Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The fallopian tube or salpinx is a tubular hollow structure measuring 11–12 cm in length that runs throughout the apex of the broad ligament and spans the distance between the uterine cornus and the ovary. It is divided into four segments: intramural (inside the uterine wall), isthmus (2–3 cm, thick-walled), ampulla (a thin-walled expanded area), and infundibulum (a trumpet-shaped ending that opens into the peritoneal cavity through the ostium and is fringed by the fimbriae ). One of the latter structures, known as the ovarian fimbria , attaches the tube to the ovary.
The inner aspect of the tube is lined by mucosa arranged in the shape of longitudinal, branching folds (known as plicae ), which merge with the fimbriae. There is good correlation between the microscopic features of this mucosa and its salpingoscopic appearance. Microscopically, the epithelium is composed of three distinct cell types: secretory , ciliated , and intercalated (peg) . Amylase is secreted by the epithelium, and its presence can be demonstrated immunohistochemically. Endocrine cells have been found only exceptionally. A low degree of proliferative activity takes place normally in the tubal epithelium, more so during proliferative/follicular phase of the menstrual cycle, compared to the secretory/luteal phase. At the time of menstruation, as well as a few days postpartum, the tubal mucosa may be normally infiltrated by neutrophils, probably as a reaction to blood and necrotic debris (“ physiologic salpingitis ”); it is accompanied by negative cultures and should not be confused with a bacterial or other infectious salpingitis.
The muscular wall (myosalpinx) is composed of an inner circular layer and an outer longitudinal layer; the isthmus near the uterotubal junction also possesses an inner longitudinal layer.
The lymphatics of the fallopian tube leave the tubal wall within the mesosalpinx, where they join efferent lymphatics from the ovary and uterus and follow the ovarian vessels to terminate in the aortic lymph nodes. Other lymphatics course within the broad ligament and drain into the interiliac nodes; a separate lymphatic channel from the ampulla of the tube follows the broad ligament to terminate in the superior gluteal lymph nodes.
The broad ligament is the peritoneal fold that supports the uterus on either side, extending from this organ to the pelvic wall. It comprises the mesovarium, mesometrium, and mesosalpinx. The broad ligament and the adjacent areas contain a variety of tubular structures related to the müllerian and wolffian systems that may give rise to grossly evident cysts. Most of these are lined by müllerian-type epithelium and have been variously referred to—depending on their specific location and presumed histogenesis—as parovarian cyst , paratubal cyst (hydatid of Morgagni) , subserosal müllerian cyst , Kobelt cyst (appendix vesiculosa) , paroöphoron cyst , epoöphoron cyst , and rete ovarii cyst. Walthard cell nests , which can also be cystic, are of a different (probably mesothelial) nature.
The round ligament is a fibrous cord attached to the superior lateral border of the uterus that passes over the external iliac vessels and the inguinal ligament to leave the abdominal cavity through the deep inguinal ring. From there, it follows the inguinal canal to anchor itself in the labium majus. It serves to orient the uterus by the fact that its insertion is anterior to that of the fallopian tube.
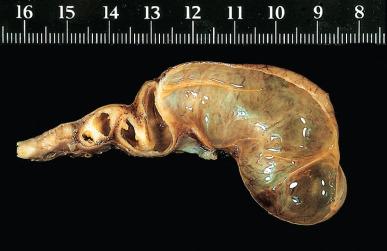
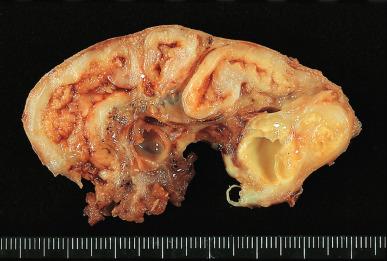
Bacterial infection of the fallopian tube is a common disease, and its incidence keeps increasing. It may follow invasive procedures (such as curettage or the insertion of intrauterine devices ), and it may accompany endometriosis, but in most cases it is due to an ascending infection, often sexually transmitted ( Fig. 34.1 ). The inflammation may result in fusion of tubal plicae and obliteration of the ostium ( Fig. 34.2A ). Obstruction of the fimbriated end and, less commonly, of the intramural or isthmic portion leads to tubal infertility. Microscopically, the trapped epithelial spaces produce a complicated gland-like pattern that can simulate malignancy (see Fig. 34.2B ).
