Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cutaneous cancers that arise from the epidermis, dermis, or adnexal structures of the eyelid. Rarely, they may be metastatic from distant sites. They include a number of histologically distinct tumors from diverse skin cell types.
Flat, eroded, or elevated lesion on the eyelid margin, eyelid skin, or brow.
Nodular and well circumscribed or irregular with indistinct borders.
Ulcerated with a central crater or benign in appearance with some telangiectatic vessels.
Slow, generally painless growth.
Dilated blood vessels.
Ectropion from skin contracture.
Firm induration.
Loss of eyelashes.
Palpable preauricular nodes.
Proptosis.
Ptosis.
Restricted ocular motility.
Thickened eyelid margin.
Malignant lesions are common around the eyes, partly because many are induced by sun exposure or develop from sun-related benign lesions. Typically, most of these are small and grow slowly, which results in minimal concern for the patient and a low index of suspicion for the physician. Although the most common malignancies rarely metastasize, they all can be very destructive locally. Any periocular lesion that shows some growth, especially when associated with chronic irritation or bleeding, should undergo a diagnostic biopsy. Confirmation of histopathology is mandatory before the patient is committed to a major resection or reconstructive procedure.
Basal cell carcinoma (BCC) is a malignant tumor derived from cells of the basal layer of the epidermis. The etiology is linked to excessive ultraviolet light exposure in fair-skinned individuals. Other predisposing factors include ionizing radiation, arsenic exposure, and scars. Although metastases are rare, local invasion is common and can be very destructive.
BCC is the most common malignant tumor of the eyelids and constitutes 85%–90% of all malignant epithelial eyelid tumors at this site. Over 99% of BCCs occur in white people; about 95% of these lesions occur between the ages of 40 and 79 years, with an average age at diagnosis of 60 years. Rarely, they also may be seen in children. BCC arises from a pluripotential stem cell in the epidermis that proliferates, amplifies, and eventually terminally differentiates. Proposed mechanisms for BCC invasion include enhanced tumor cell motility and collagenase content. Having had one BCC is a prognostic factor for the development of additional lesions.
Up to 50%–60% of BCCs affect the lower eyelid. The medial canthus is involved 25%–30% of the time. The upper eyelid is involved nearly 15% of the time, and the lateral canthus is only rarely involved (5%). On the basis of their histopathological presentation, BCCs may be classified into six basic types:
Nodular-ulcerative
Pigmented
Morphea or sclerosing
Superficial
Fibroepithelioma
Infundibulocystic
Micronodular and basosquamous are two additional descriptors with primarily histopathological as well as therapeutic and prognostic significance.
The nodular type of BCC, the most common lesion, has the classical appearance of a pink or pearly papule or nodule with overlying telangiectatic vessels. As the nodule grows in size, central ulceration may occur, surrounded by a rolled border ( Fig. 12.8.1 ). This appearance is often described as a “rodent ulcer.”
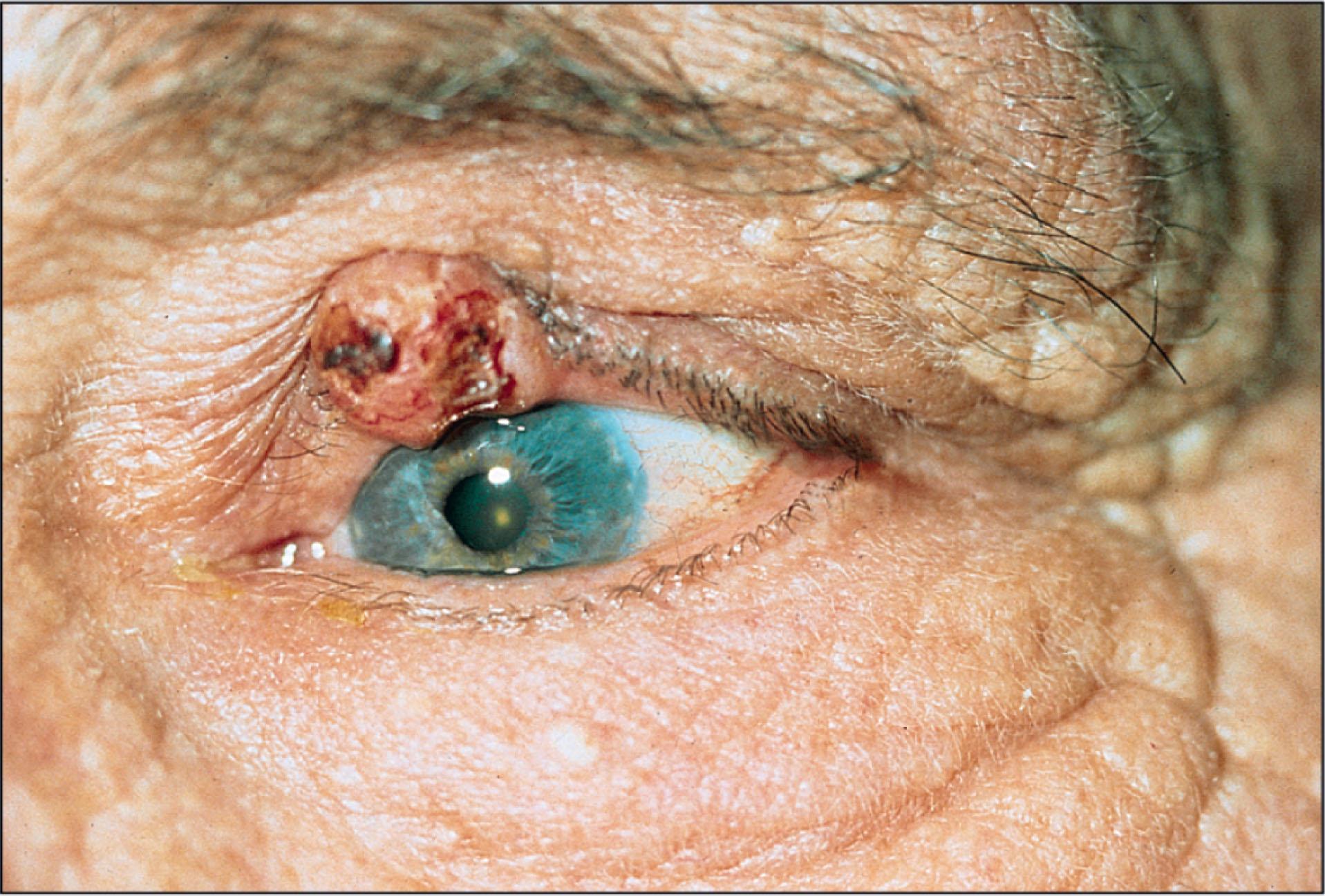
The pigmented BCC is similar to the noduloulcerative type in morphology but with brown or black pigmentation. These lesions represent the most common pigmented malignancy on the eyelids and may resemble malignant melanoma.
The morphea or sclerosing type of BCC appears as a flat, indurated, yellow–pink plaque with ill-defined borders. It may simulate a blepharitis or dermatitis. Because it has a flat appearance, it may not be as clinically noticeable as others. However, this form of BCC is aggressive and may invade the dermis deeply. It characteristically occurs in the medial canthal region and may invade the paranasal sinuses and orbit.
Superficial BCC appears as an erythematous, scaling patch with a raised pearly border. Fibroepithelioma BCC presents as a pedunculated or sessile smooth, pink nodule and primarily arises on the trunk rather than the eyelid.
Infundibulocystic BCC appears as a well-circumscribed pearly papule commonly found on the head and neck of the elderly. Basosquamous types represent well-defined nodular or superficial BCC overlying an invasive front showing BCC as well as squamous cell carcinoma histological features. Micronodular BCC presents as an erythematous macule or thin papule and histologically presents as multiple small aggregates of basaloid cells within the dermis.
The diagnosis of BCC is initially made from its clinical appearance, especially with the noduloulcerative type with its raised pearly borders and central ulcerated crater. Definitive diagnosis, however, can be made only on histopathological examination of biopsy specimens.
The differential diagnosis of BCC and of other periocular malignant lesions may be divided into several categories: other malignant lesions, premalignant lesions, benign adnexal tumors and cysts, and inflammatory and infectious conditions ( Table 12.8.1 ; see Chapter 12.14 ). In many cases the diagnosis depends on histopathology.
