Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The nervous system is affected in many familial tumor or “cancer predisposition” syndromes. The genetic and clinicopathologic features are summarized in Table 22.1 . Autosomal dominant inheritance is most common, and the majority of these disorders result from inactivation of known tumor suppressor genes. Often, the disease-causing gene is inherited from the sperm or egg of one parent, although new germline mutations are also common. This latter “sporadic” form of the disease is unassociated with a family history, but is nevertheless transmissible to future offspring, serving as the initiation point of the inherited or familial form of disease. All cells in an afflicted patient with a germline mutation harbor one mutant allele. The exception to this is in the rare example of somatic mosaic patients who carry a mutation in only limited segments of their body. In the setting of one germline mutant allele, only one additional inactivating somatic alteration (deletion, mutation, etc.) is needed in the remaining wildtype allele for tumor initiation. Given this predisposing mutation, patients with familial tumor disorders are often at even greater risk than the general population for developing secondary neoplasms from mutagenic agents, such as radiation and chemotherapy, making their treatment all the more challenging. Also, accurate diagnosis of these inherited disorders is critical, given that not only the patient but also family members are at risk of subsequent disease. Clinical or genetic screening (or both) of the latter can distinguish those who need specialized medical care and lifelong surveillance plans for early tumor detection from those where such time-consuming, anxiety-provoking, and expensive strategies are not necessary.
| Syndrome | Synonyms | Inheritance | OMIM ID | Gene | Cytoband | Protein and Major Functions | Nervous System Manifestations | Cutaneous Manifestations | Other Organ Manifestations |
|---|---|---|---|---|---|---|---|---|---|
| Neurofibromatosis type 1 | Peripheral neurofibromatosis, von Recklinghausen disease | AD | 162200 | NF1 | 17q11 | Neurofibromin: suppressor of Ras signaling | Neurofibromas, malignant peripheral nerve sheath tumors, optic nerve glioma, pilocytic or diffuse astrocytomas | Café-au-lait macules, axillary and inguinal freckling | Iris hamartomas (Lisch nodules), osseous lesions, pheochromocytoma, gastrointestinal stromal tumors, juvenile myelomonocytic leukemia, juvenile xanthogranuloma, melanoma, duodenal carcinoid, medullary thyroid carcinoma |
| Neurofibromatosis type 2 | Central neurofibromatosis | AD | 101000 | NF2 | 22q12 | Merlin: mediates signaling between cell membrane and actin cytoskeleton | Bilateral vestibular schwannomas, nonvestibular schwannomas, meningioma, meningioangiomatosis, spinal ependymoma, glial microhamartomas, cerebral calcifications | Café-au-lait macules (rare) | Posterior lens opacities, retinal hamartomas |
| Schwannomatosis | Congenital neurilemmomatosis | AD | 162091 | SMARCB1 | 22q11 | INI1/SNF5/BAF47: subunit of Swi/Snf chromatic remodeling complex | Nonvestibular schwannomas, meningiomas | None | None |
| 615670 | LZTR1 | 22q11 | LZTR1: Golgi associated protein of unknown function | ||||||
| Tuberous sclerosis complex | Bourneville disease | AD | 191100 | TSC1 | 9q34 | Hamartin: complexes with tuberin to regulate PI3K-mTOR signaling | Cortical hamartomas (tubers), subependymal nodules, subependymal giant cell astrocytoma, radial glioneuronal heterotopias | Facial angiofibromas (“adenoma sebaceum”), hypomelanotic macules, shagreen patches, subungual fibromas | Retinal hamartoma, cardiac rhabdomyomas, adenomatous polyps of the intestine, cysts of the lung and kidney, lymphangioleiomyomatosis, renal angiomyolipoma, bone cysts, dental enamel pits, gingival fibromas, chordoma |
| 613254 | TSC2 | 16p13 | Tuberin: complexes with hamartin to regulate PI3K-mTOR signaling | ||||||
| Von Hippel-Lindau disease | Cerebelloretinal hemangioblastomatosis | AD | 193300 | VHL | 3p25 | VHL: regulates HIF-1, VEGF, and erythropoietin | Hemangioblastoma | None | Retinal hemangioblastoma, clear cell renal cell carcinoma, pheochromocytoma, pancreatic serous cystadenoma and neuroendocrine tumors, visceral cysts, endolymphatic sac tumor of inner ear, papillary cystadenoma of epididymis and broad ligament |
| Constitutional mismatch repair deficiency | Mismatch repair cancer syndrome, Turcot syndrome, brain tumor polyposis syndrome, type 1 | AR | 276300 | MLH1 | 3p22 | DNA mismatch repair enzymes | Glioblastoma (ultramutated) | Café-au-lait macules | Hematologic malignancy (predominantly T cell lymphoma), adenomatous intestinal polyps and colorectal cancer |
| 120435 | MSH2 | 2p21 | |||||||
| 614350 | MSH6 | 2p16 | |||||||
| 614337 | PMS2 | 7p22 | |||||||
| Lynch syndrome | HNPCC, Muir-Torre syndrome, Turcot syndrome, brain tumor polyposis syndrome, type 1 | AD | 609310 | MLH1 | 3p22 | DNA mismatch repair enzymes | Glioblastoma (hypermutated) | Sebaceous adenomas and carcinomas | Adenomatous intestinal polyps, colorectal carcinoma, endometrial carcinoma, upper urothelial tract carcinoma, gastric carcinoma, hepatobiliary carcinomas |
| 120435 | MSH2 | 2p21 | |||||||
| 614350 | MSH6 | 2p16 | |||||||
| 614337 | PMS2 | 7p22 | |||||||
| Familial adenomatous polyposis type 1 | Gardner syndrome, Turcot syndrome, brain tumor polyposis syndrome, type 2 | AD | 175100 | APC | 5q21 | APC: regulates WNT pathway signaling | Medulloblastoma (WNT-activated) | Epidermal inclusion cysts | Adenomatous intestinal polyps, colorectal carcinoma, desmoid-type fibromatosis, lipomas, osteomas, thyroid cancer, hepatoblastoma |
| Nevoid basal cell carcinoma syndrome | Gorlin syndrome, Gorlin-Goltz syndrome | AD | 109400 | PTCH1 | 9q31 | Patched-1 and SUFU: regulate sonic hedgehog signaling pathway | Medulloblastoma (SHH activated), calcification of falx cerebri, meningioma | Basal cell carcinomas, palmar and plantar pits | Odontogenic keratocysts, cardiac and ovarian fibromas, skeletal abnormalities |
| SUFU | 10q24 | ||||||||
| Cowden disease | Multiple hamartoma syndrome | AD | 158350 | PTEN | 10q23 | PTEN: phosphatase that negatively regulates PI3K-mTOR signaling pathway | Dysplastic gangliocytoma of the cerebellum (Lhermitte-Duclos disease), megalencephaly, ganglioneuromas | Facial trichilemmomas, acral keratoses | Hamartomatous intestinal polyps, lipomas, follicular carcinoma and other thyroid lesions, breast carcinoma, endometrial carcinoma |
| Li-Fraumeni syndrome | AD | 151623 | TP53 | 17p13 | p53: transcriptional regulator of cell cycle progression, DNA damage repair, and survival | Glioblastoma, medulloblastoma, choroid plexus carcinoma | None | Breast carcinoma, bone and soft tissue sarcomas, adrenocortical carcinoma, leukemia, Wilms tumor | |
| Carney complex | LAMB syndrome, NAME syndrome | AD | 160980 | PRKAR1A | 17q24 | PRKAR1A: regulates cAMP signaling | Psammomatous melanotic schwannoma, somatotrophic pituitary adenoma | Spotty pigmentation (“lentigines”), myxomas, epithelioid blue nevi | Cardiac myxoma, pigmented nodular adrenocortical dysplasia, fibromyxoma of breast, ductal breast adenoma, large cell calcifying Sertoli cell tumor, thyroid carcinoma, osteochondromyxomas |
| Rhabdoid tumor predisposition syndrome | AD | 609322 | SMARCB1 | 22q11 | INI1/SNF5/BAF47: subunit of Swi/Snf chromatic remodeling complex | Atypical teratoid/rhabdoid tumor | None | Malignant rhabdoid tumor (predominantly renal), small cell carcinoma of the ovary of hypercalcemic type | |
| 613325 | SMARCA4 | 19p13 | BRG1: subunit of Swi/Snf chromatic remodeling complex | ||||||
| DICER1 syndrome | Pleuropulmonary blastoma familial tumor and dysplasia syndrome | AD | 601200 | DICER1 | 14q32 | DICER1: controls microRNA processing | Pineoblastoma, pituitary blastoma | None | Pleuropulmonary blastoma, cystic nephroma, multinodular goiter, embyronal rhabdomyosarcoma, Wilms tumor, intraocular medulloepithelioma |
