Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
the advent of fetal intervention introduced the concept of surgically correcting or ameliorating known congenital defects in utero. With improvements in prenatal imaging and surgical techniques, fetal interventions have grown to include diagnoses associated with intrauterine demise and significant postnatal morbidity. The goal of fetal intervention is to improve the probability that the fetus will develop normally with minimal postnatal morbidity. Increasingly, advances have changed some procedures from open in utero interventions, which are associated with significant maternal risk, to percutaneous or fetoscopic techniques, thus improving the maternal risk/benefit ratio while diminishing postoperative uterine contractions associated with open procedures.
Fetal surgery often requires the anesthesiologist to care for two or more patients at once, all with distinctive and, at times, conflicting requirements. The first is the mother who can express her level of discomfort, who can be monitored directly, and to whom drugs can be administered easily. The second (and possibly third) is the fetus. For the latter, detecting pain depends solely on indirect evidence, monitoring is limited at best, administering drugs is more complicated, and there is the possibility of long-term effects from procedures and drugs administered during early development. The anesthesiologist is required to provide both maternal and fetal anesthesia and analgesia while ensuring both maternal and fetal hemodynamic stability; a plan must be prepared to resuscitate the fetus if problems occur during the intervention.
Fetal interventions have been successfully performed with various anesthetic techniques; both maternal and fetal anesthetic requirements must be considered and may, in fact, be quite different. With some endoscopic interventions, the site of surgical intervention is not innervated; thus the fetus may not sense a noxious stimulus, and its anesthetic requirements are presumably minimal. Nevertheless, fetal immobility remains essential to procedural safety and success. Other interventions may require that a needle be inserted into the fetus, which may elicit a noxious stimulus and possibly even cause pain. Open procedures can produce significant noxious stimuli. In addition to surgical demands, each mother and fetus exhibit a unique physiologic, pharmacologic, and pathophysiologic profile; the anesthesiologist must evaluate the advantages and disadvantages of each anesthetic technique and select the safest approach.
Local anesthesia is almost exclusively used to insert the trocar for percutaneous procedures. The most obvious advantage is maternal safety because the mother receives no intravenous (IV) medications. The disadvantages of this technique include increased risk of injury to the unanesthetized, nonparalyzed fetus, the absence of analgesia for the fetus, and no uterine relaxation. Patients who receive tocolytic therapy or those with polyhydramnios and uterine contractions may be at even further risk of worsening contractions with this approach.
IV sedation involves the maternal administration of benzodiazepines, opioids, and occasionally low-dose hypnotic agents. Advantages include possible provision of anesthesia and analgesia to the fetus via transplacental transfer of drugs, as well as decreased maternal anxiety and pain. Depending on the amount and effect of the drugs administered, this sedation may increase the mother's risk of aspiration because of an unprotected airway; this technique is also devoid of uterine relaxation.
Neuraxial techniques (spinal, epidural, or combined spinal and epidural anesthesia) have been used with fetoscopic techniques and, rarely, without an adjunct general anesthetic for open techniques. A T4 sensory-level blockade is required for most surgical uterine manipulations. Neuraxial techniques provide neither uterine relaxation nor analgesia or anesthesia for the fetus.
The addition of IV sedation to regional anesthesia may provide the fetus with analgesia/anesthesia via placental drug transfer. Although IV fentanyl, propofol, and benzodiazepines can be administered to patients receiving regional anesthesia, they may place the mother at increased risk of bradyarrhythmias, respiratory depression, and pulmonary aspiration; the need for a T4 sensory block may produce alterations in respiratory mechanics in addition to those associated with pregnancy. In addition, the level of sympathetic blockade is often two to six levels greater than the sensory level. Hence, a T4 sensory block may completely block cardiac accelerator fibers (T1–T4); severe bradyarrhythmias and cardiac arrest have been reported. When IV agents with vagotonic properties are administered in this clinical setting, the risk of significant bradyarrhythmias may be increased.
General anesthesia with high-dose inhalational anesthetics (generally desflurane) provides both maternal and fetal anesthesia and dose-dependent uterine relaxation even in patients who have received tocolytic therapy for premature uterine contractions. Caution must be exercised as the required depth of maternal anesthesia necessary to provide adequate uterine relaxation may produce maternal hypotension with resultant uteroplacental insufficiency and fetal cardiovascular insufficiency. Particular attention must be paid to maintenance of maternal blood pressure in the normal or perhaps slightly supranormal range.
