Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Oocyte meiosis is arrested in prophase I from the fetal period until a luteinizing surge (LH) preceding ovulation. With the LH surge, the oocyte completes meiosis I associated with a decrease to 23 chromosomes with diploid (2 N ) DNA quantity and extrusion of the first polar body. With fertilization, meiosis II is completed with separation of sister chromatids resulting in 23 chromosomes with haploid (1 N ) DNA content and extrusion of the second polar body.
Implantation is a complex process necessitating hormones of estrogen and progesterone, cytokines such as growth factors and interleukins along with prostaglandins. During implantation extravillous trophoblast invade the endometrium to anchor the pregnancy and to remodel the spiral arteries to make the placenta a high-flow, low-resistance organ. Villous trophoblast are in contact with maternal blood in the intervillous space for gas and nutrient transfer.
Human chorionic gonadotropin (hCG) is secreted by syncytiotrophoblast and functions to maintain steroid production by the corpus luteum through interaction with the LH receptor. Other functions may include promotion of angiogenesis in the uterus, myometrial relaxation, inhibition of immune interaction at the uteroplacental interface, stimulation of fetal testosterone production and mediation of hyperemesis through receptors in the brain.
Genetic sex is determined at the time of conception. Male differentiation is determined by expression of the SRY (sex-determining region Y) gene found on the short arm of the Y chromosome. SRY protein is a transcription factor and expression is unique to the Sertoli cell of the developing testis. SRY induces expression of another transcription factor, SOX9, which is also obligatory for male sex differentiation. A loss of function mutation of either SRY or SOX9 results in XY sex reversal in which genetic men are phenotypic women. Several genes regulate SRY/SOX9 expression including WT1 (Wilms’ tumor suppressor 1) and SF1 (steroidogenic factor 1). Although ovarian formation can only occur in the absence of SRY/SOX9, there are unique genes necessary for development. FOXL2 encodes a transcription factor necessary for granulosa cell expansion. BMP15, located on the X chromosome, and GDF9 on chromosome 5 encode growth factors expressed in oocytes required for granulosa cell proliferation.
Renal and internal genital development are closely related. Under the influence of testosterone, the primordial renal mesonephros (wolffian ducts) differentiate into the vas deferens, epididymis, and seminal vesicles, while the paramesonephric ducts (müllerian ducts) are suppressed because of the secretion and action of antimüllerian hormone (AMH), also known as müllerian Inhibitory Substance (MIS), by Sertoli cells. In the absence of MIS, the wolffian ducts regress and the müllerian ducts differentiate into the fallopian tubes, uterus, and cervix.
Accompanying video for this chapter is available on ExpertConsult.com .