| Multiple endocrine neoplasia type 1 | Wermer syndrome | AD | 131100 | MEN1 | 11q13 | Menin: regulation of multiple signaling proteins including c-Jun | Pituitary adenoma, ependymoma | Nodular lipomas, collagenomas, facial angiofibromas | Parathyroid hyperplasia or adenomas, pancreatic neuroendocrine tumors, bronchial carcinoids, duodenal carcinoids, thymic carcinoids, adrenocortical adenomas, gastric enterochromaffin-like adenomas |
| Melanoma-astrocytoma syndrome | AD | 155755 | CDKN2A | 9p21 | p16 INK4a : negative regulator of kinases CDK4/6 governing cell cycle progression | Diffuse astroctyomas, pleomorphic xanthoastrocytoma, nerve sheath tumors, meningioma | Dysplastic nevi, melanoma | Panreatic adenocarcinoma | |
| Noonan syndrome | AD | 163950 | PTPN11 | 12q24 | Shp2: regulates Ras-Raf-MAPK signaling pathway | Pilocytic astrocytoma, rosette-forming glioneuronal tumor, pilomyxoid astrocytoma | None | Numerous congenital anomalies including short stature and facial dysmorphism, cardiac defects, juvenile myelomonocytic leukemia, granular cell tumors, giant cell lesions of small bones | |
| l -2-Hydroxyglutaric aciduria | AR | 236792 | L2HGDH | 14q21 | l -2-hydroxyglutarate dehydrogenase: metabolic enzyme regulating by-products of Kreb cycle | Diffuse gliomas, macrocephaly, subcortical leukoencephalopathy, cerebellar and brainstem atrophy | None | None | |
| Retinoblastoma | AD | 180200 | RB1 | 13q14 | pRB: transcriptional co-repressor governing cell cycle progression | Pineoblastoma | None | Retinoblastoma, osteosarcoma, secondary radiation-induced malignancy | |
| Fanconi anemia | AR | 605724 | BRCA2 | 13q13 | BRCA2 and PALB2: homologous recombination of DNA double-strand breaks | Medulloblastoma | None | Numerous congenital anomalies, bone marrow failure, myelodysplastic syndrome, acute myeloid leukemia, Wilms tumor | |
| 610832 | PALB2 | 16p12 | |||||||
| BAP1 tumor predisposition syndrome | AD | 614327 | BAP1 | 3p21 | BAP1: deubiquitinase and epigenetic regulator | Rhabdoid meningioma | Melanocytic neoplasms, basal cell carcinoma | Malignant mesothelioma, uveal melanoma, clear cell renal cell carcinoma | |
| Familial clear cell meningioma syndrome | AD | 607174 | SMARCE1 | 17q21 | BAF57: subunit of Swi/Snf chromatin remodeling complex | Clear cell meningioma | None | None | |
| Enchondromatosis | Ollier disease, Maffucci syndrome | Sporadic | 166000 | IDH1 | 2q34 | Isocitrate dehydrogenase-1: metabolic enzyme of Kreb cycle | Diffuse gliomas (IDH1-mutant) | Spindle cell hemangiomas | Enchondromas, chondrosarcomas, visceral hemangiomas, juvenile granulosa cell tumor of ovary |
| Sturge-Weber syndrome | Encephalofacial angiomatosis | Sporadic | 185300 | GNAQ | 9q21 | Gqα: regulates cAMP signaling | Meningeal angiomatosis | Port-wine stain (“nevus flammeus”) | Glaucoma |
| McCune-Albright syndrome | Polyostotic fibrous dysplasia, Mazabraud syndrome | Sporadic | 174800 | GNAS | 20q13 | Gsα: regulates cAMP signaling | Somatotrophic pituitary adenoma | Café-au-lait macules | Polyostotic fibrous dysplasia, sexual precocity, nodular hyperplasia or adenomas of endocrine glands, intramuscular myxomas |
In the most common syndromes, such as neurofibromatosis type 1 (NF1) and tuberous sclerosis complex (TSC), roughly half of patients present with a positive family history, while the other half are sporadic. Very rarely, two tumor syndromes occur in the same individual or family, including combined NF1 and TSC, von Hippel-Lindau (VHL) with NF1, and VHL plus multiple endocrine neoplasia type 1 (MEN1). The genes responsible for specific neoplasms in familial tumor syndromes are often the same as those inactivated in their sporadic counterparts. For instance, the NF2 gene is somatically mutated or deleted in the vast majority of sporadic schwannomas and meningiomas, just as inactivation of the NF2 gene is seen in the same tumors from NF2 patients. Nevertheless, this rule is not hard and fast. For example, germline defects in the NF1 gene account for pilocytic astrocytomas arising in NF1 patients, but usually not in those obtained from nonsyndromic patients.
NF1 is a common tumor predisposition syndrome, with highly variable clinical severity and manifestations that are influenced by age and degree of penetrance. It has also been referred to as peripheral neurofibromatosis (central neurofibromatosis being NF2) or von Recklinghausen disease, although NF1 has become a more specific and accepted term.
NF1 was first described in detail by Friedrich von Recklinghausen in 1882, although patients with classic traits of this disorder were described in the medical literature more than a century before, including artistic renditions consistent with NF1, dating back to at least the 15th century. The inherited nature of NF1 was initially recognized by Thomson in 1900 and reported in greater detail by Crowe and colleagues in 1956. The first set of diagnostic clinical criteria was published in 1988 based on a consensus conference at the National Institutes of Health (NIH). Until this time, there was great confusion between NF1 and NF2, with the two distinct disorders often lumped together. The NF1 gene was subsequently identified in 1990.
NF1 is one of the most common familial tumor syndromes and occurs at an estimated incidence of 1 in 3000. Given that it is autosomal dominant, males and females are affected equally. All ethnic groups are susceptible, and the prevalence is slightly higher in children than in adults, likely reflecting a slight increased mortality in the latter group.
A definitive diagnosis of NF1 requires at least two of the following :
six café-au-lait macules (>1.5 cm in postpubertal individuals, >0.5 cm in prepubertal individuals)
axillary or groin freckling
more than one plexiform or more than two nonplexiform neurofibromas
“optic glioma” (pilocytic astrocytoma)
more than two Lisch nodules (iris hamartomas)
dysplasia/absence of the sphenoid bone or dysplasia/thinning of long bone cortex
NF1 in a first-degree relative
By adulthood, nearly all patients with NF1 manifest café-au-lait spots, as lightly pigmented, flat, approximately symmetrical cutaneous patches ( Fig. 22.1A ). These may already be present in the newborn as the initial manifestation, but usually increase in size and number until puberty. Freckling of the intertriginous zones of the axilla or groin is another cutaneous lesion that develops later in roughly half of patients. About 90% of individuals with NF1 develop Lisch nodules by adulthood ( Fig. 22.1B ), and therefore, ophthalmologic examination is diagnostically useful. Orthopedic pathology includes dysplasias of the skull, spine, and long bones, variably leading to macrocephaly, absence of the sphenoid, vertebral scalloping, scoliosis, postfracture pseudoarthrosis of the tibia or other long bones, and long-bone thinning. Optic gliomas affect 15% to 20% of NF1 patients, mostly those younger than 7 years of age; the majority are clinically indolent and do not require surgical intervention, although a subset are progressive and may cause major morbidity.
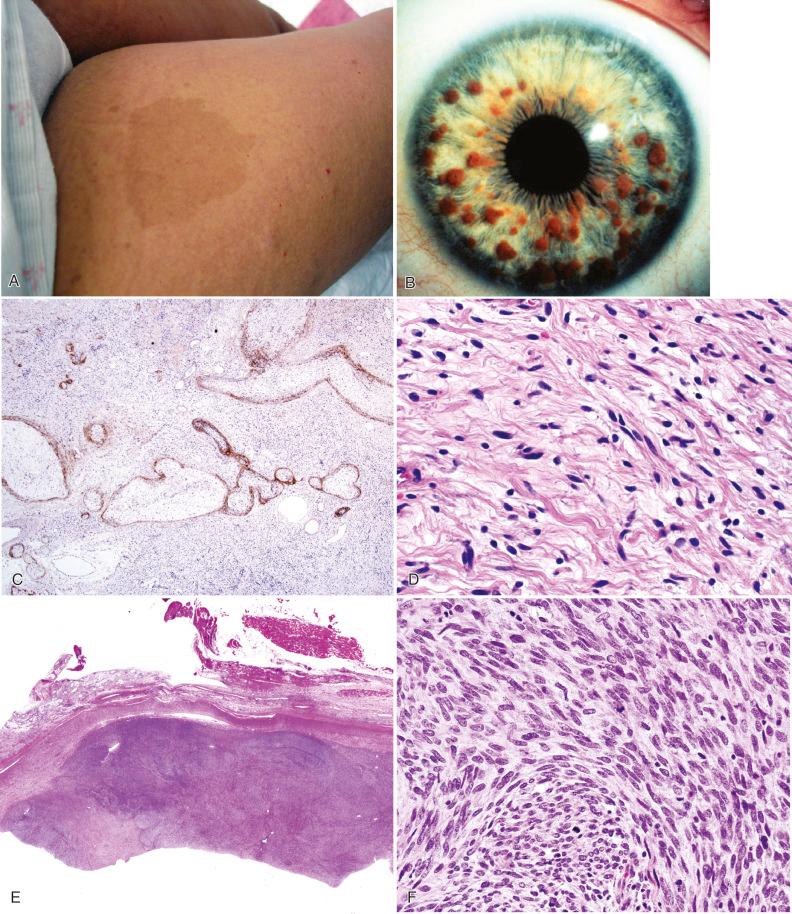
In addition to cutaneous neurofibromas, NF1-associated tumors (see Table 22.1 ) of the nervous system include :
multiple neurofibromas, particularly in paraspinal locations where they are often associated with significant peripheral neuropathy or compressive myelopathy
plexiform neurofibroma, most often affecting a large, deep nerve or plexus ( Fig. 22.1C and D )
malignant peripheral nerve sheath tumor (MPNST; Fig. 22.1E–G )
optic pathway glioma (mostly pilocytic astrocytoma; Fig. 22.1H–J )
diffuse astrocytomas, World Health Organization (WHO) grades II to IV
Systemic tumors include rhabdomyosarcoma, juvenile chronic myeloid leukemia, juvenile xanthogranuloma, gastrointestinal stromal tumor (GIST), duodenal carcinoid, melanoma, C cell hyperplasia, medullary thyroid carcinoma, other carcinomas, and pheochromocytoma. Both nervous system and systemic tumors are associated with considerable morbidity and increased mortality.
