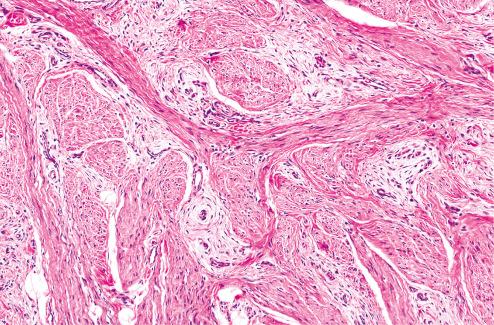
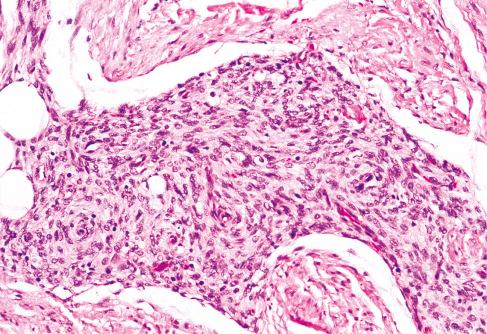
Loosely textured areas consisting chiefly of immature small, round, or stellate cells in a myxoid matrix ( Figs. 8.5 to 8.7 )
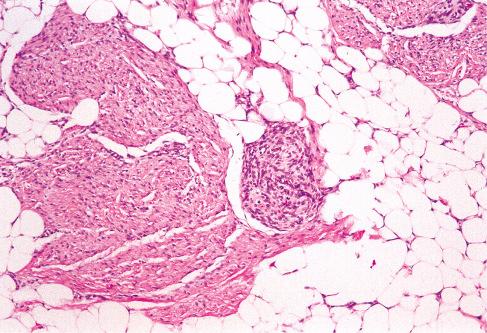
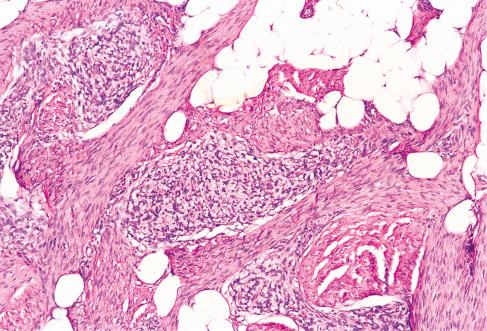
Varying amounts of interspersed mature fat, which may be present at only the periphery of the lesion or may be the major component
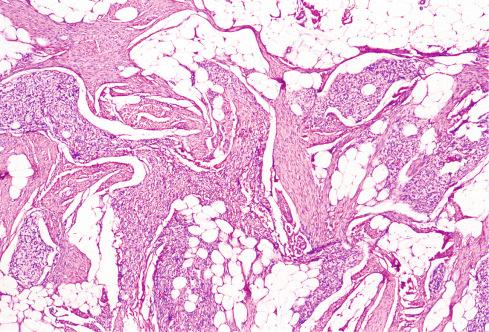
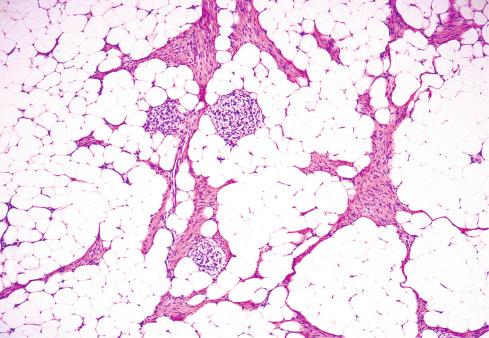
Despite the lack of clear boundaries between the fat in the tumor and that in the surrounding subcutis, the fat is an integral part of the lesion. In many cases, its total amount exceeds many times the amount of fat normally present in the surrounding subcutis. In some cases, the immature small round cells in the myxoid foci are oriented around small veins.