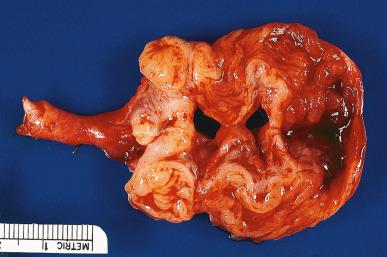
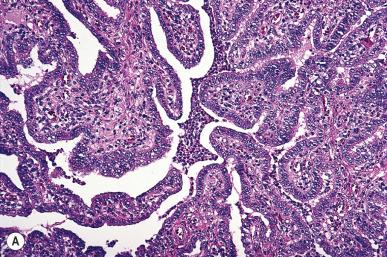
The lumen is often distended and filled with secretions or pus ( pyosalpinx ) ( Fig. 34.3 ). Massive intraluminal hemorrhage may lead to the formation of hematosalpinx , a rare condition that needs to be distinguished from the much more common form secondary to ruptured tubal pregnancy. In chronic inflammatory cases, the tubal wall is markedly fibrotic, and serosal adhesions are prominent. The inflammatory exudate often spreads to the ovary, resulting in the formation of a tubo-ovarian abscess and obliteration of the pelvic anatomic relationships ( Fig. 34.4 ). The rupture of such an abscess may lead to localized or generalized peritonitis, and it is recommended that fluid collections of greater than 3 cm be drained laparascopically. Hydrosalpinx is generally regarded as the end stage of a purulent salpingitis in which pus has been reabsorbed and replaced by a transudate of plasma. The external gross appearance has been likened to that of a retort ( Fig. 34.5 ). Exceptionally, the involvement is limited to the intramural portion of the tube. The wall is thin and fibrotic, with atrophy or even disappearance of the smooth muscle wall. The epithelium is flat and focally absent.
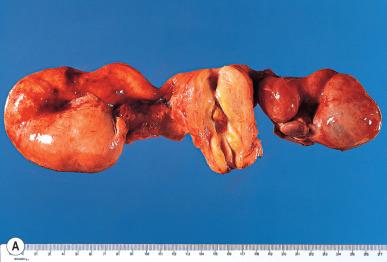
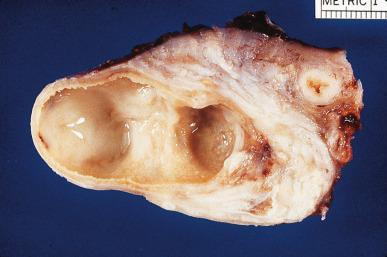
Pelvic inflammatory disease (PID) is the generic term used for inflammatory processes of this region in which the fallopian tube is the epicenter of the inflammation. Neisseria gonorrhoeae and chlamydia account for most cases. Polymicrobial tuboperitoneal infection (resulting from Bacteroides fragilis , peptostreptococci, peptococci, and other organisms) is responsible for most of the remaining cases. In established tubo-ovarian abscesses, the most common agents recovered are coliform organisms. N. gonorrhoeae was isolated only once in a series of 93 cases by Mickal et al. This finding, however, does not rule out its role as a possible initiating factor before superinfection has occurred, in view of the fact that isolation of N. gonorrhoeae is inversely proportional to the number of episodes of salpingitis.
Tuberculosis of the tube develops by the hematogenous route. Most patients are young, and infertility is common. In advanced cases, both tubes are replaced by caseous tuberculous masses ( Fig. 34.6 ). There is often extreme proliferation of the tubal mucosa in association with granulomatous inflammation, and this may lead to a mistaken diagnosis of carcinoma. The endometrium is concomitantly involved in approximately 80% of cases.
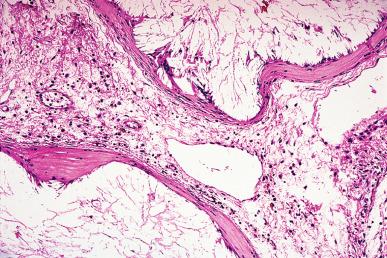
Granulomatous inflammation of the tubes can also be produced by Schistosoma , Oxyuris vermicularis , Actinomyces , Coccidioides immitis , and other organisms. Sarcoidosis and Crohn disease may be accompanied by tubal involvement.
Foreign bodies introduced for diagnostic or therapeutic measures can induce a bizarre granulomatous response ( Fig. 34.7 ). Reaction to the lipiodol that used to be introduced in the Rubin test may be so proliferative as to resemble a neoplasm. The push of a uterine sound may drive lubricant into the tube, causing lipoid granulomas .
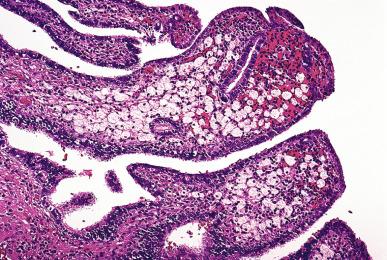
Xanthogranulomatous (pseudoxanthomatous) salpingitis is characterized by an expansion of the tubal plicae by an infiltrate of foamy histiocytes ( Fig. 34.8 ). The distinction some authors make between xanthogranulomatous and pseudoxanthomatous salpingitis is probably unwarranted. Some cases may be due to endometriosis, and, therefore, the term “salpingitis” may be inaccurate. Accordingly, some authors refer to it as pseudoxanthomatous salpingiosis .
