Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Atherosclerosis is a disease in which plaque builds up inside of the artery wall, causing the thickening and narrowing of arteries, thereby reducing blood supply to the end organs. Plaque is made up of fatty substances, cholesterol, cellular waste products and debris, calcium, fibrin, inflammatory cells, and platelets. , It can affect any artery in the body, including arteries in the heart, brain, kidneys, and legs.
Atherosclerosis is the result of nonresolving interactions of inflammation, oxidative stress, lipid deposition, and genetic predisposition. In addition, a novel concept for atherosclerosis risk implicates a lack of endothelial progenitor cell (EPC)-dependent arterial repair in the development of the disease, secondary to the exhaustion of repair-competent EPCs. Molecular evidence derived from genetic techniques indicates that atherosclerotic lesions may begin to form as arterial repair fails, rather than merely following arterial injury. Thus, chronic arterial injury may overwhelm the ability of EPCs and other vascular progenitor cells to maintain arterial homeostasis, particularly when EPCs capable of arterial repair become exhausted. Imbalance between arterial damage and repair leads to atherosclerosis ( Fig. 15.1 ). ,
Cholesterol is a fat-like substance used by cells to synthesize hormones, vitamin D, and substances that help with food digestion. However, elevated plasma cholesterol levels build up within the arterial walls and increase the risk of atherosclerosis. All nucleated cells can synthesize the enzymes to produce cholesterol, and the rate-limiting enzyme in this pathway is 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase. The product of HMG-CoA reductase, mevalonic acid, appears in plasma and urine and may be used as a guide to cholesterol synthetic activity. Post-synthesis, cholesterol is then transported in the bloodstream in small packages called lipoproteins . There are two main kinds of lipoproteins: low-density (LDL) and high-density lipoprotein (HDL). HDL carries cholesterol from other parts of the body to the liver, where it is removed from the body. Higher levels of HDL lower a person’s chances for getting CAD. LDL carries cholesterol to cells with LDL receptor (LDLR), which are then internalized and degraded. However, patients with familial hypercholesterolemia (FH) have high levels of plasma LDL due to loss of function mutations that result in an inability to remove the LDL from the bloodstream. Atherosclerosis is ultimately a universal disease of aging, with compounding additional risk factors. Of the major genetic risk factors, family history is the most significant independent one. FH, familial hypertension, familial diabetes, and familial obesity are at the top of the list of heritable and genetic risk factors. Moreover, these multiple heritable and genetic risk factors can cluster, in many cases, in families. Although FH is the best-characterized heritable risk factor in familial arteriosclerosis, it explains only a small percentage of disease susceptibility. Most of the clinical atherosclerotic diseases result from the interactions of multiple genetic and environmental factors, none of which usually can cause disease by itself.
FH is a monogenic dyslipidemia characterized by an accumulation of low-density lipoprotein (LDL) in the plasma that results in severe hypercholesterolemia with variable expression of pathognomonic traits such as: tendinous xanthomas, arcus corneae, and xanthelasma. Eventually FH patients develop precocious atherosclerotic cardiovascular disease (ASCVD) events such as myocardial infarction, stroke, and limb ischemia.