Some tumors may have hyalinized zones with cracking artifact , imparting a pseudoangiomatous appearance resembling giant cell fibroblastoma ( Fig. 8.8 ). These areas were found in 30% of cases in the Al-Ibraheemi study and in more than 50% of cases in the Saab study. Adding to this confusion is that these pseudoangiomatous areas stain for CD34, a marker also consistently expressed in giant cell fibroblastoma. Unlike giant cell fibroblastoma, PDGFRB rearrangements have not been found in fibrous hamartoma of infancy.
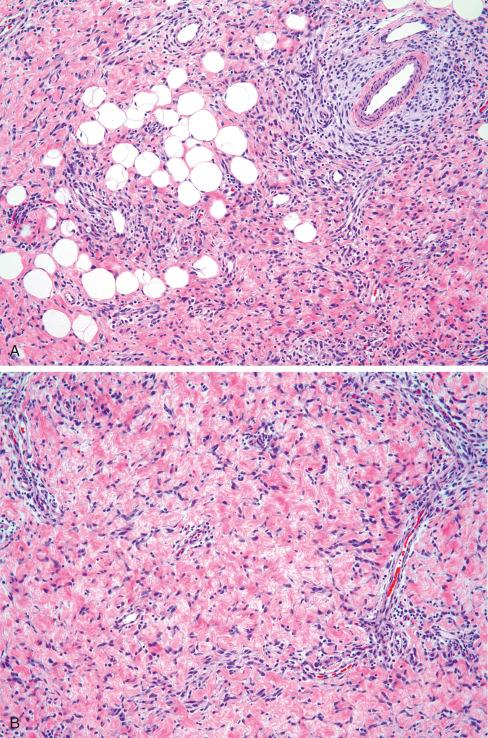
Interestingly, two cases of fibrous hamartoma of infancy with sarcomatous foci have been described. These areas are characterized by high cellularity, high nuclear grade, and brisk mitotic activity ( Fig. 8.9 ). At the molecular level, those cases with sarcomatous features were found to be hyperdiploid/near-tetraploid, with both loss of heterozygosity of chromosomes 1p and 11p, and loss of 10p, chromosome 14, and a large portion of chromosome 22.
On immunohistochemistry (IHC), smooth muscle actin (SMA) stains the spindled cells in the trabecular component, with only rare expression of desmin. The primitive myxoid matrix stains for CD34, with variable expression of actins. As mentioned previously, the pseudoangiomatous foci stain for CD34.
In 2016, Park et al. found 12 of 12 cases of fibrous hamartoma of infancy to harbor EGFR exon 20 insertion/duplication mutations, which were not found in 10 cases of other pediatric fatty tumors. This alteration was subsequently reported in a case of fibrous hamartoma of infancy with a predominant pseudoangiomatous pattern.
In most cases, the organoid pattern characteristic of fibrous hamartoma of infancy is readily recognized, so the lesion is not difficult to distinguish from other entities. On occasion, when the myofibroblastic area is predominant, the lesion may be difficult to distinguish from lipofibromatosis, the recently described lipofibromatosis-like neural tumor, diffuse myofibromatosis, and calcifying aponeurotic fibroma. Lipofibromatosis (detailed later) is characterized by either primitive cells or mature fibroblastic spindle-shaped cells that infiltrate the subcutis and skeletal muscle with fascicular growth, lacking the characteristic organoid pattern of fibrous hamartoma. Lipofibromatosis-like neural tumor (also described later) has features similar to lipofibromatosis but shows more cytologic atypia and nuclear hyperchromasia, S-100 protein immunoreactivity, and characteristic NTRK1 gene rearrangements. Diffuse myofibromatosis , typically nodular or multinodular, is characterized by light-staining nodules separated by or associated with hemangiopericytoma-like vascular areas. Calcifying aponeurotic fibroma may grow in the same trabecular manner, especially during its earliest phase, when there is still little or no calcification. However, the older age of the children and the distal extremity location should permit an unequivocal diagnosis.