On T2-weighted magnetic resonance imaging (MRI), most children between 4 and 10 years of age develop focal, typically transient, but diagnostically useful increases in signal intensity referred to as “unidentified bright objects” (UBOs). These are thought to correspond to vacuolated myelin in the basal ganglia, brainstem, and cerebellum, although they are almost never biopsied. Apparent diffusion coefficients (ADC) are also commonly elevated, both in the UBOs and in normal-appearing white matter, suggesting increased water content. White matter volumes are often increased, contributing to the megalencephaly sometimes noted in NF1.
The radiographic and gross appearances of NF1-associated tumors are essentially identical to those encountered in sporadic cases (see Fig. 22.1H ), and the reader is referred to the individual sections covering these topics. However, there is a considerable challenge in following NF1 patients with large deep plexiform neurofibromas or numerous neurofibromas for potential malignant transformation. [ 18 F]2-fluoro-2-deoxy- d -glucose positron emission tomography (FDG PET) and PET computed tomography (PET-CT) are emerging as useful tools for detecting early malignant transformation and for guiding needle biopsies to the most likely sites of transformation in otherwise large highly heterogeneous tumors. One potentially difficult challenge, however, is that smaller, earlier forms of progression may be biopsied more often than in the past, leading to diagnostic difficulties associated with “atypical neurofibromas” and “low-grade MPNSTs” (see Chapter 15 ).
Grossly, plexiform neurofibromas are characterized by “rope-like” expansions of multiple nerve fascicles resembling a “bag of worms.” Regions of malignant transformation to MPNST often appear more fleshy, heterogeneous, and invasive, often with foci of necrosis, hemorrhage, and chalky calcifications. However, foci of malignant change are not always grossly visible, and therefore, neurofibroma resection specimens should be well sampled. Lifetime risks of developing MPNST in the setting of NF1 have been estimated at roughly 10% to 15%, with patients harboring large germline deletions ( microdeletion syndrome; see Genetics section) at even greater risk and suffering more severe disease manifestations in general.
Neurofibromas in NF1 patients are similar to sporadic types (see Chapter 15 ), with a few exceptions. The presence of paraspinal intraneural neurofibromas (fusiform expansion of the nerve), particularly multiple tumors, is essentially diagnostic of NF1. Similarly, both plexiform neurofibroma (see Fig. 22.1C and D ) and massive diffuse-appearing soft tissue neurofibromas are virtually restricted to NF1. MPNSTs may be seen arising de novo, in transition from a pre-existing plexiform neurofibroma, or rarely from other nerve sheath tumors. Most are identical to sporadic counterparts, but some report a higher frequency in NF1 patients of subtypes such as malignant Triton tumor (MPNST with rhabdomyosarcomatous differentiation), glandular MPNST, and nerve sheath tumors with angiosarcoma. Although the majority are obvious high-grade malignancies, the earliest stages of malignant transformation within a neurofibroma may be hard to recognize. They typically show increased cell density with enlarged, overlapping, hyperchromatic nuclei ( Fig. 22.1F ). The presence of mitotic figures is also helpful in making this distinction, along with special stains such as Ki-67, S-100 protein ( Fig. 22.1G ), SOX10, p16, and H3K27me3 (see Chapter 15 ).
Most “optic gliomas” have classic histopathologic features of pilocytic astrocytoma, although they show a greater likelihood to invade the optic nerve parenchyma ( Fig. 22.1I ) and overlying leptomeninges, as well as to secondarily induce meningothelial hyperplasia ( Fig. 22.1J ). These often surprisingly indolent tumors often stop growing or even regress on their own. Therefore, the majority are never biopsied, and those requiring biopsy are almost “atypical” by definition. Other astrocytomas that develop in NF1 include pilocytic astrocytomas of the cerebellum and, especially, the brainstem. The latter have similarly been reported to be mostly indolent, even though many appear surprisingly infiltrative on histology.
Patients with NF1 also have increased risks of developing malignant gliomas. Such tumors are most often encountered in adults presenting with cerebral masses. While the low-grade astrocytomas including pilocytic astrocytomas that arise in NF1 are essentially histologically and clinically identical to their sporadic counterparts, the diffuse higher-grade astrocytomas that arise in NF1 may be histologically diverse and challenging to classify. Whether a subset of these tumors might represent anaplastic transformation of a pre-existing pilocytic astrocytoma rather than de novo high-grade astrocytomas is uncertain, but may be suggested by a radiographic lesion that was previously stable over several years that suddenly expanded. The histologic presence of a component of the tumor resembling conventional pilocytic astrocytoma in an otherwise high-grade astrocytoma with microvascular proliferation and/or necrosis may warrant a designation of “pilocytic astrocytoma with anaplasia” or “high-grade astrocytoma arising in the setting of NF1” rather than glioblastoma, especially given that NF1 patients diagnosed with glioblastoma have been demonstrated to have longer survival times than nonsyndromic patients in some studies. A recent study on the histopathology of NF1-associated diffuse astrocytic neoplasms demonstrated frequent ATRX loss and p53 overexpression, but lack of IDH1 R132H mutant protein immunoreactivity in all cases.
In severe or fully developed cases of NF1, the diagnosis is often straightforward. However, the diagnosis is often challenging at the initial phases of early childhood or for patients with mild manifestations. In such cases, an accurate diagnosis requires a careful clinical examination, including skin and eye. Occasionally, the first clue is the radiologic or pathologic detection of a plexiform neurofibroma or other characteristic tumor types. For families with known mutations, targeted gene sequencing, and microdeletion testing may be diagnostically useful, although in general, molecular diagnosis is laborious, expensive, and not as sensitive as one would like.
NF1 is an autosomal dominant disorder, although half of cases are sporadic, representing new mutations. Disease penetrance is nearly complete, but diagnosis may still be challenging, due to a wide variability in severity and the frequency of new mutations. Most of the latter occur in one of the parents during oogenesis or spermatogenesis. The estimated 1 per 10,000 alleles per generation mutation rate is among the highest, likely due to the large size of the NF1 gene on chromosome 17q11.2 (roughly 350 Kb, including 59 exons). It is also for this reason that mutation analysis is difficult, since there are no consistent “mutational hotspots.” It is interesting, however, that about 5% to 10% of cases are due to a 1.5-Mb deletion involving the entire NF1 gene; this microdeletion syndrome is generally associated with more severe disease, including facial dysmorphism, mental retardation, more numerous neurofibromas, and an enhanced risk of MPNST development. Patients with mosaic or segmental forms of NF1 have only portions of their body involved.
The NF1 transcript includes three alternatively spliced isoforms (exons 9a, 23a, and 48a), variably expressed in a tissue-specific and differentiation-associated manner. The gene encodes a widely expressed 220- to 250-kDa protein, known as neurofibromin. Neurofibromin loss activates K-RAS, further stimulating various mitogenic and growth-regulatory signaling pathways. Whereas NF1-associated pilocytic astrocytomas and neurofibromas are monogenic neoplasms that result from biallelic inactivation of the NF1 gene (one allele inactivated by germline mutation with the second allele inactivated by a somatic event), malignant transformation to high-grade astrocytoma or MPNST involves the acquisition of somatic alterations in additional genes. In MPNST, these commonly include TP53 mutation, CDKN2A deletion, and mutation of either SUZ12 or EED, which encode subunits of the PRC2 complex that regulates methylation at lysine-27 of the histone H3 tail.
Given the highly complex multidisciplinary needs of NF1 patients, they are often best served with treatment and careful regularly scheduled follow-up examinations at academic centers that have an established team of specialists specifically trained to serve this patient population. Changes in symptoms deserve prompt attention. In particular, newly developed pain at the site of a known plexiform neurofibroma is worrisome for possible malignant transformation to MPNST. As a group, the expected life span for NF1 patients is only mildly reduced. However, the prognosis varies greatly and depends on multiple factors, including severity of disease. Increased mortality is most strongly related to the development of MPNST, gliomas, and leukemias. Small molecule inhibitors of MEK such as trametinib and selumetinib have recently shown significant efficacy in the treatment of NF1-associated plexiform neurofibromas and represent a promising new targeted therapy for NF1 patients.
NF2 is a tumor predisposition syndrome that primarily affects the central and peripheral nervous systems. It is characterized by both neoplastic and hamartomatous proliferations of Schwann cells (schwannomas and schwannosis), meningothelial cells (meningiomas and meningioangiomatosis), and glia (ependymomas and glial microhamartomas). Synonyms include bilateral acoustic neurofibromatosis and central neurofibromatosis, although NF2 is the preferred term. Similarly, a common clinical synonym for vestibular schwannomas is “acoustic neuroma,” but this is a misnomer in two ways, since typically the vestibular branch is affected and the Schwann cell proliferation is neoplastic in origin, rather than reactive as the term “neuroma” implies.