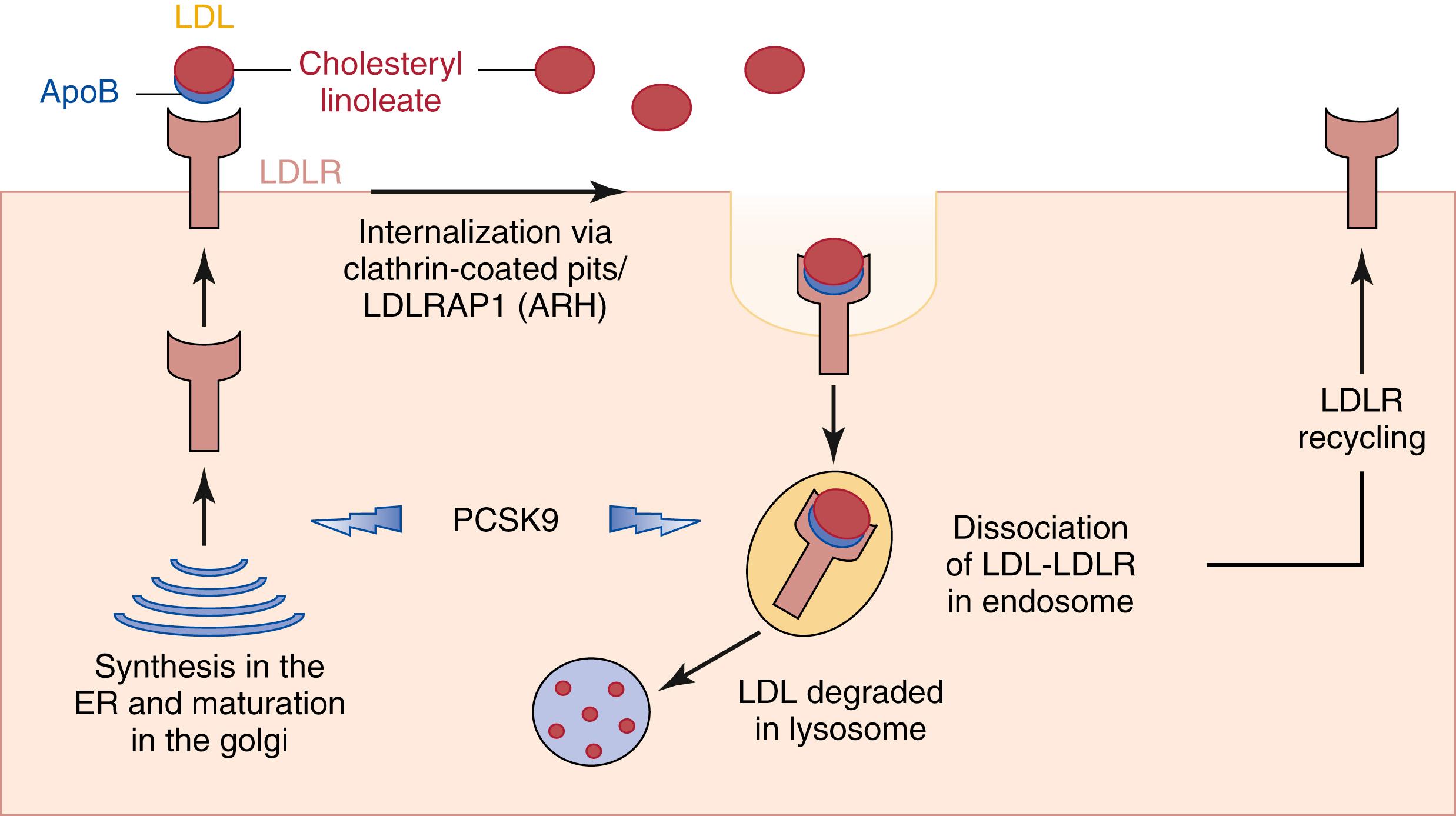
FH is an autosomal dominant disease caused by mutations in the genes encoding the LDL receptor (LDLR) , which normally removes LDL from the circulation; apolipoprotein B (ApoB), which is the part of LDL that binds with the receptor; or proprotein convertase subtilisin/kexin type 9 (PCSK9), which induces degradation of LDLR; and the adaptor protein, or autosomal recessive hypercholesterolemia (ARH) protein (LDLRAP), which facilitates the clearance of circulating LDL ( Fig. 15.2 ). Mutations in these genes result in loss of function of these molecules and contribute to elevated plasma levels of LDL. People who have one abnormal copy (are heterozygous ) in one of three genes (LDLR, ApoB, PCSK9) may have premature CAD at the age of 30 to 40. Having two abnormal copies (being homozygous ), either biallelic pathogenic variants in one of these known genes or one pathogenic variant in each of two different genes, may result in severe CAD in childhood.
Heterozygous FH (HeFH) is more common and accounts for 60% to 80% of FH, with a prevalence estimated at 1 per 200 to 500 of persons with FH. Persons with untreated FH are at an approximately 20-fold increased risk for CAD. Untreated men are at a 50% risk for a fatal or nonfatal coronary event by age 50 years; untreated women are at a 30% risk by age 60 years. In contrast, homozygous FH (HoFH) is incredibly rare with a prevalence of about 1 per 160,000 to 1 million. Most individuals with HoFH experience severe CAD by their mid-20s. The rate of death or coronary bypass surgery by adolescence is high. Severe aortic stenosis is also common. ,
As previously discussed, several mutations in genes regulating LDL homeostasis result in FH. One such mutation is the LDLR, a well-characterized cell surface transmembrane protein that binds LDL, which then becomes internalized as a complex. Once in the endosome, the LDLR dissociates from the lipoprotein during acidification while the LDL proceeds to the lysosome for enzymic degradation into its constituent cholesterol, fatty acids, and amino acids. The dissociated LDLR can be retransported back to the cell surface and recycles approximately 150 times before degradation.