Awareness of the characteristic organoid pattern also facilitates distinction from infantile fibrosarcoma and embryonal rhabdomyosarcoma. Because some fibrous hamartomas of infancy occur in the scrotal region, the spindle cell form of embryonal rhabdomyosarcoma enters the differential diagnosis; however, this lesion generally occurs in older children and is composed of cells with more cytologic atypia and myogenin immunoreactivity.
It is important to recognize and distinguish fibrous hamartoma of infancy from other forms of fibromatosis because it is a benign lesion that, despite its focal cellularity, is usually cured by local excision. Up to 16% locally recur, but recurrences are nondestructive and are generally cured by local reexcision. Follow-up (median: 8 months) in 52 patients showed only 2 with local recurrence and no metastases. Because of the presence of extensive local disease in one patient (10-month-old female) with sarcomatous foci, a forequarter amputation was necessary. Additional cases of fibrous hamartoma of infancy with sarcomatous foci and clinical follow-up are needed before the clinical significance of sarcomatous histology can be determined.
Inclusion body fibromatosis, also known as infantile digital fibromatosis, is a distinctive fibrous proliferation of infancy. It is characterized by occurrence in the fingers and toes, a marked tendency for local recurrence, and the presence of characteristic inclusion bodies in the cytoplasm of the neoplastic fibroblasts. In 1957, Jensen et al. reported seven patients whose presentations were consistent with this entity but referred to these lesions as digital neurofibrosarcoma in infancy . Enzinger subsequently reported seven cases in 1965 as infantile dermal fibromatosis .
Most patients present with a firm, broad-based, hemispheric or dome-shaped, nontender nodule with a smooth, glistening surface that is skin colored or pale red. It is usually small, rarely exceeding 2 cm in greatest diameter. Almost all lesions are noted within the first 3 years of life, with most recognized by 1 year. Up to one-third of cases are already present at birth. In the Laskin study, the 57 patients ranged in age from newborn to 10 years (median: 12 months) at surgery. Rare examples have been described in older children, adolescents, and even in adults. Unlike most other forms of fibromatosis, the condition has a roughly equal gender distribution, but no evidence of familial tendency. This lesion has been identified in patients with terminal osseous dysplasia (also known as digitocutaneous dysplasia) and pigmentary defects, a rare, lethal X-linked dominant disease that has been linked with mutations of the FLNA gene. However, it is unclear if the fibroblastic lesions in these patients are identical to inclusion body fibromatosis because the cells lack the characteristic inclusions and do not stain for smooth muscle actin.
The nodules are more often found in the fingers than the toes and usually are located on the sides or dorsum of the distal or middle phalangeal joints, especially of the third, fourth, and fifth digits. Although the thumb can be affected, cases involving the great toe have not been reported. The lesions may be single or multiple and often affect more than one digit of the same hand or foot. Occasionally, they involve both the fingers and the toes of the same patient. Very few cases have been described as occurring in areas other than the hands and feet. Purdy and Colby reported a case with typical eosinophilic perinuclear inclusions in the upper arm of a 2½-year-old child near an old injection site. Pettinato et al. described two cases of extradigital inclusion body fibromatosis in the breasts of 24- and 53-year-old women.
Although pain and tenderness are not typical symptoms, associated functional impairment or joint deformities may be present, including lateral deviation or flexion deformities of the adjacent joints, which typically remain unchanged after surgical removal of the lesions.