The first well-documented case of NF2 was published by the Scottish surgeon James Wishart in 1822. His 21-year-old patient presented with progressive bilateral deafness and a protruding occipital tumor. He developed fatal septicemia after surgical removal of the latter, and autopsy revealed multiple dural, cranial nerve, and spinal nerve root tumors. Today, similar patients with this more severe form of disease (vestibular and nonvestibular tumors) are referred to as having the Wishart variant of NF2. In 1930, Drs. Gardner and Frazier documented a family with five generations of hereditary deafness due to bilateral vestibular schwannomas. This milder familial form associated with minimal nonvestibular manifestations is now known as the Gardner variant. As discussed in the prior NF1 section, the overlap in central nervous system, peripheral nervous system, and cutaneous manifestations of NF1 and NF2 caused great confusion for decades, such that these two very different genetic disorders were often lumped under the single designation of neurofibromatosis or von Recklinghausen disease. Clear guidelines for distinguishing these two disorders were created at an NIH consensus meeting in the late 1980s. The NF2 gene was identified simultaneously by two different labs in 1993.
NF2 has a birth incidence of roughly 1 in 25,000. All ethnic groups are at risk, and the sexes are affected equally. The average age of onset is roughly 22 years, although there is a wide range depending on multiple factors, including severity of disease. Some patients present during childhood, and onset beyond the sixth decade is exceptional.
Bilateral vestibular nerve schwannoma represents the single pathognomonic signature of NF2. However, given that many patients lack a family history and initially present with other features, criteria were modified to enhance sensitivity. Thus, a diagnosis of NF2 can also be made when any of the following are met :
a first-degree relative with NF2 plus either a single vestibular schwannoma or two of the following: meningioma, nonvestibular schwannoma, ependymoma, neurofibroma, or posterior subcapsular lens opacity
a unilateral vestibular schwannoma and two of the following: meningioma, schwannoma, ependymoma, neurofibroma, or posterior subcapsular lens opacity
multiple meningiomas plus one vestibular schwannoma or any two of the following: nonvestibular schwannoma, ependymoma, neurofibroma, or cataract
Nearly all NF2 patients develop vestibular schwannomas, the most common presenting feature; however, nonvestibular schwannomas are also common. Unlike the deep-seated neurofibromas of NF1, NF2-associated schwannomas rarely involve deep nerves in a plexiform manner, and they have nearly no potential for malignant transformation. Paraspinal schwannomas often arise from microscopic precursors or “Schwann cell tumorlets,” that may stud the spinal rootlets and proximal nerves; these and reactive Schwann cell proliferations are often associated with peripheral neuropathy. Overlapping with NF1, café-au-lait spots are seen in about 43% of patients, but they usually number fewer than six. Most cutaneous lesions are schwannomas (seen in 60% to 70%), with only rare neurofibromas encountered. Cataracts afflict about a third of patients and may represent the presenting feature in children, the latter often having the severe Wishart form of NF2. Multiple meningiomas are also common in the latter cohort of NF2 patients, often associated with considerable morbidity. Meningioangiomatosis, a malformative meningothelial and vascular proliferation, presents as a plaque-like leptomeningeal or cortical mass. In contrast to the sporadic counterpart that nearly always presents with seizures, the vast majority of NF2-associated cases are asymptomatic. About 5% to 10% of NF2 patients develop gliomas, almost exclusively spinal ependymomas. The majority involve the cervical cord and are surgically treatable, slow-growing, well-demarcated masses. NF2 patients with the more severe Wishart phenotype may develop multiple ependymomas.
Neuroimaging is a common modality for making the diagnosis of NF2. The sine qua non is bilateral vestibular schwannomas ( Fig. 22.2A ). If NF2 is suggested, however, thin MRI cuts of the internal auditory meatus region should be ordered to identify small vestibular schwannomas. Otherwise, the neoplasms of NF2 are radiologically and grossly identical to those that arise sporadically.
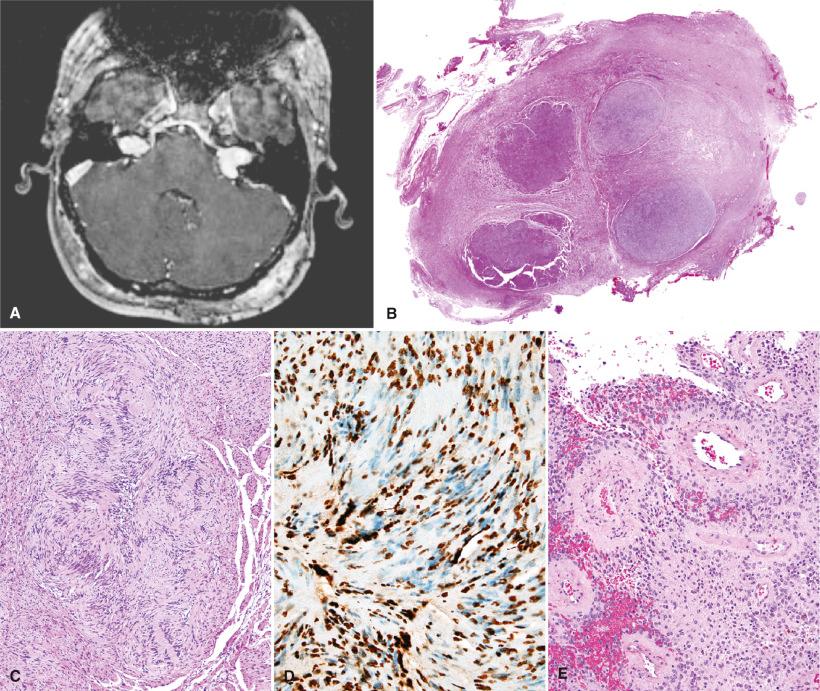
NF2-associated neoplasms are generally similar to their sporadic counterparts (see Chapter 15 ) with a few exceptions. For instance, vestibular schwannomas are more likely to be multicentric, have a multinodular (“cluster of grapes”) appearance ( Fig. 22.2B and C ) due to confluence of smaller clonal proliferations, show slightly higher proliferative indices, and are more likely to invade the parent nerve. Nonvestibular schwannomas are similarly more infiltrative, with entrapped neurofilament-positive axons. Also, plexiform schwannomas of the skin or head and neck region may be associated with either NF2 or schwannomatosis. Such tumors are often mistaken for plexiform neurofibromas. In contrast, most deep plexiform schwannomas of peripheral nerve are unassociated with either NF1 or NF2. Schwannosis is a benign Schwann cell proliferation occurring at the dorsal root entry zones or in the cord proper. It may be seen as a sporadic finding, but is more commonly encountered in NF2 patients. It is not yet clear whether or not these proliferations are clonal, although most favor a reactive origin.
Meningioma is the second most common tumor type in NF2. The fibroblastic and transitional variants are reportedly more common in some series, although this may reflect an element of misdiagnosis of schwannomas as fibrous meningiomas. These two tumor types can look remarkably similar, especially in the setting of NF2 (see Differential Diagnosis and Ancillary Diagnostic Studies sections). As in the sporadic counterpart, a wide histologic and clinical spectrum may be encountered in NF2-associated meningiomas (see Chapter 13 ). However, large series suggest an increased frequency of high-grade subtypes ( Fig. 22.2E ). Furthermore, whereas only a rare adult meningioma patient has NF2, as many as 40% of children with meningiomas suffer from this genetic disorder. Occasionally, meningioma is the presenting feature. Therefore, the diagnosis of meningioma in a child should prompt an evaluation for possible NF2, especially in the setting of multiple tumors.
Meningioangiomatosis (MA) is a rare plaque-like mass of the cortex or leptomeninges, characterized by variably cellular meningothelial and fibroblastic cell proliferations in association with a prominent small-vessel vasculopathy ( Fig. 22.2F ). Depending on the proportionate contributions of each element, MA may initially resemble a vascular malformation, perivascular fibrosis, or a meningioma. There is minimal proliferative activity, consistent with its presumed hamartomatous or malformative nature. Reactive and neurodegenerative changes are common within the entrapped or adjacent brain parenchyma. In NF2 patients, MA differs from its sporadic counterpart by its frequent multicentricity and the common finding of glial microhamartomas in nearby cortex or subcortical gray matter. The latter consist of clusters of dysplastic glial cells with sometimes bizarre multinucleate forms ( Fig. 22.2G ).
NF2-associated spinal ependymomas are histologically identical to sporadic counterparts (see Chapter 8 ). Occasional pilocytic astrocytomas have also been reported, but may represent misdiagnosed tanycytic ependymoma.
Occasionally, NF2 is confused with NF1 due to the presence of café-au-lait macules or misdiagnosis of schwannomas as neurofibromas. However, careful attention to both clinical and histologic features should resolve this issue. For example, the presence of radiologically detected masses consistent with vestibular schwannomas or meningiomas would make the diagnosis of NF1 highly suspect. Histologically, the Antoni B regions of schwannomas often resemble neurofibroma, but the cells are often larger in schwannomas and a careful search will often reveal foci of compact Antoni A pattern, tumor encapsulation, or Verocay bodies. Often, there is also considerable morphologic overlap between schwannoma and the fibroblastic variant of meningioma. Although most cases show classic features, whorl formation in schwannomas and “Verocay body”–like palisading in meningiomas are occasionally encountered. Psammoma bodies may be rare, but argue in favor of meningioma. Nevertheless, this distinction is sometimes only resolved by immunohistochemistry. Lastly, NF2 must be distinguished from schwannomatosis (see next section), although the latter is usually characterized by nonvestibular schwannomas and these patients lack other NF2-associated manifestations.