The adjunctive administration of remifentanil and propofol infusions may reduce the necessary concentration of inhalational anesthetic required without compromising uterine relaxation. With such regimens, maternal hypotension and subsequent fetal depression may be avoided. An additional benefit of this combined approach may be the contribution of remifentanil and propofol to fetal anesthesia, as both readily cross the placenta without known reductions in placental blood flow.
A combined regional and general anesthesia technique is often used for open procedures, as well as for patients with anterior placentas, in whom externalization of the uterus for safe trocar insertion is anticipated. In addition to providing the advantages of both the regional and the general anesthetic techniques listed previously, this method allows for postoperative pain control. The physical window for trocar insertion is often smaller in this patient cohort, necessitating either externalization of the uterus or extreme lateral decubitus position. Externalization of the uterus requires a larger surgical incision than for standard cesarean sections, conferring benefit from epidural anesthesia. However, perceived intraoperative benefits (e.g., reduced requirements for inhalational agents) may be offset by the necessity to provide adequate uterine relaxation.
Maternal anesthetic techniques that do not include inhalational anesthetics may not provide adequate analgesia and/or anesthesia for the fetus. However, fetal analgesia and anesthesia may also be accomplished by delivery of anesthetics and analgesics directly to the fetus. Potential methods include transplacental, direct intramuscular, direct intravascular, and intraamniotic administration; each route of administration has advantages and disadvantages that can have a direct impact on overall outcome.
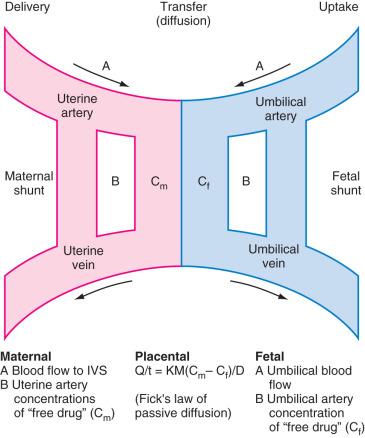
Many fetal interventions (open or endoscopic) use transplacental drug administration to provide anesthesia and analgesia for both mother and fetus ( Table 38.1 ). Many, but not all, drugs cross the placenta in accordance with Fick's law of passive diffusion ( Fig. 38.1 ). Lipid solubility, the pH of both maternal and fetal blood, the degree of ionization, protein binding, perfusion, placental area and thickness, and drug concentration are factors that influence the extent of transplacental drug diffusion. The most obvious disadvantage with this approach is that the mother must be exposed to every drug that the fetus is intended to receive, often at large concentrations, to achieve adequate drug concentrations in the fetus. In addition, the uptake of drugs may be impaired if there is reduced placental blood flow. This has implications for successful anesthesia and analgesia both in terms of the delivered fetal dose and the time interval that must be allowed from maternal administration to the start of the fetal intervention. All inhalational anesthetics cross the placental barrier, but uptake in the fetus is slower than in the mother. However, this is offset by the reduced minimum alveolar concentration (MAC) for anesthesia in the fetus, resulting in a similar onset of anesthesia as in the mother. Fetal anesthesia is also important to reduce the fetal stress response, which, through catecholamine release, can reduce placental blood flow and exacerbate any asphyxia.
| Drugs That DO NOT transfer | Drugs That DO transfer |
|---|---|
| Glycopyrrolate | Atropine |
| All neuromuscular blocking drugs | Ephedrine |
| Insulin | Esmolol, labetalol |
| Heparin | Benzodiazepines |
| Propofol | |
| Ketamine | |
| Opioids b | |
| Inhalational anesthetics | |
| Local anesthetics c |
a The major mechanism of transfer is passive diffusion of largely lipid-soluble, nonionized substances with low molecular weight (<500 D). Bulk flow, pinocytosis, and passage through the intervillous spaces are negligible sources of reliable transfer.
b Epidural or intrathecal opioids, to a lesser extent, generally produce minimal neonatal effects.
c Fetal acidosis produces higher fetal/maternal local anesthetic drug ratios because binding of hydrogen ions to the nonionized form causes trapping of the local anesthetic in the fetal circulation.
Intramuscular (IM) injection involves inserting a needle under ultrasound guidance into a fetal extremity or buttocks. Unlike umbilical cord injection, the noxious stimulus to the fetus by the IM injection stimulates the fetal stress response. Although the bleeding risk from an IM injection is less than that with intravascular injections, there remains a risk of bleeding and injury from the needle itself. Furthermore, if the fetus is already stressed, blood will be diverted away from muscle (the site of drug administration) and toward the fetal heart and brain. In this case, it may be impossible to estimate the time course for the drug to be absorbed from the IM site.