The defect of LDLR results in accumulation of LDL in the bloodstream, leading to FH. Numerous defects in the LDLR have been divided into six classes, depending on the impact of the mutation on the presence of mRNA, receptor maturation in cells, disparity between LDL and immunoglobulin binding on the cell surface, and LDLR degradation and trafficking. These LDLR classes of mutations affected the synthesis, cellular transport, ligand binding capacity, ability to internalize, recycle, and localize to the basolateral membrane ( Table 15.1 ). Although defective hepatic LDL uptake is the main and most direct consequence, other metabolic perturbations may contribute to the metabolic characteristics and accelerated atherosclerotic disease associated with FH.
| Class | Functional Defect |
|---|---|
| 1 | Largely due to promoter mutations, leave no detectable miRNA and no detectable LDLR synthesis |
| 2 | Absent (Class 2A) or impaired (Class 2B) maturation of LDLR protein |
| 3 | Normal LDLR synthesis, however impaired LDL binding due to mutations in the binding domain of LDLR |
| 4 | LDLR mature normally and can bind LDL; however, cannot cluster in coated pits on cell surface and thus are unable to internalize upon ligand binding |
| 5 | LDLR receptors have inability to recycle and are rapidly degraded |
| 6 | Failure of directing LDLR to basolateral membrane in polarized cells |
The defective apoB100 is another common reason for FH. Compared with the numerous mutations found in the LDLR and its pathway, very few mutations have been reported in apoB100 that result in disruption of ligand binding of LDL to the LDLR. Individuals with heterozygous familial binding defective apoB100 (FDB) exhibit cholesterol levels of about 300 mg/dL due to defective LDL clearance. In homozygous FDB, there appears to be no significant gene dose effect. Two homozygotes for FDB were reported to have cholesterol concentrations in the range of heterozygotes for LDLR mutations. The binding affinity for LDLR of very low density lipoprotein (VLDL) was normal in these subjects, whereas LDL affinity was 10% of normal.
An alternative mechanism for FH is mutations in the PCSK9 gene, which have been known to cause autosomal dominant hypercholesterolemia. This gain of function mutation increases the affinity of the PCSK9 protein for the LDLR, which results in diminished LDL–LDLR complex dissociation in exosomes, decreased LDLR recycling and increased LDLR degradation, all together resulting in fewer LDLR available on the cell-surface for LDL plasma clearance, thus resulting in FH.
The APOE , gene is another location for potential FH mutations. Mutations in ARH, the adaptor protein, can impair the internalization of LDLR and thus LDL plasma clearance. , This particular type of FH is a rare, autosomal recessive variant that is less severe than the LDLR mutations seen in more common HoFH phenotype.
While FH, as a monogenic dyslipidemia, results in large increases in LDL levels, the variations in the clinical presentation cannot altogether be explained by their distinct mutations. Thus there are likely other genes acting on LDL levels. Whole exome sequencing studies have demonstrated that single nucleotide polymorphisms (SNPs) can also affect LDL levels.