Immunostains for S-100 protein, SOX10, collagen IV, epithelial membrane antigen (EMA), and somatostatin receptor 2a (SSTR2a) are useful for distinguishing NF2-associated schwannomas from fibrous meningiomas. Schwannomas are typically EMA negative, diffusely S-100 protein and SOX10 positive, and display abundant pericellular collagen IV (or laminin) staining, representing basement membrane deposition. The latter may also be seen with a reticulin stain (although deposition within areas of collagen should not be overinterpreted). In contrast, roughly 80% of meningiomas show at least focal EMA positivity, virtually all express SSTR2a, they are SOX10 negative, and there is minimal intercellular basement membrane formation. The majority of fibrous meningiomas are S-100 protein positive, although it is typically a patchy rather than diffuse pattern. Lastly, the schwannomas encountered in both NF2 and schwannomatosis usually show a distinctive mosaic pattern (i.e., partial loss) of INI-1 immunoreactivity (see Fig. 22.2D ). In contrast, nonsyndromic schwannomas typically show retained expression throughout.
NF2 is a highly penetrant autosomal dominant syndrome; approximately half of cases are sporadic with de novo gene mutations and no associated family history. Somatic mosaics represent as many as 25% to 30%, often with too few mutation-bearing cells in the blood for detection by standard techniques. The NF2 gene is a classic tumor suppressor located on 22q12.2. It covers about 110 Kb of genomic DNA and includes 1 alternatively spliced and 16 constitutive exons. At least two protein isoforms have been identified. The NF2 gene is expressed in most organs, including the nervous system. Expression levels of the protein merlin (also referred to as schwannomin ) are particularly high during development. Merlin is a member of the cytoskeletal-associated protein 4.1 family. Point mutation resulting in truncated protein is the most common mechanism for gene inactivation. With multiple testing approaches, gene mutations are detectable in up to 100% of the familial and 60% of the sporadic cases.
NF2 patients suffer significant morbidity, including progressive deafness from vestibular schwannomas and, in severe cases, other central nervous system (CNS) deficits and craniospinal neuropathies (including blindness) from multiple meningiomas. The mortality rate is also considerably higher in NF2 than NF1, with most patients dying of their disease, sometimes as early as the fourth decade. Risk factors for premature death include young age at diagnosis, multiple intracranial meningiomas, lack of medical care in a specialty center, and nonsense or frameshift NF2 mutations. Nonetheless, there is marked clinical variability, with the two ends of the spectrum referred to as the Gardner (mild) and the Wishart (severe) types. The Gardner variant typically presents in adulthood with bilateral vestibular schwannomas; the Wishart variant often presents in childhood, typically with vestibular schwannomas and additional neoplasms, such as meningiomas. Nonsense and frameshift mutations of the NF2 gene are usually seen in the Wishart variant. As with NF1, the complexities of clinical care for NF2 patients are often best served at specialized multidisciplinary centers.
Schwannomatosis, also known as congenital neurilemmomatosis, has recently been defined as the presence of multiple nonvestibular schwannomas in the absence of other diagnostic NF2-associated features, such as neuroimaging evidence of bilateral vestibular schwannomas, germline NF2 mutation, or first-degree relative with NF2. Since vestibular schwannomas may take time to form, the diagnosis of schwannomatosis becomes more confident with advancing age; for example, it is extremely rare for NF2 patients older than 45 years of age to not have at least one vestibular schwannoma. Also, at least one schwannoma should be confirmed by histology before this disorder is diagnosed. Schwannomatosis has sometimes been referred to as the “third form of neurofibromatosis.”
Schwannomatosis has a relatively short history. The first example was described by a Japanese group in 1973, but the term schwannomatosis was originally proposed by MacCollin and colleagues in 1996. Subsequent studies linked this disorder to a locus on 22q centromeric to the NF2 gene. The SMARCB1 gene was implicated in 2007, and the LZTR1 gene was subsequently implicated in SMARCB1 wildtype cases in 2014.
Although the precise incidence of schwannomatosis is still unknown, data suggest that it may be as common as NF2, accounting for as many as 2.4% to 5% of all resected schwannomas. The majority of cases are sporadic, although familial examples have also been reported.
Schwannomatosis patients often present with multiple peripheral nerve or spinal nerve root schwannomas. By definition, vestibular schwannomas are not a component, although rare unilateral exceptions have been reported, and intracranial schwannomas are uncommon in general. In contrast to NF2, these schwannomas are typically distinctly painful, rather than manifesting with localized neurologic deficits. Segmental forms account for up to a third of all cases and involve only a single limb, side of the body, or region of the spine. Diagnostic criteria for schwannomatosis were established in 2011 as follows :
Molecular criteria for definite schwannomatosis:
two or more schwannomas or meningiomas (pathology proven) and genetics studies of two tumors with LOH for chromosome 22 and two different somatic NF2 mutations, or
one schwannoma or meningioma (pathology proven) and SMARCB1 germline mutation.
Clinical criteria for definite schwannomatosis:
two or more schwannomas (at least one pathology proven) and no bilateral vestibular schwannomas by thin-slice MR imaging, or
one schwannoma or meningioma (pathology proven) and first-degree relative affected by schwannomatosis.
Other than their multicentricity, the tumors of schwannomatosis are radiologically and grossly indistinguishable from their sporadic counterparts ( Fig. 22.3 ).
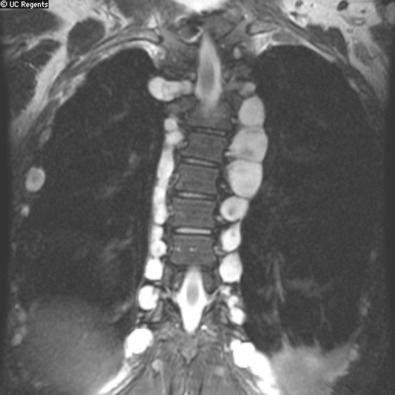
In most cases, the schwannomas associated with schwannomatosis have a prominent myxoid stroma but no other distinctive morphologic features in comparison to their sporadic counterparts (see Chapter 15 ), although there is at least one potential immunohistochemical difference (see Ancillary Diagnostic Studies section). Also, the presence of multiple tumors or tumorlets along a single nerve suggests the possibility of segmental schwannomatosis. Plexiform schwannomas have been reported in the setting of both schwannomatosis and NF2, although most are sporadic.
The main differential diagnostic consideration for schwannomatosis is neurofibromatosis type 2. The segmental or mosaic form of NF2 may be particularly difficult to exclude, since vestibular schwannomas may not develop in such patients. Genetic studies may be required to distinguish between these two possibilities.
Immunohistochemical and ultrastructural studies on the schwannomatosis-associated schwannomas show the same features as sporadic examples, with one exception. Whereas most, but not all sporadic schwannomas retain uniform nuclear positivity for INI1 (BAF47), schwannomatosis-associated neoplasms commonly display a mosaic pattern of immunoreactivity, with patchy loss of expression (see Fig. 22.2D ). Complicating matters further, however, NF2-associated schwannomas often show this same mosaic pattern of immunoreactivity. Therefore, this stain is helpful in distinguishing syndromic from sporadic schwannomas, but not in separating schwannomatosis from NF2.
Although the majority of schwannomatosis cases are sporadic (i.e., de novo mutations), familial cases have also been reported and follow an autosomal dominant pattern of inheritance. By definition, patients with schwannomatosis do not have germline NF2 gene mutations. However, genetic studies of resected schwannomas show that inactivating somatic NF2 gene mutations are nonetheless common. Linkage analyses further suggested that a second, more centromeric gene on chromosome 22q predisposes to this somatic NF2 instability, thereby implying a unique “four-hit mechanism” of tumorigenesis. Germline mutations in the SMARCB1 gene located at 22q11.23 encoding INI1 (a.k.a. BAF47 or SNF5), a subunit of the Swi/Snf chromatin remodeling complex, were identified in several familial cases of schwannomatosis. These SMARCB1 mutations in schwannomatosis are typically nontruncating missense variants or in-frame deletions that result in synthesis of a protein with altered function, in contrast to the truncating nonsense and frameshift germline mutations that are typically found in individuals affected by rhabdoid tumor predisposition syndrome (see separate section). More recently, germline mutations in the LZTR1 gene located at 22q11.21 have been identified in multiple families with schwannomatosis lacking germline variants in either NF2 or SMARCB1, in whom the term schwannomatosis type 2 has been suggested.
The mainstay of treatment for schwannomatosis is surgical resection. Clinical symptoms are primarily those of pain, which can occasionally be disabling. Other than that, however, the prognosis is excellent, and there is no known increase in mortality for these patients.
Tuberous sclerosis complex (TSC) is a familial disorder of hamartomas and benign neoplasms, involving the CNS and other organs (see Table 22.1 ). TSC is rarely referred to as “Bourneville disease,” “Bourneville-Pringle syndrome,” or “epiloia.”
The first documented case of TSC was reported by Friedrich von Recklinghausen in 1862. He described an infant with cardiac rhabdomyoma and “sclerotic” brain lesions, but did not appreciate the pathogenic relationship between them. The term tuberous sclerosis was first coined by Désiré Bourneville in 1880, based on the gross resemblance of the firm cortical lesions (i.e., “tubers”) to potatoes. The many other manifestations were slowly clarified over the next century. Formal diagnostic criteria were most recently revised in 2012, based on the recommendations of a consensus committee. The TSC1 and TSC2 genes were first cloned in 1997 and 1993, respectively.