Several areas of medical investigation have brought increased attention to the processes of fertilization and embryonic development, including teratology, stem cell research, immunogenetics, and assisted reproductive technology (ART). The preimplantation, implantation, and embryonic stages of development in the human can now be studied because of the development of newer techniques and areas of research. This chapter considers the processes of oocyte meiosis, fertilization and early cleavage, implantation, development of the genitourinary system, and sex differentiation.
The oocyte is a unique and extremely specialized cell. The primordial germ cells in both males and females are large eosinophilic cells derived from endoderm in the wall of the yolk sac. These 700 to 1300 cells migrate to the germinal ridge by way of the dorsal mesentery of the hindgut by ameboid action by 5 to 6 weeks. Oogenesis begins with the replication of the diploid oogonia through mitosis to produce primary oocytes, reaching a peak number of 600,000 (confidence interval [CI]: 70,000 to 5,000,000) at 18 to 22 weeks of gestation. Through apoptosis, the numbers decline to about 360,000 (CI: 42,000 to 3,000,000) at menarche ( ). As can be seen, there is a large variance among individuals and a direct correlation between the number of fetal oocytes and the age of menopause. The maximum rate of fetal apoptosis occurs between 14 and 28 weeks gestation. Accelerated apoptosis is seen in Turner syndrome resulting in few oocytes at birth.
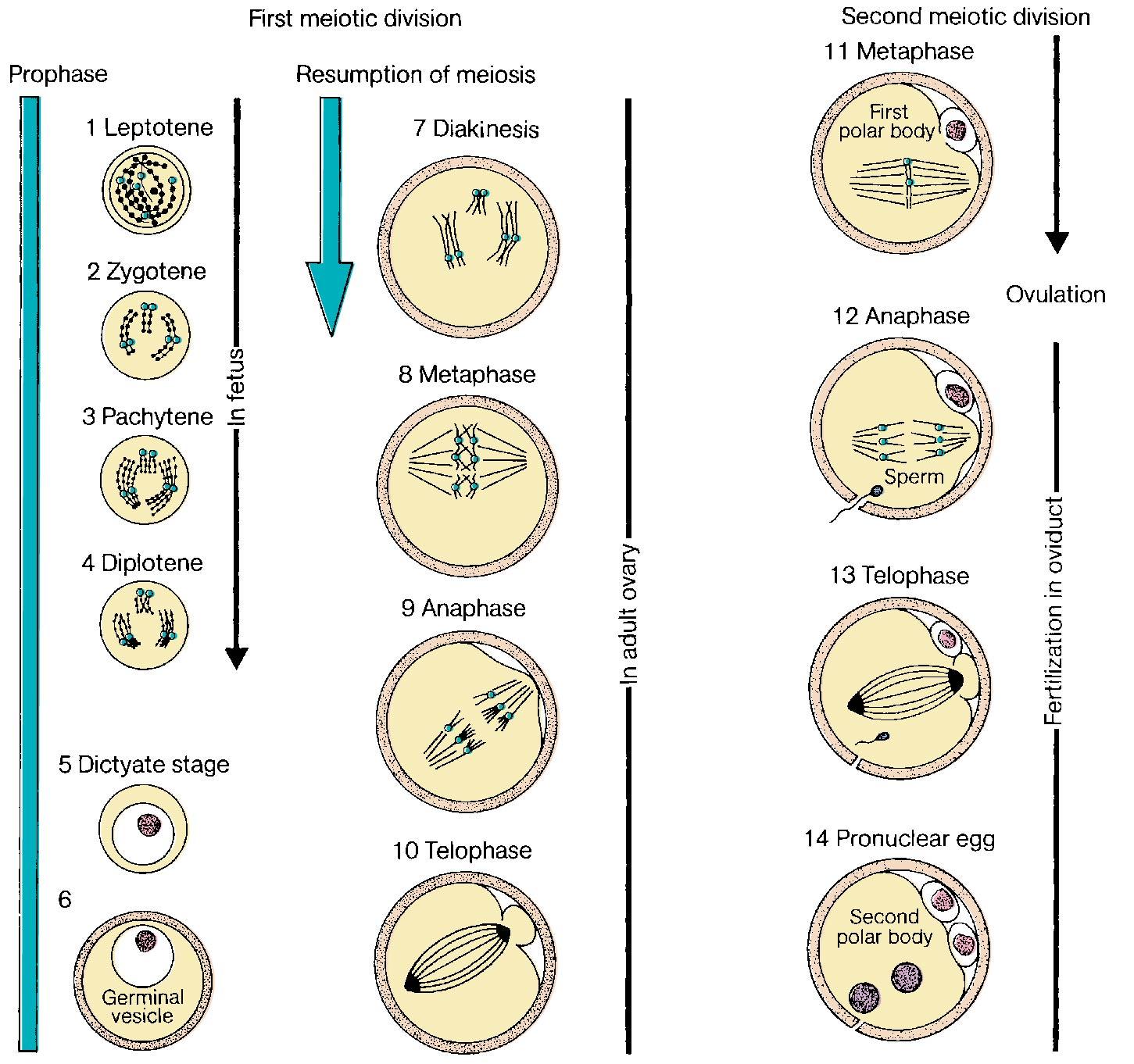
The meiotic process actually begins at 10 to 12 weeks gestation and is the mechanism by which diploid organisms reduce their gametes to a haploid state so that they can recombine again during fertilization to become diploid organisms. In humans this process reduces 46 chromosomes to 23 chromosome structures in the gamete. The haploid gamete contains only one chromosome for each homologous pair of chromosomes, so that it has either the maternal or paternal chromosome for each pair, but not both. Meiosis is also the mechanism by which genetic exchange is completed through chiasma formation and crossing over ( recombination ) between homologous chromosome pairs. Two meiotic cell divisions are required to produce haploid gametes. In the human female, oogonia enter meiosis in “waves” ( Fig. 1.1 ), that is, not all oogonia enter meiosis at the same time.
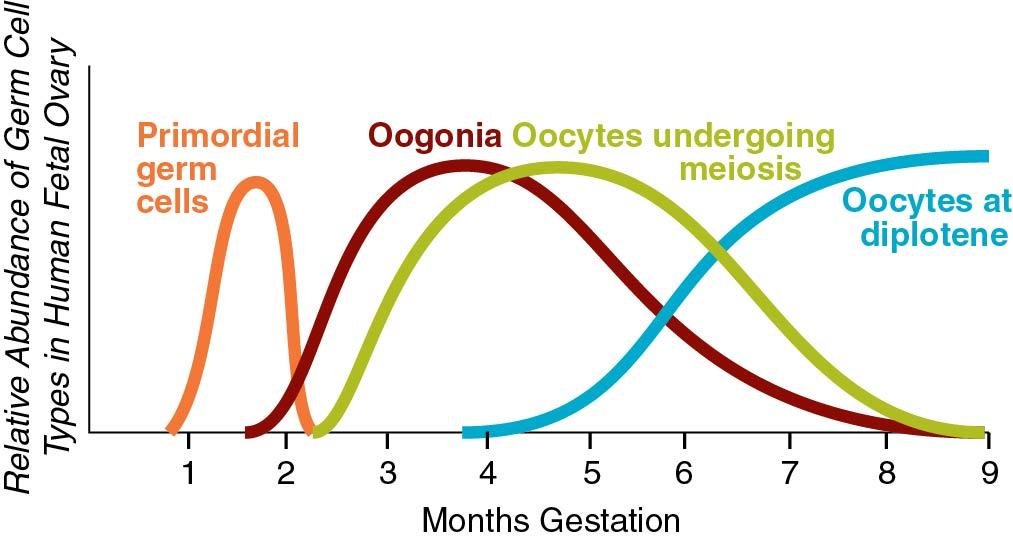
Meiosis initiation is dependent on mesonephric-produced retinoic acid ( ). Oocytes in the first substage of prophase, leptotene, are found in the human fetal ovaries as early as 10 weeks’ gestation. With increasing gestational age, greater proportions of oocytes in later stages of meiosis may be observed, and by the end of the second trimester of pregnancy, the majority of oocytes in the fetal ovaries have cytologic characteristics that are consistent with the diplotene/dictyotene substages of prophase I of meiosis I (the stage at which the oocytes are arrested until ovulation) ( Fig. 1.2 ).