Hypertension is a well-known risk factor for atherosclerosis. Over time, excessive pressure can damage the arteries, making them more vulnerable to the continued inflammation that results in plaque build-up associated with atherosclerosis. Hypertension tends to be familial and is likely to be the consequence of an interaction between genetic and environmental factors. About 30% to 50% of the variance in blood pressure readings is attributable to genetic heritability and about 50% to environmental factors. The heritability of blood pressure frequently ranges about 50% to 70% in twins, with considerably lower estimates (around 20% to 25%) in family studies. In spite of substantial efforts and progress in elucidating the genetic basis for heritable hypertension, the genetic factors contributing to common forms of hypertension remain largely unknown. The genetics of hypertension is complex, with no known single gene playing a major role but rather many genes, each with mild effects, reacting to different environmental stimuli and thus contributing to elevated blood pressure. Early studies in hypertension identified specific enzymes, channels, and receptors implicating sodium handling in the regulation of blood pressure, including genes involved in the renin–angiotensin–aldosterone system (RAAS), controlling blood pressure and saltwater homeostasis, proteins in hormonal regulation of blood pressure (enzymes and receptors of the mineralo- and glucocorticoid pathways), and proteins coded by genes involved in the structure and/or regulation of vascular tone (endothelins and their receptors). RAAS plays an important role in regulating blood pressure and during hypertensive crises ( Fig. 15.3 ). The enzyme renin acts on angiotensinogen (AGT) to generate angiotensin I (Ang I). Ang I is further converted to angiotensin II (Ang II) by angiotensin-converting enzyme (ACE). Ang II exerts its effects by binding to two major types of receptors – AT1R and AT2R. Functions like vasoconstriction, cellular proliferation, cellular hypertrophy, fibrosis, atherosclerosis, antinatriuresis, and the release of aldosterone, endothelin, norepinephrine, and vasopressin are initiated by the binding of Ang II to AT1R. In addition, Ang II directly stimulates vascular smooth muscle cell (SMC) growth and extracellular matrix production. Studies with spontaneously hypertensive rats indicate that raised blood pressure stimulates the expression of platelet-derived growth factor (PDGF), a potent mitogen for SMC. Furthermore, both renin receptors (recently identified) and functionally active Ang II-derived peptides like Ang 1 to Ang 7 have been shown to play pathologic roles in the development of hypertension. Both linkage and association studies have provided strong evidence for the role of the AGT gene in hypertension in Caucasian and African/Caribbean populations as well as in pregnancy-induced hypertension.