TSC is the second most common hereditary tumor syndrome of the nervous system, with an incidence of roughly 1 in 6000 births. There are no known ethnic or gender predispositions.
TSC is a highly penetrant genetic disorder, although the clinical severity is extremely variable, with prenatal diagnosis of severe cases representing one extreme and subtle disease that goes undiagnosed for decades (sometimes only after genetic testing) at the other. TSC nearly always involves the CNS. The classic Vogt triad of facial angiofibromas, epilepsy, and mental retardation is only encountered in 30% to 40%, with roughly half of TSC patients having normal intelligence. CNS pathology is seen in almost all patients and is characterized by cortical tubers, subependymal nodules, subependymal giant cell astrocytoma (SEGA; also called subependymal giant cell tumor ), and radial glioneuronal heterotopias. Neurologic manifestations are roughly linked to the location, number, and size of tubers; they include epilepsy (80% to 90%), mental retardation (50%), and autism (25%). Signs of elevated intracranial pressure suggest obstructive hydrocephalus associated with SEGA and represent an indication for surgery. Similarly, tubers are sometimes resected for seizure control.
Cutaneous manifestations include multiple facial angiofibromas (previously called “adenoma sebaceum” ) and subungual fibromas (“Koenen tumors”). However, hypomelanotic macules (“ashleaf spots”) are the most common and earliest clues. Also common but less specific are shagreen patches and fibrous plaques of the forehead. Other common extraneural manifestations include cardiac rhabdomyomas, pulmonary lymphangioleiomyomatosis, angiomyolipomas of the kidney, and renal cysts. Hamartomatous rectal polyps, bone cysts, and gingival fibromas are less common. The 2012 revised diagnostic criteria list major and minor criteria as follows:
Major criteria: three or more facial angiofibromas ( Fig. 22.4A and B ), cardiac rhabdomyoma ( Fig. 22.4C ), retinal nodular astroglial hamartomas ( Fig. 22.4D ), cortical tubers ( Fig. 22.4E–G ), subependymal nodules or SEGA ( Fig. 22.4E, H–J ), two or more subungual fibromas, three or more hypomelanotic macules, shagreen patch, lymphangioleiomyomatosis, and two or more renal angiomyolipomas
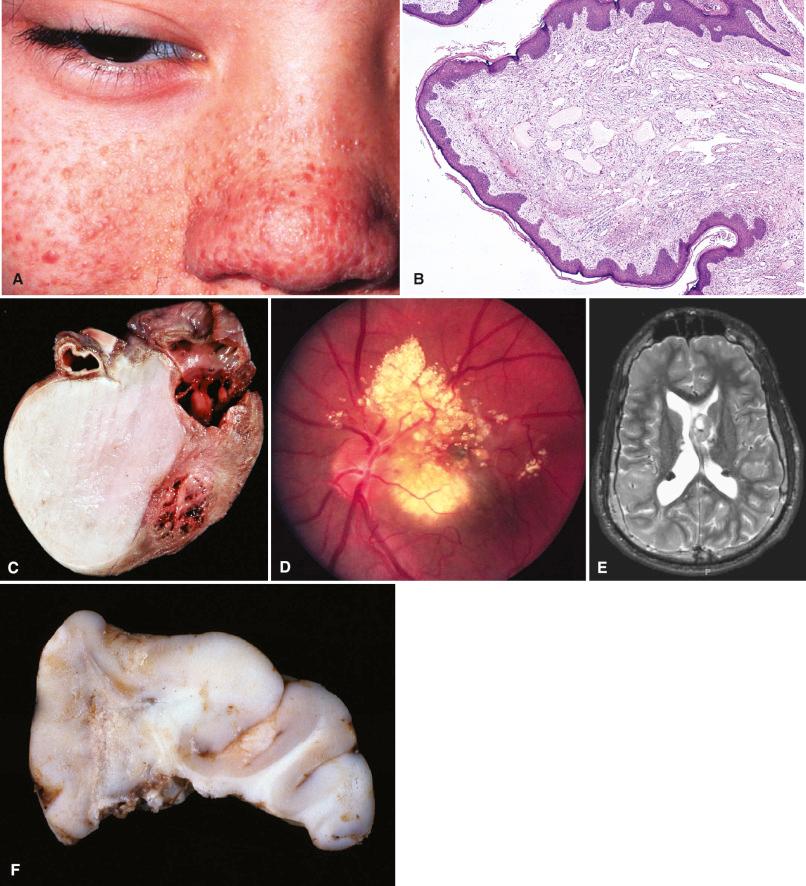
Minor criteria: dental enamel pits, gingival fibromas, rectal hamartomatous polyps, retinal achromic patches, multiple renal cysts, nonrenal hamartomas, and “confetti” skin lesions
Definite TSC is diagnosed if either two major features or one major and two minor features are present. Possible TSC is applied to those cases with one major feature or two or more minor features.
Radiology plays a critical role in both the diagnosis and routine follow-up of TSC patients. Cortical tubers are detected by CT or MRI in roughly 90% of cases. About half are calcified, and some undergo cystic change. In adults, they are often hypodense to isodense on T1-weighted and hyperintense on T2-weighted/fluid-attenuated inversion recovery (FLAIR) MRI sequences ( Fig. 22.4E ). In children, this pattern is reversed due to the high water content of the adjacent unmyelinated white matter. Spectroscopy typically shows decreased N -acetyl aspartate and increased myoinositol peaks. Diffusion tensor imaging (DTI) and PET have been shown to be useful in distinguishing epileptogenic from nonepileptogenic tubers. Those that initiate seizures usually show a higher apparent diffusion coefficient (ADC) and lower fractional anisotropy (FA) values on DTI, with hypometabolism on FDG-PET. Radial glioneuronal heterotopias have similar imaging characteristics to tubers and are best visualized on MRI. Subependymal nodules form multiple small intraventricular projections ( Fig. 22.4E ), with calcifications detected on CT. Their detection may be particularly useful for making the diagnosis of TSC in family members with subtle or silent clinical manifestations. In comparison, a SEGA is typically greater than 1 cm in size and enhances with gadolinium on MRI ( Fig. 22.4E ).
Cortical tubers are protuberant, tan-white gyral expansions that feel firm or rubbery when large and markedly gliotic (“potato-like”), typically associated with blurred gray-white junctions ( Fig. 22.4F ). Those with extensive calcifications are chalky or gritty on cut surface. Radial glioneuronal heterotopias show similar features but extend through the white matter in a linear or wedge-shaped fashion, with the apex in the latter situation pointing toward the ventricular surface. Other tubers and heterotopias are grossly subtle or unremarkable, being appreciated only on microscopic examination. Subependymal nodules or “candle gutterings” grossly resemble wax drippings, which may be numerous and line the ventricular walls, but only become symptomatic when they enlarge to form a SEGA, with subsequent obstruction of the foramen of Monro leading to secondary hydrocephalus.
The classic CNS lesions of TSC are tubers ( Fig. 22.4G ), subependymal nodules, SEGAs, radial glioneuronal heterotopias, and, rarely, agenesis of the corpus callosum, schizencephaly, or cerebellar dysplasia. Cortical tubers involve cortex or subcortical white matter (or both); they are composed of enlarged, dysmorphic glioneuronal elements with associated astrocytosis and variable calcification ( Fig. 22.4G ), the latter two features accounting for their grossly firm texture (i.e., “sclerosis”). The most distinctive element is the “balloon cell” with its neuron-like nucleus (round with vesicular chromatin and large nucleolus), combined with eccentrically placed, glassy pink astrocyte-like cytoplasm. Neuronomegaly is also common, consisting of large mildly dysmorphic ganglion cells with variably coarse or clumped Nissl substance ( Fig. 22.4G ). In the developing fetus with TSC, many dysmorphic cells are often seen in the deep white matter, consistent with migrational as well as maturational abnormalities, with the dysplastic cells getting stuck at various points along the peripheral passage from germinal matrix outward ( Fig. 22.4H and I ). This pattern ultimately results in radial glioneuronal heterotopias, yielding dysplastic glioneuronal cells, potentially all the way from the cortex down to the periventricular region.
The subependymal nodules and SEGAs are histologically indistinguishable and are similarly characterized by atypical, enlarged glioneuronal cells ( Fig. 22.4H and J ), often with dystrophic calcification. The cells are epithelioid, spindled, or gemistocyte-like, forming “sweeping fascicles” and ependymoma-like perivascular pseudorosettes, including a variably fibrillar background from thin cellular processes (see Chapter 7 as well). Like the dysplastic cells of tubers, they often combine neuron-like nuclei with astrocyte-like cytoplasm. In contrast to diffuse gliomas that occasionally project into the ventricular lumen, SEGAs are well demarcated, displaying a sharp interface with the adjacent brain parenchyma. Mitotic figures and even necrosis are occasionally seen, but have no ominous connotations. Intratumoral mast cells are also common and may be numerous ( Fig. 22.4J ). Retinal astroglial hamartomas are present in roughly half the cases ( Fig. 22.4D ) and may undergo calcification and cystic degeneration. Most are asymptomatic, although visual impairment can occur if there is macular involvement or secondary hemorrhage.