Intravascular fetal drug administration ensures immediate fetal drug delivery, and no additional dosing calculations are necessary because placental perfusion does not alter dosing. Intravascular access can be obtained via the umbilical cord (which is not innervated), larger fetal veins (e.g., hepatic vein), or intracardiac, as the specific intervention dictates. One advantage of administering drugs via the umbilical vein is the ability to provide analgesia before the surgical insult. Neuromuscular blocking drugs (NMBDs), analgesics, and vagolytic agents, as well as resuscitation drugs, can be given with assurance of immediate access to the fetal circulation. This method is also useful when alterations in peripheral blood flow occur (i.e., a “central sparing response”), which diminishes the blood distribution to sites of potential IM access.
Establishing intravascular access in the fetus requires inserting a needle in a fetus that is often not sedated from maternally administered agents. The needle may injure the moving fetus, and there is a risk of bleeding from the fetus, umbilical cord, and placenta. Uncontrolled bleeding could impair the surgical view and it places the fetus and mother in jeopardy because an open hysterotomy may be necessary to control the bleeding. Establishing access with the umbilical cord vessels may also produce vascular spasm, potentially compromising fetal perfusion.
Intraamniotic fentanyl, sufentanil, thyroxine, vasopressin, and digoxin have been safely administered in pregnant large-animal models with only minimal drug detected in the mother. If the safety and efficacy of this method of drug delivery hold true in human trials, intraamniotic drug administration may become the preferred method for fetal drug delivery; however, at present, this approach is not currently a part of routine clinical practice.
In the context of fetal interventions, there are two important causes of respiratory morbidity to consider: insufficient amniotic fluid and prematurity. With both, the timing of the insult in terms of the stage of lung development is critical to estimating the degree of likely morbidity. Deficiency of amniotic fluid may result from prelabor premature rupture of the amniotic membranes (PPROM), which may be spontaneous or iatrogenically induced either directly through trauma or by introducing infection into the uterus. Small amniotic fluid volume may also be secondary to reduced fetal urine output, from either poor renal function (e.g., with renal agenesis or urinary tract obstruction) or growth restriction secondary to placental insufficiency. Amniotic fluid deficiency contributes to pulmonary hypoplasia. In general, the likelihood of pulmonary insufficiency is inversely related to gestational age at membrane rupture, a long latency to delivery, and the amount of residual amniotic fluid. The risk is relatively small if PPROM occurs after 24 weeks gestation, as demonstrated by one series of fetuses with PPROM before 26 weeks that reported pulmonary hypoplasia in 27% of fetuses. In contrast, in fetuses less than 25 weeks gestation with severe oligohydramnios that persists for more than 2 weeks after PPROM, the predicted neonatal mortality exceeds 90%.
Studies in sheep show that oligohydramnios causes spinal flexion, which compresses the abdominal contents, displacing the diaphragm upward and thus compressing the developing lungs. This increase in the pressure gradient between the lungs and the amniotic cavity causes a net loss of lung fluid through the trachea, preventing lung expansion. Lung fluid produced in the airways is thought to act as a stent for the developing lungs. Normally, it passes out through the trachea and is either swallowed or passes into the amniotic cavity. Ligation of the trachea causes lung hyperplasia or ipsilateral lung hyperplasia if a main bronchus is ligated. Experimental drainage of amniotic fluid in animals has been shown to result in pulmonary hypoplasia. Later restoration of amniotic fluid prevents the onset of pulmonary hypoplasia. There is evidence to support amnioinfusion in humans to maintain fluid volumes around the fetus after PPROM in an effort to improve lung development.
Surfactant is a complex of phospholipids secreted by type II alveolar cells that reduces lung surface air tension, thereby preventing the lungs from collapsing at low volumes. Glucocorticoids, thyroid hormone, and β-adrenergic agonists stimulate surfactant synthesis. Surfactant is first detected in the lungs around 23 weeks gestation, but mature levels necessary for unassisted ventilation are not present until about 34 weeks. The degree of lung maturity can be evaluated by amniocentesis using the lecithin/sphingomyelin ratio or, more recently, by the lamellar body count. Acceleration of surfactant synthesis may be achieved with corticosteroids administered to the mother.