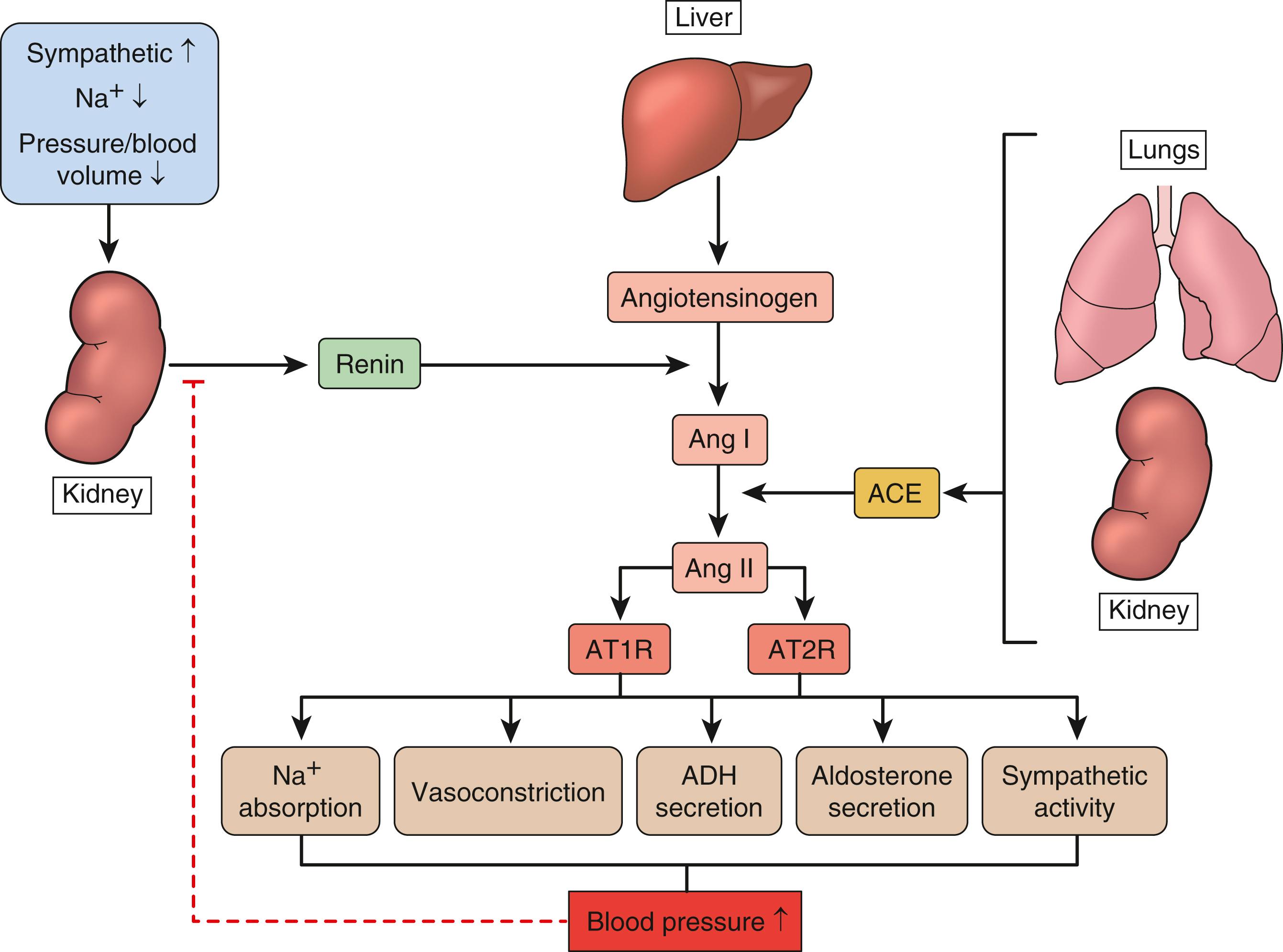
The kidney is known to be the dominant, long-term regulator of blood pressure, highlighting the need for an appreciation of its pathophysiology in considering genetic factors potentially contributing to hypertension. Moreover, pheochromocytoma is a rare cause of hypertension that may be familial.
Cardiovascular disease (CVD) is more prevalent in type 1 and type 2 diabetes (T1D and T2D) and continues to be the leading cause of death among adults with diabetes. T2D, commonly referred to as insulin resistance, is partly inherited.
Insulin resistance is also associated with the enhanced generation of reactive oxygen species (ROS). In addition, insulin resistance decreases NO production, and increases the release of free fatty acids from adipose tissue. Increased circulating levels of free fatty acids may impair endothelial function and induce low-grade inflammation. , Hyperinsulinemia augments VLDL synthesis in liver; increases cholesterol transport/synthesis in cultured arterial SMCs; stimulates the proliferation of arterial SMCs; augments collagen synthesis and turns on multiple genes involved in inflammation. , Although insulin receptors are expressed on endothelial cells, vascular SMCs, and macrophages, it remains unclear whether the vascular insulin receptors contribute directly to the vascular pathology of metabolic insulin resistance.
A subset of insulin-resistant individuals develop non-insulin-dependent diabetes mellitus (NIDDM), characterized by an insufficient insulin secretory capacity to maintain normal glucose levels. A genome scan identifies a major NIDDM susceptibility locus, designated NIDDM1, on chromosome 2q. Using linkage disequilibrium mapping, scientists have shown that an intronic polymorphism in the calpain-10 gene is strongly associated with the disease. A second locus for diabetes has been identified on chromosome 1q. It is interesting to note that the chromosome 1 locus was initially linked with familial combined hyperlipidemia (FCH) in a set of Finnish families, and that FCH overlaps with diabetes.
Advances in genotyping technology have facilitated rapid progress in large-scale genetic studies. Genome-wide associated studies (GWAS) and linkage analyses have identified greater than 120 genetic variants associated with T2D and diabetes-related traits. Most of these variants are noncoding variants; therefore, their functional consequences are challenging to investigate. However, many of the variants identified to date regulate insulin secretion and not insulin action in insulin-sensitive tissues.
