Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Blood reaches the cortex through larger arterioles, then disperses through the bulk of the tissue through capillaries, and then coalesces back into venules to exit the brain tissue. Much of the gas and nutrient exchange occurs at the capillary level, so that understanding blood flow at this scale is important for both normal and disease physiology. Advancements in optical microscopy now enable researchers to assess flow in even the tiniest vessels of the cerebrovasculature. The smallest capillaries are just large enough to let red blood cells squeeze through (about 3 μm diameter in mice) so that they can be visualized easily by optical methods. In brain, however, most of the vessels are in the depth of the tissue, so it was not until the development of two-photon microscopy that studies of blood flow in the capillaries became common . Since then, several other methods have been developed that can image with sufficient depth and spatial resolution to be useful in studies of capillary structure and function. Two-photon microscopy (also known as multiphoton microscopy, two-photon excited fluorescence microscopy, and two-photon laser scanning microscopy) has become a powerful experimental tool for in vivo studies of not only blood flow, but also many other cellular measurements, and hence it is commonly available. This chapter will describe some of the experimental and measurement techniques used to measure capillary blood flow, focusing on two-photon approaches.
In models including mice and rats, two-photon microscopy enables imaging of fluorescent labels with better than 1-μm spatial resolution deep in semiopaque tissues such as the brain. For in vivo studies, fluorescent labels can be endogenous or exogenous dyes, but many studies take advantage of the specificity of using genetic strategies to label particular cells or structures. For brain, a window of either glass or merely thinned bone is produced above the region of interest. The easiest way to label the blood vessels is an intravenous injection (usually in rodents via the tail or saphenous vein or retro-orbitally) of dextran-conjugated dyes ( Fig. 71.1A ) . The length of time the dye stays in the circulation depends on the size of the dextran; 50–100 μL of 5% w/v dye conjugated to a dextran of 70 kDa dissolved in saline lasts several hours in mice. Dextran-conjugated dyes are available in many colors, so it is possible to choose a color that does not overlap other indicators in the experiment. In adult brain, normal blood vessels, including individual capillaries, are anatomically stable for many months. Because the pattern of branches is unique, the vessels provide a convenient way of repeatedly finding the same capillaries, neurons, or other cells. One can start with the large vessels, which are easy to see on the brain surface, and follow the branches down to specific capillaries reliably across weeks to months of imaging (analogous to following a city map to find a specific address).
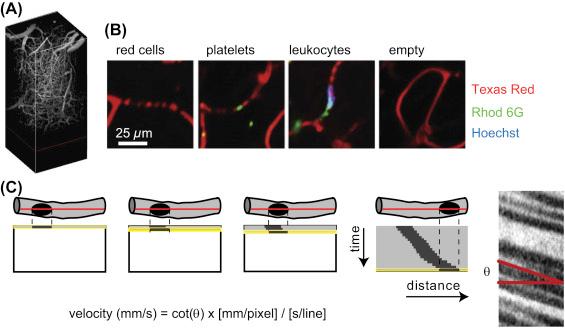
Circulating cells, including red and white blood cells, do not take up the dextran-conjugated dye, so they appear as dark patches within the vessels ( Fig. 71.1B ). Previous studies have also used red blood cells that were extracted, labeled in vitro, and reinjected, but this complicated procedure is not necessary with two-photon imaging. Since red blood cells are the vast majority of cells in circulation, most blood flow speed measurements described here rely on the red blood cells. In fact, the occasional white blood cell often temporarily decreases the blood flow speed in a capillary, but no extra label needs to be added to see this effect because these cells also show up as dark regions against the fluorescent dye.
Other fluorescent labeling techniques can also be used to label additional types of cells. Transgenic mice may have monocytes or other cells labeled by fluorescent protein expression. In some cases, labeled cells are taken from a donor animal and injected into a recipient in adoptive transfer. Several exogenous indicators also provide convenient ways to label particular cell types ( Fig. 71.1B ). An intravenous injection of rhodamine 6G (0.05 mL of 0.1% w/v per mouse) labels several types of white blood cells, including monocytes, neutrophils, and some lymphocytes, as well as platelets . The DNA-binding dye Hoechst 33342 (0.05 mL of 0.5% w/v per mouse) can also be injected in the circulation with little effect in healthy animals. It will not label cells in the healthy brain because it does not cross the blood–brain barrier, but the nuclei in circulating cells such as white blood cells will fluoresce at blue wavelength. Since platelets do not have nuclei, Hoechst will not label platelets and provides a convenient way to distinguish thrombi in capillaries from leukocyte plugs when using rhodamine 6G .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here