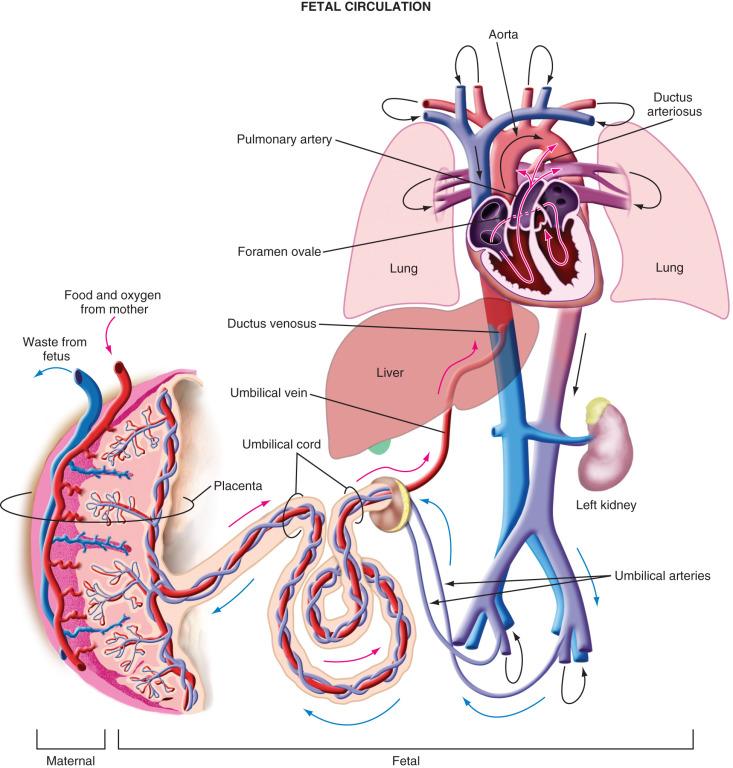
The differences between the fetal and postnatal circulations are complex ( Fig. 38.2 ). In the fetal circulation, oxygenated blood returns from the placenta via the umbilical veins and ductus venosus (bypassing the liver) into the right atrium. At 20 weeks, 30% of the umbilical venous return (40–60 mL/kg per minute) is shunted through the ductus venosus. This flow decreases over the second half of gestation as hepatic blood flow increases so that by term only 20% of umbilical venous return (<20 mL/kg per minute) is shunted through the ductus venosus (see Figs. 18.1 and 18.2 ). Hypoxia and hemorrhage increase the resistance in the liver, shunting a greater proportion of blood toward the brain and heart through the ductus venosus. The proportion of blood that perfuses the liver, which exits 15% less saturated in oxygen, rejoins the ductus venosus blood in the inferior vena cava. However, this deoxygenated blood has less kinetic energy and flows more slowly into the right atrium toward the right ventricle. The greater-velocity oxygenated blood from the ductus venosus is preferentially directed through the foramen ovale into the left side of the heart and out through the aortic arch to the developing head and upper body. The integrity of the foramen ovale is thus imperative. Blood returning from the placenta along the umbilical vein is 80% to 85% saturated. Despite this streaming within the right atrium, some mixing does occur, resulting in blood that is 65% saturated in the ascending aorta. The blood in the left ventricle, however, is 15% to 20% more saturated than the blood in the right ventricle. Most of the deoxygenated blood in the right ventricle bypasses the high-resistance pulmonary vasculature to enter the ductus arteriosus, and from there the descending aorta to supply the lower body, or pass through the umbilical arteries for reoxygenation in the placenta. In contrast to extrauterine life, when the two ventricles function in series and thus have equal outputs, before birth they function in parallel. Their outputs, therefore, do not have to be equal and, in fact, are not. In the third trimester, the right side of the heart has a greater output, as determined by Doppler ultrasonography studies, showing a 28% greater stroke volume than the left side.
The fetal heart rate (FHR) is maintained above the intrinsic rate of the sinoatrial node by a combination of vagal and sympathetic inputs, as well as circulating catecholamines. FHR decreases throughout gestation, accompanied by an increase in stroke volume as the heart grows. Hypoxic stress in late gestation produces a reflex bradycardia, with a normal heart rate or tachycardia developing a few minutes later. The chemoreceptor reflex nature of the bradycardia is demonstrated by its abolition after section of the sheep carotid sinus nerves. The later tachycardia is a result of an increase in plasma catecholamines causing β-adrenergic stimulation. Hemorrhage can also produce increases in FHR, probably via a baroreceptor reflex.