Although some studies have suggested that obesity is not an important contributing cause of atherosclerosis and CAD, , recent evidence indicates that obesity is a major contributor to CVD risk and mortality. Obesity exhibits considerable metabolic overlap with the abnormalities present in individuals with T2D and is associated with other cardiovascular factors such as hypertension, hyperlipidemia, and insulin resistance. ,
Obesity is a complex disease resulting from the interaction of a wide variety of hereditary and environmental factors. Clustering of cases within a family, the congruence of body weight in monozygotic twins, and the discovery of genes associated with obesity all suggest genetic involvement in obesity. Obesity risk is 2–8 times higher for a person with a family history as opposed to a person with no family history of obesity, and an even higher risk is observed in cases of severe obesity. Occurrences of monogenic types of obesity are evidence that obesity may be caused by genetic mutations. Eight monogenic genes and four polygenic genes have been associated with obesity. The cloning of the mouse ob gene and its human homologue, leptin, has proved to be a paradigm for the field, resulting in the identification of many genes involved in the regulation of appetite, food intake, and energy expenditure via the leptin–melanocortin pathway ( Fig. 15.4 ). These variants account for some 5% of morbid human obesity and include leptin and its receptor, the α-melanocortin-stimulating hormone receptor (MC4R), , pro-opiomelanocortin (POMC), and prohormone convertase-1. Moreover, the association of additional genes with obesity has recently been identified by GWAS ( SH2B1, KCTD15, MTCH2, NEGR1, and BDNF ) with dietary intake and nutrient-specific food preferences. This is in line with the fact that food intake-related parameters are heritable and are strongly correlated with body mass index (BMI).
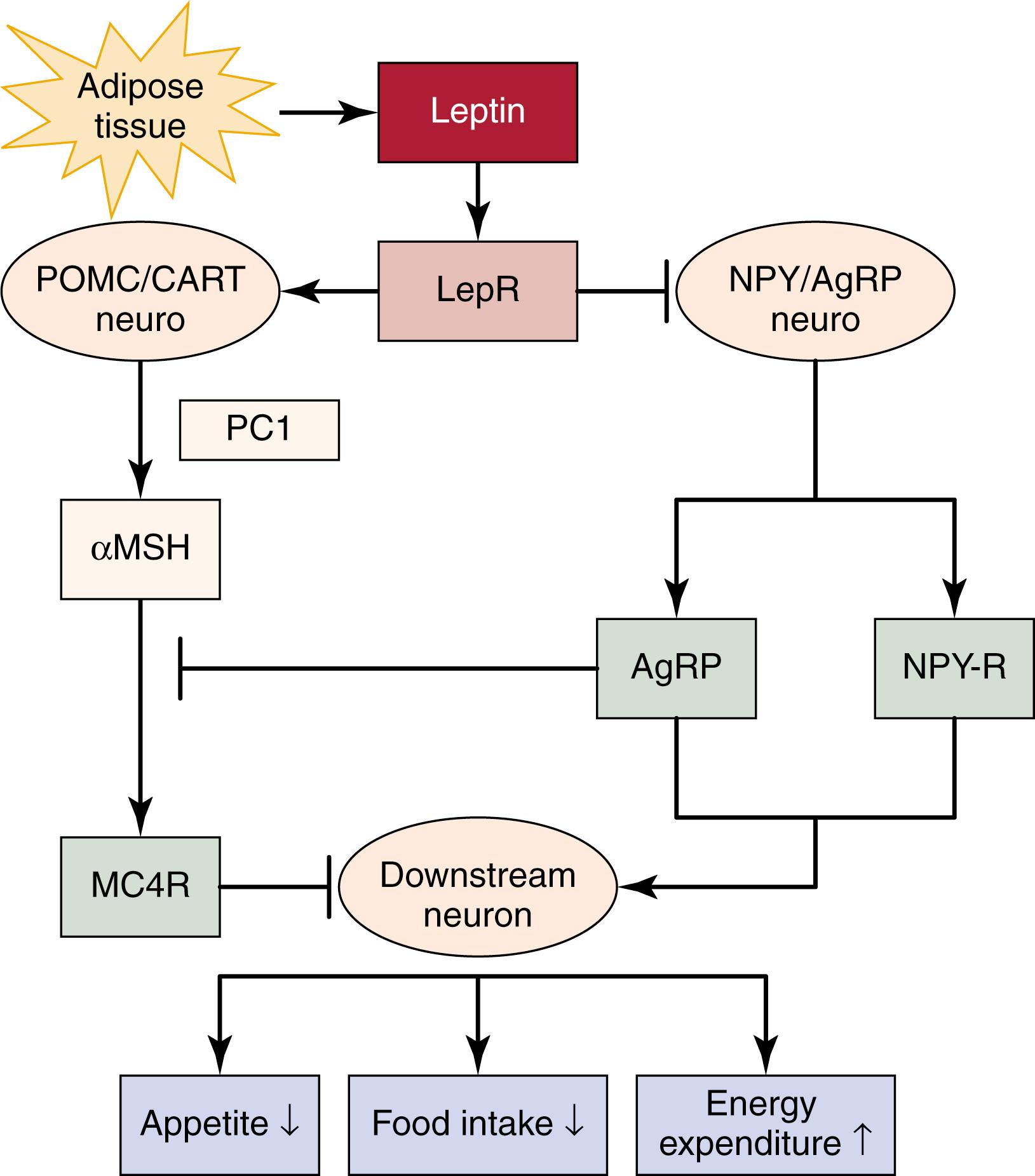
Other genes such as fat mass and obesity-associated (FTO); PCSK1; and catenin, beta-like 1 (CTNNBL1) are also associated with obesity. In addition, more than 150 loss-of-function coding mutations have been associated with monogenic obesity, and two infrequent gain-of-function coding polymorphisms (V103I and I251L) have been associated with protection from obesity. ,
PAD is a disease characterized by reduced blood flow to the lower extremities, most often because of atherosclerosis and can be considered a marker of widespread atherosclerosis. However, PAD is a distinct subtype of atherosclerotic vascular disease that differs from CAD and CVD in its clinical manifestations. Thrombus formation resulting from acute rupture or erosion of a vulnerable plaque in the coronary or cerebral arterial beds leads to acute events such as myocardial infarction (MI) or stroke. Such acute events are relatively uncommon in PAD, and symptoms most often result from progressive arterial narrowing because of ongoing atherogenesis. The underlying reasons for the differences remain unknown. It is therefore likely that risk factors, both genetic and environmental, and the intermediate biochemical pathways through which they act contribute differently to PAD than to CAD or CVD. Although traditional cardiovascular risk factors have been associated with the development of PAD, relatively few genetic variants that influence susceptibility to PAD have been discovered. This may be partly because of the greater clinical and genetic heterogeneity in PAD. Phenotypic heterogeneity seems to be a major challenge in investigating the genetic basis of PAD.