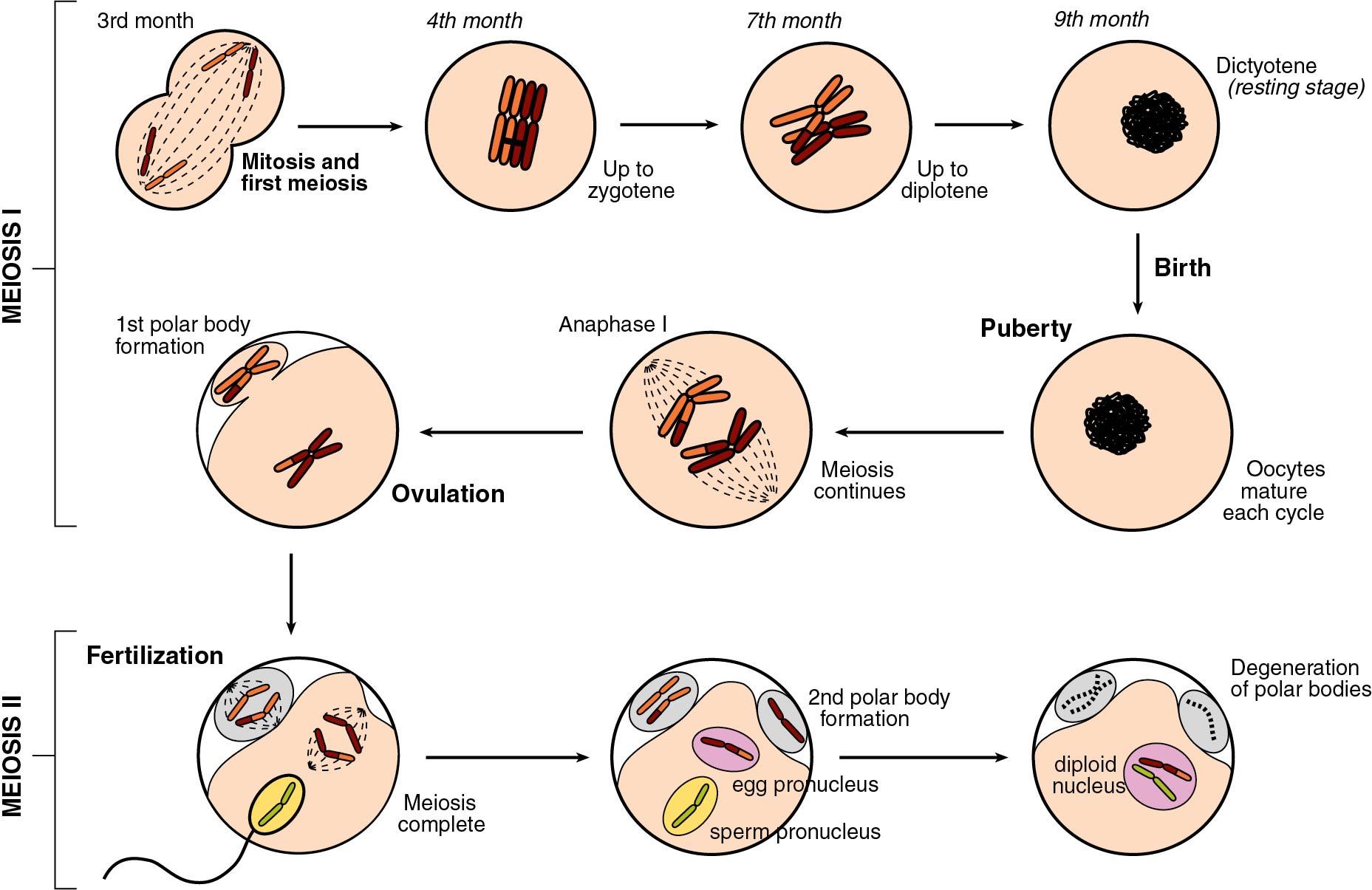
Meiosis is preceded by interphase I during which DNA replication occurs, thus transforming the diploid oogonia with a DNA content of 2 N to an oocyte with a DNA content of 4 N. Meiosis is defined in two stages. The first, known as the reduction division (division I, or meiosis I), initiates in the fetal ovaries but is then arrested and completed at the time of ovulation.
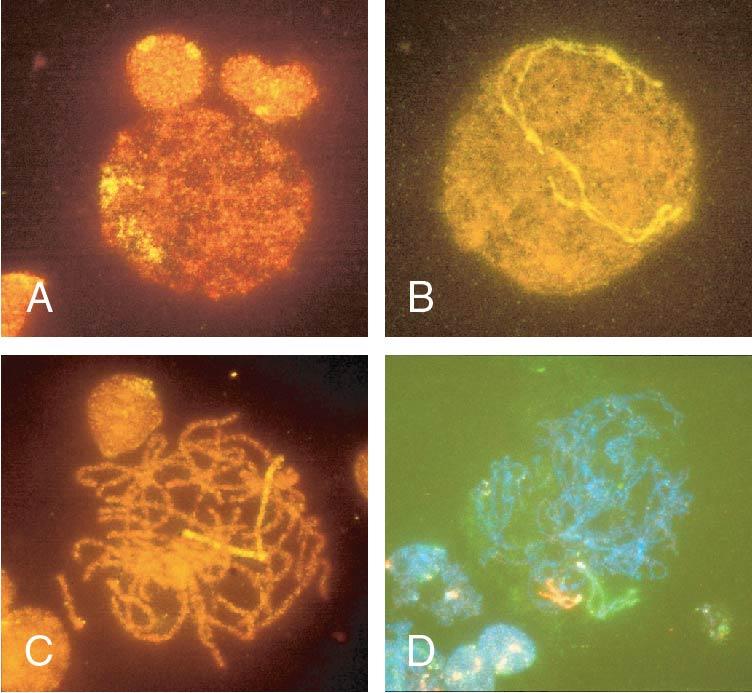
Meiosis I starts with prophase I (prophase includes leptotene, zygotene, pachytene, and diplotene), which occurs exclusively during fetal life and sets the stage for genetic exchange that ensures genetic variation in our species ( Fig. 1.3 ). More oocytes are found in the leptotene stage of prophase then in the other three stages of zygotene, pachytene, and diplotene in the fetal ovary. Leptotene is proportionately the most abundant of all the prophase I substages in early gestation. Cells in this meiotic phase are characterized by a large nucleus with fine, diffuse, string-like chromatin evenly distributed within the nucleus ( Fig. 1.3 A). Chromatin of homologous pairs occupies “domains” and does not occur as distinct linear strands of chromosomes. The zygotene substage is defined by the initiation of pairing, which is characterized by the striking appearance of the synaptonemal complex formation in some of the chromosomes ( Fig. 1.3 B). There is cytologic evidence of chromosome condensation and linearization, and the chromatin is seen as a fine, stringlike structure. The pachytene substage is the most easily recognizable period of the prophase and is characterized by clearly defined chromosomes that appear as continuous ribbons of thick beadlike chromatin ( Fig. 1.3 C). By definition, this is the substage in which all homologues have paired. In this substage the paired homologues are structurally composed of four closely opposed chromatids and are known as a tetrad. The frequency of oocytes in pachytene increases with gestational age and peaks in the mid-second trimester of pregnancy (about 20 to 25 weeks’ gestation). The diplotene substage is a stage of desynapsis that occurs as the synaptonemal complex dissolves and the two homologous chromosomes pull away from each other. However, these bivalents , which are composed of a maternally and a paternally derived chromosome, are held together at the centromere and at sites of chiasma formation that represent sites where crossing over has occurred ( Fig. 1.3 D). In general, chiasma formation occurs only between chromatids of homologous pairs and not between sister chromatids. Usually, one to three chiasma occur for each chromosome arm. Oocytes at this stage of prophase I constitute the majority of third-trimester fetal and newborn ovaries. Diplotene merges with diakinesis, the last substage of meiosis I, and is a stage of transition to metaphase, lasting many years in the humans.