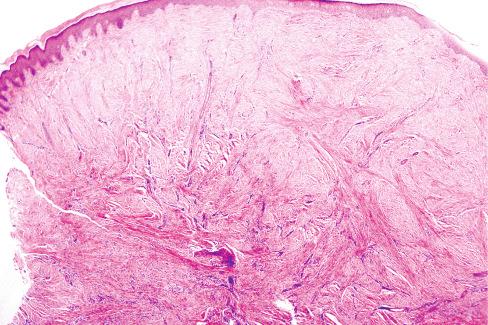
The excised lesions are small, firm masses that are covered on one side by intact skin and have a solid white cut surface ( Fig. 8.10 ). The microscopic appearance varies little, consisting of a uniform proliferation of fibroblasts surrounded by a dense collagenous stroma ( Fig. 8.11 ). The lesions are poorly circumscribed and extend from the epidermis into the deeper portions of the dermis and subcutis, typically surrounding the dermal appendages. The overlying epidermis is usually minimally altered, with slight hyperkeratosis or acanthosis.
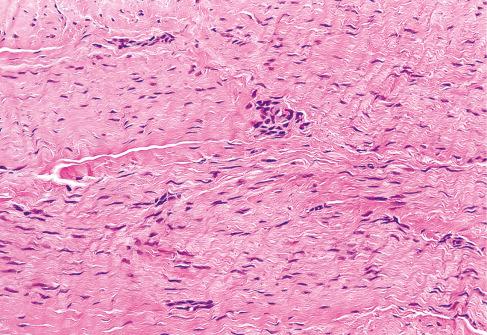
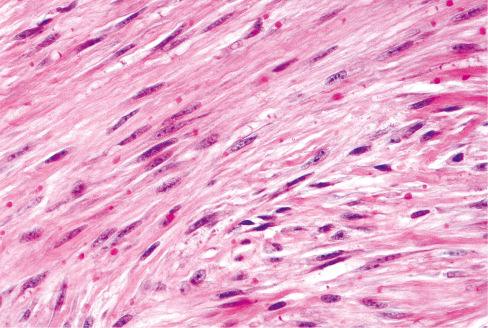
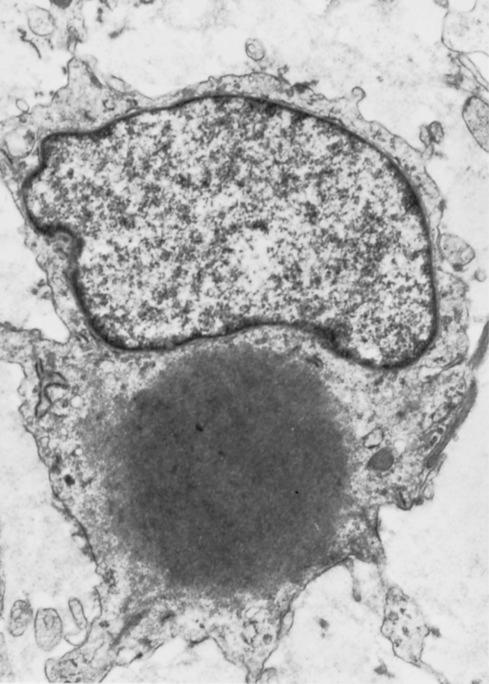
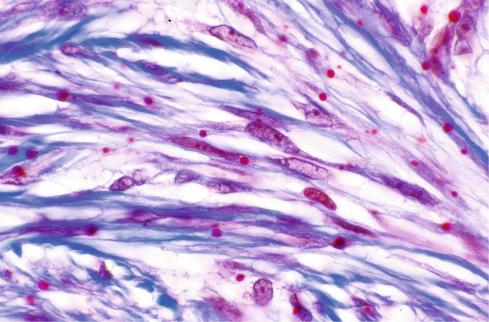
The most striking feature of the tumor is the presence of small, round inclusions in the cytoplasm of the constituent spindle cells. The number of inclusions varies from case to case. Some are numerous and easily detected, whereas others are scarce and difficult to find with hematoxylin-eosin–stained slides. Typically, these inclusions are situated close to the nucleus, from which a narrow clear zone often separates them ( Fig. 8.12 ). They are eosinophilic and resemble erythrocytes, except for their more variable size (3-15 μm), intracytoplasmic location, and lack of refraction. Ultrastructurally the inclusions correspond to localized collections of non–membrane-bound, granular fibrillary material contiguous with the rough endoplasmic reticulum ( Fig. 8.13 ). Numerous histochemical preparations can be used to highlight these inclusions; they stain a deep red with Masson trichrome stain ( Fig. 8.14 ), but they do not stain with periodic acid–Schiff (PAS), Alcian blue, or colloidal iron stains. The spindle cells have pale, eosinophilic cytoplasm and elongated nuclei with fine chromatin. Mitotic figures are rare. Hayashi et al. suggested a relationship between the age of the lesion and the number of inclusion bodies found, with younger lesions having more inclusion bodies and less fibrosis, and the opposite with older lesions.