| Simulating Lesion | BCC | SCC | SGC | MM |
|---|---|---|---|---|
| Basal cell carcinoma | X | X | ||
| Malignant melanoma | X | |||
| Sebaceous cell carcinoma | X | X | ||
| Squamous cell carcinoma | X | X | ||
| Squamous cell carcinoma in situ | X | X | ||
| Actinic keratosis | X | X | ||
| Radiation dermatitis | X | |||
| Keratoacanthoma | X | X | ||
| Cavernous hemangioma | X | |||
| Cutaneous horns | X | |||
| Dermoid and sebaceous cysts | X | |||
| Eccrine and apocrine cysts | X | |||
| Inverted follicular keratosis | X | |||
| Nevus cell and nevocellular nevi, pigmented lesions of epidermal and dermal melanocyte origin | X | |||
| Papillomatous lesions | X | X | X | |
| Pseudoepitheliomatous hyperplasia | X | |||
| Seborrheic keratosis nevus | X | X | ||
| Trichilemmoma | X | |||
| Blepharitis | X | X | ||
| Chalazion | X | X | ||
| Eczema | X | |||
| Fungal infections | X | |||
| Hordeolum | X | |||
| Psoriasis | X | |||
| Seborrheic dermatitis | X | |||
| Superior limbic keratoconjunctivitis | X | |||
| Verruca vulgaris | X | |||
| Conjunctival hemorrhage | X |
Basal cell nevus syndrome (Gorlin–Goltz syndrome) is inherited as an autosomal-dominant disorder with high penetrance and variable expressivity. Basal cell nevus syndrome is rare, occurring in less than 1% of individuals with BCC. The group of clinical findings described in 1960 as a syndrome by Gorlin and Goltz includes the following:
Multiple BCCs affecting the face, trunk, and extremities
Cysts of the jaw (odontogenic keratocysts)
Skeletal abnormalities (e.g., bifid ribs)
Neurological abnormalities (e.g., mental retardation, ectopic calcification, cerebellar medulloblastoma)
Endocrine disorders (e.g., ovarian cysts and testicular disorders)
Palmar and plantar pits also develop in young adulthood. The BCCs in this syndrome typically develop at puberty and have a predilection for the periorbital region and face. Multiple lesions occur with a high rate of recurrence. Other rare BCC syndromes include Bazex syndrome, linear unilateral basal cell nevus, and Rombo syndrome.
Also, BCC may be associated with albinism, xeroderma pigmentosum, and nevus sebaceous.
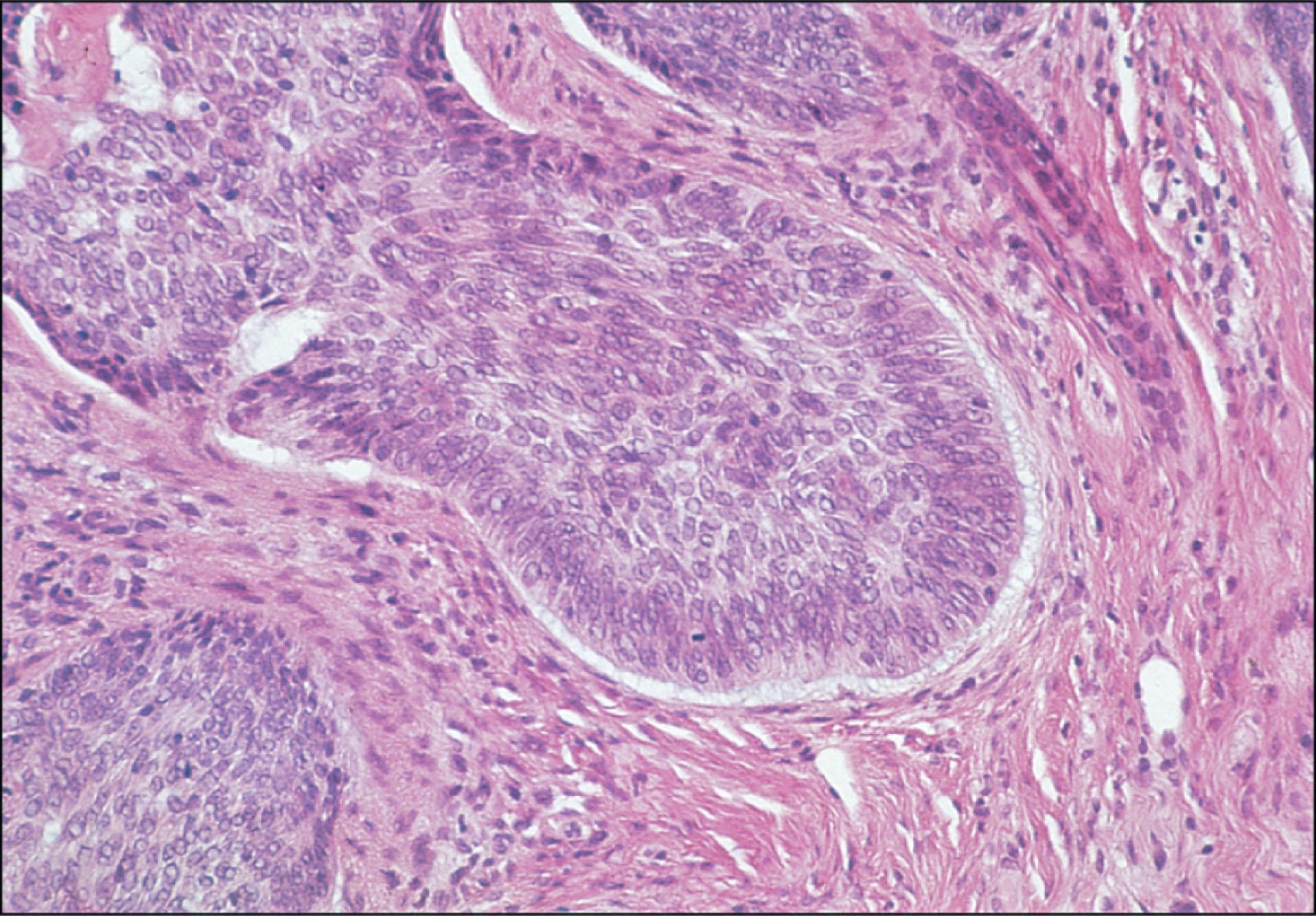
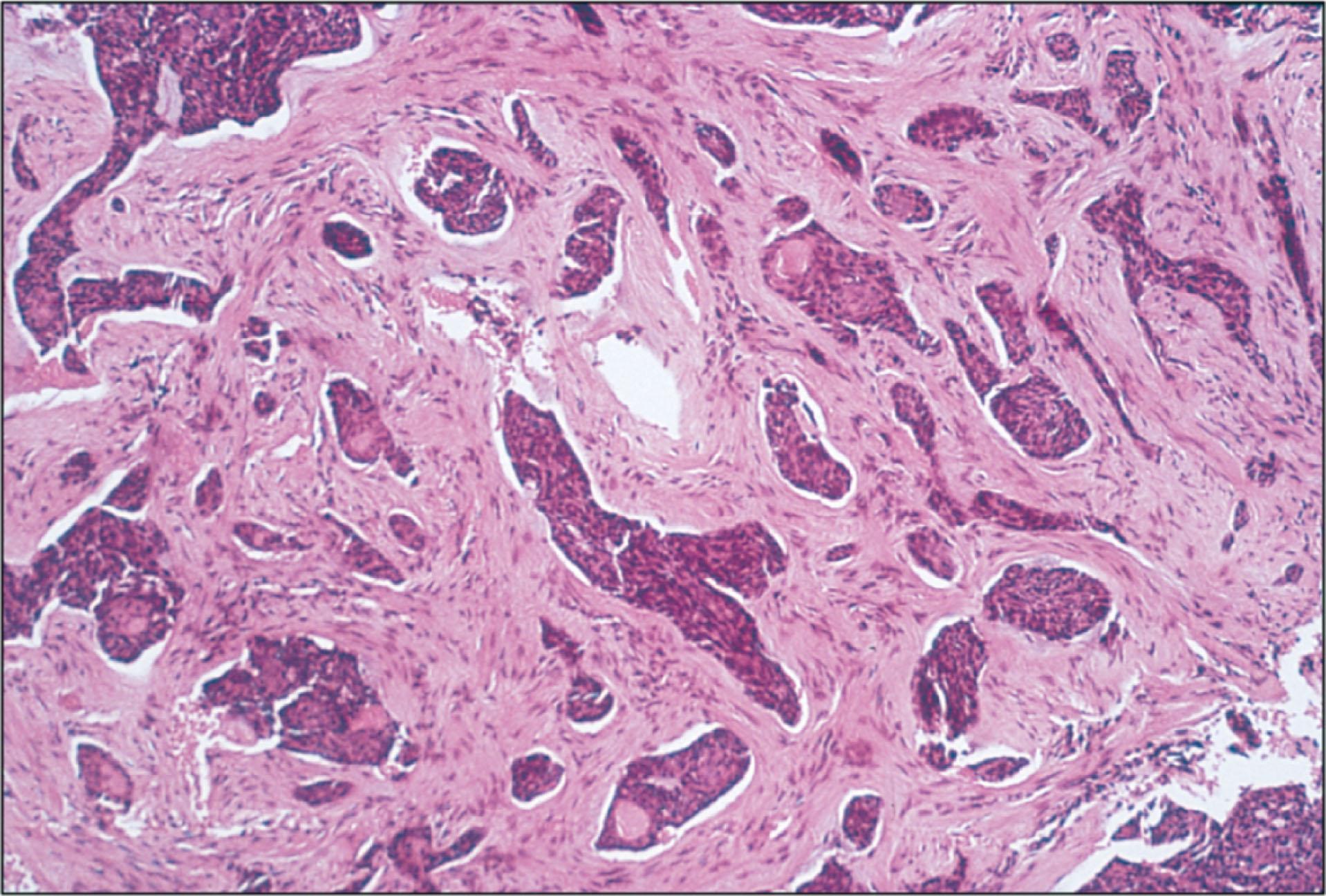
The BCCs may be grouped as either undifferentiated or differentiated by their histopathological appearance. The typical histopathology of an undifferentiated BCC consists of nests, lobules, and cords of tumor cells with peripheral palisading of cells and stromal retraction ( Fig. 12.8.2 ). Undifferentiated BCCs include the solid noduloulcerative, morphea or sclerosing, pigmented, superficial, and fibroepithelioma forms. The morphea or sclerosing form is characterized by strands of proliferating, malignant basal cells in a fibrous stroma ( Fig. 12.8.3 ).