During puberty, folliculogenesis includes progression of the follicle, consisting of the oocyte and granulosa cells from primordial to antral, which is characterized by granulosa cell proliferation, development of gonadotropin receptors, and expression of enzymes for sex steroid production ( ). It takes approximate 85 days for a follicle to mature to the point of ovulation. There is no change in the chromosome stage during folliculogenesis.
Meiosis I resumes with the surge of luteinizing hormone before ovulation completing metaphase, anaphase, and telophase. The result is two daughter cells, which are diploid (2 N ) in DNA content but contain 23 chromosome structures, each containing two closely held sister chromatids. One daughter cell, the oocyte, receives the majority of the cytoplasm, and the other becomes the first polar body. The polar body is located in the perivitelline space between the surface of the oocyte (oolemma) and the zona pellucida (ZP).
Meiosis II is rapid, with the oocyte advancing immediately to metaphase II, where the sister chromatids for each chromosome are aligned at the equatorial plate, held together by spindle fibers at the centromere. With sperm penetration, meiosis II is completed with extrusion of the second polar body yielding a haploid oocyte (1N) that is entered by a haploid (1N) sperm ( Fig. 1.4 ).
Aneuploidy in embryos is the most common cause of miscarriage and certain chromosomal abnormalities in live births, including Down syndrome (trisomy 21). The majority of the time these originate from an abnormal oocyte, increasing with age, and are more likely to affect chromosomes with short p arms (acrocentric). These chromosome segregation errors occur predominantly during meiosis I and are more common in the oocyte compared with the sperm. This is associated with deficient formation of chiasma between homologous chromosomes associated with DNA crossover (recombination) sites. Defective sites lead to less tension between homologous chromosomes, making segregation errors more likely as the spindles (microtubules) attached to the kinetochore protein complex adjacent to the centromere pull chromosomes toward the centrioles ( ).
The clinical importance of meiotic spindle integrity was evident during the development of oocyte cryopreservation. Oocyte freezing is becoming more common for fertility preservation in women with medical conditions, such as cancer, for which chemotherapy and/or radiation therapy may result in ovarian failure, and in women of increasing reproductive age. The original technique for oocyte freezing was referred to as slow