On IHC the spindled cells stain consistently for actin, but results have varied for actin immunoreactivity of the inclusion bodies themselves. Most of the earlier studies using formalin-fixed tissues were unable to demonstrate actin staining. However, actin staining of the inclusion bodies has been demonstrated using alcohol-fixed tissue as well as potassium hydroxide and trypsin-pretreated, formalin-fixed tissue. In the Laskin study, the cells consistently expressed calponin, SMA, desmin, and CD99. Most also expressed CD117, but stains for cytokeratins, estrogen, and progesterone receptors were negative. The Laskin study also indicated that rare, nuclear expression of beta-catenin may be present (2/11 = 18%), but a subsequent series by Thway et al. did not confirm this observation (0/6). Henderson et al. reported calponin immunoreactivity of the intracytoplasmic inclusions.
The exact nature of the inclusions is not clear. Because of the resemblance of these inclusions to the viroplasm of fibroblasts infected with Shope fibroma virus , Battifora and Hines proposed a possible viral etiology. The IHC and ultrastructural findings strongly suggest that the inclusions are related to the intracellular bundles of microfilaments and represent densely packed masses of actin microfilaments. The occurrence of extradigital posttraumatic lesions that are histologically indistinguishable from those on the digits and antecedent trauma or surgery related to digital lesions suggests that trauma may stimulate development of the lesion. Inclusion bodies identical to those found in inclusion body fibromatosis have also been described in a variety of other tumors, including benign phyllodes tumor and fibroadenoma of the breast and endocervical polyps.
Although a significant percentage of these lesions recur locally, the ultimate prognosis is excellent. Recurrences usually appear at the same site within a few weeks or months after the initial excision. Although there is an initial period of growth, if watched long enough, many lesions regress spontaneously. In the Laskin study, 28 of 38 (74%) cases with follow-up information persisted or locally recurred at a median of 4 months after surgery. Most authors advocate conservative treatment because there is no evidence of aggressive behavior or malignant transformation. Some advocate a watch-and-wait approach following a diagnosis, given the high rate of spontaneous regression. Intralesional injection of corticosteroids or fluorouracil instead of surgical excision has also been advocated. Mohs micrographic surgery may be effective in minimizing the risk of local recurrence. Deformities and contractures develop in some patients, regardless of whether the lesions are removed surgically, and surgical correction of contractures and functional changes are sometimes necessary.
Juvenile hyaline fibromatosis (JHF) is another rare hereditary disease that bears a superficial resemblance to myofibromatosis but differs by its cutaneous distribution of the tumor nodules, the histologic picture, and associated clinical features. Murray first described the condition in 1873 as “molluscum fibrosum in children,” thought to represent an unusual variant of neurofibromatosis. Whitfield and Robinson offered a follow-up report of these three cases in 1903, but amazingly, no further reports occurred until 1962, when Puretic et al. reported a case under the name mesenchymal dysplasia . A variety of terms were used in subsequent reports, but Kitano coined the term juvenile hyaline fibromatosis , which has become the preferred term. For many years, the terms juvenile hyaline fibromatosis and infantile systemic hyalinosis (ISH) were used interchangeably, but the exact relationship between these two entities was unclear. Although they share a number of characteristics in common, there are some significant clinical differences. Genetic data (described later) clearly link these two diseases along a spectrum.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here