Cardiac output in the fetus is determined largely by heart rate. The combined ventricular output of the left and right ventricles in the human fetus is 450 mL/kg per minute. During development, the ability of the fetus to increase stroke volume is limited by a reduced proportion of functioning contractile tissue and a limited ability to increase the heart rate because of a relatively reduced β-adrenergic receptor density and immature sympathetic drive. If the blood volume is reduced by hemorrhage, the heart cannot compensate by increasing stroke volume, or conversely, if volume is increased, the walls are less able to distend and cardiac efficiency is reduced (although this second effect is reduced substantially by the huge, relatively compliant placental circulation). Thus the only mechanism by which the fetus can increase its cardiac output is to increase its heart rate. Despite this homeostatic limitation, the fetus is able to withstand significant hemorrhage. Studies have shown that the fetal lamb can restore arterial blood pressure and heart rate very quickly after acute loss of 20% of its blood volume, without any measurable disturbance in acid-base balance. Even after a 40% reduction in blood volume, the ovine fetal blood pressure recovers to normal within 2 minutes and the heart rate within 35 minutes. Oxygen delivery to the brain and heart is maintained secondary to vascular redistribution ( central sparing effect ) and blood volume replacement from the placenta and extravascular space, with 40% of the hemorrhaged loss being corrected within 30 minutes. The development of acidemia indicates that the fetus is unable to compensate; acidosis shifts the oxygen dissociation curve to the right, thereby decreasing fetal hemoglobin oxygen saturation but improving release of oxygen from hemoglobin. Blood flow during periods of asphyxia increases more than 100% to the brainstem but only 60% to the cerebral hemispheres.
The fetus exists in an environment of low oxygen tension, with arterial oxygen partial pressure (Pa o 2 ) being approximately one-fourth that of the adult. The maximum Pa o 2 of umbilical venous blood is approximately 30 mm Hg. The affinity of fetal hemoglobin for oxygen is modulated in utero by two principal factors: fetal hemoglobin and 2,3-diphosphoglycerate (2,3-DPG). The hemoglobin oxygen dissociation curve is shifted to the left because of fetal hemoglobin (hemoglobin F), thereby increasing the affinity for oxygen. In addition, 2,3-DPG is present and might be expected to shift the oxyhemoglobin dissociation curve to the right, decreasing the affinity of the fetal hemoglobin for oxygen and favoring oxygen unloading. However, 2,3-DPG appears to only exert approximately 40% of the effect on fetal hemoglobin as it does on adult hemoglobin, thereby preserving a net leftward shift on the oxyhemoglobin dissociation curve. Thus for any given Pa o 2 , the fetus has a greater affinity for oxygen than does the mother. The P50 (the Pa o 2 at which hemoglobin is 50% desaturated) is approximately 27 mm Hg for the adult and 20 mm Hg for the fetus. The concentration of 2,3-DPG increases with gestation, as does the concentration of hemoglobin A ; the greater hemoglobin concentration (18 g/dL) results in a greater total oxygen-carrying capacity.
Oxygen supply to fetal tissues depends on a number of factors ( Table 38.2 ). First, the mother must be adequately oxygenated. Second, there must be adequate flow of well-oxygenated blood to the uteroplacental circulation. This blood flow may be reduced from maternal hemorrhage (reduced maternal blood volume) or compression of the inferior vena cava (reduced venous return), which increases uterine venous pressure, thus reducing uterine perfusion. Additionally, aortic compression reduces uterine arterial blood flow. Care must be taken to position the mother in such a way as to prevent aortocaval compression whether by airplaning the table 15 degrees left or by turning the patient to the left decubitus position and then rolling her back to a 15 degrees left tilt. The surgical incision of hysterotomy itself reduces uteroplacental blood flow by as much as 73% in sheep, whereas fetoscopic procedures with uterine entry have no effect.
| Causes of Impaired Uteroplacental Blood Flow/Oxygenation | Causes of Impaired Umbilical Blood Flow/Fetal Circulatory Redistribution |
|---|---|
| Reduced maternal oxygenation/hemoglobin concentration | Umbilical vessel spasm |
| Maternal hemorrhage | Reduced fetal cardiac output |
| Aortocaval compression | Fetal hemorrhage/reduced hemoglobin concentration |
| Drugs reducing uterine blood flow | Fetal hypothermia |
| Uterine trauma | Impaired uteroplacental blood flow/oxygenation |
| Uterine contractions | Umbilical cord kinking |
| Placental insufficiency (PET, IUGR) | |
| Polyhydramnios: pressure effect | |
| Maternal catecholamine production increasing uteroplacental vascular resistance | Fetal catecholamine production increasing fetoplacental vascular resistance |
Even if the uterine circulation is adequate, the fetus still depends on uteroplacental blood flow and umbilical venous blood flow for tissue oxygenation. Care must be taken not to interrupt umbilical vessel blood flow by manipulation or kinking the cord, which can cause vasospasm. Umbilical vasoconstriction can also occur as part of a fetal stress reaction resulting from a release of fetal stress hormones ( Fig. 38.3 ). Increases in amniotic fluid volume increase amniotic pressure and impair uteroplacental perfusion. Placental vascular resistance may increase, thereby increasing fetal cardiac afterload via a surge in fetal catecholamine production stimulated by surgical stress. Fortunately, animal studies suggest that adverse effects on the arterial blood gas in the fetus do not occur until uteroplacental perfusion has been reduced by 50% or more.