freezing , which was subsequently replaced by vitrification. Freezing involves removal of intracellular water so that ice crystals will not form during freezing, which may disrupt organelles. With slow freezing, cryoprotectants such as dimethyl sulfoxide (DMSO) and ethylene glycol are allowed to permeate the cell, replacing the water, as the oocyte is slowly cooled at 1°C to 2°C/min to –196°C and stored in liquid nitrogen. In contrast, vitrification involves the use of higher concentrations of cryoprotectant and very rapid cooling at 15,000°C to 30,000°C/min. With slow freezing there is a slow change from liquid to solid, whereas vitrification consists of immediate solidification of the cryoprotectant into a glasslike consistency. With human oocytes, vitrification causes much less spindle damage, resulting in higher oocyte survival rates.
In most mammals, including humans, the egg is released from an ovary in the metaphase II stage ( Fig. 1.5 ). When the egg enters the fallopian tube, it is surrounded by a cumulus of granulosa cells (cumulus oophorus) and intimately surrounded by a clear ZP. Within the ZP are both the egg and the first polar body. Meanwhile, spermatozoa are transported through the cervical mucus and the uterus and into the fallopian tubes.
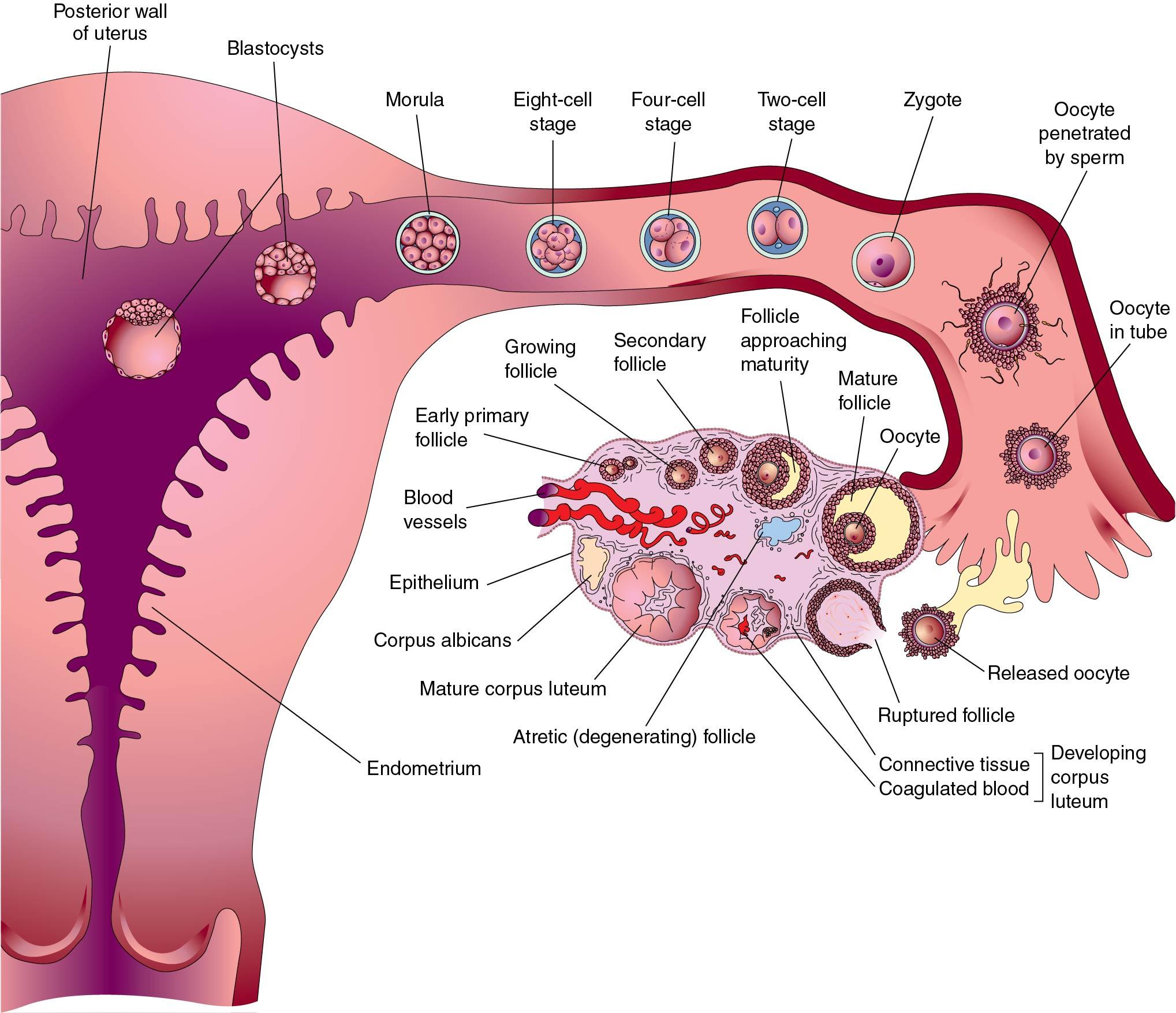
Although 20 to 200 million sperm may enter the vagina during intercourse, only 1 in 25,000 will make it to the fallopian tubes ( ). This journey involves processes of capacitation, chemotaxis, hyperactivated motility, and acrosome reaction ( Fig. 1.6 ). Capacitation precedes all other changes and involves initial removal of cholesterol from the plasma membrane altering the permeability and fluidity. This allows influx of calcium and bicarbonate, with many downstream effects, such as increased cyclic adenosine monophosphate (cAMP), protein tyrosine phosphorylation, and activation of protein kinases. A function of capacitation is to allow localization of protein complexes in the head of the sperm that will subsequently bind the ZP. Chemotaxis is shown by a greater number of sperm in the ampullary portion of the fallopian tube containing a cumulus-oocyte complex (COC) compared with the side lacking a COC. In vitro, follicular fluid acts as a chemoattractant, possibly because of progesterone, but the exact responsible constituents of the fluid continue to be debated ( ). Hyperactivated motility involves increased vigorous movement of the sperm to penetrate the cumulus (granulosa) cells surrounding the oocyte and is most likely caused by progesterone. A major action of progesterone