The adenoid and metatypical or basosquamous are the most common differentiated forms. These tumors differentiate toward glandular structures with mucinous stroma. They exhibit morphological features between those of basal cell and squamous cell carcinoma. The metatypical BCCs are more aggressive and invasive, with a higher recurrence rate and potential for metastasis.
The goal of therapy is the complete removal of tumor cells with preservation of unaffected eyelid and periorbital tissues. Although nonsurgical treatments such as cryotherapy, electrodesiccation, and laser ablation are advocated by some, surgical therapy is the generally accepted treatment of choice for the removal of BCCs. Some BCCs, especially the morphea and multicentric types, may extend far beyond the area that is apparent clinically. Recurrences are generally more aggressive, infiltrative, and destructive than the primary tumor. Therefore histological monitoring of tumor margins is essential. Mohs’ micrographic surgery and excisional biopsy with frozen section control are the two basic techniques available. An incisional biopsy may be performed before definitive treatment to confirm the clinical suspicion of BCC.
Mohs’ micrographic surgery provides the highest cure rate with the most effective preservation of normal tissue. Tissue is excised in layers that provide a three-dimensional mapping of the excised tumor. These layers are processed as frozen sections and viewed under the microscope. Any areas of residual tumor are identified, and the map is used to direct additional tumor excision. This technique is particularly useful for morphea and multicentric-type BCCs in the medial canthal region, which may exhibit subclinical extension to orbital bone or sinuses. Mohs’ micrographic surgery technique is somewhat limited if the tumor has extended to the plane of orbital fat. In addition, it requires the collaboration of a Mohs’ surgeon trained in both the surgical technique and interpretation of the dermatopathology.
Excisional biopsy with frozen section control is also an effective way to remove BCCs and can be performed by the ophthalmologist. Several studies have reported no recurrences after excision of BCCs with frozen section monitoring. However, following simple excisional biopsy without frozen section control, recurrence rates of up to 50% have been reported.
Eyelid reconstruction should be performed within 2–3 days after tumor excision. Various reconstructive surgical techniques may be used, depending on the location and size of the residual defect.
Radiation therapy is generally not recommended in the initial treatment of periocular BCCs. However, it may be useful in the treatment of advanced or recurrent lesions in the medial canthal region or elsewhere. Doses are in the range of 4000–7000 cGy. Radiation therapy is less effective in treating morphea BCCs, with the likelihood of BCC recurrence following radiotherapy being higher than that for the previously described surgical techniques. A recurrence rate of 12% was noted in one series following radiation therapy. Surgical management is very difficult after radiation treatment of an affected area. Radiotherapy complications include skin atrophy and necrosis, madarosis, cicatricial entropion and ectropion, dry eye syndrome, cataract, and corneal ulceration. Radiation therapy is contraindicated in basal cell nevus syndrome and is associated with significant complications in patients who have scleroderma or acquired immunodeficiency syndrome (AIDS).
Cryotherapy is often used to treat BCCs outside the periorbital area but also has been shown to be effective for eyelid tumors, with a recurrence rate of less than 1%. Around the eyelids, it may result in eyelid notching and malpositions, symblepharon formation with fornix foreshortening, and pigmentary changes. It is associated with a higher recurrence rate than the surgical approaches. Cryotherapy is contraindicated in lesions greater than 1 cm in diameter, medial canthal lesions, morphea-like lesions, and recurrent BCC.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here