Inhalational anesthetics may cause maternal vasodilatation and thus, in theory, could cause or exacerbate preexisting fetal hypoxia. Studies of anesthetics in hypoxic ovine fetuses have shown that isoflurane exacerbates preexisting acidosis. Isoflurane also attenuates the usual vascular redistribution response to fetal hypoxia, but this is offset by a reduction in cerebral oxygen demand. The net effect is that the balance of cerebral oxygen supply and demand is unaffected. β-Adrenergic blockade, however, renders the fetus less able to cope with asphyxia. Compared with controls, these fetuses have a smaller increase in heart rate, cerebral blood flow, and cardiac output and recover from acidosis more slowly.
By the beginning of the second trimester, the spinal cord is largely formed; development of the brain and spinal cord begins as early as postconception week 3. Neural crest cells migrate laterally to form peripheral nerves from about 4 weeks, with the first synapses between them forming a week later. Synapses within the spinal cord develop from about 8 weeks gestation, suggesting the first spinal reflexes may be present at this time. Neuronal development is maximum between 8 and 18 weeks gestation. The first neurons and glial cells develop in the ventricular zone (an epithelial layer) along which the newly formed neurons migrate out in waves to form the neocortex. Synaptogenesis occurs after neural proliferation, first in peripheral structures and, second, more centrally from approximately 20 weeks; this process depends in part on sensory stimulation.
The development of the nociceptive apparatus proceeds in parallel with the development of the basic central nervous system. The first essential requirement for nociception is the presence of sensory receptors, which develop first in the perioral area at around 7 weeks gestation. From here, they develop in the rest of the face and in the palmar surfaces of the hands and soles of the feet from about 11 weeks gestation. By 20 weeks, they are present throughout all of the skin and mucosal surfaces. The nociceptive apparatus is initially involved in local reflex movements at the spinal cord level without higher cortical integration. As these reflex responses become more complex, they in turn involve the brainstem, through which other responses, such as increases in heart rate and blood pressure, are mediated. However, such reflexes to noxious stimuli have not been shown to involve the cortex and, thus, are not thought to be available to conscious perception. The nature of fetal consciousness itself is complicated, both physiologically and philosophically, and a discussion of such is beyond the scope of this chapter. However, there is a working consensus that there must be electrical activity in the cerebral cortex for consciousness to be present. It appears that, far from being “switched on” at any one moment, consciousness evolves in a gradual process that has been likened to a “dimmer switch,” making attribution of fetal consciousness to any particular developmental moment a difficult undertaking.
When considering the effects of noxious stimuli on the developing fetus and the rationale for fetal anesthesia and analgesia, we must consider not just the humanitarian need to alleviate the possible distress of pain sensation, but also whether being subjected to surgical stress during early development might cause permanent alterations in physiology. This concept is known as programming, defined as “the process whereby a stimulus or insult at a critical, sensitive period of development has permanent effects on structure, physiology, and metabolism .” Studies in rats and nonhuman primates have shown that the numbers of hippocampal and hypothalamic glucocorticoid receptors in the offspring of antenatally stressed animals were permanently reduced. This attenuates the negative feedback response, resulting in increased basal and stress-induced cortisol concentrations in the offspring that persist into adulthood. Behavioral changes, such as poor coping behaviors, have also been observed.
The goal during any fetal intervention is to optimize fetal well-being by avoiding fetal hypoxia and hypothermia while optimizing stable fetal hemodynamics. It is essential that the physiologic response of the fetus to anesthetic and surgical stresses be understood and addressed to avoid the known detrimental effects of stress on an already compromised fetus. However, access to the fetus is limited at best and the technologies for continuous intraoperative and postoperative fetal vital sign monitoring are still in development.