is to increase calcium influx into the sperm, with multiple downstream effects. Likely the progesterone concentration increases as the sperm approaches the egg, resulting in more aggressive motility. When the egg is reached, receptor complexes on the outer most plasma membrane bind to specific ZP glycoprotein receptors (primarily ZP 3). These interactions are very species specific. Human sperm can only bind to the ZP of human, baboon, and gibbon oocytes. Binding results in fenestrations forming between the plasma membrane and the underlying acrosome membrane, releasing enzymes, including acrosin (a serine protease), to locally degrade the ZP.
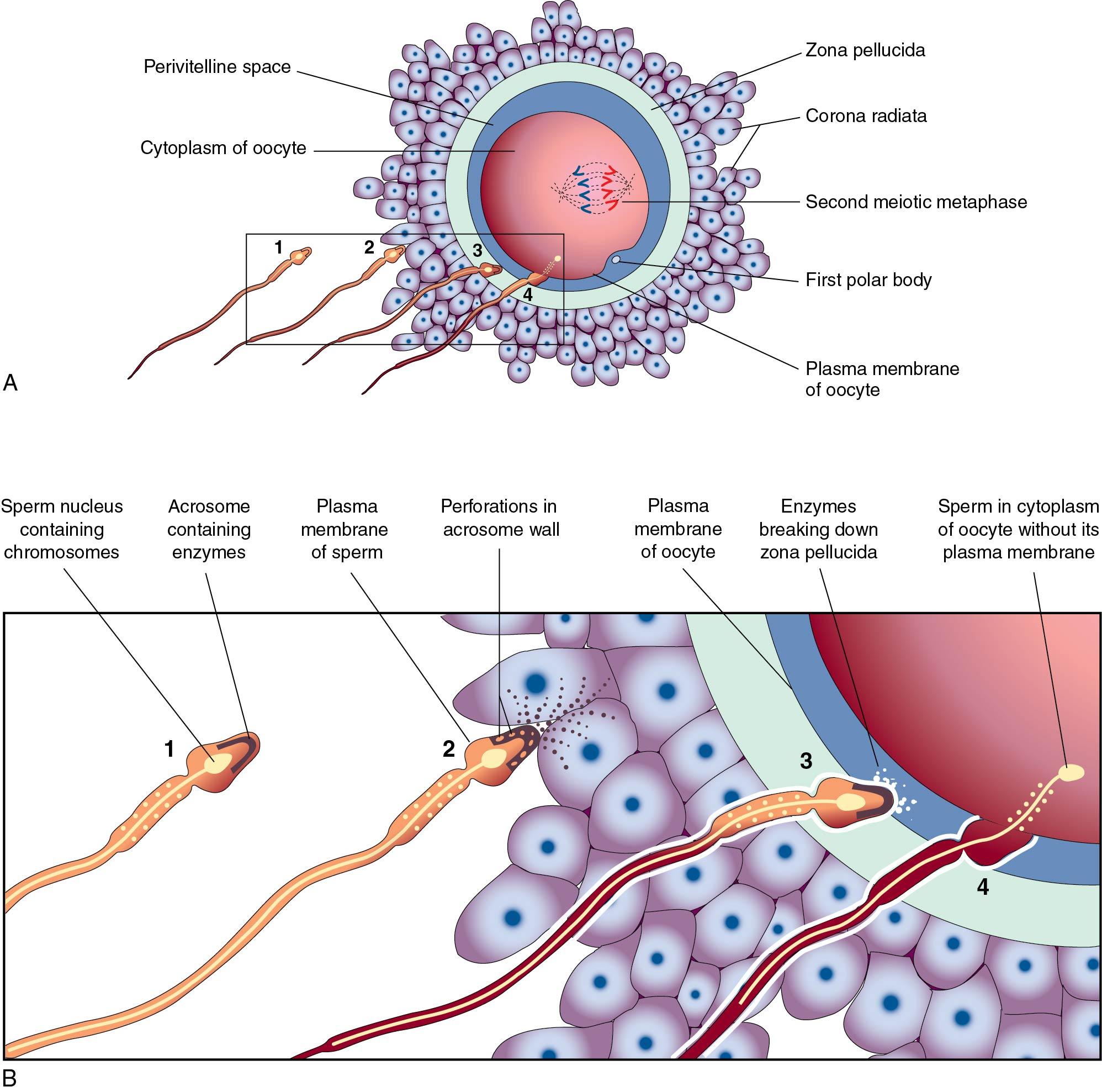
Because many sperm may initially bind the ZP, a mechanism must be in place to prevent fertilization by more than one sperm (polyspermia). With initial binding of the sperm membrane to the oolemma, a calcium-dependent release of cortical granules occurs. Cortical granules are vesicles containing protein made during oogenesis and located in the periphery of the cell. Contents are released into the perivitelline space and modify ZP proteins and enlarge the perivitelline space to prevent sperm entry. With sperm entry, the oocyte completes its second meiotic division, casting off the second polar body into the perivitelline space.
The majority of a single sperm enters the oocyte, and this is indeed the case during intracytoplasmic sperm injection (ICSI) for infertility. Only the centrioles and the nucleus survive, whereas mitochondria in the midpiece and tail are destroyed. The sperm centrioles interact with α-tubulin from the oocyte to form a microtubule network for migration of pronuclei and subsequent separation of chromosomes during the first mitosis ( ). Thus mitochondria are of maternal origin and centrioles are paternal.
Early cell division (cleavage) is not synchronous and varies in time ( Fig. 1.7 ). Time intervals from two pronuclei to two cells, two cells to three cells, three cells to four cells, and four cells to five cells are 26 hours, 12 hours, 0.8 hours, and 14 hours, respectively, as determined with time-lapse photography during in vitro fertilization (IVF) ( ). A significant number of fertilized oocytes do not complete cleavage for a number of reasons, including failure of appropriate chromosome arrangement on the spindle, specific gene defects that prevent the formation of the spindle, and environmental factors. Importantly, teratogens acting at this point are usually either completely destructive or cause little or no effect. Twinning may occur by the separation of the two cells produced by cleavage, each of which has the potential to develop into a separate embryo. Twinning may occur at any stage until the formation of the blastocyst (blast) because each cell is totipotent. Both genetic and environmental factors are probably involved in the causation of twinning.