Giant cell arteritis is occasionally found in the tubes, ovaries, and uterus of postmenopausal patients, either as an isolated finding (most frequently) or as a manifestation of a generalized immune-mediated disease.
Torsion of the fallopian tube and ovary is usually secondary to inflammation or tumor, but occasionally it develops in a previously normal organ, the appearance at surgery being that of a hemorrhagic infarct. This phenomenon can occur in adults, as well as in infants and children. In children, torsion of the normal adnexa is about one-third as common as torsion of an ovarian cyst or tumor. If the operation (which can be carried out laparoscopically) is done early enough, untwisting of the adnexa may lead to full recovery. Without surgical intervention, cases may resolve spontaneously or result in a necrotic and calcified mass, which may later detach from the uterus. It has been suggested that this complication, when occurring in previously normal adnexa, is due to the fact that the infundibulopelvic ligament extends along the edge of the ovarian ligament so that the tube and ovary hang on a very narrow stalk.
The incidence of tubal pregnancy has increased in recent times. It is often the consequence of chronic salpingitis, which leads to inflammatory destruction of the lining folds and retention of the ovum. Congenital tubal abnormalities, functional tubal disturbances, and salpingitis isthmica nodosa are responsible for a minority of the cases. A history of infertility is associated with an increased risk of tubal pregnancy.
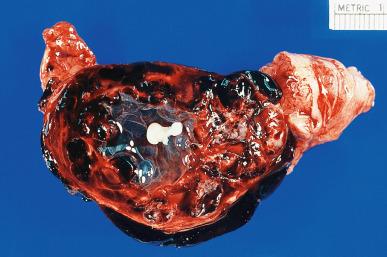
In tubal pregnancy the gestational sac is completely made up of tubal tissue, with no participation from the ovarian or intraligamentary tissues. Following implantation of the ovum in the tubal epithelium (usually in the ampullo-isthmic or midtubal portion), chorionic villi and extravillous (intermediate) trophoblasts can grow predominantly intraluminally or penetrate into the wall, just as they do in the uterus, except that the wall here is much thinner. Trophoblastic invasion of muscle and vessels is a common finding of no clinical significance. Hydropic changes and polar trophoblastic proliferation can occur; they should not be overdiagnosed as hydatidiform mole. Changes resembling atherosclerosis may be seen in tubal arteries at the site of implantation, analogous to those occurring in the uterus in orthotopic pregnancy. The fallopian tube epithelium may undergo clear cell hyperplasia. Although a few tubal pregnancies have gone to term, the usual outcome is abortion. The maternal vessels rupture into the gestational sac and cause hematosalpinx ( Fig. 34.9 ). In the presence of a large hematosalpinx, it may be difficult to identify the products of gestation; numerous blocks from the intratubal blood clot should be taken for this purpose. Tubal rupture can occur (usually near the end of the second month) because of destruction of the tubal wall by the invading trophoblast, and this may result in severe intra-abdominal hemorrhage. This rupture may result in a brisk reactive proliferation of the mesothelium, with formation of papillae and psammoma bodies. These changes should be recognized as reactive and not misinterpreted as metastases or implants from an ovarian serous neoplasm. The necrotic trophoblastic tissue may be retained for a long time and appear as hyalinized ghost outlines of chorionic villi. On occasion, the appearance of these retained tissues corresponds to that of so-called placental site nodule , as described in the uterine corpus.
The usual treatment for tubal pregnancy is salpingectomy or laparoscopic salpingostomy (evacuation of the tubal implantation with preservation of the tube). Conservative laparoscopic procedures for tubal ectopic pregnancy can result in extratubal trophoblastic implants accompanied by persistent beta human chorionic gonadotropin (β-hCG) postoperative serum titers. Exceptionally, omental deposits of fetal parts are seen as a sequela of salpingectomy for ruptured ectopic pregnancy.
Uterine curettings in the presence of a viable tubal pregnancy show gestational endometrium, sometimes associated with the Arias-Stella reaction. When trophoblastic elements are not obvious in routinely stained sections from the curettings, some authors have suggested the utility of immunohistochemical stains for hCG, human placental lactogen (hPL), and keratin in the confirmation of an intrauterine pregnancy; however, we have not found this useful in routine practice. After death of the embryo or fetus there may be expulsion of the endometrial decidual cast, regeneration of the epithelium, and reestablishment of the cyclic pattern. Therefore, the presence of a proliferative, secretory, or menstrual endometrium in a patient with an adnexal mass does not rule out the possibility of an ectopic pregnancy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here