A hysterotomy is not needed for many surgical interventions; thus the fetus remains within the uterus, often making access for direct monitoring impossible. Even for those fetuses that are partially delivered for an invasive procedure, monitoring is obtainable only intermittently and is frequently unreliable because the fetus must remain within a fluid environment during the procedure. These obstacles make it difficult to directly apply the available monitors. Current methods for assessing fetal well-being include FHR monitoring, direct measurement of fetal blood gases, fetal electrocardiography, fetal pulse oximetry (FPO), fetal echocardiography, and Doppler ultrasonography of fetal cerebral blood flow.
Currently, FHR monitoring with Doppler ultrasonography is the standard for the intrapartum assessment of fetal well-being. FHR monitoring is also used perioperatively during fetal interventions. The FHR is documented before maternal induction of anesthesia to (1) serve as a baseline for comparison and (2) reassure the perinatologist, surgeon, and anesthesiologist that the fetus is stable. The FHR may be continuously monitored intraoperatively by fetal echocardiography and with intermittent palpation of the umbilical cord in open cases. The most commonly used induction agents for anesthesia (propofol and thiopental) rapidly cross the placenta and thus also rapidly reach the fetus at appropriate doses. The inhalational anesthetics also cross the placenta, but their uptake occurs more slowly in the fetus than in the mother. These anesthetics decrease FHR and FHR variability. Although it is reassuring if the FHR is within the normal range for the gestational age, fetal bradycardia is a reliable indicator of fetal distress that needs to be immediately addressed.
With the advent of minimally invasive fetal endoscopic surgery, new problems in monitoring have surfaced. The fetus is no longer physically accessible to the surgical team, and the trocars currently used for fetoscopic surgery prevent applying radiotelemetric probes. Currently, fetoscopic or cardiac intervention use direct visualization of the heart with fetal echocardiography, which gives an accurate estimation of the FHR. Although very beneficial, the continuous use of fetal echocardiography requires the presence of a skilled ultrasonographer working in the operative field.
In suspected cases of fetal compromise during an open intervention, fetal blood can be obtained from capillary vessels, a peripheral vein, a central vein, or a puncture of the umbilical vessels. Vascular access is difficult in the fetus because of its small size and friable tissue. Puncture of the umbilical vessels can cause umbilical cord spasm, hematoma, and even fetal death. Hence umbilical cord manipulation should be reserved for circumstances when no other options are available. During an endoscopic intervention, access to the fetal circulation is possible through puncture of the umbilical vessels. With most fetal cardiac interventions, a needle and/or catheter is placed directly through the fetal myocardium, allowing access for blood samples; only a very small sample should be withdrawn because of the small circulating fetal blood volume.
Several groups have used fetal electrocardiography analysis to determine whether changes in time interval (PR and RR interval) and signal morphology (T/QRS ratio) correlate with fetal or neonatal outcome. Studies in animals and humans have shown that under normal conditions, there is a negative correlation between the PR interval and the FHR: as the FHR slows, the PR interval lengthens, and as the FHR increases, the PR interval shortens. The opposite relationship occurs in acidemic infants. During periods of fetal compromise, it is hypothesized that the sinoatrial node and the atrioventricular node respond differently. Periods of mild hypoxemia induce increases in epinephrine levels, which increase the FHR and shorten the PR interval. However, with periods of prolonged hypoxemia, the oxygen-dependent calcium channels of the sinoatrial node demonstrate reduced sensitivity to epinephrine, thus decreasing the FHR. The fast sodium channels of the atrioventricular node are unaffected by the reduction in the oxygen supply, whereas the increased levels of epinephrine shorten the PR interval. As a result, the relationship between the PR interval and FHR changes from negative to positive. Measurements of this relationship have been divided into short-term and long-term measures. The short-term measure or the conduction index can be intermittently positive over brief intervals without an adverse outcome. However, a prolonged positive conduction index (>20 minutes) has been associated with an increased risk of fetal acidemia.
Standard pulse oximeters use the transmission and absorption of light through a vascular bed to a photodetector on the opposite side of the tissue. However, the development of reflectance oximetry allows measurement of oxygen saturation from light-emitting diodes that are positioned next to each other on the same skin surface and absorption is determined from the light that scatters back to the tissue surface ; any fetal condition that decreases vascular pulsations (e.g., hypotension, vasoconstriction, shock, or strong uterine contractions) can produce inaccurate oximetry readings. Because direct contact of the oximeter must be made with the fetal skin surface, anything that interferes with light transmission or skin adhesion (e.g., fetal or maternal movement, vernix caseosa, caput succedaneum) can influence the signal quality and accuracy of the oximeter. Oximetry readings also vary in relation to the site of sensor application; several studies have found reduced baseline oxygen saturation values with the use of the oxygen sensor on the fetal buttock compared with the fetal head.