It is important to note that tubers are histologically identical to focal cortical dysplasia, type IIb (see Chapter 25 ), the only difference being that the latter are typically single and sporadic (i.e., no other signs of TSC). Since a resected “focal cortical dysplasia, type IIb” may be the first manifestation of TSC, a diagnostic comment in the pathology report is warranted, stating that clinical workup is needed to exclude the alternative interpretation of tuber. Often, a careful examination of the neuroimaging reveals tubers or subependymal nodules in patients with TSC. Also, the histology of SEGA may initially appear alarming, especially if viewed in the absence of appropriate clinical and radiologic information. The presence of gemistocyte-like cells, fibrillar processes, perivascular pseudorosettes, and occasional mitoses could easily misguide the unwary observer to a diagnosis of malignant astrocytoma or ependymoma. Useful clues to the correct diagnosis of SEGA include history of TSC or other MRI features of TSC (present in nearly every case), a purely intraventricular location, sharp demarcation from adjacent brain, and a mixed glial and neuronal phenotype. The diagnosis of a SEGA is virtually pathognomonic of TSC, although rare patients show no other manifestations. Whether these represent sporadic forms of SEGA or a forme fruste of TSC is unclear.
Immunohistochemistry may be useful in TSC-associated lesions. Dysplastic cells often stain variably with glial fibrillary acidic protein (GFAP), including the balloon cells of tubers and heterotopias, as well as the tumor cells of SEGA. However, they may also show positivity for neuronal markers, such as synaptophysin, neurofilament protein, and NeuN. Due to this dual glial and neuronal phenotype, some prefer the term subependymal giant cell “tumor,” rather than “astrocytoma.” Large dysplastic neurons (“neuronomegaly”) usually show neurofilament-positive cell bodies. Other cell types stain for neither glial nor neuronal markers, possibly representing more primitive elements. Distinctive CD34-immunoreactive cells with highly ramified processes are also seen in a subset of tubers ( Fig. 22.4K ); these cells have similarly been proposed to represent some sort of progenitor phenotype. Ultrastructurally, dysplastic cells may also show combined glial and neuronal features. In SEGAs, tumor nuclei may be TTF-1 positive and mast cells can be highlighted using an immunostain for c-kit/CD117 ( Fig. 22.4L ). The sharp interface with adjacent brain is demonstrable with a stain for neurofilament, which highlights axons only at the periphery of the tumor.
TSC is an autosomal dominant disorder, with roughly 70% of cases being sporadic (new mutations with no family history); about 2% of patients have a mosaic form. TSC results from germline mutation in one of two associated genes. TSC1 on 9q34 encodes for the hamartin protein, while TSC2 on 16p13.3 encodes for the tuberin protein, the latter showing partial homology to the Rap1 activator Rap1-GAP. Over 1000 mutations have been identified, with TSC2 mutations being most common in sporadic cases. Mutations are detectable in 80% to 90% of patients; nearly all TSC1 mutations are truncating, but about 20% of TSC2 mutations are missense. TSC2 mutations are generally associated with the more severe phenotype, particularly in regard to CNS involvement. Compared with other TSC-associated tumors, classic second hits (e.g., loss of heterozygosity) are not always found in tubers, suggesting an alternative mechanism of inactivation.
Both hamartin and tuberin proteins are widely expressed and form heterodimers that function in the PI3K signaling pathway. The tuberin-hamartin complex inhibits mTOR and affects cell size regulation, differentiation, proliferation, and cellular migration. These functions potentially explain some of the observed lesions, including heterotopias and immature, enlarged glioneuronal cells with increased proliferative potential. Studies of SEGA tumor cells, giant cells in tubers, and the balloon cells in focal cortical dysplasia show analogous genotypic and phenotypic features, suggesting the possibility of a common CNS progenitor, likely from the periventricular region.
Therapy in TSC has traditionally consisted mostly of symptomatic management. The γ-aminobutyric acid inhibitor, vigabatrin, may be useful for treating infantile spasms, whereas epileptogenic tubers benefit from surgical resection. Similarly, SEGA was commonly treated surgically, especially when associated with obstructive hydrocephalus. However, treatment with the mTOR inhibitor, rapamycin (or everolimus), has been shown to cause tumor regression of SEGA and renal angiomyolipomas and was FDA approved in August 2012 for the treatment of SEGA in patients with TSC. The most common cause of morbidity and mortality in TSC is CNS disease, although cardiac, renal, and pulmonary lesions are also significant sources. Rare examples of malignancy, such as renal cell carcinomas (RCCs), malignant angiomyolipomas, high-grade astrocytomas, and chordomas have also been associated with TSC, as have various vascular complications, such as intracranial aneurysms.
Von Hippel-Lindau disease (VHL) is a familial disorder predisposing patients to cysts and hypervascular neoplasms of multiple organs, including the CNS, eye, kidney, adrenal medulla, pancreas, inner ear/temporal bone, and epididymis. Less commonly utilized synonyms include angioblastomatosis, cerebelloretinal hemangioblastomatosis, retinocerebellar angiomatosis, and retinocerebellar angiophakomatosis. “Lindau tumor” is the older term for cerebellar hemangioblastoma. Synonyms of the VHL-associated endolymphatic sac tumor have included “Heffner tumor,” aggressive papillary middle ear tumor (APMET), papillary adenoma or adenocarcinoma of temporal bone, and low-grade adenocarcinoma of probable endolymphatic sac origin.
Several examples of blindness due to vascular retinal lesions were reported in the 1800s. However, the German ophthalmologist Eugene von Hippel was the first to designate them as “retinal angiomatosis” in 1904. The Swedish pathologist Arvid Lindau later described the histologically identical tumor in the cerebellum in 1926. He also recognized its association with the retinal counterpart and the hereditary predilection. Later work by Bailey, Cushing, and Fulton in the United States supported his hypothesis, and they subsequently coined the currently utilized term, von Hippel-Lindau disease. The VHL gene was first cloned in 1993.
The incidence of VHL has been estimated at 1 in 30,000 to 50,000 live births, with no known sex or race predilections.
As with NF1 and NF2, VHL is a complex multiorgan disease with considerable morbidity and mortality, which is most effectively treated at multidisciplinary specialty centers.
VHL-associated disease includes:
retinal hemangioblastomas (40%–60%; Fig. 22.5A )
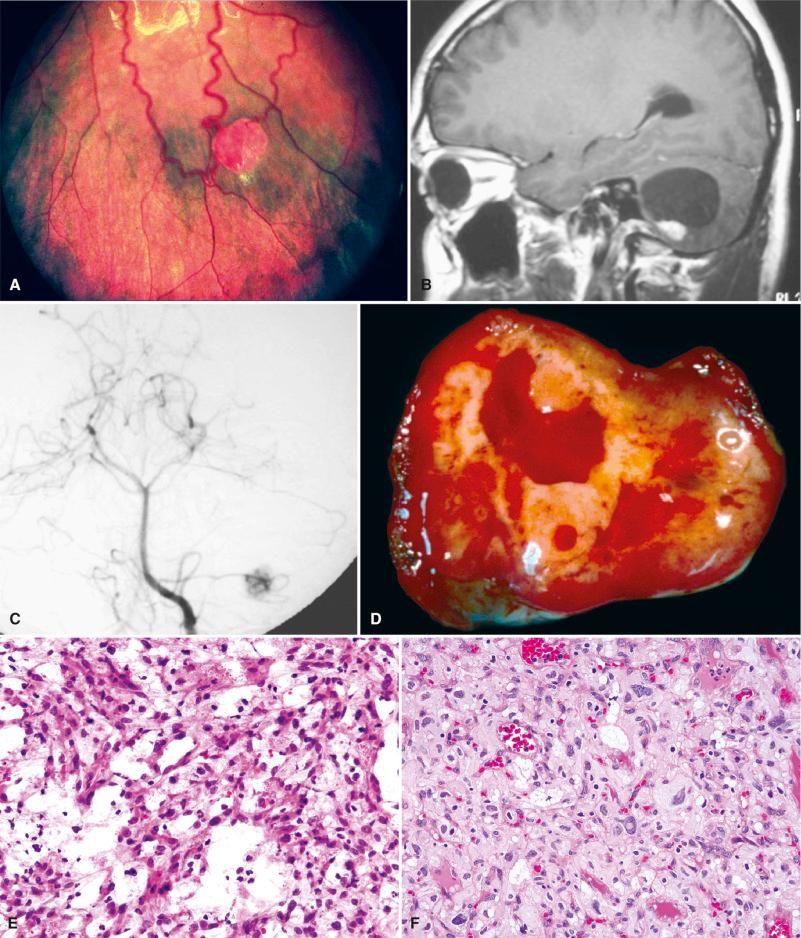
CNS hemangioblastomas (60%–80%; Fig. 22.5B–J )
endolymphatic sac tumors (2%–11%; Fig. 22.5K–N )
pheochromocytomas (10%–25%)
pancreatic cysts or islet tumors (60%–80%)
renal cysts and RCCs (30%–60%)
papillary cystadenomas of the epididymis and uterine broad ligament (20%–60%)
In the presence of a positive family history, the diagnosis of VHL requires only one CNS or retinal hemangioblastoma (HB), an RCC, or a pheochromocytoma. Those without a family history must have multiple HBs or one HB and a second characteristic lesion. VHL families are broadly divided into type 1 (no pheochromocytomas) and type 2 (with pheochromocytoma) variants. The type 2 category is further stratified into types 2A (HBs with low risk of RCC), 2B (HBs with high risk of RCC), and 2C (pheochromocytomas only). Most VHL lesions can be identified by radiologic techniques, but ophthalmologic examination for retinal HB is considered the least invasive early-detection approach for patients at risk. The penetrance for VHL is age dependent, with mean ages of clinical presentation roughly as follows: endolymphatic sac tumor (22 years), retinal HB (25 years), CNS HB (33 years), pheochromocytoma (30 years), and RCC (39 years). There is over 90% penetrance by 65 years of age.