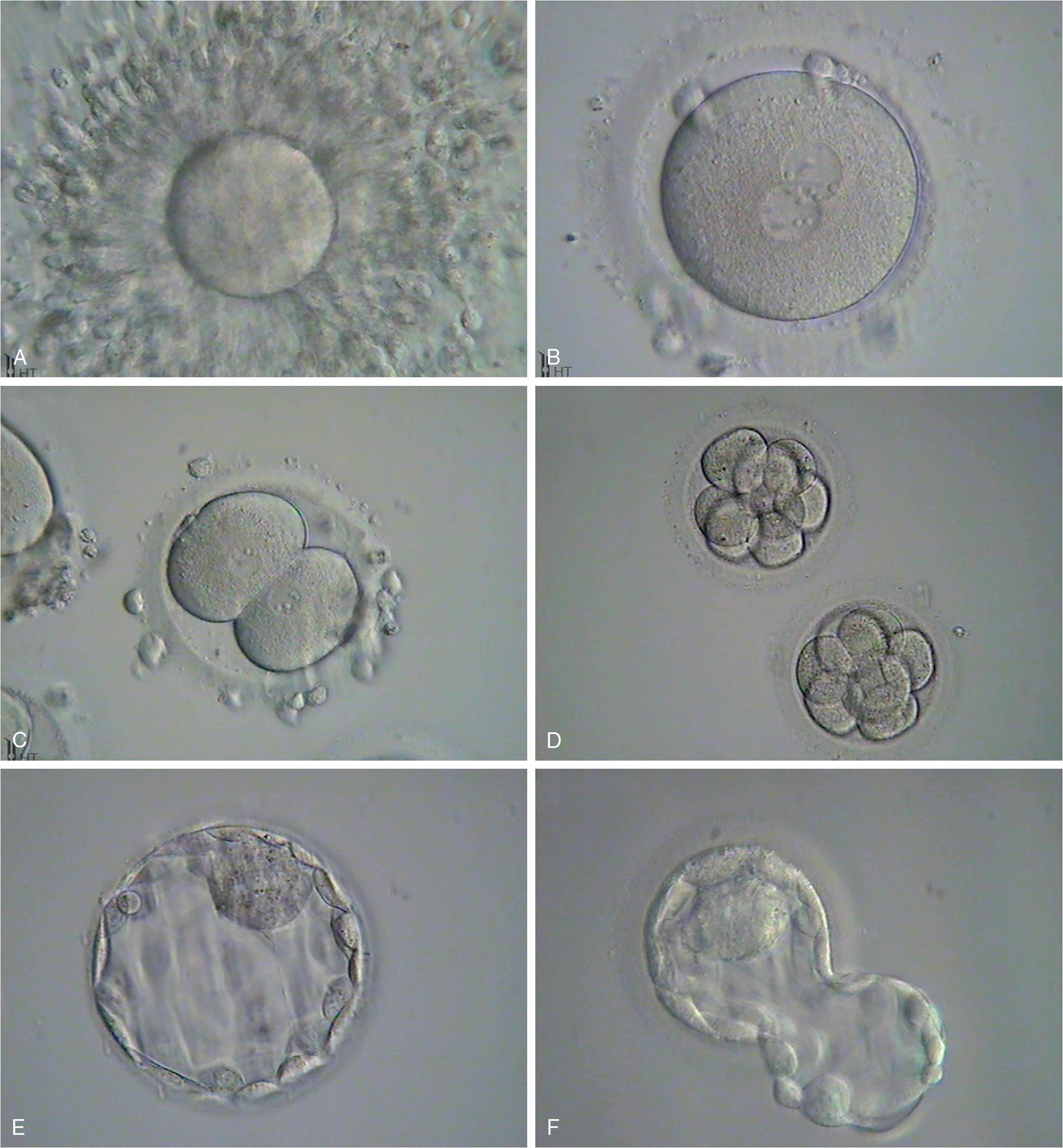
After fertilization the zygote (term for a fertilized egg) has a diameter of 83 to 105 μm and undergoes rapid mitotic division to reach the next stage of approximately 16 cells called a morula. The cells of the zygote and early cleavage embryo are considered totipotent because they are capable of producing all human tissue types (embryonic and extraembryonic). After 4 to 5 days traversing the fallopian tube, the embryo arrives in the uterine cavity at the blastocyst (blast) stage. The blast is characterized by a cavity (blastocoele) and differentiation of cells into the trophectoderm (TE), which will ultimately produce the fetal membranes and placenta, and the inner cell mass (ICM) that will produce the fetus. The cells in the blastocyst are referred to as pluripotent, meaning cells have differentiated into a group that can only yield embryonic cells and a group that can only yield extraembryonic cells. During IVF the blast forms 5 days after fertilization with a diameter of 155 to 265 μm consisting of about 40 TE cells and 20 ICM cells. In the human, implantation generally takes place 3 days after the embryo enters the uterus. The development of the blast with the separation of the ICM from the developing TE together make up the first stage of differentiation in the embryo. Differentiation within the ICM proceeds fairly rapidly, and if separation of cells and twinning occur at this point, the twins may be conjoined in some fashion.
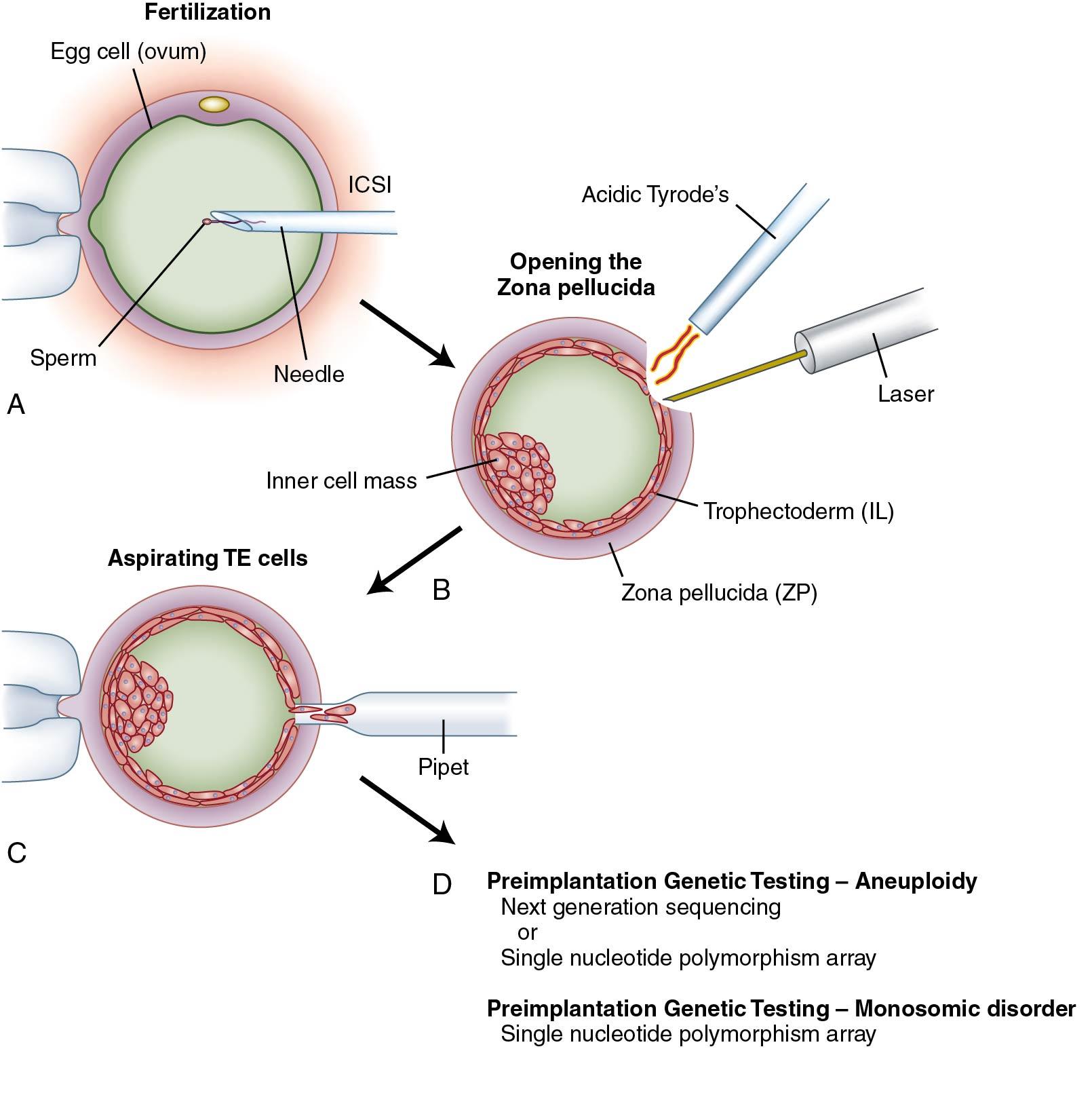
Advances in ART and genetics now provide practitioners assess to the early embryo for preimplantation genetic testing (PGT). This includes PGT for monogenic/single-gene disorders (PGT-M), testing for aneuploidy (PGT-A), and testing for chromosome structural rearrangements (PGT-SR) such as translocations ( Fig. 1.8 ). This technique involves removal of up to 10 (typically 5 to 10) TE cells from the day 5 blast for analysis. For PGT-M of single-gene disorders, DNA is extracted from the cells and the mutation analyzed by polymerase chain reaction (PCR) amplification or single nucleotide polymorphism (SNP) microarray. For PGT-A and PGT-SR, analysis of DNA is performed with comparative genomic hybridization (CGH) array or partial genomic sequencing (next generation sequencing).