The development of a 735-/890-nm wavelength system (compared with the older 660-/890-nm system) has improved the accuracy in monitoring arterial oxygen saturation (FSa o 2 ) in the fetus. With the normal range of FSa o 2 of 30% to 70% in the middle of the oxygen-hemoglobin dissociation curve, small changes in pH or oxygen partial pressure exert large changes in FSa o 2 . FPO can also identify an acidotic fetus. Increased concentrations of both the hydrogen ion and 2,3-DPG cause a rightward shift of the oxygen dissociation curve (Bohr effect) such that a chronically acidemic or hypoxemic fetus will have a reduced FSa o 2 even though the P o 2 is within normal limits.
Near-infrared spectroscopy is a monitoring modality for continuous measurement of mixed-vascular oxygenation of tissues. Using an optical probe to assess light wavelengths in the 650- to 1000-nm range, this tool is able to provide data about tissues several centimeters deep and as such has been particularly useful for assessing cerebral oxygenation as well as detecting changes in fetal tissue oxygenation noninvasively through the maternal abdominal wall in real time in a sheep model. While this monitor has yet to gain widespread clinical use during fetal surgery, in animal models of fetal surgery, near-infrared spectroscopy measurements have been shown to correlate closely with umbilical venous oxygenation.
When technically feasible, fetal echocardiography should be available to assess fetal myocardial contractility and function, heart rate, intravascular volume status, and amniotic fluid volume. We have also used echocardiography to correctly identify proper endotracheal tube placement during an EXIT procedure (see later discussion) ; a sterile sleeve is placed over the ultrasonographic probe, which is then placed over the fetal chest.
Antepartum Doppler ultrasonography studies of the fetal circulation in cases of intrauterine growth restriction with presumed hypoxia have shown a compensatory redistribution, with an increase in peripheral vascular resistance in the fetal body and placenta and a compensatory reduction in peripheral vascular resistance in the fetal brain, producing a brain-sparing effect. Intrapartum Doppler ultrasonography and FPO have verified the brain-sparing response in the presence of intrapartum arterial hypoxemia (FSa o 2 <30% for 5 minutes or more), as reflected by increased mean flow velocity in the fetal middle cerebral artery. Preliminary studies of the middle cerebral artery pulsatility index in minimally invasive procedures, such as fetal blood sampling, transfusion, shunt insertion, tissue biopsy, and ovarian cyst aspiration, have demonstrated significant cerebral hemodynamic responses (decreases in the middle cerebral artery pulsatility index) in fetuses that underwent procedures involving transgression of the fetal body. This response was not noted in the fetuses undergoing procedures at the noninnervated placental cord insertion.
Although not yet advocated for routine intrapartum management, it has been suggested that the combination of reduced arterial oxygen saturation and increased cerebral blood flow may indicate an ominous phase during labor. The redistribution of the fetal circulation is not an unlimited protective mechanism, and with persistent cerebral hypoxia, the active vasodilation of the cerebral vessels may fail, leading to disastrous consequences for the fetus.
There is an increase in metabolic demand of both the mother and the fetus, and this, along with anatomic and hormonal influences, accounts for the changes in maternal pulmonary physiology ( Table 38.3 ). Pregnancy results in progressive increases in maternal oxygen consumption and minute ventilation, along with a decreased residual volume and functional residual capacity. The increased metabolic demands and anatomic changes can make adequate oxygenation and perfusion of the parturient and the fetoplacental unit a challenge during maternal general anesthesia. During periods of apnea or hypoventilation, the parturient is prone to rapid development of hypoxia and hypercapnia. Even after adequate preoxygenation, the Pa o 2 in an apneic anesthetized parturient decreases about 8 mm Hg more rapidly per minute than in a comparable nonpregnant women. Acidosis rapidly develops from hypoxia during difficult airway situations because of a decreased buffering capacity during pregnancy. The decreased pulmonary oxygen stores and increased oxygen consumption make parturients more susceptible than nonpregnant women to the consequences of airway mismanagement.
|
Not all physiologic changes of pregnancy are deleterious to the performance of anesthesia. For example, both the induction of and emergence from anesthesia with inhalational anesthetics occur faster in parturients than in nonpregnant women because the combination of increased alveolar ventilation and decreased functional residual capacity speeds the rate at which denitrogenation occurs and at which inspired and alveolar concentrations of inhalational anesthetics reach equilibrium ; a faster induction, coupled with a decreased MAC, predisposes the parturients to relative anesthetic overdose and severe hypotension.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here