Solitary and especially multiple HBs are diagnostic hallmarks of VHL. Roughly 75% are infratentorial, mainly involving the cerebellum. The other 25% are found in the spinal cord, brainstem, and lumbosacral nerve roots. Supratentorial HBs are extremely rare. Of interest, only about 25% to 30% of cerebellar HBs occur in the setting of VHL, whereas this percentage rises to 80% for spinal cord tumors. During the course of the disease, additional hemangioblastomas often develop, either at the same or different sites. Symptoms are most often correlated with enlargement of the cyst, and there may be alternating periods of growth and quiescence that are difficult to predict clinically. Occasional CNS HBs produce sufficient erythropoietin to induce polycythemia. The endolymphatic sac tumor (ELST) is an aggressive neoplasm involving the petrous temporal bone and cerebellopontine angle. Roughly 10% to 15% of all patients with ELST have VHL, and screening for other diagnostic lesions is therefore appropriate, especially if bilateral. It is a common cause of hearing loss, tinnitus, and vertigo in VHL patients, although early detection of small tumors reduces the risk of hearing loss.
Given the characteristic features, neuroimaging (and systemic imaging) plays an important role in both the diagnosis and routine surveillance of VHL patients. The vast majority of VHL-associated tumors, including HBs, are markedly hypervascular on angiography and avidly contrast enhancing (see Fig. 22.5B and C ). The classic appearance of cerebellar HB on head MRI is a well-demarcated cystic tumor with a mural contrast-enhancing mass ( Fig. 22.5B ). However, any combination of solid, cystic, and hemorrhagic components may be encountered. Feeding or draining blood vessels may appear as “flow voids” on MRI. On high-resolution CT, ELSTs are often associated with geographic or “moth-eaten” petrous temporal bone destruction with a peripheral rim of calcification ( Fig. 22.5K ). They often enhance and extend into the cerebellopontine angle on MRI ( Fig. 22.5L ).
HBs are slow-growing (WHO grade I) tumors of uncertain histogenesis. They are well-demarcated, highly vascular tumors with varying proportions of capillary proliferation (predominant in reticular variant ), fibrosis, and epithelioid clear to foamy stromal cells (predominant in cellular variant; see Fig. 22.5F ). The stromal cells commonly show degenerative nuclear atypia, but this has no prognostic significance. Intratumoral mast cells are also common, and roughly 10% show foci of extramedullary hematopoiesis (EMH), likely related to the production of erythropoietin by stromal cells ( Fig. 22.5G ). The adjacent brain parenchyma often shows piloid gliosis with compact proliferations of astrocytic cells containing numerous Rosenthal fibers. No specific histologic features have been identified that distingish VHL-associated HBs from those that are sporadic. ELSTs are characterized by a bone-destructive, papillary epithelial tumor ( Fig. 22.5M ), lined by a single layer of bland flattened cuboidal to columnar epithelial cells with clear to eosinophilic cytoplasm. They are often focally cystic and have sclerotic bands of intervening collagen. Some contain glands or thyroid-like follicles with dense proteinaceous material resembling colloid ( Fig. 22.5N ). Nuclear pseudoinclusions may be prominent. Foci of secondary hemorrhage, hemosiderin, cholesterol debris, and inflammation are typical.
The common reticular (85% to 90% of cases) and uncommon cellular (10% to 15%) variants of HB were described earlier, and some investigators have found increased GFAP positivity, Ki-67 labeling index, and risk of recurrence in the cellular HB. Otherwise, however, both are considered histologically benign (WHO grade I).
The differential diagnosis between HB and metastatic RCC can be challenging, because (1) there is considerable morphologic overlap between the two lesions, (2) both tumors are common in VHL patients, and (3) RCC frequently metastasizes to the CNS (or even to a preexisting HB), both sporadically and in the context of VHL. Nevertheless, the stromal cells of HBs have a typically foamy or vacuolated cytoplasm that is usually not seen in RCC. The common nuclear hyperchromasia and atypia may lead to misinterpretation of a malignant diagnosis, such as diffuse astrocytoma at the time of frozen section (see Fig. 22.5E ), although the typical “cyst with a mural-enhancing nodule” on imaging would strongly argue against that possibility. The fact that Rosenthal fibers associated with pilocytic gliosis may be seen at the periphery of the tumor may cause further confusion with pilocytic astrocytoma at frozen section. Fortunately, this mistake has little clinical significance since the surgical approach to both of these tumors is essentially identical. Because these tumors are so vascular, the neurosurgeon may also provide a diagnostic clue if he or she mentions difficulty controlling the bleeding. The tumors are often superficial and may involve the leptomeninges or dura as well, potentially mimicking meningioma clinically and the angiomatous (vascular) variant of meningioma histologically (see Chapter 13 ). Unusual locations (brainstem, spinal cord, or nerve root) or multifocal disease favors a diagnosis of VHL-associated over one of sporadic tumors.
The differential diagnosis for ELST includes choroid plexus papilloma, middle ear adenoma/adenocarcinoma, carcinoid of the middle ear, salivary gland choristoma, and metastatic carcinoma (including thyroid, RCC, and lung). However, the immunohistochemical profile of ELST is distinctive (see following discussion). Additionally, meningioma and paraganglioma are often in the radiologic differential, but they are easily distinguished by histologic criteria.
The intracytoplasmic lipid in HBs can be helpful in distinguishing these tumors from glial neoplasms, particularly at the time of frozen section. This can be detected with oil red O stain or toluidine blue (see Fig. 22.5H ). They are also reticulin rich ( Fig. 22.5I ), separating them from most of their reticulin-poor clear cell mimics. Immunohistochemistry with traditional epithelial markers such as cytokeratin and EMA and glioneuronal markers such as neuron-specific enolase, GFAP, and S-100 protein are helpful in highlighting RCC cells and the stromal cells of HB, respectively. However, these immunostains are not entirely specific. For example, HB, particularly the more cellular subtypes, may rarely be reactive for epithelial antigens. The angiomatous (vascular) variant of meningioma, another common differential diagnostic consideration, is typically positive for EMA as well. RCCs, particularly those with rhabdoid and sarcomatous features, may show S-100 protein immunoreactivity. Immunohistochemical markers that are helpful in distinguishing HB from RCC include inhibin alpha and CD10. Whereas immunostaining for inhibin is usually negative in RCC, its expression is almost uniformly seen in the stromal cells of HB ( Fig. 22.5J ). In contrast, the majority of RCCs are CD10 positive, whereas HBs are negative. An additional marker of HB stromal cells of potential utility is D2-40.
ELSTs are typically positive for cytokeratins (including CK7, but not CK20), EMA, S-100 protein, GFAP, and NSE. This immunoprofile distinguishes ELSTs from the common differential diagnostic considerations listed in the prior section. In contrast to metastatic thyroid carcinoma, ELSTs are negative for thyroglobulin and TTF-1. As with most VHL-associated tumors, they are also immunoreactive for vascular endothelial growth factor (VEGF).
VHL is an autosomal dominant disorder, with roughly 20% of patients presenting as sporadic cases with no family history. The VHL tumor suppressor gene maps to chromosome 3p25 and includes three highly conserved exons. It encodes a 30-kDa protein, pVHL, which is widely expressed, and a second 19-kDa isoform resulting from internal translational initiation. Both isoforms have tumor suppressor activity. The pVHL multiprotein complex regulates a number of hypoxia-inducible factor (HIF) transcripts, including the HIFA, VEGF, and EPO (erythropoietin) genes. In normoxic conditions, the pVHL complex targets HIF for degradation, whereas in the setting of hypoxia or the loss of pVHL function, HIF levels remain elevated. The resulting stimulation of angiogenic factors, such as VEGF and platelet-derived growth factor B (PDGF-B), could explain the typical hypervascularity of VHL-associated tumors.
Over 300 germline mutations of the VHL gene have been detected in VHL families. Somatic mutations are similarly seen in about 30% of sporadic CNS HBs and 50% of clear cell RCCs. There are fairly consistent genotype-phenotype associations, such that the VHL type 2 families with pheochromocytomas usually harbor missense mutations, whereas VHL type 1 families typically harbor deletions or nonsense mutations. Germline mutations are detectable in blood from nearly all patients, with the rare exception of mosaic patients. The mechanism for somatic loss of the wildtype allele in tumors and benign cysts is highly variable, ranging from small deletions to complete monosomy of chromosome 3. While genomic analyses have determined that biallelic inactivation of VHL is typically the solitary genetic alteration in both syndromic and sporadic hemangioblastomas, VHL-associated RCC harbor additional somatic alterations.
Until recently, life expectancy was roughly 50 years, with death commonly resulting from complications of RCC and CNS HBs; however, modern screening and surveillance programs have led to earlier detection, with lower morbidity and mortality figures.
Tumors are variably treated with surgery, vascular embolization, and stereotactic radiosurgery (e.g., “gamma knife”). Treatment with the anti-VEGF antibody bevacizumab has been shown to cause regression of retinal and CNS HB, and small molecule inhibitors of the VEGF receptor (e.g., sorafenib) are FDA-approved for the treatment of clear cell RCCs including those arising in patients with VHL. ELST is considered a low-grade malignancy with a high risk of local recurrence in the absence of radical resection. However, rare examples of drop metastasis, secondary meningitis, and death due to disease have also been reported.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here