Implantation consists of apposition, attachment, and invasion. This very complex process has redundancy and involves multiple factors, including ovarian hormones, cytokines, transcription factors, growth factors, and extracellular matrix proteins (ECM) ( Table 1.1 ). These factors are produced by both the endometrium and the embryo. Communication between the embryo and the endometrium is key. Implantation occurs 7 to 10 days after ovulation, corresponding to cycle days 21 to 24 of an idyllic 28-day cycle with ovulation on day 14. During apposition the human embryo is oriented with the ICM and polar TE (the TE next to the ICM) adjacent to the endometrium.
| Event | Days After Ovulation |
|---|---|
| Zona pellucida disappears | 4-5 |
| Blastocyst attaches to epithelial surface of endometrium | 6 |
| Trophoblast erodes into endometrial stroma | 7 |
| Trophoblast differentiates into cytotrophoblastic and syncytial trophoblastic layers | 7-8 |
| Lacunae appear around trophoblast | 8-9 |
| Blastocyst burrows beneath endometrial surface | 9-10 |
| Lacunar network forms | 10-11 |
| Trophoblast invades endometrial sinusoids, establishing a uteroplacental circulation | 11-12 |
| Endometrial epithelium completely covers blastocyst | 12-13 |
| Strong decidual reaction occurs in stroma | 13-14 |
For attachment to the endometrium, the embryonic cells must first be expelled from the surrounding ZP during the process of “hatching.” Hatching involves the rupture of the ZP in one small area as opposed to a general dissolution of the entire ZP. This may involve both hydrostatic pressure from inside the ZP and from zonalytic proteases produced by the TE and endometrium. These cysteine proteases, named cathepsins, are essential for hatching. Attachment of the embryonic cells to the endometrial cells involves cell adhesion proteins, integrins, and ECM proteins such as fibronectin, laminin, and collagen. Integrins are cell surface proteins that bind extracellular matrix proteins and are expressed on both the luminal epithelium and TE.
Next, invasion of TE cells occurs by penetration between the luminal epithelial cells, through the basement membrane and into the stroma of the endometrium. These initial TE cells form the extravillous trophoblasts (EVTs), which invade the inner third of the myometrium for anchoring and into the spiral arteries for remodeling. During spiral artery remodeling, endovascular EVT disorganize and partially replace the smooth muscle wall and the vascular endothelial cells. Proliferation of endovascular EVT leads to plugging and obstruction of the decidual spiral arteries, resulting in a decrease in blood flow and oxygen tension. A low oxygen setting promotes proliferation and transformation of cytotrophoblast to syncytiotrophoblast. Before 8 weeks’ gestation, nutrition to the embryo is derived from endometrial gland secretion and plasma seeping through the obstructed spiral arteries into the intervillous space. With continued remodeling of the spiral arteries, patency is reestablished and maternal blood cells enter the intervillous space around 9 weeks’ gestation with a rise in oxygen tension. Lack of adequate EVT invasion and spiral artery remodeling is a key feature in preeclampsia, intrauterine growth restriction, and stillbirths.
The idea of low oxygen tension during early embryo development has been explored with IVF. With a limited number of trials, culturing embryos in 5% oxygen as opposed to 20% oxygen results in a modest increase in implantation rate ( ).
Villous trophoblast form fingerlike projections extending into the intervillous space and that are surrounded by maternal blood. Syncytiotrophoblast form the outer layer with an underlying layer of precursor cytotrophoblast surrounding matrix containing capillaries, fibroblasts, and macrophages (Hofbauer cells). Cytotrophoblast become less numerous as pregnancy progresses.
Blood levels of the pregnancy hormone human chorionic gonadotropin (hCG) can be detected within 48 hours of implantation. Regular hCG is produced by the syncytiotrophoblast of placental villi. Blood levels peak at 56 to 68 days, reach a nadir at 18 weeks, and then remain fairly consistent until delivery. Gonadotropin-releasing hormone (GnRH) produced in the cytotrophoblast induces expression of hCG. In spontaneous pregnancies hCG can be detected 9 days after follicle rupture observed by ultrasound. In IVF pregnancies the hormone can be found 8 days after embryo transfer. The hCG levels rise exponentially up to 8 weeks from the last menstrual period (LMP), but the doubling time increases as the level increases. For example, in a conception cycle with ovulation on cycle day 14, the doubling time from cycle days 25 to 37 for hCG is 1.6 days and from days 38 to 44 is 2.3 days ( ). The doubling time is independent of the number of gestations, although the absolute hCG level is higher for multiple pregnancies.
The classic action of regular hCG is maintenance of the corpus luteum (CL) by binding the luteinizing hormone (LH) receptor for continued estrogen and progesterone production. Yet other identified actions include promotion of angiogenesis in the uterus, myometrial relaxation, inhibition of immune interaction at the uteroplacental interface, stimulation of fetal testosterone production, and mediation of hyperemesis through receptors in the brain.
Hyperglycosylated hCG (H-hCG) is produced by EVT. H-hCG is key in promoting angiogenesis and cell invasion and correspondingly is found in the early first trimester. The protein does not activate the LH receptor and does not preserve CL function. Instead it appears to function via the transforming growth factor beta (TGF-β) receptor. Low levels of H-hCG indicate poor EVT development and are associated with spontaneous abortion and early preeclampsia ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here