PAD is complex and heterogeneous; it is not a uniform entity. Two broad subtypes of PAD, proximal and distal, are associated with distinct risk factor and comorbidity profiles. Female sex, smoking, hypertension, and dyslipidemia are more significantly associated with proximal disease, whereas older age, male sex, and diabetes are more significantly associated with distal, small vessel disease. The candidate genes include β-fibrinogen , apoB , endothelial nitric oxide synthase (eNOS), methylene tetrahydrofolate reductase , G protein β3, α-adducin , IL-6 , and glutathione S-transferase . However, any reported associations between variants in these genes and PAD have not been confirmed in independent cohorts or in GWAS. Moreover, genetic analyses do not appear to be as useful as in CAD in determining progression of the disease or its tendency to remain stable.
A linkage analysis using a 10-cM genome-wide scan in 272 PAD patients from 116 extended families has identified a region on chromosome 1 between 100 and 110 cM (logarithm of the odds score, 3.93; P = 1.04 × 10 −5 ) associated with PAD. Several candidate genes (in pathways of inflammation, coagulation, lipid metabolism, blood pressure regulation, and vascular matrix regulation) for atherosclerosis are present under the linkage signals, but the causal variants cannot be identified. Association studies using the GWAS approach identified only a few loci having weaker associations with PAD.
Similar to CAD, several Mendelian disorders are associated with PAD. These include familial lipoprotein disorders, such as chylomicronemia resulting from mutations in the lipoprotein lipase gene and FH, , hyperhomocysteinemia, and pseudoxanthoma elasticum. In a family history study including 2296 PAD cases and 4390 controls, the prevalence of a family history of PAD was significantly higher among patients with PAD than in controls, resulting in a doubling of the odds of the presence of PAD in those with family history of PAD. The association is stronger in individuals less than 68 years of age and in those with a greater number of affected relatives. In another study conducted in a twin population, the odds ratio of having PAD among individuals whose twins had PAD compared with individuals whose twins did not have PAD was 17.7 for monozygotic twins and 5.7 for dizygotic twins.
Although the critical roles of genetic factors in atherosclerotic vascular disease have increasingly been recognized, the specific etiology in many cases remains unknown, with nongenetic risk factors believed to be important contributors. It is now generally considered that atherosclerosis results from a variety of genetic causes, with added epigenetic modulation and the interaction of genetic and environmental factors. None of these triggers is sufficient to cause atherosclerosis on its own.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here